Abstract
Objective
Eating earlier in the daytime to align with circadian rhythms in metabolism enhances weight loss. However, it is unknown whether these benefits are mediated through increased energy expenditure or decreased food intake. We therefore performed the first randomized trial to determine how meal timing affects 24-hour energy metabolism when food intake and meal frequency are matched.
Methods
Eleven overweight adults practiced both early time-restricted feeding (eTRF; eating from 8 am-2 pm) and a control schedule (eating from 8 am-8 pm) each for four days. On the fourth day, 24-hour energy expenditure and substrate oxidation were measured by whole-room calorimetry, in conjunction with appetite and metabolic hormones.
Results
eTRF did not affect 24-hour energy expenditure (Δ=10±16 kcal/day; p=0.55). Despite the longer daily fast (intermittent fasting), eTRF decreased mean ghrelin levels by 32±10 pg/ml (p=0.006), made hunger more even-keeled (p=0.006), and tended to increase fullness (p=0.06-0.10) and decrease the desire to eat (p=0.08). eTRF also increased metabolic flexibility (p=0.0006) and decreased the 24-hour non-protein respiratory quotient (Δ=−0.021±0.010; p=0.05).
Conclusions
Meal timing interventions facilitate weight loss primarily by decreasing appetite rather than by increasing energy expenditure. eTRF may also increase fat loss by increasing fat oxidation.
Keywords: energy expenditure, appetite, clinical nutrition, fat oxidation
INTRODUCTION
The circadian system orchestrates metabolism in a 24-hour cycle, giving rise to rhythms in energy expenditure (1, 2), appetite (3, 4, 5, 6), insulin sensitivity (7, 8, 9), and other metabolic processes (7). Many of these processes—including insulin sensitivity (7, 8, 9) and the thermic effect of food (10, 11, 12)—peak in the morning or around noontime. Increasingly, studies in both rodents and humans show that eating out of sync with these circadian rhythms—by eating during the biological night—promotes weight gain and metabolic dysfunction (13, 14, 15, 16, 17).
Conversely, eating in sync with circadian rhythms by eating early in the daytime appears to reduce body weight and improve metabolic health. In a seminal randomized controlled trial, Jakubowicz and colleagues (18) tested whether following the popular adage of “eating breakfast like a king, lunch like a prince, and dinner like a pauper” affects weight loss. They found that women randomized to eat a large breakfast lost 5.1 kg more weight in a 12-week period than those who were prescribed an isocaloric diet but ate a small breakfast (8.7±1.4 kg vs. 3.6±1.5 kg, respectively) (18). Since then, several other interventional and cohort studies have reported that shifting food intake to earlier during the daytime increases weight loss (19, 20, 21, 22, 23, 24).
However, the mechanism(s) driving these weight loss effects are unknown. Some studies suggest that the primary mechanism is decreased appetite. Limiting food intake to an 8- to 11-hour period during the daytime (20, 23, 25) and eating a large breakfast and small dinner (18, 26, 27) decrease appetite and/or food intake in humans, with only one trial reporting an exception (6). Other studies suggest that the primary mechanism is increased energy expenditure. In rodents, eating within an 8-hour period early in their activity period increases 24-hour energy expenditure, in parallel with changes reflecting enhanced brown adipose tissue thermogenesis (28). In addition, the thermic effect of food (TEF)—the energy expended in metabolizing and storing ingested calories—is higher in humans in the morning due to the circadian system (10, 11, 12), so eating earlier in the daytime should increase energy expenditure. Indeed, one trial in Spanish adults reported that shifting the timing of lunch from 16:30 h to 13:00 h increased postprandial energy expenditure; however, the measurements were performed over only a four-hour period (29). A more recent, three-arm study in humans found that skipping dinner increases 24-hour energy expenditure by 91 kcal/day (30). However, the order of the control arm was not randomized, food intake and meal frequency were not matched among groups, and physical activity (measured via step count) was non-significantly higher by 10% in the dinner-skipping arm.
We therefore performed the first randomized controlled feeding trial to determine whether meal timing affects 24-hour energy expenditure under conditions of matched food intake and meal frequency. We compared a form of meal timing called early time-restricted feeding (eTRF) (31) to eating at typical American mealtimes (32). eTRF combines two different meal timing strategies: (1) shifting the majority of food intake to earlier in the daytime to align with circadian rhythms in metabolism and (2) daily intermittent fasting (time-restricted feeding, defined here as fasting for ≥14 hours/day); eTRF is tantamount to eating dinner in the mid-afternoon and fasting for the rest of the day (31). The primary endpoint was 24-hour energy expenditure, while secondary endpoints included substrate oxidation, subjective appetite, and metabolic hormones. Based on pre-clinical studies, we hypothesized that eTRF would increase 24-hour energy expenditure.
METHODS
Participants
The trial was conducted at Pennington Biomedical Research Center (PBRC; Baton Rouge, LA), approved by its IRB, and preregistered on ClinicalTrials.gov (NCT02247076). Participants were recruited between November 2014 - August 2016. Generally healthy adults aged 20-45 years old were eligible to participate if they had a body mass index (BMI) between 25-35 kg/m2 (inclusive); a body weight between 68-100 kg; a regular bedtime between 21:30-24:00 h; and a regular menstrual cycle (if female). The exclusion criteria are provided in the Supporting Information. All participants provided written informed consent and received a stipend for their participation.
Study Design
This study was designed as a randomized, crossover, isocaloric controlled feeding trial. Participants were randomized to follow either the control schedule (eat between 8:00-20:00 h; 12-hour daily eating period)—which is similar to the median reported breakfast and dinner times for American adults (32)—or an eTRF schedule (eat between 08:00-14:00 h; 6-hour daily eating period) for 4 days and then to cross over to the other arm after a 3.5-5 week washout period. A 6-hour daily period was selected both to maximize differences relative to the control arm and because it was hypothesized to be the smallest eating window that could be sustained long-term by humans. On Days -3 to 3, participants were required to go to bed between 21:30 h and 24:00 h and to maintain a regular bedtime. On Days 1-2 of each arm, participants followed either the control schedule or the eTRF schedule on their own (Figure 1). On Days 3-4, participants continued to follow their assigned schedule but ate only food provided by study staff while under supervision. Meals for the control schedule were served at 08:00 h, 14:00 h, and 20:00 h, while meals for the eTRF schedule were served at 08:00 h, 11:00 h, and 14:00 h. The three daily meals (50% carbohydrate, 35% fat, 15% protein) were matched across arms and were designed to meet weight-maintenance energy requirements under sedentary conditions. To do so, we used an energy expenditure equation based on weight, height, age, sex, and ethnicity, which was developed for use in 24-hour respiratory chamber studies (33). This equation was used as-is on Day 4 but multiplied by an activity factor of 1.11 for Day 3 to account for greater physical activity in free-living conditions. No other food or beverages—except for water and non-caffeinated, noncaloric drinks—were allowed. To ensure compliance, participants ate their three provided meals in the PBRC kitchen on Day 3 but were otherwise in an outpatient setting. On Day 4, participants completed a 24-hour inpatient stay in a respiratory chamber with concomitant measurement of appetite and metabolic hormones. All women performed respiratory chamber testing during the luteal phases of their menstrual cycle. Neither the participants nor study staff were blinded.
Figure 1.
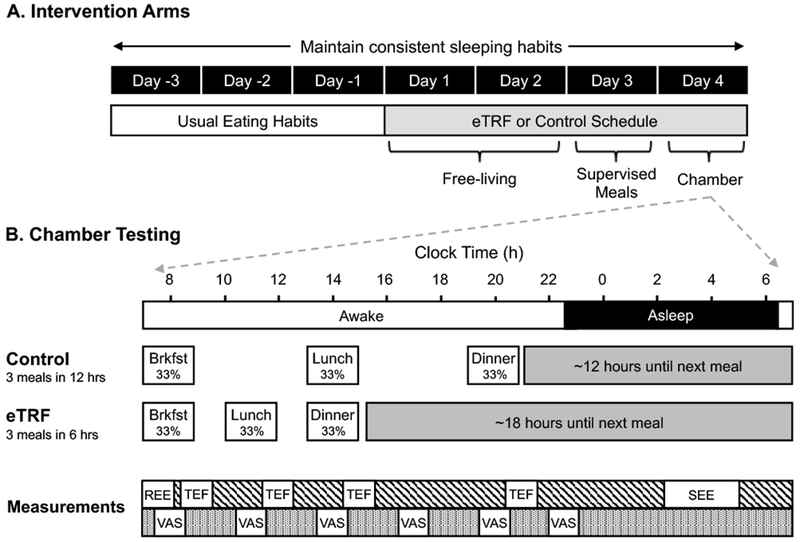
Study Protocol. (A) Each intervention arm consisted of 4 days of early time-restricted feeding (eTRF) or the control eating schedule. On Day 4 of each cycle, participants ate 3 identical meals according to their assigned schedule while spending 24 hours in a respiratory chamber. (B) Chamber measurements were performed as illustrated below and included resting energy expenditure (REE), the thermic effect of food (TEF), sleeping energy expenditure (SEE), and Visual Analog Scales (VAS) to measure appetite, energy levels, and awakeness.
Respiratory Chamber
To measure energy expenditure and substrate oxidation (34), participants resided in a respiratory chamber for 24 hours, starting at 07:00 h on Day 4. While in the chamber, participants consumed three identical isocaloric meals (consisting of a strawberry yogurt smoothie with whey protein, skim milk, and a peanut butter and jelly sandwich) following the same schedule as on Day 3 (Figure 1B). Participants were required to lay supine and motionless from 07:00-08:00 h for measurement of resting energy expenditure and from 08:45-09:30 h, 11:45-12:30 h, 14:45-15:30 h, and 20:45-21:30 h to measure TEF (Figure 1). Participants slept from approximately 22:30 h (lights off) to 06:30 h (lights on). Energy expenditure was calculated using the Weir equation with urinary nitrogen excretion data. Data were analyzed for the whole 24-hour period; in hourly increments (e.g., 01:30-02:29 h, 02:30-03:29 h); for the daytime (08:00 – 19:59 h); and for the nighttime (20:00 – 07:59 h). Sleeping energy expenditure was calculated between 02:00-04:59 h for all minutes when physical activity as measured by radar was <1%. Resting energy expenditure was calculated as the average energy expenditure between 07:35 – 07:55 h, while postprandial energy expenditure for the TEF calculation was measured during the period 60-85 minutes (a 25-minute period) following each meal. Since postprandial energy expenditure peaks around ~1-1.5 hours post-meal and then steadily declines over the subsequent 5-6 hours, using only the value measured in the early postprandial period would overestimate TEF. Since data from Segal et al. (35) indicate that the mean incremental energy expenditure is approximately half the peak value, the area-under-the-curve (AUC) for the TEF calculation was calculated as 1/2 the measured value χ 6 hoursEnergy expenditure from spontaneous physical activity (SPA) was calculated as the difference between total 24-hr energy expenditure and the y-intercept from the linear regression of energy expenditure versus % physical activity, using the data averaged in 15-minute intervals (34). Metabolic flexibility—the ability to switch between oxidizing different substrates—was defined as the difference between the maximum and minimum values of the non-protein Respiratory Quotient (npRQ) when averaged in one-hour intervals.
Serum and Urine Chemistry
Blood was drawn for measurement of leptin, active ghrelin, peptide YY3-36 (PYY), and glucagon-like peptide 1 (GLP-1) in the fasting state at 20:00 h on Day 3 (evening draw) and immediately after exiting the chamber at ~07:30 h on Day 5 (morning draw). Assays were performed as described in the Supporting Information. Mean values were derived by averaging together morning and evening values.
Subjective Appetite and Energy Levels
Participants rated their hunger, desire to eat, capacity to eat, fullness, stomach fullness, energy levels, sleepiness, and perceived body temperature using visual analog scales (VAS; 0-100 mm scale). The VAS survey was administered about every three hours for a total of six times: at 08:00, 11:00, 14:00, 17:00, 20:00, and 22:30 h. Surveys administered at the same time as a scheduled meal were filled out before eating the meal. Daily mean values of the eight indices were calculated as the AUC value divided by measurement duration in hours, while the diurnal amplitude for each index was calculated as the difference between the maximum and minimum values.
Statistical Methods
Statistical analyses were performed as two-tailed tests in SAS (version 9.4; Cary, NC) with α=0.05. Power analysis indicated that 10 individuals were needed to have 80% power to detect an 80 kcal/day difference in 24-hr energy expenditure (the primary endpoint), based on unpublished data indicating a within-subjects standard deviation of σ=79 kcal/day. Randomization was stratified by sex in blocks of four. Additional information on the randomization and missing data are provided in the Supporting Information. Baseline data and the data for each arm are reported in the text as raw mean ± SD, while the treatment effects or differences between arms (denoted Δ) are reported as least squares mean ± SEM. The treatment effects and associated p-values were computed using linear mixed models with heterogeneous compound symmetry, with participants as the random effect and the treatment, period, sequence, and BMI as fixed effects, using the Satterthwaite method for calculating degrees of freedom. Error bars in the figures are presented as SEMs for visual clarity.
RESULTS
Participant Characteristics
A total of 331 adults were screened for the study, 18 participants were randomized, and 11 completed the intervention (Figure 2). Of the 7 participants who withdrew, 4 withdrew for family or personal reasons, 2 withdrew because of scheduling conflicts, and 1 was withdrawn for an unrelated medical reason (life-altering car accident). The eleven adults (7 men/4 women; 64% African-American/27% Caucasian/9% Other) who completed the intervention had a mean age of 32±7 years, a mean BMI of 30.1±2.7 kg/m2, a mean fasting glucose of 92±5 mg/dl, and a mean blood pressure of 112±9 / 77±5 mm Hg. Energy Balance. Each of the 10 completers ate the same amount of food while following the two meal timing schedules (2,180±270 kcal/day), and participants were 100% compliant, with no weigh-backs in either arm (Δ=0±0 kcal/day; p=1.00). Although we intended to feed participants eucaloric diets, participants were less active in the chamber than we expected (physical activity level of 1.16±0.10), likely due to the mandated rest periods for measuring TEF, and consequently, were in positive energy balance on both the eTRF and control schedules (270±109 vs. 283±125 kcal/day; Δ=−11±16 kcal/day; p=0.52). Participants’ mean body weight upon entering the respiratory chamber was the same in both arms (89.8±8.8 vs. 90.1±8.9 kg; p=0.32). However, while in the chamber, the eTRF arm had a Δ=0.2±0.1 kg decrease in weight relative to the control arm (p=0.05), likely due to glycogen depletion from the extended daily fasting.
Figure 2.
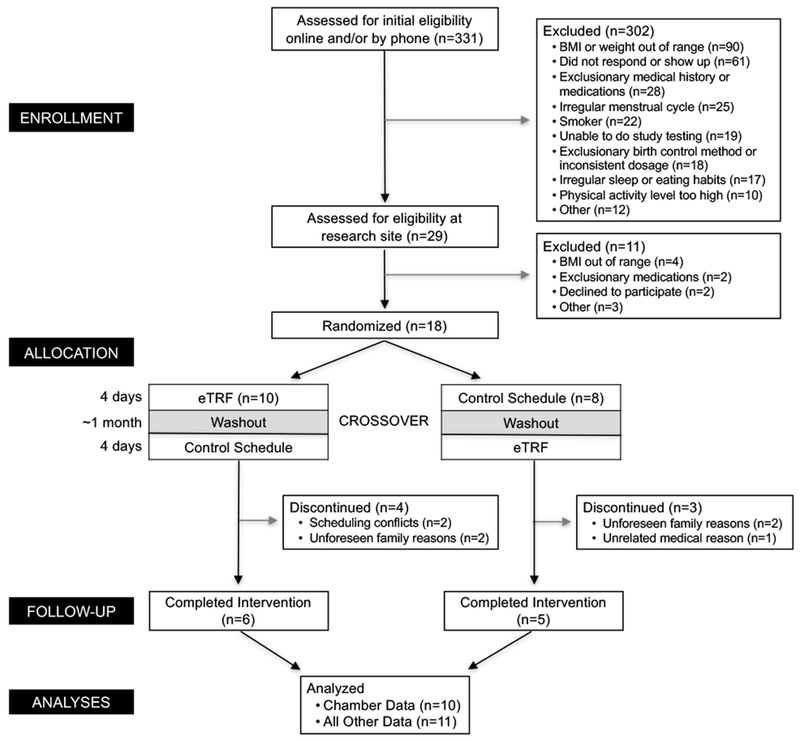
Participant Flow Diagram.
Energy Expenditure
Energy balance data is provided in the Supporting Information. Figure 3A shows the hourly values of energy expenditure for the two eating schedules. Relative to the control schedule, eTRF increased energy expenditure for a 6-hour period from 10:30-16:29 h (p≤0.007) but decreased it across a 4-hour period from 19:30-23:29 h (p≤0.01) and a 2-hour period from 1:30-3:29 h (p≤0.003). As shown in Figure 3B, most of these differences could be attributed to changes in TEF. eTRF increased TEF following the second (p=0.0008) and third meals of the day (p<0.0001) but not the first meal (p=0.93), translating into a Δ=2.4±0.7% absolute increase in mean TEF values (p=0.003) or a Δ=0.061±0.011 kcal/min increase in postprandial energy expenditure (p=0.0003). In contrast, resting energy expenditure (p=0.57) and spontaneous physical activity (180±82 vs. 168±90 kcal/day; Δ=11±9 kcal/day; p=0.23) were unaffected (Figure 3C). As a result, daytime energy expenditure was higher in the eTRF arm by Δ=56±15 kcal/12 hours (p=0.003); however, this was offset by a decline in energy expenditure by Δ=−46±6 kcal/12 hours during the nighttime (p<0.0001) and while sleeping (p=0.01). In aggregate, although eTRF did increase 24-hour energy expenditure in 8 out of 10 participants (distribution of individual values shown in Figure S1), eTRF did not significantly affect 24-hour energy expenditure (1910±285 vs. 1897±266 kcal/day; Δ=10±16 kcal/day; p=0.55).
Figure 3.
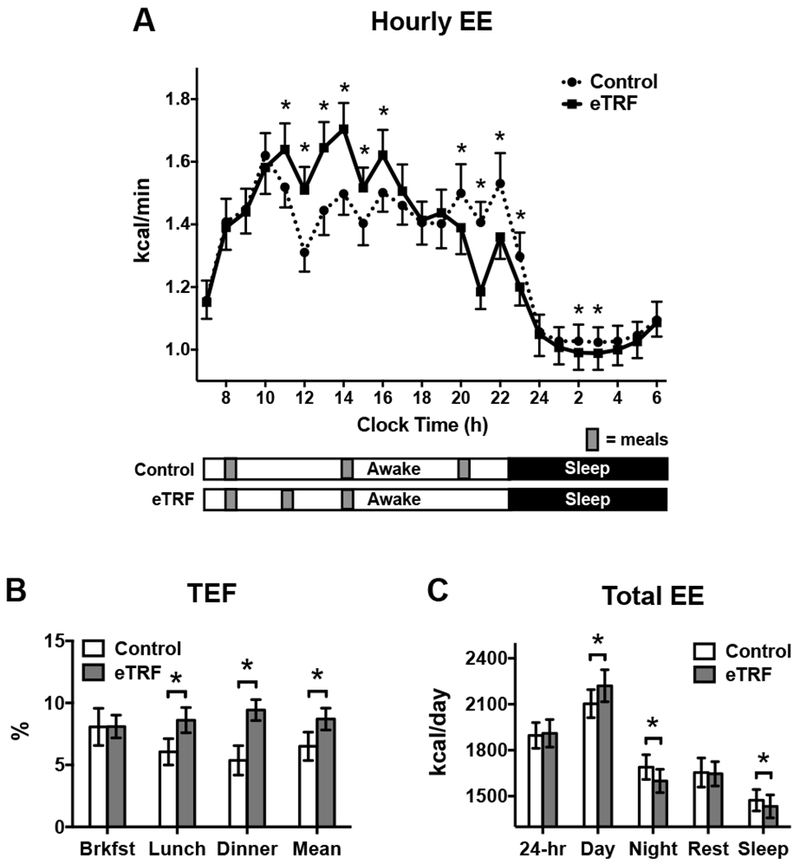
(A) Hourly values of energy expenditure (EE) differed between the early time-restricted feeding (eTRF) and control arms, primarily in the postprandial periods for lunch and dinner. (B) eTRF increased the thermic effect of food (TEF) at lunch, at dinner, and when averaged across the three meals but not at breakfast. (C) 24-hour EE was similar in the eTRF and control arms. Although eTRF increased EE during the daytime, this was offset by a decrease in EE at nighttime and during sleep. Resting energy expenditure (“Rest”) was not different between arms. *P ≤ 0.05.
Substrate Oxidation
As shown in Figure 4, eTRF increased 24-hour protein oxidation by Δ=13±4 g/day (85±15 vs. 71±11 g/day; p=0.009). Because eTRF caused some participants’ RQ values to drop below 0.71 at night and we did not measure ketone excretion, we could not directly quantify fat oxidation and instead report the npRQ. eTRF decreased npRQ nearly continuously across a 12-hour period from 20:30-08:29 h (all p≤0.08 with only two exceptions; Figure 5A). In contrast, eTRF increased npRQ for only 2 hours in the middle of the day (12:30-14:29 h; p≤0.001). In aggregate, eTRF decreased the 24-hr npRQ by Δ=−0.021±0.010 (0.776±0.047 vs. 0.799±0.049; p=0.05; Figure 5B). These differences were driven by a lower npRQ at nighttime (0.729±0.050 vs. 0.778±0.050; Δ=−0.046±0.010; p=0.0007), while sleeping (Δ=−0.031±0.013; p=0.03), and while fasting in the morning (Δ=−0.036±0.014; p=0.02). There were no differences in the daytime npRQ (p=0.72) or the postprandial npRQ following any of the three meals (p≥0.71; data not shown). As shown in Figure 5C, the decrease in npRQ at nighttime in the TRF arm translated into greater 24-hour metabolic flexibility (0.209±0.046 vs. 0.166±0.048; Δ=0.041±0.009; p=0.0006).
Figure 4.
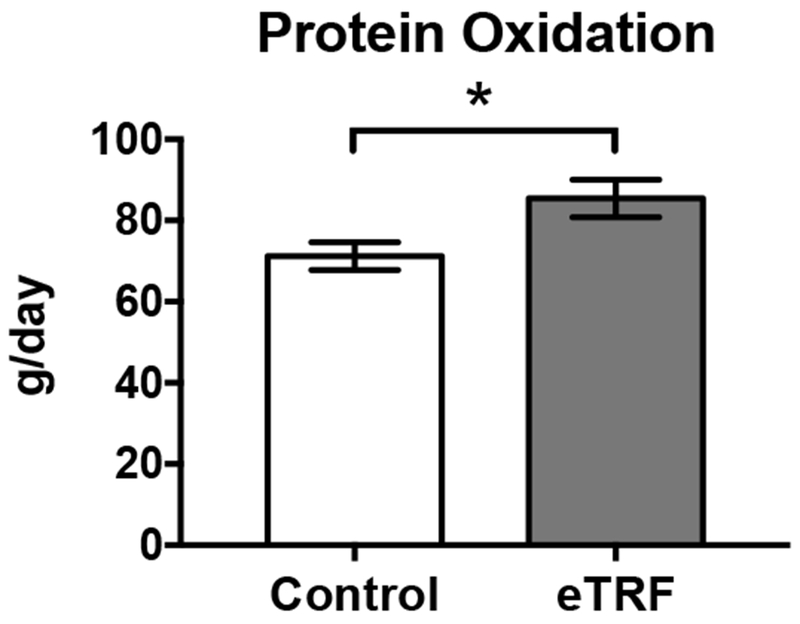
Early time-restricted feeding (eTRF) increased 24-hour protein oxidation. *P ≤ 0.05
Figure 5.
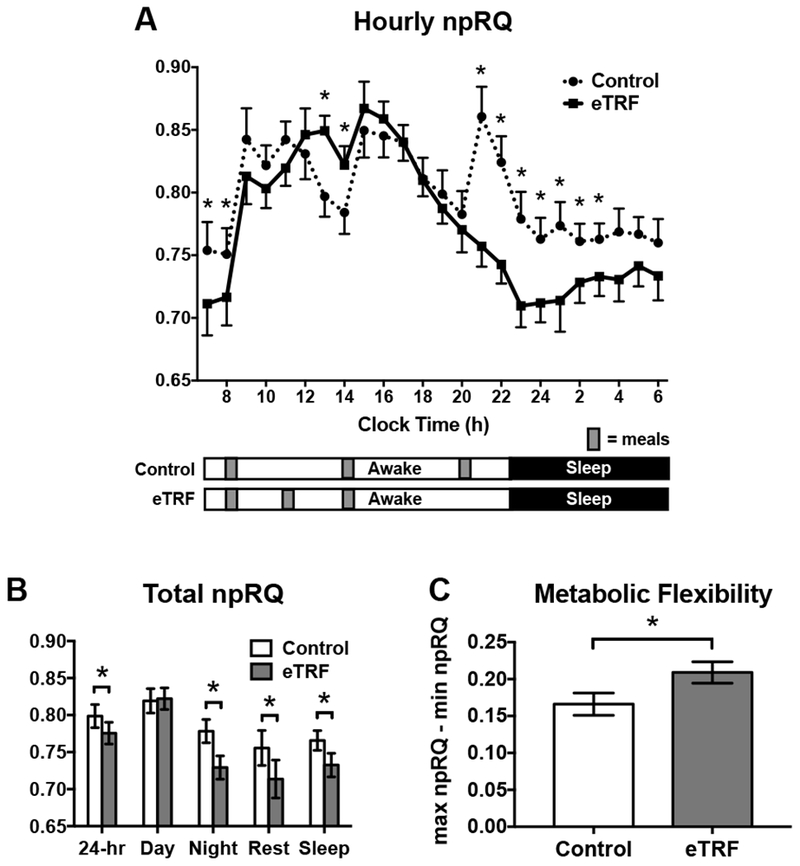
(A) Hourly values of the non-protein respiratory quotient (npRQ) differed between the early time-restricted feeding (eTRF) and control arms, particularly at nighttime. (B) eTRF decreased the 24-hr npRQ, and this was driven by decreases in npRQ during the nighttime, while sleeping, and while fasting in the morning (“Rest”). (C) eTRF increased metabolic flexibility, which was defined as the difference between the maximum and minimum hourly values of npRQ *P ≤ 0.05
Metabolic Hormones
As shown in Figure 6, eTRF decreased morning levels of active ghrelin (Δ=−43±15 pg/ml; p=0.009), leptin (Δ=−4±1 ng/ml; p=0.01) and GLP-1 (Δ=−0.8±0.3 pmol/ml; p=0.008) but did not affect PYY (p=0.25). In the evening, eTRF tended to decrease active ghrelin (Δ=−22±12 pg/ml; p=0.09) and increased PYY (Δ=17±6 pg/ml; p=0.02) but did not affect leptin (p=0.18) or GLP-1 (p=0.36). In aggregate, eTRF lowered mean levels of ghrelin by Δ=−32±10 pg/ml (p=0.006) and tended to decrease mean values of leptin (Δ=−5±2 ng/ml; p=0.07).
Figure 6.
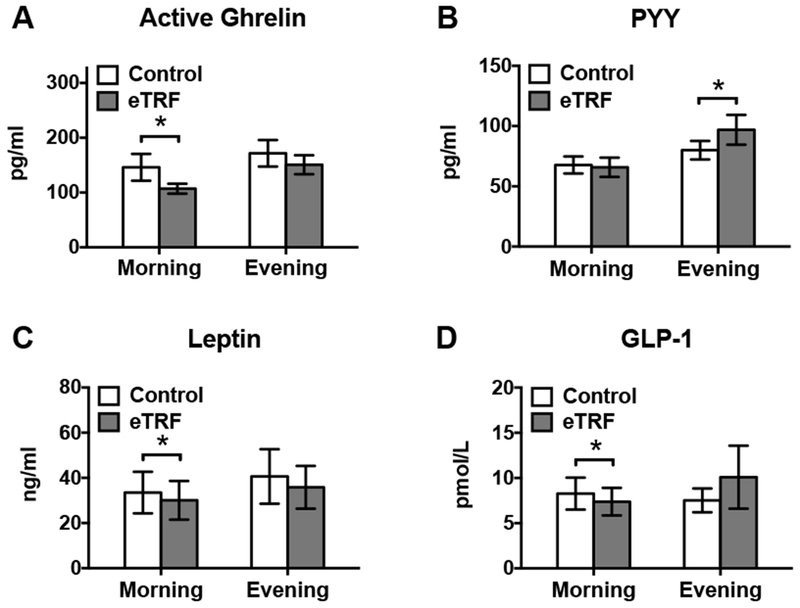
Early time-restricted feeding (eTRF) decreased fasting levels of (A) active ghrelin, (C) leptin, and (D) GLP-1 in the morning, while it (A) tended to decrease active ghrelin and (B) increased PYY in the evening. When averaged together, eTRF lowered mean active ghrelin levels and tended to increase mean leptin values (data not shown). * P ≤ 0.05
Appetite and Energy Level by VAS
Temporal data on subjective appetite, energy levels, sleepiness, and perceived body temperature are provided in Figure 7, while the daily mean, maximum, and minimum values for each index are displayed in Figure 8. eTRF altered temporal patterns of all 8 indices. Most notably, eTRF decreased several facets of appetite in the middle of the day (11:00 - 17:00 h; p≤0.05) but increased all 5 appetite indicators late in the evening at bedtime (22:30 h; all p≤0.007). eTRF did not affect appetite at breakfast (08:00 h; p=0.09 for capacity to eat but p≥0.39 for all others indices) or in the middle of the evening (20:00 h; p≥0.27). In aggregate, eTRF tended to decrease the mean desire to eat by Δ=5±2 mm (p=0.08) and to slightly increase mean fullness (Δ=3±2 mm; p=0.10) and stomach fullness (Δ=3±2 mm; p=0.06) while awake. It also reduced or tended to reduce the diurnal amplitudes in hunger (Δ=−10±3 mm; p=0.006) and the desire to eat (Δ=−9±5 mm; p=0.09), respectively. All other daily mean values and diurnal amplitudes (data not shown) were unaffected (p≥0.14).
Figure 7.
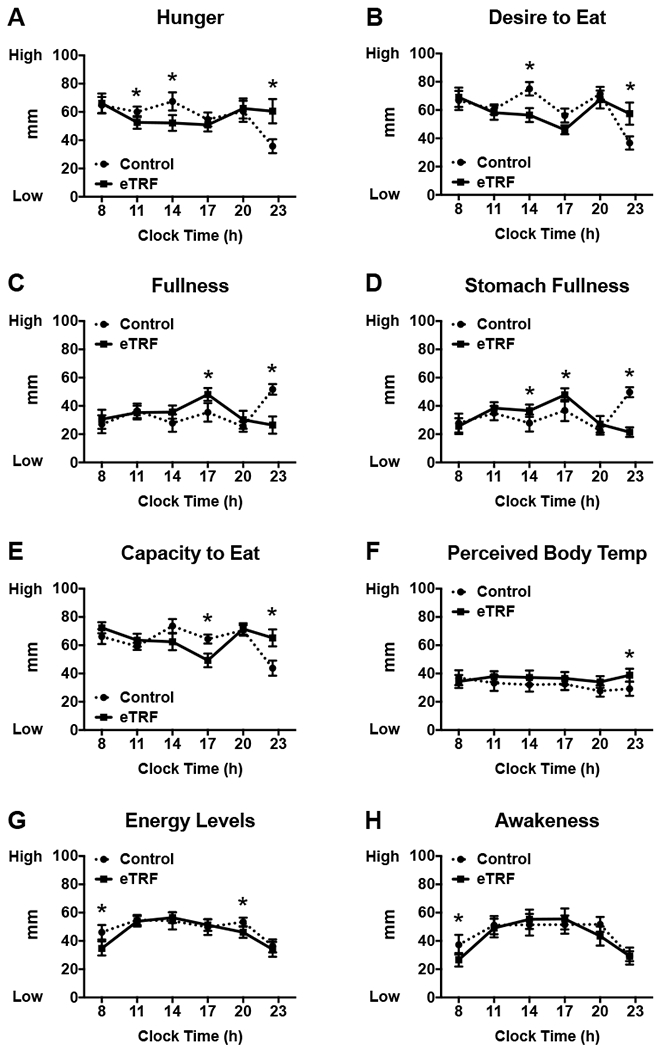
Early time-restricted feeding (eTRF) modified temporal patterns of (A-E) appetite, (F) perceived body temperature, (G) energy levels, and (H) awakeness across the waking day. *P ≤ 0.05
Figure 8.
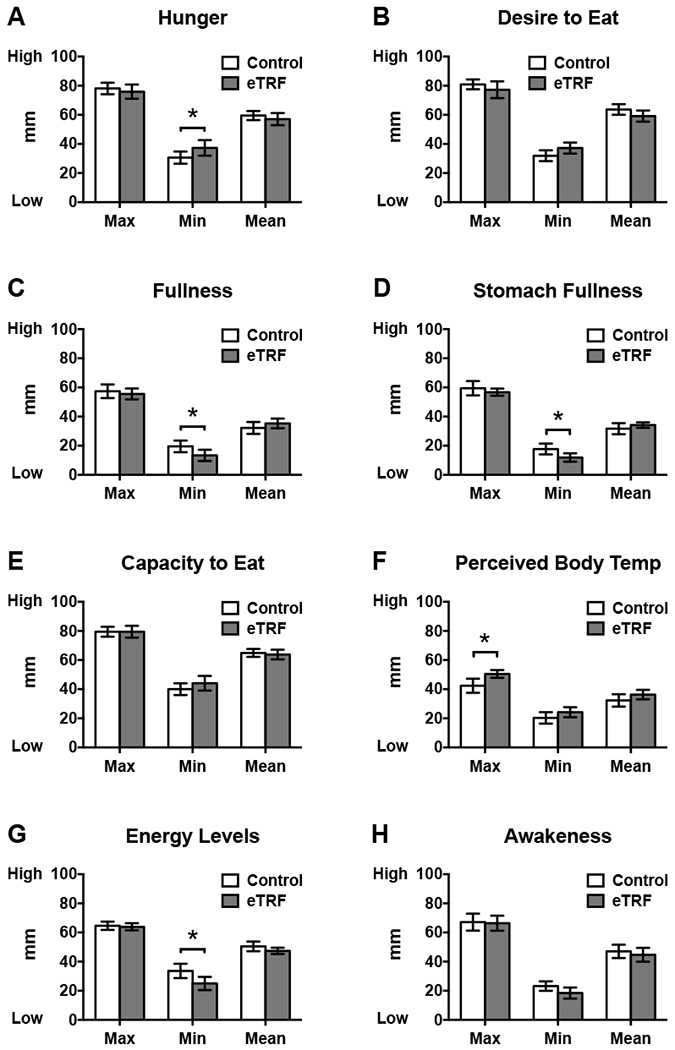
Early time-restricted feeding (eTRF) (A) increased minimum hunger levels, (B) tended to decrease mean desire to eat, (C) tended to increase mean fullness, (D) decreased minimum stomach fullness but tended to increase mean stomach fullness, (F) increased the maximum perceived body temperature, and (G) decreased minimum energy levels. The daily means and ranges for all other facets of appetite and energy levels were similar. * P ≤ 0.05
Adverse Events
There was only one adverse event possibly related to the study intervention: 1 participant (who later withdrew from the trial) reported nausea and vomiting while on the control schedule.
DISCUSSION
Eating earlier in the daytime—to align with circadian rhythms in metabolism—appears to reduce body weight (18, 19, 20, 21, 22, 23). However, the mechanisms behind these effects in humans are unknown. Some trials imply that the primary mechanism is increased energy expenditure (18, 21, 22, 29, 30)—conferring a metabolic advantage—whereas others report decreased food intake and/or appetite (18, 20, 25, 26, 27). However, prior studies measured food intake by self-report or had other limitations, such as not measuring energy expenditure over a 24-hour period.
We therefore conducted the first mechanistic trial to determine the effects of meal timing on 24-hour energy metabolism while matching both food intake and meal frequency to the control schedule. In contrast to rodent studies (28), we found that meal timing did not substantially increase 24-hour energy expenditure in humans. There were temporal differences in energy expenditure, but they arose mostly at times when meals were served in one study arm but not the other, suggesting that such differences arose from asymmetries in whether participants were in absorptive versus postabsorptive states. We speculate that early time-restricted feeding (eTRF) may have increased energy expenditure in rodents but not in humans because (a) brown fat activity is a greater fraction of energy expenditure in rodents than humans; (b) the rodents following eTRF had increased food-seeking behaviors, which boosted their activity energy expenditure; (c) the rodents’ timing and duration of sleep differed between the eTRF and control groups; and/or (d) rodent energy expenditure was normalized by body weight, rather than by fat-free mass and fat mass as recommended (36). Our data are in agreement with a prior study finding that skipping breakfast does not negatively affect 24-hour energy expenditure (37) but in conflict with a second study reporting that skipping breakfast and skipping dinner both modestly increase energy expenditure, relative to eating three meals per day (30). However, the latter three-arm study was only partially randomized, and there was no difference in energy expenditure between skipping breakfast and skipping dinner, which suggests that the circadian timing of food intake does not significantly affect 24-hour energy expenditure. Corroborating our null results for energy expenditure, a 5-week isocaloric controlled feeding study found that eTRF did not affect body weight when food intake was matched to the control arm (31). Together, this suggests that reduced food intake—which was detected in some (20, 23, 25, 27) but not other (18, 21, 22, 24) studies relying on self-report—explains the weight loss from eating earlier in the day.
Although eTRF did not increase 24-hour energy expenditure in our study, it did increase TEF as estimated from data collected during the early postprandial period. This is consistent with data showing that TEF is higher in the morning than the evening due to the circadian system (10, 11, 12). The increase in TEF in the eTRF arm was also likely due to the overlap in postprandial periods, which likely means we overestimated TEF values during lunch and dinner in the eTRF arm. Nonetheless, extrapolating the observed increase in postprandial energy expenditure out to a 24-hour day (with some reasonable assumptions about the temporal profile of TEF) suggests that eTRF should have increased 24-hour energy expenditure by no more than ~20-40 kcal/day, which is roughly in line with the Δ=10±16 kcal/day difference we observed. Although we were not powered to detect differences in 24-hour energy expenditure as small as ~20-40 kcal/day, such small differences are considered clinically insignificant.
eTRF did affect substrate oxidation. eTRF increased 24-hour protein oxidation and and decreased the 24-hour npRQ, particularly at nighttime. The increase in protein oxidation was likely due to enhanced gluconeogenesis associated with extended fasting. It will be important to measure protein balance or body composition in future studies to determine whether eTRF negatively affects lean mass. Similarly, we suspect that the decrease in the 24-hour npRQ is indicative of enhanced fat oxidation andis likely due to eTRF’s prolonged daily fasting period, rather than its circadian effects (38). Although two prior studies reported conflicting results on whether prolonged daily fasting (skipping breakfast or dinner) affects 24-hour fat oxidation (30, 37), the studies had the limitation that they did not extend the fasting duration on the day prior to testing and therefore may have underestimated fat oxidation. By contrast, two longer-term studies—including one on time-restricted feeding—suggest that intermittent fasting may boost fat loss (39, 40). However, such results are best viewed as suggestive, and more definitive trials are needed to test whether eTRF and other intermittent fasting interventions improve fat loss. It is also worth noting that we observed npRQ values below 0.71 at nighttime in some participants. RQ values below 0.71 can arise when ketones are produced at higher rates than they are oxidized or when gluconeogenesis is accompanied by storage of glucose in muscle (41). However, since we did not measure ketone production or gluconeogenesis, whether this happened is speculative, and we cannot completely rule out other possibilities (41). Nonetheless, the overall temporal effects on substrate oxidation also improved metabolic flexibility, which may independently reduce the risk of obesity and type 2 diabetes (42).
We also investigated the effects of meal timing on metabolic hormones and appetite. We found that eTRF lowered mean values of the hunger hormone ghrelin (mostly in the morning), as well as increased levels of the satiety hormone PYY in the middle of the evening. Our data concords with a prior study reporting that 12 weeks of eating breakfast like a king and dinner like a pauper reduces the active ghrelin AUC throughout the waking day (18). eTRF also tended to decrease the mean desire to eat and increase fullness across the waking day. These improvements in appetite are corroborated by other meal timing studies that report reductions in subjective appetite and/or food intake (18, 20, 25, 26, 27). It is also important to underscore that contrary to expectations, we found no evidence that daily intermittent fasting increases mean appetite levels—at least not when the energy intake is matched to the control arm. Surprisingly and paradoxically, eTRF reduced swings in hunger (i.e., reduced the diurnal amplitude of hunger), making hunger levels more even-keeled throughout the day, which may reduce overeating or binge eating.
Despite the rigor of our study design, our trial has some limitations. First, our trial was powered to detect an 80 kcal/day difference in energy expenditure (Cohen’s d=1.0); we cannot rule out that eTRF may more modestly increase energy expenditure by ~20-40 kcal/day. Second, we were able to draw blood only at two times of day rather than frequently during the 24-hour period, which limits the interpretation of our metabolic hormone data. Third, our intervention duration was short (only 4 days), which may not be long enough for metabolic and/or circadian adaptation to occur. Fourth, we did not fully characterize our participants’ habitual sleep and dietary habits at baseline. Lastly, our sample size was both relatively small and skewed towards men. While we expect our results to be qualitatively generalizable, the effect sizes may be modulated by covariates such as biological sex, due to known sex differences in substrate availability during fasting (43).
In sum, the circadian timing of food intake does not appear to significantly affect 24-hour energy expenditure. Instead, our data suggest that meal timing interventions facilitate weight loss primarily by suppressing appetite. Aligning food intake with circadian rhythms may therefore be a powerful strategy for reducing appetite and losing weight. The subset of meal timing interventions that involve intermittent fasting—such as time-restricted feeding—may confer additional metabolic advantages by improving metabolic flexibility and increasing 24-hour fat oxidation. Further research is needed to determine the effects meal timing on energy and fat metabolism.
Supplementary Material
Study Importance Questions.
What is already known about this subject?
Eating earlier in the daytime—to be in sync with circadian rhythms in metabolism— enhances weight loss.
In rodents, these effects are mediated through both increased energy expenditure and decreased food intake.
However, the mechanisms in humans are unknown.
What does this study add?
We performed the first randomized controlled trial to investigate the effects of meal timing on 24-hour energy metabolism when both food intake and meal frequency are matched.
Early time-restricted feeding (eTRF)—a form of intermittent fasting that involves eating dinner in the mid-afternoon and fasting for the rest of the day—did not affect 24-hour energy expenditure but increased metabolic flexibility, decreased the 24-hour nonprotein respiratory quotient, reduced the hunger hormone ghrelin, and reduced the desire to eat.
Meal timing interventions likely facilitate weight loss in humans by reducing food intake and enhancing fat oxidation, not by increasing energy expenditure.
ACKNOWLEDGMENTS
We deeply thank our study participants and the staff at PBRC. Study materials—including the study protocol and statistical analysis plan—can be obtained by emailing the corresponding author. Any sharing of de-identified study data will be governed by a data transfer agreement.
Funding: This work was supported by an Early Career Research Grant from The Obesity Society (CMP); two career development grants (CMP) under center grants KL2TR001419 from the National Center for Advancing Translational Sciences and U54GM104940 from the National Institute of General Medical Sciences, which supports the Louisiana Clinical and Translational Science Center (LA CaTS); and a Nutrition Obesity Research Center (NORC) Grant P30DK072476 entitled “Nutrition and Metabolic Health Through the Lifespan” (ER). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsors played no role in the design, conduct, analysis, or interpretation of data.
Footnotes
Disclosure: RAB, EP, DSH, and CMP declared no conflicts of interest. ER reports past consulting activities with Energesis Pharmaceuticals and Amway and personal fees from serving on the Nutrilite Scientific Advisory Board, outside the submitted work. This research was funded by The Obesity Society, which operates the journal Obesity. Since ER is the Editor-in-Chief of Obesity, he recused himself from being involved in any way in the review process for this manuscript.
REFERENCES
- 1.Krauchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol 1994;267: R819–829. [DOI] [PubMed] [Google Scholar]
- 2.Spengler CM, Czeisler CA, Shea SA. An endogenous circadian rhythm of respiratory control in humans. J Physiol 2000;526 Pt 3: 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheer FA, Morris CJ, Shea SA. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring) 2013;21: 421–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens DS, Macdonald I, Benton D, Sytnik N, Tucker P, Folkard S. A preliminary investigation into individual differences in the circadian variation of meal tolerance: effects on mood and hunger. Chronobiol Int 1996; 13: 435–447. [DOI] [PubMed] [Google Scholar]
- 5.Sargent C, Zhou X, Matthews RW, Darwent D, Roach GD. Daily Rhythms of Hunger and Satiety in Healthy Men during One Week of Sleep Restriction and Circadian Misalignment. Int J Environ Res Public Health 2016;13: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, et al. Meal Timing Regulates the Human Circadian System. Curr Biol 2017;27: 1768–1775 e1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poggiogalle E, Jamshed H, Peterson CM. Circadian Regulation of Glucose, Lipid, and Energy Metabolism in Humans. Metabolism 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. The Journal of clinical investigation 1991;88: 934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrasco-Benso MP, Rivero-Gutierrez B, Lopez-Minguez J, Anzola A, Diez-Noguera A, Madrid JA, et al. Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB J 2016;30: 3117–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romon M, Edme JL, Boulenguez C, Lescroart JL, Frimat P. Circadian variation of diet-induced thermogenesis. Am J Clin Nutr 1993;57: 476–480. [DOI] [PubMed] [Google Scholar]
- 11.Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, Scheer FA. The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity (Silver Spring) 2015;23: 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bo S, Fadda M, Castiglione A, Ciccone G, De Francesco A, Fedele D, et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int J Obes (Lond) 2015;39: 1689–1695. [DOI] [PubMed] [Google Scholar]
- 13.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17: 2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry 2014;26: 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res 2012;199: 337–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 2009; 106: 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibi M, Masumoto A, Naito Y, Kiuchi K, Yoshimoto Y, Matsumoto M, et al. Nighttime snacking reduces whole body fat oxidation and increases LDL cholesterol in healthy young women. Am J Physiol Regul Integr Comp Physiol 2013;304: R94–R101. [DOI] [PubMed] [Google Scholar]
- 18.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013;21: 2504–2512. [DOI] [PubMed] [Google Scholar]
- 19.Keim NL, Van Loan MD, Horn WF, Barbieri TF, Mayclin PL. Weight loss is greater with consumption of large morning meals and fat-free mass is preserved with large evening meals in women on a controlled weight reduction regimen. J Nutr 1997; 127: 75–82. [DOI] [PubMed] [Google Scholar]
- 20.Gill S, Panda S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 2015;22: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz-Lozano T, Vidal J, de Hollanda A, Scheer FA, Garaulet M, Izquierdo-Pulido M. Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clin Nutr 2016;35: 1308–1314. [DOI] [PubMed] [Google Scholar]
- 22.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37: 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr Healthy Aging 2018;4: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahleova H, Belinova L, Malinska H, Oliyarnyk O, Trnovska J, Skop V , et al. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: a randomised crossover study. Diabetologia 2014;57: 1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeCheminant JD, Christenson E, Bailey BW, Tucker LA. Restricting night-time eating reduces daily energy intake in healthy young men: a short-term cross-over study. Br J Nutr 2013;110: 2108–2113. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovitz HR, Boaz M, Ganz T, Jakubowicz D, Matas Z, Madar Z, et al. Big breakfast rich in protein and fat improves glycemic control in type 2 diabetics. Obesity (Silver Spring) 2014;22: E46–54. [DOI] [PubMed] [Google Scholar]
- 27.Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutr Res 2014;34: 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 2012; 15: 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandin C, Scheer FA, Luque AJ, Avila-Gandia V, Zamora S, Madrid JA, et al. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int J Obes (Lond) 2015;39: 828–833. [DOI] [PubMed] [Google Scholar]
- 30.Nas A, Mirza N, Hagele F, Kahlhofer J, Keller J, Rising R, et al. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am J Clin Nutr 2017;105: 1351–1361. [DOI] [PubMed] [Google Scholar]
- 31.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab 2018;27: 1212–1221 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kant AK, Graubard BI. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005-2010. Am J Clin Nutr 2014;100: 938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam YY, Redman LM, Smith SR, Bray GA, Greenway FL, Johannsen D, et al. Determinants of sedentary 24-h energy expenditure: equations for energy prescription and adjustment in a respiratory chamber. Am J Clin Nutr 2014;99: 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. The Journal of clinical investigation 1986;78: 1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segal KR, Gutin B, Nyman AM, Pi-Sunyer FX. Thermic effect of food at rest, during exercise, and after exercise in lean and obese men of similar body weight. The Journal of clinical investigation 1985;76: 1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, et al. A guide to analysis of mouse energy metabolism. Nat Methods 2011. ;9: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi F, Ogata H, Omi N, Nagasaka S, Yamaguchi S, Hibi M, et al. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obes Res Clin Pract 2014;8: e201–298. [DOI] [PubMed] [Google Scholar]
- 38.Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG 3rd, et al. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity (Silver Spring) 2018;26: 254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med 2016;14: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr 2013;110: 1534–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schutz Y, Ravussin E. Respiratory quotients lower than 0.70 in ketogenic diets. Am J Clin Nutr 1980;33: 1317–1319. [DOI] [PubMed] [Google Scholar]
- 42.Goodpaster BH, Sparks LM. Metabolic Flexibility in Health and Disease. Cell Metab 2017;25: 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Browning JD, Baxter J, Satapati S, Burgess SC. The effect of short-term fasting on liver and skeletal muscle lipid, glucose, and energy metabolism in healthy women and men. J Lipid Res 2012;53: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


