Abstract
With advances in genetic testing and its common usage, the field of precision medicine has exploded in the field of oncology. The National Cancer Institute is uniquely positioned to lead in this area of research through its wide network of investigators, partnerships with pharmaceutical companies in drug development, and laboratory capabilities. It has developed a portfolio of trials as part of a Precision Medicine Initiative, that uses various basket/umbrella designs to increase the understanding of treatment of cancer through genetic selection and targeted therapies. This article describes these trials, ALCHEMIST, LungMAP, NCI/NRG ALK Trial, MPACT, NCI-MATCH, and pediatric MATCH, and their contributions to the area of precision medicine.
Keywords: ALCHEMIST, LungMAP, ALK, MPACT, NCI MATCH, Pediatric MATCH, Precision Medicine
Introduction
Cancer treatment has evolved over the decades from use of non-specific, cytotoxic chemotherapy and radiation to a more targeted approach based on molecular abnormalities in each patients’ tumor. With the advent of genomic technology, the field of precision medicine has blossomed especially in the field of oncology. Precision medicine uses information about the genes, proteins, and other features of a person’s cancer to diagnose or treat their disease1. These targeted therapy trials have until recently been organized around specific histologies such as breast or lung cancer and not strictly around genetic alterations in a tumor agnostic fashion. Whether targeted therapies known to be effective in some tumor histologies (e.g., trastuzumab in ERBB-2 positive breast cancer)2, might be effective in another tumor histologies has only begun to be explored. More recently, ‘basket trials’ that evaluate multiple histologies treated with a targeted therapy directed against a specific genetic alteration, have been employed with some success3. Larotrectinib, which targets NTRK fusions, has recently received accelerated FDA approval for a tumor-agnostic indication due to high response rates in multiple histologies4.
Since 2016, the National Cancer Institute (NCI) had developed a portfolio of trials utilizing the basket/umbrella designs in collaboration with its large network of investigators. Two of the larger trials, Lung Master Protocol (LungMAP) and Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial (ALCHEMIST) involved lung cancer as this was the one of the first histologies to show the importance of targeting mutations eg. EGFR or ALK. One of the nation’s largest precision-medicine histology-agnostic trial is the NCI-Molecular Analysis for Therapy Choice (NCI-MATCH) trial. Molecular Profiling-based Assignment of Cancer Therapy (MPACT) was initiated prior to NCI MATCH. Though addressing a different question, it pioneered the tumor mutation assignment assay that is used in NCI-MATCH. This same approach is being tested in solid tumors in children, adolescents and young adults through the NCI-Children’s Oncology Group (COG) Pediatric MATCH trial. This article will explore in more detail these NCI sponsored basket/umbrella trials.
ALCHEMIST, LungMAP, NCI/NRG ALK
ALCHEMIST and LungMAP were the first NCI-sponsored precision medicine ‘Master Protocol’ clinical trials for patients with lung cancer. These trials were the direct result of a NCI sponsored NCI-FDA (Food and Drug Administration) workshop that brought together the NCI Thoracic Malignancies Steering Committee, the FDA, researchers, as well as industry and government stakeholders to discuss issues and challenges in the era of biomarker-driven clinical trials. These trials needed to facilitate rapid assessment of targeted agent activity to generate the data to get drug approval5 utilizing novel biomarkers discovered through The Cancer Genome Atlas (TCGA) program6,7. Unprecedented effectiveness of the targeted agents such as EGFR inhibitors (erlotinib) and ALK inhibitors (crizotinib) in late stage lung cancer patients with EGFR or ALK gene alterations at the time had led to their FDA approval.8,9.
ALCHEMIST
ALCHEMIST (NCT02194738) was developed to determine whether or not the addition of the targeted therapies erlotinib and crizotinib (FDA-approved for lung cancer in metastatic disease) could improve overall survival in patients with early stage non-squamous, non-small cell lung cancer (NSCLC)10 (Fig 1). This trial was a collaborative effort of two of NCI’s National Clinical Trials Network (NCTN) groups with Alliance Oncology leading the screening (A1512160) and erlotinib treatment trial (A081105) and the Eastern Cooperative Oncology Group- American College of Radiology Imaging Network (ECOG-ACRIN) leading the crizotinib treatment trial (E4512).
Fig. 1.
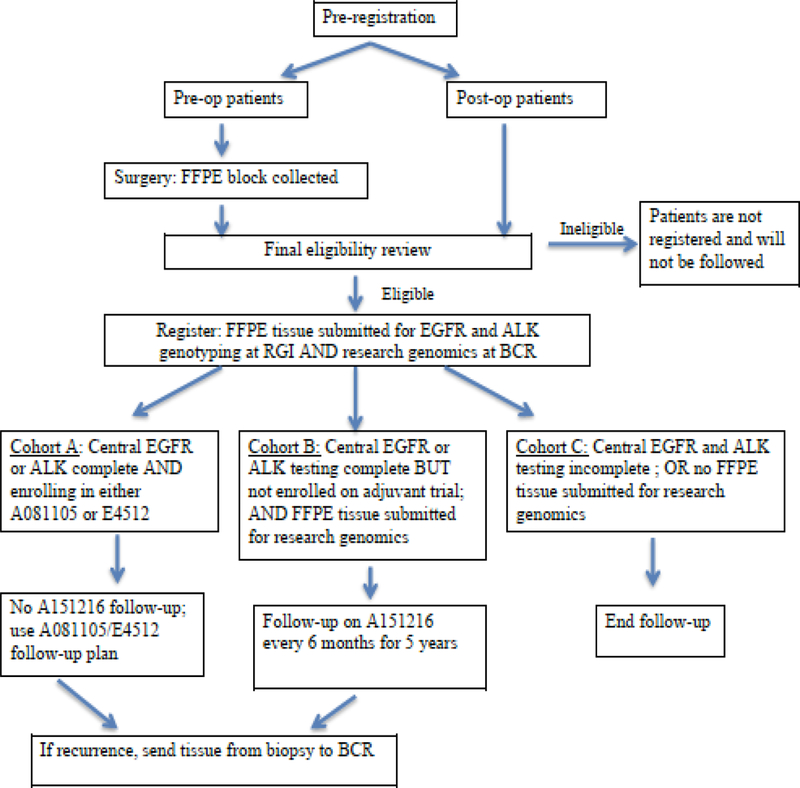
Original ALCHEMIST Schema.
To accrue enough patients with EGFR and ALK mutations, approximately 8,300 patients with early stage lung cancer needed to be screened. Currently, all patients on the screening protocol are followed, regardless of whether or not they enrolled on treatment studies, to define the epidemiological, clinical, and biologic/molecular behavior of tumors that do not harbor the targeted molecular alterations. Tumor tissue and blood are collected from all patients for genomic analysis. The approval of immunotherapy agents in lung cancer led to a major amendment of ALCHEMIST in 2015. A Phase 3 trial of checkpoint inhibitor nivolumab versus observation in patients with resected early stage lung cancer after standard therapy (EA5142) was launched led by ECOG-ACRIN. In addition, patients with squamous cell lung cancer were now eligible to enroll in the new immunotherapy trial (Fig. 2)
Fig. 2.
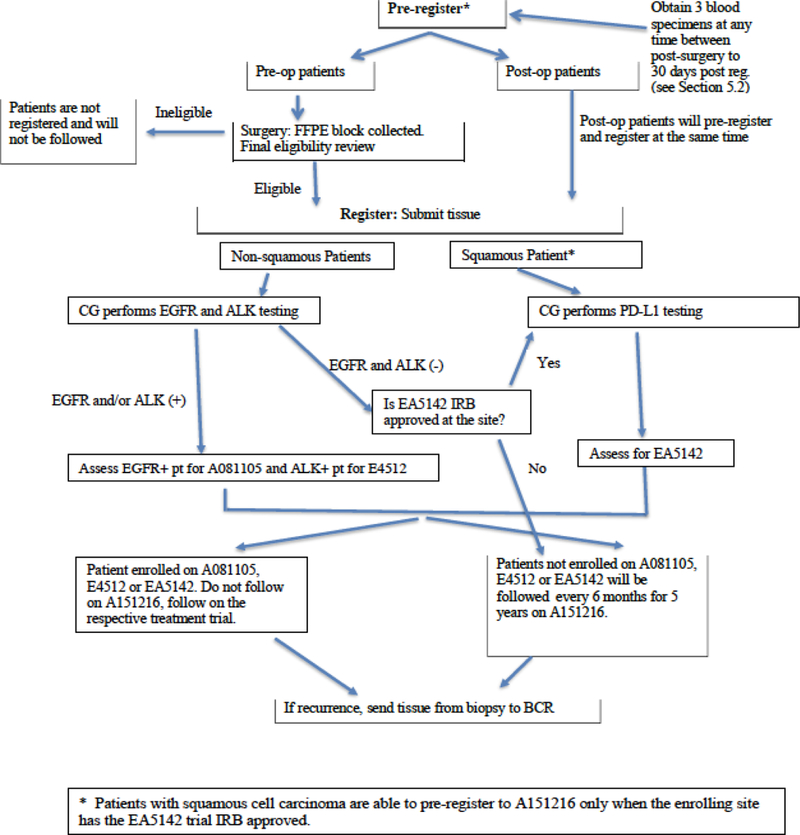
Current ALCHEMIST Schema.
Several challenges arose with ALCHEMIST, including a higher than expected drop off rate of patients after registration; preliminary review noted that one of most commonly reported reasons was the inability for patients to continue with more therapy after undergoing thoracic surgery, adjuvant chemotherapy, and or chemo-radiotherapy. Inclusion of placebo was also noted to be one of the hindering factors.
The ALCHEMIST accrual rate is over 100 patients per month with over 4500 patients screened to date. The immunotherapy arm has had a robust accrual rate and is expected to complete accrual before the end of 2019 allowing other chemo-immunotherapy trials to be considered.
LungMAP
The LungMAP (NCT03851445) study was launched in 2014 as a phase II/III trial to register patients with squamous cell lung cancer who had progressed after a platinum-based chemotherapy. This study is a public-private partnership effort led by the South West Oncology Group (SWOG), NCI, FDA, and the Foundation for the NIH (FNIH) which includes multiple pharma partners. LungMAP launched with four biomarker-driven sub-studies based on targets known to be druggable from The Cancer Genome Atlas (TCGA) data in squamous cell lung cancer6. Patients who did not have any of those alterations were enrolled in the non-match sub-study evaluating durvalumab, an anti–PD-L1 immunotherapy agent (Fig. 3)
Fig. 3.
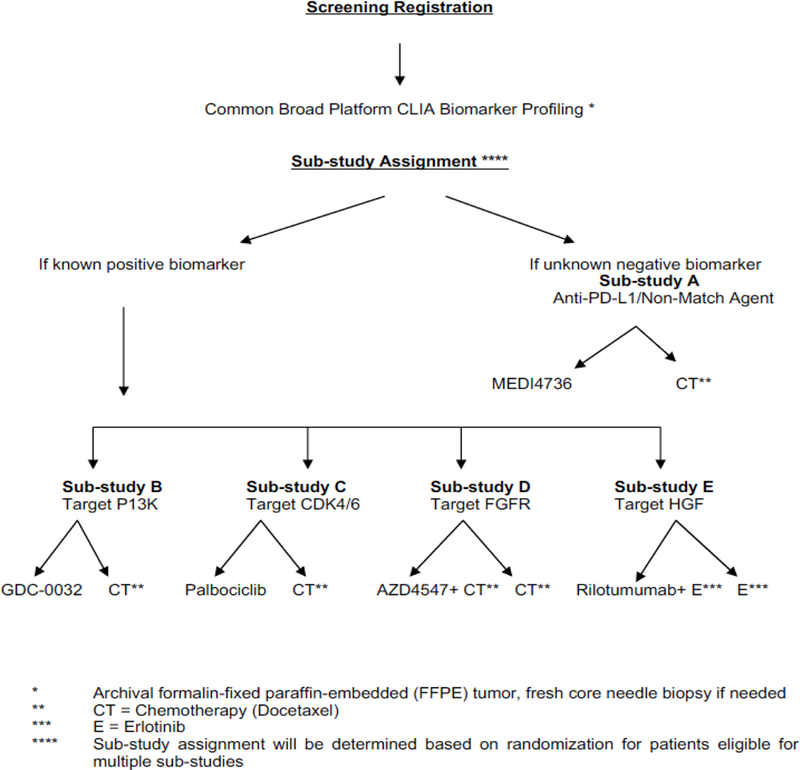
Original LungMap Schema.
Initially, the first three sub-studies included docetaxel as a randomized standard-of-care control arm, however, modifications to LungMap became quickly necessary with the approval of immunotherapy agents for lung cancer. With the approval of immunotherapy in second line, the control arm of docetaxel became obsolete making it necessary to remove docetaxel as the control arm. The trial was modified from a phase 2/3 trial to one that includes both phase 2 and phase 3 sub-studies. The phase 2 studies allow investigational agents to be evaluated as single-arm trials to improve the efficiency in assessing clinical benefit of the drugs in the immune-refractory population. It also became apparent that immunotherapies were beneficial in the treatment of lung cancer patients irrespective of patient histology. Because of this finding, patients with all NSCLC histologies are eligible for LungMap and new immunotherapy and chemotherapy combinations will be tested in the immunotherapy-refractory population (Fig. 4).
Fig. 4.
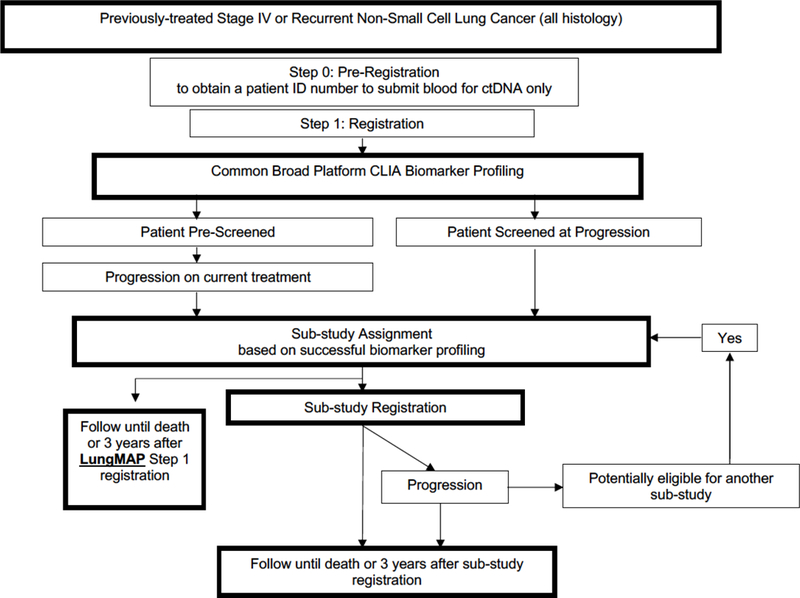
Current LungMap Schema.
NCI/NRG ALK Protocol
The NCI-NRG ALK protocol was just activated to study clinical benefit of 2nd or 3rd generation ALK inhibitors in patients who develop progression after a second-generation ALK inhibitor. Treatment will be based on genetic alterations identified in the tumor biopsy. The trial will be comparing several ALK inhibitors including lorlatinib, ceritinib, alectinib, brigatinib, ensartinib, and crizotinib vs. chemotherapy (pemetrexed plus cisplatin, and or carboplatin) in these patients with ALK resistance mutations. Another aim of this trial is to compare ctDNA to the tissue biopsy in the first 200 patients. If after consultation with the Center for Devices and Radiological Health branch of the FDA ctDNA is found to have concordance with tumor tissue, it will replace the tumor biopsy.
Molecular Profiling-based Assignment of Cancer Therapy (NCI-MPACT)
This trial was initiated in 2014 to answer the question of whether patients with advanced, refractory solid tumor cancers are more likely or not to benefit from targeted agents specific to their tumor’s actionable mutations of interest (aMOIs) (NCT01827384)11,12. It is a multicenter, double-blind, randomized trial comparing response rates for patients with an aMOI in one of 3 genetic pathways (DNA repair, PI3K, or RAS/RAF/MEK) in those treated with agents targeting the selected pathway (experimental arm) versus those treated with the same agents not targeted to that pathway (control arm) (Fig. 5). Subjects undergo tumor biopsy and genetic analysis in a Clinical Laboratory Improvement Amendment (CLIA) certified laboratory to determine study eligibility; those with an aMOI are randomized 2:1 to a drug identified to work on their tumor’s aberrant pathway or a study drug not targeting that pathway. Study drugs were initially 1) AZD 1775 and carboplatin or veliparib and temozolomide for the DNA repair pathway; 2) everolimus for the PI3K pathway; or 3) trametinib for the Ras Pathway. At disease progression, control-arm patients could cross over to a study drug matching their aMOI.
Fig. 5.
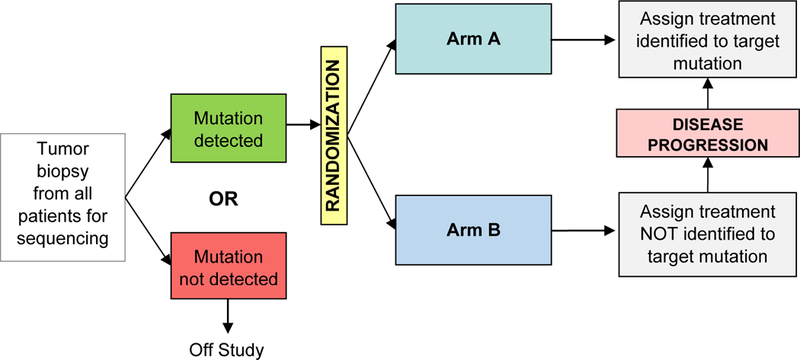
NCI MPACT Schema.
At the time of its design, the driver mutations were not well defined, and the decision was made to use pathway aberrations instead of specific variations. Choice of drug was blinded to the investigators. Due to the blinded nature of this trial and the commonplace usage of genomic testing in the last few years, it became very difficult to maintain true blinding and accrue at a reasonable rate; therefore, MPACT was amended to employ a non-blinded, non-randomized design and is ongoing.
Molecular Analysis for Therapy Choice (NCI-MATCH)
In April 2013, the NCI and ECOG-ACRIN began development of a signal-finding precision medicine trial for adult patients (solid tumor, lymphoma, or myeloma) with treatment-refractory malignancies or for whom no standard of care therapy was available. The resulting NCI Molecular Analysis for Therapy Choice (NCI-MATCH) (NCT0246506) trial opened in August 2015 as the largest precision medicine trial to date evaluating targeted therapy in a large scale, multi-arm, signal-finding trial based on molecular abnormalities in a tumor-agnostic fashion13. With the help of a large NCI sponsored national networks, the NCTN and NCI Community Oncology Research Program (NCORP) of over 1100 academic and community sites coordinated by ECOG-ACRIN, this trial accrued over 6000 patients in under 2 years14.
The objective of NCI-MATCH is to investigate the activity of genomically-targeted treatments across common and less common tumor types with one treatment option per genetic alteration. The original accrual goal was based on reported prevalence of these somatic alterations from TCGA and the International Cancer Genome Consortium although these databases are based upon primary tumors not previously treated with systemic therapies and not advanced disease possibly treated with multiple lines of therapy. This led to over predicating the prevalence rates for many of the targeted alterations. Patient’s tumor biopsies were tested using targeted next generation sequencing (NGS) analysis with an investigational device exemption (IDE). The panel consisted of 143 genes and was complemented by immunohistochemistry for PTEN, MLH1, MSH2, and Rb 15,16. Actionable molecular alterations were those that, at minimum, were associated with preclinical evidence linking the alteration with drug activity. Patients were then assigned to one of the available treatment sub-protocols (Fig. 6) by a computational platform (MATCHBOX) (Fig. 7). Single drugs or combinations were evaluated for inclusion if they targeted molecular alterations with an estimated prevalence of at least 1.5%, had a defined phase 2 dose, and met criteria for clinical activity (at least N of 1). The primary objective for each subprotocol was the overall response rate; secondary objectives included progression free survival (PFS) at 6 months, progression free survival, toxicity assessment and evaluation of predictive biomarkers. The target accrual for each arm was 35 patients and some arms with rapid accrual were increased to 70 patients.
Fig. 6.
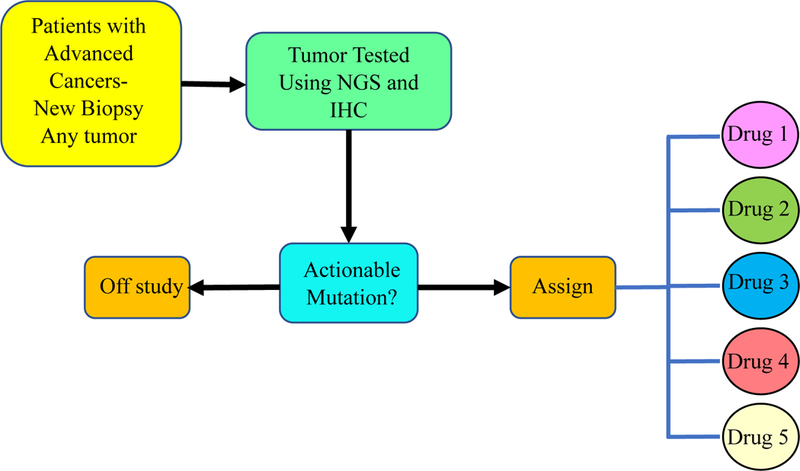
NCI MATCH Schema.
Fig. 7.
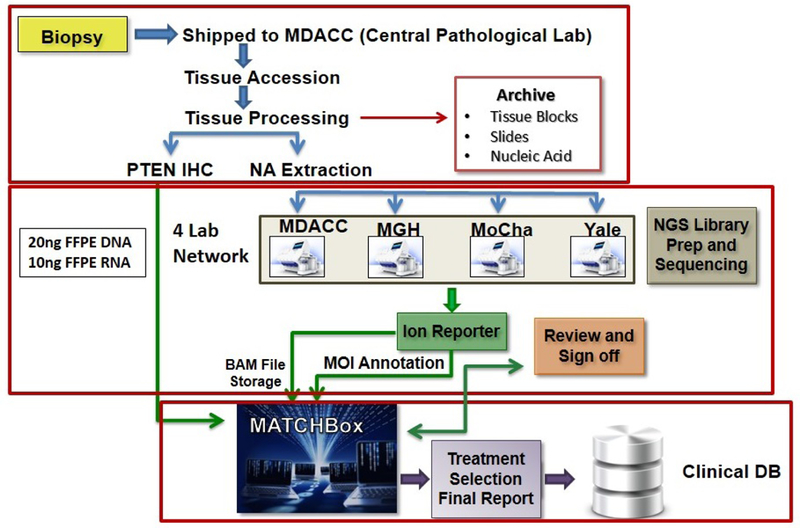
NCI MATCH Workflow.
To evaluate the trial structure, resources, and workflow, to determine whether the initial projections for patient accrual, disease distribution, alteration detection, and treatment assignment were accurate, and to make any needed adjustments, a mandated interim analysis suspended accrual after 500 patients from Nov 2015 - May 2016. The interim analysis showed 739 tissue specimens were received from 795 registered patients with equal gender representation; the majority were from white patients17. Two-thirds of the patients were referred by NCORP. The screening far exceeded the anticipated 50 patients per month, averaging 80 specimens—this robust rate of accrual overwhelmed the initially planned resources, increasing the median turnaround time from receipt of biopsy to results for assignment from 14 to 36 days in October 2015. Implementation of a higher throughput NGS platform, an increase in sample processing personnel, and improvement in tissue acquisition decreased the time of receipt of tissue to assignment back to ~14 days. In addition, the initial match rate was 12% due the limited number of arms (10); 14 additional treatment arms were added prior to reopening the trial, which has now expanded to 35. It is anticipated that a total of 39 treatment arms will be open by the third quarter 2019 (Table 1).
Table 1.
Anticipated assignment rate and expected sub-protocol enrollment with screening accrual of 5000 patients, based on mutations frequencies and tumor histology in NCI-MATCH interim analysis results in 645 patients17.
| Target (subprotocol) | Expected Assignment Rate % | Expected Enrollment |
|---|---|---|
| PIK3CA mutation (I) | 4.0 | 89 |
| MET amplification (C1) | 0.9 | 21 |
| CCND1 amplification (Z1B) | 3.6 | 79 |
| ERBB2 mutation (B) | 0.8 | 20 |
|
FGFR1/2/3 mutation/amplification /translocation (W) |
2.9 |
65 |
| BRAF V600 (H) | 0.8 | 19 |
| PTEN expression loss (P) | 2.5 | 55 |
| SMO/PTCH1 mutation (T) | 0.6 | 14 |
| ERBB2 amplification (Q) | 1.7 | 44 |
| MTOR mutation (L) | 0.3 | 7 |
| NF1 (S1) | 1.9 | 41 |
| BRAF non V600 mutation (R) | 0.3 | 8 |
| CDK4/6 amplification (Z1C) | 1.7 | 38 |
| EGFR T790M or other rare mutation (E) | 0.2 | 4 |
| TSC½ mutation (M) | 1.2 | 28 |
| ALK translocation (F) | 0.2 | 4 |
| AKT1 mutation (Y) | 1.2 | 28 |
| cKIT mutaion (V) | 0.2 | 3 |
| NRAS mutation (Z1A) | 1.2 | 28 |
| NF2 mutation (U) | 1.1 | 26 |
| PTEN mutation (N) | 1.1 | 24 |
Request for fine needle aspirate (FNA) simultaneously with the required biopsy was added as FNA rescued a proportion (7%) of biopsy specimens that may not have yield usable material. In addition, a support desk at both ECOG-ACRIN operations office for clinical questions and at the central specimen receipt center MD Anderson Cancer Center (MDACC) for laboratory issues was implemented. These changes led to a 93% successful rate of tumor molecular profiling (5560/5962 submitted samples), decreased median turnaround time, and increased profiling capacity from an expected throughput of 50 per month to 100–150 per week. The changes also led to an overall match rate of 18% and enrollment rate to an arm of 69% of assigned patients. Despite screening over 6000 patients, accrual was only completed for approximately 1/3 of the arms, largely due to the rarity of some mutations. This led to the Outside Laboratory/Rare Variant Initiative, which allows selected commercial and academic labs to refer to NCI-MATCH patients who are undergoing genomic testing as part of standard of care. Under this Outside Laboratory /Rare Variant Initiative, many of the uncommon and rare arms have now completed accrual.
NCI-MATCH with its large network of over 1,100 clinical sites, 30+ arms addressing many of the targetable genomic alterations in cancer, along with the highly skilled laboratories and the efficient matching system, MATCHBOX, have allowed this trial to be available to a large number of cancer patients who otherwise would not have had access to these investigational agents. This highly successful collaboration between ECOG-ACRIN, the NCI, and pharmaceutical partners has already begun to report results, many of which look promising with nearly 30% of arms having met the primary endpoint. One of the exciting findings was that common tumors (NSCLC, breast cancer, prostate cancer and colorectal cancer) comprised only 37.5% of tumors whereas less common/rare tumors represented the majority. In addition, the experience treating patients with less favorable responses leaves us with important lessons about the target and the therapy. Finally, the rich resource of genomic information to be gathered from whole exome and RNA sequencing of the tumors will provide the research community with many important insights into cancer and its mechanisms of resistance to therapy.
Pediatric MATCH
Despite improvements in outcomes for children and adolescents with cancer over the past five decades, recurrent and refractory disease are rarely curable. Precision medicine is being tested in the setting of pediatric oncology through the collaboration of NCI and -COG in the Pediatric MATCH trial (NCT03155620). Through collaboration and support from the NCI, Children’s Oncology Croup (COG), the only pediatric cooperative group of the NCTN, is combining the information gained from precision medicine studies in adults with cancer as well as molecular profiling studies from pediatric solid tumors and applying it in this national collaborative study. Pediatric MATCH is a histology agnostic trial in which treatment arm eligibility is based on a list of predefined genomic aberrations(s) that are thought to be driver mutations for tumors and targeted by the investigational agent for each treatment arm. Pediatric MATCH is unique in that not only is the tumor tissue screened for molecular aberrations, but patients are able to be treated with the investigational agent if they have a match within a single study18 (Fig. 8). The tumor tissue is analyzed using the same analytically validated next-generation sequencing targeted assay across more than 140 genes as used in NCI-MATCH study19. Treatment assignment is based on a rules-based system which has not been used in other pediatric trials and provides the advantage of predefining treatment based on the presence of a molecular aberration, availability of agents within the context of the trial, and negates assignment bias because all patients with predefined actionable variant are assigned a given treatment.
Fig. 8.
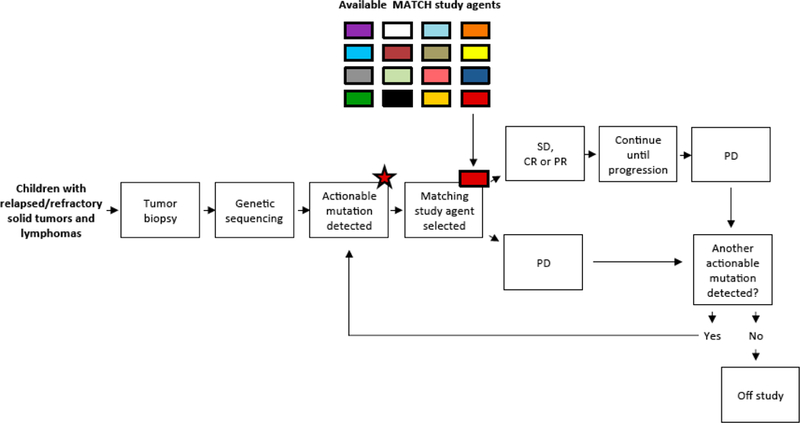
Schema for COG-NCI Pediatric MATCH study (APEC1621). Figure is as appears in a prior publication18 and is used with permission from Oxford University Press.
Pediatric MATCH is available through approximately 200 COG institutions scattered throughout the US to all children, adolescents, and young adults aged 1 to 21 years with a refractory or recurrent solid tumor (including non-Hodgkin lymphoma (NHL), central nervous system tumors, and histiocytoses). To enroll onto the study, tumor tissue must be available from the time of progression or recurrence. The only exception is for patients with intrinsic brain stem gliomas who can instead submit the diagnostic biopsy. Patients must have measurable disease and no other therapeutic options available. Treatment is offered to participants in whose tumor specimen a molecular aberration is detected that is addressed by a treatment arm in the trial. The primary endpoint is objective response rate and to determine the proportion of pediatric patients whose tumors have pathway alterations that can be targeted by existing agents. A total of 20 patients are enrolled on each treatment arm (or stratum within a treatment arm) depending on the genomic variant used to identify patients; if 3 or more responses are observed, the agent will be considered of interest for further development. Enrollment to a treatment arm may be expanded if activity is seen with a particular agent. A patient can continue treatment if the tumor is stable or shows a response, but if the tumor progresses then the patient may be eligible to enroll on another treatment arm if the tumor has additional genetic aberrations targeted by another treatment arm.
Pediatric MATCH opened to enrollment on July 24, 2017 with a goal to screen between 1000–1500 participants. Currently the study is enrolling approximately 250 patients per year. The study opened with 7 treatment arms and over the following year, 3 more arms were added for a total of 10 (Table 2). Additional treatment arms are in various stages of development. Results of the tissue analysis are returned to the treating oncologist within a median of 15 days.
Table 2.
Current Pediatric MATCH treatment arms.
| Agent Class | Treatment Arm |
|---|---|
| APEC1621-A | Larotrectinib (Vitrakvi, Bayer, NJ) |
| APEC1621-B | Erdafitinib (Balversa, Janssen, NJ) |
| APEC1621-C | Tazemetostat (EPZ-6438, Epizyme, MA) |
| APEC1621-D | LY3023414 (Lilly, IN) |
| APEC1621-E | Selumetinib (AZD6244, AstraZeneca, London, UK) |
| APEC1621-F | Ensartinib (X396, Xcovery, FL) |
| APEC1621-G | Vemurafenib (Zelboraf, Roche, Basel, Switzerland) |
| APEC1621-H | Olaparib (Lynparza, AstraZeneca, London, UK) |
| APEC1621-I | Palbociclib (Ibrance, Pfizer, CT) |
| APEC1621-J | Ulixertinib (BVD-523, BioMed Valley Discoveries, MO) |
The molecular targets and study drugs selected for the trial were identified and prioritized by the Pediatric MATCH Target and Agent Prioritization (TAP) committee consisting of representatives from COG disease committees, FDA and NCI20. Criteria used to prioritize the target and agent pairs included the frequency of the alterations in the target in pediatric cancers, strength of the evidence linking the target to activity of the agent, whether the target can be detected with the testing platform, clinical and preclinical data for the specific agent, and other ongoing or planned biomarker-defined clinical studies. For an agent to be considered for inclusion as a treatment arm, the agent must have demonstrated activity against tumors with a genomic alteration and have an established adult phase 2 dose. The same levels of evidence used for drug selection in NCI MATCH were applied to Pediatric MATCH17. It is not required that an agent have a completed pediatric phase I study if a pediatric formulation is available, although the appropriately sized capsules or tablets are required for dosing pediatric patients. As a result of these broader criteria, half of the treatment arms currently open in Pediatric MATCH are testing drugs never formally tested before in pediatric patients.
There are several unique aspects of Pediatric MATCH as compared to NCI-MATCH that are worth highlighting. When developing Pediatric MATCH, questions arose as to the need for a separate study rather than expanding NCI-MATCH to children and adolescents. Although expanding NCI-MATCH would have been a much more efficient approach, the molecular aberrations both in quantity and specific types differ between the solid tumors occurring in children and in adults. The number of molecular aberrations seen in pediatric tumors is much lower than the experience in adults, and as a result the projected matching rate when designing Pediatric MATCH was set at 10% which was much lower than the projected rate on NCI-MATCH trial of 20–25%. Additionally, the variants identified in tumors occurring in adults are different from those in children which is underscored by the fact that there was no overlap in drug treatment arms between Pediatric MATCH and NCI-MATCH when Pediatric MATCH opened. The timing of the biopsy is another difference from NCI-MATCH. The chances are greater of finding a targetable abnormality in a patient’s tumor if the tissue is obtained after recurrence or progression and near the time of analysis. To provide trial access to as many children and adolescents as possible, balanced with the risks associated with biopsies, there are no time restrictions between the biopsy to enrolling on the study. Drug availability is another challenge with Pediatric MATCH since many of the recurrent aberrations identified in pediatric tumors do not have drugs available to target the aberration. Because of the small number of pediatric cancers and even smaller molecular subgroups of tumors, unfortunately, there is a paucity of drug development in this area. One last difference from NCI-MATCH is that germline DNA is collected at the time of tissue submission on all patients enrolled on Pediatric MATCH. Clinical genomics laboratories interpret the germline findings and provide a report back to the treating oncologist as to the origin of the variants identified in the tumor and whether they are pathogenic or likely pathogenic germline variants in cancer susceptibility genes. The results of the germline analysis will not be used for treatment assignment and are not meant to provide a comprehensive cancer susceptibility evaluation. However, the treating oncologist can then decide whether formal genetic testing should be recommended to the family. Pediatric MATCH is designed to explore and provide precision medicine in the pediatric cancer patients.
Conclusions
Based on the unique position of the NCI, its collaborating networks, and its capabilities, NCI is leading the way investigating the new frontier of precision medicine. The collection of NCI-sponsored basket/umbrella trials are designed to answer a diverse set of questions. The common elements of these trials are accessibility to large numbers of patients, options for patients agnostic to histology, public-private partnerships with large number of pharmaceutical and testing laboratories, and extensive network of investigators and sites through NCTN and NCORP. Precision medicine trials focused on specific cancer genetic alterations across multiple histologies have not been accessible to most patients due to the complexity of designing such large-scale trials considering the number of “driver” mutations and the treatment arms needed. The rapid recruitment of cancer patients seen in NCI-MATCH trial reflects the awareness and strong interest of the cancer patient population in precision medicine and the unmet need for such trials in patients with rare tumors. The flexibility in design to change with the rapidly changing landscape in oncology today ensures that trial designs will continue to be important to move the field forward.
References
- 1.NCI launches trial to assess the utility of genetic sequencing to improve patient outcomes. 2014. (Accessed April 14, 2019, at https://dctd.cancer.gov/majorinitiatives/NCI-sponsored_trials_in_precision_medicine.htm#h05.)
- 2.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N Engl J Med 2001;344:783–92. [DOI] [PubMed] [Google Scholar]
- 3.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med 2015;373:726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA approves larotrectinib for solid tumors with NTRK gene fusions. 2018. (Accessed April 14, 2019, at https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions-0.)
- 5.Malik SM, Pazdur R, Abrams JS, et al. Consensus Report of a Joint NCI Thoracic Malignancies Steering Committee: FDA Workshop on Strategies for Integrating Biomarkers into Clinical Development of New Therapies for Lung Cancer Leading to the Inception of “Master Protocols” in Lung Cancer. J Thorac Oncol 2014;9:1443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Cancer Genome Atlas Research N, Collisson EA, Campbell JD, et al. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heger M TCGA Lung Cancer Study IDs Potential Drug Targets in Majority of Cases. GenomeWeb 2012. [Google Scholar]
- 8.Cohen MH, Johnson JR, Chen Y-F, Sridhara R, Pazdur R. FDA Drug Approval Summary: Erlotinib (Tarceva®) Tablets. Oncologist 2005;10:461–6. [DOI] [PubMed] [Google Scholar]
- 9.Malik SM, Maher VE, Bijwaard KE, et al. U.S. Food and Drug Administration Approval: Crizotinib for Treatment of Advanced or Metastatic Non–Small Cell Lung Cancer That Is Anaplastic Lymphoma Kinase Positive. Clin Cancer Res 2014;20:2029–34. [DOI] [PubMed] [Google Scholar]
- 10.Govindan R, Mandrekar SJ, Gerber DE, et al. ALCHEMIST Trials: A Golden Opportunity to Transform Outcomes in Early-Stage Non–Small Cell Lung Cancer. Clin Cancer Res 2015;21:5439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen AP, Williams M, Kummar S, et al. Feasibility of molecular profiling based assignment of cancer treatment (MPACT): A randomized NCI precision medicine study. J Clin Oncol 2016;34:2539. [Google Scholar]
- 12.Lih C-J, Sims DJ, Harrington RD, et al. Analytical Validation and Application of a Targeted Next-Generation Sequencing Mutation-Detection Assay for Use in Treatment Assignment in the NCI-MPACT Trial. J Mol Diagn 2016;18:51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCI-MATCH study EAY131. 2019. (Accessed April 14, 2019, at https://ecog-acrin.org/nci-match-eay131.)
- 14.L H, A C, P OD, et al. Update on the “NCI-Molecular Analysis for Therapy Choice (NCI-MATCH/EAY131)’ precision medicine trial. EORTC 2017, #B80 (Poster Presentation) 2017. [Google Scholar]
- 15.Lih C-J, Harrington RD, Sims DJ, et al. Analytical Validation of the Next-Generation Sequencing Assay for a Nationwide Signal-Finding Clinical Trial: Molecular Analysis for Therapy Choice Clinical Trial. J Mol Diagn 2017;19:313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoury JD, Wang W-L, Prieto VG, et al. Validation of Immunohistochemical Assays for Integral Biomarkers in the NCI-MATCH EAY131 Clinical Trial. Clin Cancer Res 2018;24:521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conley BA, Gray R, Chen A, et al. Abstract CT101: NCI-molecular analysis for therapy choice (NCI-MATCH) clinical trial: interim analysis. Can Res 2016;76:CT101-CT. [Google Scholar]
- 18.Allen CE, Laetsch TW, Mody R, et al. Target and Agent Prioritization for the Children’s Oncology Group—National Cancer Institute Pediatric MATCH Trial. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons D, Janeway K, Patton D, et al. Identification of targetable molecular alterations on the NCI-COG Pediatric MATCH trial. Presented at ASCO Annual Meeting 2019; abstract #10011. [Google Scholar]
- 20.Allen CE, Parsons DW, Janeway K, et al. Target and Agent Prioritization for the Children’s Oncology Group—National Cancer Institute Pediatric MATCH Trial. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]


