Abstract
Flavodiiron proteins (FDPs) constitute a group of modular enzymes widespread in Bacteria, Archaea and Eukarya. Synechocystis sp. PCC 6803 has four FDPs (Flv1-4), which are essential for the photoprotection of photosynthesis. A direct comparison of light-induced O2 reduction (Mehler-like reaction) under high (3% CO2, HC) and low (air level CO2, LC) inorganic carbon conditions demonstrated that the Flv1/Flv3 heterodimer is solely responsible for an efficient steady-state O2 photoreduction under HC, with flv2 and flv4 expression strongly down-regulated. Conversely, under LC conditions, Flv1/Flv3 acts only as a transient electron sink, due to the competing withdrawal of electrons by the highly induced NDH-1 complex. Further, in vivo evidence is provided indicating that Flv2/Flv4 contributes to the Mehler-like reaction when naturally expressed under LC conditions, or, when artificially overexpressed under HC. The O2 photoreduction driven by Flv2/Flv4 occurs down-stream of PSI in a coordinated manner with Flv1/Flv3 and supports slow and steady-state O2 photoreduction.
Research organism: Other
Introduction
A-type flavodiiron proteins (Flvs or FDPs) were originally identified in strict and facultative anaerobes among Bacteria, Archaea and Protozoa and were considered to function in O2 and/or NO detoxification (Wasserfallen et al., 1998; Gonçalves et al., 2011; Folgosa et al., 2018). All FDPs share two conserved structural domains: the N-terminal metallo-β-lactamase-like domain, harboring a non-heme diiron center, where O2 and/or NO reduction takes place; and the C-terminal flavodoxin-like domain, containing a flavin mononucleotide (FMN) moiety. The structures of FDPs in anaerobic prokaryotes and eukaryotic protozoa have been resolved as homooligomers (dimer or tetramer comprised of two dimers) arranged in a ‘head-to-tail’ configuration, so that the diiron center of one monomer and the FMN of the other monomer are in close proximity to each other, which ensures rapid electron transfer between the two cofactors.
C-type FDPs, specific to oxygenic photosynthetic organisms, hold an additional flavin-reductase-like domain, coupled with extra cofactors (Romão et al., 2016; Folgosa et al., 2018). Synechocystis sp. PCC 6803 (hereafter, Synechocystis) possesses four genes encoding FDPs: sll1521 (Flv1), sll0219 (Flv2), sll0550 (Flv3) and sll0217 (Flv4). Recently resolved crystal structure of truncated Flv1 from Synechocystis revealed a monomeric form with a ‘bent’ configuration, however the organization of the additional flavin-reductase-like domain and the oligomeric structure remain unclear (Borges et al., 2019). Photosynthetic FDPs first gained attention in 2002, when recombinant Synechocystis Flv3 protein was shown to function in O2 reduction to water without producing ROS (Vicente et al., 2002). Later, it was demonstrated that Synechocystis Flv1 and Flv3 proteins function in vivo in the photoreduction of O2 downstream of Photosystem (PS) I (Helman et al., 2003). Since then, extensive research has been performed to reveal the crucial function of Flv1 and Flv3 (and their homologs, FLVA and FLVB in other photosynthetic organisms) as a powerful sink of excess photosynthetic electrons. This safeguards PSI and secures the survival of oxygenic photosynthetic organisms under fluctuating light intensities (Allahverdiyeva et al., 2013; Gerotto et al., 2016; Chaux et al., 2017; Jokel et al., 2018) or under short repetitive saturating pulses (Shimakawa et al., 2017). The Flv1- and Flv3-mediated light-induced alternative electron transport to O2 was named as the Mehler-like reaction, being a widespread pathway, operating in nearly all photosynthetic organisms from cyanobacteria up to gymnosperms, but lost in angiosperms (Allahverdiyeva et al., 2015; Ilík et al., 2017).
The Flv2 and Flv4 proteins are encoded by an operon, together with a small membrane protein, Sll0218. The flv4-sll0218-flv2 (hereafter flv4-2) operon is strongly induced in low inorganic carbon, Ci, (atmospheric 0.04% CO2 in air, LC) and high light conditions (Zhang et al., 2009). The operon structure is highly conserved in the genome of many β-cyanobacteria (Zhang et al., 2012; Bersanini et al., 2014). The flv4-2 operon-encoded proteins have been reported to function in photoprotection of PSII by acting as an electron sink, presumably transporting electrons from PSII or the plastoquinone (PQ) pool to an unknown acceptor (Zhang et al., 2009; Zhang et al., 2012; Bersanini et al., 2014; Chukhutsina et al., 2015). Since flv2, sll0218 and flv4 are co-transcribed, the contribution of each single protein of the operon to PSII photoprotection has been difficult to dissect. Recent data examining distinct and specific roles of the Flv2/Flv4 heterodimer and the Sll0218 protein (using a set of different mutants deficient only in Sll0218 or in Flv2 and Flv4) demonstrated that the majority of observed PSII phenotypes were actually due to the absence of Sll0218, thus leading to the conclusion that Sll0218 contributes to PSII repair and stability (Bersanini et al., 2017). However, the exact donor and acceptor of the Flv2 and Flv4 proteins have not yet been identified in vivo and possible cross-talk between all four FDPs has yet to be revealed, thus limiting our understanding of the function of FDPs on a cellular level.
In this work, to shed light on the in vivo function of Flv2 and Flv4 and to clearly separate the function of the Flv1/Flv3 heterooligomer from that of Flv2/Flv4, we employed a specific set of FDP mutants. These were: (i) the ∆flv1/∆flv3 mutant, deficient in both Flv1 and Flv3 proteins (Allahverdiyeva et al., 2011); (ii) ∆flv2 which does not express the Flv2 protein but retains a low amount of Flv4 and WT levels of Sll0218 (Zhang et al., 2012); (iii) ∆flv4 which is deficient in the accumulation of all three flv4-2 operon proteins (Zhang et al., 2012); (iv) ∆sll0218 which lacks the small Sll0218 protein, but expresses the Flv2 and Flv4 proteins (Bersanini et al., 2017); (v) ∆flv3/∆flv4 which is deficient in all four FDPs, whereby the absence of Flv3 results in a strong decrease in Flv1 (Mustila et al., 2016) and the inactivation of ∆flv4 affects the expression of the whole flv4-2 operon (Zhang et al., 2012); and, finally (vi) the flv4-2 operon overexpression strain, flv4-2/OE, expressing high amounts of Flv2, Flv4 and Sll0218 (Bersanini et al., 2014).
Here, we provide in vivo evidence for Flv2/Flv4 mediated O2 photoreduction in one of the most frequently studied cyanobacterial model organisms, Synechocystis. Unlike the powerful and rapid response proteins, Flv1 and Flv3, the Flv2 and Flv4 proteins are dispensable for survival under fluctuating light intensities. The expression of flv4 and flv2 under LC was found to be regulated by the pH of the growth media, with significant downregulation observed under strongly alkaline pH conditions. Results from this study provide important insights into the response of photosynthetic organisms to changes in Ci and how they regulate the availability of electron sinks.
Results
Extent and kinetics of the Mehler-like reaction in cells acclimated to low (LC) and high Ci (HC) conditions
Application of membrane inlet mass spectrometry (MIMS) with 18O-enriched oxygen allows differentiation between photosynthetic gross O2 production and O2 uptake under illumination. The flv4-2/OE cells, accumulating high amounts of Flv2, Sll0218 and Flv4 both in LC and HC (>1% CO2 in air, HC) conditions (Bersanini et al., 2014), demonstrated substantially higher O2 photoreduction rates compared to respective WT cells (Figure 1A, B and D). The Flv3 protein level was similar in flv4-2/OE and wild-type (WT) cells grown under both LC and HC (Figure 1C), strongly supporting the in vivo contribution of flv4-2 operon proteins to O2 photoreduction during illumination. Gross O2 evolution rates of flv4-2/OE and WT cells grown under LC did not differ significantly from each other. However, a significant increase in the gross O2 evolution rate was observed in HC grown flv4-2/OE cells (Figure 1—source data 2).
Figure 1. O2 reduction rates and Flv3 and Flv4 protein accumulation in cells grown in low (LC) and high CO2 (HC).
(A, B) O2 reduction rate of WT, flv4-2/OE and (D) the M55 mutant (∆ndhB) was recorded in darkness (gray background) and under illumination (white background). The experiment was conducted in three independent biological replicates and a representative plot is shown. (Figure 1—source data 1). (C) Immunoblot detection of Flv3 and Flv4 in WT and flv4-2/OE. Pre-cultures were grown in BG-11, pH 8.2 under 3% CO2 (HC) for 3 days, after that cells were harvested and resuspended in fresh BG-11, pH 8.2 at OD750 = 0.2. The experimental cultures were grown under HC or under LC. For the MIMS experiments the cells were harvested and resuspended in fresh BG-11, pH 8.2 at 10 µg Chl a mL−1. O2 photoreduction was recorded during the transition from darkness to high-light intensity of 500 µmol photons m−2s−1. In order to create comparable conditions for MIMS measurements, LC-grown cells were supplemented with 1.5 mM NaHCO3 prior to the measurements. Independent experiments performed on WT cells grown in BG-11 lacking Na2CO3, but supplied with 1.5 mM NaHCO3 prior to MIMS measurement showed no significant difference in O2 photoreduction rates (Figure 1—figure supplement 2), thus allowing confident comparison of the MIMS results. Different phases of O2 photoreduction kinetics are indicated as {I}, {II}, {III}. 50% WT, corresponds to 1:2 diluted WT total protein sample.
Figure 1—figure supplement 1. O2 reduction rates under high CO2.
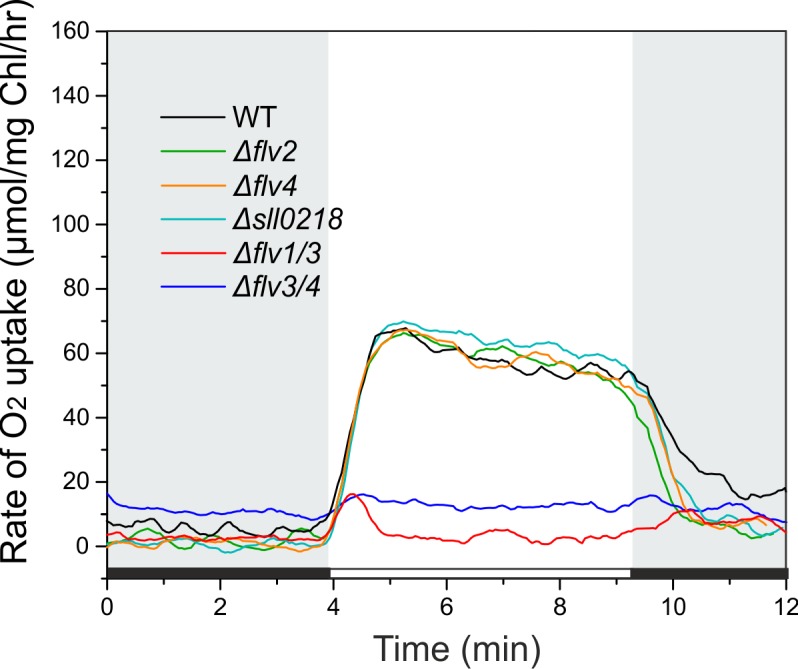
Figure 1—figure supplement 2. O2 reduction rates during the dark-to-light transition of WT cells with and without addition of 1.5 mM NaHCO3 prior MIMS measurements.
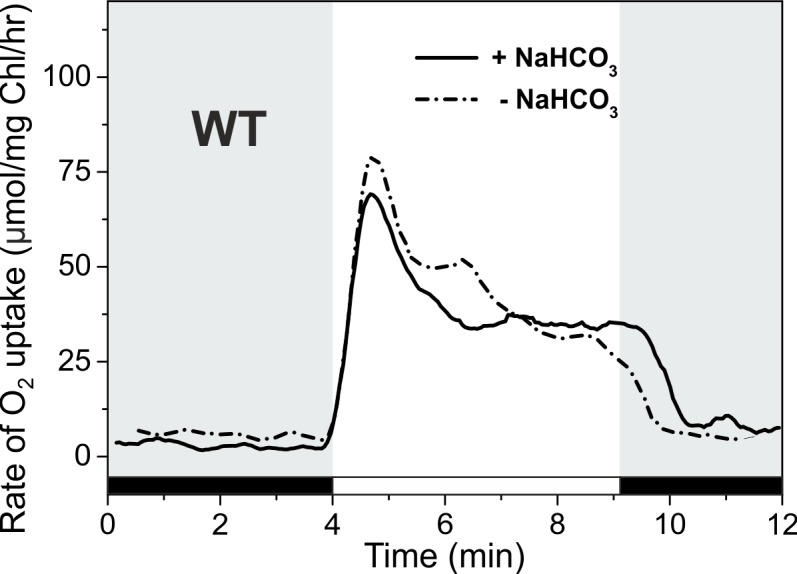
As reported earlier, the Ci level has a remarkable effect on the expression of FDPs at both transcript and protein level: Flv2, Flv4 and Flv3 have been shown to be strongly upregulated under LC (Zhang et al., 2009; Wang et al., 2004; Battchikova et al., 2010), and down-regulated upon a shift to HC (Zhang et al., 2009; Hackenberg et al., 2009; Figure 1C). Nevertheless, a direct comparison of the efficiency and kinetics of the Mehler-like reaction in HC- and LC-acclimated cells has not been reported, thus the contribution of different FDPs to O2 photoreduction has been difficult to assess. Our initial approach to evaluating the contributions of the different FDPs was based on determining the activity of the Mehler-like reaction in Synechocystis cells grown under LC and HC (3% CO2) conditions, at pH 8.2.
After a shift from darkness, WT cells demonstrated a rapid light-induced O2 uptake under both LC and HC conditions (59 ± 6.4 and 56 ± 6.4 µmol O2 mg Chl a−1 h−1, respectively). This fast induction phase is designated as {I} in Figure 1A and B. Yet, the kinetics of O2 photoreduction in LC-grown cells differed from those grown under HC. In the LC-grown WT cells, the fast induction phase {I} was followed by a clear biphasic quenching of O2 reduction, namely by the strong decay phase {II}, which continued for about one minute, followed by a quasi-stable state, phase {III} (~33 ± 5.9 µmol O2 mg Chl a−1 h−1) during illumination. Contrasting this, in HC-grown WT cell, the light-induced O2 reduction rate achieved in phase {I} declined only slightly during the first 2–3 min (from ~56 ± 7.7 to~48 ± 6.3 µmol O2 mg Chl a−1 h−1). Thereafter, the rate remained relatively steady for at least 5 min (Figure 1B) of illumination. In flv4-2/OE cells, grown both in LC- and HC, light-induced O2 reduction was stronger that in the WT. Nevertheless, the kinetic phases of O2 photoreduction in flv4-2/OE cells resembled those of respective WT cells, being relatively stable under HC and demonstrating a strong biphasic quenching under LC.
Upon a shift from darkness to light, the ∆flv2 and ∆flv4 mutants grown under HC conditions demonstrated a similar O2 photoreduction pattern as the WT (Figure 1—figure supplement 1). A negligible amount of Flv2 and Flv4 protein in the WT cells grown under HC (Zhang et al., 2009; Zhang et al., 2012; Figure 1C) explains their lack of contribution to the Mehler-like reaction. The near absence of any light-induced O2 reduction in the ∆flv3/∆flv4 and ∆flv1/∆flv3 mutants (Figure 1—figure supplement 1) confirms that the small amount of the Flv1/Flv3 heterodimers (decreased Flv3 protein accumulation in HC compared to LC conditions, Figure 1C), is responsible for the constant Mehler-like reaction under the HC condition (Helman et al., 2003).
To uncover the reason for the fast decay of O2 photoreduction observed under LC conditions (Figure 1A), we first tested putative competition between the NAD(P)H:quinone oxidoreductase (NDH-1) complex and FDPs for available photosynthetic electrons. The NDH-1 complex is a powerful machinery utilizing electrons for cyclic electron transport (CET) around PSI, CO2 uptake and respiration under LC conditions (Zhang et al., 2004; Schuller et al., 2019). To this end, O2 photoreduction was measured in the M55 mutant (ΔndhB), which is deficient in the hydrophobic NdhB subunit (Ogawa, 1991) and thus lacks all NDH-1 complexes (Zhang et al., 2004). The M55 mutant cells (grown under LC, pH 8.2 conditions) demonstrated a fast induction of O2 photoreduction (phase I) similar to the WT, which continued at steady-state, lacking the second phase of O2 photoreduction after the dark-to-light transition (Figure 1D). Importantly, the M55 mutant showed a slow induction (see phase I of gross O2 evolution in Figure 1—source data 2) and considerably lower gross O2 evolution rate compared to the WT cells (see phase III of gross O2 evolution in Figure 1—source data 2). This suggests that a steady-state O2 photoreduction in M55 is not due to increased electron flow from PSII. The lack of a strong second phase in O2 photoreduction kinetics resembles the situation in WT cells grown under HC (Figure 1B; Figure 1—figure supplement 1), where the expression of the NDH-1 complex is strongly reduced, and thus suggests competition for electrons between the NDH-1 complexes and FDPs under LC conditions.
The extent and kinetics of the Mehler-like reaction are strongly dependent on the pH and carbonate concentration of the growth medium
The pH and the presence of carbonate in the growth medium were evaluated as possible modulators of the extent and kinetics of the Mehler-like reaction and the accumulation of FDPs under LC conditions. Standard BG-11 medium containing sodium carbonate (Na2CO3) at a final concentration of 0.189 mM was used for all growth experiments, other than those indicated to be Ci limited. In these experiments, performed under atmospheric CO2, Ci limitation was achieved by omitting Na2CO3 from the BG-11 growth media.
The effect of pH
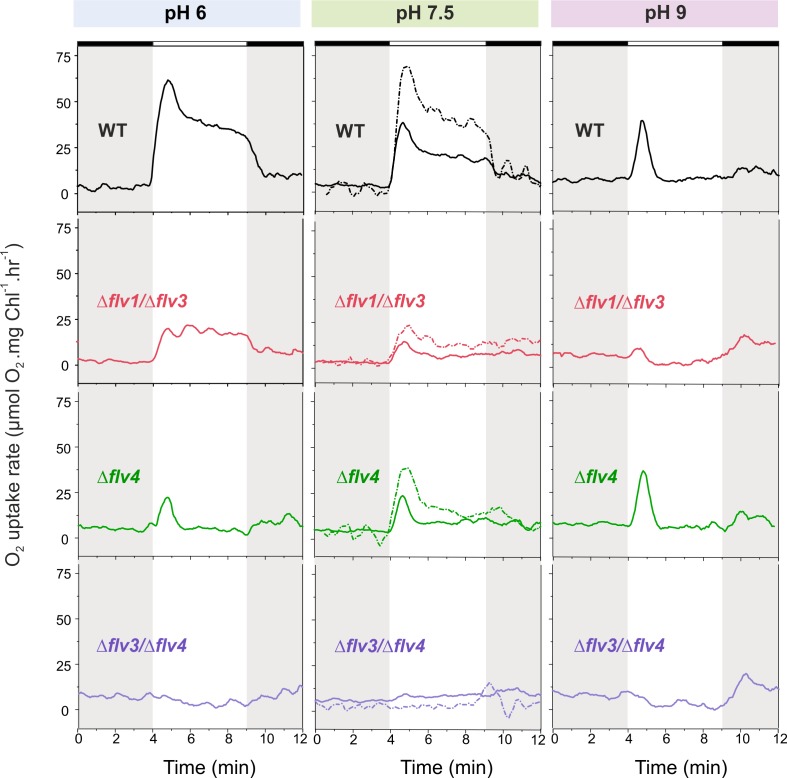
The WT cells grown at pH 9 demonstrated a strong but only transient Mehler-like reaction: the O2 photoreduction rate reached its maximum during the first 30 s of illumination, then quickly dropped (within 1 min) to the initial level of dark O2 uptake (Figure 2, right panel). Similarly to the WT, the ∆flv4 mutant cells demonstrated only a transient O2 photoreduction upon illumination. There was no significant O2 photoreduction detected for ∆flv1/∆flv3 and ∆flv3/∆flv4 mutants grown at pH 9.
Figure 2. O2 reduction rates of WT and FDP mutants grown at different pH levels.
O2 reduction rate was recorded in darkness (gray background) and under illumination with actinic white light at an intensity of 500 µmol photons m−2 s−1 (white background). Pre-cultures were grown in standard BG-11 medium (containing Na2CO3 at a final concentration of 0.189 mM) under HC for 3 days at different pH levels. For MIMS experiments, cells were shifted to LC at OD750≈0.2 (same pH) and grown for 4 days before measurements. Exceptions were: (i) pH 6 experimental cultures were inoculated from pH 8.2 pre-cultures; and (ii) pH 7.5 pre-culture was shifted to LC in standard BG-11 containing Na2CO3 at a final concentration of 0.189 mM or in BG-11 without Na2CO3 (dotted line ‘- Na2CO3’). The experiment was conducted in three independent biological replicates (except experiment at pH 6 with n = 2 independent biological replicates) and a representative plot is shown. (Figure 2—source data 1). In order to create comparable conditions for MIMS measurements, all cells were supplemented with 1.5 mM NaHCO3 prior to the measurements.
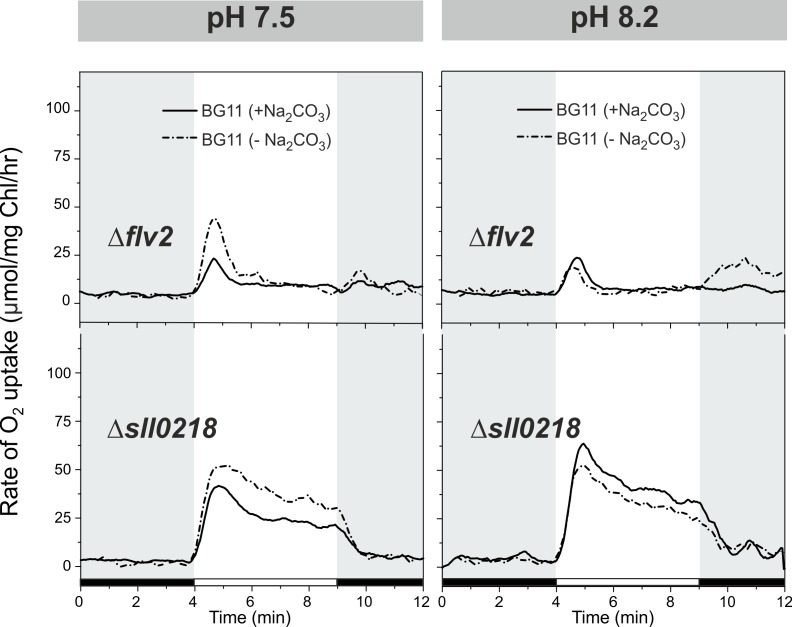
Figure 2—figure supplement 1. O2 photoreduction rates of the ∆flv2 and ∆sll0218 mutants grown at LC pH 7.5 and 8.2 with and without Na2CO3.
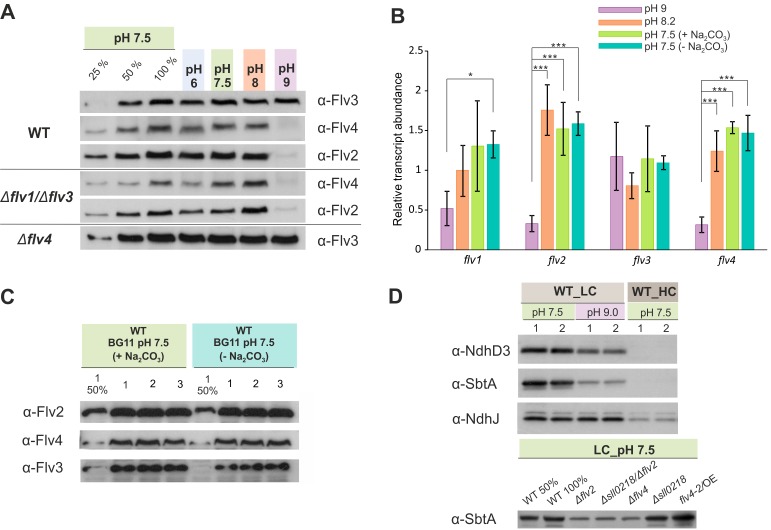
Immunoblotting using specific antibodies showed that, as for WT cells grown under HC (Figure 1D), Flv2 and Flv4 proteins were almost undetectable in the WT grown under LC at pH 9 (Figure 3A).
Figure 3. The effect of the pH of growth medium on the protein and transcript accumulation.
(A, B) The effect of the pH and (B, C) sodium carbonate in the growth medium (A, C) on the protein and (B) transcript levels of FDP. (D) Protein immunoblots demonstrating the accumulation of bicarbonate transporter (SbtA) and NDH-1 subunits (NdhD3 and NdhJ) in the cells grown at different pH and CO2 concentration. Cells were pre-grown at different pH levels (+Na2CO3) under HC for 3 days, harvested, resuspended in fresh BG-11 (pH maintained), adjusted to OD750≈0.2 and shifted to LC for 4 days. At pH 7.5, the cells were grown at LC in the presence (+ Na2CO3, at final concentration of 0.189 mM) or in the absence (- Na2CO3) of sodium carbonate (B, C). Transcript abundance is presented as mean ± SD, n = 2–4 biological replicates, asterisks indicate a statistically significant difference to the WT (*p<0.05; ***p<0.001) (Figure 3—source data 1). Numbers 1–3 indicate different biological replicates. 25% and 50% correspond to 1:4, 1:2 diluted total protein sample, and 100% indicates undiluted total protein sample.
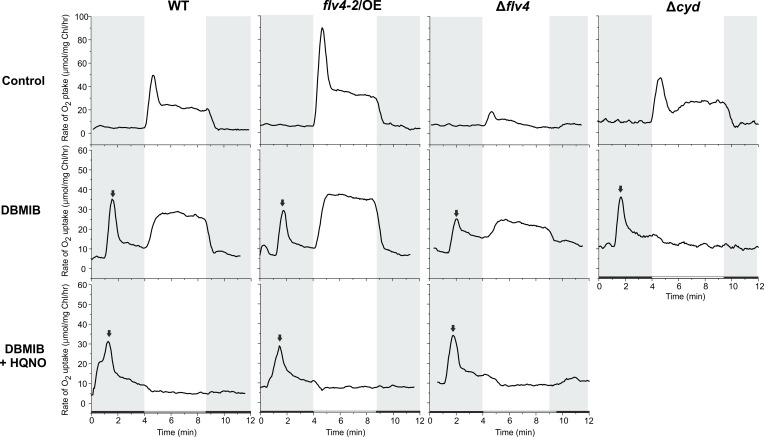
Figure 3—figure supplement 1. O2 uptake in the WT, flv4-2/OE, ∆flv4 and ∆cyd mutant.
In line with protein data, the transcript levels of both flv2 and flv4 were significantly down-regulated in the cells grown at pH 9 (Figure 3B), suggesting a pH-dependent transcriptional regulation of flv4 and flv2. This is consistent with earlier transcriptional profiling experiments reporting downregulation of flv2 and flv4 transcripts after transferring Synechocystis from pH 7.5 to pH 10 (Summerfield and Sherman, 2008). Importantly, the accumulation of Flv3 was not affected at pH 9. These results strongly suggest that the conspicuous but transient O2 photoreduction observed in the WT and ∆flv4 mutant cells at pH 9 originates mainly from the activity of Flv1/Flv3 heterodimer.
The WT cells grown at pH 6, at pH 7.5 (Figure 2, left and middle panels, respectively) and at pH 8.2 (Figure 1A) demonstrated a rapid induction of O2 reduction (phase {I}) followed by a biphasic decay during illumination: a fast decay phase (phase {II}) and a quasi-stable phase (phase {III}) (Figures 1A and 2). The highest O2 photoreduction rate was observed in the WT cells grown at pH 6 (Figure 2).
Importantly, the ∆flv1/∆flv3 mutant also showed residual O2 photoreduction: only a small O2 uptake was noticeable at pH 7.5, whereas at pH 6 the O2 photoreduction rate was substantial and constant during 5 min of illumination (Figure 2). Unlike the ∆flv1/∆flv3 mutant, both the ∆flv2 (Figure 2—figure supplement 1) and ∆flv4 (Figure 2) mutants showed a strong transient O2 photoreduction phase, peaking around the first 30 s of illumination and decaying quickly thereafter. This occurred at all tested pH levels. These results together with those demonstrating highly increased rates of O2 photoreduction in the overexpression strain flv4-2/OE (Figure 1B) collectively confirm the in vivo involvement of both Flv2 and Flv4 proteins in O2 photoreduction. The O2 photoreduction kinetics of the ∆sll0218 mutant resembled that of the WT (Figure 1—figure supplement 1 and Figure 2—figure supplement 1), indicating that the Sll0218 protein does not contribute to the Mehler-like reaction under the HC and LC conditions studied here. These results led us to exclude the ∆sll0218 mutant from any further experiments included in this section.
The data presented above allowed us to make preliminary conclusions about the origin of the different kinetic phases of O2 photoreduction. Since a transient O2 photoreduction was characteristic for the WT, ∆flv2 and ∆flv4 cells, but almost undetectable for ∆flv1/∆flv3, it is conceivable that the Flv1/Flv3 heterodimer is mostly responsible for the strong and transient O2 uptake during dark-light transitions, whilst Flv2/Flv4 contributes to steady-state O2 photoreduction under LC (see ∆flv1/∆flv3 particularly at pH 6, Figure 2). The complete lack of O2 photoreduction in the ∆flv3/∆flv4 mutant (representing deficiency of all four FDPs) is in line with this hypothesis. Importantly, there was no significant difference in the gross O2 evolution rates observed between the wild-type and the FDP mutants (Figure 1—source data 2).
It is not only FDPs, but also distinct variants of the NDH-1 complex as well as HCO3- transporters (Zhang et al., 2004) which are known to respond to CO2 and pH levels of the growth medium. Immunoblotting was performed to evaluate the abundances of NdhD3, representing a low Ci-inducible NDH-1MS complex, and SbtA, a high-affinity low Ci-inducible Na+/HCO3- transporter, in WT and different mutants under conditions used for the MIMS experiments.
As expected, in WT cells grown at pH 7.5, NdhD3 and SbtA were not detected under HC conditions, but both proteins were strongly accumulated in LC (Figure 3D). However, in LC conditions, the increase in alkalinity of the growth medium to pH 9 resulted in markedly lower levels of NdhD3 and SbtA accumulation compared to those observed at pH 7.5. The effect was more pronounced in the case of SbtA. Interestingly, the ∆flv2 and ∆flv4 mutants demonstrated a decrease of SbtA accumulation compared to WT even at pH 7.5 in LC, whereas in flv4/OE SbtA remained at the same level as in WT (Figure 3D).
The expression of the SbtA protein closely followed the changes in the expression of Flv2 and Flv4 proteins under all growth conditions, suggesting that Flv2/Flv4 and the Ci uptake mechanisms, particularly the inducible high-affinity Na+/HCO3- transporter, share a common regulatory pathway of protein expression.
Unlike the growth media at pH 6–8.2, the Ci-pool at pH 9 contains an additional species, CO32-. It is possible that a small amount of CO32- in the external growth medium acts as a signal to trigger the regulation of flv2 and flv4 expression via antisense RNA as1-flv4 and the master transcription factors, ndhR or cmpR (Eisenhut et al., 2012). Considering that the double negative charge of CO32- prevents its diffusion through the cell membrane, and the fact that an active carbonate uptake transporter is currently unknown, we cannot yet consider CO32- to be an internal sensor. To gain further insight to the carbonate effect on O2 photoreduction, MIMS experiments were performed on FDP mutants grown in BG-11 medium in the presence (0.189 mM) and absence of sodium carbonate.
The effect of sodium carbonate
Culturing the cells without Na2CO3 at pH 7.5 clearly enhanced O2 photoreduction in the WT and all studied FDP mutants (Figure 2, middle panel). Despite such a clear variation in O2 photoreduction rates in the WT, no significant difference in gene transcript (Figure 3B) and protein levels (Figure 3C) of FDPs were observed in the presence or absence of Na2CO3.
FDP induced O2 photoreduction does not occur at PSII or PQ-pool level
In order to establish where in the electron transport chain the Flv2/Flv4 heterodimer-related O2 photoreduction occurs, we focused on the flv4-2/OE mutant (grown at LC, pH 7.5, without carbonate). This mutant showed especially high accumulation of Flv2 and Flv4 proteins and a higher O2 photoreduction rate than the WT (Figure 1). When linear electron transport was blocked at Cytochrome b6f (Cyt b6f) level using DBMIB as an inhibitor (Draber et al., 1970; Yan et al., 2006), both the WT (Ermakova et al., 2016) and flv4-2/OE mutant cells demonstrated a strong light-induced O2 uptake (Figure 3—figure supplement 1). As expected, in the Δcyd mutant the light-induced O2 uptake was not detected in the presence of DBMIB (Ermakova et al., 2016), Figure 3—figure supplement 1). The addition of HQNO, an inhibitor of Cytochrome bd quinol oxidase (Cyd) (Pils et al., 1997) and Cyt b6f (Fernández-Velasco et al., 2001) to the DBMIB-treated WT and flv4-2/OE completely eliminated O2 photoreduction. These results confirmed that Cyd was solely responsible for the observed O2 photoreduction occurring at the PQ-pool level.
Growth phenotype of FDP deletion mutants under fluctuating light intensities
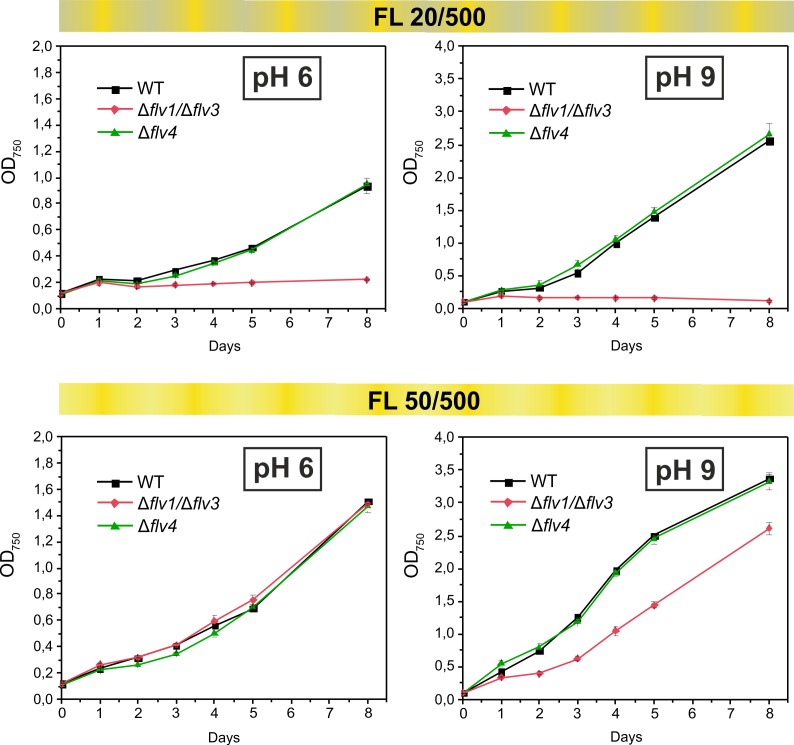
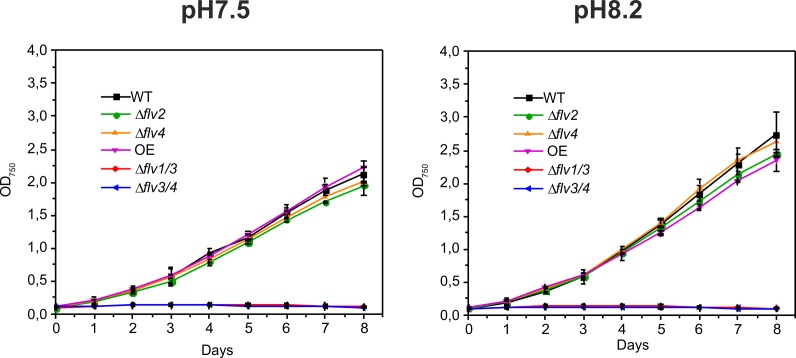
We have previously demonstrated that the Flv1/Flv3 heterodimer enables cell growth under fluctuating light, by functioning in the Mehler-like reaction as an efficient electron sink (Allahverdiyeva et al., 2013). However, the results of the current study clearly suggest an additional involvement of the Flv2/Flv4 heterodimer in the Mehler-like reaction, particularly under conditions of LC and at pH values of 8.2 or lower (Figures 1 and 2). These findings led us to more precisely examine the combined effects of the pH of the growth medium and the fluctuating growth light conditions (FL) on the growth performance of various FDP mutants. To this end, both severe (FL20/500, when 20 µmol photons m−2 s−1 background light was interrupted every 5 min by 30 s light pulse intensity of 500 µmol photons m−2 s−1) and mild (FL50/500, when 50 µmol photons m−2 s−1 background light was interrupted every 5 min by 30 s light pulse intensity of 500 µmol photons m−2 s−1) fluctuating lights were applied at different levels of pH. In line with our previous work, the ∆flv1/∆flv3 mutant (also ∆flv3/∆flv4) failed to grow under severe (FL20/500) light fluctuations, independent of the pH of the growth medium (Figure 4; Figure 4—figure supplement 1). Differently to the severe FL20/500 condition, under mild fluctuating light (FL50/500), the ∆flv1/∆flv3 mutant demonstrated slower growth than the WT under alkaline pH (pH 9, Figure 4 and pH 8.2 (Mustila et al., 2016), Figure 4—figure supplement 1)). Growth was similar to the WT at pH 7.5 (Mustila et al., 2016), Figure 4—figure supplement 1) and pH 6 (Figure 4). Importantly, the ∆flv4 mutant grew similarly to the WT at all studied pH levels, both under mild and severe FL conditions (Figure 4). The ∆flv2, ∆sll0218 and flv4-2/OE mutants also demonstrated similar growth to the WT under severe FL20/500 at pH 7.5 and 8.2 (Figure 4—figure supplement 1).
Figure 4. Growth curves of the different FDPs mutants under fluctuating light intensities.
Pre-cultures were grown in BG-11 medium under HC for 3 days illuminated with constant light of 50 µmol photons m−2 s−1. The cells pre-grown at pH 9 or pH 8.2 (for experimental culture at pH 6) were harvested, resuspended in fresh BG-11 (pH 9 or 6), adjusted to OD750 = 0.1 and shifted to LC. Experimental cultures were grown under FL 20/500 or 50/500 regime for 8 days. The experiment was conducted in two independent biological replicates and average values was plotted.
Figure 4—figure supplement 1. Growth curves of the different FDP mutants under fluctuating light intensities (FL20/500 - 20 μmol photons m−2s−1 background light is interrupted with 30 s of 500 μmol photons m−2s−1 light every 5 min).
The results above strongly suggest that, in contrast to the Flv1/Flv3-originated Mehler-like reaction, Flv2/Flv4-driven O2 photoreduction is not essential for the survival of cells under fluctuating light.
Effect of increasing light intensities on the Mehler-like reaction
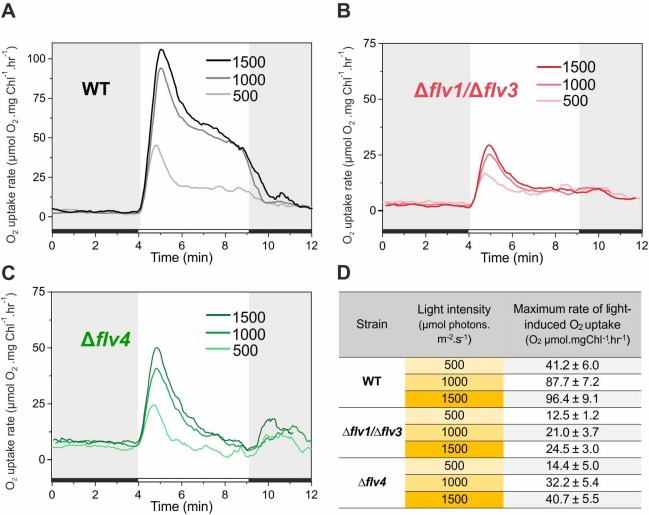
In order to assess the response of the O2 photoreduction to different light intensities, the WT, ∆flv4 and ∆flv1/∆flv3 mutant cells were illuminated with 500, 1000 and 1500 µmol photons m−2 s−1 white light (Figure 5). Under LC conditions, increasing the light intensity from 500 to 1000 µmol photons m−2 s−1 resulted in a two-fold increase of the maximum O2 photoreduction rate in the WT (Figure 5A and D). The further increase (1500 µmol photons m−2 s−1) only slightly enhanced (2.3-fold) the maximum O2 photoreduction rate, suggesting that the applied light intensity was nearly saturating. Likewise, the ∆flv4 mutant demonstrated about 1.9- and 2.3-fold enhancements of the maximum rate of transient light-induced O2 reduction under 1000 and 1500 µmol photons m−2 s−1, respectively (Figure 5C and D). Contrasting this was the results of the ∆flv1/∆flv3 mutant, which showed lesser responses to increasing light intensities (1.6- and 1.8-fold enhancement in the maximum rate at 1000 and 1500 µmol photons m−2 s −1, respectively) (Figure 5B and D). It is important to note that both the ∆flv4 and ∆flv1/∆flv3 mutants accumulate nearly the WT level of the Flv3 or Flv4/Flv2 proteins, respectively (Zhang et al., 2009; Mustila et al., 2016). Moreover, increasing light intensity from 500 to 1500 µmol photons m−2 s−1 also resulted in enhancement of the O2 photoreduction rate in the WT cells grown under HC (Figure 5—figure supplement 1).
Figure 5. Rates of O2 reduction in response to increasing light intensity in WT, ∆flv1/∆flv3 and ∆flv4 mutant cells (A, B, C, respectively).
O2 reduction rate was recorded in darkness (gray background) and under illumination with actinic white light intensities of 500, 1000 and 1500 µmol photons m−2 s−1 (white background). In order to create comparable conditions for MIMS measurements, all cells were supplemented with 1.5 mM NaHCO3 prior to the measurements. Pre-cultures were grown in BG-11 medium (pH 7.5) under 3% CO2 (HC) for 3 days and then shifted to LC (atmospheric 0.04% CO2 in air) at OD750 = 0.2 and pH 7.5 for 4 days. For MIMS measurements, cells were harvested and resuspended in fresh BG-11 medium at a Chl a concentration of 10 µg mL−1. (D) Maximum rate of light-induced O2 uptake (O2 µmol mgChl a−1 hr−1) of WT, ∆flv1/∆flv3 and ∆flv4 mutant cells at different light intensities applied. The experiment was conducted in three independent biological replicates and a representative plot is shown (Figure 5—source data 1).
Figure 5—figure supplement 1. Rates of O2 reduction in response to increasing light intensity in WT and ∆flv1/∆flv3 mutant cells grown under 3% CO2 (HC).
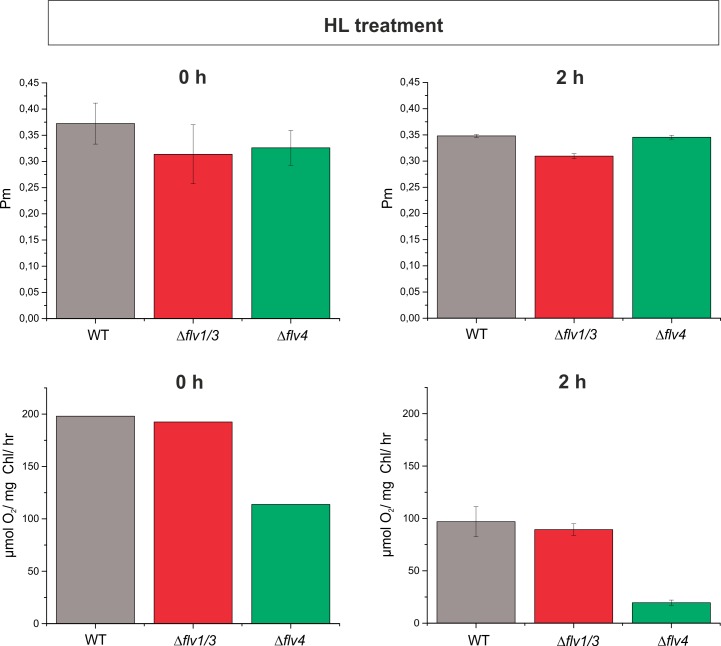
Figure 5—figure supplement 2. The maximum oxidisable amount of P700 (Pm) and PSII activity of the WT, ∆flv1/∆flv3 and ∆flv4 mutant cells.
The fast and transient response of ∆flv4 mutant cells to drastic increases in light intensity (Figure 5C) confirmed the high capacity of Flv1/Flv3-related O2 photoreduction to act as an electron sink. These results explain the essential role of Flv1/Flv3, unlike Flv2/Flv4, for the survival of cells under fluctuating light intensities. Intriguingly, both the fast induction phase {I} and quasi-stable phase {III} of O2 photoreduction rates of the WT were greater than the sum of the individual O2 photoreduction rates from ∆flv1/∆flv3 and ∆flv4, implying a strong enhancement of O2 photoreduction by various oligomer activities in the presence of all four FDPs.
Echoing trends seen in O2 photoreduction rates, gross O2 evolution rates of the WT strongly enhanced with increasing light intensities (1.6- and 1.8-fold increase in 1000 and 1500 µmol photons m−2 s−1, respectively), whereas the Δflv4 mutant showed only limited increases of gross O2 evolution rates (1.3- and 1.5-fold in 1000 and 1500 µmol photons m−2 s−1, respectively), and Δflv1/∆flv3 O2 evolution rates were already at maximum levels under the lowest light intensity of 500 µmol photons m−2 s−1 (Figure 1—source data 2). It is worth mentioning that, neither the Δflv1/∆flv3 nor Δflv4 mutant achieved a steady-state gross O2 evolution during the 5 min of illumination: Δflv1/∆flv3 demonstrated gradual increase, whereas Δflv4 showed gradual decrease in gross O2 evolution. Next, PSII (O2 evolving activity monitored in the presence of artificial electron acceptor, DMBQ) and PSI (maximum oxidizable amount of P700, Pm) activities were measured in cells grown under moderate light (50 µmol photons m−2 s−1) and exposed to high light (1500 µmol photons m−2 s−1) for 2 hr. After 2 hr of high light treatment, ∆flv1/∆flv3 showed no significant difference in the maximum oxidizable amount of P700 (Pm) and PSII activity compared to the WT and Δflv4 mutant (Figure 5—figure supplement 2). This is in line with previous studies proving that other photoprotective mechanisms are able to replace Flv1/Flv3 (Zhang et al., 2009) unless the cells experience abrupt fluctuations in light intensity (Allahverdiyeva et al., 2013). It has already been shown that a strong high light (1500 µmol photons m−2 s−1) causes slightly slow growth and a short high light treatment decreases PSII activity in the Δflv4 mutant compared to the WT (Figure 5—figure supplement 2; Zhang et al., 2009; Bersanini et al., 2014; Bersanini et al., 2017). Importantly, Δflv4 demonstrated a Pm level comparable to that of the WT after 2 hr of high-light treatment. This suggests the importance of the Flv2/Flv4 driven steady-state O2 photoreduction in photoacclimation, by the prevention of PSII photodamage caused by the over-reduction of the photosynthetic chain.
The functional expression of FDPs is highly modulated by Ci conditions and light penetration
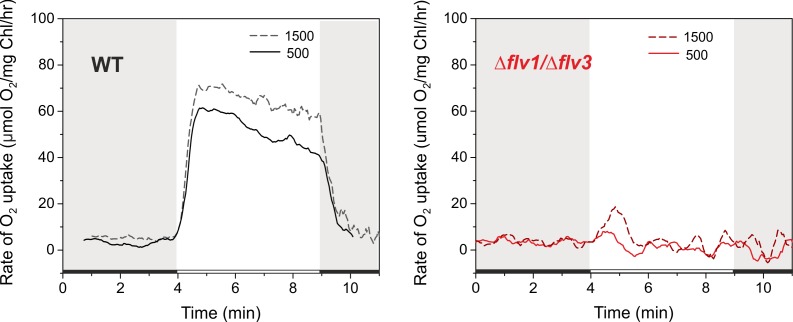
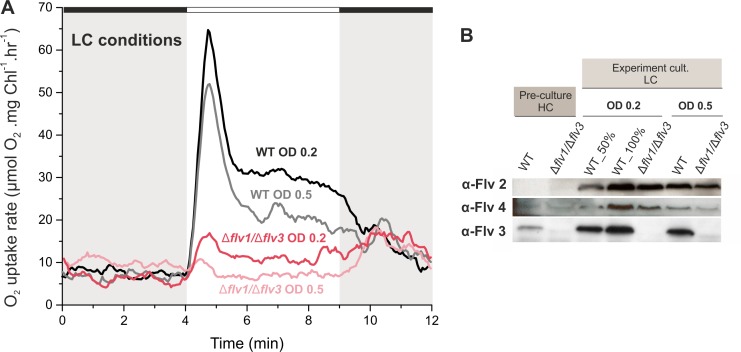
The inoculum size (starting OD750 value) determines the extent of light penetration upon starting a cultivation. In previous studies, cells were pre-grown in HC, then harvested at late logarithmic phase and inoculated in fresh BG-11 (pH 8.2) at OD750≈0.4–0.5, before shifting to LC for the next 3 days (Allahverdiyeva et al., 2011; Allahverdiyeva et al., 2013; Ermakova et al., 2016). To ensure better light penetration of the cultures and to improve the acclimation of cells to the conditions used in this study, the experimental WT and ∆flv1/∆flv3 cultures were inoculated at a low OD750≈0.1–0.2 and then cultivated for 4 days (instead of 3 days in previous studies). The WT cells grown under LC from a lower OD (OD750≈0.2) demonstrated notably higher O2 uptake during illumination, compared to the cells shifted to LC at OD750≈0.5 (Figure 6A). Importantly, the ∆flv1/∆flv3 mutant cells shifted to LC at a lower OD (OD750≈0.2) also demonstrated a residual steady-state O2 photoreduction activity.
Figure 6. Effect of inoculum size on the O2 photoreduction and accumulation of FDPs in the WT and Δflv1/Δflv3 mutant cells.
(A) Rates of O2 uptake measured by MIMS during darkness (gray background) and under illumination with actinic white light at an intensity of 500 µmol photos m−2s−1 (white background). In order to create comparable conditions for MIMS measurements, all cells were supplemented with 1.5 mM NaHCO3 prior to the measurements. (B) Protein immunoblots showing the relative accumulation of different FDPs in the WT and Δflv1/Δflv3 mutant cells. Pre-cultures were grown in BG-11 (pH 8.2) under HC until late logarithmic phase (OD750≈2.5), then harvested and inoculated in fresh BG-11 under LC at OD750 = 0.2 for 4 days or OD750 = 0.5 for 3 days. The experiment was conducted in three independent biological replicates and a representative plot is shown in (A). WT_50% corresponds to 1:2 diluted total protein sample and 100% to undiluted total protein sample.
Immunoblot analysis using specific FDP antibodies showed that the WT cells transferred from HC to LC at OD750 = 0.2 accumulated higher amount of the Flv2, Flv3 and Flv4 proteins compared to the cells shifted to LC at OD750 = 0.5 (Figure 6B). A similar trend was also observed in the ∆flv1/∆flv3 mutant, which accumulated more Flv2 and Flv4 when cultivated at LC from OD750 = 0.2. This is in line with previous results showing that the accumulation of flv2 and flv4 transcripts in Synechocystis (upon a shift from HC to LC, Zhang et al., 2009) and vegetative cell-specific flv1A and flv3A transcripts in Anabaena sp. PCC 7120 (upon a shift from dark to light, Ermakova et al., 2013) strongly depended on light intensity.
The results above highlight that Ci and light penetration upon a shift of cells from pre-culture conditions to different experimental conditions highly modulate the functional expression of FDPs.
Discussion
The Flv2/Flv4 heterodimer contributes to the Mehler-like reaction when naturally expressed under LC conditions or artificially overexpressed under HC
By characterizing Synechocystis mutants specifically affected in the accumulation of various FDPs, we show here that Flv2 and Flv4, together with Flv1 and Flv3 proteins, are involved in O2 photoreduction in vivo. Until recently, it has generally been accepted that the Flv1/Flv3 proteins safeguard PSI under both HC and LC conditions (Allahverdiyeva et al., 2013), whereas proteins encoded by the flv4-2 operon and being highly expressed under LC, function in the photoprotection of PSII, presumably by directing excess electrons from PSII to an as yet unknown acceptor (Zhang et al., 2009; Zhang et al., 2012; Shimakawa et al., 2015). The possibility of an Flv2/Flv4 contribution to O2 photoreduction in vivo was neglected due to a lack of evidence for light-induced O2 uptake in ∆flv1 and/or ∆flv3 mutants (Helman et al., 2003; Allahverdiyeva et al., 2011; Allahverdiyeva et al., 2013). Thus, Flv1 and Flv3 were assumed to be solely responsible for the Mehler-like reaction. Recently, it was demonstrated that Synechocystis Flv4 expressed in E. coli is capable of NADH-dependent O2-reduction in vitro (Shimakawa et al., 2015). However, the reported reaction rate was extremely low (almost residual) compared to the activity of FDP for example from anaerobic protozoa (Di Matteo et al., 2008) and the enzyme showed no affinity to NADPH. A similar scenario was previously presented for the Flv3 protein, where in vitro studies performed on recombinant Synechocystis protein led to a claim that Flv3 functions as a homodimer in NADH-dependent O2 reduction (very low affinity to NADPH) (Vicente et al., 2002), whilst subsequent study with ∆flv1-OEflv3 (or ∆flv3-OEflv1) mutants clearly demonstrated that homooligomers of Flv3 (or Flv1) do not function in O2 photoreduction in vivo (Mustila et al., 2016). Such discrepancies between the in vitro and in vivo results suggest that the in vitro assays conducted thus far have apparently failed to take into full consideration all the complex intracellular interactions, for example the involvement of Fed or FNR as an electron donor for FDPs, or the in vitro experiments do not necessarily demonstrate the processes occurring in vivo.
In this study, we provide compelling evidence for the in vivo contribution of Flv2/Flv4 to O2 photoreduction by applying 18O-labeled-oxygen and real-time gas-exchange measurements to distinct FDP deletion mutants. The inactivation of flv2 or flv4 is shown to result in a substantial decrease of O2 photoreduction in the mutants compared to the WT, while the overexpression of the flv4-2 operon increases the rate of O2 photoreduction approximately two-fold. In addition, the possibility that the small protein Sll0218 contributes to the Mehler-like reaction is excluded (Figure 1—figure supplement 1, compare Figure 2—figure supplement 1 and Figure 2).
It is noteworthy that both the ∆flv2 (deficient in Flv2 but retaining a low amount of Flv4) and ∆flv4 (deficient in both Flv2 and Flv4) mutants showed similar inhibition of O2 photoreduction rates, thus supporting the function of Flv2/Flv4 as a heterodimer in the Mehler-like reaction. The existence of the Flv2/Flv4 heterodimer has been proved biochemically in Synechocystis (Zhang et al., 2012). Nonetheless, our data do not exclude the possibility that Flv2/Flv2 and/or Flv4/Flv4 homooligomers are also involved in processes other than O2 photoreduction. Such a situation occurs with the Flv1 and Flv3 proteins, which contribute as homooligomers to the photoprotection of cells under fluctuating light conditions, probably via an unknown electron transport and/or regulatory network (Mustila et al., 2016).
The complete elimination of light-induced O2 reduction in WT cells grown at pH 8.2 (Ermakova et al., 2016) or at pH 7.5 (Figure 3—figure supplement 1) in the presence of electron-transport inhibitors DBMIB (blocks Qo site of Cytb6f; Roberts and Kramer, 2001) and HQNO (blocks Qi site of Cytb6f; Fernández-Velasco et al., 2001 and also Pils et al., 1997) suggests that FDP-driven O2 photoreduction (neither by Flv1/Flv3 nor by Flv2/Flv4) does not occur at the PSII or PQ-pool level. This conclusion is also supported by the fact that, differently to the WT and mutants deficient in FDPs, the ∆cyd mutant does not exhibit a light induced O2 uptake in the presence of DBMIB (Ermakova et al., 2016; Figure 3—figure supplement 1).
From the results discussed above, it can be concluded that both the Flv1/Flv3 and Flv2/Flv4 heterodimers have capacity to drive the Mehler-like reaction, functioning downstream of PSI.
The Flv1/Flv3 heterodimer drives a strong and steady-state O2 photoreduction under HC
It is generally accepted that under LC conditions, the slowing down of the Calvin-Benson cycle leads to a build-up of reduced stromal components (Cooley and Vermaas, 2001; Holland et al., 2015), which would stimulate the Mehler reaction to dissipate excess electrons (Ort and Baker, 2002). However, under HC conditions, the Mehler reaction would be expected to direct relatively low electron flux to O2. In this study, we provide evidence that HC-grown WT cells are capable of equally high O2 photoreduction as respective LC-grown WT cells, and that cells are capable of maintaining the steady-state activity at least during the first 5–10 min of illumination (Figure 1A). Compared to the WT, a drastically lower O2 photoreduction rate is observed in the ∆flv1/∆flv3 and ∆flv3/∆flv4 mutants grown in HC, confirming that O2 uptake under these conditions is mostly due to the Flv1/Flv3-driven Mehler-like reaction (Figure 1—figure supplement 1).
It is important to note that the O2 photoreduction capacity of Synechocystis generally correlates with the abundance of FDPs (Figures 1 and 6). However, protein abundance is not the only factor that determines O2 photoreduction capacity. Indeed, despite strong and steady-state O2 photoreduction, HC-grown cells demonstrate nearly undetectable levels of Flv2 and Flv4 and low amount of Flv3, compared to levels observed under LC conditions. Furthermore, the increase in O2 photoreduction rates (Figure 2, middle panel) obtained by omitting sodium carbonate from the BG-11 growth media at pH 7.5, does not correlate with any significant change in transcript and protein levels of the FDPs, thus suggesting a possible redox regulation of the enzyme activity.
Under LC, the Flv1/Flv3 heterodimer is a rapid, strong and transient electron sink whereas Flv2/Flv4 supports steady-state O2 photoreduction
The Mehler-like reaction of WT cells grown under LC at pH 6–8.2 exhibits triphasic kinetics of O2 photoreduction originating from the activity of both Flv1/Flv3 and Flv2/Flv4 heterodimers (Figure 2). In this study, we were able to unravel the contribution of Flv1/Flv3 and Flv2/Flv4 heterodimers to the O2 photoreduction kinetics: Flv1/Flv3 is mainly responsible for the rapid transient phase, whereas Flv2/Flv4 mostly contributes to the slow steady-state phase.
The almost complete absence of Flv2 and Flv4 proteins in WT cells grown under LC at pH 9 provides an excellent model system, where the Mehler-like reaction is naturally driven solely by the Flv1/Flv3 heterodimer, as is also the case under HC conditions. However, in contrast to HC-grown cells, where Flv1/Flv3 can drive a steady-state O2 photoreduction, the cells grown under LC at pH 9 demonstrate strong but only transient O2 photoreduction, which decays during the first 1–2 min of illumination (Figure 2). The identical O2 photoreduction kinetics of the WT cells grown at pH 9 (accumulating Flv3 but lacking both the Flv2 and Flv4 proteins) and the ∆flv4 mutant (accumulating Flv3 but lacking Flv4 and also Flv2), together with the complete absence of O2 photoreduction in the ∆flv3/∆flv4 mutant demonstrate that under LC, the Flv1/Flv3 heterodimer contributes to the Mehler-like reaction in a fast and transient manner (Figure 2). A similar conclusion was previously suggested for Synechocystis (Allahverdiyeva et al., 2013) and for the FlvA and FlvB proteins in Physcomitrella patens (Gerotto et al., 2016) and Chlamydomonas reinhardtii (Chaux et al., 2017; Jokel et al., 2018).
The sole contribution of Flv2/Flv4 to the Mehler-like reaction is clearly demonstrated as a steady-state O2 photoreduction by the ∆flv1/∆flv3 mutant grown under LC at pH 6 (Figure 2), whilst the same mutant cells grown at pH 7.5 and 8.2 show only residual steady-state O2 photoreduction. It is important to note that the Flv2/Flv4 heterodimer, when expressed, can readily contribute to O2 photoreduction under HC, as demonstrated by the flv4-2/OE strain (Figure 1), thus excluding all redox and structural hindrances for Flv2/Flv4 to function in O2 photoreduction under HC. However, such a contribution is naturally abolished in WT cells grown under high levels of CO2 by the down-regulation of the flv4-2 operon (Zhang et al., 2009; Zhang et al., 2012).
The rate of the Mehler-like reaction in WT cells exceeds the cumulative O2 photoreduction driven solely by Flv1/Flv3 (observed in ∆flv4) and Flv2/Flv4 (observed in ∆flv1/∆flv3). This demonstrates that all four FDPs are required for an efficient Mehler-like reaction in WT cells upon growth under LC (except at pH 9). A complex interaction between FDPs possibly arises from a coordinated inter-regulation of Flv1/Flv3 and Flv2/Flv4 heterodimers and on the possible occurrence of some active Flv1-4 oligomers (Figure 7). Despite detection of homotetrameric organization of Synechocystis Flv3 in vitro (Mustila et al., 2016), the direct biochemical demonstration of homo- or heterotetramer structures and function in vivo is still missing.
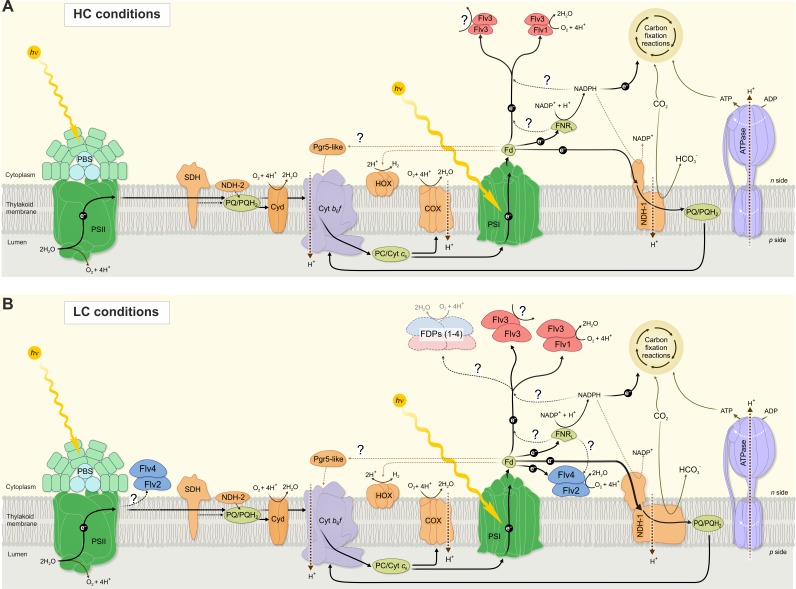
Figure 7. A schematic drawing of photosynthetic light reactions and alternative electron transport routes.
(A) A steady-state Mehler-like reaction in HC is carried out by the low-abundant, yet catalytically efficient Flv1/Flv3 heterodimer. The Flv3/Flv3 homooligomer is involved in photoprotection as an electron valve with unknown acceptor or as a component of a signaling/regulating network (Mustila et al., 2016). (B) In LC-grown cells the two pairs of FDP heterodimers are involved in the Mehler-like reaction: Flv1/Flv3 mainly drives rapid and transient O2 photoreduction and Flv2/Flv4 operates relatively slowly and provides a steady-state background O2 photoreduction. The soluble Flv1/Flv3 heterodimers function as an immediate acceptor of electrons presumable from reduced Fed, whereas association of Flv2/Flv4 with the thylakoid membrane (and/or Flv1/Flv3) is controlled by pmf and Mg2+. Several oligomeric forms of FDPs are hypothesized to exist, including a heterotetramer comprising different FDP protein compositions. The higher abundance of total NDH-1 complexes and FDPs oligomers in LC conditions, compared to HC conditions, is represented by larger size of the protein complexes.
The growth inhibition of ∆flv1/∆flv3 cells under severe fluctuating light conditions (FL 20/500) at pH 8.2 (Allahverdiyeva et al., 2013), pH 7.5 (Mustila et al., 2016), pH 6 and pH 9 (Figure 5) demonstrate the essential role of Flv1 and/or Flv3 during drastic changes of light intensity, whereas Flv2 and Flv4 are dispensable under the same conditions (Figure 4, Figure 4—figure supplement 1). Here, we demonstrate that the crucial importance of Flv1/Flv3 heterodimers is based on their high capacity to rapidly and effectively respond to increasing light intensities (Figure 5). By adjusting their O2 photoreduction activity, the Flv1/Flv3 heterodimer works as an efficient and fast sink of electrons, whereas the responsiveness of Flv2/Flv4 is relatively limited and the heterodimer mostly functions on a slow time-scale in steady-state O2 photoreduction.
The intracellular location of these enzymes may partially contribute to the difference in O2 photoreduction: Flv1 and Flv3 are soluble cytosolic proteins able to quickly associate with soluble Fed and direct electrons towards O2 photoreduction. In line with this, the possible interaction between Synechocystis Flv1, Flv3 and Fed (Hanke et al., 2011), Flv3 and Fed9 (Cassier-Chauvat and Chauvat, 2014), Chlamydomonas reinhardtii FLVB and FED1 (Peden et al., 2013) have been reported. The Flv2/Flv4 heterodimer, specific for cyanobacteria, was suggested to bind to the thylakoid membrane upon increases in Mg2+ concentration on the cytoplasmic surface of the thylakoid membrane when lights are turned on (Zhang et al., 2012). It is likely that the association of Flv2/Flv4 with the membrane enhances electron transfer from Fed (or FNR) to Flv2/Flv4 and would probably result in a delayed and limited O2 photoreduction activity by Flv2/Flv4. However, the possibility that FDPs accept electrons from different and specific Fed paralogs cannot be excluded.
Traffic downstream of PSI affects the FDP-mediated Mehler-like reaction
Unlike WT cells demonstrating biphasic decay kinetics of O2 photoreduction under LC conditions (Figure 1A and Figure 2), the M55 mutant (deficient in NDH-1 mediated CET, CO2 uptake and respiration) (Ohkawa et al., 2000) shows steady-state O2 photoreduction, similar to the HC-grown WT (Figure 1B and D). This suggests that the strongly upregulated NDH-1 complex under LC in Synechocystis (Zhang et al., 2004) contributes to a rapid quenching of O2 photoreduction (Figure 1A, phase {II}) by efficient withdrawal of electrons from reduced Fed. Under such circumstances, the low but steady-state activity of the Flv2/Flv4 heterodimer is likely to be important for keeping linear electron transport in an oxidized state. This would explain why the PQ-pool is more oxidized in the presence of Flv2/Flv4 and more reduced in its absence, indirectly affecting PSII activity (Zhang et al., 2012; Bersanini et al., 2014) and Chukhutsina et al., 2015). Thus, by allocating different roles for FDPs between the two pairs of heterodimers (Flv1/Flv3 and Flv2/Flv4), the cells are well positioned to respond appropriately to changing Ci levels as well as to abrupt changes in light intensity, in a coordinated and energetically efficient manner.
Unlike prokaryotic cyanobacteria, chlorophytic algae (e.g. Chlamydomonas reinhardtii) and mosses rely not only on the FDP-driven pathway, but also harbor the PROTON GRADIENT REGULATION5 (PGR5)/PGR5‐LIKE PHOTOSYNTHETIC PHENOTYPE 1 (PGRL1) pathway which operates concomitantly to protect the cells under fluctuating light. It is noteworthy, however, that the PGR5/PGRL1 machinery in Chlamydomonas reinhardtii is neither fast nor strong enough to mitigate acceptor-side pressure under highly fluctuating light intensities. To complement this deficiency, the FDP-mediated pathway is indispensable for coping with sudden increases in light intensity (Jokel et al., 2018). Interestingly, the introduction of Physcomitrella patens FDPs rescues a fluctuating light phenotype of the PGR5 Arabidopsis thaliana mutant (Yamamoto et al., 2016; Yamamoto and Shikanai, 2019), and alleviates PSI photodamage in the PGR5-RNAi, crr6 (defective in NDH-dependent CET) and the PGR5-RNAi crr6 double mutants of Oryza sativa by acting as a safety valve under fluctuating light and substituting for CET without competing with CO2 fixation under constant light (Wada et al., 2018). Moreover, the expression of Synechocystis Flv1 and Flv3 in tobacco plants enhances photosynthetic efficiency during dark-light transitions by providing an additional electron sink (Gómez et al., 2018). Although data on Flv2/Flv4 proteins expressed in angiosperms is not yet available, our results collectively suggest that the FDP pathway(s) is important to consider in future high-yield crop development and microbial cell factories.
The question of how FDPs avoid competition with CO2 fixation is an interesting one. Relevant mechanisms may include post-transcriptional modifications of the FDPs, such as phosphorylation (Angeleri et al., 2016), and/or pmf based regulation systems.
Figure 7 provides a summary scheme of our understanding of the function and interaction of the different FDPs and their oligomers in photoprotection of the photosynthetic apparatus in the model cyanobacterium Synechocystis sp. PCC 6803. The importance of the available Ci species in the function and accumulation of FDPs is emphasized by separate schemes for the HC and LC growth conditions.
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Synechocystis sp. PCC 6803) | WT, Wild-type | Williams, 1988 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | ∆flv2 | Zhang et al., 2012 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | ∆flv4 | Zhang et al., 2012 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | ∆flv1/∆flv3 | Allahverdiyeva et al., 2011 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | ∆flv3/∆flv4 | Helman et al., 2003 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | ∆sll0218‐flv2 | Helman et al., 2003 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | flv4-2/OE | Bersanini et al., 2014 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | ∆sll0218 | Bersanini et al., 2017 | ||
| Antibody | α-Flv2 (rabbit polyclonal) | AntiProt, against amino acids 521–535 of Synechocystis Flv2 | (1:500) | |
| Antibody | α-Flv3 (rabbit polyclonal) | AntiProt, against amino acids 377–391 of Synechocystis Flv3 | (1:2000) | |
| Antibody | α-Flv4 (rabbit polyclonal) | AntiProt, against amino acids 412–426 of Synechocystis Flv4 | (1:500) | |
| Antibody | α-NdhD3 (rabbit polyclonal) | Eurogentec, against amino acids 185 to 196 and 346 to 359 of Synechocystis NdhD3 | (1:1000) | |
| Antibody | α-SbtA | Kind gift from T. Ogawa, against amino acids 184 to 203 of Synechocystis SbtA | (1:5000) | |
| Antibody | α-NdhJ | Kind gift from J. Appel | (1:1000) | |
| Antibody | Secondary antibody, Amersham ECL Rabbit IgG, HRP-linked F(ab')₂ fragment (from donkey) | GE Healthcare | NA9340-1ML | (1:10000) |
| Commercial assay or kit | Amersham ECL Western Blotting Detection Reagent | GE Healthcare | RPN2209 | |
| Commercial assay or kit | iScript cDNA Synthesis Kit | BioRad, USA | Cat. #170–8891 | |
| Commercial assay or kit | iQ SYBR Green Supermix | BioRad, USA | Cat. #170–8882 | |
| Software, algorithm | qbase + software | Biogazelle, Zwijnaarde, Belgium - www.qbaseplus.com |
Strains and culture conditions
The glucose-tolerant Synechocystis sp. PCC 6803 was used as wild type (WT) strain (Williams, 1988). The FDP inactivation mutants ∆flv2, ∆flv4 (Zhang et al., 2012), and the double mutants ∆flv1/∆flv3 (Allahverdiyeva et al., 2011), and ∆flv3/∆flv4 (Helman et al., 2003), ∆sll0218‐flv2 (Helman et al., 2003) have been described previously. The flv4-2/OE and ∆sll0218 mutants were described in Bersanini et al. (2014); Bersanini et al. (2017).
Pre-experimental cultures were grown at 30°C in BG-11 medium, illuminated with continuous white light of 50 µmol photons m−2 s−1 (growth light: GL), under air enriched with 3% CO2 (high carbon: HC). BG-11 medium was buffered with 20 mM 2-(N-morpholino) ethanesulfonic acid (MES, pH 6.0), 20 mM HEPES-NaOH (pH 7.5), 10 mM TES-KOH (pH 8.2) or 10 mM N-Cyclohexyl-2-aminoethanesulfonic acid (CHES, pH 9.0), according to the pH of the experimental condition. Pre-cultures were harvested at logarithmic growth phase, inoculated in fresh BG-11 medium at OD750 = 0.2 (or OD750 = 0.5 when mentioned), measured with and shifted to low CO2 (atmospheric 0.04% CO2 in air, LC). OD750 was measured using Lambda 25 UV/VIS spectrometer (PerkinElmer, USA). HC experimental cultures were inoculated at OD750 = 0.1 and kept at HC for 3 days. During experimental cultivation, cells were grown under continuous GL at 30°C with agitation at 120 rpm and without antibiotics. For growth curves, cells pre-cultivated under continuous GL and HC were collected, inoculated at OD750 = 0.1 and shifted to LC under a light regime with a background light of 20 µmol photons m−2 s−1 interrupted with 500 µmol photons m−2 s−1 for 30 s every 5 min (FL 20/500) or 50 µmol photons m−2 s−1 interrupted with 500 µmol photons m−2 s−1 for 30 s every 5 min (FL 50/500). The standard BG-11 medium used in this work contains sodium carbonate (Na2CO3) at a final concentration of 0.189 mM and only when mentioned the sodium carbonate was omitted from the growth medium.
Absence of contamination with heterotrophic bacteria was checked by dropping liquid culture on LB and R2A agar plates and kept at 30°C.
Isolation of total RNA and Real-time quantitative PCR (RT-qPCR)
Total RNA was isolated from exponentially growing Synechocystis by hot-phenol method previously described (Tyystjärvi et al., 2001). After removing any residual genomic DNA, the RNA concentration and purity were measured with a NanoDrop spectrophotometer (Thermo Scientific, USA). RNA integrity was verified by agarose gel electrophoresis.
Complementary DNA was synthesized from 1 μg of purified RNA using the iScript cDNA Synthesis Kit (BioRad, USA) according to the manufacturer’s protocol. Synthesized cDNA was diluted four-fold and used as template for the RT-qPCR. The samples for RT‐qPCR were labeled by iQ SYBR Green Supermix (BioRad, USA) to detect accumulation of amplicons in 96-well plates. The primers to detect transcripts of flv1 and flv2 as well as for the reference genes rnpB and rimM are described in Mustila et al. (2016). The forward and reverse primers for flv3 were 5’-CAACTCAATCCCCGCATTAC-3’ and 5’-CAGTGGAGATTCGGAGCACT-3’ and for flv4 5’-ACGATGCCTGGAGTCAAAAC-3’ and 5’-GGGTATCCGCCACACTTAGA-3’. The PCR protocol was as follows: 3 min initial denaturation of cDNA at 95°C, followed by 40 cycles of 95°C for 10 s, annealing in 57°C for 30 s and extension in 72°C for 35 s. A melting curve analysis was performed at the end. Relative changes in the gene expression were determined using the qbase + software by Biogazelle. One-way ANOVA analysis performed with SigmaPlot was used to determine significant changes in gene expression.
MIMS experiments
In vivo measurements of 16O2 (mass 32) and 18O2 (mass 36) exchange was performed using a Membrane-inlet mass spectrometry (MIMS) as described previously in Mustila et al. (2016). Cells were harvested, adjusted to 10 µg Chl a mL−1 in fresh BG-11 medium and acclimated for 1 hr to the same experimental conditions as was applied for the cultivation.
Protein isolation, electrophoresis and immunodetection
Total cell extracts and the soluble fractions of Synechocystis cells were isolated as described (Zhang et al., 2009). Proteins were separated by 12% (w/v) SDS-PAGE containing 6 M urea and transferred onto a PVDF membrane (Immobilion-P; Millipore, Germany) and immunodetected by protein specific antibodies. Horseradish peroxidase (HRP) conjugated secondary antibody (anti-rabbit IgG from donkey) was used for recognizing the primary antibodies and Amersham ECL Western Blotting Detection Reagent (GE Healthcare) was used for the visualization of the antibodies.
Acknowledgements
The authors would like to thank Dr. Lauri Nikkanen and Dr. Natalia Battchikova for critical reading of the manuscript. Dr. Duncan Fitzpatrick is acknowledged for technical assistance and maintenance of MIMS.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Eva-Mari Aro, Email: evaaro@utu.fi.
Yagut Allahverdiyeva, Email: allahve@utu.fi.
Jürgen Kleine-Vehn, University of Natural Resources and Life Sciences, Austria.
Detlef Weigel, Max Planck Institute for Developmental Biology, Germany.
Funding Information
This paper was supported by the following grants:
Suomen Akatemia 315119 to Yagut Allahverdiyeva.
NordForsk 82845 to Eva-Mari Aro, Yagut Allahverdiyeva.
Koneen Säätiö 7fa491 to Yagut Allahverdiyeva.
Suomen Akatemia 307335 to Eva-Mari Aro.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing—original draft, Writing—review and editing.
Conceptualization, Validation, Investigation, Methodology, Writing—original draft, Writing—review and editing.
Conceptualization, Investigation, Writing—review and editing.
Conceptualization, Investigation, Writing—review and editing.
Conceptualization, Resources, Funding acquisition, Writing—review and editing.
Conceptualization, Resources, Supervision, Funding acquisition, Validation, Investigation, Visualization, Methodology, Writing—original draft, Project administration, Writing—review and editing.
Additional files
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files.
References
- Allahverdiyeva Y, Ermakova M, Eisenhut M, Zhang P, Richaud P, Hagemann M, Cournac L, Aro EM. Interplay between flavodiiron proteins and photorespiration in Synechocystis sp. PCC 6803. Journal of Biological Chemistry. 2011;286:24007–24014. doi: 10.1074/jbc.M111.223289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva Y, Mustila H, Ermakova M, Bersanini L, Richaud P, Ajlani G, Battchikova N, Cournac L, Aro EM. Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. PNAS. 2013;110:4111–4116. doi: 10.1073/pnas.1221194110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva Y, Isojärvi J, Zhang P, Aro EM. Cyanobacterial oxygenic photosynthesis is protected by flavodiiron proteins. Life. 2015;5:716–743. doi: 10.3390/life5010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeleri M, Muth-Pawlak D, Aro EM, Battchikova N. Study of O-Phosphorylation sites in proteins involved in Photosynthesis-Related processes in Synechocystis sp. strain PCC 6803: application of the SRM approach. Journal of Proteome Research. 2016;15:4638–4652. doi: 10.1021/acs.jproteome.6b00732. [DOI] [PubMed] [Google Scholar]
- Battchikova N, Vainonen JP, Vorontsova N, Keranen M, Carmel D, Aro EM. Dynamic changes in the proteome of Synechocystis 6803 in response to CO(2) limitation revealed by quantitative proteomics. Journal of Proteome Research. 2010;9:5896–5912. doi: 10.1021/pr100651w. [DOI] [PubMed] [Google Scholar]
- Bersanini L, Battchikova N, Jokel M, Rehman A, Vass I, Allahverdiyeva Y, Aro EM. Flavodiiron protein Flv2/Flv4-related photoprotective mechanism dissipates excitation pressure of PSII in cooperation with phycobilisomes in cyanobacteria. Plant Physiology. 2014;164:805–818. doi: 10.1104/pp.113.231969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersanini L, Allahverdiyeva Y, Battchikova N, Heinz S, Lespinasse M, Ruohisto E, Mustila H, Nickelsen J, Vass I, Aro EM. Dissecting the photoprotective mechanism encoded by the flv4-2 Operon: a Distinct Contribution of Sll0218 in Photosystem II Stabilization. Plant, Cell & Environment. 2017;40:378–389. doi: 10.1111/pce.12872. [DOI] [PubMed] [Google Scholar]
- Borges PT, Romão CV, Saraiva LM, Gonçalves VL, Carrondo MA, Teixeira M, Frazão C. Analysis of a new flavodiiron core structural arrangement in Flv1-ΔFlR protein from Synechocystis sp. PCC6803. Journal of Structural Biology. 2019;205:91–102. doi: 10.1016/j.jsb.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Cassier-Chauvat C, Chauvat F. Function and regulation of ferredoxins in the Cyanobacterium, Synechocystis PCC6803: Recent Advances. Life. 2014;4:666–680. doi: 10.3390/life4040666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaux F, Burlacot A, Mekhalfi M, Auroy P, Blangy S, Richaud P, Peltier G. Flavodiiron proteins promote fast and transient O2 Photoreduction in Chlamydomonas. Plant Physiology. 2017;174:1825–1836. doi: 10.1104/pp.17.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukhutsina V, Bersanini L, Aro EM, van Amerongen H. Cyanobacterial flv4-2 Operon-Encoded proteins optimize light harvesting and charge separation in photosystem II. Molecular Plant. 2015;8:747–761. doi: 10.1016/j.molp.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Cooley JW, Vermaas WF. Succinate dehydrogenase and other respiratory pathways in thylakoid membranes of Synechocystis sp. strain PCC 6803: capacity comparisons and physiological function. Journal of Bacteriology. 2001;183:4251–4258. doi: 10.1128/JB.183.14.4251-4258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo A, Scandurra FM, Testa F, Forte E, Sarti P, Brunori M, Giuffrè A. The O2-scavenging flavodiiron protein in the human parasite Giardia intestinalis. The Journal of Biological Chemistry. 2008;283:4061–4068. doi: 10.1074/jbc.M705605200. [DOI] [PubMed] [Google Scholar]
- Draber W, Trebst A, Harth E. On a new inhibitor of photosynthetic electron-transport in isolated chloroplasts. Zeitschrift Für Naturforschung B. 1970;25:1157–1159. doi: 10.1515/znb-1970-1018. [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Georg J, Klähn S, Sakurai I, Mustila H, Zhang P, Hess WR, Aro EM. The antisense RNA As1_flv4 in the Cyanobacterium synechocystis sp. PCC 6803 prevents premature expression of the flv4-2 operon upon shift in inorganic carbon supply. Journal of Biological Chemistry. 2012;287:33153–33162. doi: 10.1074/jbc.M112.391755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakova M, Battchikova N, Allahverdiyeva Y, Aro EM. Novel heterocyst-specific flavodiiron proteins in Anabaena sp. PCC 7120. FEBS Letters. 2013;587:82–87. doi: 10.1016/j.febslet.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Ermakova M, Huokko T, Richaud P, Bersanini L, Howe CJ, Lea-Smith DJ, Peltier G, Allahverdiyeva Y. Distinguishing the roles of thylakoid respiratory terminal oxidases in the Cyanobacterium Synechocystis sp. PCC 6803. Plant Physiology. 2016;171:1307–1319. doi: 10.1104/pp.16.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Velasco JG, Jamshidi A, Gong XS, Zhou J, Ueng RY. Photosynthetic electron transfer through the cytochrome b6f complex can bypass cytochrome f. The Journal of Biological Chemistry. 2001;276:30598–30607. doi: 10.1074/jbc.M102241200. [DOI] [PubMed] [Google Scholar]
- Folgosa F, Martins MC, Teixeira M. Diversity and complexity of flavodiiron NO/O2 reductases. FEMS Microbiology Letters. 2018;365:1–8. doi: 10.1093/femsle/fnx267. [DOI] [PubMed] [Google Scholar]
- Gerotto C, Alboresi A, Meneghesso A, Jokel M, Suorsa M, Aro EM, Morosinotto T. Flavodiiron proteins act as safety valve for electrons in Physcomitrella patens. PNAS. 2016;113:12322–12327. doi: 10.1073/pnas.1606685113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez R, Carrillo N, Morelli MP, Tula S, Shahinnia F, Hajirezaei MR, Lodeyro AF. Faster photosynthetic induction in tobacco by expressing cyanobacterial flavodiiron proteins in chloroplasts. Photosynthesis Research. 2018;136:129–138. doi: 10.1007/s11120-017-0449-9. [DOI] [PubMed] [Google Scholar]
- Gonçalves VL, Vicente JB, Saraiva LM, Teixeira M. Flavodiiron proteins and their role in cyanobacteria. In: Peschek G, Obinger C, Renger G, editors. Processes of Cyanobacteria. Dordrecht: Springer; 2011. pp. 631–653. [DOI] [Google Scholar]
- Hackenberg C, Engelhardt A, Matthijs HC, Wittink F, Bauwe H, Kaplan A, Hagemann M. Photorespiratory 2-phosphoglycolate metabolism and photoreduction of O2 cooperate in high-light acclimation of Synechocystis sp. strain PCC 6803. Planta. 2009;230:625–637. doi: 10.1007/s00425-009-0972-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke GT, Satomi Y, Shinmura K, Takao T, Hase T. A screen for potential ferredoxin electron transfer partners uncovers new, redox dependent interactions. Biochimica Et Biophysica Acta (BBA) - Proteins and Proteomics. 2011;1814:366–374. doi: 10.1016/j.bbapap.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Helman Y, Tchernov D, Reinhold L, Shibata M, Ogawa T, Schwarz R, Ohad I, Kaplan A. Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Current Biology. 2003;13:230–235. doi: 10.1016/S0960-9822(03)00046-0. [DOI] [PubMed] [Google Scholar]
- Holland SC, Kappell AD, Burnap RL. Redox changes accompanying inorganic carbon limitation in Synechocystis sp. PCC 6803. Biochimica Et Biophysica Acta (BBA) - Bioenergetics. 2015;1847:355–363. doi: 10.1016/j.bbabio.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Howitt CA, Vermaas WF. Quinol and cytochrome oxidases in the Cyanobacterium Synechocystis sp. PCC 6803. Biochemistry. 1998;37:17944–17951. doi: 10.1021/bi981486n. [DOI] [PubMed] [Google Scholar]
- Ilík P, Pavlovič A, Kouřil R, Alboresi A, Morosinotto T, Allahverdiyeva Y, Aro EM, Yamamoto H, Shikanai T. Alternative electron transport mediated by flavodiiron proteins is operational in organisms from cyanobacteria up to gymnosperms. New Phytologist. 2017;214:967–972. doi: 10.1111/nph.14536. [DOI] [PubMed] [Google Scholar]
- Jokel M, Johnson X, Peltier G, Aro EM, Allahverdiyeva Y. Hunting the main player enabling Chlamydomonas reinhardtii growth under fluctuating light. The Plant Journal. 2018;94:822–835. doi: 10.1111/tpj.13897. [DOI] [PubMed] [Google Scholar]
- Mustila H, Paananen P, Battchikova N, Santana-Sánchez A, Muth-Pawlak D, Hagemann M, Aro E-M, Allahverdiyeva Y. The Flavodiiron Protein Flv3 Functions as a Homo-Oligomer During Stress Acclimation and is Distinct from the Flv1/Flv3 Hetero-Oligomer Specific to the O2 Photoreduction Pathway. Plant and Cell Physiology. 2016;1767:1468–1483. doi: 10.1093/pcp/pcw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T. A gene homologous to the subunit-2 gene of NADH dehydrogenase is essential to inorganic carbon transport of Synechocystis PCC6803. PNAS. 1991;88:4275–4279. doi: 10.1073/pnas.88.10.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Pakrasi HB, Ogawa T. Two types of functionally distinct NAD(P)H dehydrogenases in Synechocystis sp. strain PCC6803. Journal of Biological Chemistry. 2000;275:31630–31634. doi: 10.1074/jbc.M003706200. [DOI] [PubMed] [Google Scholar]
- Ort DR, Baker NR. A photoprotective role for O(2) as an alternative electron sink in photosynthesis? Current Opinion in Plant Biology. 2002;5:193–198. doi: 10.1016/S1369-5266(02)00259-5. [DOI] [PubMed] [Google Scholar]
- Peden EA, Boehm M, Mulder DW, Davis R, Old WM, King PW, Ghirardi ML, Dubini A. Identification of global ferredoxin interaction networks in Chlamydomonas reinhardtii. Journal of Biological Chemistry. 2013;288:35192–35209. doi: 10.1074/jbc.M113.483727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pils D, Gregor W, Schmetterer G. Evidence for in vivo activity of three distinct respiratory terminal oxidases in the Cyanobacterium synechocystis sp. strain PCC6803. FEMS Microbiology Letters. 1997;152:83–88. doi: 10.1111/j.1574-6968.1997.tb10412.x. [DOI] [PubMed] [Google Scholar]
- Roberts AG, Kramer DM. Inhibitor "double occupancy" in the Q(o) pocket of the chloroplast cytochrome b6f complex. Biochemistry. 2001;40:13407–13412. doi: 10.1021/bi015774m. [DOI] [PubMed] [Google Scholar]
- Romão CV, Vicente JB, Borges PT, Frazão C, Teixeira M. The dual function of flavodiiron proteins: oxygen and/or nitric oxide reductases. JBIC Journal of Biological Inorganic Chemistry. 2016;21:39–52. doi: 10.1007/s00775-015-1329-4. [DOI] [PubMed] [Google Scholar]
- Schuller JM, Birrell JA, Tanaka H, Konuma T, Wulfhorst H, Cox N, Schuller SK, Thiemann J, Lubitz W, Sétif P, Ikegami T, Engel BD, Kurisu G, Nowaczyk MM. Structural adaptations of photosynthetic complex I enable ferredoxin-dependent electron transfer. Science. 2019;363:257–260. doi: 10.1126/science.aau3613. [DOI] [PubMed] [Google Scholar]
- Shimakawa G, Shaku K, Nishi A, Hayashi R, Yamamoto H, Sakamoto K, Makino A, Miyake C. FLAVODIIRON2 and FLAVODIIRON4 proteins mediate an oxygen-dependent alternative electron flow in Synechocystis sp. PCC 6803 under CO2-limited conditions. Plant Physiology. 2015;167:472–480. doi: 10.1104/pp.114.249987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakawa G, Ishizaki K, Tsukamoto S, Tanaka M, Sejima T, Miyake C. The liverwort, Marchantia, drives alternative electron flow using a flavodiiron protein to protect PSI. Plant Physiology. 2017;173:1636–1647. doi: 10.1104/pp.16.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield TC, Sherman LA. Global transcriptional response of the alkali-tolerant Cyanobacterium Synechocystis sp. strain PCC 6803 to a pH 10 environment. Applied and Environmental Microbiology. 2008;74:5276–5284. doi: 10.1128/AEM.00883-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyystjärvi T, Herranen M, Aro EM. Regulation of translation elongation in cyanobacteria: membrane targeting of the ribosome nascent-chain complexes controls the synthesis of D1 protein. Molecular Microbiology. 2001;40:476–484. doi: 10.1046/j.1365-2958.2001.02402.x. [DOI] [PubMed] [Google Scholar]
- Vicente JB, Gomes CM, Wasserfallen A, Teixeira M. Module fusion in an A-type flavoprotein from the Cyanobacterium Synechocystis condenses a multiple-component pathway in a single polypeptide chain. Biochemical and Biophysical Research Communications. 2002;294:82–87. doi: 10.1016/S0006-291X(02)00434-5. [DOI] [PubMed] [Google Scholar]
- Wada S, Yamamoto H, Suzuki Y, Yamori W, Shikanai T, Makino A. Flavodiiron protein substitutes for cyclic electron flow without competing CO2 Assimilation in Rice. Plant Physiology. 2018;176:1509–1518. doi: 10.1104/pp.17.01335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Postier BL, Burnap RL. Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. Journal of Biological Chemistry. 2004;279:5739–5751. doi: 10.1074/jbc.M311336200. [DOI] [PubMed] [Google Scholar]
- Wasserfallen A, Ragettli S, Jouanneau Y, Leisinger T. A family of flavoproteins in the domains archaea and Bacteria. European Journal of Biochemistry. 1998;254:325–332. doi: 10.1046/j.1432-1327.1998.2540325.x. [DOI] [PubMed] [Google Scholar]
- Williams JG. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. In: Packer L, Glazer A. N, editors. Methods in Enzymology. Vol. 167. Academic Press; 1988. pp. 766–778. [DOI] [Google Scholar]
- Yamamoto H, Takahashi S, Badger MR, Shikanai T. Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nature Plants. 2016;2:16012. doi: 10.1038/nplants.2016.12. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Shikanai T. PGR5-Dependent cyclic electron flow protects photosystem I under fluctuating light at donor and acceptor sides. Plant Physiology. 2019;179:588–600. doi: 10.1104/pp.18.01343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Kurisu G, Cramer WA. Intraprotein transfer of the quinone analogue inhibitor 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone in the cytochrome b6f complex. PNAS. 2006;103:69–74. doi: 10.1073/pnas.0504909102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Battchikova N, Jansen T, Appel J, Ogawa T, Aro EM. Expression and functional roles of the two distinct NDH-1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis sp PCC 6803. The Plant Cell. 2004;16:3326–3340. doi: 10.1105/tpc.104.026526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Allahverdiyeva Y, Eisenhut M, Aro EM. Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLOS ONE. 2009;4:e5331. doi: 10.1371/journal.pone.0005331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Eisenhut M, Brandt AM, Carmel D, Silén HM, Vass I, Allahverdiyeva Y, Salminen TA, Aro EM. Operon flv4-flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. The Plant Cell. 2012;24:1952–1971. doi: 10.1105/tpc.111.094417. [DOI] [PMC free article] [PubMed] [Google Scholar]