Abstract
Background and Goals:
Despite published clinical guidelines, substantive data underlying the approach to the management of hospitalized ulcerative colitis (UC) patients failing outpatient therapy are lacking. Variability in practice is therefore not uncommon and may impact clinical outcomes. The degree of variability, however, is not well-studied. Our aim was to evaluate variability in management of the hospitalized UC patient to inform future efforts targeting care optimization for this high-risk population.
Study:
An internet survey was distributed among inflammatory bowel disease providers, which included: (1) nonvignette-based questions assessing provider demographics, experience, and practice setting; (2) diagnostic and therapeutic practice patterns based on a vignette of a hospitalized UC patient. Descriptive and univariate analyses were performed.
Results:
Ninety-one percent of eligible individuals were included. Nearly 97% endorsed confidence in management of hospitalized UC patients. In general, 83% initiate intravenous corticosteroids (IVCS) as initial therapy, whereas 17% initiate infliximab (IFX) (+ / −IVCS). At IVCS failure in the vignette, 74% initiated IFX, 15% increased IVCS dose, 7% initiated cyclosporine, and 4% chose colectomy. Of those choosing IFX, 65% chose 5 mg/kg as the initial dose, whereas the remainder chose 10 mg/kg. Twenty-eight percent gave an additional IFX 5 mg/kg and 7% gave an additional 10 mg/kg dose to the patient in the vignette not responding to 5 mg/kg.
Conclusions:
Even among experienced inflammatory bowel disease providers, there is significant practice pattern variability in the management of hospitalized UC patients. Future efforts should target this variability. Adjunctively, prospective trials are needed to guide appropriate therapeutic algorithms, especially with respect to positioning and optimally dosing IFX in this population.
Keywords: ulcerative colitis, infliximab, clinical care pathway
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) of the colon characterized by intermittent flares of disease which, if severe, may warrant hospitalization and escalation of UC therapy. Unfortunately, approximately 20% of patients ultimately require definitive surgical management (ie, colectomy).1 Even in our current era of effective IBD therapies and specialized centers, disease mortality in the face of a severe UC flare can approach 5%, especially if emergent colectomy is needed.2 Despite published clinical guidelines for the management of hospitalized UC patients failing outpatient medical management,3 high-quality corroborative data are lacking. As such, variability in clinical practice is not uncommon and may impact outcomes in this high-risk population. The degree of management variability is not well studied.
Intravenous corticosteroids (IVCS) remain the first-line medical therapy for hospitalized UC patients failing outpatient medical management. In the absence of a rescue strategy, patients failing IVCS have a high rate of colectomy, reaching 38% to 47% in some series.4,5 For those in whom 3 to 5 days of appropriately dosed IVCS fails, escalation of medical management to second-line rescue therapy—IV cyclosporine (CsA) or infliximab (IFX)—may be appropriate, with colectomy reserved for patients who are either not candidates for or who do not respond to second-line therapy. Although effective, there are both patient-related and disease-related factors associated with failure of second-line therapy including delay in initiation, improper initial and/or subsequent dosing, as well as UC too advanced for medical therapy.6-8
Owing to safety concerns and the need for dose titration with CsA,9-12 IFX is often the favored second-line rescue therapy. That said, there is little clarity in positioning this agent with respect to indication, timing, and dosing within our current guidelines.3 Although effective, there are no published studies investigating the optimal initial IFX dose in patients hospitalized with severe UC. We now know that factors such as higher inflammatory burden,6,7 higher circulating and colorectal mucosal levels of tumor necrosis factor-a (“antigen sink” theory),7 more extensive disease,7,8 and fecal losses of drug,13,14 among other factors contribute to altered pharmacokinetics and higher clearance of IFX in the hospitalized UC patient compared with the non-hospitalized patient with lesser disease severity. Accordingly, a single dose of IFX 5 mg/kg may not achieve the therapeutic levels needed for clinical response and may account for a portion of IFX failures in this population.8 Although there are no prospective trials comparing initial dosing regimens in severe hospitalized UC patients, anecdotally, administering a higher upfront IFX dose (eg, 10 mg/kg) or at an accelerated dosing interval (eg, repeat 5 mg/kg dose within 7 d) is not uncommon.15
Nuances in the management of the hospitalized severe UC patient—notably with respect to choice of second-line therapy and appropriate dosing in the absence of robust comparative trials—invite variability into the delivery of care for this high-risk population. Decreasing variability in care has been consistently associated with improved outcomes and even cost efficiency. Implementation of clinical care pathways in other clinically well-defined conditions, ranging from surgeries to stroke to congestive heart failure, has been associated with decreased variability in management and, subsequently, improved outcomes.16-18 We therefore designed a survey assessing practice pattern variability in the management of the hospitalized UC patient among IBD providers at a high-volume metropolitan-based IBD referral center to assess the need for efforts targeting care optimization for this high-risk population, such as a clinical care pathway.
MATERIALS AND METHODS
Study Design
We designed an internet-based survey [SurveyMonkey] with 2 main areas—(1) nonvignette-based questions assessing: provider type and clinical experience, patient focus (adult and/or pediatric), number of hospitalized UC patients failing outpatient medical management cared for in the past year and provider’s comfort level in treating this population, members of the inpatient team managing hospitalized UC patients, as well as institution-specific questions with respect to management; (2) diagnostic and therapeutic practice patterns based on a clinical vignette of a 32-year-old hospitalized UC patient. This case-vignette was piloted on a small focus-group of IBD providers to ensure it appropriately represented the hospitalized UC patient. We used linked questions liberally in the survey design, such that subsequent questions and clinical scenarios were based on the provider’s selection in the preceding question. After the clinical vignette, participants were asked questions relating to a clinical care pathway. All answers were anonymous and participation was voluntary. Only gastroenterology (GI) fellows with more than 1 year of experience at Mount Sinai Hospital (Manhattan) and GI faculty who care for IBD patients were included for analysis. Starting in the first year, GI fellows employed at Mount Sinai Hospital (Manhattan) spend a significant amount of time as the primary provider on our inpatient IBD service with an average daily census of 10 to 15 IBD patients. Responses were collected between September 28, 2015 and November 16, 2015.
After obtaining Institutional Review Board approval from each site within the Mount Sinai Health System [Mount Sinai Hospital (Manhattan), Mount Sinai Beth Israel, Mount Sinai Brooklyn, Mount Sinai Queens, Mount Sinai St Luke’s, Mount Sinai Roosevelt], we distributed the survey by email to appropriate GI fellows and faculty who are established providers at our high-volume IBD referral center.
Clinical Vignette
A 32-year-old woman with a 7-year history of UC, previously maintained on oral and rectal mesalamine, presents with 8 bloody bowel movements daily, with at least 2 nocturnally, and occasional passage of blood and mucous alone despite initiation of prednisone 40 mg per oral 7 days before admission. She has subjective fevers, with admission physical exam notable for low-grade temperature (38.11C), mild tachycardia, and mild left lower quadrant tenderness, but with an otherwise normal exam. Labs are only notable for white blood cell count 12×109/L, hemoglobin 8.2 g/dL (baseline 11.7 g/dL), albumin 2.9 g/dL, and C-reactive protein (CRP) 30 mg/L (normal < 0.8 mg/L). In the vignette, she is initially treated with methylprednisolone 20 mg IV every 8 hours, but fails to respond by day 3. Respondents are asked for their next step in management. If they choose to increase the dose of IVCS, the patient is clinically unchanged at day 5 and they are prompted to choose alternative management. If and when second-line rescue therapy (IFX or CsA) is initiated, a reassessment of the patient at 72 hours after initiation is provided and the respondent is prompted to choose their next step in management. Colectomy is an option in each answer stem.
Vignette-based questions were divided into 2 main parts to: (1) identify providers’ initial management for a UC patient failing outpatient medical therapy and their therapeutic decision-making process when this patient fails an adequate IVCS trial; and (2) identify how providers assess responsiveness to second-line medical therapy and their subsequent therapeutic decision-making process, such as additional medical therapy or recommending colectomy.
Statistical Analyses
Data were collected and collated in SurveyMonkey and exported to Microsoft Excel (version 2011). Descriptive and univariate analyses were performed in Excel as well as Stata. Fisher exact test was used to compare categorical variables; P-value <0.05 was considered statistically significant.
RESULTS
Respondent Characteristics, Practice Setting, and General Practice Patterns
Thirty of 33 (91%) eligible individuals responded and were included for analysis. Of the respondents, 27.3% were second year, third year, or advanced IBD fellows (n = 9). Of the faculty respondents (n = 21), 76% (n = 16) were faculty at least 5 years posttraining. Almost all providers (n = 29) felt comfortable with their management of hospitalized UC patients (Table 1).
TABLE 1.
Practice Setting and Clinical Experience of Respondents (n = 30)
| Variable | Respondents [n/N (%)] |
|---|---|
| Practice setting | 22/30 (73) |
| Mount Sinai Hospital (Manhattan) | |
| Training level | |
| Z2nd-year GI fellow | 9/30 (30) |
| GI attending <5 y posttraining | 5/30 (17) |
| GI attending Z5 y posttraining | 16/30 (53) |
| Experience with managing HUC patients* | |
| Currently manage (or in the past 2 y have managed) HUC patients | 29/30 (97) |
| Level of agreement with the statement, “I feel comfortable managing a HUC patient in the inpatient setting” | |
| Strongly agree | 20/29 (69) |
| Somewhat agree | 8/29 (28) |
| Neutral | 0/29 (0) |
| Somewhat disagree | 1/29 (3.5) |
| Strongly disagree | 0/29 (0) |
| Within the past year, have managed | |
| > 10 HUC patients | 13/29 (45) |
| 5-10 HUC patients | 7/29 (24) |
| <5 HUC patients | 9/29 (31) |
| No. colorectal surgeons who routinely operate on HUC patients at the institution | |
| Z3 surgeons | 23/29 (79) |
| 1-2 surgeons | 4/29 (14) |
| 0 surgeons | 2/29 (7) |
| I’m not sure | 0/29 (0) |
| Members of inpatient clinical team caring for HUC patients | |
| GI attending | 27/30 (93) |
| GI fellow | 25/30 (86) |
| Medicine residents | 22/30 (76) |
| Surgical attending | 19/30 (66) |
| NP and/or PA | 14/30 (48) |
| Nutritionist | 12/30 (41) |
| Social worker | 9/30 (31) |
| Pharmacist | 7/30 (24) |
| Other (free text) | 0/30 (0) |
| Primary inpatient team caring for HUC patients | |
| GI/medicine service | 28/29 (97) |
| Surgery service | 0/29 (0) |
| Other (free text): “Both GI and surgical services” | 1/29 (3) |
Hospitalized UC patient failing outpatient medical therapy.
GI indicates gastroenterology; HUC, hospitalized UC; NP, nurse practitioner; PA, physician assistant; UC, ulcerative colitis.
Initial Management of UC Patients Refractory to Outpatient Therapy (Nonvignette Based)
According to nearly all respondents (97%), hospitalized UC patients are managed primarily on the GI/medicine services as opposed to the surgical service. Forty-six percent routinely consult surgery within 24 to 48 hours of admission, whereas the remaining only consult the surgical service if there is clinical worsening or no improvement with an adequate trial of medical therapy. Once hospitalized, 83% initiate IVCS as initial therapy, whereas the remaining would initiate IFX (+ / −IVCS). Ninety-five percent of respondents send the pre–antitumor necrosis factor workup (ie, hepatitis serologies, tuberculosis testing) on admission if not done within the past 6 months and/or pre-CsA labs (ie, cholesterol panel, complete metabolic panel including magnesium). Of those initiating IVCS, 83% would continue therapy for 3 days before deeming a patient steroid-refractory, with no respondent waiting more than 5 days before making this determination. Seventy-five percent order antibiotics only if there are signs/symptoms concerning for infection, whereas the remaining 25% routinely order antibiotics in any hospitalized UC patient. Sixty percent routinely order imaging on admission and 70% perform flexible sigmoidoscopy within 48 hours of admission, with 45% of these being performed on the day of admission (Table 2).
TABLE 2.
Management Decisions of Providers (Nonvignette Based), n = 30
| Management Decision | Respondents [n/N(%)] |
|---|---|
| Surgical consult | |
| Only if clinical worsening or no improvement with medical therapy | 14/28 (50) |
| Routinely within 24 h of admission | 5/28 (18) |
| Routinely within 48 h of admission | 8/28 (29) |
| Recommend colectomy as an alternative to initial trial of inpatient medical therapy in HUC patients who failed outpatient therapy | |
| Yes | 16/29 (55) |
| No | 6/29 (21) |
| Other (free text): “It depends (eg, prior dysplasia history)” | 7/29 (24) |
| Choice of initial medical therapy in HUC patients (if pursued) | |
| IV corticosteroids | 24/29 (83) |
| Other | 5/29 (17) |
| Infliximab | 100 |
| Cyclosporine | 0 |
| Adalimumab | 0 |
| Vedolizumab | 0 |
| Free text | 0 |
| No. days of clinical nonresponse to IV steroids before considering a patient “steroid-refractory” and changing management (d) | |
| 1 | 0/30 (0) |
| 3 | 25/30 (83) |
| 5 | 5/30 (17) |
| 7 | 0/30 (0) |
| 10 | 0/30 (0) |
| Routinely order imaging on admission in HUC patients | |
| Yes, always | 12/20 (60) |
| No, not routinely | 6/20 (30) |
| Other (free text): “It depends on the clinical scenario and prior images” | 2/20 (10) |
| Routinely order antibiotics on admission in HUC patients | |
| Yes, always | 5/20 (25) |
| No, not routinely | 4/20 (20) |
| Only if they have signs/symptoms concerning for infection | 11/20 (55) |
| Other (free text) | 0/20 (0) |
| Day on which FS is performed in HUC patients | |
| On admission (HD1) | 9/20 (45) |
| On HD2 | 5/20 (25) |
| On HD3 | 2/20 (10) |
| Only if the patient fails to respond to current inpatient IBD therapy | 1/20 (5) |
| I do not routinely perform FS on such patients | 0/20 (0) |
| Other (free text): “Depends on when their prior FS was” | 3/20 (15) |
FS, flexible sigmoidoscopy; HD, hospital day; HUC, hospitalized UC; IBD, inflammatory bowel disease.
Vignette-based Responses and Practice Patterns
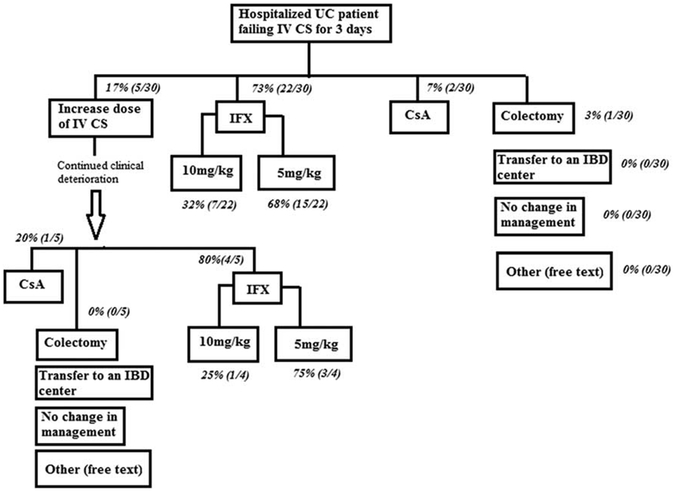
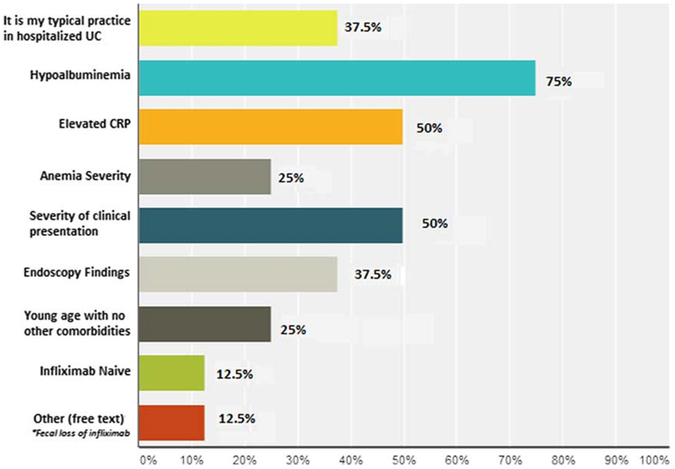
On admission, 75% of respondents would check for enteric pathogens including Clostridium difficile and simultaneously escalate to IV therapy before stool study results, whereas the remainder would await negative stool studies before escalating therapy. At the point of failing IVCS, 74% of respondents initiated IFX, 15% increased IVCS dose, 7% initiated CsA, and 4% chose colectomy (Fig. 1). Of those choosing IFX, 65% chose 5 mg/kg as the initial dose, whereas the remainder chose 10 mg/kg. Those choosing 10 mg/kg based their decision on hypoalbuminemia (75%), CRP (50%), symptom severity (50%), and endoscopy (37.5%), with 37.5% responding that dosing IFX 10 mg/kg is their typical practice in hospitalized UC patients (Fig. 2). Initial IFX dosing choice (5 vs. 10 mg/kg) was independent of level of training, that is fellows compared with attendings (P = NS).
FIGURE 1.
Clinical decision making in a hospitalized UC patient (clinical vignette). CS indicates corticosteroids; CsA, cyclosporine; IBD, inflammatory bowel disease; IFX, infliximab; UC, ulcerative colitis.
FIGURE 2.
Reasons for choosing IFX 10 mg/kg as initial dose compared with 5 mg/kg. CRP, C-reactive protein; IFX, infliximab; UC, ulcerative colitis.
In the setting of persistent symptoms 72 hours after initial IFX dose 5 mg/kg, 53% chose to continue monitoring with no changes in management, whereas 33% chose to give an additional IFX 5 mg/kg, and 7% chose to give an additional 10 mg/kg dose; 1 respondent chose discharge planning and no respondent chose colectomy. In contrast, of those choosing IFX 10 mg/kg as the initial dose, 25% chose to stay the course, 12.5% chose to give an additional IFX 10 mg/kg (and not 5 mg/kg), and 12.5% chose colectomy; 1 respondent in this group chose discharge planning. There was no difference among fellows and attendings with respect to their choice to continue current management versus administering additional IFX (P = NS). The 2 respondents choosing discharge planning for the patient in the vignette with ongoing evidence of inflammation despite IFX were both GI attendings with at least 5 years of experience posttraining. Of those choosing IFX, only 17% would check serum IFX/anti-IFX antibody (antibodies to infliximab) levels after first IFX infusion. Notably, none withheld further IFX dosing while awaiting these results.
Implementation of a Clinical Care Pathway
After describing and defining a clinical care pathway in the question stem, 93% favored its implementation, citing benefits of improved quality of care (96%), decreased variability in medical management (88%), length of stay (64%), readmission rate (52%), and earlier surgical consultation (52%).
DISCUSSION
In this survey of providers across a high-volume IBD referral center with nearly universal self-reported comfort and experience in managing hospitalized UC patients, we found marked variability in the management of the severe UC patient failing first-line medical therapy with IVCS.
Aside from dose and duration of IV steroids, the role of antibiotics, and when to consider CsA, guidelines for the medical management of acute UC necessitating hospitalization are limited and reflect the paucity of concrete data in this high-risk population. Robust data guiding therapeutic decision-making in the face of IVCS failure are particularly limited3 and manifest as significant practice pattern variability, as we have shown in the present study.
Rescue therapies include IFX and CsA, with 74% of our respondents choosing the former and only 8.6% the latter. Consistent with our findings, CsA use is limited by provider experience, adverse effect profile, frequent lab draws, as well as continuous infusion and need for daily CsA levels to allow titration in the acute setting. That said, there is little clarity in current guidelines for the positioning IFX in the management of hospitalized UC patients with respect to indication, timing, and dosing strategy. For example, in patients failing 3 to 5 days of maximal medical therapy, the American College of Gastroenterology guidelines suggest surgical referral, consideration of CsA, or “perhaps infliximab.”3 Although treatment failure is high in patients requiring rescue therapy, the efficacy of CsA and standard-dosage IFX in IVCS-refractory UC is at least comparable1,5; there are no trials, however, comparing CsA with either accelerated or higher initial dosing of IFX. Importantly, surprisingly few providers ultimately chose colectomy for the patient presented in the vignette, despite the high treatment failure rates reported in the literature.1,3,19 To date, there are no prospective randomized clinical trials comparing outcomes in patients choosing colectomy over initial trial of medical therapy in severe UC.
The patient in the clinical vignette had factors predicting lower response to second-line rescue therapy including elevated CRP and hypoalbuminemia, both of which have been consistently correlated with accelerated IFX clearance and lower serum drug levels.20-23 Yet, the same standard induction regimen of IFX 5 mg/kg at week 0, 2, and 6 studied in the landmark Active Ulcerative Colitis Trials (ACT) 1 and 2 for nonhospitalized, mild-moderate UC patients is the same dosing regimen approved for severe hospitalized UC patients requiring rescue medical therapy.24 Although there was a trend toward improved outcomes in the 10 mg/kg IFX group compared with the 5 mg/kg group at 30 weeks, this did not reach statistical significance.25 Even so, extrapolating nonsignificance between dosing strategies in the hospitalized severe UC patient would be inappropriate given the differences between outpatient and inpatient patient populations. Because there is a direct relationship between serum drug level and therapeutic efficacy in UC,8 a single infusion of IFX 5 mg/kg may not achieve the therapeutic levels needed for clinical response and may indeed be a reason for “drug failure” in the hospitalized UC patient receiving IFX. Although a recent retrospective study suggested accelerated IFX induction (3 doses of 5 mg/kg IFX within median 24 d) was associated with lower colectomy rate in the short term, there was no benefit in the long term.26 There are currently no prospective trials comparing IFX dosing regimens with respect to efficacy and safety in the hospitalized UC patient.
This lack of data is reflected in the marked variability of IFX dosing regimens among our experienced cohort. Notably, in a survey distributed to a more heterogeneous cohort which broadly included members of both the Crohn’s and Colitis Foundation of American Clinical Research Alliance and active members of the International Organization for Inflammatory Bowel Disease, variability in the IFX dosing regimen for severe UC was also reported.15 Descriptive details of this cohort were not provided in the study. In our cohort, with a substantially higher response rate (over 80%), those who chose IFX 10 mg/kg based their decision on factors associated with accelerated IFX clearance. Over one third of providers favored accelerated dosing of IFX, administering an additional 5 or 10 mg/kg within 3 days, even in patients who had received 10 mg/kg initially. In Herfarth et al15 survey, the majority (68%) based their choice of accelerated IFX dosing on clinical severity [as opposed to CRP elevation (10%), IFX level (7%), or hypoalbuminemia (2%)]. As noted, there are no published data supporting additional IFX dosing within a shorter interval, nor corroborating its safety. Serum IFX levels may prove useful in guiding management (ie, repeat IFX dosing if serum levels are low vs. earlier colectomy if IFX levels are adequate, yet there is continued clinical deterioration), although currently there are no data supporting their use in the acute setting. Further research on IFX dosing in this population with altered IFX pharmacokinetics, as well as the associated safety profile, is clearly needed to inform and clarify our current guidelines and therapeutic algorithms for the management of the hospitalized UC patient.
The results of our survey, with over 90% response rate, highlighted significant practice pattern variability even among experienced providers across a single metropolitan-based IBD referral center. Because of this variability, even providers proficient in IBD favor implementation of a clinical care pathway as one way to decrease variability and improve clinical outcomes. The main goal of a clinical care pathway is to reduce variability in management, as reduced variation in care has been associated with improved clinical outcomes, patient satisfaction, decreased medical errors and reduced health care costs, while preserving and even improving quality of care.16-18,27,28 Independent of further research focused on optimizing the management of hospitalized UC patients failing outpatient medical therapy, the implementation of a clinical care pathway alone would likely be advantageous from the broader perspective of minimizing variability to realize the improved outcomes that have been achieved in other chronic conditions such as congestive heart failure. Adjunctive research evaluating the impact (or lack thereof) of a clinical care pathway on outcomes and quality measures in this high-risk population is currently under way. Although our findings do warrant external validation, that others have separately described variability in this patient population15 suggests that our findings are applicable on a broader scale.
Footnotes
The authors declare that they have nothing to disclose.
REFERENCES
- 1.Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380:1909–1915. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein C, Ng S, Lakatos P, et al. A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis. 2013;19:2001–2010. [DOI] [PubMed] [Google Scholar]
- 3.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523. quiz 524. [DOI] [PubMed] [Google Scholar]
- 4.Truelove S, Jewell D. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet. 1974;1:1067–1070. [DOI] [PubMed] [Google Scholar]
- 5.Jarnerot G, Rolny P, Sandberg-Gertzen H. Intensive intravenous treatment of ulcerative colitis. Gastroenterology. 1985;89:1005–1013. [DOI] [PubMed] [Google Scholar]
- 6.Kevans D, Murthy S, Iacono A, et al. Sa2031 accelerated clearance of serum infliximab during induction therapy for acute ulcerative colitis is associated with treatment failure. Gastroenterology. 2012;142:S-384–S-385. [Google Scholar]
- 7.Ordas I, Mould D, Feagan B, et al. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. 2012;91: 635–646. [DOI] [PubMed] [Google Scholar]
- 8.Seow C, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. [DOI] [PubMed] [Google Scholar]
- 9.Blomberg B, Jarnerot G. Clinical evaluation and management of acute severe colitis. Inflamm Bowel Dis. 2000;6:214–227. [DOI] [PubMed] [Google Scholar]
- 10.Arts J, D’Haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis. 2004;10:73–78. [DOI] [PubMed] [Google Scholar]
- 11.Moskovitz D, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4:760–765. [DOI] [PubMed] [Google Scholar]
- 12.Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128:1805–1811. [DOI] [PubMed] [Google Scholar]
- 13.Gibson D, Doherty G. Fecal infliximab loss. Gastroenterology. 2015;149:1989. [DOI] [PubMed] [Google Scholar]
- 14.Brandse J, van den Brink G, Wildenberg M, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology. 2015;149:350–355. e2. [DOI] [PubMed] [Google Scholar]
- 15.Herfarth H, Rogler G, Higgins P. Pushing the pedal to the metal: should we accelerate infliximab therapy for patients with severe ulcerative colitis? Clin Gastroenterol Hepatol. 2015;13:336–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panella M, Marchisio S, Di Stanislao F. Reducing clinical variations with clinical pathways: do pathways work? Int J Qual Health Care. 2003;15:509–521. [DOI] [PubMed] [Google Scholar]
- 17.Kitchiner D, Davidson C, Bundred P. Integrated care pathways: effective tools for continuous evaluation of clinical practice. J Eval Clin Pract. 1996;2:65–69. [DOI] [PubMed] [Google Scholar]
- 18.Bundred PE. Clinical pathways. A practical tool for specifying, evaluating and improving the quality of clinical practice. Med J Aust. 1999;170:54–55. [DOI] [PubMed] [Google Scholar]
- 19.Kornbluth A, Marion J, Salomon P, et al. How effective is current medical therapy for severe ulcerative and Crohn’s colitis? An analytic review of selected trials. J Clin Gastroenterol. 1995;20:280–284. [DOI] [PubMed] [Google Scholar]
- 20.Cacheux W, Seksik P, Lemann M, et al. Predictive factors of response to cyclosporine in steroid-refractory ulcerative colitis. Am J Gastroenterol. 2008;103:637–642. [DOI] [PubMed] [Google Scholar]
- 21.Rowe F, Walker J, Karp L, et al. Factors predictive of response to cyclosporin treatment for severe, steroid-resistant ulcerative colitis. Am J Gastroenterol. 2000;95:2000–2008. [DOI] [PubMed] [Google Scholar]
- 22.Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259. [DOI] [PubMed] [Google Scholar]
- 23.Fasanmade A, Adedokun O, Olson A, et al. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48:297–308. [DOI] [PubMed] [Google Scholar]
- 24.Rutgeerts P, Sandborn W, Feagan B, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. [DOI] [PubMed] [Google Scholar]
- 25.Sandborn W, Rutgeerts P, Feagan B, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137:1250–1260. quiz 1520. [DOI] [PubMed] [Google Scholar]
- 26.Gibson D, Heetun Z, Redmond C, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:330–335. e1. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine Committee on Quality of Health Care in America Kohn LT, Corrigan J, Donaldson M In: To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000:1–8. [PubMed] [Google Scholar]
- 28.Weizman A, Nguyen G. Quality of care delivered to hospitalized inflammatory bowel disease patients. World J Gastroenterol. 2013;19:6360–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]