Abstract
Management of obesity and decompensated cirrhosis in those requiring liver transplantation (LT) is a challenging dilemma. Because of concerns for perioperative complications, many centers avoid transplant in those with a body mass index (BMI) greater than 40 kg/m2. Bariatric surgery is associated with increased risk attributable to complications of portal hypertension, including variceal rupture. Therefore, weight loss and LT options are limited. Several new classes of weight loss drugs are commercially available, including the anoretic, lorcaserin. This case illustrates the successful use of lorcaserin in a morbidly obese individual with decompensated cirrhosis evaluated for LT listing.
Case
A 64-year-old male with cryptogenic cirrhosis likely secondary to nonalcoholic steatohepatitis (NASH) was presented for LT evaluation. His medical history included nonbleeding esophageal varices, diuretic-controlled edema without ascites, and remote hospitalization for hepatic encephalopathy. His Model for End-Stage Liver Disease (MELD) score was 15. Additionally, he was morbidly obese (BMI of 50.3 kg/m2) and had diabetes with peripheral neuropathy. The patient also reported long-standing struggle with weight loss and hyperphagia, but otherwise denied any high-risk habits or significant alcohol history. Medications included stable doses of spironolactone, furosemide, lactulose, rifaximin, propranolol, and omeprazole. Physical exam revealed asterixis and trivial lack of awareness consistent with grade 1 encephalopathy. The abdomen was obese and mild lower extremity edema was present.
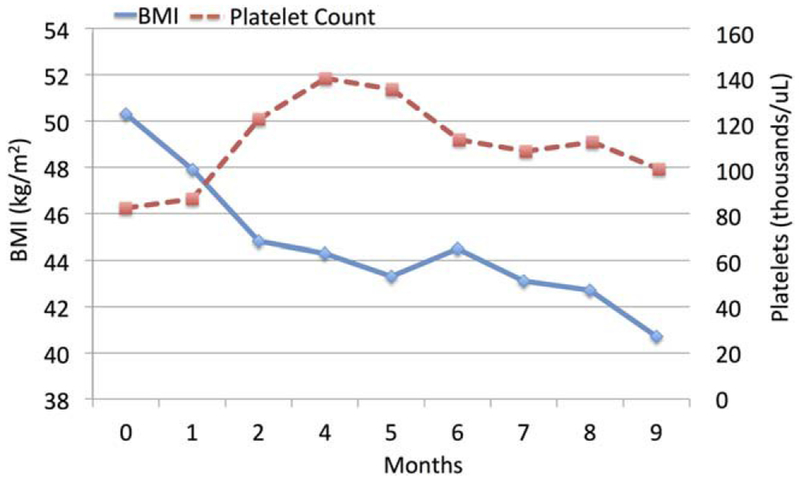
The patient was accepted for transplant listing pending demonstration of meaningful weight loss over a 6-month period. Our center considers patients with a BMI >40 on an individual basis. The patient was referred to a nutritionist with dietary recommendations and given an exercise plan. Additionally, lorcaserin 10 mg twice-daily (BID) was initiated. Within 24 hours of starting lorcaserin, the patient reported lightheadedness; however, symptoms resolved by the next day and he continued with the medication. Otherwise, no adverse events were noted on treatment. Over the next months, the patient’s weight was monitored on a regular basis (see Fig. 1).
FIG. 1.
Response to treatment with lorcaserin for a 9-month period. The patient’s BMI and platelet count (dashed line) both showed improvement.
While on lorcaserin, the patient lost 26.1 kg and improved his BMI and laboratory values compared to baseline (see Table 1). Cardiac echocardiogram showed preserved cardiac function and normal valvular structure. No significant changes were noted on a follow-up exam after 9 months of lorcaserin. The patient was deemed a suitable LT candidate.
TABLE 1.
Laboratory Results at Baseline and Follow-up Month 9 (End of Follow-up)
| Baseline | Month 9 | |
|---|---|---|
| BMI, kg/m2 | 50.3 | 40.7 |
| MELD score | 15 | 11 |
| Platelet count, 3103 u/L | 83 | 100 |
| Albumin, g/dL | 2.7 | 3.4 |
| Total bilirubin, mg/dL | 1.8 | 1.4 |
| Creatinine, mg/dL | 1.4 | 0.96 |
| International normalized ratio | 1.3 | 1.27 |
| Hemoglobin A1C, % | 5 | 4.7 |
| Aspartate aminotransferase, U/L | 50 | 33 |
| Alanine aminotransferase, U/L | 35 | 26 |
| High-density lipoprotein, mg/dL | 38 | 55 |
| Low density lipoprotein, mg/dL | 82 | 101 |
Discussion
NASH is the second-leading indication for LT.(1) Meanwhile, 35%-77% of morbidly obese patients may have NASH.(2) The collision of these two diseases leads to higher reported risk of mortality and morbidity post-LT,(3) causing many centers to avoid transplant in patients with BMI >40. Thus, those who present with cirrhosis and morbid obesity have a dire unmet medical need. New strategies are needed to transition patients to transplant or potentially rescue hepatic function.
Lorcaserin is a highly selective 5-HT2c (serotonergic) receptor agonist that is suspected to work in the brain by activating proopiomelanocortin and promote weight loss through satiety.(4) In the 1-year-long BLOSSOM trial, 47% of patients treated with lorcaserin 10 mg BID had at least 5% body weight loss compared to 25% of patients in the placebo arm.(5) Echocardiographic valvulopathy was rare in both the treatment and control arm of the study.
Despite the enthusiasm for lorcaserin, its role and long-term effectiveness in NASH, especially with advanced liver disease, is unknown. Whether the weight loss achieved with this agent can persist and aid in resolution of NASH also still requires further study. Despite these questions, lorcaserin may be helpful as a bridge to bariatric surgery or LT in those NASH patients who may otherwise be excluded because of morbid obesity.
Abbreviations:
- BID
twice-daily
- BMI
body mass index
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- NASH
nonalcoholic steatohepatitis
Footnotes
Potential conflict of interest: Nothing to report.
REFERENCES
- 1).Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547–555. [DOI] [PubMed] [Google Scholar]
- 2).Seki Y, Kakizaki S, Horiguchi N, Hashizume H, Tojima H, Yamazaki Y, et al. Prevalence of nonalcoholic steatohepatitis in Japanese patients with morbid obesity undergoing bariatric surgery. J Gastroenterol 2016;51:281–289. [DOI] [PubMed] [Google Scholar]
- 3).Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology 2002;35:105–109. [DOI] [PubMed] [Google Scholar]
- 4).Shukla AP, Kumar RB, Aronne LJ. Lorcaserin Hcl for the treatment of obesity. Expert Opin Pharmacother 2015;16:2531–2538. [DOI] [PubMed] [Google Scholar]
- 5).Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM Trial. J Clin Endocrinol Metab 2011;96:3067–3077. [DOI] [PubMed] [Google Scholar]