SUMMARY
Background
Although optimal medical management of acute severe ulcerative colitis (UC) is ill-defined, infliximab has become a standard of care. Accumulating evidence suggests an increased rate of infliximab clearance in patients with acute severe UC and a reduced colectomy rate with an intensified infliximab induction regimen.
Aim
To assess the strength of the current evidence for the relationship between infliximab pharmacokinetics, dosing strategies and disease behaviour in patients with acute severe UC.
Methods
We systematically searched MEDLINE and conference proceedings from 2000 to 2016 for relevant articles describing the pharmacokinetics of infliximab in acute severe UC and/or infliximab dose intensification strategies in acute severe UC. Eligible articles described randomised controlled trials, and cohort, cross-sectional, and case–controlled studies.
Results
Of 400 citations identified, 76 studies were eligible. Increased infliximab clearance occurs in patients with acute severe UC, and is driven by the total inflammatory burden and leakage of drug into the colonic lumen. Several cohort studies suggest that infliximab dose intensification is beneficial to at least 50% of acute severe UC patients and the results of case–controlled studies indicate that an intensified infliximab dosing regimen with 1–2 additional infusions in the first 3 weeks of treatment could reduce the early (3-month) colectomy rate by up to 80%, although these data require prospective validation.
Conclusions
Uncontrolled studies suggest a benefit for infliximab dose optimisation in patients with acute severe UC. A randomised controlled trial in acute severe UC patients comparing a personalised infliximab dose-optimisation strategy with conventional dosing is a research priority.
INTRODUCTION
Ulcerative colitis (UC) is a chronic, relapsing inflammatory disease of the colon and rectum resulting from an inappropriate immune response against environmental factors, including luminal antigens, in a genetically predisposed host.1 In the majority of patients, only the rectum or the left colon is affected, resulting in rectal bleeding, diarrhoea, tenesmus and lower abdominal cramps. Notwithstanding that the disease has a mild- to-moderate course in the majority of patients, approximately 20%–25% develop at least one severe acute exacerbation requiring hospitalisation.2 Acute severe UC is a potentially life-threatening condition that requires early recognition and timely intensive treatment. A diagnosis of acute severe UC can be made using the modified criteria of Truelove and Witts, and is defined by the presence ≥6 bloody stools per day and at least one sign of systemic toxicity including a pulse rate >90 bpm, temperature >37.8 °C, haemoglobin < 10.5 g/dL and/or an erythrocyte sedimentation rate >30 mm/h.3
Patients with acute severe UC should be hospitalised for electrolytes and nutritional support, and timely initiation of medical therapy.4 Sixty years after the seminal observations by Truelove and Witts, intravenous corticosteroids remain first-line medical therapy for acute severe UC. However, 30%–40% of patients fail corticosteroid therapy and need rescue therapy with ciclosporin or infliximab.5, 6 Failure rates of these secondary therapies can regrettably reach 40%–50% in the short-term (within 3 months) and 70% in the long-term (within 3 years), necessitating colectomy in approximately 45% of patients within 5 years.7 These results require a critical re-evaluation of the approach to medical rescue therapy. Specifically, there is considerable uncertainty regarding the optimal dosing approach for infliximab following presentation with refractory acute severe UC. In a survey of members of the Crohn’s and Colitis Foundation of American Clinical Research Alliance and the International Organization for Inflammatory Bowel Disease, 76% of respondents indicated use of an intensified dosing regimen for acute severe UC, either through increased infliximab concentration (>5 mg/kg per dose) and/or an accelerated dosing schedule.8 Some respondents indicated this regimen was standard practice, but most indicated empirical infliximab dosing according to disease severity, serum C-reactive protein (CRP) and albumin concentrations and/or serum infliximab concentrations.8 These practices are inconsistent with current evidence-based recommendations that do not support these approaches or recommend intensified infliximab dosing regimens for patients with corticosteroid-refractory acute severe UC.4, 9, 10 In this article, we review the current literature on the efficacy and safety of an intensified infliximab dosing regimen in acute severe UC based on available pharmacokinetic and clinical data, and synthesise the evidence to propose considerations for a randomised controlled trial designed to compare a dose-optimisation strategy with a standard of care approach.
METHODS
Search strategy and study selection
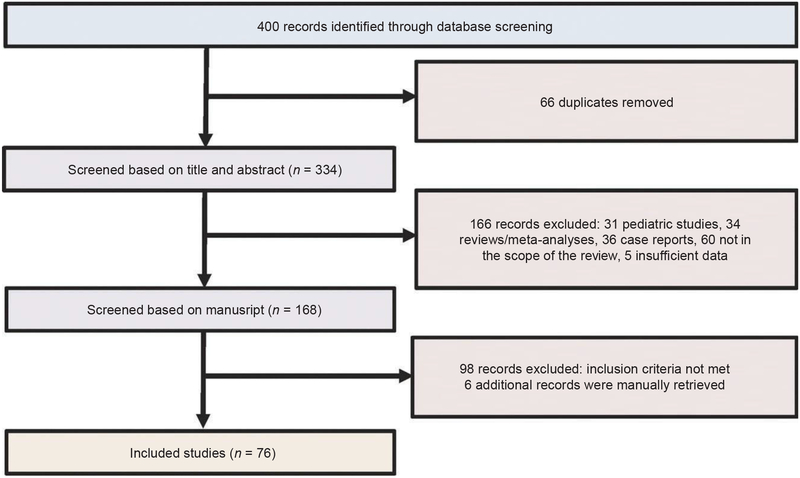
We searched MEDLINE (Ovid), EMBASE (Ovid) and CENTRAL (The Cochrane library) databases for records from 2000 to 2016, without language restriction. The following search strategy was used: 1. severe*.mp; 2. (acute* AND severe*).mp; 3. 1 and 2; 4. colitis.ti; 5. infliximab.ti; 6. 3 and 4 and 5. Bibliographies of relevant studies, review articles and meta-analyses were hand-searched to identify additional studies. This search yielded a total of 400 citations of which 66 were identified as duplicates and removed (Figure 1). Randomised controlled trials (RCTs), cohort, case–control and cross-sectional studies were eligible provided they reported on pharmacokinetics of infliximab in adult acute severe UC patients, on prognostic markers for acute severe UC outcome, or on the use of infliximab treatment intensification strategies in acute severe UC patients. Seventy-six citations were eligible for analysis (Figure 1).
Figure 1 ∣.
Flow diagram. The search strategy identified 400 references of which 66 were duplicates and removed. Two hundred sixty-four of the remaining 334 records were excluded because they did not meet the eligibility criteria or provided insufficient information for analysis. Six supplemental references were manually retrieved, resulting in a total of 76 records included for analysis.
RESULTS
Outcomes for acute severe UC patients receiving infliximab rescue therapy
The first randomised controlled trial of infliximab therapy in corticosteroid-refractory acute severe UC was conducted by Sands et al. in 2001 who reported superior clinical, biochemical and endoscopic responses to infliximab compared to placebo.11 However, the small sample size (11 patients) precluded any definitive conclusions. A subsequent placebo-controlled trial by Jarnerot et al. found a lower 90-day colectomy post-randomisation in moderate-to-severe corticosteroid-refractory UC patients (N = 45) given a single 5 mg/kg infliximab infusion compared with those assigned to placebo (67% vs. 29%, P = 0.017).12 A recent comprehensive review that summarised the efficacy data of infliximab for acute severe UC from both observational and randomised studies reported short-term (3 months) response rates ranging from 50% to 83%, and long-term (1–5 years) rates ranging from 50% to 65%. Short-term colectomy rates ranged from 0% to 50% and long-term rates ranged from 35% to 50% in the infliximab groups.7
Two randomised studies comparing infliximab to ciclosporin for acute severe UC found no important differences in the efficacy or safety of these agents.5, 6 A meta-analysis of studies that investigated infliximab and ciclosporin as rescue therapy in corticosteroid-refractory UC also found no significant differences between infliximab and ciclosporin in RCTs, in contrast to earlier observational studies that favored infliximab (5 mg/kg) over ciclosporin as a rescue therapy, and which demonstrated both a higher treatment response rate and a reduced 3-month colectomy rate with infliximab therapy.13 This meta-analysis found no difference between infliximab and ciclosporin with regard to the incidence of drug-related adverse events (14.1% and 12.8%), post-operative complications (15.9% and 11%), or mortality (1.0% and 0.9%).13.
Based on data from these RCTs, current guidelines do not recommend one option over the other,14-16 although the 2012 Canadian guidelines state that ciclosporin should only be given to azathioprine-naive patients.9 In practice, however, many gastroenterologists consider infliximab the preferred agent on the basis of ease of administration and lack of nephrotoxicity.
Despite the availably of these two treatment options, high short-term colectomy rates support a need for improved outcomes for patients with acute severe UC. For example, 45% (120 of 270) of patients required colectomy during follow-up in the UK CONSTRUCT trial, with the majority of these surgeries occurring within in the first month post-discharge.6 In the previously described study by Jarnerot et al., the median time to operation in the infliximab group was 8 days,12 and in a large Swedish cohort of hospitalised corticosteroid-refractory acute severe UC most infliximab treatment failures necessitating colectomy were observed during the first 14 days of treatment.17 These data suggest that opportunities for treatment optimisation and to avoid colectomy are greatest in the days and weeks following presentation.
Rationale for an intensified infliximab dosing regimen in acute severe UC
Pharmacokinetic data.
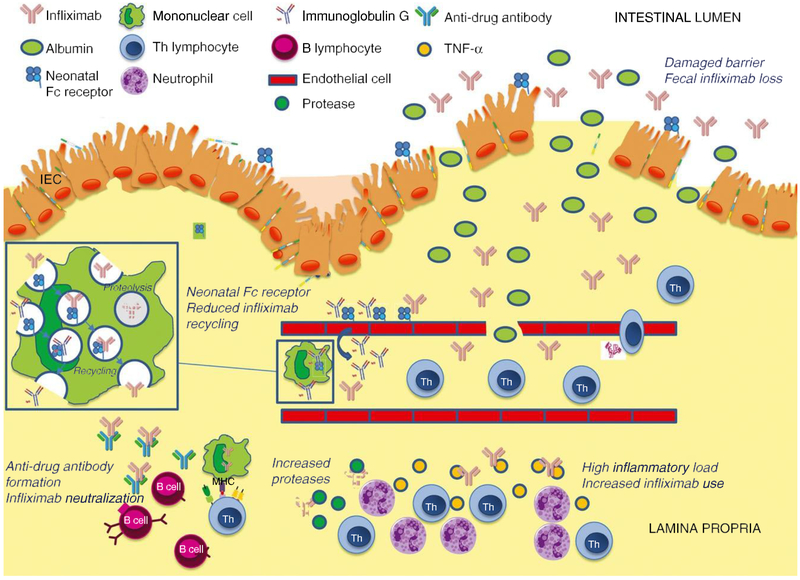
Monoclonal antibodies are large molecules that are cleared by pinocytosis and gradual acidification in endosomes, followed by fusion with lysosomes and proteolysis by the mononuclear cells of the reticuloendothelial system (Figure 2).18, 19 This pathway is mainly regulated through Fc gamma receptors that have been identified to play a role in response to tumour necrosis factor-a (TNF-a) antagonist therapy.20 A recycling mechanism exists for immunoglobulin G antibodies, which bind to the neonatal Fc receptor at low pH. This drug-receptor complex is subsequently carried back to the cell surface and dissociates at neutral pH. As a result, the immunoglobulin G antibody escapes degradation in the lysosome and returns to the serum.18 Therapeutic immunoglobulin G1 antibodies such as infliximab participate in this recycling mechanism, resulting in longer drug half-lives. Factors influencing the clearance rate of infliximab in patients with acute severe UC are shown in Figure 2.
Figure 2 ∣.
Pharmacokinetic mechanisms that influence the clearance rate of infliximab in patients with acute severe UC. High inflammatory burden results in elevated amounts of TNF-a, which serve as a sink for infliximab, and results in the formation of immune complexes.24 Phagocytosis and proteolytic degradation of these immune complexes is facilitated by an increase in the number of phagocytic mononuclear cells and the presence of activated matrix metalloproteinases in the inflamed mucosa.18, 25 A severely damaged mucosal barrier results in efflux of infliximab into the colonic lumen and faecal loss of the drug.28 The destruction of the colonic epithelium results in reduced availability of the neonatal Fc receptor which, together with increased saturation of neonatal Fc receptor binding sites by high concentrations of endogenous immunoglobulin G in the setting of inflammation, decreases neonatal Fc receptor-mediated recycling of infliximab.18 Increased clearance of infliximab leads to low drug concentrations which may enhance immunogenicity and facilitate the formation of anti-drug antibodies due to loss of high zone tolerance.32 These anti-drug antibodies further promote the degradation of infliximab.
Total inflammatory burden:
Serum and mucosal TNF-a concentrations correlate with disease activity in UC.21-23 The clearance of infliximab is influenced by the amount of soluble and transmembrane TNF-a, present in both the systemic circulation and mucosa. This “antigen sink” results in formation of immune complexes that, depending on their size and composition, are subject to phagocytosis.24 The phagocytosis and proteolytic degradation of the immune complexes may be further facilitated by upregulation of the number of phagocytic mononuclear cells in response to systemic inflammation and increased activity of reticuloendothelial system macrophages.18 The presence of activated matrix metalloproteinases in the inflamed mucosa further facilitates breakdown of infliximab.25 A high concentration of endogenous immunoglobulin G that exists in the setting of severe inflammation may saturate neonatal Fc receptor binding sites, thereby disabling the recycling of the monoclonal antibody.18 These neonatal Fc receptor binding sites are also found on the intestinal epithelium that facilitates recovery of luminal immunoglobulins. The extensive epithelial destruction present during acute severe UC may thus further decrease the availability of the neonatal Fc receptor salvage recycling system resulting in lower serum drug concentrations.26.
Faecal loss:
It is well recognised that substantial protein loss occurs in acute severe UC as a result of damage to the epithelial barrier. This phenomenon contributes to the high incidence of hypoalbuminemia observed in these patients.27 Brandse et al. demonstrated that relatively high concentrations of infliximab can be detected in the faeces of IBD patients, especially in those with severe disease.28 A similar phenomenon has been observed in patients with Crohn’s disease (CD); in a series of 13 patients, faecal concentrations of infliximab and adalimumab were highest in patients with severely active disease and inversely correlated with serum infliximab concentrations.29 Faecal loss of infliximab presents early, with the highest faecal concentrations seen in the first days following initiation of therapy.28.
Anti-drug antibodies:
The presence of high serum concentrations of TNF antagonists during induction therapy may protect against formation of anti-drug antibodies.30, 31 Increased clearance of infliximab in acute severe UC patients may result in low serum drug concentrations and promote the formation of anti-drug antibodies, leading to an inadequate response. Evidence to support this concept comes from a small pharmacokinetic study of patients with moderate-to-severe UC who received induction therapy with infliximab.32 Seven of the 19 patients in this study developed anti-drug antibodies as early as day 18 (median, 28 days; interquartile range, 18–42 days). The majority of these patients (5 of 7) had a high inflammatory burden as evidenced by a baseline serum CRP concentration > 50 mg/L. In a retrospective cohort study that compared infliximab trough concentrations and anti-drug antibody formation in patients with acute severe UC to those with moderate UC (N = 16 for both groups), there was a non-significant trend for higher anti-drug antibody concentrations in acute severe UC patients (1.2 ± 4 lg/mL as compared to 3.4 ± 5.7 lg/mL-eq, P = 0.06).33 Finally, in a retrospective cohort of 115 patients with moderate-to-severe UC, 23 patients underwent sequential measurement of trough infliximab concentrations during induction therapy. Among 13 of 23 patients with undetectable serum infliximab concentrations, 77% (10/13) had developed anti-drug antibodies by week 14.34.
Additional patient factors:
Patient-related factors that influence the clearance rate of infliximab include gender (more rapid clearance in males) and bodyweight (more rapid clearance in patients with high bodyweight but also in patients with a bodyweight <40 kg).35, 36.
Clinical data.
Accumulating evidence suggests that the theoretical pharmacokinetic mechanisms proposed above translate into increased infliximab clearance, reduced infliximab trough concentrations and poor outcomes in patients with acute severe UC as compared to patients with less severe disease, and that infliximab dose intensification strategies have the potential to overcome these pharmacokinetic challenges (Table 1).
Table I ∣.
Summary of the most compelling evidence supporting dose optimisation of infliximab for acute severe UC
| Reference | Design | Patients | Relevant finding |
|---|---|---|---|
| Kohn et al., 200744 | Retrospective multicentre cohort | Hospitalised with acute severe UC or a moderately severe attack of UC (N = 83 | Multiple infliximab infusions reduce 2-month colectomy rate compared to a single infusion (5% vs. 35%, P = 0.001). |
| Seow et al., 201034 | Retrospective single-centre cohort | Moderate-to- severe UC (N = 115) | An undetectable serum trough infliximab concentration during induction treatment is associated with a nine-fold risk of colectomy (odds ratio 9.3; 95% CI 2.9 to 29.9; P 0.001) at a median of 5.3 months. |
| Kevans et al., 201442 | Prospective single-centre cohort | Acute severe UC (N = 13) | An undetectable serum trough infliximab trough concentration during induction treatment is associated with treatment failure (with or without colectomy) at 30 weeks (no P value provided). |
| Gibson et al., 201550 | Single-centre case-control | Acute severe UC (N = 50) | An accelerated infliximab induction regimen reduces the need for colectomy during induction treatment (6.7% vs. 40% for the standard 0, 2, and 6 week regimen, P = 0.039). |
| Arias et al., 201547 | Retrospective single-centre cohort | Moderate-to-severe UC (N = 285) | A week 14 serum trough infliximab trough concentration > 2.5 lg/mL predicts colectomy-free survival (P = 0.034) at a median of 64.4 months. |
| Govanni et al., 201651 | Single-centre case-control | Acute severe UC (N =57) | An accelerated infliximab induction regimen reduces the need for colectomy within the first 90 days (12.1% vs 47.1% for the standard 0, 2 and 6 week regimen, P = 0.01). |
| Choy et al., 201652 | Retrospective multicentre cohort | Acute severe UC (N= 41) | No difference in 3- and 12-month colectomy rate between the standard 0, 2 and 6 week, and an accelerated infliximab induction regimen despite a higher baseline CRP and CRP/albumin ratio (both predictors of poor outcome) in patients that received the accelerated induction regimen. |
| Brandse et al., 201632 | Prospective single-centre cohort | Moderate-to-severe UC (N = 19) | A high baseline serum CRP (≥ 50 mg/L) and low serum albumin (≤ 25 g/L) concentration correlate with low infliximab drug exposure during induction treatment which in turn is associated with a lack of endoscopic response at week 6 (P = 0.03). |
| Ungar et al., 201633 | Multicentre case-control | Acute severe or moderately severe UC (N = 32) | Lower infliximab trough concentrations at week 14 in acute severe UC patients as compared to patients with moderately active disease (P = 0.007). |
Association between UC severity and increased drug clearance:
Many studies have reported an association between severity of UC and infliximab treatment failure, indirectly suggesting that a conventional dose (5 mg/kg) insufficiently targets the inflammation associated with severe UC.37-41 In a small prospective cohort study from Brandse et al., high baseline serum CRP (>50 mg/L), and low serum albumin (<35 g/L) concentrations and extensive colitis were independently associated with exposure to lower infliximab concentrations from weeks 0 to 632. In a recently published retrospective study, infliximab and anti-drug antibody concentrations of hospitalised patients with acute severe UC were compared with those of patients with moderate-to-severe active UC 2 weeks after the first, or scheduled infusion (5 mg/kg). Patients with acute severe UC had significantly lower week 2 infliximab concentrations than those with less severe disease.33 In a small series of patients with acute severe UC patients who had undergone serial measurement of infliximab trough concentrations during induction treatment, 8 of 13 patients had undetectable infliximab concentrations.42.
Results from the recently published ATLAS study further support the association between inflammatory bowel disease (IBD) severity and clearance of TNF antagonists. This study evaluated the pharmacokinetics of TNF antagonists in 30 patients with active IBD (6 UC, 24 CD; 18 treated with adalimumab, 12 treated with infliximab). While tissue concentrations of the TNF antagonists generally correlated with the degree of mucosal inflammation, low drug concentrations were seen in biopsies taken from severely inflamed mucosa, suggesting increased clearance in these patients. In addition, a much lower drug-to-TNF ratio was seen in moderate-to-severely inflamed tissue compared to less inflamed tissue, indicating that there was insufficient drug to neutralise the local TNF burden in patients with more severe disease.21 The potential that high local concentrations of TNF might act as a drug “sink” was demonstrated by Olsen et al. who found an inverse correlation between TNF-a gene expression in the colorectal mucosa and the clinical outcome of infliximab induction therapy in 59 patients with moderate-to-severe UC.43.
Association between infliximab dosing, exposure and clinical outcomes in acute severe UC:
The first study to demonstrate an association between clinical outcomes in acute severe UC and intensity of infliximab dosing was a retrospective multicentre study conducted in 83 acute severe UC patients.44 In this study, the only variable independently associated with early colectomy (within 2 months) was the number of infliximab infusions. There was a 35% colectomy rate after a single infusion compared with a 5% colectomy rate after multiple infusions. This finding was confirmed in another retrospective study from Denmark.45 In a meta-analysis that compared infliximab and ciclosporin rescue therapy in patients with acute severe UC, infliximab appeared to be superior for prevention of colectomy at both 3- and 12 months (odds ratios of 0.38 and 0.33, respectively) when studies using only single-dose infliximab were excluded from the analysis.13.
Evidence linking infliximab concentrations with clinical outcomes in patients with moderate-to-severe UC was initially reported by Seow et al. who retrospectively evaluated 23 patients who had received sequential measurement of infliximab trough levels during infliximab induction therapy. Patients with detectable serum infliximab concentrations at week 6 had significantly higher rates of clinical and endoscopic remission (69% vs. 15% and 27% vs. 8% respectively) and a lower 24-week colectomy rate (7% vs. 55%; P = 0.001) than those with undetectable infliximab concentrations.34 Similar findings were reported in a small series of 13 acute severe UC patients by Kevans et al., in whom undetectable infliximab serum concentrations during induction treatment were associated with long-term (30 weeks) infliximab anti-drug antibody formation and treatment failure (with or without colectomy).42.
Other retrospective studies have reported specific cut-points for serum infliximab concentrations that are associated with an increased risk of colectomy in UC patients. In a retrospective study from 3 referral centres that included 99 biologic-na€ıve UC patients with a primary nonresponse to infliximab, an infliximab concentration <16.5 lg/mL at week 2 was identified as an independent predictor of colectomy within a median follow-up period of 3.2 years.46 In another retrospective single-centre study, infliximab serum concentrations >2.5 lg/mL at week 14 predicted colectomy-free survival with a median follow-up period of 5 years.47.
Evidence for a benefit of infliximab dose optimisation in patients with acute severe UC.
Dose increase:
The possibility of improved efficacy with infliximab dose intensification originated from post hoc analyses of the ACT 1 and 2 studies.48 In these two placebo-controlled trials that compared infliximab (5 mg/kg or 10 mg/kg) to placebo in 728 ambulatory patients with moderate-to-severe UC, only the 10 mg/kg was shown to reduce the need for colectomy through 54 weeks.48 Furthermore, patients with an infliximab trough serum concentration in the lowest quartile of the distribution were less likely to achieve clinical response/remission and/or mucosal healing49 than patients in the other quartiles, independent of randomised infliximab dose (5 mg/kg or 10 mg/kg).49 This observation indicates that complex factors determine the infliximab dose-exposure relationship, and that these factors may better predict trough concentrations and clinical outcomes in individual patients than dose group assignment in clinical trials. This observation also suggests that a subset of patients with high rates of drug clearance may benefit from intensified infliximab dosing regimens.
Intensified dosing of infliximab:
In a retrospective single-centre analysis of 50 corticosteroid-refractory acute severe UC patients, Gibson et al. observed markedly lower early colectomy rates compared to historical values following the introduction of an intensified infliximab dosing regimen (three 5 mg/kg doses within a median period of 24 days) as standard practice in their institution. Colectomy rates during the induction period were 6.7% in patients who received intensified infliximab dosing (N = 15) and 40% in the historical cohort (N = 35, P = 0.039 for the comparison). Lower colectomy rates persisted after induction therapy in the group of patients who received intensified infliximab dosing; the colectomy rate with standard infliximab dosing was 51.4% at a median of 2.4 years of follow-up in the historical cohort and 27% in patients who received intensified infliximab dosing at a median follow-up of 1.6 years.50
In another retrospective study by Govani and colleagues, the 90-day colectomy rate was compared in 17 acute severe UC patients treated with intensified infliximab dosing (an additional infusion at day 3 for patients with CRP ≥70 mg/L) and 40 patients who had received a conventional infliximab dosing regimen.51 Average baseline serum CRP and albumin concentrations did not differ between the two groups. The intensified infliximab regimen was associated with a lower 90-day colectomy rate (47% vs. 12.5%, P = 0.01). Post-operative readmission rates were numerically higher in the patients treated with the intensified regimen (57.1% vs. 20% for the conventional regimen), however, post-operative complications (abscess, urinary or respiratory infections and rectal stump complication) were similar.51.
A retrospective multicentre study compared outcomes in nine acute severe UC patients who had received intensified infliximab dosing (three infusions within a median of 20 days) with 26 patients who had received standard dosing. No difference in 3- or 12-month colectomy rate was observed between the two groups of patients. However, intensified dosing in this study was selectively administered to patients with higher CRP and CRP/albumin ratios in whom an increased colectomy rate was expected.52.
Some additional support for the use of intensified dosing comes from anecdotal case reports on poorly responding acute severe UC patients rescued from colectomy with intensified infliximab dosing.28, 32 Although these observational data are compelling, the hypothesis that dose intensification in patients with acute severe UC is superior to standard dosing requires validation in a randomised trial.
Combination therapy with an immunosuppressive.
The SUCCESS trial conducted by Pannacione et al. showed that the combination of infliximab and azathioprine is a more effective therapy than either infliximab or azathioprine alone in patients with moderate-to-severe UC.53 Concomitant use of an immunosuppressive with infliximab in acute severe UC may reduce infliximab clearance by suppressing proteolysis and clearance by mononuclear cells of the reticuloendothelial system and by preventing immunogenicity and anti-drug antibody formation.54.
Co-administration of an immunosuppressive agent with infliximab was associated with a reduced risk of colectomy (odds ratio 0.34; 95% CI 0.121–0.963 P = 0.022) in a multicentre retrospective study that included 187 UC patients initiated on infliximab.41 Finally, in a small nonplacebo-controlled clinical trial that randomised 26 patients with fulminant UC to infliximab or control, none of the patients in the infliximab group who underwent late (after 7 days) colectomy had used azathioprine during infliximab maintenance therapy, whereas most of the patients (12/14) without colectomy had used this medication.55 In summary, azathioprine should be added to infliximab therapy for acute severe UC as soon as clinically possible. However, this practice should be avoided in the acute phase when oral tolerability of the patients is low and azathioprine-related side-effects, such as pancreatitis or bone-marrow suppression, would be detrimental.
Infliximab as a first-line therapy for acute severe UC?
Limited data are available comparing the relative efficacy of infliximab to corticosteroids as a first-line treatment for acute severe UC. A pilot trial randomised 13 acute severe UC patients to receive three infliximab (5 mg/kg) infusions or high-dose intravenous corticosteroids (1.5 mg/kg prednisolone, tapered to 1 mg/kg after 2 weeks followed by a weekly reduction of 5 mg).56 The primary endpoint was a decrease of > 5 points from the baseline in the modified Truelove and Witts severity score, and an overall score < 10 points.56 Similar outcomes were observed in both groups at 3 and 13 weeks. The small sample size of this trial precludes any conclusion regarding the relative efficacy of the two agents.
Similarly, there are no data on the combined use of corticosteroids and infliximab as first-line treatment. A randomised open label study () designed by the Groupe d’Etude Therapeutique des Affections Inflammatoires Digestives has been designed to compare the combination of azathioprine and infliximab with the combination of azathioprine and corticosteroids in patients with acute severe UC. The proposed primary endpoint of the study is treatment failure at week 52 (defined as the absence of steroid free remission and/or mucosal healing at week 52, colectomy or death between day 0 and week 52, infliximab withdrawal in the azathioprine and infliximab group or infliximab introduction in the azathioprine and corticosteroids group).
Safety of intensified infliximab dosing regimens.
There is little evidence to indicate that patients treated with higher doses of infliximab or those with greater drug exposure (higher serum concentrations) are at an increased risk of side-effects such as serious infection. This observation, which is consistent with those observed with other monoclonal antibodies in IBD and other immune diseases, is likely a result of the high target specificity of these agents, such that increased exposure does not result in engagement of “off target” mechanisms. In the TREAT registry, which prospectively evaluated 6273 CD patients treated with infliximab, no increased safety signal was observed at 5 years in patients who had received dose escalation from 5 mg/kg to 10 mg/kg.57 In addition, no association was found between infliximab serum trough concentrations and serious infection in the ACT trials.49 Some notable exceptions exist. In a RCT that compared the safety of methotrexate monotherapy with methotrexate and infliximab (3 mg/kg or 10 mg/kg) combination therapy in patients with active rheumatoid arthritis, the 10 mg/kg induction regimen was associated with an increased risk of serious infections through week 22 (18/361 patients) as compared to the 3 mg/kg regimen (6/360 patients) (relative risk 3.1; 95% CI 1.2–7.9, P = 0.013). The vast majority of these infections were of the respiratory tract (including tuberculosis).58.
In addition, in a long-term safety study involving 107 patients with chronic obstructive pulmonary disease who had previously been enrolled in a 44-week phase two trial and had received at least 1 dose of infliximab, an increased mortality risk was observed in the group of patients randomised to treatment with 5 mg/kg infliximab (hazard ratio 1.83; 95% CI: 0.72, 4.66) compared to the group of patients randomised to treatment with 3 mg/kg infliximab (hazard ratio 1.05; 95% CI: 0.37, 3.00). The average duration of drug exposure in the combined infliximab treatment groups was 18.6 weeks.59. Finally, an association between increased infliximab dose and increased risk of serious infections (pneumonia, abscesses, catheter sepsis, systemic fungal infections) was identified in a retrospective analysis of 86 patients with CD who had received high-dose infliximab therapy.60 A serious infection rate of 1.87, 8.53 and 13.76 per 100 patient-years was observed in patients that received less then, equal to and more than 2.5 mg/kg infliximab/week, respectively.60 These data, although limited, warrant caution and increased vigilance when prescribing infliximab dose intensification in acute severe UC patients who already carry a high overall burden of complications and are usually receiving concomitant corticosteroids and immunosuppressives.
Perspectives on infliximab dose-optimisation trials in patients with acute severe UC.
As previously described, evidence from pharmacodynamic and pharmacokinetic studies indicate that increased infliximab clearance exists in patients with acute severe UC. Results of uncontrolled and largely retrospective series suggest considerable reduction in colectomy rates with an intensified infliximab induction schedule, although at a potentially increased risk of serious infections. Based on the results of a survey of North American and international specialists, many clinicians use some form of infliximab treatment intensification for acute severe UC in routine clinical care, although approaches vary widely from dose escalation, dose acceleration or a combination of these approaches.8 These observations underscore the uncertainty amongst the clinical community regarding infliximab dosing in acute severe UC and show that clinical equipoise currently exists. A randomised trial comparing infliximab dosing regimens would inform clinical practice and either substantiate or refute the findings from aforementioned observational studies. However, a clinical trial to inform a more personalised approach to dosing has considerable challenges and several valid approaches to its design.
We are aware of one trial (PREDICT-UC) currently underway () that compares the standard (5 mg/kg at week 0, 2 and 6) infliximab regimen with an accelerated induction regimen (5 mg/kg at week 0, 1 and 3) and a combined accelerated induction and dose increase regimen (10 mg/kg at week 0 and 1) in patients with acute severe UC. The primary endpoint of this trial is 90-day colectomy rate. This is an important trial which will hopefully determine whether an intensified induction regimen can improve outcomes for patients with acute severe UC. However, this trial will not determine if this approach should be used for all acute severe UC patients. Lower risk patients may attain benefit from standard induction infliximab dosing, and would thus avoid the increased costs and potential risks associated with infliximab dose intensification. Thus, an opportunity exists to personalise infliximab dosing based upon risk factors for colectomy. Furthermore, not all patients with acute severe UC are likely to exhibit higher rates of infliximab clearance, and it is therefore also logical to restrict dose intensification to those with inadequate drug exposure.
Population:
Based on the available evidence, an enrichment strategy to include acute severe UC patients who are most likely to benefit from an infliximab dose optimisation is an attractive approach to a clinical trial of treatment strategies for acute severe UC. While several indices have been developed to predict corticosteroid failure in acute severe UC,61-64 no predictive index for assessment of response to rescue treatment currently exists. However, several objective markers have been investigated in this regard.
Serum CRP:
Several reports have underscored the value of CRP concentration at admission and infliximab initiation as risk stratification for acute severe UC patients. A CRP concentration >29 mg/L at the first infliximab infusion was predictive of colectomy in a retrospective study,45 and a high baseline CRP concentration (≥ 30 mg/L) was independently associated within the risk of colectomy within the first 3 months from the first infliximab infusion in a prospective multicentre study.65 Collectively, these findings suggest a CRP concentration ≥ 30 mg/L is an appropriate cut-off to select acute severe UC patients for a therapeutic strategy trial.
Serum albumin:
Serum albumin may be used as a biomarker of proteolysis, the efficiency of the neonatal Fc receptor salvage recycling system and the amount of faecal protein loss.66 As such, it may be an indirect predictor of the infliximab clearance rate and the need for dose optimisation. Gibson et al. observed that a serum albumin concentration > 22 g/L at infliximab initiation, which was present in more than half (26/50) of the acute severe UC patients in their study, was associated with a reduced colectomy rate (relative risk: 0.84, 95% CI: 0.75–0.95, P = 0.003).50 Sambuelli et al. and Lees et al. reported higher optimal cut-offs (30 g/L and 34 g/L, respectively), with a colectomy hazard ratio of 3.15 (95% CI 1.15 to 8.62, P = 0.0026) at a mean follow-up of 19.4 months and 12.0 (95% CI 1.28–112.7) at day 90, respectively, below these concentrations.67, 68.
Serum CRP/albumin ratio:
A CRP/albumin ratio of 1.75 at day 3 of admission was associated with a need for colectomy (sensitivity of 72%, specificity of 75%) in a retrospective cohort of 129 patients with acute severe UC.69 The 3-year colectomy rate was 20% for CRP/albumin ratio <1.75 and 50% for a ratio >1.75. In this study, day 3 CRP/albumin ratio was a more accurate predictor of subsequent 30-day colectomy than day 3 albumin alone (area under the curve 0.78 vs. 0.70).69
Faecal calprotectin:
Calprotectin is a neutrophil derived protein that correlates with intestinal inflammation. One study of 90 acute severe UC patients found that a faecal calprotectin concentration > 1922 lg/g could predict the need for colectomy with an area under the curve of 0.65.70 However, an important disadvantage of faecal calprotectin is its high variability in patients with active UC, which precludes its use as the basis for therapeutic strategies.71
Radiological indices:
Small bowel distention on plain abdominal radiography during the acute admission predicts an increased risk of colectomy.72 Pilot data in 22 patients with acute severe UC undergoing unprepared MRI demonstrated that the total colonic inflammatory score had a positive predictive value for colectomy, although availability of this modality in the acute setting is a substantial limitation to this approach.73.
Endoscopic lesions:
Severe endoscopic lesions have been associated with infliximab failure and colectomy in acute severe UC patients. In a large retrospective cohort of 113 acute severe UC patients, 27 of 81 patients (33.3%) with severe endoscopic lesions at baseline (defined as the presence of deep ulcers and/or spontaneous bleeding) underwent colectomy within 12 months compared to 2 of 32 patients (6.2%) without severe endoscopic lesions.65
Interventions:
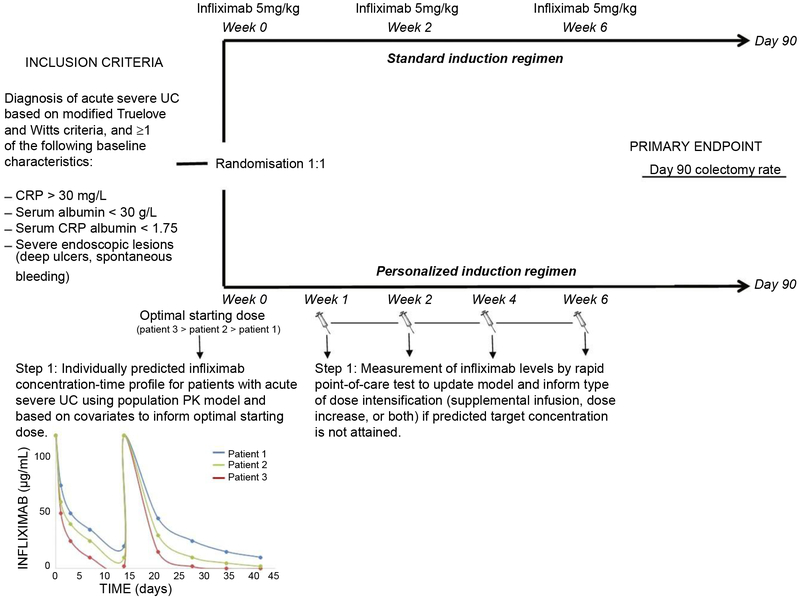
Both serum CRP and infliximab concentrations have been associated with the risk of colectomy during infliximab induction treatment, however, the proposed cut-offs differ widely amongst studies.32, 44, 74, 75 Given the multiple factors influencing infliximab drug exposure, a personalised approach to initial and subsequent dosing should be informed by a population-based pharmacokinetic model that would account for dynamic changes in parameters such as serum CRP and albumin concentrations (Figure 3). This model would ideally identify the optimal target drug concentrations that should be measured with rapid point-of-care tests to allow for subsequent and timely dose adjustments (Figure 3).
Figure 3 ∣.
Proposed framework for the design of a randomised controlled trial in acute severe UC comparing a personalised infliximab dose-optimisation strategy with conventional dosing in-patients with at least 1 poor prognostic factor (primary outcome; 90-day colectomy rate). The personalised arm would be informed by a population-based PK model that allows for adjustment of the first dose of infliximab in order to obtain the predicted and desired target drug concentration. Subsequent dose adjustment could be performed at various timepoints (the authors propose weeks 1, 2, 4 and 6) based on the results of rapid (≤ 20 minute) point-of-care tests.
Outcomes:
Colectomy is the most relevant clinical endpoint for acute severe UC clinical trials. The vast majority of colectomies in acute severe UC patients occur in the first weeks to months.12 As such, the 90-day colectomy rate is an appropriate primary endpoint for an interventional trial. Based upon retrospective data, a 40%–50% relative reduction in colectomy rates at 90 days (e.g., from approximately 20% to 10%) would be a clinically important difference.13 Secondary endpoints would include clinical response at day 7, a relapse between day 7 and day 90, incidence of severe adverse events, and mortality.
CONCLUSIONS
In summary, increased understanding of the pharmacokinetics of infliximab and emerging clinical data from observational studies indicate that some patients with acute severe UC have increased infliximab clearance, resulting in low serum infliximab concentrations and suboptimal clinical response. Case–controlled studies and case-series indicate that an intensified infliximab induction regimen may markedly reduce short-term colectomy rates after acute severe UC. A RCT is now needed to compare 90-day colectomy rates between a standard of care approach and a personalised infliximab regimen (ideally guided by serum CRP and infliximab drug concentrations) in acute severe UC patients, to inform clinical practice and guidelines.
ACKNOWLEGEMENTS
Declaration of personal interests: PH has received consulting fees from Abbvie and Takeda; speaker’s fees from Ferring, Falk Pharma, Vifor Pharma, Tillotts Pharma, Chiesi, Takeda and Abbvie: GN has received speaker’s fees from Abbvie and Merck. NVC is a Postdoctoral Fellow of the Research Foundation -Flanders (FWO), Belgium (grant number 1260714N), and has received consulting fees from UCB Pharma, Pfizer and Takeda. DL has no relevant disclosures. CP is an employee of Robarts Clinical Trials and has no relevant disclosures. LMS has no relevant disclosures. NN has received consultant, speaker, or research grants from Abbvie, Takeda, Janssen, Ferring, Actavis, and Lupin. RK has received consulting fees from AbbVie, Janssen, and Takeda Pharma. PD is supported by the-NIDDK training grant 5T32DK007202 and has no relevant disclosures. BGL is an employee of Robarts Clinical Trials and has received speaker’s fees from Mitsubishi Tanabe Pharma Corporation. WJS has received grant support from Exact Sciences, the American College of Gastroenterology, and the Broad Foundation; grant support and personal fees from Receptos, Amgen, Prometheus Laboratories, AbbVie, Boehringer Ingelheim, Takeda, Atlantic Pharmaceuticals, Janssen, Bristol-Myers Squibb, Genentech, Pfizer, and Nutrition Science Partners; and personal fees from Kyowa Hakko Kirin, Millennium Pharmaceuticals, Celgene Cellular Therapeutics, Santarus, Salix Pharmaceuticals, Catabasis Pharmaceuticals, Vertex Pharmaceuticals, Warner Chilcott, Gilead Sciences, Cosmo Pharmaceuticals, Ferring Pharmaceuticals, Sigmoid Biotechnologies, Tillotts Pharma, Am Pharma BV, Dr. August Wolff, Avaxia Biologics, Zyngenia, Ironwood Pharmaceuticals, Index Pharmaceuticals, Nestle, Lexicon Pharmaceuticals, UCB Pharma, Orexigen, Luitpold Pharmaceuticals, Baxter Healthcare, Ferring Research Institute, Amgen, Novo Nordisk, Mesoblast Inc., Shire, Ardelyx Inc., Actavis, Seattle Genetics, MedImmune (AstraZeneca), Actogenix NV, Lipid Therapeutics Gmbh, Eisai, Qu Biologics, Toray Industries Inc., Teva Pharmaceuticals, Eli Lilly, Chiasma, TiGenix, Adherion Therapeutics, Immune Pharmaceuticals, Celgene, Arena Pharmaceuticals, Ambrx Inc., Akros Pharma, Vascular Biogenics, Theradiag, Forward Pharma, Regeneron, Galapagos, Seres Health, Ritter Pharmaceuticals, Theravance, Palatin, Biogen, and the University of Western Ontario (owner of Robarts Clinical Trials). GDH has received consulting fees from AbbVie, ActoGeniX NV, Amgen, AM-Pharma BV, Boehringer-Ingelheim, ChemoCentryx, Centocor/Jansen Biologics, Cosmo Technologies, Elan/Biogen, EnGene Inc, Ferring Pharmaceuticals, Gilead Sciences, Given Imaging, GSK, Merck Research Laboratories, Merck Serono, Millenium Pharmaceuticals, Novo Nordisk, NPS Pharmaceuticals, PDL Biopharma, Pfizer, Receptos, Salix Pharmaceuticals, Schering Plough, Shire Pharmaceuticals, Sigmoid Pharma Ltd, Teva Pharmaceuticals, Tillotts Pharma AG, and UCB Pharma; research grants from AbbVie, GSK, Falk, Janssen, Merck, and Given Imaging; and lecture/speakers bureaux fees from AbbVie, Jansen, Merck, Takeda, UCB, and Shire. BGF has received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma AG, AbbVie, Novartis Pharmaceuticals, Centocor Inc., Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix, and Wyeth Pharmaceuticals Inc.; consulting fees from Millennium Pharmaceuticals, Merck, Centocor Inc., Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, AbbVie, Astra Zeneca, Serono, Genentech, Tillotts Pharma AG, Unity Pharmaceuticals, Albireo Pharma, Given Imaging Inc., Salix Pharmaceuticals, Novonordisk, GSK, Actogenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand. VJ has received consulting fees from AbbVie and Sandoz; speakers fees from Takeda and Janssen.
Footnotes
Guarantor of the article: Vipul Jairath.
Declaration of funding interests: None.
LINKED CONTENT
This article is linked to Choy and De Cruz and Jairath et al papers. To view these articles visit https://doi.org/10.1111/apt.13980 and https://doi.org/10.1111/apt.14019.
REFERENCES
- 1.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: Current state of the art. Nat Rev Gastroenterol Hepatol 2016; 13: 13–27. [DOI] [PubMed] [Google Scholar]
- 2.Domenech E, Manosa M, Cabre E. An overview of the natural history of inflammatory bowel diseases. Dig Dis 2014; 32: 320–7. [DOI] [PubMed] [Google Scholar]
- 3.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955; 2: 1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis SP, Stange EF, Lemann M, et al. European evidence-based Consensus on the management of ulcerative colitis: current management. J Crohns Colitis 2008; 2: 24–62. [DOI] [PubMed] [Google Scholar]
- 5.Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 2012; 380: 1909–15. [DOI] [PubMed] [Google Scholar]
- 6.Williams JG, Alam MF, Alrubaiy L, et al. Comparison Of iNfliximab and ciclosporin in STeroid Resistant Ulcerative Colitis: pragmatic randomised Trial and economic evaluation (CONSTRUCT). Health Technol Assess 2016; 20: 1–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seah D, De Cruz P. Review article: the practical management of acute severe ulcerative colitis. Aliment Pharmacol Ther 2016; 43: 482–513. [DOI] [PubMed] [Google Scholar]
- 8.Herfarth HH, Rogler G, Higgins PD. Pushing the pedal to the metal: should we accelerate infliximab therapy for patients with severe ulcerative colitis? Clin Gastroenterol Hepatol 2015; 13: 336–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bitton A, Buie D, Enns R, et al. Treatment of hospitalized adult patients with severe ulcerative colitis: toronto consensus statements. Am J Gastroenterol 2012; 107: 179–94. [DOI] [PubMed] [Google Scholar]
- 10.Chen JH, Andrews JM, Kariyawasam V, et al. Review article: acute severe ulcerative colitis - evidence-based consensus statements. Aliment Pharmacol Ther 2016; 44:127–44. [DOI] [PubMed] [Google Scholar]
- 11.Sands BE, Tremaine WJ, Sandborn WJ, et al. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis 2001; 7: 83–8. [DOI] [PubMed] [Google Scholar]
- 12.Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005; 128: 1805–11. [DOI] [PubMed] [Google Scholar]
- 13.Narula N, Marshall JK, Colombel JF, et al. Systematic review and meta-analysis: infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am J Gastroenterol 2016; 111: 477–91. [DOI] [PubMed] [Google Scholar]
- 14.Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 2012; 6: 991–1030. [DOI] [PubMed] [Google Scholar]
- 15.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: american College Of Gastroenterology Practice Parameters Committee. Am J Gastroenterol 2010; 105: 501–23. [DOI] [PubMed] [Google Scholar]
- 16.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011; 60: 571–607. [DOI] [PubMed] [Google Scholar]
- 17.Sjoberg M, Magnuson A, Bjork J, et al. Infliximab as rescue therapy in hospitalised patients with steroid-refractory acute ulcerative colitis: a long-term follow-up of 211 Swedish patients. Aliment Pharmacol Ther 2013; 38: 377–87. [DOI] [PubMed] [Google Scholar]
- 18.Ordas I, Mould DR, Feagan BG, et al. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012; 91: 635–46. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2008; 84: 548–58. [DOI] [PubMed] [Google Scholar]
- 20.Levin AD, Wildenberg ME, van den Brink GR. Mechanism of action of anti-TNF therapy in inflammatory bowel disease. J Crohns Colitis 2016; 10: 989–97. [DOI] [PubMed] [Google Scholar]
- 21.Yarur AJ, Jain A, Sussman DA, et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut 2016; 65: 249–55. [DOI] [PubMed] [Google Scholar]
- 22.Gardiner KR, Halliday MI, Barclay GR, et al. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut 1995; 36: 897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen T, Goll R, Cui G, et al. Tissue levels of tumor necrosis factor-alpha correlates with grade of inflammation in untreated ulcerative colitis. Scand J Gastroenterol 2007; 42: 1312–20. [DOI] [PubMed] [Google Scholar]
- 24.Gibiansky L, Gibiansky E. Target-mediated drug disposition model: approximations, identifiability of model parameters and applications to the population pharmacokinetic-pharmacodynamic modeling of biologics. Expert Opin Drug Metab Toxicol 2009; 5: 803–12. [DOI] [PubMed] [Google Scholar]
- 25.Biancheri P, Brezski RJ, Di Sabatino A, et al. Proteolytic cleavage and loss of function of biologic agents that neutralize tumor necrosis factor in the mucosa of patients with inflammatory bowel disease. Gastroenterology 2015; 149: 1564–74. [DOI] [PubMed] [Google Scholar]
- 26.Hornby PJ, Cooper PR, Kliwinski C, et al. Human and non-human primate intestinal FcRn expression and immunoglobulin G transcytosis. Pharm Res 2014; 31: 908–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beeken WL, Busch HJ, Sylwester DL. Intestinal protein loss in Crohn’s disease. Gastroenterology 1972; 62: 207–15. [PubMed] [Google Scholar]
- 28.Brandse JF, Van Den Brink GR, Wildenberg ME, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology 2015; 149: 350–5. [DOI] [PubMed] [Google Scholar]
- 29.Chaparro MGI, Bujanda L, et al. Loss of anti-TNF drugs into feces and its impact on anti-TNF serum levels and clinical response in Crohn’s Disease (CD) patients. Gastroenterology 2016; 150: S421. [Google Scholar]
- 30.Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007; 56: 1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baert F, Kondragunta V, Lockton S, et al. Antibodies to adalimumab are associated with future inflammation in Crohn’s patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut 2016; 65: 1126–31. [DOI] [PubMed] [Google Scholar]
- 32.Brandse JF, Mathot RA, van der Kleij D, et al. Pharmacokinetic features and presence of antidrug antibodies associated with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol 2016; 14: 251–8. [DOI] [PubMed] [Google Scholar]
- 33.Ungar B, Mazor Y, Weisshof R, et al. Induction infliximab levels among patients with acute severe ulcerative colitis compared with patients with moderately severe ulcerative colitis. Aliment Pharmacol Ther 2016; 43: 1293–9. [DOI] [PubMed] [Google Scholar]
- 34.Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010; 59: 49–54. [DOI] [PubMed] [Google Scholar]
- 35.Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis 2014; 20: 2247–59. [DOI] [PubMed] [Google Scholar]
- 36.Ternant D, Aubourg A, Magdelaine-Beuzelin C, et al. Infliximab pharmacokinetics in inflammatory bowel disease patients. Ther Drug Monit 2008; 30: 523–9. [DOI] [PubMed] [Google Scholar]
- 37.Jurgens M, Laubender RP, Hartl F, et al. Disease activity, ANCA, and IL23R genotype status determine early response to infliximab in patients with ulcerative colitis. Am J Gastroenterol 2010; 105: 1811–9. [DOI] [PubMed] [Google Scholar]
- 38.Armuzzi A, Pugliese D, Danese S, et al. Long-term combination therapy with infliximab plus azathioprine predicts sustained steroid-free clinical benefit in steroid-dependent ulcerative colitis. Inflamm Bowel Dis 2014; 20: 1368–74. [DOI] [PubMed] [Google Scholar]
- 39.Ebrahim V, Wu X, Shen B. Predictors of poor outcome of infliximab therapy in ulcerative colitis. Am J Gastroenterol 2014; 109: S485. [Google Scholar]
- 40.Park SH, Yang SK, Hong SM, et al. Severe disease activity and cytomegalovirus colitis are predictive of a nonresponse to infliximab in patients with ulcerative colitis. Dig Dis Sci 2013; 58: 3592–9. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Salazar L, Fernandez N, Sanchez-Ocana R, et al. Predictors of bad response to infliximab in Ulcerative Colitis patients (ECIA study ACAD). J Crohn’s Colitis 2015; 9: S330. [Google Scholar]
- 42.Kevans D, Murthy SJ, Iacono A, Silverberg MS, Greenberg GR. Accelerated clearance of serum infliximab during induction therapy for acute ulcerative colitis is associated with treatment failure. Gastroenterology 2012; 142: S–384. [Google Scholar]
- 43.Olsen T, Goll R, Cui G, et al. TNF-alpha gene expression in colorectal mucosa as a predictor of remission after induction therapy with infliximab in ulcerative colitis. Cytokine 2009; 46: 222–7. [DOI] [PubMed] [Google Scholar]
- 44.Kohn A, Daperno M, Armuzzi A, et al. Infliximab in severe ulcerative colitis: short-term results of different infusion regimens and long-term follow-up. Aliment Pharmacol Ther 2007; 26: 747–56. [DOI] [PubMed] [Google Scholar]
- 45.Mortensen C, Caspersen S, Christensen NL, et al. Treatment of acute ulcerative colitis with infliximab, a retrospective study from three Danish hospitals. J Crohns Colitis 2011; 5: 28–33. [DOI] [PubMed] [Google Scholar]
- 46.Papamichael K, Rivals-Lerebours O, Billiet T, et al. Long-term outcome of patients with ulcerative colitis and primary non-response to infliximab. J Crohns Colitis 2016; 10: 1015–23. [DOI] [PubMed] [Google Scholar]
- 47.Arias MT, Vande Casteele N, Vermeire S, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2015; 13: 531–8. [DOI] [PubMed] [Google Scholar]
- 48.Sandborn WJ, Rutgeerts P, Feagan BG, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology 2009; 137: 1250–60. [DOI] [PubMed] [Google Scholar]
- 49.Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014; 147: 1296–307. [DOI] [PubMed] [Google Scholar]
- 50.Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2015; 13: 330–5. [DOI] [PubMed] [Google Scholar]
- 51.Govani SM, Waljee AK, Stidham RW, et al. Accelerated dosing of infliximab prevents colectomy within 90 days in only half of patients with severe ulcerative colitis. Gastroenterology 2016; 150: S106. [Google Scholar]
- 52.Choy MC, Seah D, Gorelik A, et al. Comparison of accelerated infliximab induction vs standard induction treatment in acute severe ulcerative colitis. Gastroenterology 2016; 150: S803. [Google Scholar]
- 53.Panccione R, Ghosh S, Middleton S, et al. Infliximab, azathioprine, or infliximab + azathioprine for treatment of moderate to severe ulcerative colitis: The UC success trial. Gastroenterology 2011; 1: S134. [Google Scholar]
- 54.Xu Z, Davis HM, Zhou H. Clinical impact of concomitant immunomodulators on biologic therapy: pharmacokinetics, immunogenicity, efficacy and safety. J Clin Pharmacol 2015; 55(Suppl 3): S60–74. [DOI] [PubMed] [Google Scholar]
- 55.Florholmen J, Olsen T, Rismo R, et al. Short- and long-term clinical outcomes of infliximab in fulminant ulcerative colitis. Ulcers 2011; 2011: 1–7. Article ID 156407. doi: 10.1155/2011/156407 [DOI] [Google Scholar]
- 56.Ochsenkuhn T, Sackmann M, Goke B. Infliximab for acute, not steroid-refractory ulcerative colitis: a randomized pilot study. Eur J Gastroenterol Hepatol 2004; 16: 1167–71. [DOI] [PubMed] [Google Scholar]
- 57.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol 2012; 107: 1409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westhovens R, Yocum D, Han J, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum 2006; 54: 1075–86. [DOI] [PubMed] [Google Scholar]
- 59.Rennard SI, Flavin SK, Agarwal PK, et al. Long-term safety study of infliximab in moderate-to-severe chronic obstructive pulmonary disease. Respir Med 2013; 107: 424–32. [DOI] [PubMed] [Google Scholar]
- 60.Hendler SA, Cohen BL, Colombel JF, et al. High-dose infliximab therapy in Crohn’s disease: clinical experience, safety, and efficacy. J Crohns Colitis 2015; 9: 266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut 1996; 38: 905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho GT, Mowat C, Goddard CJ, et al. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther 2004; 19: 1079–87. [DOI] [PubMed] [Google Scholar]
- 63.Lindgren SC, Flood LM, Kilander AF, et al. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol 1998; 10: 831–5. [DOI] [PubMed] [Google Scholar]
- 64.Seo M, Okada M, Yao T, et al. An index of disease activity in patients with ulcerative colitis. Am J Gastroenterol 1992; 87: 971–6. [PubMed] [Google Scholar]
- 65.Monterubbianesi R, Aratari A, Armuzzi A, et al. Infliximab three-dose induction regimen in severe corticosteroid-refractory ulcerative colitis: early and late outcome and predictors of colectomy. J Crohns Colitis 2014; 8: 852–8. [DOI] [PubMed] [Google Scholar]
- 66.Fasanmade AA, Adedokun OJ, Olson A, et al. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther 2010; 48: 297–308. [DOI] [PubMed] [Google Scholar]
- 67.Sambuelli A, Gil AH, Negreira SM, et al. Predictive factors for colectomy in severe corticosteroid (CS) refractory ulcerative colitis (UC) treated with infliximab (IFX). Gastroenterology 2012; 142: S362. [Google Scholar]
- 68.Lees CW, Heys D, Ho GT, et al. A retrospective analysis of the efficacy and safety of infliximab as rescue therapy in acute severe ulcerative colitis. Aliment Pharmacol Ther 2007; 26: 411–9. [DOI] [PubMed] [Google Scholar]
- 69.Gibson D, Hartery K, Doherty J, et al. CRP/albumin Ratio: a novel predictor of early colectomy in acute severe ulcerative colitis. Gastroenterology 2016; 150: S560. [Google Scholar]
- 70.Ho GT, Lee HM, Brydon G, et al. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am J Gastroenterol 2009; 104: 673–8. [DOI] [PubMed] [Google Scholar]
- 71.Calafat M, Cabre E, Manosa M, et al. High within-day variability of fecal calprotectin levels in patients with active ulcerative colitis: what is the best timing for stool sampling? Inflamm Bowel Dis 2015; 21: 1072–6. [DOI] [PubMed] [Google Scholar]
- 72.Chew CN, Nolan DJ, Jewell DP. Small bowel gas in severe ulcerative colitis. Gut 1991; 32: 1535–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hafeez R, Punwani S, Pendse D, et al. Derivation of a T2-weighted MRI total colonic inflammation score (TCIS) for assessment of patients with severe acute inflammatory colitis-a preliminary study. Eur Radiol 2011; 21: 366–77. [DOI] [PubMed] [Google Scholar]
- 74.Brandse JF, Jansen JM, Baars PA, et al. Serum CRP is a better early marker for response to infliximab induction therapy than fecal calprotectin in patients with moderate to severe ulcerative colitis. J Crohn’s Colitis 2014; 8: S210–1. [Google Scholar]
- 75.Beswick L, Van Langenberg DR, Rosella O, et al. The predictive value of early serum infliximab, crp and faecal calprotectin levels post-first infliximab rescue dose for acute severe colitis: ‘Day 1 to 3 is key’. Gastroenterology 2015; 148: S848–9. [Google Scholar]