Abstract
Hepatic steatosis, the accumulation of lipids within hepatocytes, is a common condition. The prevalence of its most frequent manifestation, nonalcoholic fatty liver disease (NAFLD), has been estimated to be as high as 35% in some populations. Currently, liver biopsy is the gold standard for the diagnosis and assessment of severity of hepatic steatosis, staging of fibrosis, and is the only modality able to differentiate bland steatosis from steatohepatitis. However, its invasiveness, significant side effect profile, and susceptibility to sampling error ultimately make it a suboptimal tool. Accordingly, focus has been placed on noninvasive radiologic techniques for hepatic fat detection and quantification. The ratio-ale, performance characteristics, and limitations of traditional noninvasive measures, including ultrasound, computed tomography, and magnetic resonance (MR) spectroscopy and imaging, are reviewed. A novel MR method, the spectrally modeled relaxation-invariant technique, overcomes the inherent weaknesses of conventional MR to diagnose and quantify hepatic steatosis over its entire range of severity. Noninvasive radiologic techniques, particularly MR, can be applied broadly, including in the diagnosis of NAFLD in asymptomatic patients with elevated serum aminotransferase levels, longitudinal monitoring of disease progression or response to treatment, population-based epidemiologic or observational studies, and drug discovery.
Hepatic steatosis is a generic term referring to lipid accumulation within hepatocytes. Nonalcoholic fatty liver disease (NAFLD), the most common form of hepatic steatosis, is the focus of this review. NAFLD encompasses a spectrum of conditions, ranging from benign steatosis to inflammatory nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis.1,2 Associated with obesity and insulin resistance, NAFLD is considered the hepatic man-ifestation of the metabolic syndrome.3
NAFLD is the most common form of chronic liver disease among adults and children in the United States and has been reported in many other parts of the world.3,4 It is considered the leading cause of asymptomatic elevations of serum aminotransferase levels and likely accounts for most cases of cryptogenic cirrhosis. The prevalence of NAFLD is estimated to be between 5% and 34% in adults5,6 and 10% in children.7 With the rise of obesity and the metabolic syndrome, NAFLD will become even more common in coming years.
Currently, liver biopsy is the gold standard for assessing the severity of hepatic fat deposition.8 However, the invasiveness of biopsy might lead to potentially serious complications, including internal bleeding, biliary leakage, hematoma formation, and infection; in fact, up to 3% of patients require hospitalization after elective biopsy.9 Post-procedural pain and high cost are other sources of patient dissatisfaction. In pediatric patients, liver biopsies are commonly performed under general anesthesia, which introduces additional risks. Liver biopsy is also subject to sampling error because only 1/50,000th of the liver is made available for histologic analysis.10 The distribution of fat deposition in the steatotic liver might be heterogeneous, and thus a single liver biopsy from one location might not adequately represent the fat burden in the entire liver.11,12 In sum, these drawbacks make liver biopsy a suboptimal modality for longitudinal monitoring of disease progression or response to treatment, and liver biopsy is not a tenable tool to establish the diagnosis of NAFLD in the tens of millions of Americans afflicted by this condition.13
Consequently, attention has shifted to noninvasive measures of hepatic fat detection and quantification. Conventional noninvasive techniques have included transabdominal ultrasonography (US),14,15 computed tomography (CT),16 and magnetic resonance (MR) spectroscopy6,17 and imaging.18,19 This article outlines the performance characteristics and deficiencies in currently used noninvasive techniques and highlights a novel MR imaging approach that accounts for the inherent confounders in traditional MR methods to more accurately quantify hepatic fat content.
Requirements of Noninvasive Techniques in Assessing Hepatic Steatosis
From the clinician’s perspective, noninvasive techniques to assess hepatic steatosis should have 2 complementary but discrete goals in addition to comparing favorably with liver biopsy with regards to cost, safety, and patient acceptance: accurate diagnosis of steatosis and, in those with steatosis, accurate grading of steatosis severity. From a technical perspective, however, there is only 1 goal: accurate fat quantification, allowing for the numeric estimation of the liver’s fat burden. The reason is that nonsteatotic livers might contain small amounts of fat histologically, and accurate quantification is essential to classify hepatic fat content as being below or above a diagnostic threshold that defines steatosis. Traditionally, only individuals with a hepatic fat content exceeding 5% are given a diagnosis of NAFLD; those with less than 5% are considered to have physiologic or incidental steatosis.20 Fat quantification removes subjectivity from the diagnosis of NAFLD, particularly in those patients with equivocal degrees of steatosis; accurate quantification in these circumstances would correctly classify whether the individual has NAFLD or incidental steatosis.
Clinical Vignette
A 64-year-old man is referred to the Hepatology Clinic for evaluation of an elevated serum ALT level of 137 U/L. He is 175 cm tall and weighs 95 kg; his body mass index is accordingly 31 kg/m2. Three months earlier, he was found to have an elevated fasting blood sugar, diagnosed with diabetes mellitus, and prescribed an oral hypoglycemic agent. His blood pressure has also been borderline elevated, although he has not required antihypertensive medications.
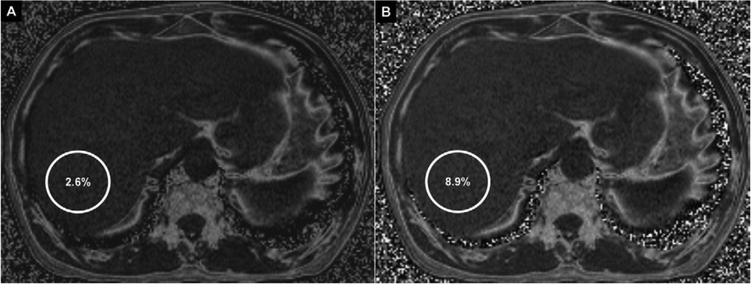
Because of a suspicion of NAFLD, the patient undergoes US, which is of low quality and fails to show the increased echogenicity generally seen with fatty hepatic deposition. Subsequently, conventional chemical shift MR imaging with the Dixon method is also unrevealing, with a hepatic fat content estimation of 2.6% (Figure 1A). The patient then returns for a repeat MR examination; this time, a novel spectrally modeled relaxation-invariant technique is used. With this method, hepatic fat content is estimated to be 8.9% (Figure 1B); MR spectroscopy agrees within 0.5% with the observed fat quantification. A liver biopsy later shows mild macrovesicular steatosis, portal inflammation, and mild portal fibrosis, consistent with NASH.
Figure 1.
Fat fraction maps of the liver at the same level with the conventional technique (A) and the novel spectrally modeled relaxation-invariant technique (B). Colocalized regions of interest are shown in the right lobe of the liver (circles). The conventional technique shows a fat fraction of 2.6% within the region of interest, which is considered nonpathologic. The novel technique shows 8.9% fat fraction in the region of interest, which meets criteria for pathologic fat deposition.
Performance and Limitations of Conventional Techniques
As demonstrated by this vignette and discussed further below, conventional noninvasive imaging techniques perform suboptimally in the diagnosis and quantification of hepatic fat.
Ultrasound
US is the most common imaging modality used to initially evaluate and diagnose hepatic steatosis because of its low cost, noninvasiveness, and widespread availability.15,21–23 The echogenicity, or brightness, of tissue depends on the degree of beam scattering by the tissue. Fat deposition in tissue accentuates scattering. Therefore, a fatty liver scatters the beam more than a nonfatty liver, and the fatty liver appears hyperechogenic; a “bright liver” is characteristic of hepatic steatosis.24 Because there is no absolute echogenicity that denotes liver fat, however, com-parison of echogenicity is required with internal structures known to be void of fat, such as the kidneys or spleen.
Fat not only scatters the US beam; it also attenuates it. US beam attenuation causes a decrease in penetration of the beam through fatty liver tissue, resulting in hypoechogenicity in the far field, or posterior darkness, and decreased diaphragmatic definition. As a result of the combination of beam scattering and attenuation, the sonographic appearance of the fatty liver tends to be bland and featureless, with suboptimal visualization of intrahepatic structures such as blood vessels and nodules.24
On the basis of these considerations, sonographic criteria for the diagnosis of liver steatosis include hepatic hyperechogenic-ity, far-field beam attenuation, and limited visualization of the diaphragm and intrahepatic structures (Figure 2). With these criteria, the reported sensitivities and specificities of US in the general population are 60%–95% and 84%–100%, respectively.24
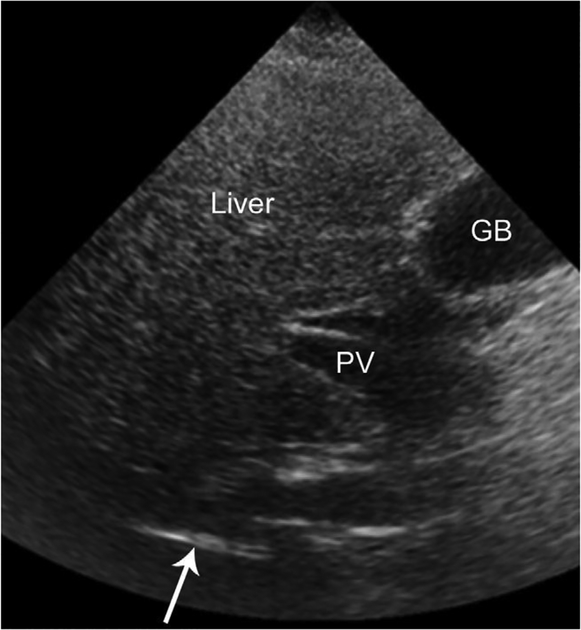
Figure 2.
Transverse US image of the liver in a 45-year-old man with fatty liver disease. The liver is hyperechoic in the near field (top half of image). Because of beam attenuation, the liver becomes progressively hypoechoic in the far field (bottom half of image). Note that only a portion of the right hemidiaphragm (arrow) is visible. Beam attenuation obscures the remainder of the hemidiaphragm. In addition, there is a paucity of intrahepatic structures. The portal vein (PV) is visible in the porta hepatis, but its intrahepatic branches are not evident. The gallbladder (GB) is incidentally noted.
The inherent weakness of US is that several factors other than fat deposition might affect hepatic echogenicity and beam attenuation. Visceral and subcutaneous adipose tissues attenuate the US beam and obscure the liver, reducing the sensitivity and specificity of US for hepatic steatosis in obese individuals to 49% and 75%, respectively.25 US is also limited in patients with small amounts of hepatic fat, with a reported sensitivity of 55% in those with less than 20% steatosis by histology.26 Hepatic fibrosis might increase hepatic echogenicity to a similar degree as fat accumulation; thus, severely fibrotic livers without steatosis might be indistinguishable from steatotic livers without fibrosis. Technical factors such as transducer frequency and instrument settings also affect hepatic echogenicity, and US is operator-dependent and nonreproducible.
Ultimately, the US assessment of hepatic fat content is based on a subjective visual assessment rather than an objective quantification of sonographic images. Objective methods to assess beam scattering and attenuation have not been validated. Steatosis is rated into a category (mild, moderate, or severe steatosis), which makes US a poor modality to assess small changes in fat content; a decrease in hepatic fat from 40% to 20%, for example, might not correspond to a reliably detected change in US echogenicity.27
Computed Tomography
Unenhanced CT has been widely used in the evaluation of fatty liver disease in adults, surpassing the performance of US in detecting and assessing the severity of hepatic steatosis. Unlike US, CT measures tissue density as a function of attenuation. Tissues that are less dense have lower attenuation and appear darker on CT imaging; a liver with fat is physically less dense than a liver without fat and therefore appears darker on CT.28 As hepatic steatosis progressively increases, the hepatic attenuation becomes lower than that of the intrahepatic vasculature, giving the appearance of a contrast-enhanced examination21 (Figure 3). Unlike US, hepatic attenuation might be objectively measured via Hounsfield units (HU); in practice, however, this absolute value of hepatic attenuation is unreliable because most CT scanners are not appropriately calibrated for this purpose.24 Therefore, most investigators prefer to compare the hepatic attenuation to an internal standard devoid of fat, such as the spleen.
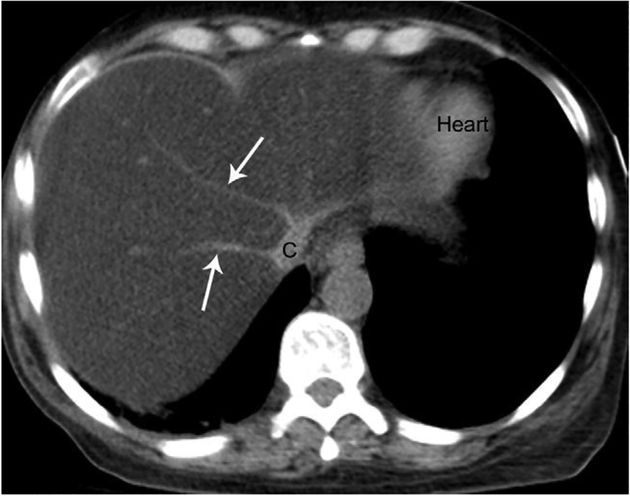
Figure 3.
Axial unenhanced CT scan through the liver in the same 45-year-old man inFigure 2. The liver is diffusely hypodense and has lower attenuation than the intrahepatic vessels, giving the appearance of a contrast-enhanced examination. The heart is incidentally noted. C, cava; arrows, hepatic veins.
Accordingly, the CT assessment of hepatic steatosis is made by determining either (1) the absolute hepatic attenuation value in HU or (2) the difference in attenuation in HU between the liver and spleen. Traditional values for hepatic steatosis have included an absolute liver attenuation of 40 HU or a difference in attenuation between the liver and spleen of less than –10 HU.28–30 At these values, the sensitivities and specificities of unenhanced CT in the detection of moderate/severe hepatic steatosis (greater than 30% on histology) have been reported to be 73%–100% and 95%–100%, respectively.24
As with US, the inherent weakness of CT is that tissue density does not depend solely on fat content. Hepatic attenuation might be affected by a variety of other factors, some immeasurable by CT, such as iron, copper, glycogen, fibrosis, or edema.31 Although unenhanced CT is a viable option for the qualitative assessment of liver fat, it is insensitive to mild steatosis, and its efficacy in quantitative assessment and detection of small changes in fat burden is clinically unacceptable.32 Finally, as a result of its reliance on ionizing radiation, CT is not feasible in longitudinal studies, particularly those involving children.
Magnetic Resonance
MR is generally considered the most definitive radiologic modality for the qualitative and quantitative assessment of fatty liver disease. It exceeds other modalities in its ability to detect mild steatosis and small changes in fat content. In fatty liver, both fat and water protons contribute to the observed MR signal but precess at different frequencies. MR techniques can exploit this precessional frequency difference to decompose the MR signal into its fat and water signal components. MR imaging resolves the signal into a structural image, providing anatomic delineation, and MR spectroscopy resolves the signal into a frequency spectrum, providing biochemical information.13
Magnetic Resonance Imaging
The most common MR imaging method for diagnosing hepatic steatosis is out-of-phase and in-phase imaging. This method acquires MR images at echo times in which fat proton and water proton signals are either out-of-phase (water and fat signals cancel) or in-phase (water and fat signals add up). By comparing the signal intensity of the liver on out-of-phase and in-phase images, the presence of fat might be inferred: signal loss on the out-of-phase images indicates the presence of fat, whereas lack of signal loss suggests the absence of fat (Figure 4).
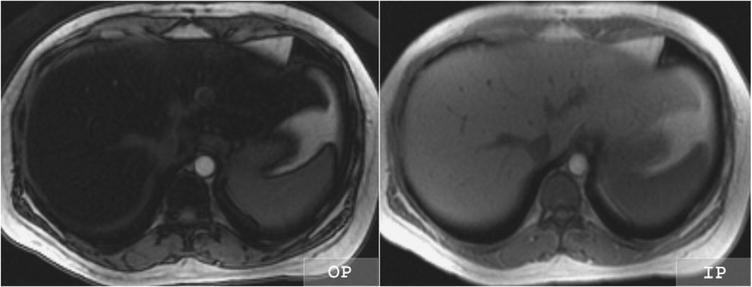
Figure 4.
Out-of-phase (OP) and inphase (IP) MR images of the liver in a 36-year-old woman with fatty liver disease. The liver loses signal intensity on the OP compared with the IP image, indicating the presence of fat and water within liver tissue voxels. A normal liver would have similar signal intensity on the OP and IP images.
Moreover, if the out-of-phase and in-phase images are acquired by using constant calibration and other scanner settings, a quantitative fat signal fraction can be calculated from the hepatic signal intensities with a simple formula: Fat signal fraction = (SIP – SOP)/(2*SIP), where S = signal intensity of liver (in arbitrary units), IP = in-phase imaging, and OP = out-of-phase imaging. This formula can be applied pixel by pixel on the image to generate a fat signal fraction map.
The performance characteristics of conventional MR imaging techniques resemble or exceed those of US and CT. MR imaging sensitivities and specificities for the detection of moderate/severe steatosis33 are greater than 80% and 95%, respectively. In addition, conventional MR imaging exceeds the other noninvasive modalities in its ability to detect mild steatosis (histologic steatosis of 5%–10%),33 where sensitivities of greater than 85% and specificities approaching 100% have been reported.
Despite its superiority and lack of radiation, MR imaging is not widely used as a result of its high charges, contraindication in patients with pacemakers or other implantable devices, and reliance on patient cooperation. The latter impediment, however, has been addressed with newer MR sequences that decrease the times required for breath-holding and the overall duration of the examination.
A more fundamental limitation is that the out-of-phase and in-phase imaging technique provides information on the fat signal fraction. Because fat and water signals might be confounded by several factors (such as T1 relaxation, T2* relaxation, and complex interference effects between the multiple spectral components of fat), the fat signal fraction might not accurately represent the fat content fraction, especially in the low fat fraction range and presence of iron. Thus, although superior to US and CT, conventional MR imaging is imperfect.
Spectrally Modeled Relaxation-Invariant Magnetic Resonance Imaging
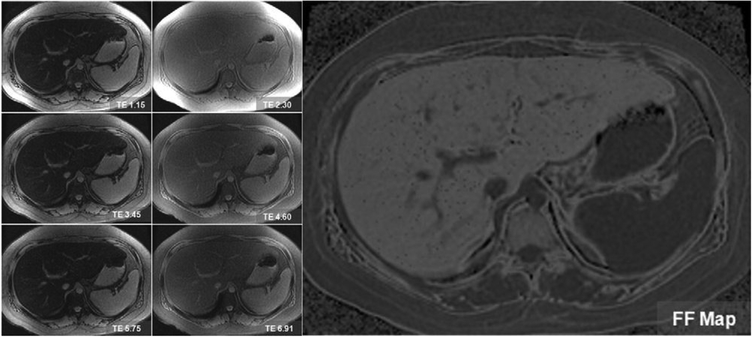
MR imaging techniques now in development account for the confounders that inherently limit conventional MR imaging to quantitatively measure hepatic fat content fraction. These techniques minimize the effects of T1 relaxation, measure and correct for the effects of T2* relaxation, and model the spectral complexity of fat. Beyond the scope of this article, the interested reader is referred to other sentinel publications that detail the related physics background.34–36 Images of the entire liver are generated in about 20 seconds. Custom-developed software generates synthetic images that display pixel by pixel the hepatic fat fraction throughout the entire liver (Figure 5). In preliminary studies, these techniques have high accuracy for the diagnosis of the entire range of steatosis severity, including mild steatosis.
Figure 5.
Spectrally modeled relaxation-invariant MR technique performed on a 53-year-old man with fatty liver disease. Source images (left panels) are obtained at serial out-of-phase and in-phase echo times. A low flip angle is used to reduce T1 effects. A synthetic fat fraction map (right) is generated from the source images by measuring and correcting for T2* and modeling fat-fat interference effects. Note that the intrahepatic blood vessels and spleen are dark on the fat fraction map because these structures are devoid of fat.
Magnetic Resonance Spectroscopy
The MR spectrum describes the intensity of MR signal as a function of precession frequency. At field strengths greater than or equal to 1.5 Tesla, the water peak at 4.7 ppm and the dominant fat peak at 1.2 ppm of –(CH2)n– can be resolved and identified as distinct spectral peaks. In the fatty liver disease, both water and fat spectral peaks are present; in the nonfatty liver, only the water peak is seen. MR spectroscopy assesses the fat-water spectrum in a single voxel of liver tissue, typically 8 cm3 (roughly 0.5% of the whole liver).
MR spectroscopy is available on all commercially produced MR scanners. It has been shown to be a valid, noninvasive measure of hepatic fat in comparison to both liver biopsy37 and biochemical assay38 and can accurately quantify hepatic fat content.39,40 In addition, it has been used in longitudinal clinical studies41,42 and for screening the general population for hepatic steatosis.6 Nevertheless, this technology is relegated to academic institutions with a dedicated research focus because of the expertise required for its implementation and analysis. As such, MR spectroscopy is not a readily available, realistic tool for hepatic fat diagnosis and quantification. Another limitation of MR spectroscopy is its restricted spatial coverage, making it prone to sampling error.
Summary of Magnetic Resonance Approaches
MR imaging and spectroscopy use the varying precessional frequencies of fat and water protons to anatomically and biochemically assess liver fat content. Beyond a qualitative assessment, MR techniques permit quantitative determination of the hepatic fat fraction to a degree that exceeds the performance of US and CT, particularly when considering cases of mild steatosis or small changes in fat content. General drawbacks of MR include limited availability, reliance on patient cooperation, and high charges of traditional examinations. Also, a lack of familiarity among clinicians forms a roadblock in the more widespread implementation of MR. One confusing aspect of MR imaging, for example, is its confusing nomenclature. The following all refer to the same conventional out-of-phase and in-phase imaging technique discussed above: chemical shift imaging, two-point Dixon method, phase shift imaging, and phase cancellation imaging. Similarly, some clinicians might not appreciate the fundamental differences between MR imaging and spectroscopy. Finally, technical shortcomings inherent to traditional out-of-phase and in-phase MR imaging fail to recognize potential confounding factors. Novel methods such as the spectrally modeled relaxation-invariant technique are being developed to account for such variables.
Role of Radiologic Modalities in the Assessment of Hepatic Steatosis
Despite attempts by some to differentiate bland steatosis from NASH,43 no imaging modalities have been validated to reliably make this distinction or grade the degree of NASH-induced fibrosis.13,44 However, a variety of noninvasive radiologic techniques do play a direct role in the diagnosis of NAFLD. NAFLD is most commonly diagnosed in asymptomatic patients after elevated screening serologic liver test results or abnormal hepatic imaging for an unrelated reason (eg, suspicion for cholelithiasis). In the absence of liver biopsy, the diagnosis of NAFLD might be assumed in patients with elevated serum aminotransferase levels and radiologic evidence for fatty deposition.25 US, CT, and traditional MR imaging have limited sensitivity for mild steatosis. Accurate identification requires MR spectroscopy or advanced MR imaging techniques currently in development to differentiate incidental from pathologic steatosis.
Except for MR spectroscopy, noninvasive techniques assess the entire liver and are thus not limited by the sampling error of liver biopsy and are advantageous for longitudinal assessment. MR imaging was used in one study for assessment of hepatic fat content to determine the efficacy of pioglitazone in the treatment of NASH; after 48 weeks, hepatic fat content was reduced on MR imaging, which correlated to histologic changes.45
Noninvasive techniques are the only plausible measures of hepatic steatosis in epidemiologic or observational studies focusing on the general population. This has been demonstrated in a population-based screening study, which reported a NAFLD prevalence of 34% by using MR spectroscopy.6 With today’s rapid MR imaging, screening of a large number of individuals can be done quickly and at lower cost.
Finally, noninvasive techniques can play a unique role in drug discovery, not only in assessing changes in fat content for NAFLD-specific interventions45 but also in detecting fat deposition as a side effect of medications intended for nonhepatic diseases. In the latter circumstance, many patients do not have underlying liver disease, making liver biopsy an inappropriate screening tool.
In summary, as technology has improved during the past few decades, radiologic modalities have been developed to noninvasively detect and assess hepatic steatosis. US and CT have generally been the modalities used for these purposes. However, MR-based methods have supplanted these modalities as a result of MR’s improved performance characteristics, particularly in detecting mild steatosis and small changes in fat content. Newer MR techniques overcome many of the inherent limitations of traditional MR imaging to quantitatively measure fat content fraction more accurately. Importantly, these new MR-based methods are rapid and can assess fat fraction in the entire liver in a single breath-hold. In principle, these techniques can be performed on routine MR scanners and can be widely implemented. Moreover, although MR imaging is usually associated with high costs, a complete quantification MR examination can be completed in 5–10 minutes without contrast agents, and it is possible to provide such examinations at a charge competitive with that of US. The increasing accuracy, convenience, and cost-effectiveness of MR make it the ideal modality for the diagnosis and quantification of hepatic steatosis. Utilization of noninvasive techniques, particularly MR, might include diagnosis of NAFLD in asymptomatic patients with elevated serum aminotransferase levels, longitudinal monitoring of disease progression or response to therapy, population-based epidemiologic or observational studies, and drug discovery.
Acknowledgments
The authors disclose the following: Supported by National Institutes of Health grants R01 DK075128 and U01 DK061734 from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Child Health and Human Development; T32 Gastroenterology Training Grant; and P60 MD00220 from the San Diego EXPORT Center, National Center of Minority Health and Health Disparities. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Dr Sirlin has a research grant from General Electric.
Abbreviations used in this paper:
- CT
computed tomography
- HU
Hounsfield unit
- MR
magnetic resonance
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
References
- 1.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 2.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467–2474. [DOI] [PubMed] [Google Scholar]
- 3.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917–923. [DOI] [PubMed] [Google Scholar]
- 4.Patton HM, Sirlin C, Behling C, et al. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J Pediatr Gastroenterol Nutr 2006;43:413–427. [DOI] [PubMed] [Google Scholar]
- 5.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol 2006;40:S5–S10. [DOI] [PubMed] [Google Scholar]
- 6.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–1395. [DOI] [PubMed] [Google Scholar]
- 7.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388–1393. [DOI] [PubMed] [Google Scholar]
- 8.Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ 2005;172:899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med 1993; 118:96–98. [DOI] [PubMed] [Google Scholar]
- 10.Maharaj B, Maharaj RJ, Leary WP, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle liver biopsy of the liver. Lancet 1986;1:523–525. [DOI] [PubMed] [Google Scholar]
- 11.Arun J, Jhala N, Lazenby AJ, et al. Influence of liver biopsy heterogeneity and diagnosis of nonalcoholic steatohepatitis in subjects undergoing gastric bypass. Obes Surg 2007;17:155–161. [DOI] [PubMed] [Google Scholar]
- 12.Machann J, Thamer C, Schnoedt B, et al. Hepatic lipid accumulation in healthy subjects: a comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn Reson Med 2006;55:913–917. [DOI] [PubMed] [Google Scholar]
- 13.Mehta SR, Thomas EL, Bell JD, et al. Non-invasive means of measuring hepatic fat content. World J Gastroenterol 2008;14: 3476–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster KJ, Dewbury KC, Griffith AH, et al. The accuracy of ultrasound in the detection of fatty infiltration of the liver. Br J Radiol 1980;53:440–442. [DOI] [PubMed] [Google Scholar]
- 15.Debongnie JC, Pauls C, Fievez M, et al. Prospective evaluation of the diagnostic accuracy of liver ultrasonography. Gut 1981;22: 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bydder GM, Kreel L, Chapman RW, et al. Accuracy of computed tomography in diagnosis of fatty liver. Br Med J 1980;281:1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longo R, Pollesello P, Ricci C, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging 1995;5:281–285. [DOI] [PubMed] [Google Scholar]
- 18.Heiken JP, Lee JK, Dixon WT. Fatty infiltration of the liver: evaluation by proton spectroscopic imaging. Radiology 1985;157: 707–710. [DOI] [PubMed] [Google Scholar]
- 19.Levenson H, Greensite F, Hoefs J, et al. Fatty infiltration of the liver: quantification with phase-contrast MR imaging at 1.5 T vs biopsy. AJR Am J Roentgenol 1991;156:307–312. [DOI] [PubMed] [Google Scholar]
- 20.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 21.Valls C, Iannacconne R, Alba E, et al. Fat in the liver: diagnosis and characterization. Eur Radiol 2006;16:2229–2308. [DOI] [PubMed] [Google Scholar]
- 22.Joseph AE, Saverymuttu SH, al-Sam S, et al. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol 1991;43:26–31. [DOI] [PubMed] [Google Scholar]
- 23.Mathiesen UL, Franzen LE, Aselius H, et al. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis 2002;34: 516–522. [DOI] [PubMed] [Google Scholar]
- 24.Charatcharoenwitthaya P, Lindor KD. Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin Liver Dis 2007;11:37–45. [DOI] [PubMed] [Google Scholar]
- 25.Mottin CC, Moretto M, Padoin AV, et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly-obese patients. Obes Surg 2004;14:635–637. [DOI] [PubMed] [Google Scholar]
- 26.Needleman L, Kurtz AB, Rifkin MD, et al. Sonography of diffuse benign liver disease: accuracy of pattern recognition and grading. AJR Am J Roentgenol 1986;146:1011–1015. [DOI] [PubMed] [Google Scholar]
- 27.Fishbein M, Castro F, Cheruku S, et al. Hepatic MRI for fat quantification: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol 2005;39:619–625. [DOI] [PubMed] [Google Scholar]
- 28.Piekarski J, Goldberg HI, Royal SA, et al. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology 1980; 137:727–729. [DOI] [PubMed] [Google Scholar]
- 29.Limanond P, Raman SS, Lassman C, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology 2004;230:276–280. [DOI] [PubMed] [Google Scholar]
- 30.Ricci C, Longo R, Gioulis E, et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol 1997;27: 108–113. [DOI] [PubMed] [Google Scholar]
- 31.Johnston RJ, Stamm ER, Lewin JM, et al. Diagnosis of fatty infiltration of the liver on contrast enhanced CT: limitations of liver-minus-spleen attenuation difference measurements. Abdom Imaging 1998;23:409–415. [DOI] [PubMed] [Google Scholar]
- 32.Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology 2006;239:105–112. [DOI] [PubMed] [Google Scholar]
- 33.Neuschwander-Tetri BA. NASH. In: Boyer TD, Wright TL, Manns MP, eds. Zakim and Boyer’s hepatology: a textbook of liver disease. 5th ed. Philadelphia: Saunders Elsevier, 2006:1045. [Google Scholar]
- 34.Bydder M, Yokoo T, Hamilton G, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging 2008;26:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussain HK, Chenevert TL, Londy FJ, et al. Hepatic fat fraction: MR imaging for quantitative measurement and display: early experience. Radiology 2005;237:1048–1055. [DOI] [PubMed] [Google Scholar]
- 36.Yu H, McKenzie CA, Shimakawa A, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging 2007;26:1153–1161. [DOI] [PubMed] [Google Scholar]
- 37.Longo R, Ricci C, Masutti F, et al. Fatty infiltration of the liver: quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol 1993; 28:297–302. [PubMed] [Google Scholar]
- 38.Rofsky NM, Fleishaker H. CT and MRI of diffuse liver disease. Semin Ultrasound CT MR 1995;16:16–33. [DOI] [PubMed] [Google Scholar]
- 39.Thomas EL, Hamilton G, Patel N, et al. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut 2005;54:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotronen A, Westerbacka J, Bergholm R, et al. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab 2007;92:3490–3497. [DOI] [PubMed] [Google Scholar]
- 41.Thomas EL, Brynes AE, Hamilton G, et al. Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol 2006;12:5813–5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297–2307. [DOI] [PubMed] [Google Scholar]
- 43.Oliva MR, Mortele KJ, Segatto E, et al. Computed tomography features of nonalcoholic steatohepatitis with histopathologic correlation. J Comput Assist Tomogr 2006;31:37–43. [DOI] [PubMed] [Google Scholar]
- 44.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiologic imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745–750. [DOI] [PubMed] [Google Scholar]
- 45.Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 2004;39:188–196. [DOI] [PubMed] [Google Scholar]