Abstract
HPV is well known as a potential cause of cervical cancer. Less well known is its link to temporal subfertility that is caused by binding of infectious virions to the spermatozoa’s head which induces sperm-DNA damage and causes a reduction in clinical pregnancy rates in women receiving HPV positive semen.
This impact on the global fertility burden remains greatly underestimated and underexplored. This risk of reduced fertility due to infectious HPV in sperm is especially important when donor sperm insemination is considered, since testing for the presence of HPV virions before use seems warranted.
We tested 514 donor sperm samples from 3 different sperm banks for 18 different HPV types.
Overall 3.9% (20/514) of tested donor sperm was positive for HPV, with different prevalence among the 3 different sperm banks (3.6% bank A, 3.1% bank B and 16.7% bank C). Also the HPV virion per spermatozoon ratio in donor samples was similar across the different sperm banks (95% CI 0,01 to 1,07 HPV virions/spermatozoon).
When HPV positive donor sperm was used, no clinical pregnancies resulted, whereas when HPV negative donor sperm was used the clinical pregnancy rate was 14.6%.
From both a cost/benefit and a safety point of view we recommend that donor sperm should always be tested for HPV before using it for insemination.
Keywords: infertility, infectious, intrauterine insemination, transient virion producing, spermatozoa, semen, donor sperm
Introduction
Although infection with human papillomavirus (HPV) is very common worldwide, until recently there was only scarce evidence linking HPV infection to reduced fertility outcomes (Garolla et al., 2016; Gizzo et al., 2014; Xiong et al., 2018).
One of the major reasons why it took so long to realize infectious HPV virions impair fertility is because formerly we often failed to see the different impact of infection and disease, HPV viruses and HPV virions (Depuydt et al., 2016a). On one hand HPV can cause the well-known but rather uncommon transformation of HPV infected dividing cells into cancer on the uterine cervix or other organs. These HPV morphotypes are incorporated into the cell’s DNA and are no longer infectious ( Depuydt et al., 2012b; 2015; 2016b). On the other hand, the more frequent free infectious HPV virions can bind the spermatozoa’s head (Foresta et al., 2011b; Kaspersen et al., 2011), induce sperm DNA damage (Boeri et al., 2019), causing temporal subfertility demonstrated by reduced clinical pregnancy rates in subfertile women receiving inseminations with HPV positive semen (Depuydt et al., 2019). It is often overlooked that the bulk of detected HPV DNA is infectious in origin (Depuydt et al., 2016a) and its impact on the global fertility burden is greatly underestimated as evidenced by a recent study that showed that the presence of HPV virions in semen significantly decrease clinical pregnancy rates in women undergoing intrauterine insemination (Depuydt et al., 2019).
Because of the absence of dividing cells in semen, the measured HPV DNA in sperm samples always originates from virions that can only be produced in non-dividing desquamating cells. Despite the fact that only 1-10 virions are sufficient to infect a basal cell and establish infection (Patterson et al., 2005), only a few authors have tested HPV prevalence in donor sperm (Kaspersen et al., 2011; D’Hauwers et al., 2012) or suggested screening of donor semen for HPV to prevent iatrogenic cervical HPV infections in the recipient (Basky G, 2000; Foresta et al., 2011a).
It has been reported before that HPV virions can physically bind to the spermatozoon’s head (Foresta et al., 2011b; Kaspersen et al., 2011) and induce sperm DNA damage (Boeri et al., 2019). In their latest guidelines, the Belgian superior health council does not recommend routine HPV testing of donor sperm, because at that time it remained unclear what the exact implications of HPV positive spermatozoa were for cell biology (Superior Health Council, 2014), indicating an update could be recommended. Although the sperm donors are selected to have good quality sperm (having children) and are labeled healthy, still a percentage can be positive for oncogenic HPV types or other HPV types that impact fertilization successes. In the past, no correlation was made on how often or how frequently HPV positive donor sperm was successful in achieving pregnancies, as compared to the HPV negative donor sperm (Kaspersen et al., 2013). We recently showed in a blinded non-interventional prospective study that women who were inseminated with HPV positive sperm had reduced clinical pregnancy rates compared to women who received HPV negative sperm.
In order to formulate a recommendation regarding HPV donor sperm testing for sperm banks, we assessed the impact of the HPV status on success rates of donor sperm by analyzing donor sperm that was used for inseminations originating from 3 different donor banks.
Methods and Materials
Study population
We measured HPV DNA in donor sperm that was destined for IUI as previously described (Depuydt et al., 2019). Briefly, depending on the capacitation procedure used (washing away the seminal plasma with capacitation medium, swim-up or density gradient), different left-over sperm fractions were obtained from donor sperm. Work-up of the 514 donor sperm samples generated 662 separate sperm fractions (151 sperm fractions after wash or density gradient and seminal plasma from 511 samples).
If in one of the sperm fractions HPV DNA was detected, the sperm sample was considered HPV positive. Donor sperm originated from one of three donor banks (A: n=365, B: n=131 and C: n=18). The study was approved by both the Institutional Review Board of the University hospital of Antwerp and the University of Antwerp (Belgian registration number: B300201733597; Eudra CT number: 2017-004791-56).
Real-time type-specific quantitative PCR (qPCR) analysis of HPV DNA in donor sperm
DNA extraction on donor sperm was performed on the ABBOTT M200sp as previously described (Depuydt et al., 2019). Each DNA extract was subjected to a clinically validated real-time quantitative PCR assay (Depuydt et al., 2012a) for the detection of 18 different HPV types: HPV6 E6, HPV11 E6, HPV16 E7, HPV18 E7, HPV31 E6, HPV33 E6, HPV35 E6, HPV39 E7, HPV45 E7, HPV51 E7, HPV52 E7, HPV53 E6, HPV56 E7, HPV58 E7, HPV59 E7, HPV66 E6, HPV67 L1 and HPV68 E7 as previously described by Micalessi et al. (2012). HPV prevalence was defined as the presence of one or more of the above mentioned HPV types. High-risk HPV infection (HR HPV) was defined as the presence of one or more of the following HPV types: HPV16, HPV18, HPV31, HPV33 HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, and HPV68 (Munoz et al., 2003), possible HR (pHR) HPV when one of the following HPV types was present: HPV type 53 and HPV type 66. Low-risk HPV infection (LR HPV) was defined as the presence of one or more of the following HPV types: HPV6, HPV11 and HPV67. A ß-globin real-time quantitative PCR was used to assess the DNA quality and to estimate the number of cells (Depuydt et al., 2006). The analytical sensitivity of the different type specific HPV qPCRs varies between 1 and 100 HPV copies/qPCR reaction (Depuydt et al., 2007; 2012a).
The HPV virion per spermatozoon ratio
The number of HPV copies (virions) per ml sperm was divided by the number of spermatozoa per ml sperm to calculate the virions to spermatozoon ratio (HPV copies/spermatozoon). When different HPV types were present in one sperm sample, the number of HPV copies per ml of sperm for each individual HPV type was added and divided by the number of spermatozoa per ml as previously described (Depuydt. et al., 2019).
Statistical analysis
Statistical analysis was performed with MedCalc® (MedCalc Software, Ostend, Belgium) (Schoonjans et al., 1995).
Results
HPV prevalence in donor sperm from 3 different sperm banks
From the 365 donor sperm samples tested from bank A, 13 tested positive for one or two HPV types (3.6%). From bank B, 4 donor sperm samples tested HPV positive out of 131 (3.1%) and from bank C, 3 samples tested positive out of 18 (16.7%; Table I).
Table I.
HPV positivity in donor sperm from 3 different sperm banks.
| Sperm bank | HPV in donor sperm | # straws | % |
|---|---|---|---|
| A | Negative | 352 | 96.4 |
| Positive | 13 | 3.6 | |
| Total | 365 | 100 | |
| B | Negative | 127 | 96.9 |
| Positive | 4 | 3.1 | |
| Total | 131 | 100 | |
| C | Negative | 15 | 83.3 |
| Positive | 3 | 16.7 | |
| Total | 18 | 100 | |
| All | Negative | 494 | 96.1 |
| Positive | 20 | 3.9 | |
| Total | 514 | 100 |
HPV negative = negative for following HPV types: HPV 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67 and 68. HPV positive = positive for one of following HPV types: HPV 16, 18, 31, 39, 51, 52, 58, 66 and 67.
HPV prevalence in donor sperm
From the 514 donor sperm samples tested, 20 (3.9%) tested positive for at least one HPV type and 2 (0.4%) tested positive for two HPV types (Table II). The majority of the detected HPV types were HR (high risk) HPV types, with HPV 31 as the most frequently detected HR HPV type (5/20; 25%). From the pHR and LR (low risk) HPV types only HPV types 66 and 67 were detected.
Table II.
Prevalence of different HPV types detected in donor sperm.
| HPV type | n | % |
|---|---|---|
| 6 | ND | 0 |
| 11 | ND | 0 |
| 16 | ND | 0 |
| 16.51 | 1 | 5.0 |
| 18 | 2 | 10.0 |
| 18.67 | 1 | 5.0 |
| 31 | 5 | 25.0 |
| 33 | ND | 0 |
| 35 | ND | 0 |
| 39 | 2 | 10.0 |
| 45 | ND | 0 |
| 51 | ND | 0 |
| 52 | 1 | 5.0 |
| 53 | ND | 0 |
| 56 | ND | 0 |
| 58 | 3 | 15.0 |
| 59 | ND | 0 |
| 66 | 1 | 5.0 |
| 67 | 4 | 20.0 |
| 68 | ND | 0 |
| Total | 20 | 100.0 |
LR HPV: low risk HPV types 6, 11 and 67; pHR HPV: possible high risk HPV types 53 and 66; HR HPV: high risk HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68; ND = not detected.
The HPV virion per spermatozoon ratio
The median HPV virion per spermatozoon ratio in HPV positive donor sperm was 0.03 (95% CI 0.01 to 1.07 HPV virions/spermatozoon). There was no difference between the median HPV virion per spermatozoon ratio of HPV positive donor sperm from the 3 different sperm banks (Figure 1). We previously showed that pregnancies can still occur for both homologous and donor sperm inseminations when the virion per spermatozoon ratio is below the threshold of 0,66 HPV virions per spermatozöon (Depuydt et al., 2019; Figure 1).
Figure 1.
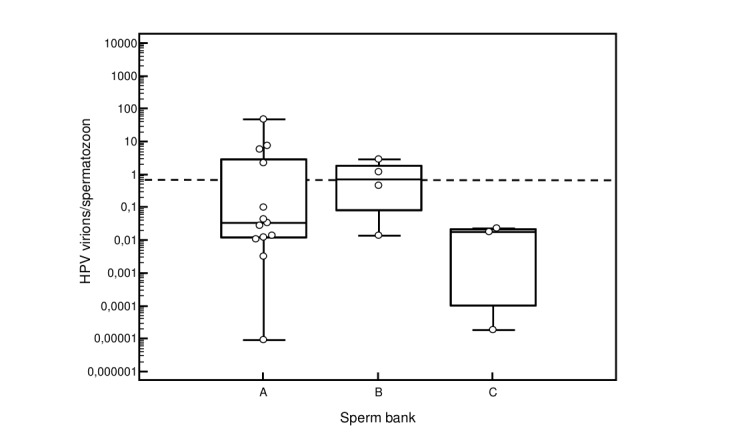
HPV virion per spermatozoon ratio in HPV positive donor sperm according to sperm bank. The dashed line represents the 0,66 HPV virions per spermatozoon cut-off above which no clinical pregnancies could be achieved when HPV positive sperm was used for IUI (ref Depuydt Fertil Steril). Following HPV types were quantified with real time qPCR: HPV 6,11,16,18,31,33,35,39,45,51,52,53,56,58,59,67 and 68. When different HPV types were present in donor sperm, the number of copies per ml of sperm for each individual HPV type was added and divided by the number of spermatozoa per ml.
Although most donor samples (14/20) had a HPV virion per spermatozoon ratio below this cut-off ratio of 0.66 HPV virions per spermatozoon, still no clinical pregnancies were achieved with HPV positive donor sperm. Whereas insemination with HPV negative donor sperm led to clinical pregnancies in 14.6% per IUI cycle.
Discussion
Our data on HPV typing of 514 semen samples from sperm donors originating from 3 different sperm banks clearly shows that HPV positive donor sperm was found in all three sperm banks. In total 20 donor sperm samples tested positive for HPV (3.9%), comparable to the 4.8% HPV prevalence previously found in the sperm bank of the University Hospital of Antwerp (D’Hauwers et al., 2012). The variation of HPV positivity of donor sperm within each of the tested sperm banks might be explained by the heterogeneity in sperm banking facilities in Belgium (Thijssen et al., 2014). Additionally, large commercial sperm banks have a greater donor pool than smaller local sperm banks and can therefore afford to select only the very best donors. This results in better sperm quality from large sperm banks which is probably linked to lower HPV sperm prevalence.
None of the inseminations from the HPV positive donors in the current study resulted in a pregnancy (Depuydt et al., 2019), as compared to 14.6% pregnancies resulting from inseminations with HPV negative donor sperm.
According to the results report generated from European registers by ESHRE for the year 2012 (Calhaz-Jorge et al., 2016) a total of 43.497 IUI with donor semen have been performed. Extrapolating from our observed frequencies, this would lead to around 1696 HPV infected donor semen samples used in IUI per year in Europe. This is however an underestimation since many cycles are not registered in the European registers because the insemination is done privately, and donor sperm can be bought by patients directly from international sperm banks due to the free movement of persons and goods in Europe.
In our opinion, donor sperm should be systematically screened for HPV, not only from a cost/benefit point of view, but also for safety reasons. Indeed since no dividing cells are present in sperm, HPV DNA detected in sperm always originates from infectious HPV virions, potentially resulting in transmission and potential infection of the inseminated women. As a consequence, most women (81%) (Depuydt et al., 2016a) will develop transient HPV virion production, prolonging their temporal subfertility, while one fifth (19%) of women will develop a clonal HPV transformed HPV infection, 1/3 of which can lead to cervix cancer (Verhelst et al., 2016). Therefore, although most induced HPV infections in those women who were infected with HPV positive donor sperm are allegedly transient or will regress in time, still a small fraction however is at risk to develop invasive cervical cancer due to insemination with HPV positive donor sperm. Because the HPV virions can bind the spermatozoa (Foresta et al., 2011b; Kaspersen et al., 2011) and efforts to use sperm preparation techniques to remove the virions were not successful (Brossfield et al., 1999; Chan et al., 1994; Olatunbosun et al., 2001), excluding sperm donations with HPV positive sperm from the banks could prevent transmission of HPV infections.
Based on these data we strongly recommend from both a cost/benefit and a safety point of view that donor sperm should always be tested for HPV prior to insemination.
Acknowledgments
This study was supported in part by AML (R14-021; R16-018), the Walking Egg non-profit organization and the Belgian National Reference Centre for HPV.
References
- 1.Basky G. Potential sperm donors should be tested for HPV. CMAJ. 2000;163:324. [PMC free article] [PubMed] [Google Scholar]
- 2.Boeri L, Capogrosso P, Ventimiglia E, et al. High-risk human papillomavirus in semen is associated with poor sperm progressive motility and a high sperm DNA fragmentation index in infertile men. Hum Reprod. 2019;34:209–217. doi: 10.1093/humrep/dey348. [DOI] [PubMed] [Google Scholar]
- 3.Brossfield JE, Chan PJ, Patton WC, et al. Tenacity of exogenous human papillomavirus DNA in sperm washing. J Assist Reprod Genet. 1999;16:325–328. doi: 10.1023/A:1020458100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calhaz-Jorge C, de Geyter C, Kupka MS, et al. Assisted reproductive technology in Europe, 2012: results generated from European registers by ESHRE. Hum Reprod. 2016;31:1638–1652. doi: 10.1093/humrep/dew151. [DOI] [PubMed] [Google Scholar]
- 5.Chan PJ, Su BC, Kalugdan T, et al. Human papillomavirus gene sequences in washed human sperm deoxyribonucleic acid. Fertil Steril. 1994;61:982–985. doi: 10.1016/s0015-0282(16)56719-3. [DOI] [PubMed] [Google Scholar]
- 6.D’Hauwers K, Tjalma W, Punjabi U, et al. Human Papillomavirus and Related Diseases - From Bench to Bedside - A Clinical Perspective. 2012. Human Papillomavirus in Donor Semen in Belgium; pp. 305–18. [Google Scholar]
- 7.Depuydt CE, Benoy IH, Bailleul EJ, et al. Improved endocervical sampling and HPV viral load detection by Cervex-Brush Combi. Cytopathology. 2006;17:374–381. doi: 10.1111/j.1365-2303.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 8.Depuydt CE, Boulet GA, Horvath CA, et al. Comparison of MY09/11 consensus PCR and type-specific PCRs in the detection of oncogenic HPV types. J Cell Mol Med. 2007;11:881–891. doi: 10.1111/j.1582-4934.2007.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Depuydt CE, Benoy IH, Beert JF. Clinical validation of a type-specific real-time quantitative human papillomavirus PCR against the performance of hybrid capture 2 for the purpose of cervical cancer screening. J Clin Microbiol. 2012a;50:4073–4077. doi: 10.1128/JCM.01231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Depuydt CE, Criel AM, Benoy IH, et al. Changes in type-specific human papillomavirus load predict progression to cervical cancer. J Cell Mol Med. 2012b;16:3096–3104. doi: 10.1111/j.1582-4934.2012.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Depuydt CE, Jonckheere J, Berth M, et al. Serial type-specific human papillomavirus (HPV) load measurement allows differentiation between regressing cervical lesions and serial virion productive transient infections. Cancer Med. 2015;4:1294–1302. doi: 10.1002/cam4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Depuydt CE, Beert J, Bosmans E, et al. Human Papillomavirus (HPV) virion induced cancer and subfertility, two sides of the same coin. FactsViewsVis Obgyn. 2016a;8:211–222. [PMC free article] [PubMed] [Google Scholar]
- 13.Depuydt CE, Thys S, Beert J, et al. Linear viral load increase of a single HPV-type in women with multiple HPV infections predicts progression to cervical cancer. Int J Cancer. 2016b;139:2021–2032. doi: 10.1002/ijc.30238. [DOI] [PubMed] [Google Scholar]
- 14.Depuydt CE, Donders GGG, Verstraete L, et al. Infectious human papillomavirus virions in semen reduce clinical pregnancy rates in women undergoing intrauterine insemination. Fertil Steril. 2019;111:1135–1144. doi: 10.1016/j.fertnstert.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Foresta C, Ferlin A, Bertoldo A, et al. Int J Androl. Human papilloma virus in the sperm cryobank: an emerging problem. 2011a;34:242–246. doi: 10.1111/j.1365-2605.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 16.Foresta C, Patassini C, Bertoldo A, et al. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PLoS One. 2011b;6:e15036. doi: 10.1371/journal.pone.0015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garolla A, Engl B, Pizzol D, et al. Spontaneous fertility and in vitro fertilization outcome: new evidence of human papillomavirus sperm infection. Fertil Steril. 2016;105:65–72. doi: 10.1016/j.fertnstert.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Gizzo S, Ferrari B, Noventa M, et al. Male and couple fertility impairment due to HPV-DNA sperm infection: update on molecular mechanism and clinical impact--systematic review. Biomed Res Int. 2014;2014:230263. doi: 10.1155/2014/230263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaspersen MD, Bungum M, Fedder J, et al. No increased sperm DNA fragmentation index in semen containing human papillomavirus or herpesvirus. Andrology. 2013;1:361–364. doi: 10.1111/j.2047-2927.2013.00067.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaspersen MD, Larsen PB, Ingerslev HJ, et al. Identification of multiple HPV types on spermatozoa from human sperm donors. PLoS One. 2011;6:e18095. doi: 10.1371/journal.pone.0018095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micalessi IM, Boulet GA, Bogers JJ, et al. High-throughput detection, genotyping and quantification of the human papillomavirus using real-time PCR. Clin Chem Lab Med. 2012;50:655–661. doi: 10.1515/cclm.2011.835. [DOI] [PubMed] [Google Scholar]
- 22.Munoz N, Bosch FX, de Sangosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 23.Olatunbosun O, Deneer H, Pierson R. Human papillomavirus DNA detection in sperm using polymerase chain reaction. Obstet Gynecol. 2001;97:357–360. doi: 10.1016/s0029-7844(00)01183-2. [DOI] [PubMed] [Google Scholar]
- 24.Patterson NA, Smith JL, Ozbun MA. Human papillomavirus type 31b infection of human keratinocytes does not require heparan sulfate. J Virol. 2005;79:6838–6847. doi: 10.1128/JVI.79.11.6838-6847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoonjans F, Zalata A, Depuydt CE, et al. MedCalc: a new computer program for medical statistics. Comput Methods Programs Biomed. 1995;48:257–262. doi: 10.1016/0169-2607(95)01703-8. [DOI] [PubMed] [Google Scholar]
- 26. Superior health councel No 9180. The role of HPV in fertility. Should male donor gametes be screened. https://www.absym-bvas.be/images/spf_sante/Conseil_superieur_de_la_sante/19099356_fr.pdf . 2014.
- 27.Thijssen A, Dhont N, Vandormael E, et al. Artificial insemination with donor sperm (AID): heterogeneity in sperm banking facilities in a single country (Belgium). Facts Views Vis Obgyn. 2014;6:57–67. [PMC free article] [PubMed] [Google Scholar]
- 28.Verhelst S, Poppe WA, Bogers JJ, et al. Serial measurement of type-specific human papillomavirus load enables classification of cervical intraepithelial neoplasia lesions according to occurring human papillomavirus-induced pathway. Eur J Cancer Prev. 2016;26:156–164. doi: 10.1097/CEJ.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 29.Xiong YQ, Chen YX, Cheng MJ. The risk of human papillomavirus infection for male fertility abnormality: a meta-analysis. Asian J Androl. 2018;20:493–497. doi: 10.4103/aja.aja_77_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


