Abstract
Anti-drug vaccines have potential as new interventions against substance use disorder (SUD). However, given the challenges seen with inter-individual variability in SUD vaccine trials to date, new interventions should ensure a robust immune response and safety profile among a diverse population. This requires accounting for sex and heritable genetic differences in response to both abused substances as well as the vaccination itself. To test response variability to our heroin-tetanus toxoid (Her-TT) immunoconjugate vaccine, we vaccinated male and female mice from several mouse strains including Swiss Webster (SW), BALB/c, and Jackson diversity mice (J:DO). Previous studies with vaccinated male SW mice demonstrated a rare hypersensitivity, resulting in mice rapidly expiring with exposure to a low dose of heroin. Our results indicate that this response is limited to only male SW mice, and not to any other strain or female SW mice. Our data suggest that this hypersensitivity is not the result of an overactive cytokine or IgE response. Vaccination was similarly effective among the sexes for each strain and against repeated heroin challenge. Inbred BALB/c and J:DO mice were found to have the best vaccine response against heroin in antinociception behavioral assay. These results highlight the importance of incorporating both male and female subjects, along with different strains to mimic diverse human populations, as new SUD vaccines are being tested.
Keywords: heroin, sex differences, strain, mouse, conjugate vaccine
Introduction
Experimental drug conjugate vaccines designed to treat substance use disorder (SUD) are new chemical immunology interventions, which have shown preclinical efficacy in animals but have not yet achieved clinical approval.[1–4] In 2018, the Centers for Disease Control (CDC) released its annual surveillance report in ‘Drug-related risks and outcomes’ and found that there was a record high in the number of overdose deaths in 2016, primarily attributed to the rise in prescription opioid abuse and heroin; opioid-associated overdose is now the leading cause of accidental death in the United States.[5]. Heroin is a schedule I drug, a highly addictive illicit opioid, and a primary player in the U.S. opioid epidemic. Current interventions such as methadone and naltrexone have not been sufficient to address the scope of the problem. Generally, 40-60% of patients who are treated for substance use disorder relapse.[5, 6] Thus, new alternatives to combat the opioid crisis are urgently needed.
A major limitation to the advancement of SUD vaccines to market is that therapeutic antibody concentrations are achieved in only a fraction of immunized patients.[7, 8] Nevertheless, this shortcoming is rarely addressed in pre-clinical studies. For example, reports of advances with heroin vaccines only have representative samples from one sex and one strain per study (Table S1). Sex is an important consideration in designing a vaccine study for the overall population, especially in regard to the general safety and efficacy of these vaccines.[9]
When interpreting data on disparities in nociception, it is important to recognize that there are sex differences in a number of physiological systems that may directly or indirectly affect measures of analgesia.[9] Immune responses also vary between sexes, and adverse effects such as respiratory depression and nausea may occur distinctively by sex.[9] Furthermore, men and women generally abuse drugs for distinct reasons, and substance use disorders may manifest differently in women and men.[10] In 2016, self-reported prevalence of prescription pain relivers was found to be 4.8% for males and 3.8% for females.[5] In murine models, relative sex differences have been previously reported in terms of their differential response to analgesic potency to opioids, including morphine. [11]
In addition to understanding the sex-related impact on active vaccination against drugs of abuse, we were also interested in examining vaccine efficacy with respect to inbred and outbred mouse strains. In terms of opioid behavioral responses, several studies have shown that brain opioid binding sites and sensitivities to morphine vary by strain.[12] This underscores the possibility of inter-experimental differences resulting from use of different backg round strains or pedigrees. Diversity outbred mice are hypothesized to be better predictors of population-wide human responses to chemical exposures due to increased genetic variability.[13]This is also consistent with observed responses to vaccine efficacy in the clinic.[7, 8] Although male and female mice have been studied independently, no SUD vaccine study to date has incorporated the use of both sexes across multiple strains within the same design. to minimize inter-experimental differences and identify whether sex and background strain are likely to have substantial impacts on SUD vaccine generalizability (Table S1).
In our research group, Taconic Swiss Webster (SW) mice are our traditional mouse models of choice due to their genetic diversity as outbred mice, their rapid availability, and affordability. While it is convenient and useful to benchmark behavioral data for new vaccine development using the same strain and sex, these narrow studies may create an unintended bias based on sex or strain in the experimental results. In order to directly assess this concern, we have now vaccinated males and females of three different strains: outbred Swiss Webster (SW), inbred BALB/c, and outbred Jackson Diversity Outbred (J:DO) mice (Table 1). We were also interested in whether heroin exposure produced any notable differences upon vaccination outcomes in mice, as patients receiving the vaccine are highly unlikely to be drug naive. Thus, an additional group of SW mice were used to address this question for each sex (Table 1). Within this study, the overall goals were to observe what effect (1) sex, (2) strain, and (3) prior exposure to heroin had on vaccine alteration of phenotypic response to heroin-induced analgesia.
Table 1.
Effects of strain and sex on lethality from a low dose of heroin (2 mg/kg) after exposure to heroin vaccine.
| Male | Female | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | Strain | Mice Expired | N | % Lethality from Drug | Mice Expired | N | % Lethality from Drug |
| Vaccinated | Taconic Swiss Webster | 6 | 64 | 9.4% | 0 | 16 | 0.0% |
| Taconic BALB/c | 2a | 16 | 0.0% | 0 | 16 | 0.0% | |
| Jackson Laboratory J:DO | 1b | 16 | 0.0% | 0 | 16 | 0.0% | |
| Charles River Swiss Websterc | 1 | 16 | 6.3% | n.a. | |||
| Nonvaccinated | Taconic Swiss Webster | 0 | 4 | 0.0% | 0 | 4 | 0.0% |
| Taconic BALB/c | 0 | 4 | 0.0% | 0 | 4 | 0.0% | |
| Jackson Laboratory J:DO | 0 | 4 | 0.0% | 0 | 4 | 0.0% | |
| Charles River Swiss Websterc | 0 | 4 | 0.0% | 0 | 4 | 0.0% | |
One mouse expired from fighting and one from heightened sensitivity to isofluorane during eye bleeds
Mouse expired from fighting
Charles River requires an 8-week quarantine, only males were run to test for vendor effect
Materials and Methods
Animals
All studies were performed in compliance with the Scripps Institutional Animal Care and Use Committee (IACUC) and all protocols adhered to the National Institute of Hea l th Guide for the Care and Use of Laboratory Animals. Male and female SW and BALB/c mice (Taconic Farms, Germantown, NY; 6-8 weeks old) and Jackson Diversity Outbred mice (J:DO, Jackson Labs, Bar Harbor, ME; 5-6 weeks old) were immunized subcutaneously (s.c.) with 40 μg of heroin-tetanus toxoid (TT) conjugate vaccines on days 0, 14, and 28. The overall vaccination schedule and behavioral experiments are detailed in Figure 1 and Figure S4. Mice were bled on day 21 and 35 using retro-orbital puncture while anesthetized under isoflurane in order to collect approximately 100-150 μL of whole blood. Groups were composed of 16 mice per sex/per strain, and 4 mice for controls per sex/per strain. Mice were group-housed in an AALAC-accredited vivarium and kept on a reverse light cycle (lights on: 9PM-9AM). Mouse weights were measured every week (female cohorts) or every other week (male cohorts) and injection site reactions were measured on the day of antinociceptive testing (Figure S5). In the follow-up study using male SW from Charles River (Wilmington, MA, 6-8 weeks old), these animals were required to undergo quarantine for eight weeks to comply with TSRI IACUC guidelines. Under quarantine, mice were vaccinated, remarked and reweighed every week.
Figure 1.
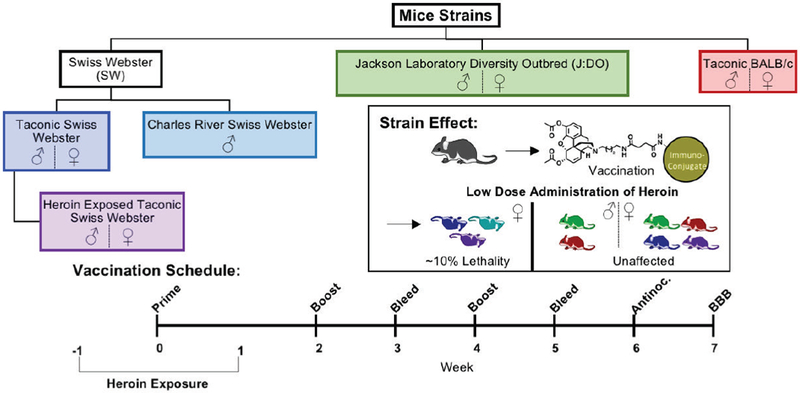
Overview of mice strains and sexes used in this study with experimental schedule.
Vaccine preparation, formulation, schedule
Heroin was obtained from NIDA Drug Supply Program. A second generation heroin hapten was synthesized according to literature procedure and utilized throughout the study (Figure 1, panel).[14, 15] After preparation of the free carboxylic acid of the hapten, the acid was activated with NHS and then mixed with bovine serum albumin (BSA) or TT (1 mg/mL) in a 1:1 w/w ratio of hapten to protein. Immunoconjugates were allowed to react at room temperature for 20 hours using gentle end-over-end mixing. Following conjugation, the solutions were dialyzed against PBS buffer (pH 7.4) using a 10K molecular weight cut off dialysis cassettes (Thermo Fisher Scientific). Vaccines were cryoprotected with glycerol (50% v/v) and stored at −80 °C. On the day of vaccination, immunoconjugates were defrosted and formulated with 40 ug CpG oligodeoxynucleotide (ODN) 1826 (Eurofins), 1 mg alum (Alhydrogel), and allowed to mix at room temperature for an hour.
Hapten copy number by MALDI-ToF
The heroin hapten density for immunoconjugates prepared in this study was quantified using MALDI-ToF and ESI-ToF MS analysis and compared to BSA according to literature procedure.[14, 15] The immunoconjugates were run through a PD MiniTrap G-10 desalting column (GE Healthcare) and then analyzed by MALDI-ToF. Spectra can be found in the supplementary information (Figures S1–3).
ELISA protocol
Anti-heroin midpoint titer for each vaccination group and nonvaccinated control mice were determined according to literature procedure as written, except for employing a final incubation time of 8 minutes with TMB and H2O2 before quenching with 2.0 M H2SO4. [14, 15]
Antibody affinity determination by SPR
The binding IC50 for mouse serum IgGs and free 6-acetylmorphine (6-AM) was determined by competitive binding assay via surface plasmon resonance (SPR) according to literature methods.[14, 15] Diluted mouse serum from day 21 and 35 were incubated with serial dilutions of heroin, 6-AM and morphine. IC50 values were determined from a 12 point 6-AM dilution curve and derived from a nonlinear fit of the binding curves in PRISM 6 (GraphPad Software, Inc).
Antinociception
Mice were tested for cumulative heroin response, administered intraperitoneally (i.p.), in suprapinal (hot plate) and spinal (tail flick) behavioral tests on week 6 as previously described.[14–16] In an attempt to prevent or minimize tissue damage, mouse paws and tails were gently placed in cool water after testing at each dose. Data are reported as ED50, which is the concentration of drug where 50% of animals within a group experience the antinociceptive effects of heroin. The ED50 values and 95% confidence intervals were determined for each antinociception test and individual treatment groups to determine ED50 values.
Repeat antinociception assay
After performing the full range of antinociception for both hot plate and tail immersion tests (heroin dosage 2 mg/kg – 18 mg/kg, i.p.) for both vaccinated and nonvaccinated controls, half of the vaccinated mice and all of the control mice (male and female SW, heroin-exposed SW, BALB/c, and J:DO) were run again twenty-four hours later. After determination of the ED50 value (from the previous day), 4 mg/kg heroin (i.p.) was administered to the mice after baseline measurements were taken before administration of drug in the absence of heroin. Upon administration of drug, the analgesia effect was measured.
Blood brain distribution experiments
Blood brain biodistribution was determined according to literature procedure with minor modifications.[17, 18] A calibration curve for using standard solutions of heroin, 6-acetylmorphine, and morphine was constructed (Figure S10). On Week 7 (3 weeks from last boost), mice were injected i.p. (4 mg/kg) with heroin. At five, fifteen, or thirty minutes following injection the animals were fully anesthetized with isoflurane and then rapidly decapitated using a sharp guillotine. The brain and trunk blood were collected. The trunk blood was collected in a 1:1 ratio with acetate buffer (0.1 M sodium acetate/0.1M acetic acid/50 g/L NaF, pH 6.0), placed on ice for several hours, centrifuged at 10,000 rpm for ten minutes.
Brain tissue was immediately homogenized using a Bullet Blender with zirconium oxide beads (0.5 mm diameter, Thomas Scientific) with acetate buffer and stored on dry ice. Afterwards the homogenate was centrifuged at 2,500 rpm for ten minutes. A 100 μL aliquot of the homogenate or plasma was added to 100 μL of spiked heroin, 6-AM, and morphine concentrations (for standard curve, made up in 85:15 ACN:MeOH) or 100 μL of 85:15 ACN:MeOH (for samples), 100 μL of d9-heroin, d3-acetylmorphine, and d3-morphine (1 μg/mL in ACN) and 300 μL of ice-cold acetonitrile/methanol (85:15). The mixture was vortexed for 30 seconds and stored in the −20 °C freezer for twenty minutes, followed by centrifugation at 2,500 rpm for ten minutes. A 450 μL aliquot was transferred to another test tube and the samples was evaporated using GENEVAC. The dried sample was taken up in acetonitrile, centrifuged at 10,000 rpm for five minutes and then transferred to vials for LC/MS analysis.[17, 18] On average, in processed brain tissue samples, heroin, 6-AM, and morphine eluted at 3.64, 3.24, and 1.40 min, respectively. For blood samples, heroin, 6-AM, and morphine eluted at 3.75, 3.46, and 1.56 min, respectively.
Cytokine expression panel of blood and brain samples
A mouse cytokine multiplexed ELISA array was performed by Quansys Biosciences’ (Logan, UT) to measure and quantify the following rodent cytokines:IL-1a, IL-1b, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-17, MCP-1, IFNγ, TNFα, MIP-1a, GMCSF, and RANTES. Using male SW mice (Taconic), a separate set of 32 mice were vaccinated with Her-TT and adjuvanted with 40 μg CpG ODN and 1 mg alum/dose. After a full vaccination schedule, mice were injected with 2.0 mg/kg heroin. Samples were tested in triplicate and with internal standards. Plasma and brain homogenates were submitted for analysis.
Results and Discussion
Investigation of heroin hypersensitivity
Prior observations in male SW mice
Previously, we observed an unexpected side effect in eight separate anti-he roin vaccine studies, incorporating a total of 147 male SW mice (Taconic), using a combination of three different carrier proteins, five adjuvants, and several formulation parameters, including either glycerol or trehalose as the cryoprotectant (Table S2). The only commonality among the different experimental parameters was the hapten itself and the sex, strain, and vendor of the animal model. This side effect can be characterized as an apparent hypersensitivity to heroin, inducing rapid lethality upon exposure to a small dose of heroin (i.p.). This outcome was found to occur between 6-25% of all vaccinated mice, depending on vaccine formulation parameters and sample size. A contingency analysis was used to determine the relative risk for each independent adjuvant and condition (Table S2 and Figure 2).
Figure 2.
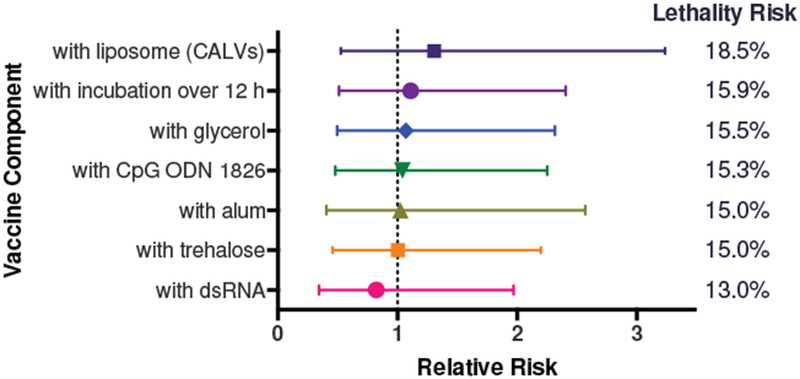
Relative and lethality risk calculated for each vaccine adjuvant or condition. Relative risk is shown below as mean ± 95% confidence interval. For the Her-TT vaccine used in this study, alum, CpG ODN 1826, glycerol, a prolonged incubation time of 1h with alum was incorporated into the vaccine formulation. Conjugatable adjuvant lipid vesicles (CALVs) were previously found to be not as effective as the combination of alum with CpG, so were not used in this study as a vaccine component.
There was no obviously increased risk associated with any single adjuvant or carrier protein component, as some studies precluded an adjuvant or carrier protein completely and the adverse reaction was still observed. In contrast, both nonvaccinated naive mice (n = 25) and mice administered vaccine vehicle or saline (n = 18) did not exhibit hypersensitivity at the 2 mg/kg dose of heroin (i.p.).[14, 15, 19] Typically, antinociception assays using heroin were performed with doses starting at 2 mg/kg (i.p.) and ending at 18 mg/kg.[14–16, 19] For additional reference, a 160 mg/kg dose of heroin (i.p.) prompted an LD50 or lower for vaccinated mice and an LD75 for nonvaccinated mice for male SW mice (Taconic) in our hands.[15] We also found that the LD50 of heroin for male SW mice (Taconic) through direct intravenous (i.v.) injection was found to be 23.7 mg/kg.[20] This illustrates the magnitude of difference between the 2 mg/kg dose of heroin that induced this hypersensitivity versus the 80-fold (i.p.) and 10-fold (i.v.) larger doses of heroin needed to trigger overdose in these mice.
It should be noted that this hypersensitive lethality is also independent of vaccine efficacy, suggesting that the underlying mechanism behind this phenomenon may be independent of antibody interaction. Moreover, one study determined the isotyping of antibodies from vaccination and found no detectable IgE response from either supersensitive or normal mice, indicating that a maladaptive immune response was not the etiology.[19] Lastly, several operators prepared vaccines, administered vaccinations, and/or administered drug. We selected a vaccine formulation that incorporated elements most likely to generate an adverse response to assess if lethality after exposure to heroin was seen in other mouse strains (Table S2 and Figure 2). This study included an inbred mouse model, BALB/c, that commonly used in anti-drug vaccine development (Table S1).[21–23]
Strain and sex-dependent hypersensitivity
In addition, to ensure an adequate sample population, we increased the sample size of our vaccine groups from n=4-6 mice per group to n=16 mice per group for each strain and sex combination (Figure 1 and Table 2). Weight change was monitored for the mice throughout the vaccination schedule as a common indicator of general health (Figure S5). Although the body weight change for male BALB/c was significantly lower than the other mouse strains, the recorded weight according to their weekly age was consistent with the average body weight information for BALB/c (Taconic Biosciences, Inc). After the full vaccination schedule and administration of heroin on the day of the antinociception assay, (Figure 1 and Figure S4), 6 of the 64 male SW mice (Taconic) expired rapidly after receiving an initial dose of 2 mg/kg (Table 2). Three of the male SW (Taconic) mice were in the heroin-exposed vaccination group, and 3 of the mice were in the heroin-naive vaccination group. Taken together, the total lethality for this specific strain, sex, and vendor was 9.4%, which was consistent with previous observations.
Table 2.
Summary of midpoint titer data and IC50 values of polyclonal antibody response to 6-acetylmorphine. Numbers represent means ± SEM for midpoint titer and means for IC50 values in nanomolar concentration. Data were tested for statistical outlier evaluation using Grubbs’ test and significant outliers were removed. For male mice, no outliers were found, but the number of mice in BALB/c and J:DO were reduced from 16 to 14 and 15, respectively due to mice expiring from fighting. For female mice at week 3, 1 individual was excluded from J:DO, and at week 3, 1 J:DO mouse and 3 heroin exposed SW were excluded.
| Sex | Strain | Midpoint Titers | IC50 (nM) | ||
|---|---|---|---|---|---|
| Week 3 | Week 5 | Week 3 | Week 5 | ||
| ♂ | Taconic Swiss Webster | 21971 ± 3477 | 23210 ± 3243 | 285 | 90.5 |
| Taconic BALB/c | 20763 ± 7000 | 22304 ± 5060 | 441 | 160 | |
| Jackson Laboratory J:DO | 26883 ± 6283 | 14744 ± 5382 | 470 | 44.2 | |
| ♀ | Taconic Swiss Webster | 55001 ± 13137 | 52537 ± 6923 | 225 | 170 |
| Taconic BALB/c | 21623 ± 2605 | 63446 ± 10357 | 791 | 180 | |
| Jackson Laboratory J:DO | 56424 ± 14679 | 59854 ± 16799 | 362 | 150 | |
One male BALB/c mouse expired during the study due to excessive fighting by cage-mates and one from an adverse reaction to isoflurane. A J:DO male mouse also expired due to excessive fighting with cage-mates. It should be noted that general aggressive behavior was noted only for males, regardless of strain; however, fighting was reduced by separating particularly aggressive males from their cage-mates. As expected, no mice in the control groups expired. Surprisingly, there was no hypersensitivity observed for vaccinated SW female mice upon exposure to a low dose of heroin (Table 1).
Effect of heroin exposure on vaccination
Previously it was mentioned that prior heroin exposure is a more likely characteristic of an SUD patient and prior opioid exposure may influence the activity of the vaccine. After exposing both female and male SW to two rounds of 2 mg/kg heroin (i.p.) two weeks apart, midpoint titers, affinity data, antinociception, repeat antinociception, and blood brain distribution experiments were performed (Figures S4–S5). Pre-exposure to heroin did not induce tolerance. For both male and female heroin exposed mice, no hypersensitivity was observed after the first inoculation and exposure to 2 mg/kg of heroin (i.p.). Based on the analyzed data (Figure S5–S8), there was no significant difference found between the two cohorts by any measure.
Charles River vs Taconic Swiss Webster mice
The hypersensitivity to heroin following Her-TT vaccination in Male SW mice prompted us to explore if this effect was present in all SW mice or just an effect of a random genetic mutation acquired from a vendor over time. To test if this hypersensitivity was related to conditions arising at a specific vendor, we acquired a separate set of 20 male Charles River SW mice, and vaccinated 16 of them. Charles River mice were under quarantine during vaccination, but their housing conditions were similar to mice out of quarantine. Charles River SW mice were vaccinated at the same age (week) as Taconic and the other strains.
After the full vaccination, Charles River SW mice were run in an antinociception assay (Figure 3). Interestingly, one mouse expired at the low 2 mg/kg dose of heroin (6.3% lethality rate), indicating that hypersensitivity is not solely a vendor effect, but in fact a general trend observed for all SW mice (Table 1). Nonvaccinated mice exhibit an increased tolerance to heroin in supraspinal response (hot plate), which is indicative of higher brain function and different receptor chemistry in the brain. However, for vaccinated mice, the efficacy of the vaccine was generally similar among the two vendor groups (Figure 3). Based on this comprehensive study, it appears that this hypersensitivity to heroin is limited to only males of the SW strain, is not observed in either sex for the two other strains and does not distinguish between heroin-exposed and heroin-naive animals.
Figure 3.
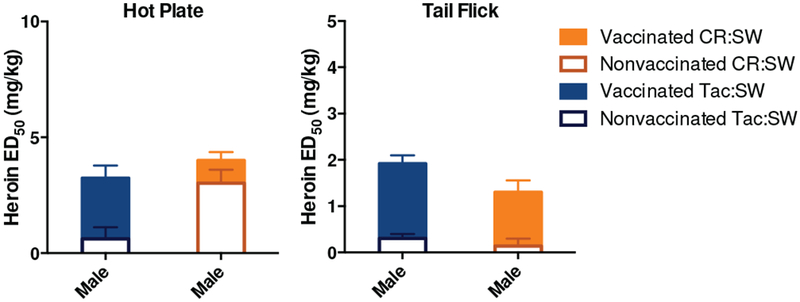
Effect of animal vendor on heroin vaccine efficacy in antinociception assays. Panel A shows results from hot plate assay. Panel B shows results from tail flick assay. Bars represent means ± SEM, where each vaccinated group is comprised of 16 mice, and control groups are comprised of 4 mice. CR:SW stands for Charles River Swiss Webster; Tac:SW stands for Taconic Swiss Webster. Tac:SW are the same data as shown in Figure 5 for males. Only males were run in this vendor study.
Mice from Taconic farms were more sensitive to morphine analgesia and toxic effect than Charles River.[24] However, the degree of tolerance observed was similar. In agreement with the literature, nonvaccinated control mice were approximately 2-fold more sensitive to heroin’s effects than Charles River, which is attributed to the greater number of brain opioid binding sites. A survey of literature revealed that although Swiss Webster are outbred, mice from Taconic Farms are supersensitive to opioids, showing lethality after administration of 50 or 100 mg/kg of morphine, s.c. [12, 24] The LD50 of morphine in these mice is 313 mg/kg, s.c., was well below the dose administered to Taconic mice. Charles Rive mice had an LD50 of 745 mg/kg and no lethality was observed at the 50 or 100 mg/kg dose of morphine. [12] This supersensitivity is thought to be caused by the increase number of opioid receptors. Based on the lethality data for morphine, before mice are vaccinated, Taconic mice are 2.2-2.4 times more sensitive to the lethal effect than compared with Charles River mice, which is consistent with the effect observed after vaccination (1.5 times more sensitive).
Cytokine panel
Cytokine production is an important physiological response following T cell activation and proliferation. We hypothesized that the supersensitivity observed in male Swiss Webster mice may have been due vaccine-induced cytokine-release syndrome or also known as a cytokine storm.[25] A cytokine storm is a systemic immune response that involves the potential release of more than 150 inflammatory mediators, including cytokines and chemokines, which precipitates system wide symptoms including shock, cell death, infection, and ultimately organ failure.[26] It is also widely documented that certain vaccines and monoclonal antibodies induce cytokine-release syndrome.[27] Although cytokines are required for several immunoregulatory activities, cytokine storm is characterized by increased levels of TNFα, IFNγ, IL-6, IL-10 (sometimes IL-2 and IL-8).[26]
To test this hypothesis, a separate batch of 32 male Swiss Webster (Taconic) mice were vaccinated with the same heroin vaccine. At week 6, mice were run at baseline and then injected i.p. with 2 mg/kg heroin. Mice exhibiting hypersensitivity were quickly anesthetized and the blood and brain samples were harvested. Samples were then submitted for testing of normal mice and hypersensitive mice. Results are shown in Figure S11–S12. The only significant difference is between IL-1b in the brain. In both mice and humans, this cytokine induces the cyclooxygenase-2 expression in the brain, which contributes to inflammatory pain hypersensitivity.[28, 29] The only other notable trend is that cytokine levels were generally elevated in the blood for control mice and elevated in brain for hypersensitive mice.
Based on our comprehensive sex and strain studies, the observed hypersensitivity to heroin is centralized to only one sex of one specific strain. Although the mechanism is not clear, we are confident that it is not the result of an IgE response, operator error, or a cytokine storm and occurs in strains from two separate vendors.
Effect of sex and strain on vaccine efficacy
Midpoint titer and affinity data
Both male and female vaccinated mice exhibited a robust polyclonal antibody response against 6-acetylmorphine, the primary psychoactive metabolite of heroin (Table 2). Generally, midpoint titers increased for both males and females between week 3 and week 5, after receiving a boost at week 4. Only J:DO males’ titer decreased substantially by week 5. Another notable observation was that female mice largely had higher titers than their male counterparts. BALB/c mice had similar responses, then diverged at week 5, with females having a higher titer of 63,446 vs. 22,304. Utilizing SPR measurements, the strains can be ranked based on highest to lowest anti-6-AM antibody affinity: J:DO > SW > BALB/c Unexpectedly, although female subjects had higher titers than male subjects, male mice developed antibodies with better affinity for 6-AM than female mice, with J:DO males exhibiting nanomolar IC50 values. Regardless, this decrease in titers has been previously observed.[30–33]
Antinociception assay
In order to gauge the efficacy of our heroin vaccine, we employed behavioral assays to assess the antinociceptive effect of heroin between vaccinated and unvaccinated animals. In agreement with affinity data, J:DO mice performed the best in the supraspinal response to heat stimuli (hot plate assay, Figure 4A). In Figure 4, the filled-in bars represent the ED50 of vaccinated mice, and unfilled bars represent nonvaccinated control mice. Response to heroin significantly differed in vaccinated and nonvaccinated J:DO male and female mice in both behavioral models. The response also differed significantly between vaccinated and nonvaccinated BALB/c mice, but only in the spinal response to heat stimuli (tail flick assay, Figure 4B).
Figure 4.

Effects of strain and sex on heroin vaccine efficacy in antinociception assays. Panel A shows results from hot plate assay. Panel B shows results from tail flick assay. Bars represent means ± SEM. A two-way ANOVA was performed for each antinociception assay, followed by a Tukey’s post hoc comparison test. * Denotes vaccine effect. *P < 0.05, **P < 0.01, ****P < 0.0001 versus control nonvaccinated mice. ### Denotes sex difference, P < 0.05 for both male (P < 0.001) and female (P < 0.0001) in tail flick by a two-way ANOVA.
Generally, males and females of the same strain performed similarly in both antinociception assays, except for J:DO response to the tail flick assay. Based on a two-way ANOVA analysis for hot plate, strain differences were observed between J:DO vs other female strains (P < 0.0001). For tail flick, a two-way ANOVA shows that strain differences were observed amongst all strains for both male (P<0.001) and female (P<0.0001). Potency values for each sex and strain are given in Figure S9, where potency ratio is defined by the ED50 of vaccinated mice normalized by ED50 of nonvaccinated control mice. Based on the potency values, J:DO females performed the best and J:DO males performed the worst. However, these potency ratio values are extremely biased on the response of nonvaccinated control mice, and obscure overall information on vaccine performance by strain. Interestingly, male J:DO mice were the least sensitive to the antinociceptive effects of heroin of all strains and sexes (Figure 4). Alternatively, female J:DO mice were extremely sensitive to heroin, therefore resulting in an extremely large potency ratio. Largely, female control mice were more sensitive to heroin than their male counterparts. Taken together, it appears that the Her-TT vaccine works the best in the inbred strain BALB/c and the outbred stain J:DO, and is the least effective in SW mice (Taconic).
Repeat antinociception assay
In a realistic scenario of heroin drug abuse, an individual with SUD is likely to engage in more than one challenge of an opioid.[34] Yet, contiguous, cumulative, daily antinociceptive testing can cause tissue damage. To model this repeated administration behavior, we included a repeat antinociception challenge. However, we only acquired two measurements: a baseline measurement and antinociception at a single dose on day two, in order to minimize potentially-confounding tissue damage. In order to determine the appropriate dosage for repeated antinociception rather than an arbitrarily assigned dose, we dosed the animals at the calculated ED50 of the vaccine. Only half of the vaccinated cohorts were run in this study, due to logistical limitations (i.e., immunizations, bleeds, assays, and experiments were split over two days due to the large number of animals required to be run in behavioral experiments see Figure S4, day 44).
Results from the repeated antinociception experiments are shown in Figure 5. Day 1 values are extrapolated from the standard antinociception assay run at baseline and at 4 mg/kg. It is apparent that mice have experienced minor tissue damage, despite efforts to mitigate damage through strict cutoff exposure times and treatment with cool water, based on the increase in average baseline times for nonvaccinated control mice over time for all mice except male SW. Despite this, it appears that the vaccine is still efficacious after repeated exposure to heroin in both assays, particularly for the BALB/c and J:DO mice. SW mice had modest efficacy over two exposures of heroin (blue bars, Figure 5)
Figure 5.
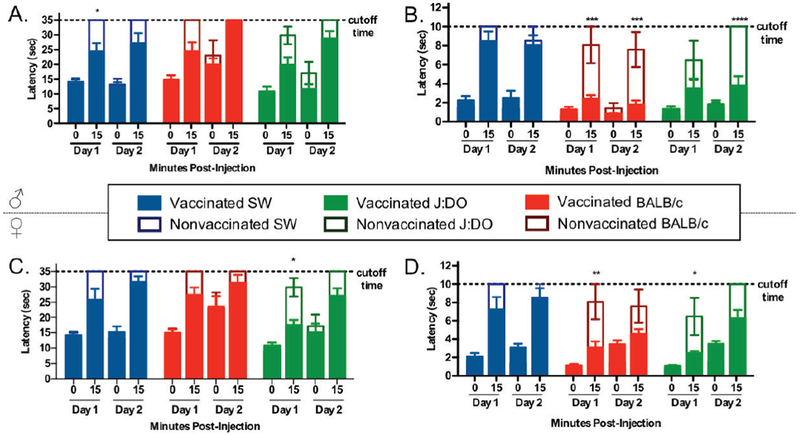
Repeated antinociception study. Panel A and C show results from hot plate assay. Panel B and D show results from tail flick assay. Solid bars represent means ± SEM for vaccinated mice (n = 8), where SW, BALB/c, and J:DO mice correspond to blue, red, and green solid bars. Clear bars represent means ± SEM for nonvaccinated control mice (n = 4), where SW, BALB/c, and J:DO mice correspond to blue, red, and green unfilled bars. Study shows that vaccines are efficacious after repeated exposure to heroin (4 mg/kg, i.p.) in both assays. A and B are males; C and D are females. * Denotes vaccine effect. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus control nonvaccinated mice by a two-way ANOVA.
A Tukey’s multiple comparisons test for male mice in the tail flick assay revealed that comparison between Day 1 and 2 at 15 minutes was not significant for any group except J:DO, but even in this case the vaccinated group performed the same and the control group performed worse, likely due to tissue damage. Both vaccinated BALB/c and J:DO were significantly less affected by heroin than SW mice at both Day 1 (P < 0.0001) and Day 2 (P < 0.0001 and P < 0.001, respectively, Tukey’s). Results from Tukey’s post hoc analysis for the hot plate assay showed that there was no significant difference between Day 1 and 2 at 15 minutes for SW mice, but there was a significant difference for BALB/c and J:DO strains, indicating either increased susceptibility to tissue damage on the hot plate or decreased vaccine efficacy. Nonvaccinated controls of BALB/c and J:DO also exhibit an increased latency time between Day 1 and 2 but these changes are not significant. Overall, it is generally true that the Her-TT vaccine remained efficacious after repeated exposure to heroin, especially in the tail flick assay (Figure 5).
Blood brain distribution study
As a terminal experiment, mice were administered 4 mg/kg heroin (i.p.) and brain tissue and blood were harvested at five, fifteen, or thirty minutes post drug administration at week 7 (day 49-50, Figure S4). Results for both males and female mouse strains are shown in Figure 6. Based on the analysis, all vaccinated mice sequestered 6-AM in the periphery (Figure 6). Female mice exhibited higher concentrations of 6-AM in both the plasma and brain tissue compared to males (Figure 6). Generally, the vaccinated strains performed similarly in blocking 6-AM from the brain. It should be noted that one limitation of this study is that drug dosing is based on the overall animal weight, however, the general size of the brain does not necessarily correlate to body size (Figure S11). For example, J:DO male mice are significantly smaller in body mass than male SW mice but are not significantly different in brain size (the same trend is observed for female SW mice compared to male SW mice). Although brain tissue is processed based on its weight, the dosing the animal receives varies based on sex or strain and may artificially inflate or deflate the concentration of 6-AM observed in processed samples.
Figure 6.
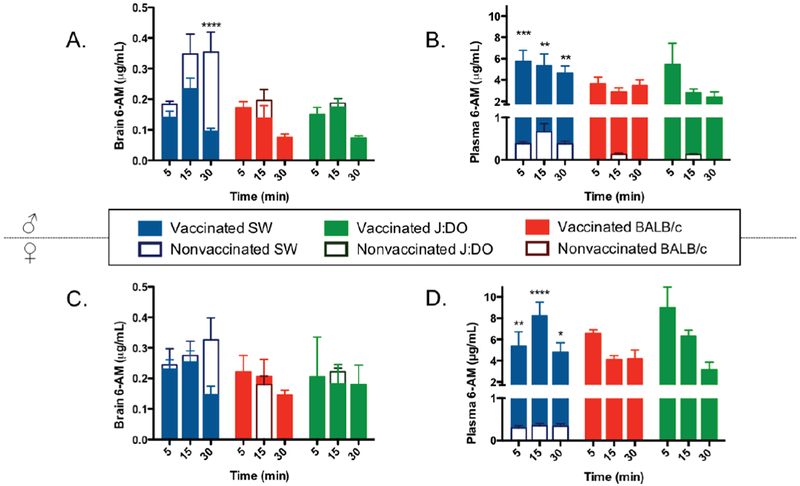
Blood brain distribution study. Panels A and B show 6-acetylmorphine concentrations in the brain and blood for males, respectively. Panels C and D show 6-AM concentrations in the brain and blood for females, respectively. Bars represent means + SEM, where each group is comprised of 4-6 mice. Samples were collected at five, fifteen, and thirty minutes post intraperitoneal injection of 4 mg/kg heroin. Filled in bars represent vaccinated mice, and clear bars represent nonvaccinated control mice. * Denotes vaccine effect. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus control nonvaccinated mice by a two-way ANOVA. Control values for female BALB/c and J:DO at 15 minutes were excluded due to limitations on quantitative assessment of 6-AM concentrations from samples collected for these groups (panel D).
Conclusion
Vaccines against drugs of abuse are new immuno-chemical interventions that may assist in the treatment and prevention of overdoses in the U.S. opioid epidemic. Although preclinical studies with animal models may not reflect true pharmacological properties encountered in humans, a concerted effort to incorporate larger samples sizes, both sexes, and multiple strains is a step in the right direction. We previously observed a rare hypersensitivity to a low dose of heroin. Based on a study incorporating both sexes, and three different strains of mice from three different vendors, we found that this hypersensitivity was localized to only male SW mice at a lethality rate of 6.3-9.4% and was not based on cytokine storm or an allergic IgE reaction. Moreover, our study shows that, males and females generally have similar responses to blockade of heroin-induced analgesia within a strain. In addition, BALB/c and J:DO mice outperformed our standard Taconic SW model in behavioral assays in both measures. Lastly, a repeated antinociception assay indicated that vaccine efficacy was maintained over multiple challenges of heroin.
Supplementary Material
Highlights.
Heroin tetanus toxoid vaccine variability was tested in both males and females in several mouse strains
Vaccinate male SW mice exhibit rare, induced hypersensitivity to low exposure of heroin.
Hypersensitivity is limited to male SW mice, and not to any other strain or female SW, and is not the result of cytokine or IgE response.
Inbred balb/c and outbred J:DO had the best response against heroin in antinociception assay.
Males and females of the same strain have generally similar response to blockade of heroin-induced analgesia
Acknowledgements
This work was supported by the National Institute of Health under grant UH3DA041146.
Abbreviations:
- NHS
N-Hydroxysuccinimide
- PBS
phosphate buffered saline
- BSA
bovine serum albumin
- TT
tetanus toxoid
- CpG ODN
CpG oligodeoxynucleotides
- s.c.
subcutaneous
- i.p.
intraperitoneal
- SPR
surface plasmon resonance
- 6-AM
6-acetylmorphine
- J:DO
Jackson Diversity:Outbred
- SUD
substance use disorder
- SW
Swiss Webster
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- [1].Ohia-Nwoko O, Kosten TA, Haile CN. Animal Models and the Development of Vaccines to Treat Substance Use Disorders. Int Rev Neurobiol. 2016;126:263–91. [DOI] [PubMed] [Google Scholar]
- [2].Pravetoni M Biologics to treat substance use disorders: Current status and new directions. Human Vaccines & Immunotherapeutics. 2016;12:3005–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kosten T, Domingo C, Orson F, Kinsey B. Vaccines against stimulants: cocaine and MA. Br J Clin Pharmacol. 2014;77:368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brimijoin S, Shen X, Orson F, Kosten T. Prospects, promise and problems on the road to effective vaccines and related therapies for substance abuse. Expert Rev Vaccines. 2013;12:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Centers for Disease Control and Prevention 2018 Annual Surveillance Report of Drug-Related Risks and Outcomes — United States Surveillance Special Report Centers for Disease Control and Prevention, US Department of Health and Human Services; Published August 31, 2018. Accessed [November 11, 2018] from https://wwwcdcgov/drugoverdose/pdf/pubs/2018-cdc-drug-surveillance-reportpdf [Google Scholar]
- [6].McLellan A, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–95. [DOI] [PubMed] [Google Scholar]
- [7].Hoogsteder PHJ, Kotz D, van Spiegel PI, Viechtbauer W, van Schayck OCP. Efficacy of the nicotine vaccine 3′ -AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: a randomized placebo-controlled trial. Addiction. 2014;109:1252–9. [DOI] [PubMed] [Google Scholar]
- [8].Kosten TR, Rosen M, Bond J, Settles M, Roberts JSC, Shields J, et al. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002;20:1196–204. [DOI] [PubMed] [Google Scholar]
- [9].Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132 Suppl 1 :S26–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–31. [DOI] [PubMed] [Google Scholar]
- [11].Kest B, Wilson SG, Mogil JS. Sex differences in supraspinal morphine analgesia are dependent on genotype. Journal of Pharmacology and Experimental Therapeutics. 1999;289:1370–5. [PubMed] [Google Scholar]
- [12].Yoburn BC, Kreuscher SP, Inturrisi CE, Sierra V. Opioid receptor upregulation and supersensitivity in mice: Effect of morphine sensitivity. Pharmacology Biochemistry and Behavior. 1989;32:727–31. [DOI] [PubMed] [Google Scholar]
- [13].French JE, Gatti DM, Morgan DL, Kissling GE, Shockley KR, Knudsen GA, et al. Diversity Outbred Mice Identify Population-Based Exposure Thresholds and Genetic Factors that Influence Benzene-Induced Genotoxicity. Environmental health perspectives. 2015;123:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bremer PT, Schlosburg JE, Banks ML, Steele FF, Zhou B, Poklis JL, et al. Development of a Clinically-Viable Heroin Vaccine. Journal of the American Chemical Society. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hwang CS, Bremer PT, Wenthur CJ, Ho SO, Chiang S, Ellis B, et al. Enhancing Efficacy and Stability of an Antiheroin Vaccine: Examination of Antinociception, Opioid Binding Profile, and Lethality. Mol Pharm. 2018;15:1062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bremer PT, Schlosburg JE, Lively JM, Janda KD. Injection route and TLR9 agonist addition significantly impact heroin vaccine efficacy. Mol Pharm. 2014;11:1075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AAK, et al. Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci. 2013;110:9036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Karinen R, Andersen JM, Ripel A, Hasvold I, Hopen AB, Merland J, et al. Determination of heroin and its main metabolites in small sample volumes of whole blood and brain t i ssue by reversed-phase liquid chromatography-tandem mass spectrometry. Journal of analytical toxicology. 2009;33:345–50. [DOI] [PubMed] [Google Scholar]
- [19].Hwang CS, Ellis B, Zhou B, Janda KD. Heat shock proteins: A dual carrier-adjuvant for an anti-drug vaccine against heroin. Bioorganic & Medicinal Chemistry. 2018;27:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Smith LC, Lin L, Hwang CS, Zhou B, Kubitz DM, Wang H, et al. Lateral Flow Assessment and Unanticipated Toxicity of Kratom. Chem Res Toxicol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jalah R, Torres OB, Mayorov AV, Li F, Antoline JF, Jacobson AE, et al. Efficacy, but not antibody titer or affinity, of a heroin hapten conjugate vaccine correlates with increasing hapten densities on tetanus toxoid, but not on CRM197 carriers. Bioconjug Chem. 2015;26:1041–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sulima A, Jalah R, Antoline JFG, Torres OB, Imler GH, Deschamps JR, et al. A Stable Heroin Analogue That Can Serve as a Vaccine Hapten to Induce Antibodies That Block the Effects of Heroin and Its Metabolites in Rodents and That Cross-React Immunologically with Related Drugs of Abuse. Journal of Medicinal Chemistry. 2018;61:329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Matyas GR, Mayorov AV, Rice KC, Jacobson AE, Cheng K, Iyer MR, et al. Liposomes containing monophosphoryl lipid A: a potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine. 2013;31:2804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yoburn BC, Sierra V, Lutfy K. Simultaneous development of opioid tolerance and opioid antagonist-induced receptor upregulation. Brain Research. 1990;529:143–8. [DOI] [PubMed] [Google Scholar]
- [25].Ponce R Adverse consequences of immunostimulation. Journal of immunotoxicology. 2008;5:33–41. [DOI] [PubMed] [Google Scholar]
- [26].Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiology and molecular biology reviews : MMBR. 2012;76:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bugelski PJ, Achuthanandam R, Capocasale RJ, Treacy G, Bouman-Thio E. Monoclonal antibody-induced cytokine-release syndrome. Expert review of clinical immunology. 2009;5:499–521. [DOI] [PubMed] [Google Scholar]
- [28].Hoozemans JJM, Veerhuis R, Janssen I, Rozemuller AJM, Eikelenboom P. Interleukin-1β induced cyclooxygenase 2 expression and prostaglandin E2 secretion by human neuroblastoma cells: implications for Alzheimer’s disease. Experimental Gerontology. 2001. ;36:559–70. [DOI] [PubMed] [Google Scholar]
- [29].Claycomb RJ, Hewett SJ, Hewett JA. Neuromodulatory role of endogenous interleukin-1β in acute seizures: possible contribution of cyclooxygenase-2. Neurobiology of disease. 2012;45:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hwang CS, Smith LC, Natori Y, Ellis B, Zhou B, Janda KD. Efficacious Vaccine against Heroin Contaminated with Fentanyl. ACS Chem Neurosci. 2018;9:1269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, Janda KD. Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew Chem Int Ed Engl. 2016;55:3772–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gooyit M, Miranda PO, Wenthur CJ, Ducime A, Janda KD. Influencing Antibody-Mediated Attenuation of Methamphetamine CNS Distribution through Vaccine Linker Design. ACS Chem Neurosci. 2016;8:468–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kimishima A, Wenthur CJ, Zhou B, Janda KD. An Advance in Prescription Opioid Vaccines: Overdose Mortality Reduction and Extraordinary Alteration of Drug Half-Life. ACS Chemical Biology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yuill MB, Zee ML, Marcus D, Morgan DJ. Tolerance to the antinociceptive and hypothermic effects of morphine is mediated by multiple isoforms of c-Jun N-terminal kinase. Neuroreport. 2016;27:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


