Abstract
Objective
We aimed to review published literature on methicillin resistant S. aureus (MRSA) in the Asia Pacific Region to document MRSA prevalence in the Region and examine the impact of variability in study design on the reported data of MRSA prevalence.
Methods
We included studies that reported MRSA prevalence between 2000 and 2016 and excluded studies if they did not contain complete information on antibiotic susceptibility testing (AST) methods. Our primary outcomes were the proportion of MRSA isolates among S. aureus isolates (resistance proportion) or among individual samples (prevalence).
Findings
We included 229 studies in 19 countries/territories in this study. There was substantial heterogeneity in both outcomes (resistance proportion: I2 = 99·6%, prevalence: I2 = 99·8%), precluding pooled averages, and meta-regression analyses revealed that these variations were explained by country income status and participant characteristics but not methodological differences in AST. We also found no significant secular changes in MRSA prevalence or resistance proportions in the Asia Pacific.
Conclusion
The resistance proportions and prevalence of MRSA infections in the Asia Pacific is comparable to those reported in other regions with no significant secular changes in the past decade. Country income status and the characteristics of the sample population explained more variations in the reported resistance proportions and prevalence of MRSA than methodological differences in AST across locations in the Region.
Keywords: Antimicrobial resistance, methicillin-resistance, S. aureus, Asia-Pacific
1. INTRODUCTION
Antimicrobial resistance (AMR) is a major public health concern globally and methicillin resistant Staphylococcus aureus (MRSA) is one of the most important pathogens worldwide, accounting for over 80,000 severe infections in the United States alone in 2011 and more than half of hospital-related Staphylococcus aureus (S. aureus) infections in most Asian countries.1, 2 In particular, the spread of MRSA infections from healthcare settings to various community settings over recent decades has raised considerable concern.3 While the prevalence of methicillin resistance among invasive S. aureus infections is reported to be on the decline in Europe and the US,4, 5 resistance remains common in Asia, where self-medication with antibiotics is common.6
The Asia Pacific region is the most populous region in the world, with one-third of the world’s population.7 With rapid urbanization, a significant proportion of people in this region live in high-density cities, which increases the risk of the development and spread of antibiotic resistance.8 Since the 1980’s, methicillin resistance detection in S. aureus in healthcare settings within Asia has increased significantly, with regional detection proportions ranging from 26% to 73% in 2011.9 This poses significant health burden on healthcare systems in the region, especially in resource limited countries where S. aureus infection frequently presents as severe or invasive diseases.10 The overall mortality from S. aureus blood infection was 48% in a study conducted in northeast Thailand.11 which is almost double the mortality rate reported in a similar study in the United States.12 Although decreasing proportions of healthcare-associated MRSA (HA-MRSA) have been reported in Taiwan and Japan since 2000, community-associated MRSA (CA-MRSA) infections are increasingly detected in the region.13 As MRSA remains an important cause of nosocomial and community infections in the region, and antibiotic susceptibility patterns may differ between HA-MRSA and CA-MRSA strains, knowledge about their respective distributions in the population is important for the treatment and management of MRSA infections.14 Besides infections, there have also been increasing reports of MRSA carriage in various population groups in the region, including in young children and adults.6 While MRSA carriers do not experience clinical symptoms as a result of carriage, they may be at higher risk of MRSA infection especially in the event of hospitalization or major invasive procedures.15 Nevertheless, epidemiological trends in methicillin-resistance among S. aureus isolates are difficult to determine in the absence of established and standardized common surveillance protocols in the region.16
Variations in published measures of methicillin-resistance in S. aureus isolates also complicate the interpretability of MRSA detection data. Typically, levels of methicillin resistance in S. aureus within a selected population are measured using isolate-based screening, in which resistance is measured as a proportion of MRSA detected among S. aureus that are successfully isolated from individual samples. This strategy is used by most established AMR surveillance networks, including the European Antimicrobial Resistance Surveillance Network (EARS-Net) and the WHO Global Antimicrobial Resistance Surveillance System (GLASS).17, 18 However, as more laboratory methods (e.g. screening agars) were made available for the detection of MRSA directly from clinical specimens, some studies today report methicillin resistance levels using a sample-based approach, where resistance is measured as a proportion of MRSA detected from all clinical samples, including samples testing negative for S. aureus. While the isolate-based and the sample-based approaches measure methicillin resistance in S. aureus in different subsets of the population, MRSA resistance quantified with the two approaches is often compared with one another and described with non-standardized terms such as “resistance”, “resistance rates”, “incidence” and “prevalence”.19, 20
Therefore, in this systematic review, we sought to document methicillin resistance in S. aureus based on published literature in the Asia Pacific region. We paid particular attention to the surveillance metrics used, and examined the impact of variability in study participants, source of infection, and laboratory methods on MRSA prevalence.
2. METHODS
2.1. Search strategy
This systematic review was conducted in accordance with the PRISMA-P 2015 guidelines.21 We systematically searched literature published through 5 December 2017 on methicillin resistance in S. aureus reported in 41 countries and regions in the Asia Pacific Region using the bibliographic databases PubMed and Embase (Appendix). Search results were catalogued using bibliographic software (Endnote version X7, Clarivate Analytics), and a database was generated to manage article screening and evaluation. In this review, the Asia Pacific Region refers to countries and regions that comprise Eastern Asia, South-Eastern Asia, and Oceania according to the definitions provided by the United Nations Statistics Division.
2.2. Study selection
Three reviewers (WWL, JW and KN) independently screened titles, abstracts, and full texts of articles. Disagreements between the reviewers were resolved by the decision of a fourth author (PW). Studies were included in this review if they were original studies that met all of the following conditions: (1) assessed methicillin resistance in S. aureus isolated from clinical specimens collected from populations in the Asia Pacific Region, (2) assessed methicillin resistance in S. aureus isolates between 2000 and 2016, (3) expressed antibiotic resistance in proportions (%) of resistant organisms, (4) were written in English, and (5) had a full text accessible to the review team (Appendix). Studies were excluded if they were (1) randomized controlled trials, reviews, case studies, opinions, or multiple publications of the same dataset (only one publication per dataset was included), (2) studies that did not state laboratory methods or standards for susceptibility testing, (3) studies that did not specify individual drug or antibiotic names used for susceptibility testing, (4) studies that included data before year 2000 that could not be disaggregated from later data, and (5) studies in which the study period was not clearly defined. The third exclusion criteria was not applicable to studies that assessed methicillin resistance in S. aureus through automated AST methods or molecular and screening methods such as resistance gene identification with polymerase chain reaction (PCR) and biochemical MRSA screening tests.
2.3. Data extraction
We extracted the following data from each included study using a standardized form: author and year of publication, country, type of study, study period, sample population, participant age range, setting, source of infection, sampling site, sample size, laboratory methods, laboratory standards for antimicrobial susceptibility testing, whether unique patient isolates were used to avoid duplication of samples, and proportion of S. aureus resistant to methicillin, cefoxitin, oxacillin, or flucloxacillin out of total samples tested (prevalence) or S. aureus isolates tested (resistance proportion) (Appendix).
2.4. Data analysis
We first described the overall search results (i.e. resistance proportions and prevalence of MRSA) for all countries with available data. In this review, we define “MRSA prevalence” as the proportion of MRSA among all tested samples, and “MRSA resistance proportion” as the proportion of MRSA among all S. aureus isolates. Articles that reported resistance proportions and prevalence for different years, sampled populations, or source of infections had individual entries recorded for each year, population subgroup (e.g. inpatients and outpatients recorded separately), and source of infection (e.g. hospital-associated infections are recorded separately from community-associated infections) or carriage. MRSA prevalence and resistance proportions were double arcsine-transformed22 and combined using a Restricted Maximum-Likelihood (REML)-based random-effects model.23, 24 Statistical heterogeneity for MRSA prevalence and resistance proportion were assessed with the I2 statistic, the value of which indicates the proportion of variation in reported estimates across studies that can be attributed to heterogeneity between these studies rather than chance.25, 26 Meta-regression analyses were conducted using multivariable mixed-effect models for studies that reported MRSA prevalence and resistance proportions, and reports on MRSA carriage were analyzed separately from MRSA infections. Covariates of interest included country gross national income (GNI) per capita, study year, participant age group, AST method, sampling site, and the use of unique patient isolates to avoid duplication of samples. The influence of sample population on the prevalence and resistance proportions of MRSA carriage, and source of infection on the prevalence and resistance proportions of MRSA infections were also investigated. All data were visualized and analyzed in R version 3.4.1 (R Development Core Team, Vienna, Austria) with the metafor package,27 as well as the Quantum GIS Geographic Information System version 2.18.7 (QGIS, Open Source Geospatial Foundation Project).28
3. RESULTS
3.1. Study characteristics
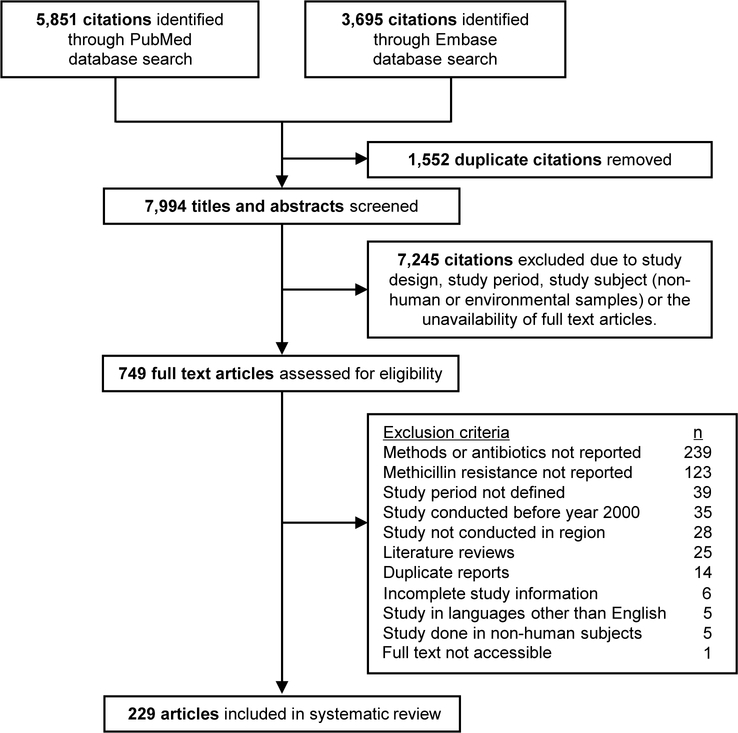
From the 9546 articles identified through our search on the PubMed and Embase databases, 7994 abstracts were screened, 749 full text articles were assessed, and 229 articles that reported MRSA prevalence and resistance proportions in 19 of the 41 selected countries/territories in the Asia Pacific region between year 2000 and 2016 were included (Figure 1). Most articles were excluded because they did not report methicillin resistance (n=123) or the methods and test antibiotics used in antibiotic susceptibility tests (n=240). Detailed study characteristics are available in the Appendix. Of the 229 included articles, 210 were published on or after year 2007 (Table 1). As MRSA prevalence and resistance proportions for different years, sample population, and source of infection in the same study were recorded as separate observations, 346 observations or data points were included in this review.
Figure 1.
Flow diagram of study selection.
Table 1.
Summary of the studies included in the analysis.
| Studies, n | |
|---|---|
| Total number of articles included | 229 |
| Publication year | |
| 2000 – 2006 | 19 |
| 2007 – 2011 | 59 |
| 2012 – 2017 | 151 |
| Observations, n | |
| Total number of observations includeda | 346 |
| Study setting | |
| Healthcare | 311 |
| Community | 25 |
| Healthcare and community (mixed) | 4 |
| Animal/livestock-related | 6 |
| Number of study sites | |
| Single site | 209 |
| 2 – 10 | 63 |
| > 10 or multiple sites | 74 |
| Sample population | |
| Inpatient | 146 |
| Outpatient | 53 |
| Healthy participants | 53 |
| Mixed | 66 |
| Not reported | 28 |
| Age group of study participants | |
| Children (0 – 17 years) | 39 |
| Adults (18 – 64 years) | 129 |
| Older adults (65 years and above) | 30 |
| All ages/not reported | 148 |
| Source of infection | |
| Hospital-associated | 53 |
| Community-associated | 60 |
| Hospital- and community-associated | 147 |
| Carriage | 83 |
| Livestock-associated | 3 |
| Measure of antibiotic resistance | |
| Resistance proportionb | 293 |
| Prevalencec | 216 |
| Laboratory method | |
| Agar dilution | 10 |
| Automated systems | 53 |
| Broth dilution | 70 |
| Chromogenic agar | 21 |
| Disk diffusion | 156 |
| Etest | 3 |
| Oxacillin agar test | 2 |
| PCR and/or molecular typing methods | 12 |
| Mixed | 19 |
| Antibiotics testedd | |
| Cefoxitin | 75 |
| Methicillin | 15 |
| Oxacillin | 226 |
| Flucloxacillin | 10 |
| More than one antibiotic tested | 26 |
| Not applicable | 45 |
| Sampling site | |
| Blood only | 47 |
| Respiratory tract only | 75 |
| Skin/Wound only | 24 |
| Mixed sites | 175 |
| Not reported | 25 |
The total number of articles included in this study differs from the total number of studies (observations) included because some articles include data for more than one country, year, sample population, or study setting.
Resistance proportion is defined as the proportion of methicillin-resistant S. aureus (MRSA) out of the total S. aureus isolates subjected to susceptibility testing for methicillin resistance.
Prevalence is defined as the proportion of MRSA out of the total number of specimens collected or patients recruited in the study.
Antibiotics used for susceptibility testing are not recorded for studies that use automated antibiotic susceptibility testing (AST) methods or genotyping and screening methods such as resistance gene identification with polymerase chain reaction (PCR) and biochemical (chromogenic) MRSA screening tests.
There were more reports of methicillin resistance as a proportion of MRSA isolates among S. aureus isolates (n=293) than among individual samples (n=216). Most observations were results from studies conducted in healthcare settings (n=311), among inpatients (n=146), and in single sites (n=209). More than 75% of the observations recorded in this review were reports of MRSA infections (n=263) and the remaining observations were of MRSA carriage (n=83). A substantial number of studies were conducted in adults (43%) while 33% of the studies included samples or isolates from individuals from all ages.
Almost half (45%) of included observations used the disk diffusion method for AST, and oxacillin was used as the test antibiotic in around two-thirds of these observations. Half of all included observations assessed antibiotic resistance of S. aureus isolated from various body sites, and the most common sampling sites were the respiratory tract (22%), blood (14%), and skin or wound (7%). The types of samples collected from the respiratory tract include sputum, nasal swabs, and throat swabs, and samples collected from the skin or wound include pus and swabs taken from sites such as the axilla and groin.
3.2. Estimates of MRSA prevalence and resistance proportions
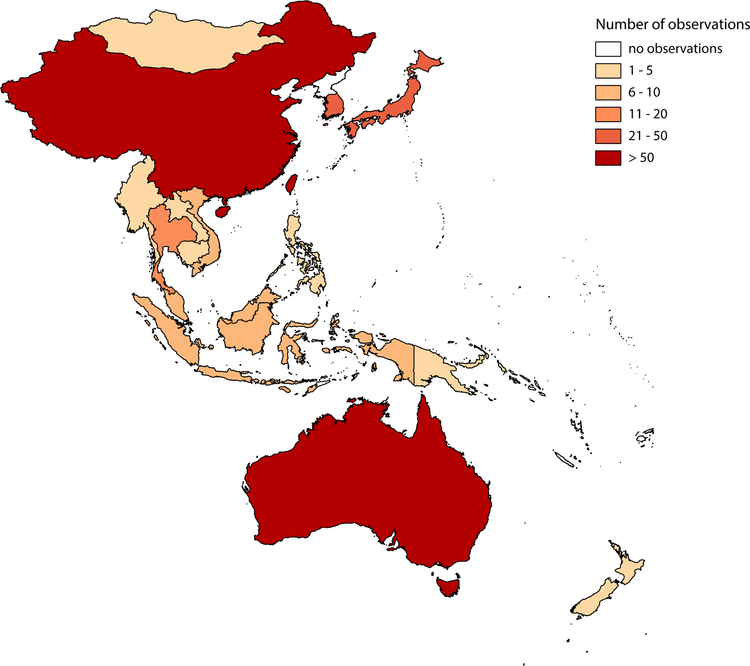
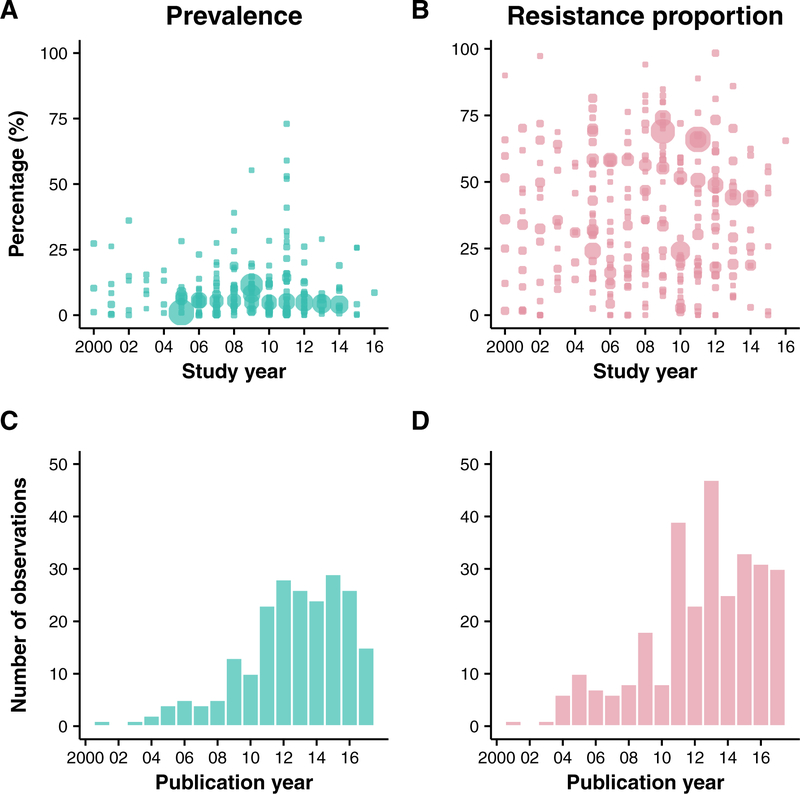
The most observations (prevalence and resistance proportions) between year 2000 and 2016 were reported from mainland China (n=74), Taiwan (n=64), Australia (n=62), South Korea (n=30), Japan (n=30), Hong Kong (n=20), and Thailand (n=15). 12 locations reported fewer than 10 observations each (Figure 2). Overall, the prevalence of MRSA infections in the 19 locations ranged from 0% to 73% between year 2000 and 2016 (Figure 3). The resistance proportions of MRSA infections ranged from 0% to 98·4% for the same period. The prevalence and resistance proportions of MRSA carriage ranged from 0% to 39·1% and 0% to 88·9% respectively. However, these proportions vary considerably between locations (Table 2).
Figure 2.
Number of observations reported for MRSA prevalence and resistance proportions in selected locations in the Asia Pacific (2000 – 2016).
Figure 3. MRSA prevalence and resistance proportions reported in Asia Pacific by year of publication.
Panel A. MRSA prevalence, defined as the proportion of MRSA among all tested samples, reported in selected countries between year 2000 and 2016. For studies that report prevalence for more than one year, the midpoint of the study is reported as the study year. Bubble sizes reflect the study sample size for each observation. Panel B. MRSA resistance proportion, defined as as the proportion of MRSA among all S. aureus isolates, reported in selected countries between year 2000 and 2016. For studies that report prevalence for more than one year, the midpoint of the study is reported as the study year. Bubble sizes reflect the study sample size for each observation. Panel C. Number of publications that report MRSA prevalence in selected countries in year 2000 – 2017. Panel D. Number of publications that report MRSA prevalence in selected countries in year 2000 – 2017.
Table 2.
Reported MRSA prevalence and resistance proportions by country (2000–2016)
| Prevalence (%) Range (min, max) |
Resistance proportion (%) Range (min, max) |
|||
|---|---|---|---|---|
| Country | Infections# | Carriage | Infections# | Carriage |
| American Samoa | 8.0* | - | 17.4* | - |
| Australia | 2.2, 26.0 | 1.21, 16.00 | 7.25, 82.50 | 3.10, 50.00 |
| Cambodia | - | 3.50, 4.10 | - | - |
| China | 0.5, 55.30 | 0, 10.50 | 2.62, 98.40 | 0, 47.83 |
| Hong Kong | 0.22, 28.00 | 0.52, 39.06 | 2.00, 84.80 | 1.12* |
| Indonesia | - | 6.04, 9.33 | - | 0, 21.43 |
| Japan | 0.70, 41.00 | 0.78, 30.20 | 24.35, 72.00 | 14.81, 88.89 |
| Korea | 0.58, 73.00 | 0, 36.10 | 10.59, 81.44 | 0, 71.92 |
| Laos | 0 | - | 0, 7.30) | - |
| Malaysia | 1.01, 32.00 | 0, 2.10 | 7.90, 60.00 | 0, 6.67 |
| Mongolia | - | - | 8.8* | - |
| Myanmar | 0.15, 2.97 | - | 4.35, 38.73 | - |
| New Zealand | 0.50, 9.00 | 8.2* | 1.54, 2.94 | 14.6* |
| Papua New Guinea | 2.6* | - | 75.0* | - |
| Philippines | 59.0* | - | 30.10, 80.00 | - |
| Singapore | 4.80, 52.00 | 1.79, 20.20 | 70.0* | - |
| Taiwan | 0, 29.00 | 0.60, 32.20 | 0, 97.30 | 5.26, 77.03 |
| Thailand | 0, 53.00 | 0, 3.60 | 0, 71.40 | 0, 6.67 |
| Vietnam | 0, 3.00 | 7.90, 8.59 | 0, 90.00 | 65.5* |
Infections include hospital-associated (HA), community-associated (CA), mixed (HA and CA), and livestock/animal-associated MRSA infections
Only one observation recorded
-: no data available
3.3. Factors that influence prevalence and resistance proportions of MRSA carriage and infections
A high degree of statistical heterogeneity was indicated in estimates of the MRSA prevalence and resistance proportion (prevalence: I2 = 99.83%, resistance proportion: I2 = 99.59%) using a restricted maximum-likelihood (REML) random-effects model. Meta-regression analyses were conducted to assess the potential association between the reported prevalence and resistance proportions of MRSA carriage and infections and study variables (including country grouped by income status (GNI per capita), study year, participant age group, AST method, sample population, source of infection, sampling site, and the use of unique patient isolates to avoid duplication of samples). Factors showing a statistically significant association with MRSA carriage or infections are presented in Tables 3 and 4, including country grouped by income status (GNI per capita), study year, and sample population groups.
Table 3.
Variables potentially associated with the prevalence and resistance proportion of MRSA carriage identified in the meta-regression analysis.
| Prevalence | Resistance proportion | |||
|---|---|---|---|---|
| β | 95% CI | β | 95% CI | |
| Study year | −0.002 | −0.011, 0.007 | 0.007 | −0.017, 0.031 |
| Age groupa | ||||
| Children (0 – 17 years) | Referent | Referent | ||
| Adults (18 – 64 years) | −0.022 | −0.010, 0.056 | −0.117 | −0.313, 0.079 |
| Older adults (≥ 65 years) | 0.097 | 0.002, 0.192 | 0.148 | −0.111, 0.407 |
| All ages/not reported | 0.019 | −0.073, 0.110 | −0.240 | −0.527, 0.046 |
| GNI per capitab | 0.021 | 0.003, 0.039 | 0.014 | −0.044, 0.072 |
| Population group | ||||
| Inpatients | Referent | Referent | ||
| Outpatients | −0.112 | −0.201, −0.022 | −0.151 | −0.403, 0.101 |
| Mixed | −0.103 | −0.306, 0.099 | 0.036 | −0.465, 0.536 |
| Healthy participants | −0.097 | −0.163, −0.030 | −0.225 | −0.423, −0.027 |
| Model characteristics | k=80, I2=98.06%, R2=24.71% | k=51, I2=97.44%, R2=14.74% | ||
The median or average of participants included in the study.
World Bank data for country gross national income per capita in 2016.79
Table 4.
Variables potentially associated with the prevalence and resistance proportion of MRSA infection identified in the meta-regression analysis.
| Prevalence | Resistance proportion | |||
|---|---|---|---|---|
| β | 95% CI | β | 95% CI | |
| Study year | 0.008 | −0.001, 0.017 | 0.001 | −0.007, 0.009 |
| Age groupa | ||||
| Children (0 – 17 years) | Referent | Referent | ||
| Adults (18 – 64 years) | 0.090 | −0.018, 0.199 | 0.038 | −0.084, 0.160 |
| Older adults (≥ 65 years) | 0.044 | −0.123, 0.211 | 0.046 | −0.132, 0.224 |
| All ages/not reported | 0.108 | −0.004, 0.220 | 0.026 | −0.100, 0.152 |
| GNI per capitab | 0.022 | 0.005, 0.039 | −0.024 | −0.041, −0.007 |
| Source of infection | ||||
| Hospital-associated | Referent | Referent | ||
| Community-associated | −0.011 | −0.112, 0.091 | −0.315 | −0.413, −0.217 |
| Hospital- or community-associated | 0.058 | −0.032, 0.148 | −0.100 | −0.183, −0.017 |
| Livestock/animal associated | −0.128 | −0.341, 0.086 | −0.417 | −0.716, −0.119 |
| Model characteristics | k=136, I2=99.84%, R2=10.86% | k=242, I2=99.47%, R2=21.47% | ||
The median or average of participants included in the study
World Bank data for country gross national income per capita in 201679
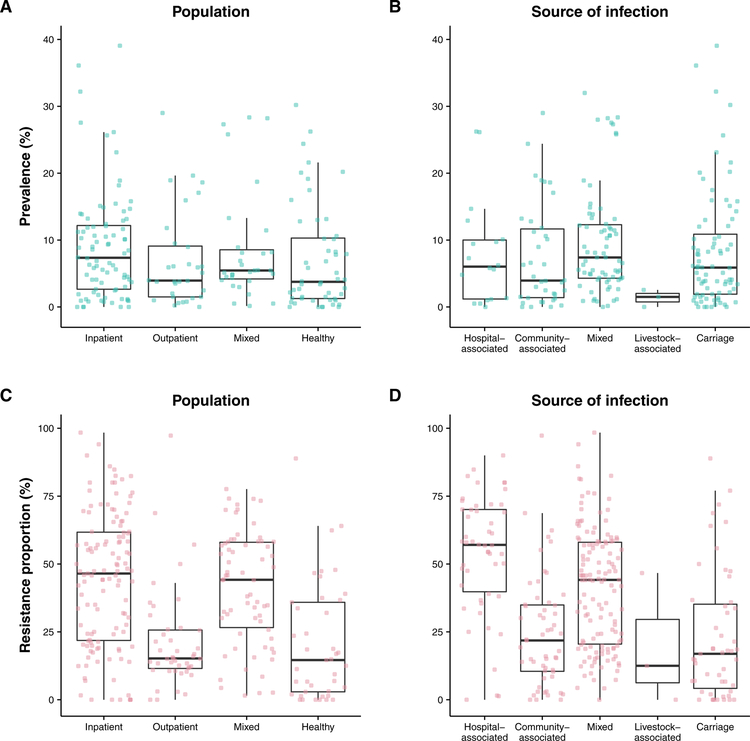
Meta-regression results show non-significant (p>0.05) temporal increases in the prevalence and resistance proportions of MRSA carriage and the resistance proportions of MRSA infections during the study period (2000 – 2016). However, temporal increases in prevalence for MRSA infections were statistically significant. In studies that reported MRSA carriage, we found significantly higher prevalence of MRSA in older adults compared with children (less than 18 years old), and significantly lower prevalence in outpatients and healthy participants compared with inpatients (Figure 4). GNI per capita was associated with increases in the prevalence of MRSA carriage and infections. However, this association was not significant for resistance proportion of MRSA carriage, and the association was significantly negative in the resistance proportion of MRSA infection. In studies that report MRSA infections, there were no statistically significant associations between the age group of participants and MRSA prevalence or resistance proportions. However, we found lower prevalence of MRSA infections in upper middle and lower middle-income countries compared with high income countries. Generally lower prevalence and resistance proportions of MRSA infections were reported in community-associated or livestock-associated infections compared with hospital-associated infections, although no statistically significant association was shown between the prevalence of MRSA infections and the source of infections.
Figure 4. MRSA prevalence and resistance proportion by source of infection and population segment.
Panel A. MRSA prevalence reported in selected countries by source of patients or persons sampled. Panel B. MRSA prevalence reported in selected countries by source of infection. Panel C. MRSA resistance proportion reported in selected countries by source of patients or persons sampled. Panel D. MRSA resistance proportion reported in selected countries by source of infection. MRSA prevalences and resistance proportions for studies with unknown source of infection or population groups are not included.
4. DISCUSSION
Methicillin resistance in S. aureus has been documented in the Asia Pacific region since the 1960’s shortly after methicillin became available for clinical use. While there has been an increase in published studies on methicillin resistance in S. aureus especially after the mid-2000s, there are substantial variations in approaches to measure resistance across the studies included in this review. As noted in previous narrative reviews, most studies are hospital-based, single-institution studies conducted predominantly in better-resourced countries in East and Southeast Asia.6, 13
Although a pooled average MRSA prevalence or resistance proportion could not be reliably estimated for the current review due to the substantial statistical heterogeneity across the included studies, the range of resistance proportions for MRSA infections and prevalence for MRSA carriage recorded in locations where data were available was consistent with previous reports.6, 9 The range of MRSA infections reported the Asia Pacific region in our study (0% - 98.4%) was comparable to resistance proportions reported in Europe (19·7% - 21.5%)18, 29 and the Middle East (12·4% - 30%),30 and lower than those reported in the United States (29% - 43.2%)31, 32 and Africa (16% - 55%).19 East Asian locations reported the highest resistance proportions (above 40%) in the region followed by South-east Asian locations (20% and 30%). MRSA prevalence reported in the Asia Pacific region are similar to those reported in other regions and are typically between 1% and 25%.33, 34
On average, we observed positive but mostly non-significant secular trends in MRSA prevalence and resistance proportions for MRSA infections or carriage in the past 16 years even when between-country differences and methodological variation such as laboratory methods and sampling sites were considered. As there is no consensus or a most appropriate metric to report levels of methicillin resistance in S. aureus infections or carriage, resistance proportions and prevalence of MRSA infections and carriage can be compared inappropriately. By definition, the prevalence of MRSA in a population, usually measured with sample-based approaches, will be lower than the resistance proportion among S. aureus isolates (usually measured with isolate-based strategies) as the former includes clinical samples that test negative for S. aureus. While prevalence and resistance proportions for MRSA infections measured by sample-based and isolate-based strategies are useful for surveillance purposes as they indicate the proportion of MRSA isolates detected in a specific population and S. aureus isolates respectively, this review indicates that these measures may be particularly vulnerable to differences in the demographic and health status of populations studied. The interpretability of either sample-based or isolate-based data is largely limited by the lack of relevance to patients and their clinical conditions.
Of the 229 studies included in this review, we observed substantial disparities in the demographic and health status of populations studied, screening and sampling policies, and study periods, similar to what Dulon et al. reported in an earlier review on MRSA prevalence in European healthcare settings,34 These factors, along with differences in study settings, AST methods and sampling sites, were often suggested as potential sources of heterogeneity in MRSA detection.34–36 This is consistent with findings from our meta-analysis, where study heterogeneity was found to account for more than 99% of the variance between study estimates of MRSA prevalence and resistance proportions.
Of all potential contributors to variability in MRSA carriage, estimates at the population level, relatively better-documented ones include age group,37, 38 and sample population groups based on health and admission status (i.e. inpatient, outpatient, and healthy people).39 In our study, we found MRSA carriage prevalence higher in adults 65 years or older compared with children below the age of 18 years. While existing literature on the differences in MRSA carriage among different age groups is inconsistent,40 the higher MRSA carriage prevalence in older adults reported in our study might be the result that most of the studies were conducted in long-term care facilities or nursing homes in the region.41–44 This is in contrast with MRSA carriage studies in children or adults, which were often conducted in community settings among healthy participants45–70. We also found MRSA carriage to be lower in outpatients and healthy participants compared with inpatients, which is congruent with the increased likelihood of exposure to resistant organisms in the hospital setting among inpatients.39
For MRSA infections, we did not find statistically significant differences in prevalence or resistance proportions across age groups. Previous studies also suggested an inconsistent pattern in MRSA infections among different age groups,71 with some studies reporting a higher prevalence of MRSA infections in older adults72 and others showing the prevalence of MRSA infections higher in children and young adults.73, 74 Nevertheless, our study suggested that MRSA resistance proportions of MRSA infections varied by source of infections. Consistent with previous studies, resistance proportions for CA-MRSA seem to be lower than HA-MRSA infections,6 reflecting the higher proportion of MRSA among S. aureus found in healthcare settings compared with community settings.
As S. aureus most commonly causes skin or soft tissue, respiratory tract, and bloodstream infections.75 Most studies included in this review reported prevalences and resistance proportions of MRSA infections in isolates sampled from one or more of these sites. We did not find statistically significant differences in prevalences or resistance proportions of MRSA infections in blood, respiratory tract, and skin or wound samples. An important caveat to this finding is that we did not record and consider the type of infection that is associated with the collection of samples for testing (e.g. blood samples collected from patients with respiratory infections are not differentiated from those collected from patients with bacteraemia).
Given the highly circumstantial nature of prevalence and resistance proportions measured by isolate-based and sample-based approaches, it may be useful to consider moving towards surveillance approaches (such as case-based surveillance) in which pathogens and resistance patterns identified through laboratory testing can be explicitly related to patients’ clinical conditions or diseases, which may provide data of clinical relevance to guide medical practice, and at the same time reduce data variability within the sample population. The levels of antibiotic resistance measured by isolate-based and sample-based strategies typically contain little information about the proportion of MRSA infections within a specific group of infections, such as skin infections or lower respiratory tract infections such as pneumonia. Studies using isolate-based measurement strategies tend to collect subsequent clinical samples for testing within a selected period while sample-based strategies tend to collect samples from groups defined by their health or hospitalization status.
In contrast to a recent study by Alvarez-Uria and colleagues that found a negative association between GNI per capita and methicillin resistance in S. aureus isolates,76 the prevalence of MRSA infections and carriage significantly increased with GNI per capita in our study. However, a significant negative association was found between GNI per capita and resistance proportions of MRSA infections. As Alvarez-Uria and colleagues included mostly data from European countries and only two countries from the Asia Pacific (Thailand and Australia),76 this may suggest that the differences in proportions of MRSA infections and carriage by country income status in Asia Pacific may be different than of Europe, in that better-resourced countries in Asia may have higher methicillin-resistance among S. aureus isolates compared with countries with less resources. However, unlike Europe, where countries with higher gross national income (GNI) per capita tend to have a lower rate of antibiotic consumption and better AMR surveillance and stewardship, this correlation is still unclear in Asia Pacific countries. Generally, better-resourced countries in Asia Pacific have more AMR surveillance and stewardship programs in place,16 but differences in antibiotic consumption between better and less well-resourced countries may be less apparent in the region compared to European countries77. For instance, in a systematic review by Morgan et al., better resourced countries in Europe reported less non-prescription use of antimicrobials in the general population than less well-resourced countries.77 This is in contrast with countries in Asia, where non-prescription use of antimicrobials in China was reported to be 36% compared with Indonesia (17%), India (18%), Vietnam (62%), and Bangladesh (86%).77
Our review has a few limitations. First, as our review was limited to data published on international bibliographic databases, the findings derived from our review may lack the insights of unpublished data from health authorities in the Asia Pacific region. However, the reported resistance proportions and prevalence of MRSA in the countries included in our study are generally comparable with data reported to the Global Antimicrobial Resistance Surveillance System.78 Secondly, the inclusion of only published literature written in English also excluded data that were presented in other languages. Although a formal quality assessment was not conducted on included studies, we have incorporated several quality-related exclusion criteria to exclude studies that are likely to be of lower quality. These include the lack of information on laboratory methods and clearly defined study periods.
In conclusion, the resistance proportions and prevalence of MRSA infections in the Asia Pacific is comparable to those reported in other regions. Although there appears to be no significant secular changes in prevalence or resistance proportions of MRSA infections and carriage in the Asia Pacific, our results highlight the greater influence of country income status and the characteristics of the sample population on these measures compared with methodological differences in AST and the need to compare and consider MRSA prevalence and resistance proportions separately as these are two distinct metrics.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Julie Au for technical assistance.
FUNDING
This study was financially supported by the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558).
Footnotes
COMPETING INTERESTS
BJC has received research funding from Sanofi Pasteur for a study of influenza vaccine effectiveness. The authors declare no other potential competing interests.
ETHICAL APPROVAL
Not required.
REFERENCES
- 1.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. [Internet]. 2013. [cited 2017 Nov 14]. Available from: https://www.cdc.gov/drugresistance/threat-report-2013/.
- 2.Jean S-S, Hsueh P-R. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011;37(4):291–5. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organisation. Global report on surveillance. Geneva: World Health Organisation; 2014. [Google Scholar]
- 4.European Centre for Disease Prevention and Control. Summary of the latest data on antibiotic resistance in the European Union: EARS-Net surveillance data. [Internet]. 2016. [cited 2017 Nov 15]. Available from: https://ecdc.europa.eu/sites/portal/files/documents/antibiotics-EARS-Net-summary-2016_0.pdf.
- 5.Klein EY, Mojica N, Jiang W, Cosgrove SE, Septimus E, Morgan DJ, et al. Trends in methicillin-resistant Staphylococcus aureus hospitalizations in the United States, 2010–2014. Clin Infect Dis. 2017;65(11):1921–3. [DOI] [PubMed] [Google Scholar]
- 6.Chen CJ, Huang YC. New epidemiology of Staphylococcus aureus infection in Asia. Clin Microbiol Infect. 2014;20(7):605–23. [DOI] [PubMed] [Google Scholar]
- 7.The World Bank. Population, total. [Internet] 2016. [cited 2018 Mar 15]. Available from: https://data.worldbank.org/indicator/SP.POP.TOTL.
- 8.Bruinsma N, Hutchinson JM, van den Bogaard AE, Giamarellou H, Degener J, Stobberingh EE. Influence of population density on antibiotic resistance. J Antimicrob Chemother. 2003;51(2):385–90. [DOI] [PubMed] [Google Scholar]
- 9.Mendes RE, Mendoza M, Banga Singh KK, Castanheira M, Bell JM, Turnidge JD, et al. Regional resistance surveillance program results for 12 Asia-Pacific nations (2011). Antimicrob Agents Chemother. 2013;57(11):5721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickerson EK, West TE, Day NP, Peacock SJ. Staphylococcus aureus disease and drug resistance in resource-limited countries in south and east Asia. Lancet Infect Dis. 2009;9(2):130–5. [DOI] [PubMed] [Google Scholar]
- 11.Nickerson EK, Wuthiekanun V, Day NP, Chaowagul W, Peacock SJ. Meticillin-resistant Staphylococcus aureus in rural Asia. Lancet Infect Dis. 2006;6(2):70–1. [DOI] [PubMed] [Google Scholar]
- 12.Fowler VG jr., Olsen MK, Corey GR, Woods CW, Cabell CH, Reller LB, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163(17):2066–72. [DOI] [PubMed] [Google Scholar]
- 13.Huh K, Chung DR. Changing epidemiology of community-associated methicillin-resistant Staphylococcus aureus in the Asia-Pacific region. Expert Rev Anti Infect Ther. 2016;14(11):1007–22. [DOI] [PubMed] [Google Scholar]
- 14.Fey PD, Said-Salim B, Rupp ME, Hinrichs SH, Boxrud DJ, Davis CC, et al. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47(1):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sollid JUE, Furberg AS, Hanssen AM, Johannessen M. Staphylococcus aureus: Determinants of human carriage. Infect Genet Evol. 2014;21(Supplement C):531–41. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization Regional Office for the Western Pacific Antimicrobial resistance in the Western Pacific Region: a review of surveillance and health systems response. Geneva: World Health Organization; 2015. [Google Scholar]
- 17.World Health Organization. Global antimicrobial resistance surveillance system (GLASS) report: early implementation 2016–2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 18.European Centre for Disease Prevention and Control Antimicrobial resistance surveillance in Europe 2009: Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: European Centre for Disease Prevention and Control; 2010. [Google Scholar]
- 19.Falagas ME, Karageorgopoulos DE, Leptidis J, Korbila IP. MRSA in Africa: Filling the global map of antimicrobial resistance. PLoS One. 2013;8(7):e68024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woolhouse M, Waugh C, Perry MR, Nair H. Global disease burden due to antibiotic resistance – state of the evidence. J Glob Health. 2016;6(1):010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann of Math Stat. 1950;21(4):607–11. [Google Scholar]
- 23.Kelley GA, Kelley KS. Statistical models for meta-analysis: A brief tutorial. World J Methodol. 2012;2(4):27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7(1):55–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J (Clin Res Ed). 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 27.Viechtbauer W Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36(3):48. [Google Scholar]
- 28.Quantum GIS Development Team. Quantum GIS Geographic Information System: Open Source Geospatial Foundation Project 2017. [cited 2017 Oct 16]. Available from: http://qgis.osgeo.org.
- 29.European Centre for Disease Prevention and Control. Summary of the latest data on antibiotic resistance in the European Union. [Internet]. 2017. [cited 2017 Nov 30]. Available from: https://ecdc.europa.eu/sites/portal/files/documents/EAAD%20EARS-Net%20summary.pdf.
- 30.Tokajian S New epidemiology of Staphylococcus aureus infections in the Middle East. Clin Microbiol Infect. 2014;20(7):624–8. [DOI] [PubMed] [Google Scholar]
- 31.Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Supplement 5):S344–S9. [DOI] [PubMed] [Google Scholar]
- 32.Haddadin A, Fappiano S, Lipsett P. Methicillin resistant Staphylococcus aureus (MRSA) in the intensive care unit. Postgrad Med J. 2002;78(921):385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarvis WR, Jarvis AA, Chinn RY. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at United States health care facilities, 2010. Am J Infect Control. 2012;40(3):194–200. [DOI] [PubMed] [Google Scholar]
- 34.Dulon M, Haamann F, Peters C, Schablon A, Nienhaus A. MRSA prevalence in European healthcare settings: a review. BMC Infect Dis. 2011;11:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohlmann R, Gatermann SG. Analysis and presentation of cumulative antimicrobial susceptibility test data – The influence of different parameters in a routine clinical microbiology laboratory. PLoS One. 2016;11(1):e0147965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kavanagh KT, Abusalem S, Calderon LE. The incidence of MRSA infections in the United States: is a more comprehensive tracking system needed? Antimicrob Resist Infect Control. 2017;6(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mainous AG 3rd, Hueston WJ, Everett CJ, Diaz VA. Nasal carriage of Staphylococcus aureus and methicillin-resistant S aureus in the United States, 2001–2002. Ann Fam Med. 2006;4(2):132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. 2008;197(9):1226–34. [DOI] [PubMed] [Google Scholar]
- 39.Zacharioudakis IM, Zervou FN, Ziakas PD, Mylonakis E. Meta-analysis of methicillin-resistant Staphylococcus aureus colonization and risk of infection in dialysis patients. J Am Soc Nephrol. 2014;25(9):2131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehraj J, Akmatov MK, Strömpl J, Gatzemeier A, Layer F, Werner G, et al. Methicillin-sensitive and Methicillin-resistant Staphylococcus aureus nasal carriage in a random sample of non-hospitalized adult population in northern Germany. PLoS One. 2014;9(9):e107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan TC, Cheng VC, Hung IF, Chan FH, Ng WC, Yuen KY. The association between methicillin resistant staphylococcus aureus colonization and mortality in Chinese nursing home older adults: a 2-year prospective cohort. J Am Med Dir Assoc. 2015;16(9):796–7. [DOI] [PubMed] [Google Scholar]
- 42.Cheng VC, Tai JW, Wong ZS, Chen JH, Pan KB, Hai Y, et al. Transmission of methicillin-resistant Staphylococcus aureus in the long term care facilities in Hong Kong. BMC Infect Dis. 2013;13:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho PL, Lai EL, Chow KH, Chow LS, Yuen KY, Yung RW. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in residential care homes for the elderly in Hong Kong. Diagn Microbiol Infect Dis. 2008;61(2):135–42. [DOI] [PubMed] [Google Scholar]
- 44.Tsao FY, Kou HW, Huang YC. Dissemination of methicillin-resistant Staphylococcus aureus sequence type 45 among nursing home residents and staff in Taiwan. Clin Microbiol Infect. 2015;21(5):451–8. [DOI] [PubMed] [Google Scholar]
- 45.Chan KS, Ling ML, Hsu LY, Tan AL. Methicillin-resistant Staphylococcus aureus throat colonization among healthcare workers during an outbreak in Singapore General Hospital. Infect Control Hosp Epidemiol. 2009;30(1):95–7. [DOI] [PubMed] [Google Scholar]
- 46.Chang CJ, Chen NC, Lao CK, Huang YC. Nasal Staphylococcus aureus and Methicillin-resistant S. aureus carriage among janitors working in hospitals in northern Taiwan. PLoS One. 2015;10(9):e0138971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen CJ, Hsu KH, Lin TY, Hwang KP, Chen PY, Huang YC. Factors associated with nasal colonization of methicillin-resistant Staphylococcus aureus among healthy children in Taiwan. J Clin Microbiol. 2011;49(1):131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen CS, Chen CY, Huang YC. Nasal carriage rate and molecular epidemiology of methicillin-resistant Staphylococcus aureus among medical students at a Taiwanese university. Int J Infect Dis. 2012;16(11):e799–803. [DOI] [PubMed] [Google Scholar]
- 49.Chen B, Dai X, He B, Pan K, Li H, Liu X, et al. Differences in Staphylococcus aureus nasal carriage and molecular characteristics among community residents and healthcare workers at Sun Yat-Sen University, Guangzhou, Southern China. BMC Infect Dis. 2015;15:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen BJ, Xie XY, Ni LJ, Dai XL, Lu Y, Wu XQ, et al. Factors associated with Staphylococcus aureus nasal carriage and molecular characteristics among the general population at a medical college campus in Guangzhou, south China. Ann Clin Microbiol Antimicrob. 2017;16(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng JJ, Xiao GG, Zhu Y, Zhou W, Wan CM. Staphylococcus aureus nasal carriage and its antibiotic resistance profiles in Tibetan school children in southwest China. HK J Paediatr. 2014;19(2):75–8. [Google Scholar]
- 52.Gong Z, Shu M, Xia Q, Tan S, Zhou W, Zhu Y, et al. Staphylococcus aureus nasal carriage and its antibiotic resistance profiles in children in high altitude areas of southwestern China. Arch Argent Pediatr. 2017;115(3):274–6. [DOI] [PubMed] [Google Scholar]
- 53.Ho PL, Lai EL, Chow KH. Carriage of meticillin-susceptible and -resistant Staphylococcus aureus by medical students in Hong Kong. J Hosp Infect. 2015;91(2):184–5. [DOI] [PubMed] [Google Scholar]
- 54.Huang YC, Su LH, Chen CJ, Lin TY. Nasal carriage of methicillin-resistant Staphylococcus aureus in school children without identifiable risk factors in northern Taiwan. Pediatr Infect Dis J. 2005;24(3):276–8. [DOI] [PubMed] [Google Scholar]
- 55.Huang YC, Su LH, Lin TY. Nasal carriage of methicillin-resistant Staphylococcus aureus among pediatricians in Taiwan. PLoS One. 2013;8(11):e82472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitti T, Boonyonying K, Sitthisak S. Prevalence of methicillin-resistant Staphylococcus aureus among university students in Thailand. Southeast Asian J Trop Med Public Health. 2011;42(6):1498–504. [PubMed] [Google Scholar]
- 57.Kuroda T, Kinoshita Y, Niwa H, Shinzaki Y, Tamura N, Hobo S, et al. Meticillin-resistant Staphylococcus aureus colonisation and infection in thoroughbred racehorses and veterinarians in Japan. Vet Rec. 2016;178(19):473. [DOI] [PubMed] [Google Scholar]
- 58.Ma XX, Sun DD, Wang S, Wang ML, Li M, Shang H, et al. Nasal carriage of methicillin-resistant Staphylococcus aureus among preclinical medical students: epidemiologic and molecular characteristics of methicillin-resistant S. aureus clones. Diagn Microbiol Infect Dis. 2011;70(1):22–30. [DOI] [PubMed] [Google Scholar]
- 59.Mat Azis N, Pung HP, Abdul Rachman AR, Amin Nordin S, Sarchio SNE, Suhaili Z, et al. A persistent antimicrobial resistance pattern and limited methicillin-resistance-associated genotype in a short-term Staphylococcus aureus carriage isolated from a student population. J Infect Public Health. 2017;10(2):156–64. [DOI] [PubMed] [Google Scholar]
- 60.Qu F, Cui E, Guo T, Li H, Chen S, Liu L, et al. Nasal colonization of and clonal transmission of methicillin-susceptible Staphylococcus aureus among Chinese military volunteers. J Clin Microbiol. 2010;48(1):64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Syafinaz AM, Nur Ain NZ, Nadzirahi SN, Fatimah JS, Shahram A, Nasir MD. Staphylococcus aureus Nasal Carriers Among Medical Students in A Medical School. Med J Malaysia. 2012;67(6):636–8. [PubMed] [Google Scholar]
- 62.Tang CS, Wang CC, Huang CF, Chen SJ, Tseng MH, Lo WT. Antimicrobial susceptibility of Staphylococcus aureus in children with atopic dermatitis. Pediatr Int. 2011;53(3):363–7. [DOI] [PubMed] [Google Scholar]
- 63.Treesirichod A, Hantagool S, Prommalikit O. Nasal carriage and antimicrobial susceptibility of Staphylococcus aureus among medical students at the HRH Princess Maha Chakri Sirindhorn Medical Center, Thailand: a cross sectional study. J Infect Public Health. 2013;6(3):196–201. [DOI] [PubMed] [Google Scholar]
- 64.McDonald LC, Lauderdale TL, Shiau YR, Chen PC, Lai JF, Wang HY, et al. The status of antimicrobial resistance in Taiwan among Gram-positive pathogens: the Taiwan Surveillance of Antimicrobial Resistance (TSAR) programme, 2000. Int J Antimicrob Agents. 2004;23(4):362–70. [DOI] [PubMed] [Google Scholar]
- 65.Tsai MS, Chen CJ, Lin TY, Huang YC. Nasal methicillin-resistant Staphylococcus aureus colonization among otherwise healthy children aged between 2 months and 5 years in northern Taiwan, 2005–2010. J Microbiol Immunol Infect. 2017. [DOI] [PubMed] [Google Scholar]
- 66.Verwer PE, Robinson JO, Coombs GW, Wijesuriya T, Murray RJ, Verbrugh HA, et al. Prevalence of nasal methicillin-resistant Staphylococcus aureus colonization in healthcare workers in a Western Australian acute care hospital. Eur J Clin Microbiol Infect Dis. 2012;31(6):1067–72. [DOI] [PubMed] [Google Scholar]
- 67.Wang HK, Huang CY, Chen CJ, Huang YC. Nasal Staphylococcus aureus and methicillin-resistant Staphylococcus aureus carriage among college student athletes in northern Taiwan. J Microbiol Immunol Infect. 2017;50(4):537–40. [DOI] [PubMed] [Google Scholar]
- 68.Williamson DA, Ritchie S, Keren B, Harrington M, Thomas MG, Upton A, et al. Persistence, discordance and diversity of staphylococcus aureus nasal and oropharyngeal colonization in school-aged children. Pediatr Infect Dis J. 2016;35(7):744–8. [DOI] [PubMed] [Google Scholar]
- 69.Yan X, Song Y, Yu X, Tao X, Yan J, Luo F, et al. Factors associated with Staphylococcus aureus nasal carriage among healthy people in northern China. Clin Microbiol Infect. 2015;21(2):157–62. [DOI] [PubMed] [Google Scholar]
- 70.Ye X, Liu W, Fan Y, Wang X, Zhou J, Yao Z, et al. Frequency-risk and duration-risk relations between occupational livestock contact and methicillin-resistant Staphylococcus aureus carriage among workers in Guangdong, China. Am J Infect Control. 2015;43(7):676–81. [DOI] [PubMed] [Google Scholar]
- 71.Ajao AO, Harris AD, Johnson JK, Roghmann MC, Perencevich EN, Schweizer ML, et al. Association between methicillin-resistant Staphylococcus aureus colonization and infection may not differ by age group. Infect Control Hosp Epidemiol. 2013;34(1):93–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elixhauser A, Claudia Steiner M. Infections with Methicillin-Resistant Staphylococcus Aureus (MRSA) in U.S. Hospitals, 1993–2005: Statistical Brief #35 2007. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US) Available from: https://www.ncbi.nlm.nih.gov/books/NBK61977/. [PubMed] [Google Scholar]
- 73.Ray GT, Suaya JA, Baxter R. Trends and characteristics of culture-confirmed Staphylococcus aureus infections in a large U.S. integrated health care organization. J Clin Microbiol. 2012;50(6):1950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khanal LK, Jha BK. Prevalence of methicillin resistant Staphylococcus aureus (MRSA) among skin infection cases at a hospital in Chitwan, Nepal. Nepal Med Coll J. 2010;12(4):224–8. [PubMed] [Google Scholar]
- 75.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG, Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alvarez-Uria G, Gandra S, Laxminarayan R. Poverty and prevalence of antimicrobial resistance in invasive isolates. Int J Infect Dis. 2016;52(Supplement C):59–61. [DOI] [PubMed] [Google Scholar]
- 77.Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis. 2011;11(9):692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Global Antimicrobial Resistance Surveillance System (GLASS). GLASS country profiles. [Internet]. 2018. [cited 2018 August 9]; 2018(August 9). Available from: http://apps.who.int/gho/tableau-public/tpc-frame.jsp?id=2004.
- 79.The World Bank. Gross national income per capita 2016, Atlas method and PPP. [Internet]. 2016. [cited 2018 Mar 16]. Available from: http://databank.worldbank.org/data/download/GNIPC.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.