Abstract
Protein homeostasis is an emerging component of schizophrenia (SZ) pathophysiology. Proteomic alterations in SZ are well-documented and changes in transcript expression are frequently not associated with changes in protein expression in SZ brain. The underlying mechanism driving these changes remains unknown, though altered expression of ubiquitin proteasome system (UPS) components have implicated protein degradation. Previous studies have been limited to protein and transcript expression, however, and do not directly test the function of the proteasome. To address this gap in knowledge, we measured enzymatic activity associated with the proteasome (chymotrypsin-, trypsin-, and caspase-like) in the superior temporal gyrus (STG) of 25 SZ and 25 comparison subjects using flourogenic substrates. As localization regulates which cellular processes the proteasome contributes to, we measured proteasome activity and subunit expression in fractions enriched for nucleus, cytosolic, and membrane compartments. SZ subjects had decreased trypsin-like activity in total homogenate. This finding was specific to the nucleus-enriched fraction and was not associated with changes in proteasome subunit expression. Interestingly, both chymotrypsin-like activity and protein expression of 19S RP subunits, which facilitate ubiquitin-dependent degradation, were decreased in the cytosol-enriched fraction of SZ subjects. Intracellular compartment-specific proteasome dysfunction implicates dysregulation of protein expression both through altered ubiquitin-dependent degradation of cytosolic proteins and regulation of protein synthesis due to degradation of transcription factors and transcription machinery in the nucleus. Together, these findings implicate proteasome dysfunction in SZ, which likely has a broad impact on the proteomic landscape and cellular function in the pathophysiology of this illness.
Introduction
Abnormal protein homeostasis, collectively called proteostasis, is an emerging component of schizophrenia (SZ) pathophysiology. Proteomics, together with studies on individual proteins, provide a growing body of evidence for proteostasis dysregulation in SZ1. Intriguingly, analyses examining both transcript and protein expression in postmortem brain within the same subjects repeatedly demonstrate that changes in transcript expression are not predictive of protein expression and vice versa2–34. Why this occurs is unknown, but proteostasis regulatory mechanisms including epigenetics, non-coding RNA, and altered protein translation are increasingly investigated to address this gap in knowledge35–37. These pathways focus on regulation of protein synthesis, but overlook the crucial role of protein degradation. The proteasome, a complex that regulates the proteome through protein degradation, is well-placed to impact abnormalities in SZ.
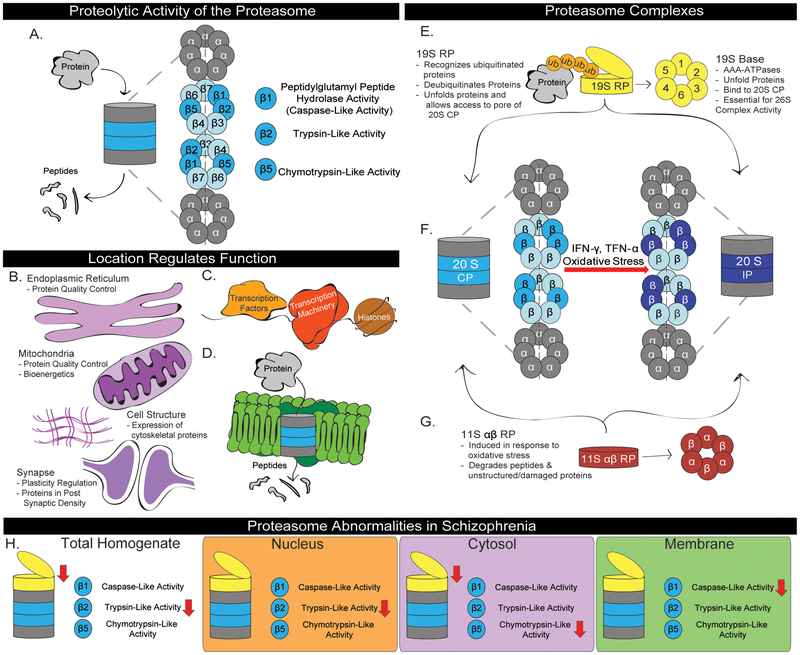
The ubiquitin proteasome system (UPS) facilitates proteostasis maintenance. It is initiated upon ubiquitin attachment, as either a monomer or polymeric chain, to substrate proteins38. Ubiquitination effects substrate localization and/or function, but is best known for targeting substrates to proteasomes39. The proteasome is a large, multicatalytic complex responsible for the majority of intracellular protein degradation38, 40. It is comprised of a core particle (CP), which performs proteolytic activity, and regulatory particles (RP) that facilitate access to the core and determine substrate specificity38(Fig.1). Distinct proteasome populations interact with different cellular processes including protein quality control, cellular bioenergetics, and cellular stress responses in the cytosol, and regulation of transcription in the nucleus39(Fig.1). Proteasomes, therefore, have not only an essential role in protein degradation but also in protein synthesis. Additionally, recent work has identified a novel neuron-specific population of proteasomes localized to extracellular membranes which degrade intracellular proteins and release peptides that appear to modulate neurotransmission in the extracellular space41(Fig.1). As such, both localization and complex expression are critical components to understanding proteasome function and impact on the cell.
Figure 1. Proteasome Function and Regulation in Schizophrenia.
Structure and function of proteasome complexes are described, including the (A) 20S CP, (E) 19S RP, (F) Immunoproteasome, and (G) 11S αβ RP. (A) In the CP, three β subunits have proteolytic activity that facilitates protein degradation through a series of cleavage events, resulting in the production of peptides38. (B-D) The proteasome interacts with various cellular processes depending on where it is in the cell. (B) In the cytosol, the proteasome regulates protein quality control, bioenergetics, cell structure and synaptic plasticity through degradation of key proteins39. (C) In the nucleus, the proteasome degrades transcription factors, removes stalled transcription machinery, and clears misfolded/damaged histones39. (D) In neurons, the proteasome associates with the membrane through interactions with transmembrane proteins41. This allows it to degrade intracellular proteins and export peptides into the extracellular space where they can interact with neurotransmitter receptors and modulate neurotransmission41. (F-G) Cellular stress is known to recruit both (F) inducible catalytic subunits that replace constituitive catalytic subunits to create the immunoproteasome and (G) the cytosolic 11S αβ RP86. (H) Abnormalities in proteasome expression and activity observed in the STG of subjects with schizophrenia. Previously, decreased expression of 19S RP subunits (Rpt1, Rpt3, and Rpt6) has been observed62 and in the current study we detected decreased trypsin-like activity in total homogenate. In fractions enriched for markers of the nucleus, cytosol, and cellular membranes we observed distinct proteasome expression and activity. Specifically, we observed decreased trypsin-like activity in the nucleus-enriched fraction, decreased chymotrypsin-like activity and 19S RP AAA-ATPase expression in the cytosol-enriched fraction, and decreased caspase-like activity in the cellular membrane-enriched fraction.
Proteasomes contribute to aspects of neural function known to be abnormal in SZ, including neurodevelopmental processes, dendritic morphology and maintenance, and bioenergetic homeostasis42–48. Additionally, NMDA receptor activity and dopaminergic signaling, pathways known to be dysregulated in SZ, influence proteasome activity, expression and localization49–51. As a cellular process connected to all of these systems, the proteasome is well-placed to both be impacted by and contribute to dysfunction in SZ.
Abnormal UPS expression in SZ supports a role for UPS dysregulation in this illness. A pathway-wide association study on single-nucleotide polymorphisms that increase risk of SZ identified ubiquitin-mediated proteolysis as a top pathway52. cDNA microarrays demonstrate abnormal transcript expression of enzymes that facilitate ubiquitin addition and removal, and proteasome subunits, in multiple brain regions53–57. UPS expression in blood is correlated with severity of positive symptoms in SZ patients58, 59. Neocortical protein expression of enzymes responsible for adding and removing ubiquitin is abnormal in SZ and in a neurodevelopmental phencyclidine mouse model of SZ at multiple developmental time points60, 61. Additionally, we have observed decreased protein expression of free ubiquitin and ubiquitinated proteins, as well as reduced expression of RP proteasome subunits, including the 19S AAA-ATPases Rpt1, Rpt3, Rpt6 and 11S RP α, in the superior temporal gyrus (STG) of SZ subjects60, 62. The STG is a brain region associated with SZ, and especially auditory hallucinations. Imaging studies have demonstrated that the STG is active during auditory hallucinations, and stimulation of the STG, either directly or in epilepsy, is sufficient to induce auditory hallucinations63–70. Reduced STG volume is frequently observed in SZ subjects, and has been repeatedly inversely associated with severity of auditory hallucinations71. Cortical volume reductions in SZ are believed to be due to reduced neuropil, including decreased soma size, dendritic arborization, and dendritic spine density72, however further characterization of molecular abnormalities in this region is necessary to understand the mechanisms underlying STG dysfunction in SZ. These previous studies suggest reduced utilization of ubiquitin to target proteins to the proteasome and expression of proteasome complexes that mediate ubiquitin- and ATP-dependent degradation. Therefore, we hypothesized that proteasome activity is decreased in the STG of SZ subjects. As localization of proteasomes determines function, we predicted changes in proteasome activity and expression may be specific to intracellular compartments. We sought to directly test these predictions by measuring proteasome activity and expression in tissue homogenate from the STG of subjects with SZ, and in subcellular fractions enriched for cytosol-, nucleus-, and membrane-associated markers.
MATERIALS AND METHODS
Subjects
Postmortem brain tissue from 25 subjects diagnosed with schizophrenia based on DSM-III-R criteria and 25 comparison subjects was obtained from the Mount Sinai/Bronx Veterans Administration (VA) Medical Center Department of Psychiatry Brain Collection through the NIH Neurobiobank (Supplemental Table 1). Assessment, consent, and postmortem procedures were performed for all subjects as has been previously described73, 74. Neurodegenerative disorders were ruled out by pathologic examination, and subjects had no history of alcoholism and/or substance abuse. We performed a power analysis, based on our previous experience with protein measures in postmortem tissue in SZ and a pilot study on activity assays in mice, to determine the sample size necessary to detect an effect size of ≥ 0.4 (α = 0.05, β = 0.02). As the number of subjects between groups was equal we performed data analyses assuming equal variance. Samples were coded to allow for experimenter blinding until data analysis. Randomization is not applicable in our design.
Tissue Preparation
Samples were obtained at autopsy, sliced into 0.8–1.0 cm slabs in the coronal plane, dissected into 1-cm3 cubes, and stored at −80°C until use73, 74. The samples used in this study were specifically dissected from the full thickness of gray matter of the left STG (BA22). Samples were manually homogenized using a glass tissue grinder in 6 µL/mg of Proteasome Homogenization Buffer (PHB; Proteasome Buffer (50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 250 µM DTT, 5 mM MgCl2, 2 mM ATP) containing 10% glycerol and phosphatase inhibitors (PhosSTOP, Roche Diagnostics, Manheim, Germany). Samples were then incubated on ice for 20 min, vortexing every 5 min. Following homogenization, samples were either stored at −80°C (Total homogenate) or fractionated into Nucleus-, Cytosol-, and Membrane-Enriched fractions. Optimization experiments and control studies used neocortical tissue from healthy control brains from the Alabama Brain Collection (ABC)75.
Intracellular Compartment Fractionation
Sequential centrifugation steps were used to create fractions enriched for specific intracellular compartments. An initial low speed spin (510xg, 5 min) was performed directly after tissue was homogenized. The supernatant (S1) was transferred to 11×35 mm Konical polypropylene centrifuge tubes (Beckman Coulter, Brea, CA, USA) for further processing, while the pellet (P1) was solubilized in 5 µL/mg of PHB to create a Nucleus-Enriched Fraction (NEF) and stored at −80°C until use. S1 was ultracentrifugated at 100,000xg (27,300 rpm; SW-60Ti rotor) for 1 h at 4°C, after which the resulting supernatant (S2) was removed and retained as a Cytosol-Enriched Fraction (CEF), and the pellet (P2) was solubilized in 2.5 µL/mg of PHB to create a Membrane-Enriched Fraction (MEF). Protein concentration of Total Homogenate and all fraction samples were assessed using a BCA assay kit (Thermo Fisher Scientific). Homogenization and fractionation of cortical tissue from an ABC subject was performed alongside the STG samples and was used as a control for intra-assay variation between separate plates and/or gels throughout the study.
Rodent Studies
We modeled PMI conditions typically seen at the time of death in humans in female 3–7 week old C57BL/6 mice. After decapitation, brains were either immediately frozen in dry ice (Hour 0, n = 3), or held at 4⁰C for progressively longer times (1, 4, 10 hours, n = 4), and then transferred to −80⁰C for storage.
Seven of the 25 subjects with SZ were off antipsychotic medication for at least 6 weeks (Median = 23.9 weeks, SD = 55.9 weeks), and the remaining subjects were on typical antipsychotics (Supplemental Table 1). Testing for the effect of antipsychotic treatment within this cohort is therefore underpowered, so we chose to model chronic antipsychotic treatment using a rodent model. Male Sprague-Dawley rats (250 g) were housed in pairs for 9 months, during which they were treated with either haloperidol decanoate (28.5 mg/kg, n = 10) or vehicle (sesame oil, n = 10) every 3 weeks, for a total of 12 intramuscular injections. The dose/duration of haloperidol treatment was chosen based on previous analysis of antipsychotic treatment in rats76, 77. The animals were killed by decapitation and the brains immediately removed, dissected on wet ice, snap frozen on dry ice, and stored at −80° C. All rodent tissue was homogenized as described above. These studies were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Sample sizes for these studies were based on power analysis from previous experiments, samples were coded for experimenter blinding until data analysis, and treatment group was randomized.
Proteasome Activity Assays
For all proteasome activity assays, 20 µg of sample was loaded in triplicate into Costar® polysterene non-treated black 96-well assay plates (Corning Incorporated, Kennebunk, ME, USA) followed by 100 µL/well of assays for chymotrypsin-like activity (CTLA; 100 µM Leu-Leu-Val-Tyr-7-Amino-4-methylcoumarin (LLVY-AMC), Sigma-Aldrich, St. Louis, MO, USA, Cat# S6510), trypsin-like activity (TLA; 100 µM Arg-Leu-Arg-AMC (RLR-AMC), EnzoLifesciences, Farmingdale, NY, USA; Cat# BML-AW9785–0005), or caspase-like activity (CLA; 200 µM Leu-Leu-Glu-AMC (LLE-AMC), EnzoLifesciences, Farmingdale, NY, USA; Cat# BML-ZW9345–0005). A Synergy HT plate reader (BioTek, Winooski, VT, USA) was used to measure fluorescence (Emission - Center Wavelength (CW): 360 nm, Full Width at Half Maximum (FWHM): 40 nm; Excitation – CW: 460 nm, FWHM: 40 nm) every 10 minutes for 4 hours while plates were kept at 37°C. Each plate contained a fraction-matched ABC subject for inter-plate normalization when measuring proteolytic activity in the SZ and comparison subjects.
To confirm these assays measured proteasome-specific enzymatic activity, control experiments were performed with ABC tissue that tested assay sensitivity to denaturing conditions (2% SDS, 8 h incubation at 100°C) and the proteasome-specific inhibitor Lactacystin (120 µM) (Figure 2A, 3C-F). After plating, samples were incubated at room temperature (RT) for 1 h before measuring proteolytic activity (Fig.2A, Fig.3C). An additional control experiment was performed in compartment-enriched fractions to assess the density at which proteolytic activity was enriched. To do this, we prepared an 8–32% continuous gradient by layering 1.5 mL of 8% glycerol on top of 1.5 mL of 32% glycerol in a 14 × 89 mm Beckman polyammor ultracentrifuge tube. The tubes were then covered in parafilm, set on their side, and incubated at RT for 2.5 h. 500 µg of tissue was layered on top of the gradient and samples underwent ultracentfiguation at 130,000xg (31,000 rpm; SW-60Ti rotor) for 22 h at 4°C. After ultracentrifugation, 200 µL of each sample was sequentially removed to make glycerol gradient fractions. Activity assays were then performed on all fractions, using a fixed volume of tissue proportional to a total of either 20 µg (CEF, MEF) or 30 µg (Total Homogenate, NEF) of initial input (Fig.3D-F).
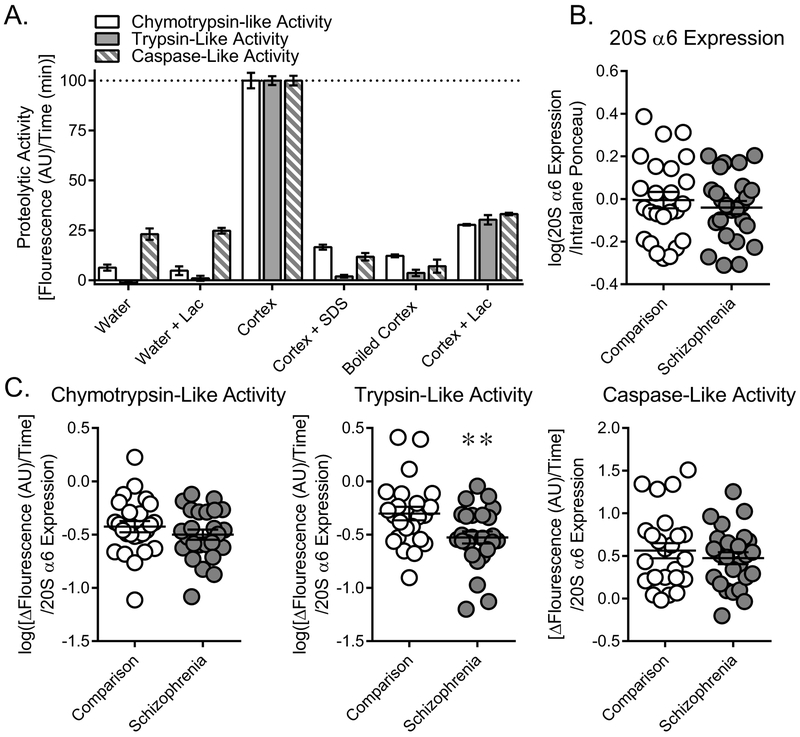
Figure 2. Trypsin-like activity is decreased in schizophrenia.
Proteasome activity in the STG of comparison and SZ subjects. (A) Flourogenic substrates specific to chymotrypsin- (LLVY-AMC), trypsin- (RLR-AMC), and caspase-like (LLE-AMC) activity were used to measure proteasome activity in human cortex. These assays were sensitive to denaturing conditions (“Boiled Cortex” – 8 h incubation at 100 °C; “+ SDS” - addition of 2% SDS, a strong detergent) and lactacystin (“+ Lac”), a proteasome-specific inhibitor, confirming that these assays reflect proteasome-specific activity. (B) Protein expression of the 20S CP α6 subunit determined from western blot analysis was unchanged in comparison and schizophrenia subjects. As this subunit is present in all proteasomes this measure reflected total proteasome content and was used to normalize subsequent activity assay data. (C) Chymotrypsin-, trypsin-, and caspase-like activity was measured in the STG of comparison and schizophrenia subjects. Proteolytic activity was determined by measuring change in fluorescence over time and normalizing that measurement to 20S α6 expression. Trypsin-like activity was decreased in schizophrenia subjects, while chymotrypsin- and caspase-like activity were unchanged. Center lines are Means ± S.E.M., ** p < 0.01
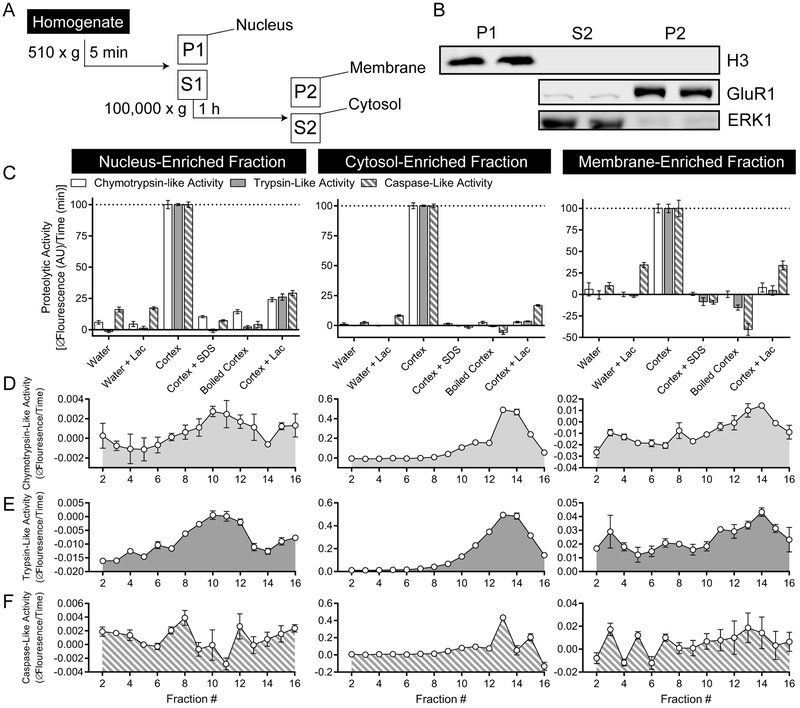
Figure 3. Active proteasome populations in intracellular compartment-enriched fractions from human cortex.
(A) Human cortex was separated into nucleus- (P1), membrane- (P2), and cytosol-enriched (S2) fractions using differential centrifugation. (B) The first spin (510 x g, 5 min) resulted in isolation of all H3 (Histone 3), a marker for the nucleus, while the second spin (100,000 x g, 1 h) separated membrane (GluR1) and cytosol (ERK1) associated markers. (C) Flourogenic substrates (LLVY-AMC, RLR-AMC, LLE-AMC) were sensitive to denaturing conditions and lactacystin in all three fractions. (D-F) 8–32% continuous glycerol gradients were used to separate proteins and complexes based on density. In all three compartment-enriched fractions, (D) chymotrypsin- (LLVY-AMC) and (E) trypsin-like activity (RLR-AMC) is enriched in low-glycerol percentage fractions, where large complexes are predicted to be. This suggests these assays measure proteolytic activity specific to proteasome complexes. (F) Caspase-like activity (LLE-AMC) is also enriched in low-glycerol fractions in the cytosol-enriched fraction, but there is no consistent pattern of enrichment in either the nucleus- or membrane-enriched fractions. Center lines are Means ± S.E.M.
Western Blot Analysis
Reducing buffer (6X: 170 mM Tris (pH 6.8), 4.5% SDS, 36% glycerol, 0.0018% bromephenol blue, 2% βME) was added to each sample, followed by a 10 min incubation at 70 °C. 10 µg of each sample was then loaded into Bolt™ 4–12% Bis-Tris Plus Gels (Invitrogen, Carlsbad, CA, USA), with fraction-matched ABC control tissue loaded on every gel. Gels underwent electrophoresis at 150 V for ~1 h, or until loading dye ran off the gel, using a Mini Gel Tank (Invitrogen, Carlsbad, CA, USA), then transferred to nitrocellulose membrane using a BioRad semi-dry transblotter (Hercules, CA). After transfer, blots were incubated in Ponceau (5% Acetic Acid, 0.1% Ponceau) for 5 min, followed by two one minute washes with tris-buffered saline (TBS) and an image was digitally acquired. Membranes were then incubated with TBS containing either 50% Li-Cor blocking buffer or 5% BSA for 1 h at RT. After blocking, blots were incubated overnight at 4°C in primary antisera diluted in TBS containing 0.1% Tween-20 (TBST) with either 50% Li-Cor buffer or 5% BSA (Supplemental Table 2). Blots were then washed with 1X TBST, incubated with secondary antibody, and washed again before being scanned on an Odyssey Infrared Imaging System (Li-Cor Biosciences) at a resolution of 169 µm and intensity level of 5.
Data Analysis
Image Studio Lite Version 5.2.5 was used to determine relative expression of each protein. Integrated signal values of proteins of interest (POI) were normalized to total protein expression as measured by the full lane value of Ponceau. Values were normalized to the expression of ABC subject POI to control for inter-blot variability and duplicate values averaged for each subject.
Activity assay flourescence values were obtained using Gen 5 (Version 1.02.8, BioTek Instruments) and plotted as a function of time to determine initial velocity. Signal intensity varied depending on flourogenic substrate and compartment, so initial velocity was determined during the period in which we consistently saw a linear slope (10–40 min: Total and CEF CTLA and TLA; 10–60 min: NEF CTLA and TLA, Total and CEF CLA; 10–90 min: NEF CLA, All MEF assays). Baseline activity, as measured by a condition containing no tissue, was subtracted from each value, followed by normalization to ABC subject activity to control for inter-plate variability. Triplicate values were then averaged for each subject.
Data were considered normally distributed if at least two tests of normality (D’Agnostino-Perason omnibus normality test, Shapiro-Wilk normality test, and Kolmogorov-Smirnov test) were not significant. Log transformation and/or the statistical removal of outliers were performed when necessary to handle non-normally distributed data; however, if these steps were not sufficient, we used comparable non-parametric tests on the original data. We used either two-tailed unpaired Student’s t-tests or Mann-Whitney U analyses to determine the effect of diagnosis, except for 19S Rpt subunits, where instead of 6 distinct bivariate analyses we performed a two-way ANOVA with diagnosis and subunit as factors. Pearson correlation coefficients were used to determine associations between protein expression and subject age, tissue pH, and PMI. When associations were found, a follow-up ANCOVA was performed to control for these covariates. All data analysis was performed using GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla, California, USA) and STATISTICA version 7 (StatSoft, Inc, Tulsa, Oklahoma, USA). For all tests we used an initial α = 0.05 followed by the Benjamini-Hochberg method to reduce risk of type I statistical errors associated with multiple comparisons78. Briefly, p-values are ranked and used to calculate the Benjamini-Hochberg critical value q = ([(individual p-value rank)/(total number of tests)]/false discover rate (FDR)). In this study, we used an FDR value of 0.20. Tests where the original p-value was less than the associated q-value were considered significant.
RESULTS
TLA is decreased in SZ
We used denaturing conditions and the proteasome-specific inhibitor lactacystin to confirm that the fluorogenic substrates for CTLA (LLVY-AMC), TLA (RLR-AMC), and CLA (LLE-AMC), measure proteasome-specific enzymatic activity in human cortex (Fig.2A). As PMI can impact protein stability and function79, 80, we tested the effect of a range of PMIs (0, 1, 4, 10 h) on proteolytic activity in mouse brain and observed no difference [CTLA, F(3,11)=0.73; TLA, F(3,11)=1.50, CLA, F(3,11)=1.48]. We then used these assays to measure proteasome activity in STG homogenates of SZ and comparison subjects, normalizing activity for each subject to expression of 20S α6, a component of all proteasome complexes and therefore a measure of total proteasome content. 20S α6 expression was not changed in SZ [t(48)=0.71] (Figure 2B). We observed decreased TLA in subjects with SZ [t(47)=2.63, p=0.01, q = 0.03], but no change in CTLA [t(48)=1.05] or CLA [t(48)=0.78] (Fig.2C).
Active proteasomes in intracellular compartment-enriched fractions from human cortex
Intracellular fractions enriched for biochemical markers of the nucleus (H3), cytosol (ERK1), and cellular membranes (GluR1) were produced by differential centrifugation (Figure 3A-B). CTLA (LLVY-AMC), TLA (RLR-AMC), and CLA (LLE-AMC) assays were sensitive to denaturing conditions and lactacystin in all fractions (Fig.3C). Furthermore, we prepared 8–32% continuous glycerol gradients to separate large complexes from small proteases and demonstrated enrichment of CTLA and TLA in low-glycerol concentration fractions, where large complexes like the proteasome are predicted to be (Fig.3D-E). CLA exhibited the same pattern in the CEF, where activity is the highest, but no distinguishable pattern was observed in either the NEF or MEF (Fig.3F). This is likely due to lower sensitivity of the LLE-AMC assay.
Intracellular compartment-specific abnormalities in proteasome activity in SZ
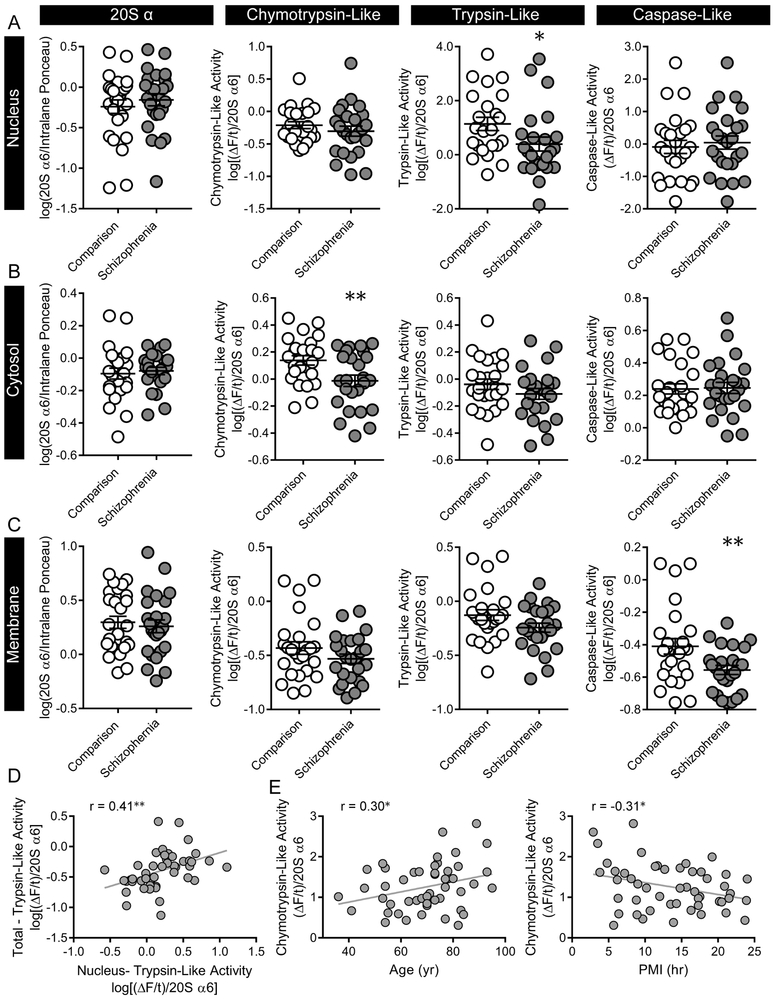
We measured proteolytic activity in the NEF, CEF, and MEF from the STG of SZ and comparison subjects. No changes in 20S α6 protein expression were observed [NEF, t(48)=0.75; CEF, t(47)=0.42; MEF, t(48)=0.46] (Fig.4A-C). The NEF demonstrated the same pattern of activity as we observed in total homogenates, with decreased TLA (U=166, p=0.02, q = 0.04), and no change in either CTLA [t(47)=0.97) or CLA (t(48)=0.47] in SZ (Fig.4A). TLA in total homogenate and the NEF were positively correlated for all subjects [r=0.41, p=0.004] (Figure 4D). In the CEF we observed decreased CTLA activity [t(47)=2.74, p=0.009, q = 0.02], but no change in TLA [t(48)=0.10] or CLA [t(48)=0.14] in SZ (Fig.4B). In the MEF we observed decreased CLA [t(48)=2.63, p=0.01, q = 0.04], but no change in CTLA [t(48)=1.37] or TLA [t(48)=1.78] (Fig.4C).
Figure 4. Intracellular compartment-specific deficits in proteolytic activity.
Proteolytic activity was measured in SZ and comparison subjects using fluorogenic substrates for chymotrypsin- (LLVY-AMC), trypsin- (RLR-AMC), and caspase-like (LLE-AMC) activity. (A-C) No differences in 20S α6 expression, an index of total proteasome content, were observed. In SZ subjects, (A) trypsin-like activity was decreased in the nucleus-enriched fraction, (B) chymotrypsin-like activity was decreased in the cytosol-enriched fraction, and (C) caspase-like activity was decreased in the membrane enriched fraction. (D) Regardless of diagnosis, trypsin-like activity in the nucleus-enriched fraction was positively correlated with total trypsin-like activity, while (E) chymotrypsin-like activity in the cytosol-enriched fraction was significantly correlated with both age and PMI. Center lines are Means ± S.E.M., *p < 0.05, **p < 0.01.
Associations with potential covariates were assessed for all activity measures, and CTLA from the CEF was positively associated with age [r=0.30, p=0.04, q = 0.006] and negatively correlated with PMI [r=−0.31, p=0.03, q = 0.006] (Fig.4E). However, neither of these findings survived Benjamini-Hochberg FDR analysis and an ANCOVA demonstrated that the effect of diagnosis on CTLA in the CEF remained when controlling for both age and PMI [ANCOVA, FDX(1,45)=7.09, p=0.01].
Intracellular compartment-specific expression of proteasome subunits
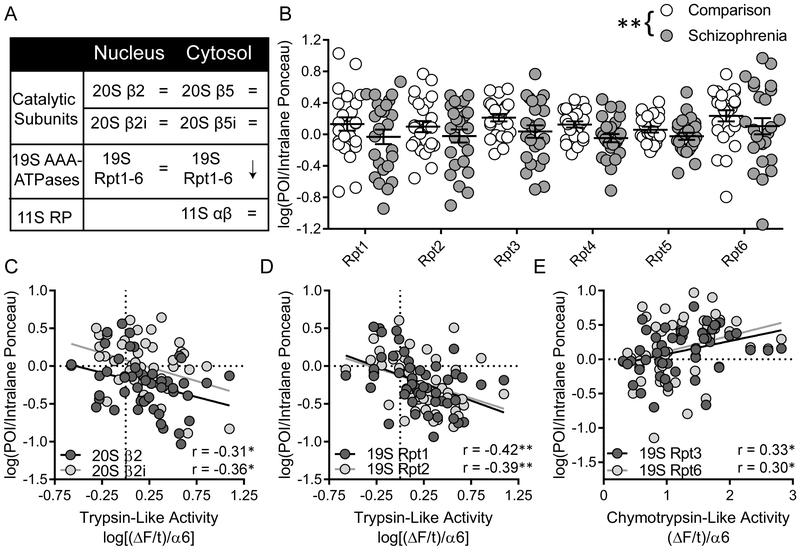
Proteasome subunit expression was measured in both the NEF and CEF (Fig.5). In the NEF, the constitutive (20S β2) and inducible (20S β2i) catalytic subunits associated with TLA were not differentially expression between comparison and SZ subjects [β2, t(48)=1.07; β2i, t(47)=0.27]. Additionally, there was no effect of diagnosis on 19S Rpt expression [FDX(1,287)=0.08, Fsubunit(5,287)=3.79, p=0.002, q = 0.007, FDX x subunit(5,287)=0.26]. Protein expression was, however, negatively correlated with TLA for several proteasome subunits, including 20S β2 [r=−0.31, p=0.03, q = 0.06], 20S β2i [r=−0.36, p=0.02, q = 0.03], 19S Rpt1 [r=−0.42, p=0.003, q =0.01] and Rpt2 [r=−0.39, p=0.007, q = 0.02] (Fig.5C-D).
Figure 5. Intracellular compartment-specific protein expression of proteasome subunits.
(A). Protein expression of proteasome subunits was measured in both the nucleus- and cytosol-enriched fractions using western immunoblotting. (B) In the cytosol-enriched fraction, 19S AAA-ATPase expression was decreased in SZ. (C) In the nucleus-enriched fraction, trypsin-like activity was negatively correlated with both the constitutive and inducible catalytic subunits, (D) as well as the 19S RP subunits Rpt1 and Rpt2. (E) The 19S RP subunits Rpt3 and Rpt6 were positively correlated with chymotrypsin-like activity in the cytosol-enriched fraction. Center lines are Means ± S.E.M., *p < 0.05, **p < 0.01
In the CEF, the constitutive (20S β5) and inducible (20S β5i) catalytic subunits associated with CTLA were measured, along with subunits from the 19S (Rpt1–6) and 11S (α, β) RPs. No change in protein expression was observed for the catalytic subunits [β5, t(48) 0.51; β5i, t(48)=0.62] or the 11S RP subunits [11S α, t(48)=0.26; 11S β, t(47)=0.12]. However, there was a significant main effect of diagnosis on 19S Rpt expression [FDX(1,288)=8.12, p=0.005, q = 0.01] (Fig.5B). As no interaction between diagnosis and subunit was observed [FDX x subunit(5,288)=0.06], this does not appear to be driven by a single subunit, but instead reflects decreased expression of all 19S AAA-ATPases. Several subunits were positively associated with brain pH [Rpt1, r=0.42, p=0.002, q = 0.006; Rpt2, r=0.42, p=0.003, q =0.01; Rpt4, r=0.36, p=0.01, q = 0.02], however an ANCOVA demonstrated that the effect of diagnosis on 19S Rpt expression remained significant when controlling for this covariate [FDX(1,287)=4.87, p=0.03]. Independent of diagnosis, expression of several 19S RP subunits was positively correlated with CTLA [Rpt3, r=0.33, p=0.02, q = 0.04; Rpt6, r=0.30, p=0.04, q = 0.07], further implicating this RP complex in CTLA abnormalities.
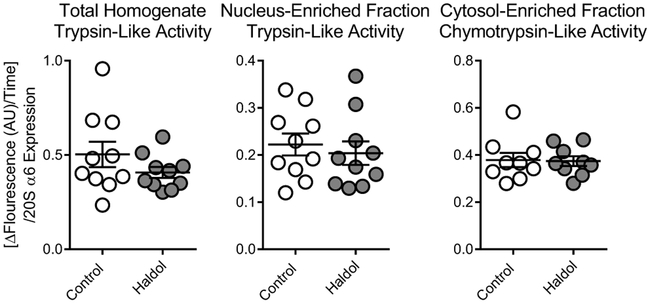
Chronic antipsychotic treatment does not change proteasome activity in rat brain
Proteolytic activities found to be abnormal in SZ were measured in aged rats chronically treated with haloperidol decanoate. No changes due to antipsychotic treatment were observed in TLA in either total homogenate [t(18)=1.31] or the NEF [t(18)=0.54], or in CTLA in the CEF [t(16)=0.13] (Fig.6).
Figure 6. Proteolytic activity after chronic antipsychotic treatment in aged rats.
Chronic (9 month) treatment with haloperidol decanoate did not lead to changes in total trypsin-like activity (RLR-AMC), trypsin-like activity in the nucleus-enriched fraction, or chymotrypsin-like activity (LLVY-AMC) in the cytosol-enriched fraction from rat brain tissue. Center lines are Means ± S.E.M.
DISCUSSION
In this study, we measured proteasome activity from the left STG in SZ and comparison subjects and observed decreased TLA in SZ in tissue homogenates. To further characterize this deficit in proteasome activity, we prepared fractions enriched for nuclei, cytosol, and extracellular membranes and found decreased TLA in the NEF and CTLA in the CEF in SZ, suggesting intracellular compartment-specific proteasome dysfunction. TLA from total tissue homogenates and the NEF were positively associated, regardless of diagnosis, suggesting that the changes we found in total tissue homogenates may be driven by nucleus-localized proteasomes. Changes in activity were not accompanied by abnormal expression of catalytic subunits, but we did observe decreased 19S Rpt expression in the CEF. These findings suggest cell compartment-specific proteasome dysfunction in SZ.
The proteasome is responsible for the majority of intracellular protein degradation40, therefore the observed deficits in proteolytic activity likely result in abnormal protein expression. Supporting this prediction, evidence for abnormal protein expression in SZ is well-documented, through both large-scale proteomic studies and analyses demonstrating changes in individual proteins1. Our intracellular compartment-specific abnormalities suggest that proteasome regulation of protein synthesis in the nucleus in addition to the proteasome’s traditional role in degradation39. Global proteasome impairment typically leads to accumulation of misfolded proteins and cell apoptosis, as is seen in neurodegenerative disorders like Alzheimer’s Diseases, Parkinson’s Disease, and Amyloid Lateral Sclerosis81. However, there is little evidence of neurodegeneration and accumulation of misfolded proteins, suggesting that the proteasomal protein degradation is not completely impaired82, 83. This is consistent with work describing consequences of inhibiting only a single type of proteasome activity. Kisselev et al.84 demonstrated that inhibition of one activity type only moderately reduced total protein degradation. However, when looking at known proteasome substrates they observed substrate-specific decreased degradation capacity unique to each activity type84. While further work characterizing the consequences of changing a single type of proteasome activity on the proteome are necessary to fully understand the potential role of proteasome dysfunction in SZ, we propose that our findings represent substrate-specific alterations in protein degradation that are distinct depending on subcellular localization.
Changes in proteasome activity are often accompanied by alterations in proteasome complex dynamics. Decreased 19S RP expression is associated with reduced proteolytic activity, while abnormal immunoproteasome subunit expression impacts each type of activity differently depending on the subunit85. In the current study, we did not observe differences in total proteasome content, as measured by 20S α6 expression, or catalytic subunit expression regardless of fraction. We did observe, however, decreased 19S Rpt expression in the CEF. This is consistent with previous work from our lab that reported decreased expression of 19S Rpt1, Rpt3, and Rpt662, and supports a theory of UPS dysfunction in SZ. While decreased proteasome activity is generally associated with ubiquitinated protein accumulation, the opposite is found in the STG of SZ subjects60. Abnormal transcript and protein expression of UPS elements have been repeatedly observed in SZ, including reduced expression of ubiquitin, ubiquitinated proteins, and enzymes associated with both ubiquitin attachment and removal53–61. These studies suggest reduced utilization of ubiquitin-dependent degradation by the proteasome, rather than a proteasome-dependent accumulation of ubiquitinated proteins. This may underlie cellular recruitment of alternative protein degradation methods, such as lysosomal or ubiquitin-independent proteasome degradation. Immunoproteasomes and uncapped CP complexes are recruited in response to oxidative stress as they are more effective at degrading oxidatively damaged proteins than 26S complexes86. While markers of oxidative stress, such as reduced expression of antioxidants, increased lipid peroxidation, and reactive oxygen species have been found in SZ brain47, 87, 88, there is little evidence in SZ of apoptosis or neurodegeneration, processes that result from increased oxidative stress81-83. Why there is increased oxidative stress but not apoptosis is currently a gap in knowledge in SZ. We propose that our findings are consistent with abnormal prioritization of ubiquitin-independent over ubiquitin-dependent degradation in SZ, and that this results in resilience to oxidative stress and prevents cell death.
While these changes in proteasome activity and expression may be beneficial to cell survival, they are also likely to disrupt other cellular processes. Neurotransmission may be particularly sensitive to proteasome dysfunction as it is dependent on coordinated regulation of multiple components including receptors, transporters, and auxiliary proteins, many of which are known proteasome substrates89. Additionally, neurotransmission is facilitated by the development, maintenance, and plasticity of dendrites, dendritic spines, and synapses90. The proteasome is known to degrade cytoskeletal elements, and studies have demonstrated that proteasomes regulate dendritic spine morphogenesis, maintenance and capacity for plasticity42–48, 90. As such, the proteasome dysfunction we observed may lead to deficits in the capacity of cells to build and regulate the structural connections necessary to receive information, to recognize input and integrate it through intracellular signaling, and to develop appropriate intercellular responses. Supporting this prediction, evidence for reduced dendritic spine density, abnormal expression of synaptic proteins, and disrupted synaptic plasticity has been repeatedly observed in multiple brain regions of SZ subjects72. Microstructural deficits including reduced soma size, decreased dendritic arborization, and reduced dendritic spine density have been observed and are proposed to underlie decreased cortical volume72. Specifically, reduced STG volume is one of the most consistent findings in SZ literature71, 91, and as such we propose a model in which proteasome dysfunction leads to disruption of STG circuit function through the destabilization of cytoarchitecture and neuronal capacity for synaptic plasticity.
The STG has long been associated with SZ and auditory hallucinations. Temporal lobe dysfunction and damage is associated with SZ-like symptoms92, 93 and stimulation of the temporal lobe induces hallucinations in both epileptic and SZ patients69, 70. Additionally, patients with epilepsy localized to the STG report similar auditory hallucinations to patients with SZ64–68, and STG volume is associated with severity of auditory hallucinations71, 91. Typically, the STG is thought to integrate information from multiple modalities to contextualize auditory information91. The theoretical role of sensory integration in differentiating between internal and external stimuli, assigning appropriate valence to stimuli, and filtering sensations to allow for focusing attention, may explain how STG dysfunction could lead to auditory hallucinations and contribute to executive function deficits. Integrating sensory information relies on the capacity of the STG to receive input from many other brain regions and coordinate information through intracortical connections91. We speculate that disrupting the capacity of cells in this region to receive, process, and respond to intercellular input due to proteasome dysfunction leads to behavioral symptoms through destabilization of the circuit underlying STG-related sensory and cognitive function. Further work characterizing how the abnormalities we observed in proteasome expression and activity impact neural function, intercellular communication, and circuit function are needed to determine the viability of our predictions.
Intriguingly, the impact of altered proteasome activity may not be limited to changes in protein expression. The proteasome degrades proteins through a series of cleavages, resulting in the production of small peptides85. While these peptides are often further degraded by other proteases, some associate with the major histocompatibility complex (MHC)85. The MHC is eventually shuttled to the cell surface where T-cells can recognize non-self antigens and instigate an immune response85. Constitutive and immunoproteasomes produce distinct populations of peptides, as do proteasomes capped by various RPs85. Peptide production depends on a balance between multiple cleavage events, and therefore the effect of proteasome inhibitors appears to have both substrate-specific impacts on peptide production and a widespread effect on the population of peptides85. The alteration in a single type of proteolytic activity in SZ may, therefore, result in differential production of peptides. This is a particularly interesting possibility as SZ has been associated with altered immune responses, increased production of autoantibodies to neural-associated proteins, and abnormalities in the MHC94.
Mechanisms known to alter proteasome complex expression overlap with processes though to be regulated by the proteasome, implicating a bidirectional association of proteasome activity and expression with these cellular processes. Specifically, NMDA receptor and dopaminergic activity induce 19S RP dissociation, while immunoproteasomes and uncapped CP complexes are recruited in response to oxidative stress and inflammation49–51, 86. The bidirectional connection of the proteasome to these cellular processes suggests that the proteasome may act as a central hub of dysfunction in SZ. Specifically, proteasomes may have the capacity to sense dysfunction of initial causative elements such as genetic dysregulation of NMDA receptor and dopaminergic activity and inflammation due to maternal immune activation49–51, 86, as well as contribute to pathophysiology through dysregulation of energy homeostasis, immune function and neurotransmission42–48, 85.
There are several limitations to consider in interpretation of this study, including potential confounding effects of aging, PMI, and treatment with antipsychotic medications on proteasomes. Proteasome function is implicated as a component of aging95. Decreased proteasome activity due to aging has been observed in a large variety of rodent and non-neural human tissues/cell types, and proteasome inhibition leads to age-related phenotypes in mice and induced senescence of human fibroblasts95–97. However, the only measure of proteolytic activity we observed associated with aging was CTLA in the CEF. Specifically, CTLA and aging were positively correlated independent of diagnosis, suggesting that activity increases with age unlike previous reports. As such, our finding may represent human brain-specific age-associated regulation of proteasomes. Alternatively, longevity is associated with higher or maintained proteasome activity in human centerrian fibroblasts and long-living queen bees98, 99. As our population is primarily elderly subjects, our sample may be biased for long-lived individuals who have increased proteasome activity as a component of increased resiliency to aging. Additionally, the advanced age of our subjects limits the conclusions that can be made from this study. SZ develops over the lifespan, and molecular changes at onset are likely not the same as those found at late stages of the illness. Further work defining how proteasomes are regulated throughout development and aging in the human brain, and how this is disrupted in SZ, is necessary to fully understand the proteasomes role in SZ pathophysiology.
There are inherent concerns with postmortem studies in SZ that include both the confounding factors of PMI and antipsychotic treatment. PMI can impact measures of protein expression and activity79, 80, and the majority of SZ subjects in this cohort were chronically treated with antipsychotics, which are known to impact cellular function in a variety of ways. We demonstrated that PMI up to 10 h does not affect proteolytic activity measures prior to examining activity in this cohort. Additionally, we found that chronic antipsychotic treatment in rats was not sufficient to reproduce abnormalities in proteolytic activity. This is consistent with previous work demonstrating no effect of chronic haloperidol treatment on expression of 19S Rpt subunits in rats62.
In summary, we examined intracellular compartment-specific proteasome activity in the STG of SZ and comparison subjects. We found decreased TLA in tissue homogenate as well as in the NEF, and reduced CTLA and 19S Rpt expression in the CEF, in SZ. These findings are consistent with abnormal regulation of proteasome function in SZ, which likely has a broad effect on the proteomic landscape and cellular function in the pathophysiology of this illness.
Supplementary Material
Footnotes
Supplementary information is available at Molecular Psychiatry’s website.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Davalieva K, Maleva Kostovska I, Dwork AJ. Proteomics Research in Schizophrenia. Frontiers in cellular neuroscience 2016; 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karson CN, Mrak RE, Schluterman KO, Sturner WQ, Sheng JG, Griffin WS. Alterations in synaptic proteins and their encoding mRNAs in prefrontal cortex in schizophrenia: a possible neurochemical basis for ‘hypofrontality’. Molecular psychiatry 1999; 4(1): 39–45. [DOI] [PubMed] [Google Scholar]
- 3.Eastwood SL, Burnet PW, Gittins R, Baker K, Harrison PJ. Expression of serotonin 5-HT(2A) receptors in the human cerebellum and alterations in schizophrenia. Synapse 2001; 42(2): 104–114. [DOI] [PubMed] [Google Scholar]
- 4.Eastwood SL, Cotter D, Harrison PJ. Cerebellar synaptic protein expression in schizophrenia. Neuroscience 2001; 105(1): 219–229. [DOI] [PubMed] [Google Scholar]
- 5.Dracheva S, Elhakem SL, McGurk SR, Davis KL, Haroutunian V. GAD67 and GAD65 mRNA and protein expression in cerebrocortical regions of elderly patients with schizophrenia. Journal of neuroscience research 2004; 76(4): 581–592. [DOI] [PubMed] [Google Scholar]
- 6.Erdely HA, Tamminga CA, Roberts RC, Vogel MW. Regional alterations in RGS4 protein in schizophrenia. Synapse 2006; 59(8): 472–479. [DOI] [PubMed] [Google Scholar]
- 7.Verrall L, Walker M, Rawlings N, Benzel I, Kew JN, Harrison PJ et al. d-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. The European journal of neuroscience 2007; 26(6): 1657–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophrenia research 2008; 104(1–3): 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Shachar D, Karry R. Neuroanatomical pattern of mitochondrial complex I pathology varies between schizophrenia, bipolar disorder and major depression. PloS one 2008; 3(11): e3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnet PW, Hutchinson L, von Hesling M, Gilbert EJ, Brandon NJ, Rutter AR et al. Expression of D-serine and glycine transporters in the prefrontal cortex and cerebellum in schizophrenia. Schizophrenia research 2008; 102(1–3): 283–294. [DOI] [PubMed] [Google Scholar]
- 11.Oni-Orisan A, Kristiansen LV, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Altered vesicular glutamate transporter expression in the anterior cingulate cortex in schizophrenia. Biological psychiatry 2008; 63(8): 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang J, LeGros RP, Louneva N, Yeh L, Cohen JW, Hahn CG et al. Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Human molecular genetics 2009; 18(20): 3851–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uriguen L, Garcia-Fuster MJ, Callado LF, Morentin B, La Harpe R, Casado V et al. Immunodensity and mRNA expression of A2A adenosine, D2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: effect of antipsychotic treatment. Psychopharmacology 2009; 206(2): 313–324. [DOI] [PubMed] [Google Scholar]
- 14.Fatemi SH, Folsom TD, Reutiman TJ, Vazquez G. Phosphodiesterase signaling system is disrupted in the cerebella of subjects with schizophrenia, bipolar disorder, and major depression. Schizophrenia research 2010; 119(1–3): 266–267. [DOI] [PubMed] [Google Scholar]
- 15.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. The American journal of psychiatry 2010; 167(12): 1479–1488. [DOI] [PubMed] [Google Scholar]
- 16.Kristiansen LV, Bakir B, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor trafficking complex in prefrontal cortex from a group of elderly patients with schizophrenia. Schizophrenia research 2010; 119(1–3): 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gigante AD, Andreazza AC, Lafer B, Yatham LN, Beasley CL, Young LT. Decreased mRNA expression of uncoupling protein 2, a mitochondrial proton transporter, in post-mortem prefrontal cortex from patients with bipolar disorder and schizophrenia. Neuroscience letters 2011; 505(1): 47–51. [DOI] [PubMed] [Google Scholar]
- 18.Sinclair D, Tsai SY, Woon HG, Weickert CS. Abnormal glucocorticoid receptor mRNA and protein isoform expression in the prefrontal cortex in psychiatric illness. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2011; 36(13): 2698–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udawela M, Scarr E, Hannan AJ, Thomas EA, Dean B. Phospholipase C beta 1 expression in the dorsolateral prefrontal cortex from patients with schizophrenia at different stages of illness. The Australian and New Zealand journal of psychiatry 2011; 45(2): 140–147. [DOI] [PubMed] [Google Scholar]
- 20.Joshi D, Fung SJ, Rothwell A, Weickert CS. Higher gamma-aminobutyric acid neuron density in the white matter of orbital frontal cortex in schizophrenia. Biological psychiatry 2012; 72(9): 725–733. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Costas E, Melendez-Ferro M, Rice MW, Conley RR, Roberts RC. Dopamine pathology in schizophrenia: analysis of total and phosphorylated tyrosine hydroxylase in the substantia nigra. Frontiers in psychiatry 2012; 3: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinclair D, Webster MJ, Fullerton JM, Weickert CS. Glucocorticoid receptor mRNA and protein isoform alterations in the orbitofrontal cortex in schizophrenia and bipolar disorder. BMC psychiatry 2012; 12: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean B, Gibbons AS, Tawadros N, Brooks L, Everall IP, Scarr E. Different changes in cortical tumor necrosis factor-alpha-related pathways in schizophrenia and mood disorders. Molecular psychiatry 2013; 18(7): 767–773. [DOI] [PubMed] [Google Scholar]
- 24.Drummond JB, Tucholski J, Haroutunian V, Meador-Woodruff JH. Transmembrane AMPA receptor regulatory protein (TARP) dysregulation in anterior cingulate cortex in schizophrenia. Schizophrenia research 2013; 147(1): 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fatemi SH, Folsom TD, Rooney RJ, Thuras PD. Expression of GABAA alpha2-, beta1- and epsilon-receptors are altered significantly in the lateral cerebellum of subjects with schizophrenia, major depression and bipolar disorder. Translational psychiatry 2013; 3: e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fatemi SH, Folsom TD, Rooney RJ, Thuras PD. mRNA and protein expression for novel GABAA receptors theta and rho2 are altered in schizophrenia and mood disorders; relevance to FMRP-mGluR5 signaling pathway. Translational psychiatry 2013; 3: e271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao R, Cousijn H, Jaffe AE, Burnet PW, Edwards F, Eastwood SL et al. Expression of ZNF804A in human brain and alterations in schizophrenia, bipolar disorder, and major depressive disorder: a novel transcript fetally regulated by the psychosis risk variant rs1344706. JAMA psychiatry 2014; 71(10): 1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matosin N, Fernandez-Enright F, Fung SJ, Lum JS, Engel M, Andrews JL et al. Alterations of mGluR5 and its endogenous regulators Norbin, Tamalin and Preso1 in schizophrenia: towards a model of mGluR5 dysregulation. Acta neuropathologica 2015; 130(1): 119–129. [DOI] [PubMed] [Google Scholar]
- 29.Udawela M, Money TT, Neo J, Seo MS, Scarr E, Dean B et al. SELENBP1 expression in the prefrontal cortex of subjects with schizophrenia. Translational psychiatry 2015; 5: e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Bueno B, Gasso P, MacDowell KS, Callado LF, Mas S, Bernardo M et al. Evidence of activation of the Toll-like receptor-4 proinflammatory pathway in patients with schizophrenia. Journal of psychiatry & neuroscience : JPN 2016; 41(3): E46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishiura K, Ichikawa-Tomikawa N, Sugimoto K, Kunii Y, Kashiwagi K, Tanaka M et al. PKA activation and endothelial claudin-5 breakdown in the schizophrenic prefrontal cortex. Oncotarget 2017; 8(55): 93382–93391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purves-Tyson TD, Owens SJ, Rothmond DA, Halliday GM, Double KL, Stevens J et al. Putative presynaptic dopamine dysregulation in schizophrenia is supported by molecular evidence from post-mortem human midbrain. Translational psychiatry 2017; 7(1): e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Udawela M, Scarr E, Boer S, Um JY, Hannan AJ, McOmish C et al. Isoform specific differences in phospholipase C beta 1 expression in the prefrontal cortex in schizophrenia and suicide. NPJ schizophrenia 2017; 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandey GN, Rizavi HS, Zhang H, Ren X. Abnormal gene and protein expression of inflammatory cytokines in the postmortem brain of schizophrenia patients. Schizophrenia research 2018; 192: 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tremolizzo L, Carboni G, Ruzicka WB, Mitchell CP, Sugaya I, Tueting P et al. An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proceedings of the National Academy of Sciences of the United States of America 2002; 99(26): 17095–17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen T, Olsen L, Lindow M, Jakobsen KD, Ullum H, Jonsson E et al. Brain expressed microRNAs implicated in schizophrenia etiology. PloS one 2007; 2(9): e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.English JA, Fan Y, Focking M, Lopez LM, Hryniewiecka M, Wynne K et al. Reduced protein synthesis in schizophrenia patient-derived olfactory cells. Translational psychiatry 2015; 5: e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annual review of biochemistry 1999; 68: 1015–1068. [DOI] [PubMed] [Google Scholar]
- 39.von Mikecz A The nuclear ubiquitin-proteasome system. Journal of cell science 2006; 119(Pt 10): 1977–1984. [DOI] [PubMed] [Google Scholar]
- 40.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 1994; 78(5): 761–771. [DOI] [PubMed] [Google Scholar]
- 41.Ramachandran KV, Margolis SS. A mammalian nervous-system-specific plasma membrane proteasome complex that modulates neuronal function. Nature structural & molecular biology 2017; 24(4): 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos AR, Mele M, Vaz SH, Kellermayer B, Grimaldi M, Colino-Oliveira M et al. Differential role of the proteasome in the early and late phases of BDNF-induced facilitation of LTP. The Journal of neuroscience : the official journal of the Society for Neuroscience 2015; 35(8): 3319–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Djakovic SN, Marquez-Lona EM, Jakawich SK, Wright R, Chu C, Sutton MA et al. Phosphorylation of Rpt6 regulates synaptic strength in hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 2012; 32(15): 5126–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erturk A, Wang Y, Sheng M. Local pruning of dendrites and spines by caspase-3-dependent and proteasome-limited mechanisms. The Journal of neuroscience : the official journal of the Society for Neuroscience 2014; 34(5): 1672–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton AM, Oh WC, Vega-Ramirez H, Stein IS, Hell JW, Patrick GN et al. Activity-dependent growth of new dendritic spines is regulated by the proteasome. Neuron 2012; 74(6): 1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Molecular psychiatry 2011; 16(9): 960–972. [DOI] [PubMed] [Google Scholar]
- 47.Rajasekaran A, Venkatasubramanian G, Berk M, Debnath M. Mitochondrial dysfunction in schizophrenia: pathways, mechanisms and implications. Neuroscience and biobehavioral reviews 2015; 48: 10–21. [DOI] [PubMed] [Google Scholar]
- 48.Moyer CE, Shelton MA, Sweet RA. Dendritic spine alterations in schizophrenia. Neuroscience letters 2015; 601: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barroso-Chinea P, Thiolat ML, Bido S, Martinez A, Doudnikoff E, Baufreton J et al. D1 dopamine receptor stimulation impairs striatal proteasome activity in Parkinsonism through 26S proteasome disassembly. Neurobiology of disease 2015; 78: 77–87. [DOI] [PubMed] [Google Scholar]
- 50.Caldeira MV, Curcio M, Leal G, Salazar IL, Mele M, Santos AR et al. Excitotoxic stimulation downregulates the ubiquitin-proteasome system through activation of NMDA receptors in cultured hippocampal neurons. Biochimica et biophysica acta 2013; 1832(1): 263–274. [DOI] [PubMed] [Google Scholar]
- 51.Ferreira JS, Schmidt J, Rio P, Aguas R, Rooyakkers A, Li KW et al. GluN2B-Containing NMDA Receptors Regulate AMPA Receptor Traffic through Anchoring of the Synaptic Proteasome. The Journal of neuroscience : the official journal of the Society for Neuroscience 2015; 35(22): 8462–8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu C, Bousman CA, Pantelis C, Skafidas E, Zhang D, Yue W et al. Pathway-wide association study identifies five shared pathways associated with schizophrenia in three ancestral distinct populations. Translational psychiatry 2017; 7(2): e1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vawter MP, Barrett T, Cheadle C, Sokolov BP, Wood WH 3rd, Donovan DM et al. Application of cDNA microarrays to examine gene expression differences in schizophrenia. Brain research bulletin 2001; 55(5): 641–650. [DOI] [PubMed] [Google Scholar]
- 54.Middleton FA, Mirnics K, Pierri JN, Lewis DA, Levitt P. Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience 2002; 22(7): 2718–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y et al. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biological psychiatry 2005; 58(2): 85–96. [DOI] [PubMed] [Google Scholar]
- 56.Chu TT, Liu Y, Kemether E. Thalamic transcriptome screening in three psychiatric states. Journal of human genetics 2009; 54(11): 665–675. [DOI] [PubMed] [Google Scholar]
- 57.Arion D, Corradi JP, Tang S, Datta D, Boothe F, He A et al. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Molecular psychiatry 2015; 20(11): 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bousman CA, Chana G, Glatt SJ, Chandler SD, May T, Lohr J et al. Positive symptoms of psychosis correlate with expression of ubiquitin proteasome genes in peripheral blood. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics 2010; 153B(7): 1336–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bousman CA, Chana G, Glatt SJ, Chandler SD, Lucero GR, Tatro E et al. Preliminary evidence of ubiquitin proteasome system dysregulation in schizophrenia and bipolar disorder: convergent pathway analysis findings from two independent samples. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics 2010; 153B(2): 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubio MD, Wood K, Haroutunian V, Meador-Woodruff JH. Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2013; 38(10): 1910–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andrews JL, Goodfellow FJ, Matosin N, Snelling MK, Newell KA, Huang XF et al. Alterations of ubiquitin related proteins in the pathology and development of schizophrenia: Evidence from human and animal studies. Journal of psychiatric research 2017; 90: 31–39. [DOI] [PubMed] [Google Scholar]
- 62.Scott MR, Rubio MD, Haroutunian V, Meador-Woodruff JH. Protein Expression of Proteasome Subunits in Elderly Patients with Schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2016; 41(3): 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Curcic-Blake B, Ford JM, Hubl D, Orlov ND, Sommer IE, Waters F et al. Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Progress in neurobiology 2017; 148: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karagulla S, Robertson EE. Phychical phenomena in temporal lobe epilepsy and the psychoses. British medical journal 1955; 1(4916): 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slater E, Beard AW, Glithero E. Schizophrenia-Like Psychoses of Epilepsy. International journal of psychiatry 1965; 1: 6–30. [PubMed] [Google Scholar]
- 66.Korzeniowski L [Diagnostic problems regarding delusion psychoses in the course of epilepsy]. Neurologia, neurochirurgia i psychiatria polska 1965; 15(6): 823–828. [PubMed] [Google Scholar]
- 67.Korzeniowski L [Diagnostic Problems Concerning Paranoic Schizophreniform Psychoses in Epilepsy]. Annales medico-psychologiques 1965; 123: 35–42. [PubMed] [Google Scholar]
- 68.Alsen V [Epilepsy and psychosis]. Der Nervenarzt 1965; 36(11): 490–493. [PubMed] [Google Scholar]
- 69.Hori H [Hallucinations by the electrical stimulation of temporal lobe]. Seishin shinkeigaku zasshi = Psychiatria et neurologia Japonica 1962; 64: 1010–1016. [PubMed] [Google Scholar]
- 70.Ishibashi T, Hori H, Endo K, Sato T. Hallucinations Produced by Electrical Stimulation of the Temporal Lobes in Schizophrenic Patients. The Tohoku journal of experimental medicine 1964; 82: 124–139. [DOI] [PubMed] [Google Scholar]
- 71.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. The American journal of psychiatry 2005; 162(12): 2233–2245. [DOI] [PubMed] [Google Scholar]
- 72.Forsyth JK, Lewis DA. Mapping the Consequences of Impaired Synaptic Plasticity in Schizophrenia through Development: An Integrative Model for Diverse Clinical Features. Trends in cognitive sciences 2017; 21(10): 760–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Powchik P, Davidson M, Haroutunian V, Gabriel SM, Purohit DP, Perl DP et al. Postmortem studies in schizophrenia. Schizophrenia bulletin 1998; 24(3): 325–341. [DOI] [PubMed] [Google Scholar]
- 74.Purohit DP, Perl DP, Haroutunian V, Powchik P, Davidson M, Davis KL. Alzheimer disease and related neurodegenerative diseases in elderly patients with schizophrenia: a postmortem neuropathologic study of 100 cases. Archives of general psychiatry 1998; 55(3): 205–211. [DOI] [PubMed] [Google Scholar]
- 75.Barksdale KA, Perez-Costas E, Gandy JC, Melendez-Ferro M, Roberts RC, Bijur GN. Mitochondrial viability in mouse and human postmortem brain. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2010; 24(9): 3590–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kashihara K, Sato M, Fujiwara Y, Ogawa T, Fukuda K, Otsuki S. Effects of intermittent and continuous haloperidol administration on the dopaminergic system in the rat brain. Yakubutsu, seishin, kodo = Japanese journal of psychopharmacology 1986; 6(2): 275–280. [PubMed] [Google Scholar]
- 77.Harte MK, Bachus SB, Reynolds GP. Increased N-acetylaspartate in rat striatum following long-term administration of haloperidol. Schizophrenia research 2005; 75(2–3): 303–308. [DOI] [PubMed] [Google Scholar]
- 78.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society 1995; 57(1): 289–300. [Google Scholar]
- 79.Fountoulakis M, Hardmeier R, Hoger H, Lubec G. Postmortem changes in the level of brain proteins. Experimental neurology 2001; 167(1): 86–94. [DOI] [PubMed] [Google Scholar]
- 80.Beckstrom H, Julsrud L, Haugeto O, Dewar D, Graham DI, Lehre KP et al. Interindividual differences in the levels of the glutamate transporters GLAST and GLT, but no clear correlation with Alzheimer’s disease. Journal of neuroscience research 1999; 55(2): 218–229. [DOI] [PubMed] [Google Scholar]
- 81.McKinnon C, Tabrizi SJ. The ubiquitin-proteasome system in neurodegeneration. Antioxidants & redox signaling 2014; 21(17): 2302–2321. [DOI] [PubMed] [Google Scholar]
- 82.Rund BR. The research evidence for schizophrenia as a neurodevelopmental disorder. Scandinavian journal of psychology 2018; 59(1): 49–58. [DOI] [PubMed] [Google Scholar]
- 83.Bradshaw NJ, Korth C. Protein misassembly and aggregation as potential convergence points for non-genetic causes of chronic mental illness. Molecular psychiatry 2018. [DOI] [PubMed] [Google Scholar]
- 84.Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. The Journal of biological chemistry 2006; 281(13): 8582–8590. [DOI] [PubMed] [Google Scholar]
- 85.Vigneron N, Van den Eynde BJ. Proteasome subtypes and regulators in the processing of antigenic peptides presented by class I molecules of the major histocompatibility complex. Biomolecules 2014; 4(4): 994–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJ. The immunoproteasome, the 20S proteasome and the PA28alphabeta proteasome regulator are oxidative-stress-adaptive proteolytic complexes. The Biochemical journal 2010; 432(3): 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boskovic M, Vovk T, Kores Plesnicar B, Grabnar I. Oxidative stress in schizophrenia. Current neuropharmacology 2011; 9(2): 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reyazuddin M, Azmi SA, Islam N, Rizvi A. Oxidative stress and level of antioxidant enzymes in drug-naive schizophrenics. Indian journal of psychiatry 2014; 56(4): 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bingol B, Schuman EM. Synaptic protein degradation by the ubiquitin proteasome system. Current opinion in neurobiology 2005; 15(5): 536–541. [DOI] [PubMed] [Google Scholar]
- 90.Hamilton AM, Zito K. Breaking it down: the ubiquitin proteasome system in neuronal morphogenesis. Neural plasticity 2013; 2013: 196848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nature reviews Neuroscience 2015; 16(9): 535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vaillant G Schizophrenia in a Woman with Temporal Lobe Arterio-Venous Malformations: An Unusual Case Report. The British journal of psychiatry : the journal of mental science 1965; 111: 307–308. [DOI] [PubMed] [Google Scholar]
- 93.Hollender MH, Hirsch SJ, Goodwin FK, Kaplan EA, Rubert SL, Watkins ES et al. Schizophrenia or temporal lobe disorder? International psychiatry clinics 1965; 2(3): 667–689. [PubMed] [Google Scholar]
- 94.Debanth M, Berk M, Leboyer M, Tamouza R. The MHC/HLA gene complex in major psychiatric disorders: Emerging roles and implications. Current Behavioral Neuroscience Reports 2018; 5(2): 179–188. [Google Scholar]
- 95.Saez I, Vilchez D. The Mechanistic Links Between Proteasome Activity, Aging and Age-related Diseases. Current genomics 2014; 15(1): 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomaru U, Takahashi S, Ishizu A, Miyatake Y, Gohda A, Suzuki S et al. Decreased proteasomal activity causes age-related phenotypes and promotes the development of metabolic abnormalities. The American journal of pathology 2012; 180(3): 963–972. [DOI] [PubMed] [Google Scholar]
- 97.Torres C, Lewis L, Cristofalo VJ. Proteasome inhibitors shorten replicative life span and induce a senescent-like phenotype of human fibroblasts. Journal of cellular physiology 2006; 207(3): 845–853. [DOI] [PubMed] [Google Scholar]
- 98.Chondrogianni N, Petropoulos I, Franceschi C, Friguet B, Gonos ES. Fibroblast cultures from healthy centenarians have an active proteasome. Experimental gerontology 2000; 35(6–7): 721–728. [DOI] [PubMed] [Google Scholar]
- 99.Hsu CY, Qiu JT, Chan YP. Cellular degradation activity is maintained during aging in long-living queen bees. Biogerontology 2016; 17(5–6): 829–840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.