Abstract
BACKGROUND
Anorectal malignant melanoma (AMM) is a rare disorder with an extremely poor prognosis. Although there is currently no consensus on the treatment methods for AMM, surgical procedures have been the most common treatment methods used until now. We recently encountered a case of AMM that we diagnosed using endoscopic submucosal dissection (ESD). To our knowledge, this is the first case of ESD for AMM, suggesting that ESD can potentially be a diagnostic and treatment method for AMM.
CASE SUMMARY
A 77-year-old woman visited our hospital with a chief complaint of anal bleeding and a palpable rectal mass. Colonoscopy revealed a 20-mm protruded lesion in the lower rectum. After obtaining biopsy specimens from the lesion, although a malignant rectal tumor was suspected, a definitive diagnosis was not made. Endoscopic ultrasonography revealed tumor invasion into the submucosal layer but not the muscular layer. Therefore, we performed an excisional biopsy using ESD. Immunohistochemical examination of the ESD-resected specimen revealed tumor cells positive for Human Melanin Black-45, Melan-A, and S-100. Moreover, the tumor cells lacked melanin pigment; thus, a diagnosis of amelanotic AMM was made. Although the AMM had massively invaded the submucosal layer and both lymphatic and venous invasion were present, we closely monitored the patient without any additional therapy on the basis of her request. Six months after ESD, local recurrence was detected, and the patient consented to wide local excision.
CONCLUSION
It is suggested that ESD is a potential diagnostic and treatment method for AMM.
Keywords: Endoscopic submucosal dissection, Anorectal malignant melanoma, Endoscopic mucosal resection, Case report
Core tip: For anorectal malignant melanoma (AMM), surgical procedures such as abdominoperineal resection and wide local excision have been the most common treatment methods used until now. We recently encountered a case in which endoscopic submucosal dissection (ESD) was used for an excisional biopsy of AMM. ESD may be effective for an early and accurate AMM diagnosis because the ESD-resected specimen could provide adequate pathological findings. In addition, as the ESD technique can yield a high en bloc resection rate, ESD may effectively treat early-stage AMM. Thus, it is suggested that ESD is a potential diagnostic and treatment method for AMM.
INTRODUCTION
Anorectal malignant melanoma (AMM) is a rare disease, accounting for less than 1% of all melanomas and approximately 1% of carcinomas arising from the anorectal region. The prognosis of AMM is extremely poor, with a median survival of 24 mo and a 5-year survival rate of 10%[1]. Clinical manifestations are non-specific[2], and the histological features of AMM overlap with those of other tumors, such as sarcoma, lymphoma, and undifferentiated carcinoma[3]. These non-specific clinical features contribute to delayed diagnosis, and almost 60% of patients are diagnosed with metastatic lesions at initial diagnosis[4]. Although surgical procedures such as abdomi-noperineal resection (APR) and wide local excision (WLE) have been the mainstay treatments for AMM[5], increasing evidence suggests that the surgical method affects the local recurrence rate but not survival outcomes[6,7]. On the other hand, less invasive therapies such as endoscopic mucosal resection (EMR) may be effective for early-stage AMM[8,9].
We recently encountered a case in which endoscopic submucosal dissection (ESD) was used for an excisional biopsy of AMM. To our knowledge, this is the first case of ESD for AMM. Given that our case suggests the efficacy of ESD as an excisional biopsy for AMM, we present this case with a brief review of the literature.
CASE PRESENTATION
Chief complaints
A 77-year-old woman visited our hospital with a chief complaint of anal bleeding and a palpable rectal mass.
History of present illness
Anal bleeding and a palpable rectal mass were observed one month before presen-tation.
History of past illness
The patient had no notable previous medical history.
Physical examination
Digital rectal examination revealed a nodular mass just above the dentate line.
Laboratory examinations
Blood analysis revealed that tumor markers, including carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9, were within the normal range (CEA level of 1.7 ng/mL and CA19-9 level of 13.9 U/mL). Complete blood count and results from blood biochemical analysis were normal.
Imaging examinations
Total colonoscopy revealed no abnormality in the colon. However, it revealed a 20-mm protruded lesion in the lower rectum just above the dentate line, which was thought to be the cause of the patient’s chief complaint (Figure 1). Narrow-band imaging with magnifying endoscopy (NBI-ME) revealed non-structural areas and some thick, unevenly sized vessels with branching and curtailed irregularity (Figure 2). Endoscopic ultrasonography (EUS) revealed the tumor invading the submucosal layer but not the muscular layer (Figure 3). Contrast-enhanced computed tomography (CECT) and fluorodeoxyglucose positron emission tomography/computed tomo-graphy (FDG PET/CT) showed no lymph nodes or distant metastases.
Figure 1.
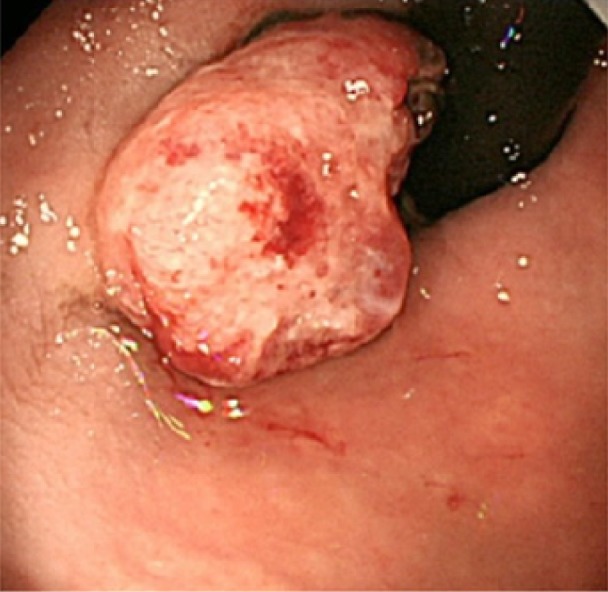
Colonoscopic findings. A 20-mm protruded lesion in the lower rectum just above the dentate line is detected.
Figure 2.
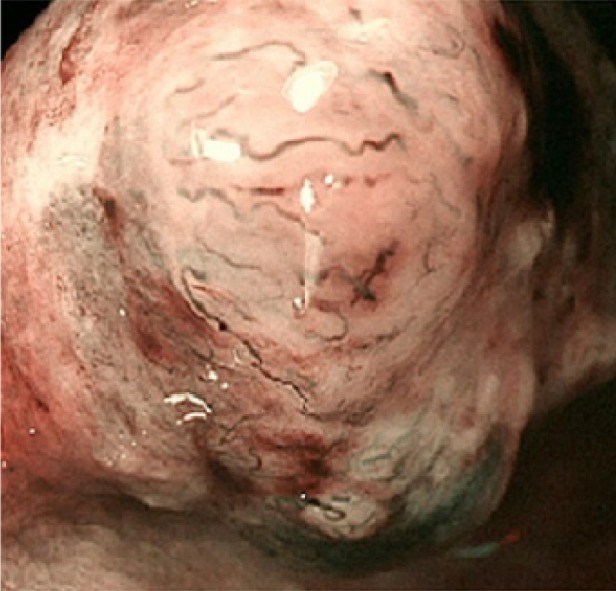
Narrow-band imaging with magnifying endoscopy findings. Non-structural areas and some thick, unevenly sized vessels with branching and curtailed irregularity are observed.
Figure 3.
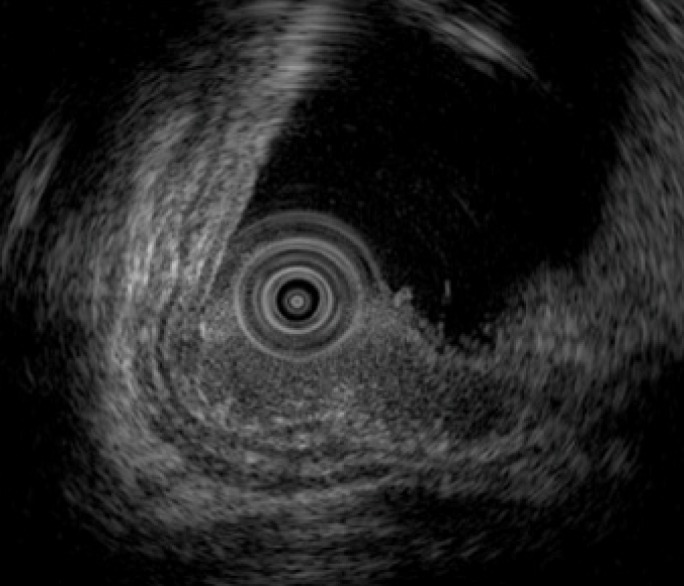
Endoscopic ultrasonography findings. Endoscopic ultrasonography image shows the lesion as a hypoechoic mass. The tumor invades the submucosal layer but not the muscular layer.
Further diagnostic work-up
Biopsy specimens of the lesion showed diffuse infiltration of round atypical cells with irregular nuclei. On the basis of the endoscopic findings and histopathological examination of the biopsy specimens, a malignant rectal tumor, including adenocar-cinoma, neuroendocrine tumor, and malignant lymphoma, was suspected. However, we could not make a definite diagnosis regardless of several sufficiently sized biopsy specimens. In addition, since this lesion was expected to have invaded the submuco-sal layer on the basis of EUS findings, endoscopic therapy alone was considered insufficient. However, considering that this lesion was located in the lower rectum, we believed that ESD would be a better first choice as an excisional biopsy than other surgical procedures that would be highly invasive. Therefore, we performed an excisional biopsy via ESD instead of re-performing biopsy using forceps. Histopatho-logical examination of the ESD-resected specimen revealed diffuse infiltration of round tumor cells with irregular nuclei (Figure 4A). Additionally, immunohisto-chemical examination revealed tumor cells positive for Human Melanin Black-45, Melan-A, and S-100 (Figure 4B-D). In addition, the tumor cells lacked melanin pigment; thus, a diagnosis of amelanotic AMM was made. Although all margins of the ESD-resected specimen were free from melanoma cells, the AMM had invaded the submucosal layer up to 7000 µm, and both lymphatic and venous invasion were noted.
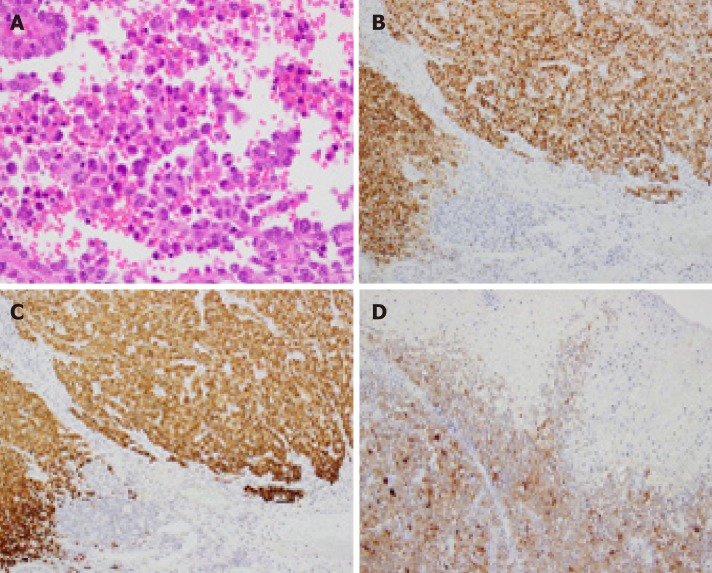
Figure 4.
Histopathological and immunohistochemical findings of the endoscopic submucosal dissection-resected specimen. A: Hematoxylin and eosin staining shows diffuse infiltration of round tumor cells with irregular nuclei. Melanin pigment is absent (magnification × 400). The tumor cells are positive for B: Human Melanin Black-45 (× 200), C: Melan-A (× 200), and D: S-100 (× 100).
FINAL DIAGNOSIS
On the basis of the histopathological findings of the ESD-resected specimen, a dia-gnosis of amelanotic AMM was made.
TREATMENT
ESD as an excisional biopsy was performed using a colonoscope (CF-Q260AI; Olympus Corporation, Tokyo, Japan), Flush Knife BT-S (DK2620J, FUJIFILM Medical, Tokyo, Japan), and high-frequency generator unit (VIO300D, Erbe Elektromedizin GmbH, Tuebingen, Germany).
First, as the tumor was located very close to the dentate line, we injected lidocaine solution into the submucosal layer to prevent the patient’s pain during the procedure. Subsequently, we started the mucosal incision from the anal side of the lesion. After creating a mucosal flap, we performed mucosal incision of the entire circumference around the lesion with an adequate margin and continued with submucosal dissection. No non-lifting sign was observed during ESD. The operation time was 62 min, and the lesion was resected en bloc without any complications. The ESD-resected specimen and the tumor measured 40 mm × 30 mm and 20 mm × 15 mm, respectively (Figure 5). The patient was discharged on postoperative day 4.
Figure 5.
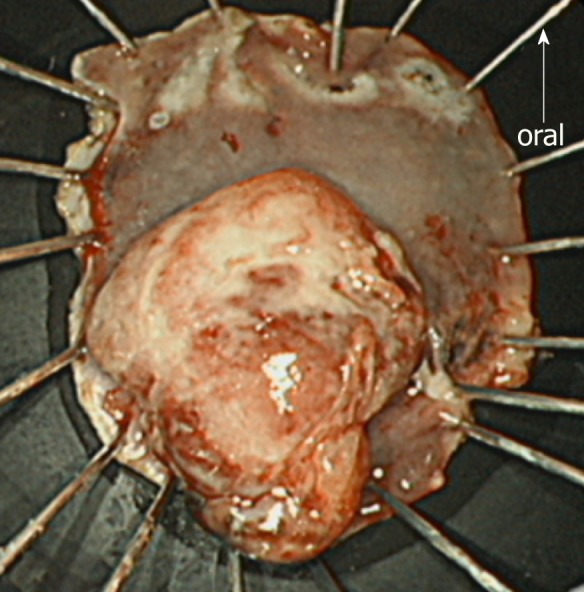
Endoscopic submucosal dissection-resected specimen. The endoscopic submucosal dissection-resected specimen is 40 × 30 mm in size. The tumor is protruded and measures 20 × 15 mm. Macroscopically, an adequate margin from the tumor, especially on the oral side, is ensured.
OUTCOME AND FOLLOW-UP
After obtaining the histopathological results of the ESD-resected specimen, we recommended additional surgical treatment, such as APR or WLE, with adjuvant chemotherapy. However, the patient and her family requested observation without any additional therapy because of the advanced age of the patient, high invasiveness and lack of proven efficacy of the suggested therapies, and a small chance of no recurrence. Therefore, we closely monitored the patient by endoscopic examination, CECT, and FDG PET/CT, as requested. During the 6-mo follow-up period, the patient was asymptomatic, and there was no evidence of recurrence (Figure 6). However, 6 mo after ESD, local recurrence was detected on endoscopic examination (Figure 7), following which the patient and her family finally consented to WLE.
Figure 6.
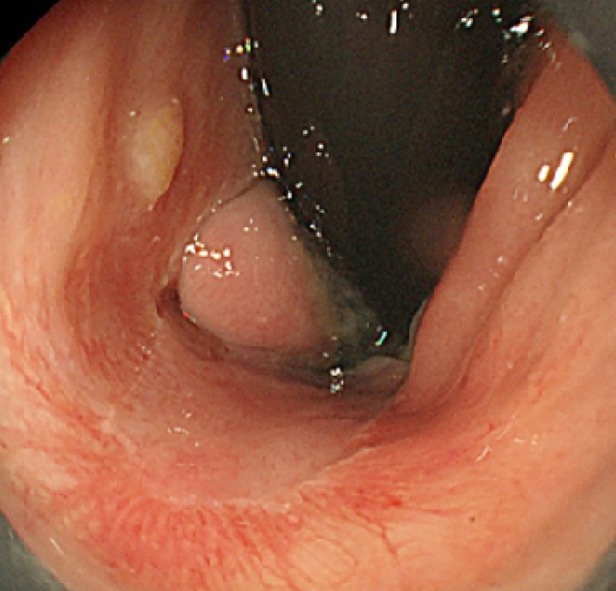
Follow-up endoscopic findings 3 mo after the endoscopic submucosal dissection. The post-endoscopic submucosal dissection ulcer is healed, and no local recurrence is detected.
Figure 7.
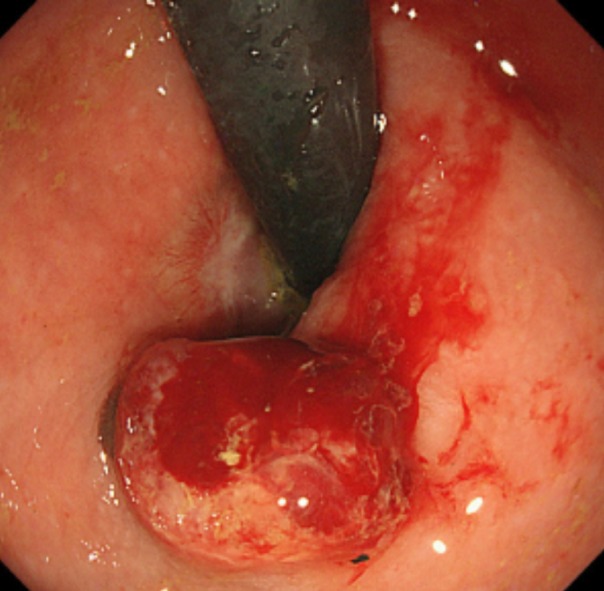
Follow-up endoscopic findings 6 mo after the endoscopic submucosal dissection. A 10-mm protruded lesion is identified about 10 mm from the oral side of the post-endoscopic submucosal dissection scar. Biopsy specimens from this lesion confirm a malignant melanoma.
DISCUSSION
AMM is a rare disorder arising from melanocytes in the mucosa around the anorectal region, and its prognosis is extremely poor[1]. The 5-year survival rate varies according to the presence of metastasis. The 5-year survival rate when AMM is confined to a local area has been reported to be 32%, whereas that in the presence of regional or distant disease decreases to 17% or 0%, respectively[10]. However, as high as 60% of patients are diagnosed with metastatic lesions at initial diagnosis[4].
Given that AMM is rare and its clinical features lack specificity, the rate of misdia-gnosis is high (57.14%–58.23%)[7,11]; this, in turn, may lead to its delayed diagnosis and poor prognosis. Further, amelanotic AMM, which accounts for approximately 20% of AMM cases[6], is more likely to be misdiagnosed. The causes for misdiagnosis include the lack of sufficient knowledge about this disease among endoscopists, the lack of specific clinical features, and the difficulty in its pathological diagnosis. Especially, an accurate diagnosis of amelanotic AMM could not be established without immuno-histochemical examination[7]. In our case, a diagnosis of amelanotic AMM was not established based on the NBI-ME, EUS, and histopathological examination of the biopsy specimens, which were performed before ESD. It was only after the excisional biopsy via ESD that we were able to diagnose amelanotic AMM. Thus, ESD was essential in establishing the diagnosis of amelanotic AMM in this particular case.
Although there is currently no consensus on the treatment methods for AMM because of its infrequency and poor biological behavior, surgical procedures such as APR and WLE have been the most common treatment methods used until now[5]. Additionally, there has been a long-standing debate regarding whether APR or WLE is better in terms of long-term survival and quality of life. Although designing a prospective, large-scale study is unrealistic because of the low incidence of AMM, some studies have suggested that there are no significant differences in survival outcomes between patients treated with APR and those treated with WLE and that APR is superior to WLE only in terms of the local recurrence rate despite the high invasiveness of APR[6,7]. Therefore, considering the extremely poor prognosis of AMM and that local recurrence could be controlled by salvage surgery, WLE may be a better first-choice surgical procedure for AMM than APR.
On the other hand, recently, only a few cases of AMM have been treated with EMR[8,9]; the advantage of this procedure is that it is much less invasive than conven-tional surgical procedures. Moreover, these cases suggest that early-stage AMM could be managed by endoscopic therapy alone. Compared with EMR, the ESD technique can yield a high en bloc resection rate for a lesion, irrespective of its size or shape[12]. Therefore, compared to EMR, ESD could more effectively treat early-stage AMM. Indeed, ESD was applied to a few cases of esophageal malignant melanomas, and long-term survival was achieved in these cases[13,14]. However, to our knowledge, this is the first case of ESD for AMM.
Unfortunately, in this particular case, as the AMM had massively invaded the submucosal layer, and as both lymphatic and venous invasion were present, the possibility of recurrence or distant metastasis was speculated to be extremely high, and ESD alone was considered insufficient therapy. Indeed, local recurrence occurred, and the patient subsequently underwent WLE. However, we were able to establish the diagnosis of AMM correctly from the ESD-resected specimen and were able to present the option of additional therapy to the patient subsequently.
Thus, although early diagnosis of AMM may contribute to definitive resection and better survival outcomes, prompt and accurate diagnosis of AMM is difficult, leading to a high incidence of locally advanced or metastatic lesions at the time of diagnosis[15]. In those cases, ESD may be effective for an early and accurate diagnosis of AMM, as the ESD-resected specimen could provide adequate pathological findings, compared to biopsy specimens, for pathologists to arrive at a diagnosis of AMM. In addition, compared to EMR, the ESD technique can yield a high en bloc resection rate. Further, it is relatively easier to perform ESD for lower rectal lesions than for lesions located in other parts of colon, and perforation does not occur as long as the lesion is located under the level of peritoneal reflection. Therefore, ESD could be a relatively safe and better endoscopic treatment method than EMR for early-stage AMM. Thus, it is suggested that ESD is a potential diagnostic and treatment method for AMM.
CONCLUSION
This case report highlights the efficacy of ESD as an excisional biopsy for AMM. This case suggests that ESD is effective in establishing a diagnosis of AMM because the ESD-resected specimen could provide adequate pathological findings to make a diagnosis of AMM. In addition, as the ESD technique can yield a high en bloc resection rate, ESD could be one of the endoscopic treatment methods for early-stage AMM. Further cases are needed to confirm the efficacy and limitations of ESD for AMM.
Footnotes
Informed consent statement: The patient was offered written explanations before the start of the treatment, and she provided consent.
Conflict-of-interest statement: The authors declare that there is no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: March 2, 2019
First decision: March 29, 2019
Article in press: May 2, 2019
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: de Moura DTH, Lim SC, Shin SJ S-Editor: Gong ZM L-Editor: A E-Editor: Wang J
Contributor Information
Shigeo Manabe, Department of Gastroenterology, Kouseikai Takeda Hospital, Kyoto 600-8558, Japan. s-manabe@takedahp.or.jp.
Yoshio Boku, Fujita Clinic, 67, Gokiya-cho, Oomiya-dori Shichijo-kudaru, Shimogyo-ku, Kyoto 600-8267, Japan.
Michiyo Takeda, Department of Gastroenterology, Kouseikai Takeda Hospital, Kyoto 600-8558, Japan.
Fumitaka Usui, Department of Gastroenterology, Kouseikai Takeda Hospital, Kyoto 600-8558, Japan.
Ikuhiro Hirata, Department of Gastroenterology, Kouseikai Takeda Hospital, Kyoto 600-8558, Japan.
Shuji Takahashi, Department of Gastroenterology, Kouseikai Takeda Hospital, Kyoto 600-8558, Japan.
References
- 1.Kong X, Liu Q, Lin G, Zhao D, Qiu H, Cui Q. The first attempt in local excision of anorectal malignant melanoma using transanal endoscopic microsurgery. Int J Clin Exp Pathol. 2015;8:11735–11740. [PMC free article] [PubMed] [Google Scholar]
- 2.Nam S, Kim CW, Baek SJ, Hur H, Min BS, Baik SH, Kim NK. The clinical features and optimal treatment of anorectal malignant melanoma. Ann Surg Treat Res. 2014;87:113–117. doi: 10.4174/astr.2014.87.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erdas E, Calò PG, Licheri S, Pomata M. Unexpected post-operative diagnosis of primary rectal melanoma. A case report. G Chir. 2014;35:137–139. [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi T, Velasco L, Zarate X, Medina-Franco H, Cortes R, de la Garza L, Gamboa-Dominguez A. Anorectal melanoma: report of three cases with extended follow-up. South Med J. 2004;97:311–313. doi: 10.1097/01.SMJ.0000091034.77040.AC. [DOI] [PubMed] [Google Scholar]
- 5.Keskin S, Tas F, Karabulut S, Yildiz I, Kiliç L, Ciftci R, Vatansever S. The role of surgical methods in the treatment of anorectal malignant melanoma (AMM) Acta Chir Belg. 2013;113:429–433. [PubMed] [Google Scholar]
- 6.Matsuda A, Miyashita M, Matsumoto S, Takahashi G, Matsutani T, Yamada T, Kishi T, Uchida E. Abdominoperineal resection provides better local control but equivalent overall survival to local excision of anorectal malignant melanoma: a systematic review. Ann Surg. 2015;261:670–677. doi: 10.1097/SLA.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 7.Che X, Zhao DB, Wu YK, Wang CF, Cai JQ, Shao YF, Zhao P. Anorectal malignant melanomas: retrospective experience with surgical management. World J Gastroenterol. 2011;17:534–539. doi: 10.3748/wjg.v17.i4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, Lee JR, Yoon HS, Jung TY, Lee EJ, Lim JG, Ko SY, Wang JH, Lee JD, Kim HY. Primary anorectal malignant melanoma treated with endoscopic mucosal resection. Intest Res. 2015;13:170–174. doi: 10.5217/ir.2015.13.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka S, Ohta T, Fujimoto T, Makino Y, Murakami I. Endoscopic mucosal resection of primary anorectal malignant melanoma: a case report. Acta Med Okayama. 2008;62:421–424. doi: 10.18926/AMO/30952. [DOI] [PubMed] [Google Scholar]
- 10.Podnos YD, Tsai NC, Smith D, Ellenhorn JD. Factors affecting survival in patients with anal melanoma. Am Surg. 2006;72:917–920. [PubMed] [Google Scholar]
- 11.Zhang S, Gao F, Wan D. Effect of misdiagnosis on the prognosis of anorectal malignant melanoma. J Cancer Res Clin Oncol. 2010;136:1401–1405. doi: 10.1007/s00432-010-0793-z. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Zhang XH, Ge J, Yang CM, Liu JY, Zhao SL. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal tumors: a meta-analysis. World J Gastroenterol. 2014;20:8282–8287. doi: 10.3748/wjg.v20.i25.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eleftheriadis N, Inoue H, Ikeda H, Onimaru M, Yoshida A, Hosoya T, Maselli R, Hamatani S, Kudo SE. Endoscopic submucosal dissection for primary malignant esophageal melanoma (with video) Gastrointest Endosc. 2013;78:359; discussion 360. doi: 10.1016/j.gie.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Wang M, Chen J, Sun K, Zhuang Y, Xu F, Xu B, Zhang H, Li Q, Zhang D. Primary malignant melanoma of the esophagus treated by endoscopic submucosal dissection: A case report. Exp Ther Med. 2016;12:1319–1322. doi: 10.3892/etm.2016.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Sun S, Liu X, Ge N, Wang G, Guo J, Liu W, Wang S. Endoscopic diagnosis of primary anorectal melanoma. Oncotarget. 2017;8:50133–50140. doi: 10.18632/oncotarget.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]