Abstract
Recently the field of cholestasis has expanded enormously reflecting an improved understanding of the molecular mechanisms underlying bile secretion and its perturbation in chronic cholestatic disease. Novel anti-cholestatic therapeutic options have been developed for patients not favorably responding to ursodeoxycholic acid (UDCA), the current standard treatment for cholestatic liver disease. Important novel treatment targets now also include nuclear receptors involved in bile acid (BA) homoeostasis like farnesoid X receptor and G protein-coupled receptors e.g., the G-protein-coupled BA receptor “transmembrane G coupled receptor 5”. Fibroblast growth factor-19 and enterohepatic BA transporters also deserve attention as additional drug targets as does the potential treatment agent norUDCA. In this review, we discuss recent and future promising therapeutic agents and their potential molecular mechanisms in cholestatic liver disorders.
Keywords: Bile acids, Drug therapy, Cholestatic liver disease, Nuclear receptor agonists
Core tip: Anti-cholestatic therapeutic options now go beyond ursodeoxycholic acid (UDCA) and target nuclear receptors regulating bile acid (farnesoid X receptor) and G protein-coupled receptor and fibroblast growth factor-19. Additionally, enterohepatic bile acid transporters and norUDCA represent potential targets in cholestatic disease.
INTRODUCTION
Cholestasis is a clinical syndrome that results from diminished bile formation by hepatocytes or impaired bile secretion at the level of cholangiocyte or obstruction of bile flow by stones (cholelithiasis) or by tumor bulk[1]. Consequently, cholestasis can be either extra- or intra-hepatic in etiology. The common causes of extrahepatic cholestatic liver disease include choledocholithiasis, tumors and parasitic infections. Several causes of intrahepatic cholestasis include immune-mediated conditions like primary biliary cholangitis, primary sclerosing cholangitis (PSC), exposure to several medications (steroids, nonsteroidal anti-inflammatory drugs, antibiotics, anti-diabetic agents) and inborn errors of cholesterol/bile acid (BA) biosynthesis and/or metabolism. Irrespective of the root cause(s), these syndromes are characterized by retention of products which under normal circumstances would be excreted into bile, particularly bile salts (BS). Cholestasis itself causes progressive bile duct injury and drives further retention of toxic hydrophobic BAs, which cause persistent and extensive damage to the bile duct. To adequately treat cholestatic injury, it is necessary to first identify and appropriately target those defective secretory mechanisms and/or remove or remediate lesions that obstruct or interfere with bile duct patency.
Chronic cholestatic disorders significantly increase the burden on health care from liver diseases. Since hepatitis C treatment has recently become tremendously successful, there is pressing need for more effective therapies to correct cholestatic liver diseases. The pathogenesis of many cholestatic liver disorders has been evolving with most research in bile formation/secretion pathways which has resulted in a change in the paradigms for treatment. Recently several molecular targets for diverse aspects of BA signaling and transport have been developed[2]. These approaches represent major developments which may lead to increased interest in exploring the novel paradigms and molecular approaches as better disease-modifying drugs in the treatment of chronic cholestasis.
Primary biliary cholangitis/cirrhosis (PBC) and PSC are prototype of chronic cholestatic liver diseases and we consider them here as model diseases to discuss the medical management of cholestasis. In the first part of this review we provide a short overview of BA synthesis, transport and regulation as an in-depth review of BAs is beyond the scope of this article. This will help the reader to better understand later described novel medical therapies from ursodeoxycholic acid (UDCA) and beyond (Table 1).
Table 1.
Novel Drugs for cholestatic liver diseases
| Class | Drug | Mechanism of action | Trial |
| Bile acid | UDCA | Increases biliary bicarbonates; Anti-apoptotic, anti-inflammatory | |
| Nor UDCA | Cholehepatic; shunting; Choleretic | NCT01755517 | |
| FXR ligands | Obetocholic acid | Reduced hepatic bile salt synthesis; Anti-inflammatory, Immunomodulator, Choleretic | POISE COBALT AESOP |
| GS9674 | NCT02854605 | ||
| LJN452/Tropifexor | NCT02516605 | ||
| FGF-19 mimetics | NGM 282 | Reduced bile acid synthesis | NCT02135536 |
| TGR5 agonist | INT-777 | Decreased bile acid pool, Choleretic, Anti-inflammatory, improves intestinal barrier | Not in trial |
| INT-767 | Not in trial | ||
| PPAR agonist | Banzfibrate | Increase biliary phospholipid concentration, Anti-inflammatory | NCT01654731 |
| MBX-8025 | NCT02609048 | ||
| Elafibrinor | NASH trials | ||
| ASBT inhibitor | A4250 | Dose dependent reduction in bile acids | |
| Maralixibat | CLARITY | ||
| GSK2330672 | |||
| Immunomodulator | FFP-104 | Anti CD40 human monoclonal IgG4 | NCT02193360 |
UDCA: Ursodeoxycholic acid; ASBT: Apical sodium-dependent bile salt transporter; FXR: Farnesoid X receptor; FGF: Fibroblast growth factor; TGR: Transmembrane G coupled receptor; PPAR: Peroxisome proliferator-activated receptor.
BILE ACID SYNTHESIS, TRANSPORT AND REGULATION
BAs act as detergents in initiating bile flow. BAs also facilitate intestinal absorption of lipids and function as signaling molecules which can activate nuclear and membrane receptors. BAs (along with phospholipids and cholesterol) represent the major constituents of bile in humans. BA synthesis is a complex metabolic pathway involving hydroxylation and modification of cholesterol. BA synthesis can be summarized into three main steps: (1) Modification of the steroid ring; (2) Cleavage of the cholesterol side chain; and (3) Conjugation with glycine or taurine. This latter step involves 2 pathways: BA synthesis the “classical” pathway produces cholic acid (CA) and chenodeoxycholic acid (CDCA), responsible for 90% of primary BA synthesis while the alternative pathway can only produce CDCA in humans[3]. Conjugation with glycine/taurine makes BAs more hydrophilic and more readily secretable in the bile. Cytochrome P450 7A1 (CYP7A1) is the rate-limiting enzyme in BA synthesis. Conjugated BAs, (mainly glycoconjugates), exist as anionic salts called “BS” under physiological conditions. These BS are stored in the gallbladder and are released into the intestinal lumen upon a meal-related gall bladder contraction. Here, they help in absorption of fat and fat-soluble vitamins. Conjugated primary BAs released into the intestinal lumen are further modified by the intestinal bacteria by deconjugation, oxidation and dehydroxylation to produce secondary BAs: Lithocholic acid (LCA) and deoxycholic acid (DCA).
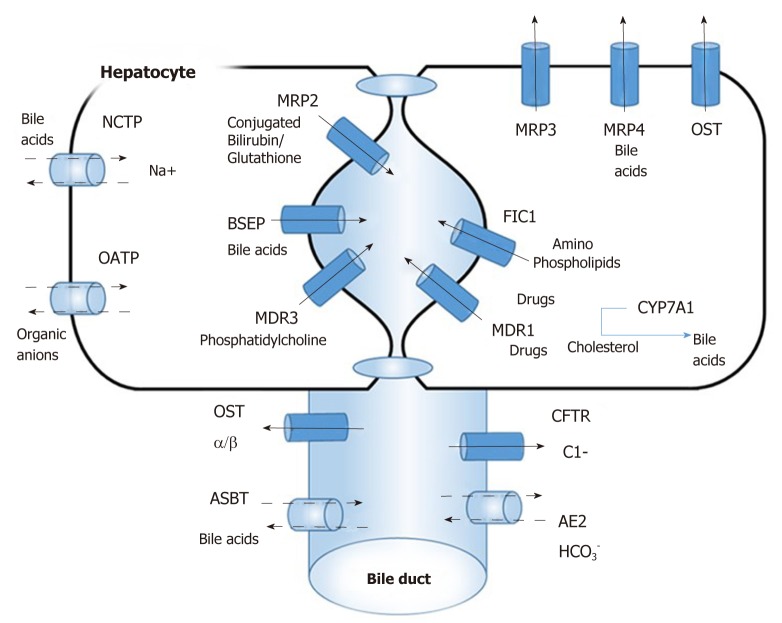
There are specific uptake and export systems for biliary compounds in liver hepatocytes and cholangiocytes[4] (Figure 1) The determinants of hepatic uptake of BAs include the sodium taurocholate co-transporting polypeptide (NTCP) and a family of multi-specific organic anion transporters (OATPs) which are mainly responsible for the first pass clearance of conjugated BAs as they are returned to the liver in portal blood. Excretion of BAs in bile canaliculi is mediated by ATP-binding cassette (ABC) transporters. Bile-salt export pump (BSEP) for excretion of monovalent BAs and conjugate export pump MRP2 for excretion of bilirubin and divalent BAs are present on canalicular membrane. The multidrug export pump MDR1 assists in the excretion of cationic drugs. The phospholipid export pump MDR3 “flops” phosphatidyl-choline from the inner to outer membrane leaflets, which forms mixed micelles together with BAs and cholesterol. Other BA export pumps, MRP3, MRP4 and organic solute transporter (OSTa/b) are present at the basolateral membrane and function as back-up pumps for alternative sinusoidal BA export. The cystic fibrosis transmembrane conductance regulator (CFTR) drives bicarbonate excretion and present only in cholangiocytes in the liver. The biliary epithelium also reabsorbs BAs via an apical Na+-dependent bile-salt transporter ASBT and the basolateral counterpart OSTa/b.
Figure 1.
Overview of bile acid transport system. Na+/ taurocholate cotransporter, organic anion transporters, Bile-salt export pump multidrug export pump MDR1, organic solute transporter OSTa/b, cystic fibrosis transmembrane conductance regulator, apical Na+-dependent bile-salt transporter apical sodium-dependent bile-salt transporter. NTCP: sodium taurocholate co-transporting polypeptide; OATPs: Organic anion transporters; BSEP: Bile-salt export pump; CFTR: Cystic fibrosis transmembrane conductance regulator; OST: Organic solute transporter; ASBT: Apical sodium-dependent bile-salt transporter.
Hepatobiliary transport systems are regulated by ligand-activated nuclear receptors (NRs) at transcriptional levels via positive and negative feedback pathways[5]. Amongst the NRs for BAs, farnesoid X receptor (FXR), is involved in the regulation of NTCP and Na+-independent hepatocellular BA uptake (OATP1B1 and OATP1B3)[6], canalicular excretion of monovalent (BSEP) and divalent BAs (MRP2), conjugated bilirubin (MRP2) and FXR also regulates the rate limiting enzyme of BA production CYP7A1[7]. The other classical ligand-activated NRs, pregnane X receptor (PXR), the constitutive androstane receptor and the vitamin D receptor also play important roles in the regulation of BAs and bilirubin transport in addition to detoxifying xenobitics[8]. In summary, BA homeostasis is tightly regulated by the negative feedback effect of BAs on the activity and expression of CYP7A1 as well as signaling via various NRs like FXR and PXR.
Inborn errors of BA biosynthesis and transport can present with chronic cholestasis with or without multisystem involvement in infant, children and adults. Most of these can be effectively treated medically if diagnosed early. These rare disorders include 3β-hydroxysteroid-Δ5-C27-steroid dehydrogenase deficiency, Δ4-3-oxosteroid 5β-reductase deficiency, sterol 27-hydroxylase deficiency, oxysterol 7α-hydroxylase deficiency, BA-CoA: Amino acid N-acyltransferase deficiency and BA-CoA ligase deficiency to name but a few[9].
UDCA: FIRST LINE THERAPY
UDCA is the accepted therapy for PBC and was the only treatment available until 2016. UDCA is a physiological BA which constitutes only a small proportion (3%) of the normal BA pool, but its proportion can increase to 40% in patients with PBC upon treatment with UDCA[10]. UDCA activates impaired hepatocellular secretion of hydrophobic BAs and stabilizes the biliary HCO3¯ “umbrella” effectively protecting the cholangiocytes from the toxic effects of hydrophobic BAs. Furthermore, UDCA also exhibits anti-apoptotic and anti-inflammatory effects[11,12]. UDCA has shown to improve biochemical and histological markers in PBC[13]. UDCA is also known to delay progression to cirrhosis and the time to liver transplantation[14]. UDCA response had also been demonstrated to reduce the risk of hepatocellular carcinoma. The accepted optimal dose of UDCA is 13-15 mg/kg/d. However, 35%-40% of PBC patients have an incomplete response to UDCA. PBC patients not responding to UDCA are known to have poor prognosis[15]. Studies have shown that the transplant-free survival rate among UDCA-treated patients is lower than control population[16], indicating that there is still a pressing need for newer therapeutic options particularly for patients not showing adequate response to UDCA.
The efficacy of UDCA in PSC patients remains a hotly debated issue. Clinical studies have shown that UDCA at therapeutic doses (15-20 mg/kg/d) improves serum liver chemistry, but is without any benefit to survival or histology[17]. Conversely, a recent study showed an adverse result in PSC patients treated with very high UDCA dosing (28-30 mg/kg/d)[18]. Therefore, at present there are no evidence-based recommendations for normal doses of UDCA in PSC, but it is known that very high-dose regimens should be avoided. UDCA is also used to treat other cholestatic conditions like intrahepatic cholestasis of pregnancy, progressive familial intrahepatic cholestasis type 3, Δ4-3-oxosteroid 5β-reductase deficiency and cystic fibrosis-associated liver disease although experimental support for UDCA efficacy in these setting is scarce. Thus, effective medical therapy for patients with these forms of chronic cholestatic liver injury is still an unmet medical need.
FARNESOID X RECEPTORS LIGANDS
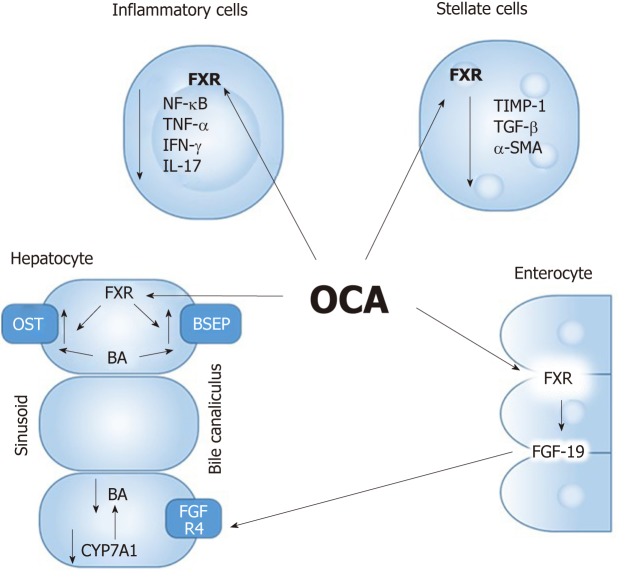
FXR was discovered in 1995 as an orphan NR[19]. Later it was found that natural BAs (CA and CDCA) are physiological ligands for the FXR receptor[20]. FXR exists as a heterodimer with the all-trans retinoic acid receptor RXR. This complex binds to the LR-1 DNA motive in the promoter region of target genes[21]. The receptor is predominantly expressed in the liver, intestine and to lesser extent in kidney, adipose tissue, muscle and adrenal glands. After a meal-induced gallbladder contraction, BAs enter the intestine and are reabsorbed in the terminal ileum where they activate FXR. FXR has a number of target genes in the intestinal cell and hepatocyte of which short heterodimer partner (SHP) and fibroblast growth factor (FGF)-19 are important. FXR regulates hepatic BA homeostasis via stimulation of SHP in the liver and activation of FGF-19 in the intestine, both inhibiting the rate-limiting enzyme cholesterol 7a-hydroxylase (CYP7A1) which causes reduced hepatic BA synthesis (Figure 2). Moreover, FXR controls BA influx (NTCP) and efflux (BSEP) systems, thereby limiting hepatic BA overload[22]. FXR also exerts positive immunosuppressive/immuno-modulatory and anti-inflammatory effects by attenuating nuclear factor of activated B cells (NF-kB)[23]. In addition, FXR may improve the intestinal epithelial barrier function under cholestatic conditions[24]. Thus, FXR activation predominantly leads to inhibition of BA synthesis with a net increase in choleresis through increase in canalicular secretion and BA reuptake inhibition.
Figure 2.
Mechanism of action of Farnesoid X receptor ligands. FXR: Farnesoid X receptor; OCA: Obetocholic acid; BA: Bile acid; FGF: Fibroblast growth factor; OST: Organic solute transporter; BSEP: Bile-salt export pump.
Obetocholic acid (6-ethyl-chenodeoxycholic acid) is a steroidal derivative of UDCA that regulates the FXR receptor and is 100 times more potent than the physiologically abundant CDCA. Obetocholic acid (OCA) has already been approved in PBC by regulatory authorities. In clinical phase III trials (POISE) both UDCA naive and UDCA non-responder PBC patients showed improvements in liver enzymes including serum levels of alkaline phosphatase (ALP) under OCA arm[25]. Half of their study population achieved the primary study endpoint which was reduction in ALP by 1.5-fold and 15% of the patients reached normal bilirubin levels (compared to only 10% in the placebo arm). Higher reductions in ALP were seen with higher doses of OCA. Gamma-glutamyltransferase, CRP and IgM immunoglobulin levels also decreased under OCA treatment. However, the study failed to show a change in the fibrosis score using transient elastography and did not find an improvement in overall quality of life as measured by PBC-40 questionnaire after 12 mo of OCA treatment.
In another study by Hirschfield et al[26], pruritus did not improve in 50% of the PBC patients, and even intensified in patients receiving 25 or 50 mg OCA. There are still some concerns related to OCA in PBC. Pruritus was most common reason for treatment discontinuation in both OCA studies which could be mitigated by dose-titration. However, development and worsening of pruritus is a barrier to treatment as most of the PBC patients have pruritus as a highly common symptom at baseline. Other adverse effects included lipid changes, predominantly reduction of potentially beneficial high density lipoprotein levels in patient treated with OCA, the clinical impact of which is unknown. Severe adverse events reported in clinical studies which occurred much less frequently included hyperbilirubinemia and hepatic decompensation. Recently, the Food and Drug Administration also issued a warning concerning severe liver injury and death related to OCA in PBC. Although not all data are yet available, it appears that deaths occurred in patients with advanced liver disease who received higher than recommended doses of OCA. So, whether the serious adverse events occurred due to OCA or because of liver disease progression remains unanswered. Another limitation includes lack of clinical data on transplant free survival and overall mortality. These questions may be answered in the “COBALT” study that is currently enrolling patients (ClinicalTrials.gov identifier: NCT02308111). OCA has also been tested in phase II PSC study (“AESOP”) involving 35 centers across United States and Italy. The preliminary results showed statistically significant reductions in ALP in the OCA-treated study arm compared to placebos[27]. As expected, the most common adverse event in the study was again pruritus. Some limitations of this study could be inclusion of patients with mainly earlier stage disease, shorter duration of therapy (up to 24 wk) and the use of ALP as surrogate endpoint for primary outcome. Other concerns about OCA use is the stimulation of FGF-19 which is produced in the terminal ileum upon activation of FXR by BS absorbed from intestine. FGF-19 is known to be pro-proliferative and pro-carcinogenic in mouse models[28]. Therefore, theoretically prolonged exposure to FGF-19 via OCA may increase risk of bile duct, gall bladder and colon cancer in PSC[29].
Other FXR agonists have been evaluated in phase I studies including the non-BA GS9674 (ClinicalTrials.gov identifier: NCT02854605) and LJN452 (ClinicalTrials.gov identifier: NCT02516605). In these studies, mild lipid changes observed in the treatment arm were not significantly different from placebo. LJN 452 (Tropifexor) is a non-BA FXR agonist engages the enterocyte as its target. Preclinical data demonstrate that LJN452 is highly selective, potent and causes dose dependent elevation of FGF-19. It is orally available, well tolerated and there is no finding of drug-related pruritus. GS9674 acts on the intestinal epithelium, resulting in the release of FGF-19, thus causing elevations of FGF-19 levels and ultimately diminishing lipogenesis, gluconeogenesis and BA synthesis[30]. Currently, both drugs are in phase II studies.
FGF-19 MIMETICS
FGF-19 is an enterokine induced after FXR activation by BAs in ileum. FGF-19 provides a negative feedback loop for BA synthesis from terminal ileum. FGF-19 is secreted into the portal circulation from enterocytes and reach the hepatocytes. At the surface of the hepatocyte, FGF-19 binds to the FGFR4/β-klotho receptor and blocks HNF4α and LRH-1 mediated transcription of CYP7A1. Blockade of CYP7A1 in turn reduces BA synthesis[31]. FGF-19 has strong metabolic effects as it suppresses lipogenesis and gluconeogenesis[32]. FGF-19 mimetics have been shown to be potent inhibitors of BA synthesis and sclerosing cholangitis in experimental animal models[33]. FGF-19 also improves fibrosis and has anti-inflammatory activities. Even if FGF-19 is ultimately reported to have proliferative and carcinogenic potential, the novel engineered FGF-19 analog (NGM 282), has been shown in animal models to retain full BA regulatory activity and to lack carcinogenic properties[34]. Still, there is no stable effective oral compounds available, hence these are all injectable drugs which raises similar concerns associated with other injectable medications like inducing antibody production and injection-site reactions. A clinical study of NGM 282 in patients with PBC not responding to UDCA showed a dose-related and dramatic drop in serum C4 which is a marker of de novo BA synthesis[35]. In this trial, NGM 282 achieved its endpoint of reduction in serum BAs in humans which in turn produced a reduction in ALP at 28 d. There was no increase in pruritus but did show an unexpected increase in non-serious GI symptoms as diarrhea, loose stools which are thought to be due to pro-secretory and pro-motility actions of NGM 282. NGM 282 in combination with UDCA has now also entered phase II testing in PBC patients (ClinicalTrials.gov identifier: NCT02135536).
TRANSMEMBRANE G COUPLED RECEPTOR 5 (TGR5) AGONIST
TGR5 is membrane bound BA specific cell surface receptor[36]. TGR 5 is expressed mainly in the liver (Kupffer cells and cholangiocytes) as well as in adipose tissue, spleen, gall bladder and colon tissues (especially intestinal L cells)[37,38]. TGR5 inhibits pro-inflammatory cytokine production and activation of macrophages and Kupffer cells in the liver in part by suppression of NF-kB signaling[39]. TGR5 is also involved in modulation of intestinal inflammation and motility. Mouse models have shown that lacking TGR5 leads to a decreased total BA pool size, increased CYP7A1 gene expression and a more hydrophobic BA composition[37]. TGR5 also improves intestinal barrier function[40].
INT-777 [6α-ethyl-23(S)-methylcholic acid] is a semisynthetic, selective and potent TGR5 agonist[41]. In animal studies, INT-777 has shown to increase bile flow and improve liver function with concomitant reductions in steatosis, suggesting its potential in NASH treatment. However, currently there are no clinical trials with INT-777 in cholestatic liver diseases as activation of TGR5 is known to aggravate pruritus in animal models, a common symptom in patients with cholestasis[42].
INT-767, a semisynthetic BA analogue, inhibits BA synthesis, stimulates bicarbonate-rich choleresis and causes immunomodulation by inhibiting NF-κB via dual FXR and TGR5 agonist actions but has a higher affinity for FXR[43]. In a mouse model of sclerosing cholangitis, INT-767 was shown to reduce liver enzymes, markers of liver inflammation and fibrosis[44]. Therefore, INT-767 appears promising therapeutic agent in treating cholestasis and it is currently entering phase I clinical trials.
Interestingly, a single-nucleotide polymorphism of TGR5 is also seen in PSC, suggesting that this receptor could be a potential therapeutic target in PSC[45]. However, a TGR5 agonist approach failed improve cholestatic liver injury in a mouse model of sclerosing cholangitis. Other concerns over the use of TGR5 agonists may arise due to some of its undesirable off-target effects like bile reflux-induced pancreatitis in animal models[46]. TGR5 mediated proliferative effects on cholangio-cytes[47] and its role in carcinogenesis (via its interaction with EGFR in gastric and esophageal adenocarcinoma[48]). Hence, TGR5 ligands, despite their therapeutic potential, do not appear to be candidates worthy of clinical development for cholestatic disorders.
PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR (PPAR) AGONIST
PPARs are NRs whose natural ligands are fatty acids and their derivatives. PPARs are present in skeletal muscles, liver, heart and GI tract[48]. PPAR-alpha response elements are prominent in the canalicular membrane phospholipid export pump (MDR3/ABCC4) and PPAR-alpha agonists cause increased biliary phospholipid concentrations which protect cholangiocytes from potentially damaging and toxic effects of BAs. PPAR-alpha also suppresses BA synthesis[49]. Furthermore, PPAR-alpha exerts anti-steatotic effects by stimulating fatty acid oxidation and has anti-inflammatory actions in the GI tract and systemically[50]. Fibrates are ligands for the PPAR-alpha and have been proposed to be beneficial as a second line therapy in PBC. A number of uncontrolled clinical studies, largely in patients with PBC, have shown significant improvements in ALP with bezafibrate, an activator of PPAR-alpha[51].
In a meta-analysis of studies comparing patients treated with UDCA plus bezafibrate vs UDCA alone, combination therapy performed better than monotherapy when considering biochemical parameters, however symptoms and overall survival were not different[52]. A recent prospective study reported potential beneficial effects of fenofibrate, a PPAR-alpha agonist among UDCA non-responders[53]. However, several potential concerns include increases in serum creatinine and bilirubin in patients with cirrhosis[54]. Currently a phase III study of bezafibrate (ClinicalTrials.gov identifier: NCT01654731) in patients with PBC with incomplete response to UDCA is under enrollment.
MBX-8025 is a selective, orally active and potent PPAR-delta agonist (ClinicalTrials.gov identifier: NCT02 609048). The results from phase II study with MBX-8025 are exciting, so far showing marked improvements in markers of cholestasis.
Elafibranor is an oral agent PPAR-alpha/delta agonist. In experimental NASH studies, elafibranor has been shown to decrease BA synthesis and to increase uptake and detoxification of BAs, via activation of PPAR-alpha in addition to its anti-inflammatory effects through NF-Kb mediated light chain enhancement of activated B cells[55]. Elafibranor has so far been evaluated in multiple phase II studies of dyslipidemia and nonalcoholic steatohepatitis. All these studies reported significant improvements in ALP and GGT levels in addition to their primary endpoints. A multicenter, randomized, double-blind, placebo-controlled, phase II study to evaluate the efficacy and safety of elafibranor in patients with PBC, non-responsive to UDCA, is currently recruiting patients.
CHOLEHEPATIC DRUG: NORUDCA
24-norursodeoxycholic acid (norUDCA) is a side chain shortened derivative of UDCA without a methylene group resulting in a resistance against side chain conjugation with taurine or glycine[56]. Nor-UDCA is passively absorbed from cholangiocytes and undergoes “cholehepatic shunting” (instead of the complete enterohepatic cycle) with induction of bicarbonate rich hypercholeresis which is key protective mechanism against BS toxicity[57]. NorUDCA also has potent anti-inflammatory, anti-proliferative and anti-fibrotic properties. NorUDCA is also more hydrophilic and consequently less toxic to hepatocytes and cholangiocytes in vitro than UDCA which may help to further reduce biliary toxicity. NorUDCA has shown to improve sclerosing cholangitis in animal models of this condition[58]. NorUDCA had been evaluated in phase II clinical trial in 116 PSC patients over 38 centers[59]. These results were promising with demonstration of pronounced reductions in ALP. NorUDCA was found to be effective in UDCA naïve and UDCA experienced-PSC patients, regardless of whether they had responses to therapy. Also, this drug was very well tolerated. Several limitations of the study were the short course of treatment, no data on FGF-19 levels and inclusion of patients with only earlier stages of disease and whose surrogate primary end point was reducing ALP.
ENTEROHEPATIC BLOCKERS
ASBT (Apical sodium-dependent BS transporter/ileal BA transporter) critically determines BA reabsorption and subsequently biliary BA concentrations. ASBT inhibitors block the uptake for BA to reduce the BA levels, thereby counteracting toxic BA-mediated liver injury and reducing pruritus. In mouse models of sclerosing cholangitis, ASBT inhibition reduced cholestatic liver injury and fibrosis by increasing fecal BA excretion, lowering toxic BA, and preserving biliary bicarbonate secretion[60]. A phase I clinical study with the ASBT inhibitor (A4250) showed dose-dependent reduction of serum BAs and FGF-19 levels in all treatment groups with major adverse events like diarrhea[61]. Maralixibat (an ASBT inhibitor) used in the CLARITY clinical trial in patients with PBC, showed significant reductions in serum BA, but was unable to show reductions in pruritus compared to placebo[62].
A recent phase II clinical trial with another ASBT inhibitor in PBC (GSK2330672) showed different results with significant reductions in pruritus but with increased gastrointestinal side effects which includes abdominal discomfort and diarrhea[63].
IMMUNOMODULATORS
FFP-104 is an anti-CD40 human monoclonal IgG4 antibody which is derived from the chimeric monoclonal antibody (ch5D12) that specifically targets human CD40. This agent is currently undergoing study in a pilot phase II, open-label, multicenter trial, to evaluate its safety and tolerability in subjects diagnosed with PBC (ClinicalTrials.gov identifier: NCT02193360). Whether combinations of these drugs with or without addition of UDCA can enhance the anti-cholestatic properties is still unknown. Currently more multicenter effort and partnerships with larger patient enrollments at both federal and commercial levels are required to improve the management of cholestatic liver diseases.
CONCLUSION
The field of treating chronic liver diseases is rapidly changing due to recent advances in understanding the molecular mechanisms of hepatocellular and cholangiocelluar cholestasis. Newer insights into the diverse roles played by BAs have led to the development of new therapeutic targets mainly for receptors and transcription factors controlling BA metabolism such as FXR, TGR5, and PPARs. In addition, several novel therapies such as FGF-19 mimetics, norUDCA, and ASBT inhibitors have been introduced as powerful and novel therapeutic options. The rapid development of more specific, safer and easily administered drugs aimed at treating cholestasis ensures even greater therapeutic success for managing chronic cholestatic liver diseases in the near future.
Footnotes
Conflict-of-interest statement: The authors declare that there is no conflict of interest regarding the publication of this paper.
Manuscript source: Invited manuscript
Peer-review started: January 10, 2019
First decision: January 30, 2019
Article in press: June 10, 2019
Specialty type: Medicine, Research and Experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali FEM, Kawy HAS, Chen CJ, Joshi D S-Editor: Dou Y L-Editor: A E-Editor: Liu JH
Contributor Information
Hrishikesh Samant, Division of Gastroenterology and Hepatology, Department of medicine, LSU health, Shreveport, LA 71103, United States; John C McDonald Transplant Center, Willis Knighton Medical Center, Shreveport, LA 71103, United States.
Wuttiporn Manatsathit, Division of Gastroenterology and Hepatology, University of Nebraska, Omaha, NE 68194, United States.
David Dies, John C McDonald Transplant Center, Willis Knighton Medical Center, Shreveport, LA 71103, United States.
Hosein Shokouh-Amiri, John C McDonald Transplant Center, Willis Knighton Medical Center, Shreveport, LA 71103, United States.
Gazi Zibari, John C McDonald Transplant Center, Willis Knighton Medical Center, Shreveport, LA 71103, United States.
Moheb Boktor, Division of Gastroenterology and Hepatology, Department of medicine, LSU health, Shreveport, LA 71103, United States.
Jonathan Steve Alexander, Department of Molecular and Cellular Physiology, Louisiana State University, School of Medicine, Shreveport, LA 71103, United States. jalexa@lsuhsc.edu.
References
- 1.Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med. 1998;339:1217–1227. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- 2.Beuers U, Trauner M, Jansen P, Poupon R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol. 2015;62:S25–S37. doi: 10.1016/j.jhep.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 4.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–S102. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 5.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 6.Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276:28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 7.Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 8.Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm. 2006;3:231–251. doi: 10.1021/mp060010s. [DOI] [PubMed] [Google Scholar]
- 9.Corso G, Dello Russo A, Gelzo M. Liver and the defects of cholesterol and bile acids biosynthesis: Rare disorders many diagnostic pitfalls. World J Gastroenterol. 2017;23:5257–5265. doi: 10.3748/wjg.v23.i29.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dilger K, Hohenester S, Winkler-Budenhofer U, Bastiaansen BA, Schaap FG, Rust C, Beuers U. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133–140. doi: 10.1016/j.jhep.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Benz C, Angermüller S, Otto G, Sauer P, Stremmel W, Stiehl A. Effect of tauroursodeoxycholic acid on bile acid-induced apoptosis in primary human hepatocytes. Eur J Clin Invest. 2000;30:203–209. doi: 10.1046/j.1365-2362.2000.00615.x. [DOI] [PubMed] [Google Scholar]
- 12.Miura T, Ouchida R, Yoshikawa N, Okamoto K, Makino Y, Nakamura T, Morimoto C, Makino I, Tanaka H. Functional modulation of the glucocorticoid receptor and suppression of NF-kappaB-dependent transcription by ursodeoxycholic acid. J Biol Chem. 2001;276:47371–47378. doi: 10.1074/jbc.M107098200. [DOI] [PubMed] [Google Scholar]
- 13.Poupon R, Chrétien Y, Poupon RE, Ballet F, Calmus Y, Darnis F. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis? Lancet. 1987;1:834–836. doi: 10.1016/s0140-6736(87)91610-2. [DOI] [PubMed] [Google Scholar]
- 14.Parés A, Caballería L, Rodés J, Bruguera M, Rodrigo L, García-Plaza A, Berenguer J, Rodríguez-Martínez D, Mercader J, Velicia R. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. UDCA-Cooperative Group from the Spanish Association for the Study of the Liver. J Hepatol. 2000;32:561–566. doi: 10.1016/s0168-8278(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 15.Lammers WJ, Hirschfield GM, Corpechot C, Nevens F, Lindor KD, Janssen HL, Floreani A, Ponsioen CY, Mayo MJ, Invernizzi P, Battezzati PM, Parés A, Burroughs AK, Mason AL, Kowdley KV, Kumagi T, Harms MH, Trivedi PJ, Poupon R, Cheung A, Lleo A, Caballeria L, Hansen BE, van Buuren HR Global PBC Study Group. Development and Validation of a Scoring System to Predict Outcomes of Patients With Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology. 2015;149:1804–1812.e4. doi: 10.1053/j.gastro.2015.07.061. [DOI] [PubMed] [Google Scholar]
- 16.Corpechot C, Carrat F, Bahr A, Chrétien Y, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology. 2005;128:297–303. doi: 10.1053/j.gastro.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, Mooney J, Sargeant C, Braaten J, Bernard T, King D, Miceli E, Schmoll J, Hoskin T, Thapa P, Enders F. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seol W, Choi HS, Moore DD. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- 20.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 22.Halilbasic E, Baghdasaryan A, Trauner M. Nuclear receptors as drug targets in cholestatic liver diseases. Clin Liver Dis. 2013;17:161–189. doi: 10.1016/j.cld.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu C, Fuchs CD, Halilbasic E, Trauner M. Bile acids in regulation of inflammation and immunity: friend or foe? Clin Exp Rheumatol. 2016;34:25–31. [PubMed] [Google Scholar]
- 24.Verbeke L, Farre R, Verbinnen B, Covens K, Vanuytsel T, Verhaegen J, Komuta M, Roskams T, Chatterjee S, Annaert P, Vander Elst I, Windmolders P, Trebicka J, Nevens F, Laleman W. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J Pathol. 2015;185:409–419. doi: 10.1016/j.ajpath.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U, Trauner M, Jones DE, Floreani A, Hohenester S, Luketic V, Shiffman M, van Erpecum KJ, Vargas V, Vincent C, Hirschfield GM, Shah H, Hansen B, Lindor KD, Marschall HU, Kowdley KV, Hooshmand-Rad R, Marmon T, Sheeron S, Pencek R, MacConell L, Pruzanski M, Shapiro D POISE Study Group. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 26.Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC, Jr, Parés A, Trauner M, Marschall HU, Adorini L, Sciacca C, Beecher-Jones T, Castelloe E, Böhm O, Shapiro D. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751–61.e8. doi: 10.1053/j.gastro.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Larusso NF, Bowlus CL, Levy C, Vuppalanchi R, Floreani A, Andreone P, Srestha R, Trotter J, Goldberg D, Rushbrook S, Hirschfield GM, Van Biene V, Penceck R, Macconell L, David S. The AESOP Trial: A Randomized, Double-Blind, Placebo-Controlled, Phase 2 Study of Obeticholic Acid in Patients with Primary Sclerosing Cholangitis. Digest Liver Dis. 2018;50:e67. [Google Scholar]
- 28.Nicholes K, Guillet S, Tomlinson E, Hillan K, Wright B, Frantz GD, Pham TA, Dillard-Telm L, Tsai SP, Stephan JP, Stinson J, Stewart T, French DM. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am J Pathol. 2002;160:2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zweers SJ, Booij KA, Komuta M, Roskams T, Gouma DJ, Jansen PL, Schaap FG. The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology. 2012;55:575–583. doi: 10.1002/hep.24702. [DOI] [PubMed] [Google Scholar]
- 30.Myers RP, Djedjos C, Kirby B, Bilin A, Khan M, Gosink J, Song Q, Srihari R. A198 Pharmacodynamic Effects of the Oral, Non-steroidal Farnesoid X Receptor Agonist GS-9674 in Healthy Volunteers. J Canadian Assoc of Gastroenterology. 2018;1:346. [Google Scholar]
- 31.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 32.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou M, Learned RM, Rossi SJ, DePaoli AM, Tian H, Ling L. Engineered fibroblast growth factor 19 reduces liver injury and resolves sclerosing cholangitis in Mdr2-deficient mice. Hepatology. 2016;63:914–929. doi: 10.1002/hep.28257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou M, Wang X, Phung V, Lindhout DA, Mondal K, Hsu JY, Yang H, Humphrey M, Ding X, Arora T, Learned RM, DePaoli AM, Tian H, Ling L. Separating Tumorigenicity from Bile Acid Regulatory Activity for Endocrine Hormone FGF19. Cancer Res. 2014;74:3306–3316. doi: 10.1158/0008-5472.CAN-14-0208. [DOI] [PubMed] [Google Scholar]
- 35.Mayo MJ, Wigg AJ, Leggett BA, Arnold H, Thompson AJ, Weltman M, Carey EJ, Muir AJ, Ling L, Rossi SJ, DePaoli AM. NGM282 for Treatment of Patients With Primary Biliary Cholangitis: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Hepatol Commun. 2018;2:1037–1050. doi: 10.1002/hep4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 37.Maruyama T, Tanaka K, Suzuki J, Miyoshi H, Harada N, Nakamura T, Miyamoto Y, Kanatani A, Tamai Y. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191:197–205. doi: 10.1677/joe.1.06546. [DOI] [PubMed] [Google Scholar]
- 38.Keitel V, Cupisti K, Ullmer C, Knoefel WT, Kubitz R, Häussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 2009;50:861–870. doi: 10.1002/hep.23032. [DOI] [PubMed] [Google Scholar]
- 39.Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011;54:1421–1432. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cipriani S, Mencarelli A, Chini MG, Distrutti E, Renga B, Bifulco G, Baldelli F, Donini A, Fiorucci S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One. 2011;6:e25637. doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pellicciari R, Gioiello A, Macchiarulo A, Thomas C, Rosatelli E, Natalini B, Sardella R, Pruzanski M, Roda A, Pastorini E, Schoonjans K, Auwerx J. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J Med Chem. 2009;52:7958–7961. doi: 10.1021/jm901390p. [DOI] [PubMed] [Google Scholar]
- 42.Lieu T, Jayaweera G, Zhao P, Poole DP, Jensen D, Grace M, McIntyre P, Bron R, Wilson YM, Krappitz M, Haerteis S, Korbmacher C, Steinhoff MS, Nassini R, Materazzi S, Geppetti P, Corvera CU, Bunnett NW. The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology. 2014;147:1417–1428. doi: 10.1053/j.gastro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, Orlandi S, Sadeghpour B, Wang XX, Jiang T, Levi M, Pruzanski M, Adorini L. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol. 2010;78:617–630. doi: 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baghdasaryan A, Claudel T, Gumhold J, Silbert D, Adorini L, Roda A, Vecchiotti S, Gonzalez FJ, Schoonjans K, Strazzabosco M, Fickert P, Trauner M. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2-/- (Abcb4-/-) mouse cholangiopathy model by promoting biliary HCO⁻₃ output. Hepatology. 2011;54:1303–1312. doi: 10.1002/hep.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perides G, Laukkarinen JM, Vassileva G, Steer ML. Biliary acute pancreatitis in mice is mediated by the G-protein-coupled cell surface bile acid receptor Gpbar1. Gastroenterology. 2010;138:715–725. doi: 10.1053/j.gastro.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62:720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 48.Cao W, Tian W, Hong J, Li D, Tavares R, Noble L, Moss SF, Resnick MB. Expression of bile acid receptor TGR5 in gastric adenocarcinoma. Am J Physiol Gastrointest Liver Physiol. 2013;304:G322–G327. doi: 10.1152/ajpgi.00263.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghonem NS, Assis DN, Boyer JL. Fibrates and cholestasis. Hepatology. 2015;62:635–643. doi: 10.1002/hep.27744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pawlak M, Baugé E, Bourguet W, De Bosscher K, Lalloyer F, Tailleux A, Lebherz C, Lefebvre P, Staels B. The transrepressive activity of peroxisome proliferator-activated receptor alpha is necessary and sufficient to prevent liver fibrosis in mice. Hepatology. 2014;60:1593–1606. doi: 10.1002/hep.27297. [DOI] [PubMed] [Google Scholar]
- 51.Cuperus FJ, Halilbasic E, Trauner M. Fibrate treatment for primary biliary cirrhosis. Curr Opin Gastroenterol. 2014;30:279–286. doi: 10.1097/MOG.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Chen K, Dai W, Xia Y, Wang F, Shen M, Cheng P, Wang C, Yang J, Zhu R, Zhang H, Li J, Zheng Y, Wang J, Lu J, Zhou Y, Guo C. Combination therapy of bezafibrate and ursodeoxycholic acid for primary biliary cirrhosis: A meta-analysis. Hepatol Res. 2015;45:48–58. doi: 10.1111/hepr.12373. [DOI] [PubMed] [Google Scholar]
- 53.Hosonuma K, Sato K, Yamazaki Y, Yanagisawa M, Hashizume H, Horiguchi N, Kakizaki S, Kusano M, Yamada M. A prospective randomized controlled study of long-term combination therapy using ursodeoxycholic acid and bezafibrate in patients with primary biliary cirrhosis and dyslipidemia. Am J Gastroenterol. 2015;110:423–431. doi: 10.1038/ajg.2015.20. [DOI] [PubMed] [Google Scholar]
- 54.Cheung AC, Lapointe-Shaw L, Kowgier M, Meza-Cardona J, Hirschfield GM, Janssen HL, Feld JJ. Combined ursodeoxycholic acid (UDCA) and fenofibrate in primary biliary cholangitis patients with incomplete UDCA response may improve outcomes. Aliment Pharmacol Ther. 2016;43:283–293. doi: 10.1111/apt.13465. [DOI] [PubMed] [Google Scholar]
- 55.Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A GOLDEN-505 Investigator Study Group. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147–1159.e5. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 56.Hofmann AF, Zakko SF, Lira M, Clerici C, Hagey LR, Lambert KK, Steinbach JH, Schteingart CD, Olinga P, Groothuis GM. Novel biotransformation and physiological properties of norursodeoxycholic acid in humans. Hepatology. 2005;42:1391–1398. doi: 10.1002/hep.20943. [DOI] [PubMed] [Google Scholar]
- 57.Dumont M, Erlinger S, Uchman S. Hypercholeresis induced by ursodeoxycholic acid and 7-ketolithocholic acid in the rat: possible role of bicarbonate transport. Gastroenterology. 1980;79:82–89. [PubMed] [Google Scholar]
- 58.Fickert P, Pollheimer MJ, Silbert D, Moustafa T, Halilbasic E, Krones E, Durchschein F, Thüringer A, Zollner G, Denk H, Trauner M. Differential effects of norUDCA and UDCA in obstructive cholestasis in mice. J Hepatol. 2013;58:1201–1208. doi: 10.1016/j.jhep.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trauner M, Fickert P, Hirschfield G, Reiter F, Altorjay I, Marschall HU, Färkkilä M, Schramm C, Spengler U, Chapman R, Bergquist A, Schrumpf E, Nevens F, Halilbasic E, Greinwald R, Proels M, Manns MP. Norursodeoxycholic acid improves cholestasis in primary sclerosing cholangitis: results of a phase II dose finding study. J Hepatol. 2016;64:S208–S209. doi: 10.1016/j.jhep.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Baghdasaryan A, Fuchs CD, Österreicher CH, Lemberger UJ, Halilbasic E, Påhlman I, Graffner H, Krones E, Fickert P, Wahlström A, Ståhlman M, Paumgartner G, Marschall HU, Trauner M. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J Hepatol. 2016;64:674–681. doi: 10.1016/j.jhep.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 61.Graffner H, Gillberg PG, Rikner L, Marschall HU. The ileal bile acid transporter inhibitor A4250 decreases serum bile acids by interrupting the enterohepatic circulation. Aliment Pharmacol Ther. 2016;43:303–310. doi: 10.1111/apt.13457. [DOI] [PubMed] [Google Scholar]
- 62.Mayo MJ, Pockros P, Jones D, Bowlus C, Levy C, Patanwala I, Bacort B, Luketic V, Vuppalanchi R, Medendorp S, Dorenbaum A, Kennedy C, Novak P, Raychaudhuri A, Goyal S, Abi-Saab W, Hirschfield GM. Clarity; a phase II, randomized, double-blind, placebo-controlled study of LUM001, a novel apical sodium- dependent bile acid transporter inhibitor, in the treatment of primary biliary cirrhosis associated with itching. J Hepatol. 2016;64:S197. [Google Scholar]
- 63.Hegade VS, Kendrick SF, Dobbins RL, Miller SR, Thompson D, Richards D, Storey J, Dukes GE, Corrigan M, Oude Elferink RP, Beuers U, Hirschfield GM, Jones DE. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114–1123. doi: 10.1016/S0140-6736(17)30319-7. [DOI] [PubMed] [Google Scholar]