Abstract
In view of the increasing life expectancy in different parts of the world, a larger proportion of elderly patients with hepatocellular carcinoma (HCC) requiring oncological treatment is expected. The clinicopathological characteristics of HCC in elderly patients and in younger patients are different. Elderly patients, in general, also have more comorbidities. Evaluation of the efficacy of different HCC treatment options in elderly patients is necessary to optimize treatment outcomes for them. Treatment modalities for HCC include hepatectomy, liver transplantation, radiofrequency ablation, transarterial chemoembolization, and molecular-targeted therapy with sorafenib. In this review, current evidence on the risks and outcomes of the different HCC treatments for elderly patients are discussed. According to data in the literature, elderly patients and younger patients benefited similarly from HCC treatments. More clinical data are needed for the determination of selecting criteria on elderly HCC patients to maximize their chance of getting the most appropriate and effective treatments. As such, further studies evaluating the outcomes of different HCC treatment modalities in elderly patients are warranted.
Keywords: Hepatocellular carcinoma, Aged, Clinical outcome, Surgery, Hepatectomy
Core tip: Elderly patients and younger patients benefited similarly from hepatocellular carcinoma (HCC) treatments. Advanced age and comorbidity are intrinsic factors in elderly HCC patients but should not preclude them from receiving treatments. Patients should be evaluated individually and treatment options should be personalized. All treatment options available to the young should be made available to the elderly. Careful assessment of clinical status, cancer stage and comorbidity is needed to ensure good treatment outcomes.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most common cause of cancer-related deaths worldwide[1]. Because of the high prevalence of hepatitis B virus infection[2], countries in eastern and southeast Asia have the highest incidence of HCC in the world[3].
Aging is a major risk factor and poor prognostic factor for most chronic diseases. Owing to the remarkable socioeconomic development and advancement of medical care, average life expectancy has increased around the world; citizens of many developed countries enjoy a lifespan of over 80 years. In the Hong Kong Special Administrative Region, the average life expectancy is the longest in the world. According to a report by Hong Kong Centre for Health Protection, the average life expectancy at birth reached 81.9 and 87.6 years for males and females respectively in 2017. Consequently, an increasing proportion of elderly patients with HCC requiring oncological treatment was expected[4]. It was estimated that the incidence of HCC will increase by approximately 59% by 2030, more than 50% of which will be in people aged 65 or above[5].
HCC in the elderly population may show different clinical and pathological characteristics when compared to the younger population. The incidence rate of HCC raises at the age of forties and decreases after eighties[6]. Aging has been shown to be associated with gradual alteration of hepatic structure and function as well as various changes in liver cells[7,8]. In the sequenced process of liver injury, aging decreases regenerative ability[7,9]. Elderly patients also have inferior cardiopulmonary function. Comorbidities such as diabetes mellitus and renal insufficiency are common among the elderly population. Aging is associated with higher severity and worse prognosis of various liver diseases due to the summation of the aforesaid factors.
Most developed countries accept the chronological age of 65 years as the definition of an elderly person[10]. However, many clinical studies about HCC in the elderly population defined elderly as over 70 years of age[11-17]. This heterogeneity will persist through the discussion in this review. The age cutoff for elderly in the literature varies from 60 to 80, while 70, 75 and 80 were the cutoffs commonly used.
Although the prevalence of hepatitis B virus infection is decreasing in our locality due to the implementation of the universal neonatal vaccination program since 1988, the need for HCC treatment in the elderly may not decrease due to the following three reasons: (1) Delay in HCC recurrence due to improved primary treatment; (2) Increasing incidence of types of liver cirrhosis that require more time to develop into HCC, e.g., non-alcoholic steatohepatitis[18]; (3) Liberal use of antiviral agents which have an effect on viral hepatitis and delay the natural course of HCC formation[19]. In view of the above, evaluation of treatment options for elderly HCC patients is of high clinical relevance. The treatment options of HCC vary according to the stage of the disease. They include hepatectomy, liver transplantation (LT), radiofrequency ablation (RFA), transarterial chemoembolization (TACE), and molecular-targeted therapy with sorafenib. However, whether or not age should be a factor of consideration in HCC treatment allocation is controversial.
The aim of this review is to give an overview of the current knowledge on HCC treatments in elderly patients and to provide available evidence on the treatment modalities for HCC. In particular, the discussion will focus on the role of each treatment in the management of elderly HCC patients and these treatments’ respective clinical impact.
HEPATECTOMY
Hepatectomy is an established therapeutic modality for HCC[20]. The mortality rate of liver resection is minimal[21] although the morbidity rate is 20%-30%[22-25]. The surgical outcome of hepatectomy depends on host liver function, HCC staging, and anesthetic risk[20]. In addition to the fact that young and old patients have histological difference in liver, elderly patients usually have more comorbidities, such as cardiovascular problems, diabetes mellitus, hypertension, and pulmonary disease. As such, they tend to suffer worse surgical outcomes[26].
Numerous studies reported the results of hepatectomy for HCC in elderly patients. However, the majority of them were retrospective observational studies and therefore the validity of these results is debatable[27-36]. There are a few meta-analyses comparing hepatectomy outcomes between the elderly and the young, and Table 1 is a summary of these meta-analyses[37-39]. In general, comparable short-term outcomes (mortality, morbidity, immediate surgical complications, etc.) between the elderly and the young were reported. Long-term outcomes (1-, 3- and 5-year overall and disease-free survival rates) were also comparable between the two groups of patients in all the meta-analyses. The results were surprising as it would have been expected that the elderly would have worse post-hepatectomy outcomes due to their impaired physiological functions. In fact, one of the analyzed studies even reported better 1-year survival in the elderly compared to the younger group (odds ratio = 0.762, P = 0.045). This could probably be explained by the more careful selecting criteria on elderly patients – lower risk for surgery and lower grade of background liver fibrosis[40]. Heterogeneity of study design and publication bias might also have led to the paradoxical result.
Table 1.
Summary table of meta-analyses comparing outcomes of hepatectomy for hepatocellular carcinoma in the elderly and young populations
| Authors (year) | Characteristics of included studies | Outcome measures and results | Conclusions |
| Hung et al[38], (2015) | 23 studies included in total 18 studies on hepatectomy for hepatocellular carcinoma (6341 patients) | Short-term outcomes Treatment complications: Comparable between the elderly and younger groups Long-term outcomes 1-, 3-, 5-yr disease-free and overall survival: Increased 1-yr overall survival in the elderly compared to the younger group Comparable 3- and 5-yr survival, disease-free survival | Hepatectomy, transarterial chemoembolization and radiofrequency ablation are safe and effective for elderly hepatocellular carcinoma patients Similar success compared to younger patients Optimal strategy depends on patient and tumor characteristics (evaluation of cancer stage and general condition is important) |
| Mizuguchi et al[37], (2014) | 16 studies included in total 5 studies on hepatectomy for hepatocellular carcinoma (1932 patients) | Short-term outcomes Morbidity and mortality: No significant differences between the elderly and younger groups | Outcome of hepatectomy depends on tumor type (hepatocellular carcinoma vs colorectal metastatic cancer) Hepatectomy is indicated in older hepatocellular carcinoma patients |
| Zhou et al[9], (2013) | 28 studies included in total 11 studies on hepatectomy for hepatocellular carcinoma (3560 patients) | Short-term outcomes Morbidity and mortality: No significant differences between the elderly and younger groups Long-term outcomes 5-yr disease-free and overall survival: No significant differences between the elderly and younger groups | Similar overall morbidity and mortality in elderly and young patients Analysis should be interpreted with caution as elderly mortality after hepatectomy has been reported to be higher in the presence of cirrhosis Age alone should not be a contraindication to hepatectomy |
There are different scoring systems for assessing elderly patients with poor physiological status, including Possum/pPossum, E-PASS, and APACHE II score. Although age is not an accurate risk factor for mortality or morbidity, it usually correlates with physiological reserve and is a surrogate marker for comor-bidities[15,41,42]. The proportion of comorbid disease (cardiovascular, cerebrovascular and renal diseases, chronic obstructive pulmonary disease, etc.) is higher in elderly patients than in their younger counterparts. Cardiopulmonary workup including echocardiography and lung function test should be performed, if necessary, for patients with a high index of suspicion of occult cardiopulmonary disease[43]. Perioperative patient evaluation by physicians and anesthetists would optimize the outcomes of hepatectomy in elderly patients.
Laparoscopic hepatectomy was introduced in 1996[44] as a minimally invasive technique with potential advantages. Its potential benefits over open hepatectomy include decreased operative blood loss, decreased pain, better cosmesis, faster recovery, fewer cardiovascular and respiratory complications, and shorter hospital stay. Many studies have shown that it could attain better perioperative out-comes[43,45-47]. Although laparoscopic hepatectomy has been shown to be a safe and effective approach to the management of liver disease[48-52], its application to elderly HCC patients with cirrhosis and other comorbidities remains unclarified. For this potentially feasible and safe alternative to open hepatectomy to benefit more patients, selecting criteria on elderly patients need to be determined.
LIVER TRANSPLANTATION
In the past, LT was seldom performed in elderly patients[53]. Since the 1990s, cases of LT in the aged population have gradually been reported and its feasibility in treating the elderly has been evaluated[54-56]. However, most of these studies used a cutoff age of 50-60 years, which would be controversial nowadays in view of the improved life expectancy worldwide. A more recent retrospective study reported a mean post-LT survival of 65 mo in 13 patients who were 75 or older at the time of LT[57].
Data in the literature about LT for elderly HCC patients was very limited since HCC is not the only indication for LT. In the several studies that evaluated the outcomes of LT in elderly patients[58-64], there were inconsistencies in the results: some reported comparable survival outcomes between older and younger recipients while some reported significantly worse survival outcomes in the elderly group. The studies quoted were all retrospective in nature with questionable validity, and the cutoff used for old age also varied in the studies, making direct comparison difficult. However, the researchers in the different studies generally agreed that age should not be the only factor in determining eligibility for LT. It is believed that other patient factors like preoperative disease severity (MELD score) and functional reserve are more important than chronological age in determining eligibility for LT[65]. Since elderly HCC patients often have a lower grade of background liver fibrosis and lower Child-Pugh scores when compared to younger HCC patients[40], LT may potentially be a treatment option for elderly HCC patients. Studies specifically evaluating LT as an oncological treatment for elderly HCC patients need to be conducted before patient selection criteria can be determined and evidence on outcomes can emerge.
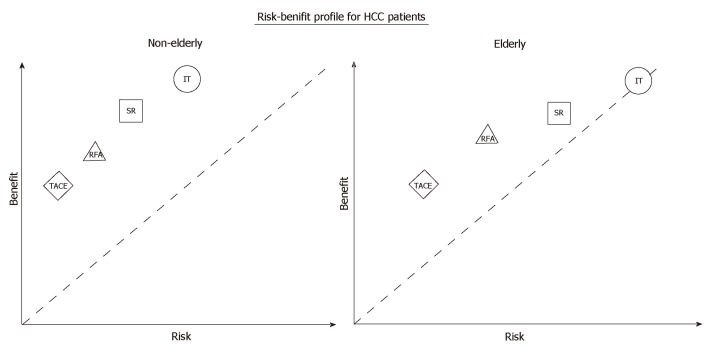
The risk-benefit profile is the most important parameter for consideration of high-risk procedures such as LT for elderly patients. Data on the benefit of LT to elderly HCC patients are lacking, but we can assume that LT, as an oncological treatment, offers the elderly and the young similar benefit, as do other HCC treatment modalities. However, as depicted in Figure 1, the operative risk is considerably elevated in the older population. Therefore, LT as a treatment option for the aged population is still subject to debate, and the upper age limit for undergoing LT is yet to be defined.
Figure 1.
Schematic diagram–risk-benefit profile comparing aged patients with young patients in hepatocellular carcinoma treatments. TACE: Transarterial chemoembolization; RFA: Radiofrequency ablation; SR: Surgical resection; LT: Liver transplantation; HCC: Hepatocellular carcinoma.
In clinical practice, LT is seldom performed on elderly patients. In Hong Kong and other Asian regions, the rates of organ donation from the deceased are low[66]. The utilization of deceased-donor liver grafts for elderly HCC patients would be subjected to evaluation based on treatment efficacy and cost-effectiveness. Donor risk would be an additional concern in the case of living-donor LT. Furthermore, elderly waitlisted patients have a higher dropout rate than their younger counterparts, mainly due to deterioration of cardiopulmonary status.
RADIOFREQUENCY ABLATION
Hepatectomy, LT and RFA are considered curative modalities for early-stage HCC. Percutaneous RFA would be beneficial to elderly patients with a poor risk profile due to avoidance of risks associated with general anesthesia. It is also less invasive and hence has fewer periprocedural risks and deteriorative effects on liver function[67]. It has become an increasingly popular treatment option for elderly HCC patients. AASLD and EASL guidelines present RFA as a potential treatment option for compensated cirrhotic patients with small HCCs < 5 cm[68,69]. However, to date, there have not been many studies about the long-term outcome of RFA in HCC patients. Percutaneous ethanol injection has been used to treat elderly HCC patients but evidence of its efficacy is limited[70,71]. RFA has largely replaced percutaneous ethanol injection for better recurrence-free survival and fewer treatment sessions[72].
In the comparison of RFA and surgical resection by postoperative outcomes, contradictory results were yielded. Peng et al[73] reported that patients having RFA had better outcomes than those having surgical resection, while Bauschke et al[74] and Yu et al[75] reported better outcomes for surgical resection. Another retrospective study by Jiang et al[76] concluded that RFA should be recommended for elderly patients (age > 65 years) with HCCs ≤ 20 mm while surgical resection would be a better treatment for HCCs of 21-50 mm in elderly patients. A meta-analysis by Hung et al[38] found that elderly and young patients shared similar survival outcomes at one year and three years after RFA. However, the elderly group had a significantly worse 5-year survival rate (odds ratio = 1.379, P = 0.01). Unlike elderly patients having surgical resection, elderly patients having RFA had poorer survival when compared with young patients. This may be due to selection bias – elderly patients with a poorer general health profile were included into the RFA arm.
TRANSARTERIAL CHEMOEMBOLIZATION
TACE is a widely used nonsurgical treatment that is considered to be effective in prolonging HCC patients’ survival[33,77-79]. It has been reported that development of peptic ulcer disease occurred in 2.5% of patients and development of liver failure occurred in 11% of patients[16]. The meta-analysis by Hung et al[38] found that elderly patients benefited more from TACE than younger patients did. Significantly better 1- and 3-year survival rates were seen in the elderly group (odds ratio = 0.664, P < 0.01 and odds ratio = 0.795, P = 0.013). Nonetheless, no significant difference in 5-year survival between the two groups of patients was observed. In general, elderly patients with HCC at an earlier stage or with a higher surgical risk are more likely to be selected to receive TACE. This may be a major reason to explain why some studies reported a better survival outcome in elderly patients after TACE.
TARGET THERAPY AND IMMUNOTHERAPY
Sorafenib represents the standard of care in the management of advanced HCC. It has been reported that sorafenib achieved similar progression-free survival and overall survival in elderly and young patients with advanced HCC[80]. However, morbidities including neutropenia, malaise and mucositis occurred more frequently in elderly patients. Dose reduction is a way to increase its tolerability[81,82]. Other common adverse effects (e.g., hand and foot syndrome and diarrhea) were reported to be similar in both populations[80]. Certain novel immunotherapy agents have been approved and introduced into clinical practice to treat HCC, e.g., anticytotoxic T lymphocyte-associated protein 4 antibody, anti-programmed cell death 1 antibody, and anti-programmed death-ligand 1 antibody. Despite limited data, immune checkpoint inhibitors may represent a potential option of nonsurgical treatment for elderly HCC patients[83].
CONCLUSION
Overall, elderly patients and younger patients benefited similarly from HCC treatments. Advanced age and comorbidity are intrinsic factors in elderly HCC patients but should not preclude them from receiving treatments. Patients should be evaluated individually and treatment options should be personalized. All treatment options available to the young should be made available to the elderly. Careful assessment of clinical status, cancer stage and comorbidity is needed to ensure good treatment outcomes. More clinical data are needed for the determination of selecting criteria on elderly HCC patients to maximize their chance of getting the most appropriate and effective treatments. As such, further studies evaluating the outcomes of different HCC treatment modalities in elderly patients are warranted.
Footnotes
Conflict-of-interest statement: None of the authors has any conflict of interest.
Peer-review started: April 1, 2019
First decision: April 30, 2019
Article in press: June 30, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carrier P, Marzano C, Shousha HI S-Editor: Yan JP L-Editor: Ze-Mao Gong E-Editor: Wu YXJ
Contributor Information
Kevin Ka Wan Chu, Department of Surgery, Queen Mary Hospital, Hong Kong, China.
Kenneth Siu Ho Chok, Department of Surgery and State Key Laboratory for Liver Research, The University of Hong Kong, Hong Kong, China. chok6275@hku.hk.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, Khin MW, Koo WH Asian Oncology Summit. Management of hepatocellular carcinoma in Asia: Consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 6.Yoon SK, Chun HG. Status of hepatocellular carcinoma in South Korea. Chin Clin Oncol. 2013;2:39. doi: 10.3978/j.issn.2304-3865.2013.11.08. [DOI] [PubMed] [Google Scholar]
- 7.Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol. 2015;31:184–191. doi: 10.1097/MOG.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Couteur DG, Warren A, Cogger VC, Smedsrød B, Sørensen KK, De Cabo R, Fraser R, McCuskey RS. Old age and the hepatic sinusoid. Anat Rec (Hoboken) 2008;291:672–683. doi: 10.1002/ar.20661. [DOI] [PubMed] [Google Scholar]
- 9.Sanz N, Díez-Fernández C, Alvarez AM, Fernández-Simón L, Cascales M. Age-related changes on parameters of experimentally-induced liver injury and regeneration. Toxicol Appl Pharmacol. 1999;154:40–49. doi: 10.1006/taap.1998.8541. [DOI] [PubMed] [Google Scholar]
- 10.Seo JH, Kim DH, Cho E, Jun CH, Park SY, Cho SB, Park CH, Kim HS, Choi SK, Rew JS. Characteristics and Outcomes of Extreme Elderly Patients With Hepatocellular Carcinoma in South Korea. In Vivo. 2019;33:145–154. doi: 10.21873/invivo.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu PH, Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW, Chiou YY, Lin HC, Huo TI. Uncompromised treatment efficacy in elderly patients with hepatocellular carcinoma: A propensity score analysis. Medicine (Baltimore) 2014;93:e264. doi: 10.1097/MD.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirici-Cappa F, Gramenzi A, Santi V, Zambruni A, Di Micoli A, Frigerio M, Maraldi F, Di Nolfo MA, Del Poggio P, Benvegnù L, Rapaccini G, Farinati F, Zoli M, Borzio F, Giannini EG, Caturelli E, Bernardi M, Trevisani F Italian Liver Cancer Group. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: A 20-year multicentre experience. Gut. 2010;59:387–396. doi: 10.1136/gut.2009.194217. [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa H, Kita R, Kimura T, Ohara Y, Takeda H, Sakamoto A, Saito S, Nishijima N, Nasu A, Komekado H, Osaki Y. Transcatheter arterial chemoembolization for intermediate-stage hepatocellular carcinoma: Clinical outcome and safety in elderly patients. J Cancer. 2014;5:590–597. doi: 10.7150/jca.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MJ, Bloom AI, Barak O, Klimov A, Nesher T, Shouval D, Levi I, Shibolet O. Trans-arterial chemo-embolization is safe and effective for very elderly patients with hepatocellular carcinoma. World J Gastroenterol. 2013;19:2521–2528. doi: 10.3748/wjg.v19.i16.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oishi K, Itamoto T, Kohashi T, Matsugu Y, Nakahara H, Kitamoto M. Safety of hepatectomy for elderly patients with hepatocellular carcinoma. World J Gastroenterol. 2014;20:15028–15036. doi: 10.3748/wjg.v20.i41.15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yau T, Yao TJ, Chan P, Epstein RJ, Ng KK, Chok SH, Cheung TT, Fan ST, Poon RT. The outcomes of elderly patients with hepatocellular carcinoma treated with transarterial chemoembolization. Cancer. 2009;115:5507–5515. doi: 10.1002/cncr.24636. [DOI] [PubMed] [Google Scholar]
- 17.Suda T, Nagashima A, Takahashi S, Kanefuji T, Kamimura K, Tamura Y, Takamura M, Igarashi M, Kawai H, Yamagiwa S, Nomoto M, Aoyagi Y. Active treatments are a rational approach for hepatocellular carcinoma in elderly patients. World J Gastroenterol. 2013;19:3831–3840. doi: 10.3748/wjg.v19.i24.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bugianesi E. Non-alcoholic steatohepatitis and cancer. Clin Liver Dis. 2007;11:191–207, x-xi. doi: 10.1016/j.cld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Cammà C, Giunta M, Andreone P, Craxì A. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: An evidence-based approach. J Hepatol. 2001;34:593–602. doi: 10.1016/s0168-8278(01)00005-8. [DOI] [PubMed] [Google Scholar]
- 20.Chu KK, Cheung TT. Update in management of hepatocellular carcinoma in Eastern population. World J Hepatol. 2015;7:1562–1571. doi: 10.4254/wjh.v7.i11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: Toward zero hospital deaths. Ann Surg. 1999;229:322–330. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook EJ, Welsh FK, Chandrakumaran K, John TG, Rees M. Resection of colorectal liver metastases in the elderly: Does age matter? Colorectal Dis. 2012;14:1210–1216. doi: 10.1111/j.1463-1318.2012.02946.x. [DOI] [PubMed] [Google Scholar]
- 23.Di Benedetto F, Berretta M, D'Amico G, Montalti R, De Ruvo N, Cautero N, Guerrini GP, Ballarin R, Spaggiari M, Tarantino G, Di Sandro S, Pecchi A, Luppi G, Gerunda GE. Liver resection for colorectal metastases in older adults: A paired matched analysis. J Am Geriatr Soc. 2011;59:2282–2290. doi: 10.1111/j.1532-5415.2011.03734.x. [DOI] [PubMed] [Google Scholar]
- 24.Mazzoni G, Tocchi A, Miccini M, Bettelli E, Cassini D, De Santis M, Colace L, Brozzetti S. Surgical treatment of liver metastases from colorectal cancer in elderly patients. Int J Colorectal Dis. 2007;22:77–83. doi: 10.1007/s00384-006-0096-7. [DOI] [PubMed] [Google Scholar]
- 25.Nagano Y, Nojiri K, Matsuo K, Tanaka K, Togo S, Ike H, Shimada H. The impact of advanced age on hepatic resection of colorectal liver metastases. J Am Coll Surg. 2005;201:511–516. doi: 10.1016/j.jamcollsurg.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Velanovich V. The effects of age, gender, race and concomitant disease on postoperative complications. J R Coll Surg Edinb. 1993;38:225–230. [PubMed] [Google Scholar]
- 27.Aldrighetti L, Arru M, Caterini R, Finazzi R, Comotti L, Torri G, Ferla G. Impact of advanced age on the outcome of liver resection. World J Surg. 2003;27:1149–1154. doi: 10.1007/s00268-003-7072-y. [DOI] [PubMed] [Google Scholar]
- 28.Cescon M, Grazi GL, Del Gaudio M, Ercolani G, Ravaioli M, Nardo B, Cavallari A. Outcome of right hepatectomies in patients older than 70 years. Arch Surg. 2003;138:547–552. doi: 10.1001/archsurg.138.5.547. [DOI] [PubMed] [Google Scholar]
- 29.Ezaki T, Yukaya H, Ogawa Y. Evaluation of hepatic resection for hepatocellular carcinoma in the elderly. Br J Surg. 1987;74:471–473. doi: 10.1002/bjs.1800740614. [DOI] [PubMed] [Google Scholar]
- 30.Fortner JG, Lincer RM. Hepatic resection in the elderly. Ann Surg. 1990;211:141–145. doi: 10.1097/00000658-199002000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lui WY, Chau GY, Wu CW, King KL. Surgical resection of hepatocellular carcinoma in elderly cirrhotic patients. Hepatogastroenterology. 1999;46:640–645. [PubMed] [Google Scholar]
- 32.Nagasue N, Chang YC, Takemoto Y, Taniura H, Kohno H, Nakamura T. Liver resection in the aged (seventy years or older) with hepatocellular carcinoma. Surgery. 1993;113:148–154. [PubMed] [Google Scholar]
- 33.Poon RT, Fan ST, Lo CM, Liu CL, Ngan H, Ng IO, Wong J. Hepatocellular carcinoma in the elderly: Results of surgical and nonsurgical management. Am J Gastroenterol. 1999;94:2460–2466. doi: 10.1111/j.1572-0241.1999.01376.x. [DOI] [PubMed] [Google Scholar]
- 34.Takenaka K, Shimada M, Higashi H, Adachi E, Nishizaki T, Yanaga K, Matsumata T, Ikeda T, Sugimachi K. Liver resection for hepatocellular carcinoma in the elderly. Arch Surg. 1994;129:846–850. doi: 10.1001/archsurg.1994.01420320072014. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Takenaka K, Matsumata T, Shimada M, Itasaka H, Shirabe K, Sugimachi K. Right hepatic lobectomy in elderly patients with hepatocellular carcinoma. Hepatogastroenterology. 1997;44:514–518. [PubMed] [Google Scholar]
- 36.Zieren HU, Müller JM, Zieren J. Resection of colorectal liver metastases in old patients. Hepatogastroenterology. 1994;41:34–37. [PubMed] [Google Scholar]
- 37.Mizuguchi T, Kawamoto M, Meguro M, Okita K, Ota S, Ishii M, Ueki T, Nishidate T, Kimura Y, Furuhata T, Hirata K. Impact of aging on morbidity and mortality after liver resection: A systematic review and meta-analysis. Surg Today. 2015;45:259–270. doi: 10.1007/s00595-014-0863-y. [DOI] [PubMed] [Google Scholar]
- 38.Hung AK, Guy J. Hepatocellular carcinoma in the elderly: Meta-analysis and systematic literature review. World J Gastroenterol. 2015;21:12197–12210. doi: 10.3748/wjg.v21.i42.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Zhang X, Zhang Z, Liu X, Wu L, Li Y, Li B. Hepatectomy in elderly patients: Does age matter? World J Surg. 2013;37:2899–2910. doi: 10.1007/s00268-013-2184-5. [DOI] [PubMed] [Google Scholar]
- 40.Miki D, Aikata H, Uka K, Saneto H, Kawaoka T, Azakami T, Takaki S, Jeong SC, Imamura M, Kawakami Y, Takahashi S, Itamoto T, Asahara T, Arihiro K, Chayama K. Clinicopathological features of elderly patients with hepatitis C virus-related hepatocellular carcinoma. J Gastroenterol. 2008;43:550–557. doi: 10.1007/s00535-008-2194-5. [DOI] [PubMed] [Google Scholar]
- 41.Cucchetti A, Ercolani G, Cescon M, Ravaioli M, Zanello M, Del Gaudio M, Lauro A, Vivarelli M, Grazi GL, Pinna AD. Recovery from liver failure after hepatectomy for hepatocellular carcinoma in cirrhosis: Meaning of the model for end-stage liver disease. J Am Coll Surg. 2006;203:670–676. doi: 10.1016/j.jamcollsurg.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Goldhill DR. Preventing surgical deaths: Critical care and intensive care outreach services in the postoperative period. Br J Anaesth. 2005;95:88–94. doi: 10.1093/bja/aeh281. [DOI] [PubMed] [Google Scholar]
- 43.Chan AC, Poon RT, Cheung TT, Chok KS, Dai WC, Chan SC, Lo CM. Laparoscopic versus open liver resection for elderly patients with malignant liver tumors: A single-center experience. J Gastroenterol Hepatol. 2014;29:1279–1283. doi: 10.1111/jgh.12539. [DOI] [PubMed] [Google Scholar]
- 44.Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc. 1996;10:758–761. doi: 10.1007/BF00193052. [DOI] [PubMed] [Google Scholar]
- 45.Belli G, Fantini C, D'Agostino A, Cioffi L, Langella S, Russolillo N, Belli A. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: Short- and middle-term results. Surg Endosc. 2007;21:2004–2011. doi: 10.1007/s00464-007-9503-6. [DOI] [PubMed] [Google Scholar]
- 46.Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamamoto S, Yamazoe S, Ohira G, Nakajima T. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2013;20:512–517. doi: 10.1007/s00534-012-0592-9. [DOI] [PubMed] [Google Scholar]
- 47.Truant S, Bouras AF, Hebbar M, Boleslawski E, Fromont G, Dharancy S, Leteurtre E, Zerbib P, Pruvot FR. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: A case-matched study. Surg Endosc. 2011;25:3668–3677. doi: 10.1007/s00464-011-1775-1. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, Li H, Liu F, Li B, Wei Y. Surgical outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma for various resection extent. Medicine (Baltimore) 2017;96:e6460. doi: 10.1097/MD.0000000000006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komatsu S, Brustia R, Goumard C, Sepulveda A, Perdigao F, Soubrane O, Scatton O. Clinical impact of laparoscopic hepatectomy: Technical and oncological viewpoints. Surg Endosc. 2017;31:1442–1450. doi: 10.1007/s00464-016-5135-z. [DOI] [PubMed] [Google Scholar]
- 50.Rhu J, Kim SJ, Choi GS, Kim JM, Joh JW, Kwon CHD. Laparoscopic Versus Open Right Posterior Sectionectomy for Hepatocellular Carcinoma in a High-Volume Center: A Propensity Score Matched Analysis. World J Surg. 2018;42:2930–2937. doi: 10.1007/s00268-018-4531-z. [DOI] [PubMed] [Google Scholar]
- 51.Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, OʼRourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schön MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619–629. doi: 10.1097/SLA.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 52.Yoon YI, Kim KH, Kang SH, Kim WJ, Shin MH, Lee SK, Jung DH, Park GC, Ahn CS, Moon DB, Ha TY, Song GW, Hwang S, Lee SG. Pure Laparoscopic Versus Open Right Hepatectomy for Hepatocellular Carcinoma in Patients With Cirrhosis: A Propensity Score Matched Analysis. Ann Surg. 2017;265:856–863. doi: 10.1097/SLA.0000000000002072. [DOI] [PubMed] [Google Scholar]
- 53.National Institutes of Health Consensus Development Conference Statement: Liver transplantation--June 20-23, Hepatology. 1984;4:107S–110S. [PubMed] [Google Scholar]
- 54.Emre S, Mor E, Schwartz ME, Katz E, Acarli K, Fukuzawa K, Miller CM. Liver transplantation in patients beyond age 60. Transplant Proc. 1993;25:1075–1076. [PubMed] [Google Scholar]
- 55.Bilbao I, Balsells J, Lazaro JL, Charco R, Murio E, Gifre E, Ruiz C, Edo A, Margarit C. Liver transplantation in patients over 60 years of age. Transplant Proc. 1995;27:2337–2338. [PubMed] [Google Scholar]
- 56.Shaw BW., Jr Transplantation in the elderly patient. Surg Clin North Am. 1994;74:389–400. [PubMed] [Google Scholar]
- 57.Taner CB, Ung RL, Rosser BG, Aranda-Michel J. Age is not a contraindication for orthotopic liver transplantation: A single institution experience with recipients older than 75 years. Hepatol Int. 2012;6:403–407. doi: 10.1007/s12072-011-9286-7. [DOI] [PubMed] [Google Scholar]
- 58.Aduen JF, Sujay B, Dickson RC, Heckman MG, Hewitt WR, Stapelfeldt WH, Steers JL, Harnois DM, Kramer DJ. Outcomes after liver transplant in patients aged 70 years or older compared with those younger than 60 years. Mayo Clin Proc. 2009;84:973–978. doi: 10.1016/S0025-6196(11)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gil E, Kim JM, Jeon K, Park H, Kang D, Cho J, Suh GY, Park J. Recipient Age and Mortality After Liver Transplantation: A Population-based Cohort Study. Transplantation. 2018;102:2025–2032. doi: 10.1097/TP.0000000000002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ikegami T, Bekki Y, Imai D, Yoshizumi T, Ninomiya M, Hayashi H, Yamashita Y, Uchiyama H, Shirabe K, Maehara Y. Clinical outcomes of living donor liver transplantation for patients 65 years old or older with preserved performance status. Liver Transpl. 2014;20:408–415. doi: 10.1002/lt.23825. [DOI] [PubMed] [Google Scholar]
- 61.Kuramitsu K, Egawa H, Keeffe EB, Kasahara M, Ito T, Sakamoto S, Ogawa K, Oike F, Takada Y, Uemoto S. Impact of age older than 60 years in living donor liver transplantation. Transplantation. 2007;84:166–172. doi: 10.1097/01.tp.0000269103.87633.06. [DOI] [PubMed] [Google Scholar]
- 62.Levy MF, Somasundar PS, Jennings LW, Jung GJ, Molmenti EP, Fasola CG, Goldstein RM, Gonwa TA, Klintmalm GB. The elderly liver transplant recipient: a call for caution. Ann Surg. 2001;233:107–113. doi: 10.1097/00000658-200101000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharpton SR, Feng S, Hameed B, Yao F, Lai JC. Combined effects of recipient age and model for end-stage liver disease score on liver transplantation outcomes. Transplantation. 2014;98:557–562. doi: 10.1097/TP.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson GC, Quillin RC, 3rd, Wima K, Sutton JM, Hoehn RS, Hanseman DJ, Paquette IM, Paterno F, Woodle ES, Abbott DE, Shah SA. Is liver transplantation safe and effective in elderly (≥70 years) recipients? A case-controlled analysis. HPB (Oxford) 2014;16:1088–1094. doi: 10.1111/hpb.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zenilman ME. Surgery in the geriatric patient: Aging, the heart, emergencies, and us. Arch Surg. 2007;142:109–110. doi: 10.1001/archsurg.142.2.109. [DOI] [PubMed] [Google Scholar]
- 66.Lo CM. Deceased donation in Asia: Challenges and opportunities. Liver Transpl. 2012;18 Suppl 2:S5–S7. doi: 10.1002/lt.23545. [DOI] [PubMed] [Google Scholar]
- 67.Hiraoka A, Michitaka K, Horiike N, Hidaka S, Uehara T, Ichikawa S, Hasebe A, Miyamoto Y, Ninomiya T, Sogabe I, Ishimaru Y, Kawasaki H, Koizumi Y, Hirooka M, Yamashita Y, Abe M, Hiasa Y, Matsuura B, Onji M. Radiofrequency ablation therapy for hepatocellular carcinoma in elderly patients. J Gastroenterol Hepatol. 2010;25:403–407. doi: 10.1111/j.1440-1746.2009.06037.x. [DOI] [PubMed] [Google Scholar]
- 68.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guy J, Kelley RK, Roberts J, Kerlan R, Yao F, Terrault N. Multidisciplinary management of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2012;10:354–362. doi: 10.1016/j.cgh.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 70.De Sio I, Castellano L, Calandra M, Persico M, Romano M, Torella R, Del Vecchio-Blanco C. Ultrasound-guided percutaneous ethanol injection: First choice for treatment of hepatocellular carcinoma in the elderly. Arch Gerontol Geriatr. 1996;22 Suppl 1:295–303. doi: 10.1016/0167-4943(96)86952-4. [DOI] [PubMed] [Google Scholar]
- 71.Kang SD, Kim JW, Jwa YJ, Choi YH, Song TJ, Bae WK, Kim NH, Kim KA, Lee JS. Treatment of hepatocellular carcinoma in elderly patients. Hepatogastroenterology. 2014;61:2001–2008. [PubMed] [Google Scholar]
- 72.Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, Sacchetto P, Gandini G, Rizzetto M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol. 2008;43:727–735. doi: 10.1080/00365520701885481. [DOI] [PubMed] [Google Scholar]
- 73.Peng ZW, Liu FR, Ye S, Xu L, Zhang YJ, Liang HH, Lin XJ, Lau WY, Chen MS. Radiofrequency ablation versus open hepatic resection for elderly patients (gt; 65 years) with very early or early hepatocellular carcinoma. Cancer. 2013;119:3812–3820. doi: 10.1002/cncr.28293. [DOI] [PubMed] [Google Scholar]
- 74.Bauschke A, Altendorf-Hofmann A, Mothes H, Rauchfuß F, Settmacher U. Partial liver resection results in a significantly better long-term survival than locally ablative procedures even in elderly patients. J Cancer Res Clin Oncol. 2016;142:1099–1108. doi: 10.1007/s00432-016-2115-6. [DOI] [PubMed] [Google Scholar]
- 75.Yu B, Ding Y, Liao X, Wang C, Wang B, Chen X. Radiofrequency ablation versus surgical resection in elderly patients with early-stage hepatocellular carcinoma in the era of organ shortage. Saudi J Gastroenterol. 2018;24:317–325. doi: 10.4103/sjg.SJG_261_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang YQ, Wang ZX, Deng YN, Yang Y, Wang GY, Chen GH. Efficacy of Hepatic Resection vs. Radiofrequency Ablation for Patients With Very-Early-Stage or Early-Stage Hepatocellular Carcinoma: A Population-Based Study With Stratification by Age and Tumor Size. Front Oncol. 2019;9:113. doi: 10.3389/fonc.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bismuth H, Morino M, Sherlock D, Castaing D, Miglietta C, Cauquil P, Roche A. Primary treatment of hepatocellular carcinoma by arterial chemoembolization. Am J Surg. 1992;163:387–394. doi: 10.1016/0002-9610(92)90039-t. [DOI] [PubMed] [Google Scholar]
- 78.Bronowicki JP, Vetter D, Dumas F, Boudjema K, Bader R, Weiss AM, Wenger JJ, Boissel P, Bigard MA, Doffoel M. Transcatheter oily chemoembolization for hepatocellular carcinoma. A 4-year study of 127 French patients. Cancer. 1994;74:16–24. doi: 10.1002/1097-0142(19940701)74:1<16::aid-cncr2820740105>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 79.Ikeda K, Kumada H, Saitoh S, Arase Y, Chayama K. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer. 1991;68:2150–2154. doi: 10.1002/1097-0142(19911115)68:10<2150::aid-cncr2820681011>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 80.Wong H, Tang YF, Yao TJ, Chiu J, Leung R, Chan P, Cheung TT, Chan AC, Pang RW, Poon R, Fan ST, Yau T. The outcomes and safety of single-agent sorafenib in the treatment of elderly patients with advanced hepatocellular carcinoma (HCC) Oncologist. 2011;16:1721–1728. doi: 10.1634/theoncologist.2011-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Montella L, Addeo R, Cennamo G, Vincenzi B, Palmieri R, Sperlongano P, Sperlongano R, Iodice P, Russo P, Del Prete S. Sorafenib in elderly patients with advanced hepatocellular carcinoma: A case series. Oncology. 2013;84:265–272. doi: 10.1159/000345558. [DOI] [PubMed] [Google Scholar]
- 82.Williet N, Clavel L, Bourmaud A, Verot C, Bouarioua N, Roblin X, Merle P, Phelip JM. Tolerance and outcomes of sorafenib in elderly patients treated for advanced hepatocellular carcinoma. Dig Liver Dis. 2017;49:1043–1049. doi: 10.1016/j.dld.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 83.El-Khoueiry AB AB. Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]