Abstract
Objective
To evaluate the effect of lymph-vascular space invasion (LVSI) on location of recurrences in Danish patients with endometrial cancer.
Methods
This national cohort study (2005–2012) included 4,380 radically operated patients (no visual tumor, all distant metastasis removed). LVSI status was recorded in 3,377 (77.1%). In stage I patients, 2.6% received adjuvant radiotherapy and 1.4% adjuvant chemotherapy. Adjusted Cox regression was used to compare actuarial recurrence rates.
Results
LVSI was present in 18.7% of 3,377 patients with known LVSI status. Of these, 7.6% stage I patients with LVSI experienced an isolated locoregional and 19.4% a non-locoregional recurrence. Compared to no LVSI, 5-year recurrence rate was higher (25.5% vs. 8.5%) in patients with LVSI and the frequency of distant recurrences was strikingly higher (stage I: 15.2% vs. 2.7%), the effect being similar across International Federation of Gynecology and Obstetrics stages and histological types. In intermediate-risk stage I patients with LVSI, 8.0% experienced an isolated locoregional recurrence compared to 20.1% with non-locoregional recurrence, giving these patients a seriously adverse risk of survival. A separate analysis in patients with recurrences demonstrated that those with LVSI had significantly more distant recurrences (55.4% vs. 29.9%) and fewer isolated vaginal recurrences (24.3% vs. 42.8%) than patients with no LVSI.
Conclusion
LVSI is a strong independent risk factor for the development of non-locoregional recurrences even in intermediate-risk stage I endometrial cancer. The non-locoregional recurrence pattern suggests a future focus for optimization of postoperative treatment in these patients.
Keywords: Endometrial Cancer, Lymph Vascular Space Invasion, Recurrences, Survival, Risk Groups
INTRODUCTION
The presence of lymph-vascular space invasion (LVSI) in endometrial cancer has been demonstrated to be significantly and independently associated with poor outcome. LVSI has been associated with decreased survival [1,2,3,4,5,6,7] and increased risk of disseminated disease at time of diagnosis [8], including pelvic and paraaortic lymph node (LN) metastasis [7,9,10,11,12]. Moreover, the risk of recurrence also seems to be increased in these patients [2,4,5,6,8,13].
LVSI is incorporated into the European Society for Medical Oncology/European Society of Gynaecological Oncology/European Society for Radiotherapy & Oncology risk stratification system, and non-staged LVSI-positive intermediate-risk stage I patients are recommended adjuvant external beam radiation therapy (EBRT) to decrease locoregional recurrence. However, no treatment is suggested that may have an effect on the risk of distant recurrences [14]. Recommendations are based on limited data on recurrence patterns in small studies [2,15,16,17,18] with a limited number of patients with LVSI (66 to 129 cases), which does not allow drawing safe conclusion with respect to risk stratification (stage, tumor type, and risk stratification in stage I disease). Therefore, knowledge of the pattern of recurrences in different stage and risk groups of tumors is an important determinant for the type of adjuvant therapy offered LVSI cases.
The Danish Gynecological Cancer Database (DGCD) covers all endometrial cancers [19]. Danish stage I patients have traditionally rarely been given postoperative radiotherapy; therefore, this gives a unique opportunity to monitor the pattern of recurrence over time in a population less likely to be biased due to the treatment given. The aim of the present study was to evaluate in detail the recurrence patterns of Danish endometrial cancer patients in relation to LVSI to help tailor future research regarding the indications and choice of postoperative adjuvant therapy.
MATERIALS AND METHODS
The validated DGCD 2005–12 includes all 4,706 prospectively registered endometrial cancers (excluding sarcomas and carcinosarcomas) diagnosed from 2005 to 2012 [19,20]. Of these, 190 patients did not undergo hysterectomy, leaving 4,516 patients to be included in the analysis of survival in the present study. For analysis of recurrence rates, another 136 non-radically operated patients with progression were excluded (non-radical defined as residual macroscopic tumor in the abdomen or distant metastasis not removed).
Because reporting was optional, LVSI status was primarily registered in only 1,944 cases; therefore, the pathology report of the remaining 2,573 case was retrieved from the pathology registry. LVSI (yes or no) was described in 1,519 cases and entered into the database, but for 1,053 cases, LVSI was not described and therefore registered as unknown, giving altogether 3,463 patients with known LVSI status. LVSI was defined as the presence of tumor cells in a space lined by endothelial cells outside the immediate invasive border and included tumor invasion in both lymph and blood vessels. Pathologists specialized in gynecological cancers evaluated 70% of all cases; the remaining 30% were evaluated by general gynecological pathologists.
As International Federation of Gynecology and Obstetrics (FIGO) stage changed during the study period, all patients were reclassified to FIGO 2009 using the data reported by the pathologist. Patients were divided into cases with known LVSI, no LVSI, and unknown LVSI. Patients were further subdivided into endometrioid and non-endometrioid tumors (clear cell, serous, and undifferentiated carcinoma defined as >10% of the special tumor type). Stage I cases were divided into 3 risk groups: 1) low-risk (grades 1 and 2 with <50% myometrial invasion), 2) intermediate-risk (grades 1 and 2 with >50% myometrial invasion or grade 3 with <50% myometrial invasion), and 3) high-risk (grade 3 with >50% myometrial invasion or non-endometrioid (clear cell, serous, and undifferentiated carcinoma if >10% of the tumor). We also examined separately the stage I patients in whom LNs were staged.
Registration and treatment of endometrial cancers in Denmark is performed according to uniform, strictly respected guidelines, and endometrial cancer treatment at each gynecological oncology center is annually evaluated with respect whether treatment has been according to these guidelines. The operating gynecologist did the primary registration of the surgical treatment. Patients were offered abdominal hysterectomy (radical hysterectomy for stage II and bilateral salpingo-oophorectomy, peritoneal washings, and intraabdominal assessment in all cases. Omentectomy was carried out in high-risk patients, and routine pelvic lymphadenectomy was recommended for all intermediate- and high-risk patients, taking into account comorbidity and high age. Some institutions routinely undertook paraaortic lymphadenectomy for high-risk cases, and all institution removed macroscopically enlarge paraaortic LNs.
Using uniform guidelines, the pathologists reported the following: histopathology types; grade of tumor; myometrial invasion (no invasion, more or less than 50%); involvement of cervix (glandular/stromal invasion), parametrium, vagina, ovaries, tubes, omentum; and number of pelvic and paraaortic LNs including number of metastatic nodes. Disease stage and risk group were reported as well as presence of tumor cells in washings.
Low- and intermediate-risk stage I cases were given no further therapy, but offered 5-years follow-up (every 3–6 months for 3–5 years). No patients received brachytherapy. EBRT was recommended for high-risk stage I patients until 2010 (mainly 50 Gy in 27 fractions) but was seldom given (14.1%). High-risk cases were offered participation in the ongoing European Network for Gynaecological Oncological Trial Groups' protocol (29 patients) or 5-years follow-up with no further treatment. Stage III–IV cases were referred to an oncology department for 6 series of adjuvant chemotherapy (CT), mainly carboplatin-paclitaxel, and rarely a combination of EBRT and CT.
Missing data in the DGCD were retrieved from patients' medical records and pathology reports using the Danish pathology database (a mandatory, nationwide database). Deaths were retrieved from the Danish Central Person Register, which contains information on all Danish residents (death and emigration). Death certificates were acquired from the cause of death register of the Danish National Board of Health, and cause of death was further checked in the medical records. All histologically verified recurrences (n=568) were obtained from the pathology database, and another 90 non-histologically verified recurrences identified by checking the medical record of patients that had subsequently died. All but 2 non-histologically verified recurrences died from their disease (1 cured by radiotherapy for vaginal recurrence without prior histological verification and another alive 6 years after full remission from positron emission tomography-verified LN and bone metastases; this latter case is doubtful, but included as not to underestimate recurrences rates).
Medical records were reviewed for patients with known recurrences to retrieve site, time, and treatment of recurrences. For the analysis of recurrence patterns, we excluded 136 patients with progressive disease at time of primary surgery (not radically operated or distant metastasis not removed). Details regarding the locations of the 657 recurrences were based on both imaging reports and patient files in 264 patients, physicians' descriptions in patient files in 381 patients, but for the remaining 12 recurrences, information was derived solely from the pathology reports. These 12 cases may have had unregistered additional recurrences.
Actuarial recurrences rates were used to compensate for differences in survivals between groups. Recurrences were divided into locoregional, including vaginal and pelvic recurrences (pelvic LNs, local spread to rectum, and bladder), and non-locoregional, abdominal (ascites and carcinomatosis; involvement of bowel, omentum, and paraaortic LNs), and distant recurrences (lung, liver, bone, brain, and LNs other than pelvic and paraaortic) [21]. To evaluate the number of isolated locoregional recurrences, recurrences were also divided into isolated locoregional (vaginal/pelvic), isolated non-locoregional (abdominal/distant), or both locoregional and non-locoregional recurrences. LN recurrences were divided into locoregional including pelvic and paraaortic LNs and extra-abdominal LNs including (inguinal, mediastinal, neck, and axillar).
Data were analyzed using Stata 11 (StataCorp LLC, College Station, TX, USA) [22]. Kaplan–Meier estimates and actuarial recurrence rates were used to compute survival and recurrence. In all, 11 of 4,516 patients had left Denmark and were lost to follow-up. Causes of death other than endometrial cancer were censored for the analysis of disease-specific survival. Recurrence-free survival was estimated using time from surgery to first recurrence, censoring patients dying from causes other than endometrial cancer. Student's t-test was used to calculate differences between means, and the Person χ2 differences between categorical parameters. Differences between survivals and actuarial recurrence rates were calculated using unadjusted and adjusted Cox regression analysis after adjustment for unknown LVSI status. The following patient characteristics were included in the analysis: LVSI status (no LVSI, LVSI, unknown LVSI), age (20–59, 60–69, 70–79, and over 80), American Society of Anesthesiologists physical status (1, 2, 3–4, and unknown 8 patients), stage, grade (grades I, II, III, serous, clear, and undifferentiated), LN resection (yes/no), and adjuvant therapy with EBRT (yes/no) or CT (yes/no) as categorical parameters. As the groups no LVSI and LVSI were different with respect to confounders, we also performed propensity-score matching on the entire population for overall, cancer-specific and recurrence-free survival with results similar to those obtained using Cox regression analysis.
No approval from The Danish Ethics Committees was needed. The study was approved by the Danish Data Protection Board (No. 2010-41-4627) and by the Danish health authorities (No. 3-3013-297/1/).
RESULTS
The mean observation time for survival was 9.2±2.2 years. For recurrences, observation time was at least 5 years. Characteristics of the included patients in relation to LVSI are given in Table 1. Adjuvant therapy was given to 16.6% (6.9% EBRT, 9.0% CT, and 0.55% both) of all 4,516 patients and 3.9% of 3,426 final stage I patient (EBRT/CT: low-risk, 1.2%/0.4%; intermediate-risk, 2.3%/1.1%; and high-risk, 14.1%/9.8%). Only 25 patients had combined chemo-radiation (high-risk: 2/306 patients; stage II: 2/461 patients; stage IIIA/B 7/292 patients; stage IIIC: 10/210 patients; and stage IV: 4/127 patients). LN resection was performed in 28.9% of all cases and in 22.4% of stage I (low-risk: 9.4%; intermediate-risk: 41.2%; and high-risk: 63.7%).
Table 1. Comparison of epidemiological, surgical, and histological characteristics of all 4,516 and for 3,426 stage I Danish endometrial cancer patients in relation to LVSI status at final histology.
| Characteristics | All stages | Stage I | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | No LVSI | LVSI | Unknown LVSI status | p-value of LVSI vs. no LVSI | All | No LVSI | LVSI | Unknown LVSI status | p-value of LVSI vs. no LVSI | ||
| No. | 4,516 | 2,760 (61.1) | 703 (15.6) | 1,053 (23.3) | 3,426 | 2,425 (70.8) | 306 (8.9) | 695 (20.3) | |||
| Age (yr) | 67.4±10.6 | 67.1±10.4 | 68.8±10.7 | 67.1±10.8 | <0.001 | 66.9±10.4 | 67.0±10.3 | 67.9±10.4 | 66.1±10.6 | 0.149 | |
| BMI | 28.5±7.0 | 28.6±7.0 | 28.0±6.7 | 28.8±7.0 | 0.045 | 28.9±7.1 | 28.7±7.0 | 29.3±7.4 | 29.5±7.2 | 0.169 | |
| ASA | 1.8±0.6 | 1.8±0.6 | 1.8±0.7 | 1.7±0.7 | 0.020 | 1.7±0.6 | 1.8±0.6 | 1.8±0.7 | 1.7±1.7 | 0.266 | |
| Histological type | <0.001 | 0.003 | |||||||||
| Endometrioid | 4,035 (89.4) | 2,537 (91.9) | 562 (79.9) | 936 (88.9) | 3,196 (93.3) | 2,267 (93.5) | 272 (88.9) | 657 (94.5) | |||
| Non-endometrioid | 481 (10.7) | 223 (8.1) | 141 (20.1) | 117 (11.1) | 230 (6.7) | 158 (6.5) | 34 (11.1) | 38 (5.5) | |||
| Risk stratification | <0.001 | <0.001 | |||||||||
| Low-risk | 2,247 (49.8) | 1,658 (60.1) | 98 (13.9) | 491 (46.6) | 2,247 (65.6) | 1,658 (68.4) | 98 (32.0) | 491 (70.7) | |||
| Intermediate-risk | 873 (19.3) | 573 (20.8) | 147 (20.9) | 153 (14.5) | 873 (25.5) | 573 (23.6) | 147 (48.0) | 153 (22.0) | |||
| High-risk | 306 (6.8) | 194 (7.0) | 61 (8.7) | 51 (4.8) | 306 (8.9) | 194 (8.0) | 61 (19.9) | 51 (7.3) | |||
| Stages II–IV | 1,090 (24.1) | 335 (12.1) | 397 (56.5) | 358 (34.0) | - | - | - | - | |||
| Nodal staging | 1,304 (28.9) | 677 (24.5) | 322 (45.8) | 305 (29.0) | <0.001 | 766 (22.4) | 512 (21.1) | 111 (36.3) | 143 (20.6) | <0.001 | |
| Pelvic | 1,297 (28.7) | 673 (24.4) | 319 (45.4) | 305 (29.0) | 764 (22.3) | 510 (21.0) | 111 (36.3) | 143 (20.6) | |||
| PA | 125 (2.8) | 51 (1.9) | 40 (5.7) | 34 (3.2) | 51 (1.5) | 37 (1.5) | 7 (2.3) | 7 (1.0) | |||
| Nodal metastasis | 231/1,304 (17.7) | 39/677 (5.8) | 130/322 (40.4) | 62/305 (20.3) | <0.001 | - | - | - | - | ||
| Adjuvant therapy | |||||||||||
| No adjuvant therapy | 3,767 (83.4) | 2,531 (91.7) | 405 (57.6) | 831 (78.9) | <0.001 | 3,291 (96.1) | 2,357 (97.2) | 269 (87.9) | 665 (95.7) | <0.001 | |
| RT | 313 (6.9) | 110 (4.0) | 108 (15.4) | 95 (9.0) | <0.001 | 87 (2.5) | 39 (1.6) | 23 (7.5) | 25 (3.6) | <0.001 | |
| CT | 408 (9.0) | 111 (4.0) | 178 (25.3) | 119 (11.3) | <0.001 | 46 (1.3) | 28 (1.2) | 13 (4.3) | 5 (0.7) | <0.001 | |
| RT+CT | 25 (0.55) | 7 (0.25) | 12 (1.7) | 6 (0.6) | - | 2 (0.06) | 1 (0.04) | 1 (0.33) | - | - | |
| Unknown | 3 (0.07) | 1 (0.04) | - | 2 (0.19) | - | - | - | - | - | - | |
| Progression | 136 (3.0) | 15 (0.5) | 71 (10.1) | 50 (4.7) | <0.001 | 0 | - | - | - | - | |
| Death <5 yr | 935 (20.7) | 390 (14.1) | 311 (44.2) | 234 (22.2) | <0.001 | 474 (13.8) | 294 (12.1) | 86 (28.1) | 94 (13.5) | <0.001 | |
| From cancer | 530 (11.7) | 158 (5.7) | 236 (33.6) | 136 (12.9) | <0.001 | 172 (5.0) | 95 (3.9) | 48 (15.7) | 29 (4.2) | <0.001 | |
| From others | 405 (9.0) | 232 (8.4) | 75 (10.7) | 98 (9.3) | 0.060 | 302 (8.8) | 199 (8.2) | 38 (12.4) | 65 (9.4) | <0.012 | |
Data are shown as mean±standard deviation or number (%). p-values using Pearson's t-test or the χ2 test.
ASA, American Society of Anesthesiologists; BMI, body mass index; CT, chemotherapy; LVSI, lymph-vascular space invasion; PA, paraaortic; RT, radiotherapy.
1. Recurrences
Five-year recurrence rates are related to LVSI status in Table 2. A significantly higher 5-year recurrence rate in LVSI-positive (all: 39.0%; stage I: 25.5%) compared to LVSI-negative cases (all: 10.3%; stage I: 8.5%) was demonstrated. This difference meant that the risk of recurrences was more than double in LVSI-positive cases for both all patients (stage I–IV) and for stage I patients only. However, most markedly the recurrence patterns were clearly different between patients with LVSI and those with no LVSI, with a strikingly higher frequency of especially distant recurrence and a tripled risk in patients with LVSI compared to no LVSI cases (all: hazard ratio [HR]=3.3, 95% confidence interval [CI]=2.5–4.5, p<0.001; stage I: HR=3.7, 95% CI=2.4–5.7; p<0.001). Of all LVSI-positive cases, 19.3% has an isolated non-locoregional recurrence (abdominal/distant), 15.5% experienced a simultaneous locoregional and non-locoregional recurrence, while only 10.5% of LVSI-positive cases had an isolated locoregional recurrence (vaginal/pelvic) compared to 3.3%, 2.6%, 4.7% for LVSI-negative cases. Moreover, LVSI was an independent prognostic factor for LN recurrence at all locations.
Table 2. Five-year recurrence rates for all and for stage I patients with LVSI, no LVSI, and unknown LVSI status at final histology.
| All stages | No LVSI | LVSI | Unknown | Unadjusted/adjusted Cox | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | Actuarial (%) | No. (%) | Actuarial (%) | No. (%) | Actuarial (%) | HR‡ (95% CI) | |||
| All patients (n=4,380) | 2,745 (62.7) | 632 (14.4) | 1,003 (22.9) | ||||||
| Total recurrence rate | 273 (10.0) | 10.3 | 234 (37.0) | 39.0 | 150 (15.0) | 15.4 | 4.7*/2.2* (1.8–2.6) | ||
| All vaginal | 140 (5.1) | 5.3 | 98 (15.5) | 18.2 | 80 (8.0) | 8.3 | 3.8*/2.3* (1.8–3.1) | ||
| All pelvic | 80 (2.9) | 3.1 | 72 (11.4) | 14.2 | 49 (4.9) | 5.4 | 5.0*/2.1* (1.5–3.1) | ||
| All abdominal | 107 (3.9) | 4.2 | 104 (16.5) | 19.7 | 62 (6.2) | 6.7 | 5.4*/2.1* (1.5–2.8) | ||
| All distant | 89 (3.2) | 3.5 | 139 (22.0) | 25.7 | 56 (5.6) | 6.3 | 8.7*/3.3* (2.5–4.5) | ||
| Site of first recurrence | |||||||||
| Isolated locoregional | 124 (4.5) | 4.7 | 54 (8.5) | 10.5 | 59 (5.9) | 6.2 | 2.3*/1.5† (1.1–2.2) | ||
| Isolated non-locoregional | 84 (3.1) | 3.3 | 99 (15.7) | 19.3 | 44 (4.4) | 4.9 | 6.6*/2.2* (1.6–3.1) | ||
| Both local & non-locoregional | 65 (2.4) | 2.6 | 81 (12.8) | 15.5 | 47 (4.7) | 5.2 | 6.8*/3.0* (2.1–4.3) | ||
| Locoregional LN recurrences | 81 (3.0) | 3.2 | 101 (16.0) | 19.4 | 49 (4.9) | 5.4 | 6.9*/2.5* (1.8–3.5) | ||
| All PL | 53 (1.9) | 2.1 | 57 (9.0) | 11.1 | 31 (3.1) | 3.4 | 5.9*/2.3* (1.5–3.5) | ||
| All PA | 53 (2.0) | 2.1 | 71 (11.2) | 14.1 | 32 (3.2) | 3.6 | 7.4*/2.7* (1.8–4.0) | ||
| Extraabdominal LN | 32 (1.2) | 1.3 | 44 (7.0) | 9.0 | 14 (1.4) | 1.6 | 7.7*/2.9* (1.7–5.0) | ||
| Stage I (n=3,426) | 2,425 (70.8) | 306 (8.9) | 695 (20.3) | ||||||
| Total recurrence rate | 201 (8.3) | 8.5 | 74 (24.2) | 25.5 | 59 (8.5) | 15.5 | 3.4*/2.3* (1.7–3.0) | ||
| All vaginal | 114 (4.7) | 4.9 | 39 (12.8) | 14.1 | 38 (5.5) | 8.4 | 3.1*/2.4* (1.7–3.6) | ||
| All pelvic | 50 (2.1) | 2.2 | 19 (6.2) | 7.3 | 15 (2.2) | 5.4 | 3.5*/2.7* (1.5–4.7) | ||
| All abdominal | 69 (2.9) | 3.0 | 32 (10.5) | 11.9 | 22 (3.2) | 6.7 | 4.3*/2.8* (1.8–4.3) | ||
| All distant | 60 (2.5) | 2.7 | 41 (13.4) | 15.2 | 17 (2.5) | 6.3 | 6.3*/3.7* (2.4–5.7) | ||
| Site of first recurrence | |||||||||
| Isolated locoregional | 104 (4.3) | 4.5 | 20 (6.5) | 7.6 | 28 (4.0) | 6.2 | 1.7†/1.4 (0.8–2.3) | ||
| Isolated non-locoregional | 57 (2.4) | 2.5 | 26 (8.5) | 10.2 | 16 (2.3) | 4.9 | 4.3*/2.3* (1.4–3.8) | ||
| Both local & non-locoregional | 40 (1.7) | 1.8 | 28 (9.2) | 10.3 | 15 (2.2) | 5.2 | 6.3*/4.6* (2.7–7.7) | ||
| Locoregional LN recurrences | 52 (2.1) | 2.3 | 23 (7.5) | 8.8 | 15 (2.2) | 5.4 | 4.0*/2.4* (1.4–4.1) | ||
| All PL | 31 (1.3) | 1.4 | 13 (4.3) | 4.8 | 9 (1.3) | 3.5 | 3.8*/2.6* (1.3–5.3) | ||
| All PA | 39 (1.6) | 1.7 | 16 (5.2) | 6.3 | 11 (1.6) | 3.6 | 3.8*/2.1† (1.2–3.9) | ||
| Extraabdominal LN | 22 (0.9) | 1.0 | 15 (4.9) | 5.9 | 3 (0.4) | 1.6 | 6.3*/4.2* (2.0–8.8) | ||
Recurrences divided into total recurrence rate, isolated locoregional, isolated non-locoregional, or both LN recurrences. Total recurrence rate: number of recurrences at each location: vaginal, pelvic, abdominal, or distant recurrences. Isolated locoregional (vaginal+pelvic), isolated non-locoregional (abdominal/distant). Locoregional LN recurrences (pelvic and aortic LN) and extra abdominal LN (inguinal, mediastinal, neck, and axillar).
CI, confidence interval; HR, hazard ratio; LN, lymph node; LVSI, lymph-vascular space invasion; PA, paraaortic; PL, pelvic lymphadenectomy.
*The p<0.001; †The p<0.05 Cox analysis testing LVSI against no LVSI using unadjusted or adjusted Cox; ‡HR adjusted for unknown LVSI status, age (20–59, 60–69, 70–79, and over 80), American Society of Anesthesiologists (1, 2, 3–5, and unknown), stage, grade (grade I, II, III, serous, clear, and undifferentiated), LN resection and adjuvant therapy with external beam radiation therapy (yes/no) or chemo (yes/no) as categorical parameters. One hundred and thirty-six patients with progression excluded from analysis of recurrences.
2. Recurrence rate in subgroups
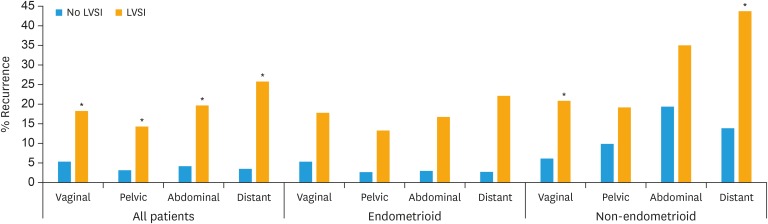
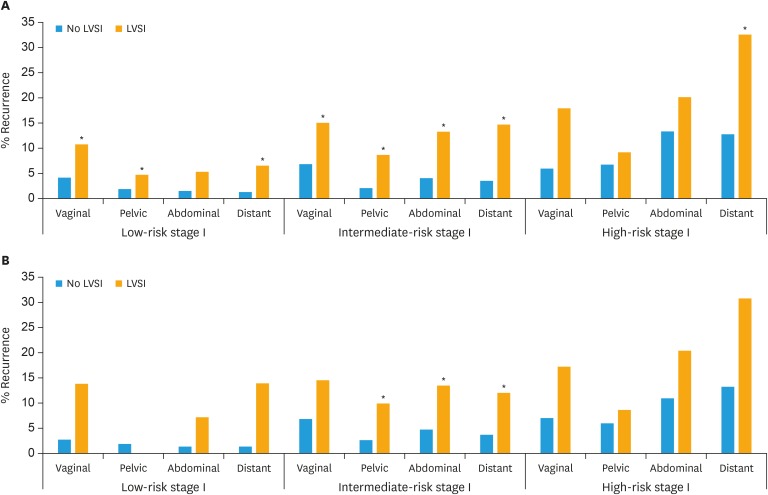
We further examined whether LVSI was an independent prognostic factor in sub-groups of patients with endometrial cancer (Table 3). LVSI was an independent significant predictor for risk of recurrence for non-endometrioid as well as endometrioid tumors and for all FIGO stages except stage IV. In low-risk stage I patients, both locoregional and non-locoregional recurrence was increased in patients with LVSI. Remarkably, for LVSI-positive cases there were higher risks of non-locoregional recurrence compared to locoregional recurrence in patients with stage I (low- and intermediate-risk), stage II and for endometrioid tumors (Tables 2 and 3, Fig. 1). LVSI was also prognostic for LN recurrences in most sub-groups (Tables 2 and 3, Fig. 2).
Table 3. Five-year recurrence rate in endometrial cancer patients with and without LVSI stratified for FIGO stages and histological types (endometrioid/non-endometrioid), risk groups, LN-negative risk groups.
| Variables | LVSI of known status | Unknown status | Recurrence rates actuarial | All Locoregional actuarial | All non-locoregional actuarial | Pelvic/paraaortic LN actuarial | Non-local LN actuarial | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No LVSI | LVSI | HR (95% CI)‡ | No LVSI | LVSI | HR (95% CI)‡ | No LVSI | LVSI | HR (95% CI)‡ | No LVSI | LVSI | HR (95% CI)‡ | No LVSI | LVSI | HR (95% CI)‡ | ||||
| All stages | 18.7 (632/3,377) | 22.9 | 10.2 | 39.0 | 2.1* (1.8–2.6) | 7.2 | 24.3 | 2.1* (1.6–2.7) | 5.8 | 31.8 | 2.6* (2.0–3.3) | 3.2 | 19.4 | 2.5* (1.8–3.5) | 1.3 | 9.0 | 2.9* (1.7–5.0) | |
| FIGO stages | ||||||||||||||||||
| Stage I | 11.2 (306/2,731) | 20.3 | 8.5 | 25.5 | 2.3* (1.7–3.0) | 6.2 | 17.1 | 2.3* (1.6–3.3) | 4.2 | 19.4 | 3.1* (2.2–4.5) | 2.3 | 8.8 | 2.4* (1.4–4.1) | 1.0 | 5.9 | 4.2* (2.0–8.8) | |
| Stage II | 31.4 (94/299) | 35.1 | 18.0 | 37.0 | 2.3* (1.4–3.7) | 14.8 | 25.9 | 1.9† (1.1–3.4) | 11.6 | 26.7 | 2.4† (1.3–4.4) | 9.6 | 16.3 | 1.6 (0.8–3.5) | 1.6 | 6.7 | 3.5 (0.8–15.9) | |
| Stage IIIA/B | 62.7 (116/185) | 30.2 | 37.7 | 55.0 | 1.7† (1.0–2.8) | 23.9 | 31.0 | 1.4 (0.7–2.7) | 32.4 | 45.1 | 1.6 (0.9–2.8) | 13.4 | 32.4 | 2.9† (1.2–6.9) | 9.7 | 14.6 | 1.1 (0.3–3.8) | |
| Stage IIIC | 74.1 (100/135) | 26.2 | 17.6 | 60.8 | 5.0* (2.1–11.7) | 2.9 | 39.4 | 17.1† (2.3–127) | 17.6 | 56.6 | 4.4* (1.8–10.4) | 8.9 | 40.7 | 6.4† (1.9–22.0) | 2.9 | 15.1 | 5.6 (0.7–47.2) | |
| Stage IV | 59.3 (16/27) | 40.0 | 49.4 | 55.0 | 2.6 (0.6–10.4) | 14.3 | 31.0 | 4.8 (0.2–123) | 40.6 | 50.9 | 2.4 (0.6–10.3) | 14.3 | 36.5 | 17.9 (0.6–521) | 14.3 | 15.4 | 2.3 (0.1–36.0) | |
| Endometrioid | 17.1 (521/3,051) | 22.8 | 8.8 | 35.4 | 2.4* (1.9–3.0) | 6.6 | 23.3 | 2.2* (1.7–2.9) | 4.2 | 27.3 | 3.1* (2.3–4.2) | 2.4 | 16.5 | 2.9* (1.9–4.3) | 0.9 | 7.5 | 3.7* (1.9–7.2) | |
| Non-endometrioid | 34.1 (111/326) | 23.8 | 27.5 | 56.7 | 1.6† (1.1–2.5) | 13.8 | 29.8 | 1.9† (1.0–3.5) | 24.3 | 53.4 | 1.6† (1.1–2.6) | 13.0 | 34.6 | 2.0† (1.1–3.7) | 6.0 | 17.4 | 2.0 (0.7–5.3) | |
| Stage I risk groups | ||||||||||||||||||
| Low-risk | 5.6 (98/1,756) | 21.9 | 5.8 | 13.7 | 2.4† (1.3–4.3) | 4.9 | 10.8 | 2.2† (1.1–4.3) | 2.1 | 8.7 | 4.2* (1.9–9.1) | 1.1 | 2.3 | 1.9 (0.4–8.1) | 0.5 | 3.3 | 6.4† (1.7–24.2) | |
| Intermediate-risk | 20.4 (147/720) | 17.5 | 11.6 | 28.1 | 2.9* (1.9–4.4) | 8.4 | 19.6 | 2.8* (1.7–4.6) | 5.4 | 20.1 | 4.5* (2.6–7.9) | 3.1 | 11.6 | 4.4* (2.1–9.1) | 1.4 | 4.8 | 3.7† (1.1–11.9) | |
| High-risk | 23.9 (61/255) | 16.7 | 22.6 | 39.8 | 1.6 (0.9–2.9) | 11.1 | 21.5 | 1.8 (0.8–4.0) | 19.4 | 36.8 | 1.9† (1.0–3.4) | 10.1 | 13.3 | 1.1 (0.4–3.1) | 4.2 | 15.9 | 3.4 (0.9–12.1) | |
| Stage I LN negative | ||||||||||||||||||
| Intermediate-risk | 23.6 (68/288) | 20.0 | 11.5 | 27.2 | 2.7* (1.6–5.9) | 7.8 | 20.0 | 3.0† (1.4–6.6) | 6.0 | 17.8 | 3.4† (1.4–8.2) | 4.5 | 13.3 | 3.9† (1.4–10.9) | 2.1 | 1.9 | 0.4 (0.04–4.2) | |
| High-risk | 17.2 (28/163) | 16.4 | 22.5 | 39.5 | 1.5 (0.7–3.1) | 10.4 | 17.6 | 1.4 (0.4–4.7) | 20.0 | 36.1 | 1.6 (0.7–3.6) | 10.4 | 13.4 | 1.1 (0.3–4.0) | 3.5 | 15.4 | 4.9 (0.9–27.1) | |
Values are presented as number (%). Recurrences divided into locoregional (vaginal/pelvic), non-locoregional including (abdominal/distant recurrences). LN recurrences furthermore divided into pelvic/paraaortic and non-local LN recurrences (inguinal/extra abdominal LN).
CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; LN, lymph node; LVSI, lymph-vascular space invasion.
*The p<0.001; †The p<0.05 LVSI against no LVSI by adjusted Cox analysis; ‡HR (95% CI) for Cox adjusted for unknown LVSI status, age (20–59, 60–69, 70–79, and over 80), American Society of Anesthesiologists (1, 2, 3–5, and unknown), stage, grade (grade I, II, III, serous, clear, and undifferentiated), LN resection and adjuvant therapy with external beam radiation therapy (yes/no), or chemo (yes/no) as categorical parameters. One hundred and thirty-six patients with progression excluded from analysis of recurrences.
Fig. 1. Five-year recurrence rates of cases with or without LVSI subdivided into endometrioid and non-endometrioid cancers. Recurrences divided into total number of recurrences at each location: vaginal, pelvic, abdominal, or distant recurrences. If a patient had recurrences at several sites, the patient can be represented more than once.
LVSI, lymph-vascular space invasion.
*The p<0.05 LVSI tested against no LVSI using adjusted Cox analysis.
Fig. 2. Five-year actuarial recurrence rates of cases with and without LVSI sub-divided into (A) Low-, intermediate-, and high-risk stage I and (B) Lymph node stage and negative low-, intermediate-, and high-risk stage I. Recurrences divided into total number of recurrences at each location: vaginal, pelvic, abdominal, or distant recurrences. If a patient had recurrences at several sites, the patient can be represented more than once.
LVSI, lymph-vascular space invasion.
*The p<0.05 testing LVSI against no LVSI using adjusted Cox analysis.
3. Intermediate-risk stage I
In intermediate-risk stage I, 20.4% had LVSI, with a total recurrence rate of 28.1% for LVSI-positive cases compared to 11.6% for LVSI-negative cases (Table 3, Fig. 2). LVSI-positive cases had the highest HR of 4.5 (95% CI=2.6–7.9; p<0.001) for non-locoregional recurrences (abdominal/distant), which was almost double the HR compared to the risk of locoregional recurrence (HR=2.8; 95% CI=1.7–4.6; p<0.001). In the subgroup (41%) of intermediate-risk patients that were LN staged and LN-negative (Table 3), LVSI status was similarly negatively related to recurrence rate and to recurrence at all locations except for extra-abdominal LN recurrence.
4. High-risk stage I
In the small group of high-risk stage I patients, 23.9% had LVSI, but the risk of recurrence was high for both LVSI-positive (39.8%) and LVSI-negative cases (22.6%). LVSI was associated with increased recurrence at all locations, but LVSI was an independently significant predictor for only non-local recurrences (Table 3, Fig. 2). The number of patients in this group was; however, small, giving lower statistical power.
5. Patients with recurrences
Table 4 shows a comparison between recurrence patterns with regard to LVSI status in patients with known recurrences. Patients with LVSI had significantly more distant recurrences (stage I: 55.4% vs. 29.9%) and fewer isolated vaginal recurrences (24.3% vs. 42.8%) compared to patients with no LVSI.
Table 4. Descriptive analysis of recurrence patterns in patients diagnosed with recurrences with respect to LVSI status.
| Variables | All staged | Stage I | |||
|---|---|---|---|---|---|
| No LVSI | LVSI | No LVSI | LVSI | ||
| No. | 273 | 234 | 201 | 74 | |
| Death before 5 yr | 154 (56.4) | 179* (76.5) | 101 (50.3) | 51† (68.9) | |
| Death from cancer before 5 yr | 142 (52.0) | 170* (72.7) | 93 (46.3) | 48† (64.9) | |
| Total recurrence rate | |||||
| All vaginal | 140 (51.3) | 98† (41.9) | 114 (56.7) | 39 (52.7) | |
| All pelvic | 80 (29.3) | 72 (30.8) | 50 (24.9) | 19 (25.7) | |
| All abdominal | 107 (39.2) | 104 (44.4) | 69 (34.3) | 32 (43.2) | |
| All distant | 89 (32.6) | 139* (59.4) | 60 (29.9) | 41* (55.4) | |
| Site of most severe first recurrence | |||||
| Vaginal | 97 (35.5) | 38* (16.2) | 86 (42.8) | 18† (24.3) | |
| Pelvic | 27 (9.9) | 16 (6.8) | 18 (9.0) | 2 (2.7) | |
| Abdominal | 60 (22.0) | 41 (17.5) | 37 (18.4) | 13 (17.6) | |
| Distant | 89 (32.6) | 139* (59.4) | 60 (29.9) | 41* (55.4) | |
| Loco- and non-locoregional | |||||
| Isolated locoregional | 124 (45.4) | 54* (23.1) | 104 (51.7) | 20* (27.3) | |
| Isolated non-locoregional | 84 (30.8) | 99† (42.3) | 57 (28.4) | 26 (35.1) | |
| Both local & non-locoregional | 65 (23.8) | 81† (34.6) | 40 (19.9) | 28† (37.8) | |
| Locoregional LN recurrences | 81 (29.7) | 101† (43.2) | 52 (25.9) | 23 (31.1) | |
| All PL | 53 (19.4) | 57 (24.4) | 31 (15.4) | 13 (17.6) | |
| All PA | 53 (19.4) | 71† (30.3) | 39 (19.4) | 16 (21.6) | |
| Both PL and PA | 25 (9.2) | 27 (11.5) | 18 (9.0) | 6 (8.1) | |
| Extra abdominal LN | 32 (11.7) | 44† (18.8) | 22 (11.0) | 15† (20.3) | |
Values are presented as number (%). Analysed for all patients and for stage I patients separately. Recurrences divided into total recurrence rate, site of most severe first recurrence, loco- and non-locoregional recurrences, and LN recurrences. Total recurrence rate: number of recurrences at each location: vaginal, pelvic, abdominal, or distant recurrences. Site of most severe first recurrence in the order distant>abdominal>pelvic>vaginal. Isolated locoregional (vaginal+pelvic), isolated non-locoregional (abdominal/distant) or both locoregional LN recurrences PL and PA LN and extra abdominal LN (inguinal, mediastinal, neck, and axillar).
LN, lymph node; LVSI, lymph-vascular space invasion; PA, paraaortic; PL, pelvic lymphadenectomy.
*The p<0.001; †The p<0.05 using the χ2 test. One hundred and thirty-six patients with progression excluded from analysis of recurrences.
6. Multivariate analysis for risk of recurrences
Supplementary Table 1 in the appendix displays the risk of recurrences in relation to LVSI (HR=2.15; 95% CI=1.76–2.62) compared to other variables. Adjuvant CT, but not EBRT, seems to decrease the risk of recurrence, but only a limited numbers of selected patients were treated, which decreases the value of this finding.
7. Survivals
Compared with no LVSI, patients with LVSI had a significantly lower overall, disease-specific and recurrence-free survival (adjusted HR approximately 2), and this was true for both endometrioid and non-endometrioid tumors (Supplementary Fig. 1). For stage I, LVSI was a significant prognostic predictor for survival in all 3 risk groups except with regard to cancer-specific and recurrence-free survival in high-risk patients, but the number of patients in this group was small (61 with LVSI of 255 with known LVSI status), giving lower statistical power (Supplementary Fig. 2).
DISCUSSION
In this national cohort, the presence of LVSI in endometrial cancer was an independent adverse predictor for the risk of developing recurrences in patients with all stages of disease and in endometrioid as well as in non-endometrioid tumors. The recurrence pattern for patients with LVSI was clearly different from that in patients with no LVSI, patients with LVSI having a high-risk of non-locoregional recurrences. Thus, in stage I low- and intermediate-risk patients, the presence of LVSI 4-doubled the risk of non-locoregional recurrences (abdominal/distant). A separate analysis of the recurrence patterns in patients with recurrences demonstrated that patients with LVSI had significantly more distant recurrences (stage I: 55.4% vs. 29.9%) and fewer isolated vaginal recurrences (24.3% vs. 42.8%) than patients with recurrence and no LVSI. The very high-risk of non-local recurrences in patients with LVSI is the main finding in the present study and needs to be considered when advising adjuvant treatment to this group of patients.
LVSI was presented in a considerable proportion (20.4%) of stage I intermediate-risk patients. In these patients, 28% had recurrences (20% non-locoregional (abdominal/distant) and only 8% isolated local recurrence), demonstrating that non-local recurrences are the most serious adverse risk of survival in patients with LVSI.
The major strengths of the present study are inclusion of an entire population, the high number of LVSI cases (n=703), and loss of only 11 of 4,516 patients followed up, thereby giving reliable recurrence and survival data. A further advantage is the Danish centralization of cancer treatment and the vast registration of data that enabled us to also have valid data on the location of recurrences. Tissue pathology was reported by a pathologist specialized in gynecological oncology in 70% of cases, ensuring highly accurate reports in the majority of cases.
A clear limitation of the present study is that 23% of cases had unknown LVSI status, which may have introduced selection bias. The number of cases with unknown LVSI status is, however, similar in the different histological subgroups and in the 3 different risk groups of stage I disease. The missing LVSI information is almost completely due to a period during which documentation of LVSI was voluntary, and some pathologists, including specialized pathologists, did not report LVSI status. Because the survival of the unknown cases is situated between the no LVSI and with LVSI cases, indicating that the group contains a mixture of cases, the group is considered comparable with the other groups. We acknowledge that data may have been less accurate with regard to the site of multiple metastases in the same patient because histological verification was not performed at all locations, and the site of metastasis therefore mainly relied on surgeons' or oncologists' descriptions of image reports. Not all patients had nodal staging, and some patients might have been understaged. However separate analysis of woman with nodal staging only changed the risk marginally. Another bias is the lack of differentiation between lymphovascular and blood vessel invasion in accordance with traditional pathological assessment [23]. Modern immunohistochemistry methods now allow differentiation between lymph vessels and blood vessels [8,15]. A recent study, examining 66 cases with vessel invasion, indicates that patients with tumor invasion into lymph vessels more often have LN metastasis at primary diagnosis, whereas cases with tumor invasion into blood vessels seem to have a higher risk of distant recurrences (12 cases). Future research should be dedicated to finding pathological/biomarkers, including refinement of the diagnosis of LVSI and differentiation between tumor invasion into blood and lymph vessels to predict risk of recurrences and guide the indication for and the type of postoperative therapy offered individual endometrial cancer patients [15].
The present study supports the vast literature demonstrating that the present of LVSI in endometrial cancer is an independent adverse predictor for overall, cancer-specific and recurrence-free survival [1,2,4,6,7,13]. To our knowledge, only a few small studies (66 to 129 LVSI cases) have previously examined the specific location of recurrences in patients with LVSI [2,15,16,17,18]. One of the largest studies examined 129 LVSI-positive cases among 926 patients with endometrioid stage I cancers, mainly intermediate-risk, but only 13 LVSI-positive cases received no postoperative adjuvant therapy. The authors demonstrated that substantial LVSI (44 patients) was the strongest independent prognostic factor for pelvic and distant recurrences [2]. In the present large cohort, these findings were confirmed for stage I intermediate-risk patients. In addition, with regard to the specific location of recurrences, we noted the special high-risk (HR=4.5) of non-local recurrences in mainly non-irradiated LVSI cases.
Endometrial cancer patients have traditionally been given postoperative EBRT to decrease vaginal and pelvic recurrences. One could argue that the increased risk in non-local recurrences found in the present study could also be a result of the Danish practice of not giving postoperative radiotherapy. Studies of post-operative radiation therapy in endometrial carcinoma have; however, demonstrated that while EBRT or vaginal brachytherapy reduces locoregional recurrences, the number distant recurrences and the recurrence-free survival are unaffected by the treatment given intermediate- and high-risk patients [24,25,26,27]. In Danish historical data, we found that the risk of recurrences seems to increase after all types of radiotherapy was omitted for intermediate-risk due to an increase in locoregional recurrences, while in a small group of high-risk patients, no effect on number of locoregional or non-locoregional recurrences could be detected after radiotherapy was omitted [21,28]. In the pioneer study of Alders et al. [26] on EBRT for stage I patients, the authors found a decrease in locoregional recurrences, but also a marginal, but non-significant, increase in distant recurrences, which explains the unchanged recurrence-free survival in patients given compared with patients not given radiotherapy. A plausible explanation for this could be that vaginal recurrence occur sooner then distant recurrence [21,29], and as many patients experience multiple sites of recurrences, the first recurrences may shift from vaginal to distant if the vaginal recurrence is prevented by radiotherapy.
Newer studies have, however, indicated that for endometrial cancers patients with a high-risk of recurrence, adjuvant CT may also reduce the risk of distant recurrences [15,30]. In a prior study, EBRT decreased pelvic but not distant recurrence in patients with LVSI, while vaginal brachytherapy did not affect pelvic recurrence, and EBRT and/or CT was suggested [2]. In the present study, only a small group of selected women had adjuvant treatment, but CT seemed to decrease recurrence, while EBRT did not. However, the findings in the present study, with a high proportion of LVSI-positive cases experiencing non-locoregional recurrences, probably due to the fact that LVSI also includes blood vessel invasion, indicate that local treatment is not sufficient in intermediate- and high-risk stage I cases, and future studies should be dedicated toward finding other treatment regimens that can reduce the risk of developing both locoregional and non-locoregional recurrences.
In conclusion, non-locoregional recurrences are the most serious adverse risk for survival in endometrial cancer cases with LVSI, and research into effective postoperative systemic adjuvant therapy should be initiated.
ACKNOWLEDGMENTS
We would like to acknowledge all the doctors, nurses, and secretaries in the Danish Departments of Gynecology, Pathology, Oncology, and Radiology for the time and effort they spent constantly keeping the database up to date and answering all our requests for data. They represent the foundation for all Danish Gynecological Cancer Database (DGCD) studies. We furthermore acknowledge the hard work of the secretary of the DGCD, and we thank Edwin Stanton Spencer for linguistic corrections.
Footnotes
Funding: The study was financially supported by the Health research fund of Copenhagen University Hospital and Hans & Nora Buchard's Fund.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Ø.G., L.T.L., H.C., D.M.
- Data curation: Ø.G., L.T.L., H.C., H.E.S.
- Formal analysis: Ø.G., L.T.L., H.C., D.M.
- Funding acquisition: Ø.G.
- Investigation: Ø.G., L.T.L.
- Methodology: Ø.G., L.T.L., H.C., H.E.S., D.M.
- Project administration: Ø.G.
- Resources: Ø.G.
- Supervision: D.M.
- Writing - original draft: Ø.G., L.T.L., H.E.S., D.M.
- Writing - review & editing: Ø.G., L.T.L., H.C., H.E.S., D.M.
SUPPLEMENTARY MATERIALS
Unadjusted and adjusted Cox regression to determine significant and independent variables on risk of recurrences in 4,380 radically operated Danish endometrial cancer patients
OS, CSS, and RFS for patients with LVSI, no LVSI, or unknown LVSI status for all patients and subdivided into non-endometrioid and endometrioid carcinomas.
OS, CSS, and RFS for patients with LVSI or no LVSI with a final pathologic diagnosis of low-, intermediate-, and high-risk stage I.
References
- 1.Loizzi V, Cormio G, Lorusso M, Latorre D, Falagario M, Demitri P, et al. The impact of lymph vascular space invasion on recurrence and survival in patients with early stage endometrial cancer. Eur J Cancer Care (Engl) 2014;23:380–384. doi: 10.1111/ecc.12115. [DOI] [PubMed] [Google Scholar]
- 2.Bosse T, Peters EE, Creutzberg CL, Jürgenliemk-Schulz IM, Jobsen JJ, Mens JW, et al. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer--a pooled analysis of PORTEC 1 and 2 trials. Eur J Cancer. 2015;51:1742–1750. doi: 10.1016/j.ejca.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Bendifallah S, Canlorbe G, Raimond E, Hudry D, Coutant C, Graesslin O, et al. A clue towards improving the European Society of Medical Oncology risk group classification in apparent early stage endometrial cancer? Impact of lymphovascular space invasion. Br J Cancer. 2014;110:2640–2646. doi: 10.1038/bjc.2014.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akhavan S, Ahmadzadeh A, Mousavi A, Gilany MM, Kazemi Z, Rahim F, et al. The impact of lymphovascular space invasion on recurrence and survival in Iranian patients with early stage endometrial cancer. World J Oncol. 2016;7:70–74. doi: 10.14740/wjon981w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cusano E, Myers V, Samant R, Sudai T, Keller A, Le T, et al. Prognostic significance of lymphovascular space invasion in the absence of lymph node metastases in early-stage endometrial cancer. Int J Gynecol Cancer. 2018;28:890–894. doi: 10.1097/IGC.0000000000001229. [DOI] [PubMed] [Google Scholar]
- 7.Jorge S, Hou JY, Tergas AI, Burke WM, Huang Y, Hu JC, et al. Magnitude of risk for nodal metastasis associated with lymphvascular space invasion for endometrial cancer. Gynecol Oncol. 2016;140:387–393. doi: 10.1016/j.ygyno.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakayama A, Kudaka W, Matsumoto H, Aoyama H, Ooyama T, Taira Y, et al. Lymphatic vessel involvement is predictive for lymph node metastasis and an important prognostic factor in endometrial cancer. Int J Clin Oncol. 2018;23:532–538. doi: 10.1007/s10147-017-1227-6. [DOI] [PubMed] [Google Scholar]
- 9.Chang SJ, Kong TW, Kim WY, Yoo SC, Yoon JH, Chang KH, et al. Lymph-vascular space invasion as a significant risk factor for isolated para-aortic lymph node metastasis in endometrial cancer: a study of 203 consecutive patients. Ann Surg Oncol. 2011;18:58–64. doi: 10.1245/s10434-010-1206-x. [DOI] [PubMed] [Google Scholar]
- 10.Vaizoglu F, Yuce K, Salman MC, Basaran D, Calis P, Ozgul N, et al. Lymphovascular space involvement is the sole independent predictor of lymph node metastasis in clinical early stage endometrial cancer. Arch Gynecol Obstet. 2013;288:1391–1397. doi: 10.1007/s00404-013-2913-x. [DOI] [PubMed] [Google Scholar]
- 11.Sari ME, Yalcin İ, Sahin H, Meydanli MM, Gungor T. Risk factors for paraaortic lymph node metastasis in endometrial cancer. Int J Clin Oncol. 2017;22:937–944. doi: 10.1007/s10147-017-1139-5. [DOI] [PubMed] [Google Scholar]
- 12.Solmaz U, Mat E, Dereli M, Turan V, Gungorduk K, Hasdemir P, et al. Lymphovascular space invasion and cervical stromal invasion are independent risk factors for nodal metastasis in endometrioid endometrial cancer. Aust N Z J Obstet Gynaecol. 2015;55:81–86. doi: 10.1111/ajo.12321. [DOI] [PubMed] [Google Scholar]
- 13.Briët JM, Hollema H, Reesink N, Aalders JG, Mourits MJ, ten Hoor KA, et al. Lymphvascular space involvement: an independent prognostic factor in endometrial cancer. Gynecol Oncol. 2005;96:799–804. doi: 10.1016/j.ygyno.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visser NC, Werner HM, Krakstad C, Mauland KK, Trovik J, Massuger LF, et al. Type of vascular invasion in association with progress of endometrial cancer. APMIS. 2017;125:1084–1091. doi: 10.1111/apm.12774. [DOI] [PubMed] [Google Scholar]
- 16.Gadducci A, Cosio S, Fabrini MG, Fanucchi A, Barsotti C, Cristofani R, et al. Patterns of failures in endometrial cancer: clinicopathological variables predictive of the risk of local, distant and retroperitoneal failure. Anticancer Res. 2011;1:3483–3488. [PubMed] [Google Scholar]
- 17.Weinberg LE, Kunos CA, Zanotti KM. Lymphovascular space invasion (LVSI) is an isolated poor prognostic factor for recurrence and survival among women with intermediate- to high-risk early-stage endometrioid endometrial cancer. Int J Gynecol Cancer. 2013;23:1438–1445. doi: 10.1097/IGC.0b013e3182a16c93. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo K, Garcia-Sayre J, Medeiros F, Casabar JK, Machida H, Moeini A, et al. Impact of depth and extent of lymphovascular space invasion on lymph node metastasis and recurrence patterns in endometrial cancer. J Surg Oncol. 2015;112:669–676. doi: 10.1002/jso.24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sørensen SM, Bjørn SF, Jochumsen KM, Jensen PT, Thranov IR, Hare-Bruun H, et al. Danish Gynecological Cancer Database. Clin Epidemiol. 2016;8:485–490. doi: 10.2147/CLEP.S99479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juhl CS, Hansen ES, Høgdall CK, Ørtoft G. Valid and complete data on endometrial cancer in the Danish Gynaecological Cancer Database. Dan Med J. 2014;1:A4864. [PubMed] [Google Scholar]
- 21.Ørtoft G, Hansen ES, Bertelsen K. Omitting adjuvant radiotherapy in endometrial cancer increases the rate of locoregional recurrences but has no effect on long-term survival: the Danish Endometrial Cancer Study. Int J Gynecol Cancer. 2013;23:1429–1437. doi: 10.1097/IGC.0b013e3182a5e77d. [DOI] [PubMed] [Google Scholar]
- 22.StataCorp. Stata statistical software: release 11. College Station, TX: StataCorp LP.; 2009. [Google Scholar]
- 23.McCluggage WG. Pathologic staging of endometrial carcinomas: selected areas of difficulty. Adv Anat Pathol. 2018;5:71–84. doi: 10.1097/PAP.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 24.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post operative radiation therapy in endometrial carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 25.Nout RA, Smit VT, Putter H, Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 26.Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;6:419–427. [PubMed] [Google Scholar]
- 27.Creutzberg CL, van Putten WL, Wárlám-Rodenhuis CC, van den Bergh AC, de Winter KA, Koper PC, et al. Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: the postoperative radiation therapy in endometrial carcinoma trial. J Clin Oncol. 2004;22:1234–1241. doi: 10.1200/JCO.2004.08.159. [DOI] [PubMed] [Google Scholar]
- 28.Ørtoft G, Hansen ES, Bertelsen K. Omitting adjuvant radiotherapy in endometrial cancer increases the rate of locoregional recurrences but has no effect on long-term survival: the Danish Endometrial Cancer Study. Int J Gynecol Cancer. 2013;23:1429–1437. doi: 10.1097/IGC.0b013e3182a5e77d. [DOI] [PubMed] [Google Scholar]
- 29.Ørtoft G, Høgdall C, Juhl C, Petersen LK, Hansen ES, Dueholm M. Location of recurrences in high-risk stage I endometrial cancer patients not given postoperative radiotherapy: a Danish gynecological cancer group study. Int J Gynecol Cancer. 2019;29:497–504. doi: 10.1136/ijgc-2018-000056. [DOI] [PubMed] [Google Scholar]
- 30.Ørtoft G, Høgdall C, Juhl C, Petersen LK, Hansen ES, Dueholm M. The effect of introducing pelvic lymphadenectomy on survival and recurrence rates in Danish endometrial cancer patients at high risk: a Danish Gynecological Cancer Group study. Int J Gynecol Cancer. 2019;29:68–76. doi: 10.1136/ijgc-2018-000023. [DOI] [PubMed] [Google Scholar]
- 31.de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Unadjusted and adjusted Cox regression to determine significant and independent variables on risk of recurrences in 4,380 radically operated Danish endometrial cancer patients
OS, CSS, and RFS for patients with LVSI, no LVSI, or unknown LVSI status for all patients and subdivided into non-endometrioid and endometrioid carcinomas.
OS, CSS, and RFS for patients with LVSI or no LVSI with a final pathologic diagnosis of low-, intermediate-, and high-risk stage I.