Abstract
Objective
To treat advanced ovarian cancer, interval debulking surgery (IDS) is performed after 3 cycles each of neoadjuvant chemotherapy (NAC) and postoperative chemotherapy (IDS group). If we expect that complete resection cannot be achieved by IDS, debulking surgery is performed after administering additional 3 cycles of chemotherapy without postoperative chemotherapy (Add-C group). We evaluated the survival outcomes of the Add-C group and determined their serum cancer antigen 125 (CA125) levels to predict complete surgery.
Methods
A retrospective chart review of all stage III and IV ovarian, fallopian tube, and peritoneal cancer patients treated with NAC in 2007–2016 was conducted.
Results
About 117 patients comprised the IDS group and 26 comprised the Add-C group. Univariate and multivariate analyses revealed that Add-C group had an equivalent effect on progression-free survival (PFS; p=0.09) and overall survival (OS; p=0.94) compared with the IDS group. Multivariate analysis revealed that patients who developed residual disease after surgery had worse PFS (hazard ratio [HR]=2.18; 95% confidence interval [CI]=1.45–3.28) and OS (HR=2.33; 95% CI=1.43–3.79), and those who received <6 cycles of chemotherapy had worse PFS (HR=5.30; 95% CI=2.56–10.99) and OS (HR=3.05; 95% CI=1.46–6.38). The preoperative serum CA125 cutoff level was 30 U/mL based on Youden index method.
Conclusions
Administering 3 additional cycles of chemotherapy followed by debulking surgery exhibited equivalent effects on survival as IDS followed by 3 cycles of postoperative chemotherapy. Preoperative serum CA125 levels of ≤30 U/mL may be a useful predictor of achieving complete surgery.
Keywords: Ovarian Neoplasm, Neoadjuvant Therapy, Cytoreduction Surgical Procedures, CA-125 Antigen
INTRODUCTION
The standard treatment for advanced ovarian cancer is primary debulking surgery (PDS) to completely resect all macroscopically visible diseases followed by taxane and platinum-based adjuvant chemotherapy; however, incomplete surgery of any macroscopic residual disease is associated with poor progression-free survival (PFS) and overall survival (OS) [1,2]. Neoadjuvant chemotherapy (NAC) and interval debulking surgery (IDS) have been proposed as alternative treatment strategies for patients with advanced ovarian cancer who were unable to undergo complete debulking surgery [3,4,5]. Several published clinical trials demonstrated that the patients received 3 or 4 cycles of NAC and adjuvant chemotherapy [3,4,5,6]. Complete surgery, that is, the complete removal of macroscopic residual disease, is associated with better survival outcome when performed using either PDS or NAC-IDS approach [1,2,3,4,5,6].
The number of gynecologic oncologists who prefer the NAC-IDS approach is increasing. However, there is no consensus regarding the optimal number of chemotherapy cycles to be administered before and after IDS. Several retrospective studies revealed the impact of the number of NAC cycles on prognosis [7,8,9,10,11,12]. Preoperative evaluation of complete surgery is necessary because it is directly linked to patients' survival outcome. Several studies have investigated the preoperative serum cancer antigen 125 (CA125) level as a predictor of achieving complete surgery by IDS [13,14,15,16,17].
In our institution, IDS was performed after 3 cycles of NAC followed by 3 cycles of postoperative chemotherapy in patients with unresectable advanced ovarian cancer by PDS. However, in patients who were not expected to achieve complete surgery by IDS, debulking surgery was performed after administering additional 3 cycles of chemotherapy without any postoperative chemotherapy (Add-C). The present study aimed to evaluate the efficacy of administering an additional 3 cycles of chemotherapy followed by debulking surgery after 3 cycles of NAC on the survival of patients with advanced ovarian cancer. Moreover, we aimed to investigate the serum CA125 levels to predict complete surgery in patients treated with NAC.
MATERIALS AND METHODS
1. The treatment strategy in our institution
The treatment strategy used in our institution is shown in Supplementary Fig. 1. Standard treatment for ovarian, fallopian tube, and peritoneal cancers was PDS with or without systemic chemotherapy. Surgery included total abdominal hysterectomy, bilateral salpingo-oophorectomy, and omentectomy with or without retroperitoneal lymphadenectomy. Maximal effort was made to achieve complete surgery. All patients received combination chemotherapy with taxane and carboplatin. NAC was used in patients with stage IV disease, poor Eastern Cooperative Oncology Group performance status (2–4), or unresectable abdominal diseases, detected on image, such as bulky upper abdominal disease and multiple peritoneal metastases. We reevaluated the resectability on image after 3 cycle of NAC. IDS was performed followed by 3 cycles of postoperative chemotherapy in patients who were expected to achieve complete surgery by IDS. However, debulking surgery was performed after administering additional 3 cycles of chemotherapy without any postoperative chemotherapy in patients with unresectable diseases (bulky upper abdominal disease and multiple peritoneal metastases). Patients at our institutions usually received 6 cycles of NAC and did not usually undergo postoperative chemotherapy. No evidence exists to suggest that more than 6 cycles of chemotherapy result in a better outcome for front-line chemotherapy for International Federation of Gynecology and Obstetrics (FIGO) stage II–IV epithelial ovarian cancer [18]. We believe that the evidence also applies to the NAC approach.
2. Data collection
This retrospective study was approved by the Institutional Review Board of the National Cancer Center Hospital (2018-120). We analyzed the medical records of patients with pathologically confirmed ovarian, fallopian tube, and peritoneal cancer who received treatment at the National Cancer Center Hospital between August 2007 and December 2016. Patients who underwent PDS, had FIGO (2014) stage I or II, received <3 cycles of NAC, and had no surgical adaptation were excluded. Patients' prognoses and data on age, serum CA125 levels before treatment and surgery, FIGO stage, clinical TNM classification, tumor histological type, operation timing, resection status after debulking surgery, and cycles of NAC and number of chemotherapy cycles (NAC and postoperative chemotherapy) were obtained from medical records. The IDS group comprised patients who were planned to undergo IDS after receiving 3 cycles of chemotherapy, while the Add-C group comprised patients who were planned to undergo additional chemotherapy after initially receiving 3 cycles of chemotherapy. Following the Japan Clinical Oncology Group (JCOG) 0602 trial, some patients were administered 4 cycles of NAC followed by IDS and 4 cycles of postoperative chemotherapy within the study period. These patients were also included in the IDS group.
3. Statistical analysis
Categorical variables were compared using the χ2 test, while continuous variables were compared using the Mann-Whitney U test. For the survival analysis, PFS was defined as the period from the date of first chemotherapy to the date of first recurrence or death of any cause. OS was defined as the period from the date of first chemotherapy to the date of death of any cause. Survival curves were constructed using the Kaplan-Meier method, and a univariate log-rank test was used to assess statistical significance. Multivariate analyses for PFS and OS were performed using Cox proportional hazard model. In patients who underwent surgery, we calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of preoperative serum CA125 levels in predicting the possibility of achieving complete surgery after NAC. Receiver operating characteristic curve (ROC) analysis was demonstrated to seek the threshold of preoperative serum CA125 levels for predicting complete surgery after NAC. The cutoff point was selected based on the Youden index method [19]. For the analyses, the level of statistical significance was set at p<0.05. All statistical analyses were performed using SPSS version 19 for Mac (IBM Corp., Armonk, NY, USA).
RESULTS
1. Study population and clinicopathological characteristics
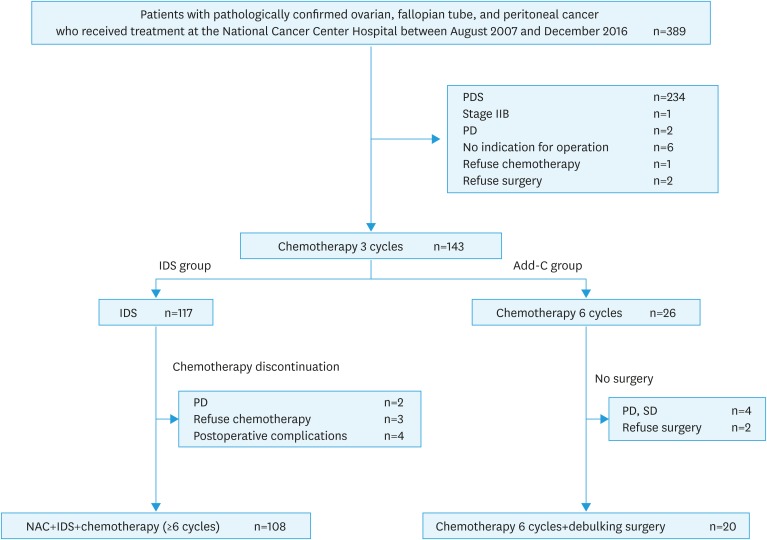
A total of 389 patients with ovarian, fallopian tube, and peritoneal cancer were identified during the study period. Of the 389 patients, 143 met the inclusion criteria and 246 were excluded because they underwent PDS, were classified as FIGO stage II, received <3 cycles of NAC, or had no surgical adaptation. Of these, 117 patients were in the IDS group and 26 in the Add-C group (Fig. 1). Nine patients in the IDS group could not complete the adjuvant chemotherapy due to progressive disease (2 patients), patients' choices (2 patients), and occurrence of postoperative complications (4 patients). Of these 4 patients, 2 had intestinal perforation, 1 had cerebellar infarction, and 1 had depression. In the Add-C group, 6 patients did not undergo debulking surgery because of progressive or stable disease (4 patients) and patients' choices (2 patients). Two patients who refused surgery were expected to achieve complete surgery. The median follow-up period was 38 months (range, 8–130 months). The patients' characteristics are summarized in Table 1. The median age was 61 years (range, 36–87 years) in the IDS group and 62 years (range, 41–78 years) in the Add-C group, with no significant difference between the 2 groups. Compared with the Add-C group, the IDS group had higher serum CA125 levels before NAC and surgery (p<0.01; p=0.04); in the IDS and Add-C group, the median serum CA125 levels before NAC were 1,410 U/mL (range, 52–18,250 U/mL) and 580 U/mL (range, 20–43,460 U/mL), and the median serum CA125 levels before surgery were 28 U/mL (range, 7–922 U/mL) and 17 U/mL (range, 5–195 U/mL). Compared with the Add-C group, the IDS group had higher proportion of patients classified as FIGO stage III (p=0.02). Several patients in the IDS and Add-C group had clinical T3 (95.7%, 96.2%), had pathological serous carcinoma (96.6%, 96.2%), and achieved complete surgery (70.1%, 69.2%), with no significant differences. About half of the patients in the IDS and Add-C group had clinical N1 (45.3%, 50.0%), but without significant differences. Most of the patients (92.3%) in the IDS group were administered ≥6 cycles of chemotherapy, while all patients in the Add-C group were administered 6 cycles of chemotherapy. No patients were administered bevacizumab as the first-line chemotherapy in both groups.
Fig. 1. Number of patients included for analysis. One hundred seventeen patients were in the IDS group and 26 in the Add-C group.
Add-C, additional chemotherapy; IDS, interval debulking surgery; NAC, neoadjuvant chemotherapy; PD, progressive disease; PDS, primary debulking surgery; SD, stable disease.
Table 1. Patients' characteristics (n=143).
| Characteristics | IDS (n=117) | Add-C (n=26) | p-value | |
|---|---|---|---|---|
| Age (yr) | 61 (36–87) | 62 (41–78) | 0.95 | |
| CA125 before NAC (U/mL) | 1410 (52–18,250) | 580 (20–4,346) | <0.01 | |
| CA125 before surgery (U/mL) | 28 (7–922) | 17 (5–195) | 0.04 | |
| FIGO stage (2014) | 0.02 | |||
| III | 69 (59.0) | 9 (34.6) | ||
| IV | 48 (41.0) | 17 (65.4) | ||
| TNM classification | 0.92 | |||
| cT1,2 | 5 (4.3) | 1 (3.8) | ||
| cT3 | 112 (95.7) | 25 (96.2) | ||
| cN0 | 64 (54.7) | 13 (50.0) | 0.66 | |
| cN1 | 53 (45.3) | 13 (50.0) | ||
| Histological type | 0.49 | |||
| Serous | 113 (96.6) | 25 (96.2) | ||
| Endometrioid | 1 (0.9) | 0 (0.0) | ||
| Clear cell | 3 (2.6) | 1 (3.8) | ||
| Resection status | 0.93 | |||
| No residual | 82 (70.1) | 18 (69.2) | ||
| Residual | 35 (29.9) | 2 (7.7) | ||
| No operation | 0 (0.0) | 6 (23.1) | ||
| Number of chemotherapy cycles* | ||||
| 3 cycles | 4 (3.4) | 0 (0.0) | ||
| 4 cycles | 2 (1.7) | 0 (0.0) | ||
| 5 cycles | 3 (2.6) | 0 (0.0) | ||
| 6 cycles | 92 (78.6) | 26 (100.0) | ||
| 7 cycles | 4 (3.4) | 0 (0.0) | ||
| 8 cycles | 12 (10.3) | 0 (0.0) | ||
| NAC | ||||
| 3 cycles | 76 (65.0) | 0 (0.0) | ||
| 4 cycles | 41 (35.0) | 0 (0.0) | ||
| 5 cycles | 0 (0.0) | 0 (0.0) | ||
| 6 cycles | 0 (0.0) | 20 (76.9) | ||
Values are presented as median (range) or number of patients (%).
Add-C, additional chemotherapy; CA125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics; IDS, Interval debulking surgery; NAC, neoadjuvant chemotherapy.
*NAC and postoperative chemotherapy.
2. Prognostic factors for PFS and OS
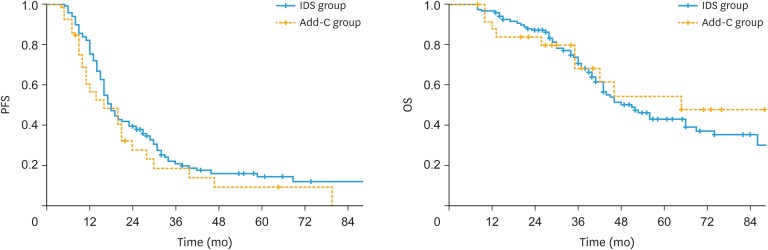
During the follow-up period, 95 (81.2%) patients had recurrence and 60 (51.3%) died in the IDS group, while 23 (88.5%) patients had recurrence and 11 (42.3%) died in the Add-C group. Kaplan-Meier estimates of PFS and OS of the IDS and Add-C group are presented in Fig. 2. The median PFS and OS of the IDS group were 18 and 51 months, respectively. By contrast, the median PFS and OS of the Add-C group were 16 and 65 months, respectively. The 5-year PFS rate and 5-year OS rate of the IDS group were 14.2% (95% confidence interval [CI]=6.9%–21.5%) and 43.0% (95% CI=32.4%–53.6%), respectively. On the contrary, the 5-year PFS rate and 5-year OS rate of the Add-C group were 9.2% (95% CI=0.0%–21.2%) and 47.8% (95% CI=23.8%–71.7%), respectively. There were no significant differences in PFS (p=0.23) and OS (p=0.70) between the IDS group and Add-C group.
Fig. 2. Kaplan-Meier estimates for PFS and OS of IDS and Add-C group. There were no significant differences in PFS (p=0.23) and OS (p=0.70) between the IDS group and Add-C group.
Add-C, additional chemotherapy; IDS, interval debulking surgery; OS, overall survival; PFS, progression-free survival.
Multivariate analysis on age, FIGO Stage, resection status after debulking surgery, number of chemotherapy cycles, and therapeutic plan revealed that resection status and number of chemotherapy cycles have significant effects on survival outcome, and an equivalent effects on PFS (hazard ratio [HR]=1.51; 95% CI=0.94–2.41) and OS (HR=1.03; 95% CI=0.53–2.01) in both groups. Patients with residual disease after surgery had worse PFS (HR=2.18; 95% CI=1.45–3.28) and OS (HR=2.33; 95% CI=1.43–3.79), whereas patients who received <6 cycles of chemotherapy had worse PFS (HR=5.30; 95% CI=2.56–10.99) and OS (HR=3.05; 95% CI=1.46–6.38) (Table 2).
Table 2. The multivariate analysis of clinicopathological factors and survival outcomes.
| Factor | PFS | OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age | |||||
| <70 | 1 | 1 | |||
| ≥70 | 0.9 (0.57–1.40) | 0.64 | 0.95 (0.53–1.71) | 0.88 | |
| FIGO stage (2014) | |||||
| III | 1 | 1 | |||
| IV | 0.85 (0.58–1.26) | 0.41 | 1.04 (0.64–1.70) | 0.87 | |
| Resection status | |||||
| No residual | 1 | 1 | |||
| Residual | 2.18 (1.45–3.28) | <0.01 | 2.33 (1.43–3.79) | <0.01 | |
| Number of chemotherapy cycles* | |||||
| ≥6 | 1 | 1 | |||
| <6 | 5.3 (2.56–10.99) | <0.01 | 3.05 (1.46–6.38) | <0.01 | |
| Therapeutic plan | |||||
| IDS | 1 | 1 | |||
| Add-C | 1.51 (0.94–2.41) | 0.09 | 1.03 (0.53–2.01) | 0.94 | |
Add-C, additional chemotherapy; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; IDS, interval debulking surgery; OS, overall survival; PFS, progression-free survival.
*Neoadjuvant chemotherapy and postoperative chemotherapy.
3. Threshold of preoperative serum CA125 levels for predicting complete surgery
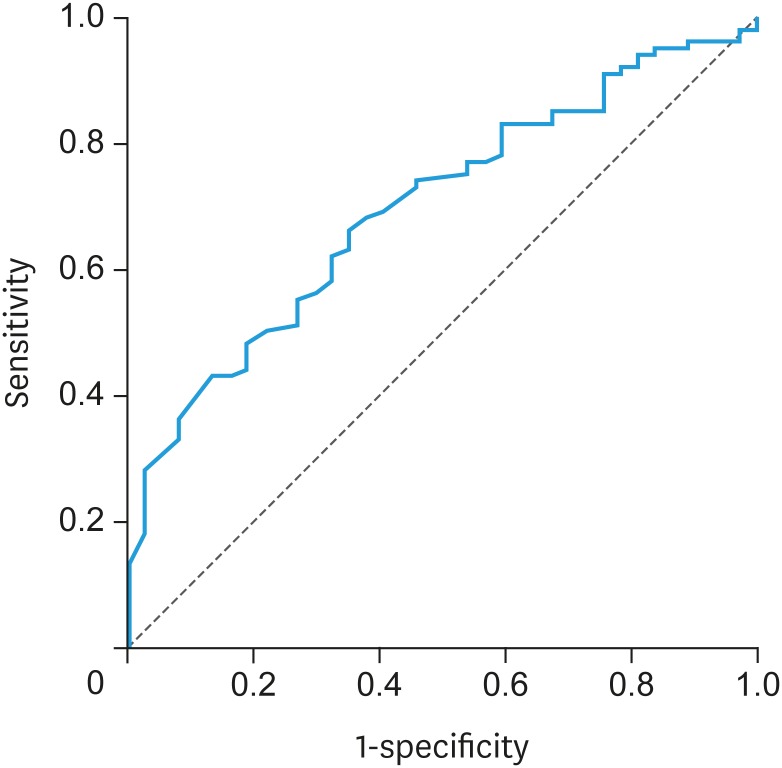
ROC curve demonstrated the threshold of preoperative serum CA125 levels for predicting complete surgery after NAC (Fig. 3). The area under the curve (AUC) was 0.70. The cutoff level was 30 U/mL based on the Youden index method. The sensitivity, specificity, PPV, NPV, and accuracy were 66.0%, 64.9%, 83.5%, 41.4%, and 65.7%, respectively (Table 3).
Fig. 3. ROC demonstrating the predictive ability of preoperative serum CA125 levels.
CA125, cancer antigen 125; ROC, receiver operating characteristic curve.
Table 3. Associations of preoperative serum CA125 levels with resection status after surgery.
| Preoperative CA125 levels | No residual | Residual | Total |
|---|---|---|---|
| ≤30 U/mL | 66 | 13 | 79 |
| <30 U/mL | 34 | 24 | 58 |
| Total | 100 | 37 | 137 |
Sensitivity, 66.0%; specificity, 64.9%; positive predictive value, 83.5%; negative predictive value, 41.4%; accuracy, 65.7%.
CA125, cancer antigen 125.
DISCUSSION
Our study showed that the effect of administering 6 cycles of chemotherapy followed by debulking surgery on the survival of patients with advanced ovarian, fallopian tube, and peritoneum cancer was equivalent to that of IDS after 3 cycles of NAC followed by 3 cycles of postoperative chemotherapy. Additionally, achieving complete surgery, regardless of the timing of surgery, and the administration of ≥6 cycles of chemotherapy were considered as favorable prognostic factors. Furthermore, our findings suggest that a preoperative serum CA125 level of ≤30 U/mL is a useful predictor of achieving complete surgery in patients with ovarian cancer treated with NAC.
Univariate and multivariate analyses revealed that administering 6 cycles of NAC followed by debulking surgery had equivalent effects on the PFS and OS compared with the IDS group. However, the association between NAC and postoperative chemotherapy cycles and prognosis of patients with advanced ovarian cancer remained unclear. Additionally, previous studies evaluating the relationship between NAC cycles and prognosis had conflicting results. Compared with ≤4 cycles of NAC, previous studies reported that ≥5 cycles of NAC had equivalent effects on PFS and OS [7,10,11], while others reported poor prognosis [9,12]. With regard to postoperative chemotherapy, Chung et al. [8] reported that >3 cycles of postoperative chemotherapy had no benefit in a group of patients who achieved complete radiological remission after 3 cycles of postoperative chemotherapy. Our treatment strategy is unique compared with these previous studies. In our institutions, patients administered with 6 cycles of NAC did not usually receive postoperative chemotherapy. The patients' characteristics including those reported in these studies were different from those of other previous studies. Compared with the Add-C group, the IDS group had higher serum CA125 levels before NAC. We believed that this difference did not influence the treatment decision because we did not refer to serum CA125 levels before NAC for surgical indication of IDS. However, there was a significant difference in serum CA125 levels before NAC between the IDS group and the Add-C group. As a result, selection bias might have influenced the survival outcomes.
Multivariate analyses revealed that complete surgery and administering ≥6 cycles of chemotherapy were independent favorable prognostic factors. Phillips et al. [10] reported that complete surgery remains a significant independent marker of survival in patients undergoing surgery even after 5 cycles of NAC. This result is similar to those reported in our study. Since complete surgery is important for patients' prognosis, performing debulking surgery in a period when complete surgery is expected may be better than performing on schedule regardless of chemotherapy responses. Chung et al. [8] reported that at least 6 cycles of total chemotherapy (NAC and postoperative adjuvant chemotherapy) is an independent prognostic factor in patients treated with NAC, IDS, and postoperative chemotherapy. This result was also similar with our findings. Administering 6 cycles of chemotherapy followed by debulking surgery had significant advantages. Several types of radical surgeries, such as bowel resection, diaphragm stripping, splenectomy, and so on, were also performed to achieve complete surgery, and only a few complications were reported. Some patients did not receive postoperative chemotherapy after undergoing IDS. In this study, postoperative chemotherapy was not administered in four patients who developed postoperative complications. In particular, 2 patients had intestinal perforation caused by IDS. Hence, 6 cycles of chemotherapy followed by debulking surgery may be suitable for patients who require radical surgery to achieve complete surgical resection.
About 37 out of 137 (27.0%) patients who underwent debulking surgery had residual disease; nevertheless, patients who were expected to achieve complete surgery based on the following factors were scheduled for surgery: patients' performance status, pelvic exam, and image findings such as computed tomography, magnetic resonance imaging, and echo. Some patients had multiple microscopic peritoneum metastases at the mesentery, which were not detected during preoperative imaging. Hence, it was difficult to completely remove such visceral multiple peritoneum metastases. Our study suggested that a preoperative serum CA125 level of ≤30 U/mL was a useful predictor of achieving complete surgery. The PPV was high (83.5%); therefore, complete surgery could be achieved if the preoperative serum CA125 level was ≤30 U/mL. Some previous studies reported CA125 cutoff value as a predictor of achieving complete surgery after NAC in patients with advanced ovarian cancer [13,14,15,16,17]. Furukawa et al. reported that the preoperative serum CA125 level of ≤20 U/mL was considered as the cutoff value that predicts complete surgical resection in patients who underwent IDS [13]. All patients in this study underwent IDS after 3 cycles of NAC. Pelissier et al. [14] reported that the preoperative serum CA125 level of ≤75 U/mL after 3 cycles of NAC was a predictor of achieving complete surgical resection in patients who underwent IDS. The specificity and the NPV were 73.6% and 68.4%, respectively [14]. All study patients underwent IDS after each cycle of NAC, and the median NAC cycle was 6. Rodriguez et al. [16] reported that patients with a preoperative CA125 level of ≤100 U/mL had a higher likelihood of achieving complete surgical resection compared with those who had a preoperative CA125 level of >100 U/mL (HR=2.6; 95% CI=1.05–6.5). Serum CA125 was measured preoperatively, and the number of NAC cycles varied in this study. The differences in optimal thresholds for CA125 may be due to the differences in patients' characteristics, such as CA125 measurement timing, NAC cycles, and the rate of complete surgery. Our date may not be sufficient to predict complete surgical resection because of low NPV (41.4%) and accuracy (65.7%). Therefore, further studies are necessary to determine other effective predictors.
The present study had several limitations. The judgement of resectability depended on physician's decision, even though it was discussed at the conference attended by some gynecologic oncologists. Thus, it may be difficult to generalize the findings of the present study. The number of patients in the Add-C group was limited. Hence, more large-scale cohort studies should be performed in the future to validate the findings of our study.
In conclusion, with regard to the treatment of advanced ovarian, fallopian tube, and peritoneum cancer, complete surgery and ≥6 cycles of chemotherapy are independent favorable prognostic factors, and preoperative serum CA125 level of ≤30 U/mL may be a useful predictor of complete surgery in patients treated with NAC. After 3 cycles of NAC, administering additional 3 cycles of chemotherapy followed by debulking surgery exhibited equivalent effects on survival as IDS followed by 3 cycles of postoperative chemotherapy, although the number of included patients in the Add-C group was small. Therefore, administering additional 3 cycles of chemotherapy followed by debulking surgery might be preferable for patients who are not expected to achieve complete surgery after 3 cycles of NAC. However, further larger-scale studies are necessary to validate the findings of the present study.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Y.Y., I.M.
- Data curation: Y.Y.
- Formal analysis: Y.Y., I.M.
- Funding acquisition: Y.Y.
- Investigation: Y.Y.
- Methodology: Y.Y., I.M.
- Project administration: M.T., K.T.
- Resources: Y.Y., I.M., U.T., S.H., U.M.,, K.T.
- Software: Y.Y.
- Supervision: M.T., K.T.
- Validation: Y.Y., I.M.
- Visualization: Y.Y.
- Writing - original draft: Y.Y.
- Writing - review & editing: Y.Y., I.M., U.T., S.H., U.M., M.T., K.T.
SUPPLEMENTARY MATERIAL
The treatment strategy used in our institution for the patients with ovarian, fallopian tube, and peritoneum cancer.
References
- 1.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 2.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur J Cancer. 2016;59:22–33. doi: 10.1016/j.ejca.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 5.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 6.Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Nakamura K, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer. 2016;64:22–31. doi: 10.1016/j.ejca.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Akladios C, Baldauf JJ, Marchal F, Hummel M, Rebstock LE, Kurtz JE, et al. Does the number of neoadjuvant chemotherapy cycles before interval debulking surgery influence survival in advanced ovarian cancer? Oncology. 2016;91:331–340. doi: 10.1159/000449203. [DOI] [PubMed] [Google Scholar]
- 8.Chung YS, Kim YJ, Lee I, Lee JY, Nam EJ, Kim S, et al. Impact of neoadjuvant chemotherapy and postoperative adjuvant chemotherapy cycles on survival of patients with advanced-stage ovarian cancer. PLoS One. 2017;12:e0183754. doi: 10.1371/journal.pone.0183754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo PE, Labaki M, Fabbro M, Bertrand M, Mourregot A, Gutowski M, et al. Impact of neoadjuvant chemotherapy cycles prior to interval surgery in patients with advanced epithelial ovarian cancer. Gynecol Oncol. 2014;135:223–230. doi: 10.1016/j.ygyno.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Phillips A, Sundar S, Singh K, Nevin J, Elattar A, Kehoe S, et al. Complete cytoreduction after five or more cycles of neo-adjuvant chemotherapy confers a survival benefit in advanced ovarian cancer. Eur J Surg Oncol. 2018;44:760–765. doi: 10.1016/j.ejso.2018.01.097. [DOI] [PubMed] [Google Scholar]
- 11.Stoeckle E, Boubli B, Floquet A, Brouste V, Sire M, Croce S, et al. Optimal timing of interval debulking surgery in advanced ovarian cancer: yet to be defined? Eur J Obstet Gynecol Reprod Biol. 2011;159:407–412. doi: 10.1016/j.ejogrb.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Deng F, Lv M, Chen X. The number of cycles of neoadjuvant chemotherapy is associated with prognosis of stage IIIc-IV high-grade serous ovarian cancer. Arch Gynecol Obstet. 2017;295:451–458. doi: 10.1007/s00404-016-4256-x. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa N, Sasaki Y, Shigemitsu A, Akasaka J, Kanayama S, Kawaguchi R, et al. CA-125 cut-off value as a predictor for complete interval debulking surgery after neoadjuvant chemotherapy in patients with advanced ovarian cancer. J Gynecol Oncol. 2013;24:141–145. doi: 10.3802/jgo.2013.24.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelissier A, Bonneau C, Chéreau E, de La Motte Rouge T, Fourchotte V, Daraï E, et al. CA125 kinetic parameters predict optimal cytoreduction in patients with advanced epithelial ovarian cancer treated with neoadjuvant chemotherapy. Gynecol Oncol. 2014;135:542–546. doi: 10.1016/j.ygyno.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Pelissier A, Roulot A, Guéry B, Bonneau C, Bellet D, Rouzier R. Serum CA125 and HE4 levels as predictors for optimal interval surgery and platinum sensitivity after neoadjuvant platinum-based chemotherapy in patients with advanced epithelial ovarian cancer. J Ovarian Res. 2016;9:61. doi: 10.1186/s13048-016-0270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez N, Rauh-Hain JA, Shoni M, Berkowitz RS, Muto MG, Feltmate C, et al. Changes in serum CA-125 can predict optimal cytoreduction to no gross residual disease in patients with advanced stage ovarian cancer treated with neoadjuvant chemotherapy. Gynecol Oncol. 2012;125:362–366. doi: 10.1016/j.ygyno.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Zeng J, Yin J, Song X, Jin Y, Li Y, Pan L. Reduction of CA125 levels during neoadjuvant chemotherapy can predict cytoreduction to no visible residual disease in patients with advanced epithelial ovarian cancer, primary carcinoma of fallopian tube and peritoneal carcinoma. J Cancer. 2016;7:2327–2332. doi: 10.7150/jca.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–vi32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 19.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The treatment strategy used in our institution for the patients with ovarian, fallopian tube, and peritoneum cancer.