Abstract
Objective
A subset of patients with recurrent ovarian cancer (ROC) may benefit from antiestrogen therapy with higher response rates reported in tumors that are strongly estrogen receptor (ER)-positive (ER+). PARAGON is a basket trial that incorporates 7 phase 2 trials investigating the activity of anastrozole in patients with ER+ and/or progesterone receptor (PR)-positive (PR+) recurrent/metastatic gynecological cancers.
Methods
Postmenopausal women with ER+ and/or PR+ ROC, who were asymptomatic and had cancer antigen 125 (CA125) progression after response to first line chemotherapy, where chemotherapy was not clinically indicated. Patients received anastrozole 1 mg daily until progression or unacceptable toxicity.
Results
Fifty-four patients were enrolled (52 evaluable). Clinical benefit at three months (primary endpoint) was observed in 18 patients (34.6%; 95% confidence interval [CI]=23%–48%). Median progression-free survival (PFS) was 2.7 months (95% CI=2.1–3.1). The median duration of clinical benefit was 6.5 months (95% CI=2.8–11.7). Most patients progressed within 6 months of starting anastrozole but 12 (22%) continued treatment for longer than 6 months. Anastrozole was well tolerated. In the exploratory analysis, ER histoscores and the intensity of ER staining did not correlate with clinical benefit rate or PFS.
Conclusion
A subset of asymptomatic patients with ER+ and/or PR+ ROC and CA125 progression had durable clinical benefit on anastrozole, with acceptable toxicity. Anastrozole may delay symptomatic progression and the time to subsequent chemotherapy. The future challenge is to identify the subset of patients most likely to benefit from an aromatase inhibitor and whether the clinical benefit could be increased by the addition of other agents.
Keywords: Ovarian Neoplasm, CA-125 Antigen, Estrogens, Aromatase Inhibitors
INTRODUCTION
There is evidence that a subset of patients with recurrent epithelial ovarian cancer (EOC), including fallopian tube and primary peritoneal cancers, may benefit from treatment with an antiestrogen [1,2,3]. The reported frequency of hormone receptor expression in EOC is variable and ranges from 6% to 77% for estrogen receptor (ESR1, ER) and from 26% to 43% for progesterone receptor (PR) [1,2,4,5,6,7,8,9,10]. A large study of almost 3,000 patients with EOC, reported that 67% were ER-positive (ER+) and 34% PR-positive (PR+), representing the most reliable data on ER/PR-positivity in EOC to date [8]. In contrast to breast cancer, the prognostic and predictive values of ER/PR status have not been established. Estrogen has been demonstrated to stimulate the growth of ovarian tumor cell lines expressing estrogen receptors and can be blocked by tamoxifen [11,12,13].
Endocrine therapy is widely used in the clinic in patients with recurrent ovarian cancer (ROC) with variable response rates reported. A Cochrane systematic review of tamoxifen in ROC reported a 10% objective response rate (ORR) and 32% disease stabilization rate, unselected by hormone-receptor status [1]. These studies comprised a heterogenous group of patients including asymptomatic patients with a rising cancer antigen 125 (CA125) as well as those with chemotherapy-resistant ROC after multiple lines of chemotherapy. A Gynecologic Oncology Group (GOG) phase 2 study reported a 13% ORR with tamoxifen in patients with platinum-resistant EOC with median response duration of 4.4 months [4,14]. GOG 198 compared tamoxifen versus thalidomide in women with ROC with Gynecologic Cancer InterGroup (GCIG)-defined CA125 progression after first-line chemotherapy. Median progression-free survival (PFS) in the thalidomide vs tamoxifen groups was 3.2 months vs 4.5 months, suggesting a greater benefit with tamoxifen [15].
There have been several phase 2 trials of aromatase inhibitors (AIs) in ROC with mixed results reported. Bowman reported a study of 60 unselected patients with ROC who received letrozole (50 evaluable), and found that ten had stable disease for at least 3 months by Response Evaluation Criteria in Solid Tumors (RECIST), but no responses were seen [6]. Another study of letrozole included patients with ER+ ROC with GCIG CA125 progression, 46% after at least two lines of treatment and 43% with platinum-resistant disease [2]. ORRs were observed in 17% by CA125 criteria (n=42) and 9% (n=33) by RECIST. Subgroup analysis indicated higher response rates in patients with high ER histoscores. The CA125 response rate was 0% in patients with tumors with histoscores of 150–199, 12% with histoscores 200–249, and 33% with histoscores 250–300 [2].
A large European Organisation for Research and Treatment of Cancer (EORTC) trial found that there was no advantage in commencing chemotherapy early in asymptomatic patients with CA125 progression alone [16]. Most patients continue to have CA125 monitoring after completing chemotherapy, although treatment is not routinely commenced in asymptomatic patients with CA125 progression and small volume disease. These patients may be offered endocrine therapy or a ‘watch and wait’ approach with further chemotherapy commenced when they become symptomatic.
Hormonal therapy is an attractive option in asymptomatic patients with CA125 progression as it is generally well tolerated, can be administered for prolonged periods, and may delay the time to subsequent chemotherapy. However, there is uncertainty regarding the proportion of patients who derive clinical benefit and ORRs have been variable probably reflecting the heterogeneous population treated. In a retrospective study of 56 ROC with asymptomatic, low-volume disease treated with tamoxifen, 42% and 19% remained on tamoxifen for >6 and >12 months, respectively, although it remains uncertain whether the delay to chemotherapy is due to benefit from tamoxifen or reflects more indolent disease [17]. Furthermore, it is unclear whether the degree of ER and PR-positivity predicts response to antiestrogens. AIs have higher response rates than tamoxifen in postmenopausal women with hormone dependent breast cancer [5] and there is preliminary data to suggest that they are also active in EOC with higher responses rates correlating with higher ER histoscores [2,6].
PARAGON is an investigator-initiated single arm open-label multicentre phase 2 trial, designed to evaluate the activity of anastrozole in postmenopausal patients with a wide range of ER+ and/or PR+, recurrent or metastatic gynecological tumors. Seven separate prospective phase 2 studies were included within this basket protocol and we report the study that included asymptomatic patients with ROC and CA125 progression.
MATERIALS AND METHODS
Anastrozole (1 mg per oral daily) was administered in asymptomatic women with EOC (including primary peritoneal cancers and cancers of the fallopian tube) with GCIG defined CA125 progression [18]. Patients were treated until disease progression, based on either CA125 or RECIST v1.1 criteria, unacceptable toxicity or physician/patient preference.
Eligible patients were postmenopausal women who had either ER+ and/or PR+ tumors by immuno-histochemical staining, based on local assessment by pathologists, with at least 10% of cells staining positive for ER and/or PR. A tumor specimen was requested for central confirmation of ER and PR expression. Patients with measurable disease (by RECIST) were allowed to participate, but the protocol stipulated that they should have small volume recurrence and no clinical indication to commence chemotherapy within the next 3 months; age ≥18; life expectancy >3 months, Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 and no previous endocrine treatment.
1. Assessments
Baseline evaluation included history, physical examination, ECOG performance status, routine blood tests, CA125, computed tomography (CT) abdomen and pelvis (with CT chest, if pulmonary metastases were present) and quality of life (QoL) forms were required within 28 days of registration. CTs were required 3-monthly; CA125 and QoL forms were required once a month for the first 3 months, and then three-monthly thereafter until progression.
At study entry, investigators nominated response assessment according to either GCIG CA125 or RECIST v1.1 (if measurable target lesion was present). A response based on GCIG CA125 was defined as at least a 50% reduction in CA125 levels from a pre-treatment sample. The response must have been confirmed and maintained for at least 28 days. Patients could be evaluated according to CA125 response criteria only if they had pre-treatment sample that was at least twice the upper limit of normal and within 2 weeks prior to starting treatment [18]. We assessed adverse events using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.0.
2. Study objectives
The primary objective of the PARAGON trial was to determine the clinical benefit rate (CBR) at 3 months (i.e. partial/complete response/stable disease). Response was determined either by RECIST v1.1 criteria and/or by GCIG CA125 criteria if no measureable disease was present at baseline [18,19]. Secondary objectives included PFS, response duration, QoL and toxicity. QoL was assessed using the EORTC Quality of Life Questionnaire Core 30 (QLQ-C30) and Functional Assessment of Cancer Therapy-Endocrine Symptoms (FACT-ES) subscale. The exploratory endpoint was to determine the association between ER histoscores and CBR.
3. Study conduct
The PARAGON study was coordinated by the National Health and Medical Research Council (NHMRC) Clinical Trials Centre, University of Sydney, in collaboration with Australian New Zealand Gynaecological Oncology Group (ANZGOG). Anastrozole was supplied by Astra Zeneca. This study was conducted according to the principles of the Declaration of Helsinki and was prospectively registered (ACTRN12610000796088).
4. Tissue microarray construction and immunohistochemical staining for ER and PR
Formalin-fixed paraffin-embedded tumor tissue was sent to a central reference laboratory for confirmation and quantitation of hormone receptor status. The study pathologist selected representative areas for coring from haematoxylin and eosin stained sections of diagnostic tumor blocks. Duplicate 1 mm cores were taken from each donor block and assembled in a recipient paraffin block by using a manual precision instrument (MTA-1; Beecher Instrument, Sun Prairie, Wisconsin, USA). Endometrium (positive control) and spleen (negative control) were included in each array. Microarray sections (4 μm) were mounted on Superfrost Plus microscope slides and dried at 37°C for 72 hours before staining.
Immunohistochemical staining using primary antibodies to ER (Ventana SP1, 1 μg/mL) and PR (Ventana 1E2, 1 μg/mL) was performed using a Ventana Benchmark Ultra autostainer according to the manufacturer' instructions (Ventana Medical Systems Inc., Tucson, Arizona, USA), visualized with diaminobenzidine and counterstained with hematoxylin. Staining was evaluated by two observers blinded to treatment response (JS and CM). Consensus scores were reported. ER positivity was based on nuclear staining intensity, define as 0=negative, 1= weak, 1.5–2= moderate, and 3= strong (see Supplementary Fig. 1); and a histoscore were determined for each core. The histoscore was the product of the percentage of the tumor within the core that had positive nuclear staining and the staining intensity (range, 0–300). The mean histoscore and staining intensity were determined for duplicate cores when present.
5. Statistical consideration
The primary endpoint was the CBR at 3 months. The study had a stopping rule to allow early termination if there was lack of efficacy. A pre-planned interim analysis after 25 evaluable subjects who had been on study for at least 3 months and received at least 2 weeks of treatment was reviewed by the Independent Data and Safety Monitoring Committee (IDSMC). The IDSMC recommended continued recruitment until a total of 50 patients.
Based on a literature review on hormonal therapies, the expected CBR at 3 months in asymptomatic EOC patients with rising CA125 was 25%. A sample size of up to 50 patients was selected providing that at least 3 patients in the first 25 patients treated experienced a clinical benefit (i.e., minimum number of responses required to be consistent with the expected CBR).
Analysis of efficacy (overall response, or clinical benefit) was performed using the proportion of patients who responded, with a 95% confidence interval (CI) for the estimates. These rates were based on both all patients receiving anastrozole for at least 2 weeks (intention-to-treat population) as well as patients who had been on study for at least 4 weeks (evaluable for response). Toxicity analyses were evaluated by treatment received. All comparisons were 2-tailed with a nominal significance level of 0.05. Analyses of PFS and duration of clinical benefit used time-to-event methods, with Kaplan-Meier survival curves constructed for graphical display, and unadjusted log-rank tests used where appropriate. Death from any cause was considered an event. 95% CIs for proportions were constructed by using the modified Wilson method [20]. The conditional binomial exact test was used to test for association between binary variables and the Cochran-Armitage test (exact) used to test for trend among binomial proportions across levels of an ordinal explanatory variable. For QoL, to compare baseline and on-study scores, changes from baseline to each time-point were calculated and paired t-tests were performed. In addition, change scores between baseline and on-study averaged scores were also computed and assessed using one-sample t-tests. Linear regression was used to compare changes in QoL scores between patients achieving a 3-month clinical benefit and those who progressed, with adjustment for the baseline score.
RESULTS
1. Patient characteristics
Eligible patients (n=54) with asymptomatic GCIG defined CA125 progression were recruited from 17 trial centers across Australia and 1 from Belgium, from February 2012 to November 2014. The mean age was 65 years (range, 38–88). About 70% of patients had a treatment free interval (TFI) of at least 6 months at study entry. CA125 was nominated as the method of disease assessment for clinical benefit in 37 patients (69%) as no measurable disease was present at baseline. Thirty-one percent had small volume measurable disease (based on RECIST v1.1) at baseline and were deemed eligible for the study. Thirty-nine out of 54 patients had sufficient tissue for central pathology review, with the remainder based on pathology reports. The majority were high grade serous carcinoma. Relevant demographic details are listed in Table 1.
Table 1. Patient characteristics at baseline (n=54).
| Characteristic | Value | ||
|---|---|---|---|
| Age (yr) | 65 (38–88) | ||
| ECOG performance status | |||
| 0 | 35 (64.8) | ||
| 1 | 19 (35.2) | ||
| Tumor grade | |||
| Grade 1 (well differentiated) | 4 (7.4) | ||
| Grade 2 (moderately differentiated) | 5 (9.2) | ||
| Grade 3 (poorly or undifferentiated) | 40 (74.1) | ||
| Unknown | 4 (7.4) | ||
| Missing | 1 (1.9) | ||
| Prior chemotherapy | 54 (100.0) | ||
| Lines of prior chemotherapy | |||
| 1 | 50 (92.6) | ||
| ≥2 | 4 (7.4) | ||
| Method of response measurement | |||
| RECIST v1.1 | 17 (31.5) | ||
| CA125 by GCIG criteria | 37 (68.5) | ||
| Histology | |||
| High grade serous | 40 (74.1) | ||
| Low grade serous | 5 (9.3) | ||
| Serous carcinoma, unknown grade | 4 (7.4) | ||
| Endometrioid | 4 (7.4) | ||
| Clear cell carcinoma | 1 (1.9) | ||
| Patients with % of tumor stained ER-positive | 52* | ||
| <50% | 8 (15.4) | ||
| 50%–89% | 14 (26.9) | ||
| 90%–100% | 30 (57.7) | ||
| ER/PR nuclear staining | 30† | ||
| ER staining (intensity) | |||
| Negative | 1 (3.3) | ||
| Weak (1+) | 3 (10.0) | ||
| Moderate (1.5–2+) | 18 (60.0) | ||
| Strong (2.5–3+) | 8 (26.7) | ||
| ER histoscores | |||
| 0–100 | 9 (30.0) | ||
| 101–200 | 13 (43.3) | ||
| 201–300 | 8 (26.7) | ||
| PR staining (intensity) | |||
| Negative | 12 (40.0) | ||
| Weak (1+) | 12 (40.0) | ||
| Moderate (1.5–2+) | 4 (13.3) | ||
| Strong (2.5–3+) | 2 (6.7) | ||
| PR histoscores | |||
| 0–100 | 28 (93.3) | ||
| 101–200 | 0 | ||
| 201–300 | 2 (6.7) | ||
Values shown are number (%) or mean (range).
CA125, cancer antigen 125; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; GCIG, Gynecologic Cancer InterGroup; PR, progesterone receptor; RECIST, Response Evaluation Criteria in Solid Tumors.
*Fifty-two patients with tissue available for ER confirmation; †ER/PR intensity and histoscores, on tissue microarray, were available in 30 patients.
2. Antitumor activity
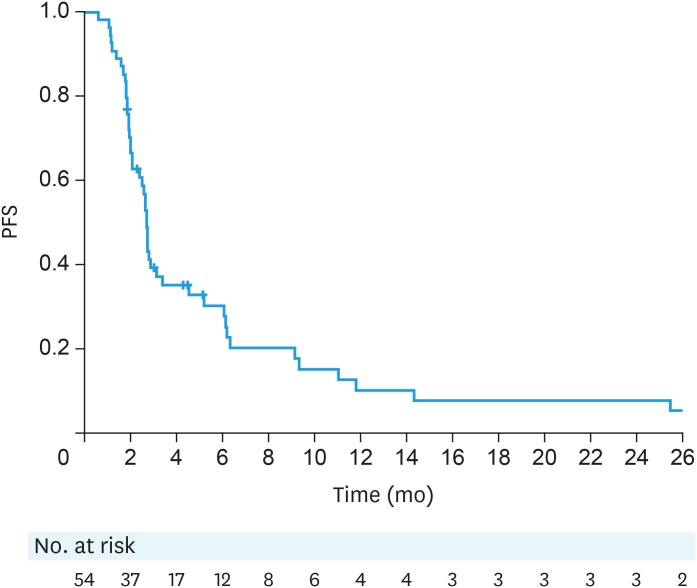
Fifty-two patients were evaluable for responses (1 patient stopped treatment at 8 weeks due to treatment-related adverse events [arthralgia] and 1 patient stopped at 9 weeks due to clinician preference). Eighteen patients (34.6%; 95% CI=23%–48%) had clinical benefit at 3 months (primary end point), including 11 (2 responses, 9 stable disease [SD]) based on CA125 and 7 (all SD) based on RECIST. The overall median PFS was 2.7 months (95% CI=2.1–3.1, Fig. 1).
Fig. 1. PFS (ITT population).
ITT, intention-to-treat; PFS, progression-free survival.
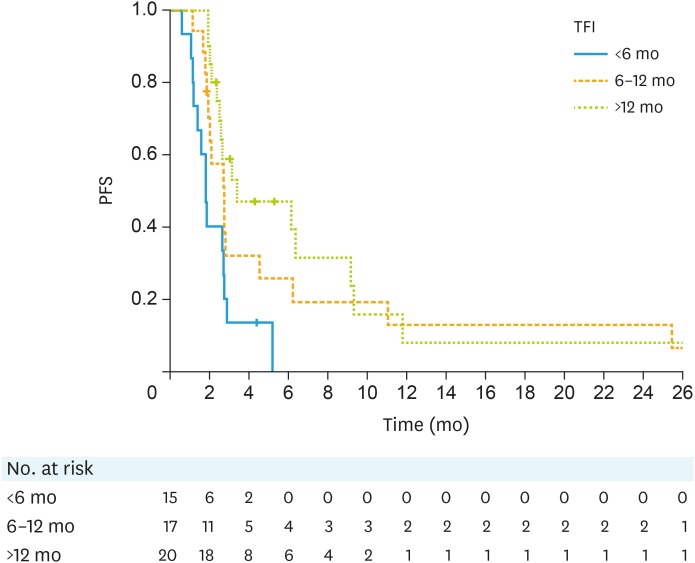
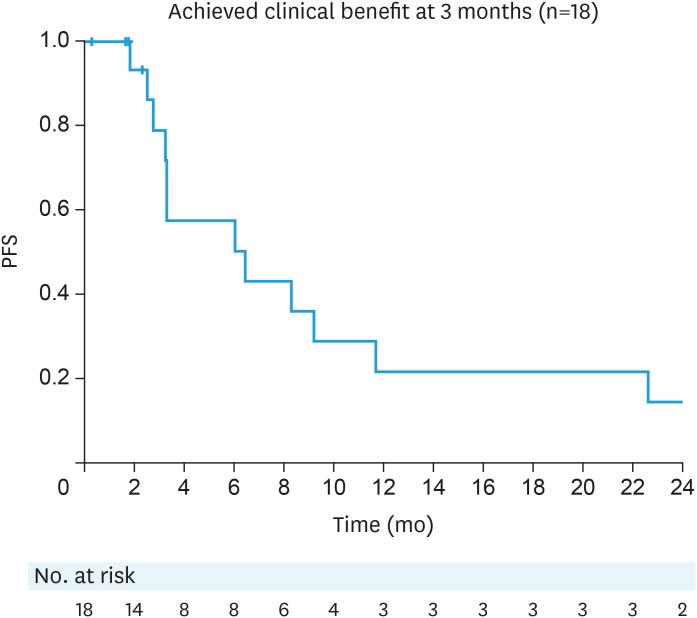
Patients who had a prior TFI of less than 6 months had a median PFS of 1.8 months (n=15; 95% CI=1.2–2.7). The median PFS in patients who had a TFI of 6 to 12 months and >12 months was 2.7 months (n=17; 95% CI=1.9–4.5) and 3.4 months respectively (n=20; 95% CI=2.4–9.2, Fig. 2). The median duration of clinical benefit was 6.5 months (95% CI=2.8–11.7, Fig. 3).
Fig. 2. PFS by TFI.
PFS, progression-free survival; TFI, treatment free interval.
Fig. 3. PFS in patients who achieved clinical benefit at 3 months. The median duration of clinical benefit was 6.5 months (95% CI=2.8–11.7). Thirteen patients have progressed (5 censored).
CI, confidence interval; CLB, clinical benefit; PFS, progression-free survival.
CBR was 46.7% (95% CI=24.8%–69.9%) (7/15 evaluable) in patients with measurable disease at baseline, and 29.7% (95% CI=17.5–45.8%) (11/37) in patients with non-measurable disease. The median PFS in patients who had measurable disease by RECIST was 3.4 months (95% CI=2.1–6.3) and 2.7 months (95% CI=1.9–2.9) in those with non-measurable disease.
3. ER and PR staining and clinical benefit
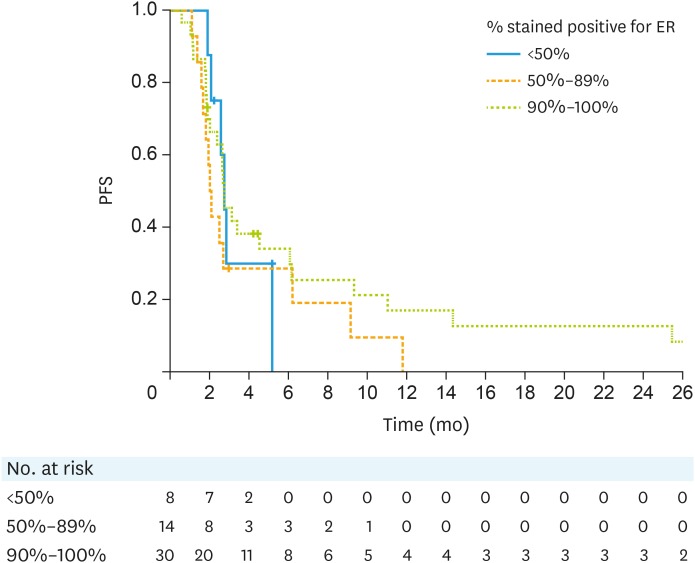
Overall, 52 patients had ER assessed and were all confirmed to be ER+. Fifty patients were evaluable for CBR at 3 months. There was no statistical significant difference in CBR and PFS by percentage of tumor stained ER-positivity. The median PFS in these groups is shown in Fig. 4.
Fig. 4. PFS by % staining positive for ER.
ER, estrogen receptor; PFS, progression-free survival.
Tumor blocks were available and suitable for inclusion in a tissue microarray (TMA), for 30 patients (Table 1). Twenty-eight patients were evaluable for CBR at 3 months. The 3-month CBR of patients with ER histoscores of 0–100 (n=8) was 25%, 101–200 (n=12) was 50% and 201–300 (n=8) was 25%. The median PFS of patients with histoscores of 0–100 was 2.1 months (n=9; 95% CI=1.6–5.2); 101–200 was 6.1 months (n=13; 95% CI=1.9–11.8) and 201–300 was 2.7 months (n=8; 95% CI=1.1–9.3; p=0.25). The 3-month CBR was 33% (1/3) in weak; 37.5% (6/16) in moderate and 25.0% (2/8) in strong ER staining intensity. The median PFS was 2.1 (n=3; 95% CI=2.0–6.2) months; 2.7 (n=18; 95% CI=1.9–11.0) months and 2.7 (n=8; 95% CI=1.1–9.3) months in patients with weak, moderate and strong ER staining.
The 3-month CBR was 41% in PR+ evaluable patients (7/17) and 27.3% in PR− patients (3/11). The median PFS was slightly longer in PR+ patients (2.7 [95% CI=2.1–6.2] months vs. 2.4 [95% CI=1.2–6.1] months). Of interest, one patient who had the high PR and ER scores was still on treatment and was progression-free at 49 months.
Twelve out of 54 patients (22%) received anastrozole for at least 6 months. Of these, 9 had tumors that were both ER+ and PR+. The characteristics of these patients are shown in Table 2. Most of these patients also had a longer TFI (>12 months) at enrolment, high-grade disease, moderate to strong ER intensity/histoscores and are PR+.
Table 2. Demographics of patients on anastrozole for >6 months (n=12).
| Characteristic | Value | |
|---|---|---|
| Mean age (yr) | 66 | |
| TFI | ||
| Missing | 2 (16.7) | |
| 6–12 months | 4 (33.3) | |
| >12 months | 6 (50.0) | |
| Distant metastases | ||
| Liver | 1 (8.3) | |
| Lung | 0 | |
| Peritoneal | 5 (41.7) | |
| Nodal | 6 (50.0) | |
| Lines of prior chemotherapy | ||
| 1 | 11 (91.7) | |
| 2 | 0 | |
| 3 | 1 (8.3) | |
| Immunohistochemistry | ||
| ER+/PR− | 3 (25.0) | |
| ER+/PR+ | 9 (75.0) | |
| ER−/PR+ | 0 | |
| Grade (at initial diagnosis) | ||
| Grade 1 (well differentiated) | 2 (16.7) | |
| Grade 2 (moderately differentiated) | 2 (16.7) | |
| Grade 3 (poorly or undifferentiated) | 8 (66.7) | |
| ER intensity* | ||
| Weak: 1 | 1 (14.3) | |
| Moderate: 1.5–2 | 4 (57.1) | |
| Strong: 2.5–3 | 2 (28.6) | |
| ER histoscore* | ||
| 0–100 | 1 (14.3) | |
| 101–200 | 4 (57.1) | |
| 201–300 | 2 (28.6) | |
| PR intensity* | ||
| Negative | 3 (42.9) | |
| Positive | 4 (57.1) | |
ER, estrogen receptor; PR, progesterone receptor; TFI, treatment free interval
*Only 7 of 12 patients who received at least 6 months of treatment had data for ER/PR.
4. Safety
Anastrozole was generally well tolerated. Most common adverse events were grade 1 which included fatigue, hot flushes and arthralgia. Grade 1, 2 and 3 arthralgia were reported in 18 (33.3%), 5 (9.3%) and 2 patients (3.7%) respectively. Other grade 3 adverse events that were thought to be disease related include vomiting (3 patients), nausea (2 patients) and anorexia (1 patient). Only 2 patients ceased treatment due to treatment-related adverse events (1 patient stopped at 8 weeks due to arthralgia, headaches and hot flushes and another stopped at 18 weeks due to arthralgia).
5. QoL
Compliance with completing QoL questionnaires was high: 96% completed QLQ-C30 questionnaires at baseline (51/ 53 patients), 87% at 1 month (45/ 52), 98% at 2 months (41/42) and 96% at 3 months (27/ 28); 46 completed QoL at both baseline and follow-up.
There were no clinically meaningful and statistically significant changes from baseline in the QLQ-C30 functioning scales at any time point or averaged over the total time on-study. Emotional and cognitive scores changed little from baseline, while patients' physical, role and social functioning scores deteriorated by averages of 2.6 points (95% CI=−6.6, 1.5), 3.1 (95% CI=−7.7, 1.6) and 1.6 points (95% CI=−5.4, 2.3) respectively. The global health scores decreased significantly by 3.7 points on average over the total time on-study (n=44; 95% CI=−7.3, −0.2; p=0.04); nausea and vomiting worsened significantly (mean change=5.4; 95% CI=1.2, 9.6; p=0.01), although these changes were clinically insignificant and were defined as small (subtle but clinically relevant) by Cocks et al. [21].
No significant changes from baseline were seen in FACT-ES total global health scores (range, 0–180) at any time point. The average change from baseline over the total on-study period was an increase of 1.6 points (n=45; 95% CI=−1.2, 4.3). For the physical well-being subscale (range, 0–28) patients scores decreased significantly by −0.9 points over the total on-study period (95% CI=−1.7, −0.1; p=0.03). Emotional well-being subscale (range, 0–24) scores improved significantly at 1 month by 1.2 points on average (n=44; 95% CI=0.2, 2.3; p=0.02); however, this was not maintained, a similar trend was displayed for the QLQ-C30 emotional functioning scale.
In patients who had clinical benefit at 3 months, QLQ-C30 total global health scores decreased by 1.4 points (n=17; 95% CI=−7.5, 4.8; p=0.64) on average over the time period from 1 to 3 months compared to a decrease of 6.0 (n=27; 95% CI=−10.6, −1.4; p=0.01) in patients who progressed (adjusted difference=2.6; 95% CI=−4.7, 9.9).
DISCUSSION
Anastrozole was associated with clinical benefit at 3 months in 34.6% of asymptomatic women with ROC and GCIG defined CA125 progression after first line chemotherapy. The median duration of clinical benefit was 6.5 months. Anastrozole was well tolerated with main side effects being grade 1 fatigue, hot flushes and arthralgia. Global measures of QoL remained stable while patients were on anastrozole.
Although chemotherapy is not usually administered to asymptomatic patients with ROC with CA125 progression and small volume recurrent disease, it is not uncommon for patients to be prescribed tamoxifen or an AI. The median lead time between CA125 progression and the development of symptoms/requiring chemotherapy in the Rustin study was 4.8 months [16]. The aim of hormonal therapy in asymptomatic patients with CA125 progression is to delay the time to recommence chemotherapy and maintain QoL in these patients who are destined to receive further chemotherapy.
The results of our study are in keeping with the study reported by Bowman et al. [6], which used letrozole in a similar population of patients. They reported a clinical benefit of 38%, including 5 out of 50 patients with >50% decrease in CA125 and 14 patients with stable disease by CA125 criteria (<25% increase or <50% decrease). The clinical benefit was 34.6% at 3 months in ER+/PR+ patients, and 25% in the subset with ER histoscores of 0–100 and 201–300 and 50% in the group with histoscore 101–200. However, contrary to the Smyth et al.'s study [2], we were unable to demonstrate any relationship between ER histoscore and response/clinical benefit.
There are a number of limitations in our study, including small sample size and ER/PR status for eligibility was based on immunohisto-staining at each institution. Central adjudication of ER/PR nuclear staining and histoscore was done retrospectively on available archival tissue blocks and we cannot confidently exclude a relationship between ER/PR histoscore and response to hormonal therapy.
It is currently hard to predict who will benefit from treatment and the challenge is to look beyond ER and PR to identify predictors of response and clinical benefit. The PFS was longer in patients with a TFI of at least 6 months compared to those with a TFI of <6 months, which reflects the fact that patients with platinum resistant disease have more aggressive cancers and a poorer prognosis.
Importantly, anastrozole was well tolerated and one of the observations in this study was the apparent difference in AI-induced musculoskeletal events in women with ROC compared to these adverse effects reported by women with breast cancer. Studies in women with early breast cancer using adjuvant AIs reported 40%–70% rates of treatment related arthralgia with 20%–30% of patients stopping treatment early due to adverse effects [22,23,24].
There is a good rationale for treating asymptomatic patients with CA125 progression with hormonal therapy to delay the need for further chemotherapy and maintain QoL. Although we found that a subset of patients do appear to benefit from anastrozole, the results of this study raise more questions rather than provide definitive answers. Why is it that only a subset of patients with ER+ EOC derive clinical benefit and that the duration of benefit is so much less than in women with advanced breast cancers? With emerging new therapeutics such as CDK 4/6 inhibitors (shown to double PFS in combination with AI) in advanced breast cancer [25] and inhibitors of PI3K/AKT/mTOR pathway (activated in approximately 70% of ovarian cancer) [26], would the addition of a CDK4/6 inhibitor or an mTOR (mammalian target of rapamycin) inhibitor to an AI be superior to an AI alone? What are the mechanisms of hormone resistance in EOC? Given the increased interest in and the potential importance of personalized targeted therapies we will continue to explore these questions in the subsequent PARAGON 2 trial, with a greater emphasis on translational research and the collection of tumor specimens where possible at recurrence and progression.
ACKNOWLEDGEMENTS
We thank the patients who participated in the trial and the clinical research nurses and data managers at all the study sites. The study was funded by Cancer Australia and was conducted by NHMRC Clinical Trials Centre, in collaboration with ANZGOG. Anastrozole was provided by AstraZeneca. We thank the Gynaecological Oncology Biobank at Westmead in Sydney, which is funded by Cancer Institute NSW (12/RIG/1-17 and 15/RIG/1-16) and is a member bank of the Australasian Biospecimens Network-Oncology, funded by NHMRC (ID310670, ID628903) for construction of tissue microarrays and the pathology laboratories for providing tissue blocks and slides.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: S.K., D.A., F.M.
- Data curation: K.P.S., O'C.R.L., S.J., S.K., D.A., M.C.
- Formal analysis: K.P.S., B.P., S.J., O'C.R.L., S.K., D.A., M.C., F.M.
- Funding acquisition: F.M.
- Investigation: K.P.S., S.J., S.K., D.A., M.C.
- Methodology: B.P., O'C.R.L., S.K., D.A., F.M.
- Project administration: K.P.S.
- Resources: B.P., O'C.R.L., S.K., D.A., F.M.
- Software: K.P.S.
- Supervision: F.M.
- Validation: K.P.S., F.M.
- Visualization: K.P.S.
- Writing - original draft: K.P.S.
- Writing - review & editing: K.P.S., B.P., O'C.R.L., G.P., B.T., S.J., A.Y., G.J., S.K., D.A., M.C., A.F., F.M.
SUPPLEMENTARY MATERIAL
ER and PR nuclear staining intensity. Expression of ER and PR on tumor samples represented on tissue microarrays. Sections (4 um) of formalin fixed paraffin embedded tumor samples were stained with ER and PR primary antibodies, visualized with diaminobenzidine (brown) and counterstained with hematoxylin (blue). Representative images illustrating nuclear staining intensity for ER (A) weak (1+), (B) moderate (1.5–2+), (C) and strong (3+) and PR (D) weak, (E) moderate, and (F) strong.
References
- 1.Williams CJ. Tamoxifen for relapse of ovarian cancer. Cochrane Database Syst Rev. 2001:CD001034. doi: 10.1002/14651858.CD001034. [DOI] [PubMed] [Google Scholar]
- 2.Smyth JF, Gourley C, Walker G, MacKean MJ, Stevenson A, Williams AR, et al. Antiestrogen therapy is active in selected ovarian cancer cases: the use of letrozole in estrogen receptor-positive patients. Clin Cancer Res. 2007;13:3617–3622. doi: 10.1158/1078-0432.CCR-06-2878. [DOI] [PubMed] [Google Scholar]
- 3.Heinzelmann-Schwarz V, Knipprath Mészaros A, Stadlmann S, Jacob F, Schoetzau A, Russell K, et al. Letrozole may be a valuable maintenance treatment in high-grade serous ovarian cancer patients. Gynecol Oncol. 2018;148:79–85. doi: 10.1016/j.ygyno.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 4.Hatch KD, Beecham JB, Blessing JA, Creasman WT. Responsiveness of patients with advanced ovarian carcinoma to tamoxifen. A Gynecologic Oncology Group study of second-line therapy in 105 patients. Cancer. 1991;68:269–271. doi: 10.1002/1097-0142(19910715)68:2<269::aid-cncr2820680209>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Mouridsen H, Gershanovich M, Sun Y, Pérez-Carrión R, Boni C, Monnier A, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–2606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 6.Bowman A, Gabra H, Langdon SP, Lessells A, Stewart M, Young A, et al. CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: identification of an endocrine-sensitive subgroup. Clin Cancer Res. 2002;8:2233–2239. [PubMed] [Google Scholar]
- 7.Papadimitriou CA, Markaki S, Siapkaras J, Vlachos G, Efstathiou E, Grimani I, et al. Hormonal therapy with letrozole for relapsed epithelial ovarian cancer. Long-term results of a phase II study. Oncology. 2004;66:112–117. doi: 10.1159/000077436. [DOI] [PubMed] [Google Scholar]
- 8.Høgdall EV, Christensen L, Høgdall CK, Blaakaer J, Gayther S, Jacobs IJ, et al. Prognostic value of estrogen receptor and progesterone receptor tumor expression in Danish ovarian cancer patients: from the ‘MALOVA’ ovarian cancer study. Oncol Rep. 2007;18:1051–1059. [PubMed] [Google Scholar]
- 9.Karagol H, Saip P, Uygun K, Caloglu M, Eralp Y, Tas F, et al. The efficacy of tamoxifen in patients with advanced epithelial ovarian cancer. Med Oncol. 2007;24:39–43. doi: 10.1007/BF02685901. [DOI] [PubMed] [Google Scholar]
- 10.Langdon SP, Smyth JF. Hormone therapy for epithelial ovarian cancer. Curr Opin Oncol. 2008;20:548–553. doi: 10.1097/CCO.0b013e3283063912. [DOI] [PubMed] [Google Scholar]
- 11.Galtier-Dereure F, Capony F, Maudelonde T, Rochefort H. Estradiol stimulates cell growth and secretion of procathepsin D and a 120-kilodalton protein in the human ovarian cancer cell line BG-1. J Clin Endocrinol Metab. 1992;75:1497–1502. doi: 10.1210/jcem.75.6.1464654. [DOI] [PubMed] [Google Scholar]
- 12.Clinton GM, Hua W. Estrogen action in human ovarian cancer. Crit Rev Oncol Hematol. 1997;25:1–9. doi: 10.1016/s1040-8428(96)00216-8. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki H, Hayakawa J, Terai Y, Kanemura M, Tanabe-Kimura A, Kamegai H, et al. Difference between genomic actions of estrogen versus raloxifene in human ovarian cancer cell lines. Oncogene. 2008;27:2737–2745. doi: 10.1038/sj.onc.1210926. [DOI] [PubMed] [Google Scholar]
- 14.Markman M, Iseminger KA, Hatch KD, Creasman WT, Barnes W, Dubeshter B. Tamoxifen in platinum-refractory ovarian cancer: a Gynecologic Oncology Group Ancillary Report. Gynecol Oncol. 1996;62:4–6. doi: 10.1006/gyno.1996.0181. [DOI] [PubMed] [Google Scholar]
- 15.Hurteau JA, Brady MF, Darcy KM, McGuire WP, Edmonds P, Pearl ML, et al. Randomized phase III trial of tamoxifen versus thalidomide in women with biochemical-recurrent-only epithelial ovarian, fallopian tube or primary peritoneal carcinoma after a complete response to first-line platinum/taxane chemotherapy with an evaluation of serum vascular endothelial growth factor (VEGF): a Gynecologic Oncology Group Study. Gynecol Oncol. 2010;119:444–450. doi: 10.1016/j.ygyno.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Rustin GJ, van der Burg ME, Griffin CL, Guthrie D, Lamont A, Jayson GC, et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010;376:1155–1163. doi: 10.1016/S0140-6736(10)61268-8. [DOI] [PubMed] [Google Scholar]
- 17.Markman M, Webster K, Zanotti K, Rohl J, Belinson J. Use of tamoxifen in asymptomatic patients with recurrent small-volume ovarian cancer. Gynecol Oncol. 2004;93:390–393. doi: 10.1016/j.ygyno.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 18.Rustin GJ, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) Int J Gynecol Cancer. 2011;21:419–423. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. [Google Scholar]
- 21.Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011;29:89–96. doi: 10.1200/JCO.2010.28.0107. [DOI] [PubMed] [Google Scholar]
- 22.Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 23.Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375:209–219. doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Presant CA, Bosserman L, Young T, Vakil M, Horns R, Upadhyaya G, et al. Aromatase inhibitor-associated arthralgia and/ or bone pain: frequency and characterization in non-clinical trial patients. Clin Breast Cancer. 2007;7:775–778. doi: 10.3816/CBC.2007.n.038. [DOI] [PubMed] [Google Scholar]
- 25.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Zeng J, Shen K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch Gynecol Obstet. 2014;290:1067–1078. doi: 10.1007/s00404-014-3377-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ER and PR nuclear staining intensity. Expression of ER and PR on tumor samples represented on tissue microarrays. Sections (4 um) of formalin fixed paraffin embedded tumor samples were stained with ER and PR primary antibodies, visualized with diaminobenzidine (brown) and counterstained with hematoxylin (blue). Representative images illustrating nuclear staining intensity for ER (A) weak (1+), (B) moderate (1.5–2+), (C) and strong (3+) and PR (D) weak, (E) moderate, and (F) strong.