Summary
Endothelial cells (ECs), which line blood and lymphatic vessels, are generally described to come from the lateral plate mesoderm despite experimental evidence for a broader source of origin, including the paraxial mesoderm (PXM). Current dogma suggests that following specification from mesoderm, local environmental cues establish the distinct molecular and functional characteristics of ECs in different vascular beds. Here we present evidence to challenge this view, showing that lymphatic EC fate is imprinted during transition through the PXM lineage. We show that PXM-derived cells form the lymphatic endothelium of multiple organs and tissues, with a more restricted contribution to blood vessel endothelium. By deleting Prox1 specifically in PXM-derived cells, we show that this lineage is indispensable for lymphatic vessel development. Collectively, our data establish lineage history as a critical determinant of EC specialization, a finding with broad implications for our understanding of vascular development and heterogeneity.
Keywords: cell lineage, lineage tracing, mesoderm, paraxial mesoderm, endothelial differentiation, endothelial heterogeneity, lymphatic, lymphangiogenesis, vasculogenesis
Graphical Abstract
Highlights
-
•
Lineage history is a key determinant of endothelial cell specialization
-
•
Endothelial cells arising from paraxial mesoderm preferentially form lymphatic vessels
-
•
Paraxial-mesoderm-derived endothelial cells are essential for lymphatic development
Following specification from mesoderm, the prevailing view is that local environmental cues control the specialization of endothelial cells in different vessel beds. Stone and Stainier show that lymphatic endothelial cell fate is imprinted much earlier as cells transition through the paraxial mesoderm.
Introduction
During the iterative process of differentiation, intrinsic and extrinsic cues establish heterogeneity at the cell, organ, and system level. A striking paradigm for this diversification can be observed in the endothelium, which differentiates from mesoderm to form arterial, venous, lymphatic, and organ-specific vessel networks (Herbert and Stainier, 2011). Although multiple cellular origins for endothelial cells (ECs) have been described (Plein et al., 2018, Reischauer et al., 2016, Klotz et al., 2015, Stanczuk et al., 2015, Nguyen et al., 2014, Wilting et al., 1995), the impact of cell lineage on EC diversification is poorly understood. The prevailing view is that tissue-derived signals, coupled to vessel subtype-specific transcriptional networks, establish molecular and functional heterogeneity as ECs invade different organs and tissues (Potente and Mäkinen, 2017).
The initial steps of vascular development take place during gastrulation as mesodermal progenitors commit to an endothelial fate. In zebrafish (Reischauer et al., 2016, Mosimann et al., 2015, Nguyen et al., 2014), chick (Wilting et al., 2000, Pardanaud et al., 1996, Couly et al., 1995, Wilting et al., 1995), and mouse (Mayeuf-Louchart et al., 2014, Hutcheson et al., 2009, Wasteson et al., 2008), ECs have been shown to derive from both lateral plate mesoderm (LPM) and paraxial mesoderm (PXM, also known as presomitic mesoderm) (Figure 1A). Additionally, erythro-myeloid progenitor cells have been reported as a source of both yolk sac and intraembryonic ECs in mouse (Plein et al., 2018). Whether ECs derived from these various lineages are functionally distinct, or preferentially contribute to different vascular beds is poorly understood. Intriguingly, transplantation experiments in avian embryos showed that while PXM-derived ECs contribute to the cardinal vein, wing bud, and perineural vascular plexus, they are unable to invade the visceral organs and are excluded from the ventral wall of the dorsal aorta (Pardanaud et al., 1996), suggesting that ECs from divergent sources may possess unique functional and molecular properties.
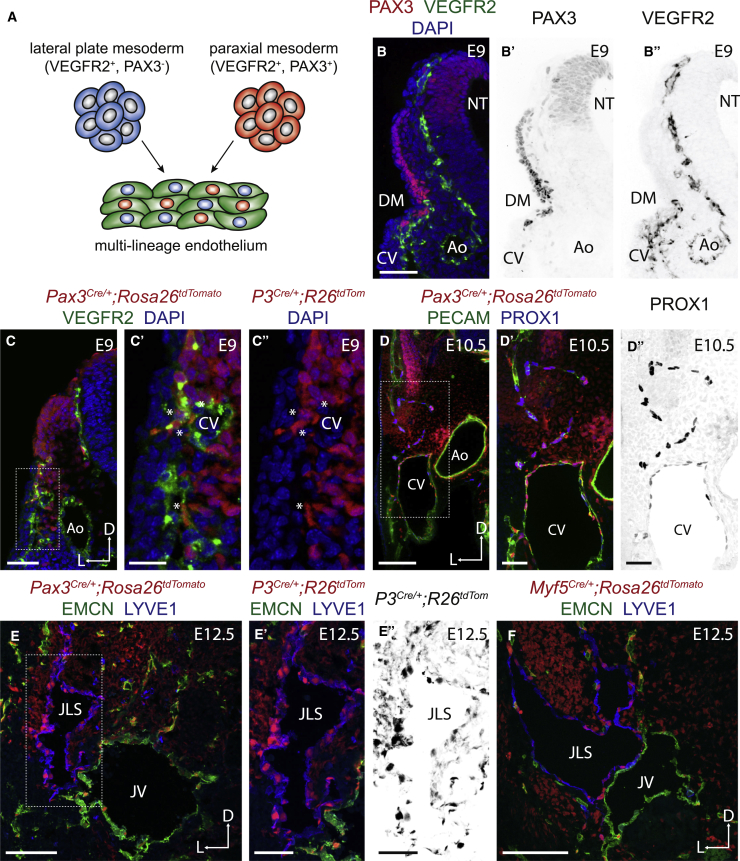
Figure 1.
Paraxial Mesodermal Cells Contribute to the Cardinal Vein and Developing Lymphatics
(A) Schematic representation of the contribution of lateral plate and paraxial mesoderm-derived cells to the developing endothelium.
(B–B″) Immunofluorescence for PAX3 and VEGFR2 on transverse cryosections.
(C–C″) Immunofluorescence for tdTomato and VEGFR2 on transverse cryosections from a Pax3Cre/+;Rosa26tdtomato embryo.
(D–D″) Immunofluorescence for tdTomato, PECAM, and PROX1 on transverse vibratome sections from a Pax3Cre/+;Rosa26tdtomato embryo.
(E–E″) Immunofluorescence for tdTomato, EMCN, and LYVE1 on transverse cryosections from a Pax3Cre/+;Rosa26tdtomato embryo.
(F) Immunofluorescence for tdTomato, EMCN, and LYVE1 on transverse cryosections from a Myf5Cre/+;Rosa26tdtomato embryo.
(CV, cardinal vein; Ao, aorta; DM, dermomyotome; NT, neural tube; JLS, jugular lymph sac; JV, jugular vein.)
Scale bars: 50 μm (B, C, E′, and E″); 20 μm (C′ and C″); 100 μm (D, E, and F).
The cellular origin of lymphatic vessels has been the subject of debate for over a century (Ulvmar and Mäkinen, 2016, Srinivasan et al., 2007, Huntington and McClure, 1910, Sabin, 1902). Live imaging in zebrafish showed that lymphatic ECs (LECs) arise from venous endothelium (Yaniv et al., 2006), and lineage tracing analyses in mouse have shown that the majority of LECs are derived from transdifferentiation of venous (Srinivasan et al., 2007), intersegmental (Yang et al., 2012), and capillary (Pichol-Thievend et al., 2018) blood ECs. Additionally, contribution of hemogenic endothelium-derived cells to lymphatics in the mesentery (Stanczuk et al., 2015) and heart (Klotz et al., 2015) has been reported in mouse. Furthermore, studies in Xenopus (Ny et al., 2005) and avian models (Wilting et al., 2006, Schneider et al., 1999) have suggested alternative nonendothelial sources of LECs, including the PXM.
Following specification from the anterior primitive streak, the PXM extends bilaterally along the anterior-posterior axis adjacent to the neural tube (Hubaud and Pourquié, 2014). Segmentation of the PXM creates the somites, transient structures that contain progenitors for a number of tissues including bone, dorsal dermis, skeletal muscle, and the endothelium of the limb (Buckingham and Rigby, 2014). In chick, clonal analyses of individual somitic cells identified bipotent precursors for the skeletal muscle and endothelium of the limb (Kardon et al., 2002), while cells coexpressing PAX3 (a marker of myogenic precursors) and VEGFR2 (vascular endothelial growth factor receptor 2) have been observed in the somite of the mouse (Mayeuf-Louchart et al., 2014, Kardon et al., 2002). Genetic lineage tracing using Cre recombinase alleles driven from the endogenous transcription start site of the Pax3 and Myf5 (myogenic factor 5) genes demonstrated a common origin for skeletal muscle and a subset of the endothelium in the mouse limb (Hutcheson et al., 2009). Furthermore, the descendants of VEGFR2-expressing cells contribute to both endothelium and skeletal muscle in mouse (Motoike et al., 2003). Collectively, these data reveal the existence of a common bipotent progenitor for skeletal muscle and endothelium.
Here, we systematically analyzed the contribution of PXM-derived cells (PXMDCs) to the endothelium of different vascular networks to determine the impact of lineage history on the terminal fate of ECs. Using Cre-loxP-based lineage tracing and high-resolution imaging, these analyses have revealed that PXMDCs are preferentially localized to the dorsal aspect of the cardinal vein before the expression of the earliest known markers of lymphatic differentiation. PXM-derived ECs selectively transdifferentiate from the cardinal vein to form LEC progenitors and subsequently give rise to the lymphatic endothelium of multiple organs and tissues, including the cardiopulmonary system, skin and liver, as well as capillary-derived LEC clusters in the embryonic skin. In addition to Pax3-lineage somitic muscle progenitors, we also identified a contribution of Myf5-lineage cells to lymphatics in the meninges and postnatal skin of the ear, and revealed the anterior second heart field (the cellular origin of a subset of head and neck muscles) as the source of a limited number of anterior jugular lymph sac, cardiac, and dermal LECs. Furthermore, by deleting Prox1 specifically in the PXM, we find this to be the only lineage competent for LEC differentiation. Collectively, our analyses identify the earliest step in the formation of the lymphatic vasculature and reveal a common progenitor for skeletal muscle and lymphatic endothelium.
Results
Paraxial Mesodermal Cells Contribute to the Cardinal Vein and Developing Lymphatics
Genetic lineage tracing has demonstrated the presence of a multipotent progenitor population in the mouse dermomyotome (a subset of the somitic PXM) that is marked by the expression of PAX3 and MYF5 (Figure S1A), and gives rise to skeletal muscle and endothelium (Buckingham and Rigby, 2014). To examine their contribution to different vascular beds, we lineage traced PXMDCs using established Cre driver lines (Pax3Cre [Engleka et al., 2005], Pax7Cre [Keller et al., 2004], Myf5Cre [Tallquist et al., 2000]) and a ubiquitously expressed lineage reporter (Rosa26tdTomato [Madisen et al., 2010]) (Figures S1B and S1C). We found that PXMDCs contribute to the endothelium of the embryonic forelimb (Figures S1D and S1D′) and gastrocnemius muscle of the adult hindlimb (Figures S1E and S1E′) confirming previous observations (Mayeuf-Louchart et al., 2014, Hutcheson et al., 2009). Analysis of transverse sections from Pax3Cre;Rosa26tdTomato embryos at E9 revealed contribution of PXMDCs to the endothelium of the perineural vascular plexus, intersegmental vessels, and the dorsolateral wall of the developing cardinal vein (Figures 1C–1C″, S1F, and S1F′). LEC progenitors are known to differentiate from the dorsolateral wall of the cardinal vein under the transcriptional control of SOX18, COUPTFII, and PROX1 (François et al., 2008, Srinivasan et al., 2007, You et al., 2005, Wigle and Oliver, 1999), and previous studies have reported a contribution of somitic cells to the lymphatic endothelium in avian embryos (Wilting et al., 2006, He et al., 2003, Schneider et al., 1999). Therefore, we investigated whether PXMDCs contribute to the lymphatic endothelium using immunofluorescence for established LEC markers. Analysis of transverse sections from Pax3Cre;Rosa26tdTomato embryos at E10.5 demonstrated that PROX1-positive LEC progenitors located within and migrating from the cardinal vein were derived from the PXM (Figures 1D–1D″). Subsequently, we found these cells migrating from the cardinal vein to form the jugular lymph sacs (JLS) (Figures 1E–1E″). To determine the spatiotemporal pattern of differentiation from the dermomyotome, we lineage traced PXMDCs using Pax7Cre (restricted to the central dermomyotome; Figure S1A) and Myf5Cre (later onset of expression during myogenic lineage commitment; Figure S1B) lines. Consistent with a previous report (Hutcheson et al., 2009), we observed contribution of the Myf5 (Figure 1F), but not the Pax7 lineage (Figures S1G and S1H), to the endothelium. Myf5 lineage cells also contributed to the embryonic lymph sacs, albeit to a lesser extent than Pax3 lineage cells (Figure 1F), indicating that commitment of PXMDC to the lymphatic endothelium is initiated before the onset of Myf5 expression in the dermomyotome. Collectively, these data indicate that LEC fate may become hard wired as cells transition through the PXM lineage.
Cardiopulmonary, Subcutaneous, and Dermal Lymphatics Are Derived from the Paraxial Mesoderm
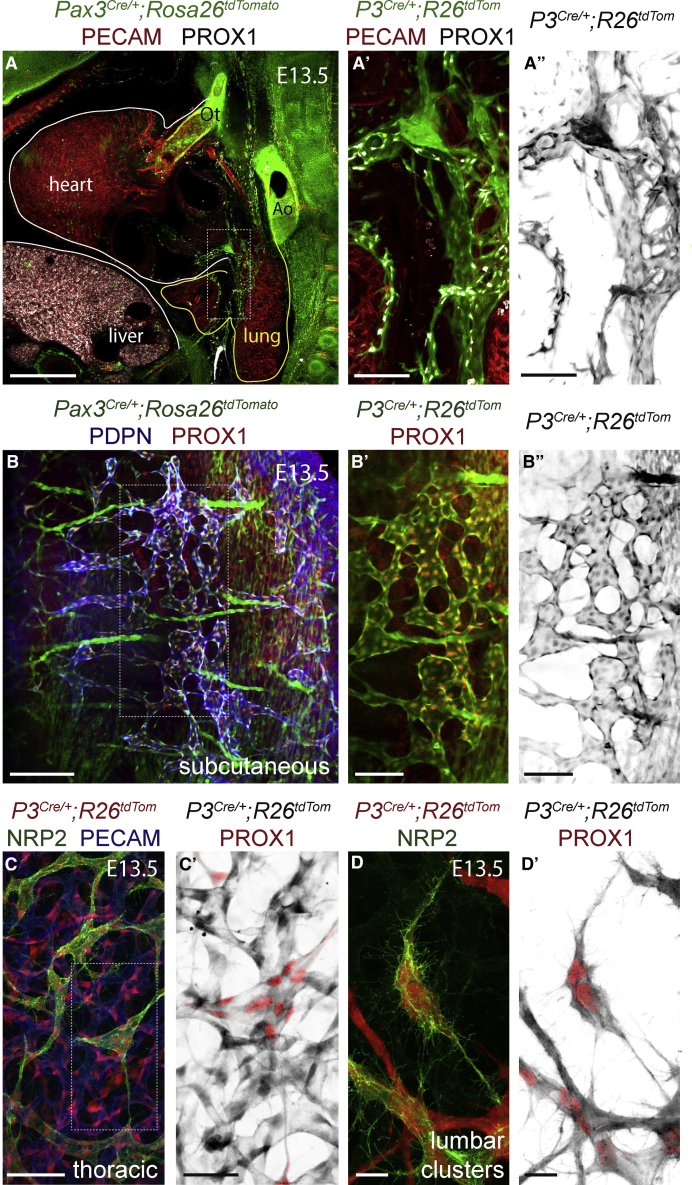
To determine their contribution to the endothelium of different lymphatic beds, we lineage traced PXMDCs and analyzed whole-mount tissues and sections at various embryonic stages. Sagittal vibratome sections of E13.5 Pax3Cre;Rosa26tdTomato embryos immunostained for PROX1 and PECAM revealed labeling of LECs migrating into the cardiopulmonary system with limited or no contribution to blood ECs (BECs) (Figures 2A–2A″). PXMDCs initially migrate onto the ventral side of the embryonic lung (Figure S2A). Imaging of fetal and adult hearts revealed that most cardiac LECs were labeled in Pax3Cre;Rosa26tdTomato animals (Figure S2B), reflecting the migration of PXMDCs from the venous endothelium, which is the source of most cardiac LECs (Klotz et al., 2015). The lymphatic vasculature of the embryonic skin has been shown to form through lymphangiogenic sprouting from the JLS in the cervicothoracic region and differentiation from the dermal capillary plexus in the lumbar dermis and the cervicothoracic midline (Pichol-Thievend et al., 2018). Sagittal vibratome sections of E13.5 Pax3Cre;Rosa26tdTomato (Figures 2B–2B″) and Myf5Cre;Rosa26tdTomato (Figures S2C–S2C‴) embryos immunostained for podoplanin (PDPN) and PROX1 revealed that subcutaneous LECs spanning the lateral lumbar region are PXM-derived. Analysis of E13.5 whole-mount skin immunostained for PECAM and NRP2 showed that the superficial thoracic dermal LECs (Figures 2C, 2C′, S3D, and S3F), which migrate from the JLS, and the clustered lumbar dermal LECs (Figures 2D, 2D′, S3E, and S3G), which arise from the dermal capillary plexus, are initially derived from the PXM. These data indicate that dermal LECs differentiate from venous and dermal capillary progenitor populations that share a common PXM origin. At later embryonic stages, PXMDCs comprise the lymphatic endothelium of the thoracic, lumbar, and sacral dermis (Figures S3H–S3K‴). However, analysis of whole-mount skin immunostained for PECAM, NRP2, and PROX1 revealed that the lymphatic endothelium of the cervical skin is a mixture of cells from Pax3+ve and Pax3-ve lineages (Figures S3A–S3B‴).
Figure 2.
Paraxial Mesodermal Cells Give Rise to the Cardiopulmonary, Subcutaneous, and Dermal Lymphatics
(A–A″) Immunofluorescence for tdTomato, PECAM, and PROX1 on a sagittal vibratome section at the level of the cardiac outflow tract from a Pax3Cre/+;Rosa26tdtomato embryo.
(B–B″) Immunofluorescence for tdTomato, PECAM, and PROX1 on a sagittal vibratome section at the level of the subcutaneous lymphatic network from a Pax3Cre/+;Rosa26tdtomato embryo.
(C–D′) Immunofluorescence for tdTomato, NRP2, and PECAM on whole-mount skin from Pax3Cre/+;Rosa26tdtomato embryos at the indicated positions and stages.
(Ao, aorta; Ot, outflow tract.)
Scale bars: 500 μm (A); 200 μm (B); 100 μm (A′ and A″); 50 μm (C); 20 μm (C′–D′).
Distinct Muscle Progenitor Populations Give Rise to Lymphatics
PAX3 labels myogenic precursors in the somitic PXM, but independent gene regulatory networks control myogenesis in the head and neck (Braun and Gautel, 2011); the contribution of Pax3 lineage cells to the lymphatic endothelium may be spatially restricted in a similar manner. While the expression of PAX3 is limited to the somitic PXM, myogenic regulatory factors such as MYF5 determine muscle fate throughout the body (Braun and Gautel, 2011). Analysis of the head lymphatics in E13.5 Myf5Cre;Rosa26tdTomato embryos revealed a contribution of Myf5 lineage cells to the lymphatic endothelium of the lower jaw (Figures S3C–S3C‴), a population of LECs that is not labeled in Pax3Cre;Rosa26tdTomato embryos. To understand whether other populations of muscle progenitors may also serve as LEC progenitors, we next analyzed the Mef2c-AHFCre line, which labels derivatives of the anterior second heart field (Verzi et al., 2005). A subset of the head and neck muscles have been shown to derive from multipotent progenitor cells that also give rise to second heart field-derived regions of the heart (Diogo et al., 2015). These muscles are labeled in Mef2c-AHFCre, but not in Pax3Cre mice (Lescroart et al., 2015). Sagittal vibratome sections of E13.5 Mef2c-AHFCre;Rosa26tdTomato embryos stained for PROX1 and PDPN revealed labeling of a small number of LECs in the anterior JLS (Figures S3D and S3D′). Furthermore, imaging of embryonic hearts immunostained for CDH5 and PROX1 showed that a minor proportion of ventral cardiac LECs are also labeled in Mef2c-AHFCre;Rosa26tdTomato animals (Figures S3E and S3E′). Moreover, analysis of whole-mount skin revealed a contribution of the Mef2c-AHF lineage to the cervicothoracic dermal capillary plexus (Figures S3F and S3F′) and lymphatic endothelium (Figures S3G and S3G′). Collectively, these data indicate that LECs may arise from multiple anatomically distinct muscle progenitor populations.
Paraxial Mesoderm-Derived Cells Form Organ-Specific Lymphatic Networks
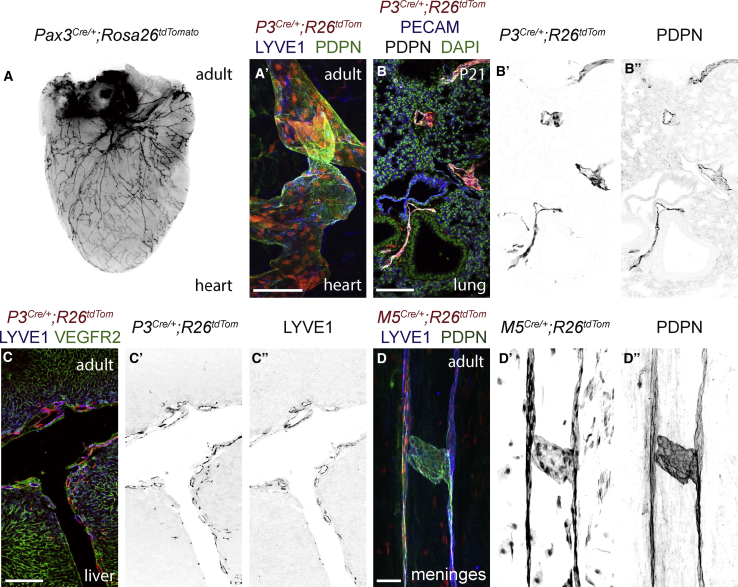
To determine their contribution to the postnatal and adult lymphatic endothelium, we lineage traced PXMDCs and analyzed whole-mount tissues and sections. Imaging of adult hearts from Pax3Cre;Rosa26tdTomato animals revealed labeling of a network of vessels at the surface of the heart (Figure 3A). Immunostaining for LYVE1 and PDPN showed that most of cardiac LECs are PXM-derived (Figure 3A′) with no contribution of PXMDCs to the coronary endothelium. Immunostaining of lung sections from Pax3Cre;Rosa26tdTomato animals showed that PXMDCs give rise to lymphatic vessels that sit proximal to the airways and major vessels (Figures 3B–3B″), with limited contribution to blood vessel endothelium, while analysis of liver tissue from Pax3Cre;Rosa26tdTomato animals showed that LECs in this tissue are PXM-derived (Figures 3C–3C″), and also revealed a contribution of PXMDCs to the hepatic blood vessel endothelium (Figure S3H). In contrast to all other visceral organs examined, we did not observe a contribution of PXMDCs to the mesenteric or intestinal lymphatics (Figures S3I and S3I′). Immunostaining of whole-mount meninges (Figures 3D–3D″) and ear skin (Figures S3J–S3K′) revealed that the LECs in these lymphatic beds are derived from a Myf5+ve muscle progenitor population with no contribution from the Pax3 lineage.
Figure 3.
Paraxial Mesoderm-Derived LECs Form the Lymphatic Vasculature of Postnatal and Adult Tissues
(A) Representative whole-mount stereoscopic imaging of the adult heart from a Pax3Cre/+;Rosa26tdtomato animal.
(A′) Whole-mount immunofluorescence for tdTomato, LYVE1, and PDPN on the adult ventricle from a Pax3Cre/+;Rosa26tdtomato animal.
(B–B″) Immunofluorescence for tdTomato, PECAM, and PDPN on cryosectioned lung tissue from a postnatal day 21 Pax3Cre/+;Rosa26tdtomato mouse.
(C–C″) Immunofluorescence for tdTomato, LYVE1, and VEGFR2 on cryosectioned liver tissue from the caudal lobe of a Pax3Cre/+;Rosa26tdtomato animal.
(D–D″) Whole-mount immunofluorescence for tdTomato, LYVE1, and PDPN on the adult meninges from a Myf5Cre/+;Rosa26tdtomato animal.
Scale bars: 100 μm (A′ and B–B″); 250 μm (C–C″); 50 μm (D–D″).
Transition through the Paraxial Mesodermal Lineage Is Essential for Lymphatic Endothelial Differentiation
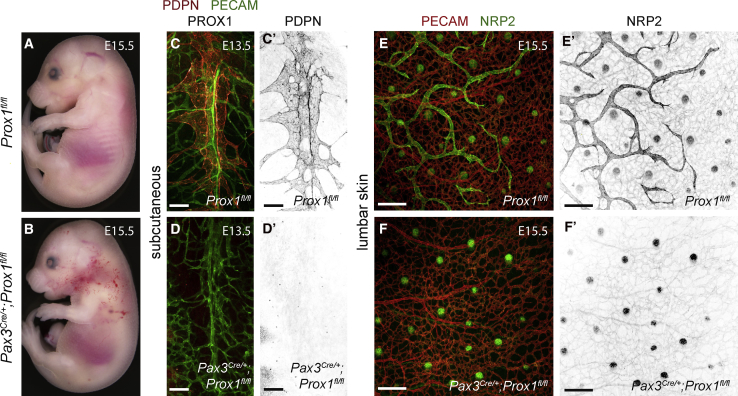
To determine the requirement for PXMDCs during the formation of the lymphatic vasculature (i.e., do cells derived from other mesodermal subtypes have the capacity to differentiate into LECs when this process is impaired in PXMDCs), we conditionally deleted the master regulator of LEC fate Prox1 (Wigle and Oliver, 1999) in the PXM lineage. For these analyses, we crossed a conditional Prox1fl allele (Martinez-Corral et al., 2015) with Pax3Cre and examined lymphatic development. Gross morphological assessment revealed that homozygous loss of Prox1 in the PXM lineage leads to subdermal edema and blood-filled lymphatic vessels in the cervical and thoracic skin at E15.5 (Figures 4A and 4B). Detailed morphological analyses of sagittal vibratome sections immunostained for PDPN, PECAM, and PROX1 showed a lack of subcutaneous LECs in the lateral lumbar region of Pax3Cre;Prox1fl/fl embryos at E13.5 (Figures 4C–4D′). Furthermore, analysis of whole-mount skin immunostained for PECAM and NRP2 revealed a complete lack of lumbar dermal lymphatics at E15.5 (Figures 4E–4F′). Conditional knock out of Prox1 in the Tie-2 lineage was previously shown to result in incomplete recombination and the presence of a dysmorphic cardiac lymphatic network (Klotz et al., 2015). Analysis of sagittal vibratome sections at the level of the cardiac outflow tract showed the presence of PXM-derived LECs that expressed low levels of PROX1 in the anterior region of the lung in Pax3Cre;Prox1fl/fl;Rosa26tdTomato embryos at E13.5 (Figures S4A–S4B′). Furthermore, analysis of whole-mount skin revealed the presence of PROX1-expressing LECs in the thoracic and cervical dermis in Pax3Cre;Prox1fl/fl;Rosa26tdTomato embryos at E15.5 (Figures S4C–S4H′), indicating that recombination of the Prox1fl allele is incomplete in certain lymphatic beds. Collectively, these data show an absence of LEC differentiation where complete recombination of Prox1 is achieved in the PXM lineage, indicating that the PXM may be the only mesodermal subtype competent for LEC differentiation.
Figure 4.
Transition through the Paraxial Mesodermal Lineage Is Essential for LEC Differentiation
Whole-mount brightfield images of Prox1fl/fl (A) and Pax3Cre/+;Prox1fl/fl embryos (B) at E15.5. Immunofluorescence for PDPN, PECAM, and PROX1 on sagittal vibratome sections at the level of the subcutaneous lymphatic network from Prox1fl/fl (C) and Pax3Cre/+;Prox1fl/fl embryos (D) at E13.5. Immunofluorescence for PECAM and NRP2 on whole-mount lumbar skin from Prox1fl/fl (E and E′) and Pax3Cre/+;Prox1fl/fl embryos (F and F′) at E15.5.
Scale bars: 100 μm (C–D′); 200 μm (E–F′).
Discussion
Our study shows that contrary to current dogma, terminal EC fate is imprinted during mesodermal differentiation and before initiation of the established endothelial genetic program. Akin to the contribution of the first and second heart fields to distinct regions of the heart (Kelly et al., 2014), and the allocation of preconfigured cells to anterior versus posterior fates in the nervous system (Metzis et al., 2018), this work reveals that ECs can be functionally subdivided based on their embryonic origin. In avian embryos, LECs have been shown to derive from both the LPM and PXM (Wilting et al., 2006, Wilting et al., 2000). While accurate lineage tracing of the cranial PXM is currently not possible because of the lack of a distinct morphological and molecular boundary with the cranial LPM (Sambasivan et al., 2011), lineage tracing with Myf5Cre indicates that distinct muscle progenitor populations give rise to LECs in the head. Furthermore, lineage tracing with Mef2c-AHFCre identified the second heart field as an additional local progenitor source for LECs in the neck and heart. Collectively, our data indicate that multiple classically defined muscle progenitor populations serve as the cellular source of LECs in distinct anatomical locations.
Our analyses show that LECs in the mouse cardiopulmonary, liver, meningeal, subcutaneous, and dermal lymphatic vessels derive from the PXM lineage, which also makes a more limited and tissue-restricted contribution to the blood vasculature; for example, PXM-derived blood ECs were rarely observed in the heart or lung, but fairly frequently in the liver. A recent study in mouse reported that yolk sac and embryonic erythro-myeloid progenitors are the cellular source of up to 60% of liver ECs (Plein et al., 2018); however, whether erythro-myeloid progenitor-derived ECs are functionally distinct from neighboring ECs was not established. Intriguingly, in an aortic endothelial injury model, a subpopulation of highly proliferative ECs was shown to drive regeneration of the endothelial layer (McDonald et al., 2018), highlighting the functional heterogeneity of neighboring ECs within individual vessels. Future studies comparing PXM-derived ECs with neighboring ECs may reveal important aspects of endothelial heterogeneity that may impact the physiological response of entire vessel networks.
Lymphatic diseases represent a significant healthcare burden with estimates of up to 300 million individuals suffering from primary or secondary lymphedema worldwide (Bellini and Hennekam, 2014). Recent genome-wide analyses have uncovered novel molecular players in the development of primary lymphedema; however, the genetic basis of more than 75% of all cases is currently unknown (Brouillard et al., 2014). Furthermore, current therapies for secondary lymphedema are palliative (Grada and Phillips, 2017). Our findings identify the earliest step in LEC differentiation, which precedes induction of LEC fate by the well-described SOX18-COUPTFII-PROX1 axis (Francois et al., 2011). It is thus likely that investigation of the molecular cues that drive specification of LECs from the PXM will help the discovery of novel causative mutations in primary lymphatic disease in humans. Furthermore, future efforts to define the signals that drive PXM to LEC differentiation in vivo may provide a framework to differentiate progenitor cells into LECs, which could be used to treat secondary lymphedema. Notably, as the roles of lymphatics in cancer, cardiovascular disease, immunity, and infection are being described in increasing depth (Mortimer and Rockson, 2014), our findings may also have broad implications for human pathophysiology.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rat anti-VEGFR2 | BD Pharmingen | Cat# 550549; RRID:AB_2132506 |
| Mouse anti-PAX3 | R&D Systems | Cat# MAB2457; RRID:AB_2159398 |
| Rat anti-CD31 (PECAM) | BD Pharmingen | Cat# 553370; RRID:AB_394816 |
| Rabbit anti-PROX1 | Reliatech GmbH | Cat# 102-PA32AG |
| Rabbit anti-PROX1 | Proteintech | Cat# 11067–2-AP; RRID:AB_2268804 |
| Rat anti-EMCN | Santa Cruz Biotechnology | Cat# SC-65495; RRID:AB_2100037 |
| Rat anti-LYVE1 | Reliatech GmbH | Cat# 103-PA50, |
| Hamster anti-PDPN | Developmental Studies Hybridoma Bank | RRID:AB_531893 |
| Goat anti-NRP2 | R&D Systems | Cat# AF567; RRID:AB_2155253 |
| Rabbit anti-ACTA2 | GeneTex | Cat# GTX100034; RRID:AB_1240408 |
| CD144 (CDH5) | BD Pharmingen, | Cat# 555289; RRID:AB_395707 |
| Alexa Fluor® 405, Goat anti-Rabbit IgG (H+L) | Thermo Fisher Scientific | Cat# A-31556; RRID:AB_221605 |
| Alexa Fluor® 488, Goat anti-Rat IgG (H+L) | Thermo Fisher Scientific | Cat# A-11006; RRID:AB_2534074 |
| Alexa Fluor® 488, Goat anti-Hamster IgG (H+L) | Thermo Fisher Scientific | Cat# A-21110; RRID:AB_141509 |
| Alexa Fluor® 488, Goat anti-Mouse IgG2a IgG (H+L) | Thermo Fisher Scientific | Cat# A-21131; RRID:AB_141618 |
| Alexa Fluor® 647, Goat anti-Rabbit IgG (H+L) | Thermo Fisher Scientific | Cat# A-21244; RRID:AB_141663 |
| Alexa Fluor® 647, Goat anti-Rat IgG (H+L) | Thermo Fisher Scientific | Cat# A-21247; RRID:AB_141778 |
| Alexa Fluor® 647, Donkey anti-Goat IgG (H+L) | Thermo Fisher Scientific | Cat# A-21447; RRID:AB_141884 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| DAPI | Sigma | Cat# D954 |
| Paraformaldehyde | Sigma | Cat# P6148 |
| Phosphate-buffered saline tablet | Sigma | Cat# P4417 |
| Triton X-100 | Sigma | Cat# X-100 |
| Tween 20 | Sigma | Cat# P1379 |
| Bovine Serum Albumin | Sigma | Cat# A9418 |
| Normal Goat Serum | Thermo Fisher Scientific | Cat# 16210072 |
| Vectashield® | Vector Laboratories | Cat# H-1000 |
| Tissue-Tek® O.C.T. compound | VWR | Cat# 25608–930 |
| Experimental models: Organisms/Strains | ||
| Pax3tm1(cre)Joe | Engleka et al., 2005 | MGI:3804315 |
| Pax7tm1(cre)Mrc | Keller et al., 2004 | MGI:3510832 |
| Myf5tm3(cre)Sor | Tallquist et al., 2000 | IMSR_JAX007893 |
| Tg(Mef2c-cre)2Blk | Verzi et al., 2005 | MGI:3639735 |
| Gt(ROSA)26Sortm9(CAG-tdTomato)Hze | Madisen et al., 2010 | IMSR_JAX007909 |
| Prox1tm1a(EUCOMM)Wtsi | Martinez-Corral et al., 2015 | MGI:5617984 |
| Oligonucleotides | ||
| Pax3 Fw | CTGCACTCGGTGTCACG | Pax3Cre genotyping |
| Pax3 mut Rev | AGGCAAATTTTGGTGTACGG | |
| Pax3 wt Rev | AAGCGAGCACAGTGCGGC | |
| Pax7 wt Fw_1 | CTCCTCCACATTCCTTGCTC | Pax7Cre genotyping |
| Pax7 wt Fw_2 | CGGCCTTCTTCTAGGTTCTG | |
| Pax7 mut Rev_1 | GCGGTCTGGCAGTAAAAACTATC | |
| Pax7 mut Rev_2 | GTGAAACAGCATTGCTGTCACTT | |
| Myf5 Fw | AACCAGAGACTCCCCAAGGT | Myf5Cre genotyping |
| Myf5 wt Rev | CGGCTCTTAAAGCAATGGTC | |
| Myf5 mut Rev | ACGAAGTTATTAGGTCCCTCGAC | |
| Cre Fw | ATTGCTGTCACTTGGTCGTGGC | Mef2c-AHFCre genotyping |
| Cre Rev | GGAAAATGCTTCTGTCCGTTTGC | Mef2c-AHFCre genotyping |
| Rosa26tdtomato wt Fw | AAGGGAGCTGCAGTGGAGTA | Rosa26tdTomato genotyping |
| Rosa26tdtomato wt Rev | CCGAAAATCTGTGGGAAGTC | |
| Rosa26tdtomato mut Fw | GGCATTAAAGCAGCGTATCC | |
| Rosa26tdtomato mut Rev | CTGTTCCTGTACGGCATGG | |
| Prox1 flox (a) | TGCTGAAGATGTTGGTTGCT | Prox1fl genotyping |
| Prox1 flox (b) | GGCTTTTCTGTTGCTGAAGG | |
| Prox1 flox (c) | CTGAACTGATGGCGAGCTCAGAC | |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Didier Y.R. Stainier (didier.stainier@mpi-bn.mpg.de).
Experimental Model and Subject Details
Animals were maintained under standard conditions and all experiments were conducted in accordance with institutional (MPG) and national (Regierungspraesidium, Darmstadt) ethical and animal welfare guidelines. All mouse lines were used as previously described (Pax3Cre (Engleka et al., 2005) (RRID:MGI:3804315), Pax7Cre (Keller et al., 2004) (RRID:MGI:3510832), Myf5Cre (Tallquist et al., 2000) (RRID:IMSR_JAX007893), Mef2c-AHFCre (Verzi et al., 2005) (MGI:3639735), Rosa26tdTomato (Madisen et al., 2010) (RRID:IMSR_JAX007909) and Prox1fl (Martinez-Corral et al., 2015) (MGI:5617984)) and maintained on a C57BL/6J background. For embryonic staging, the morning of the vaginal plug was considered E0.5. For analyses at E10.5 and earlier, staging was performed by counting somite pairs, and at E12.5 and later by timing of the vaginal plug.
Method Details
Immunostaining and Imaging of Mouse Tissues
The following antibodies were used for immunofluorescence staining of cryosections, vibratome sections and whole mount tissues: VEGFR2 (1:200), PAX3 (1:100), CD31 ((PECAM) 1:100), PROX1 (1:200), PROX1 (1:100), EMCN (1:50), LYVE1 (1:250), PDPN (1:20), NRP2 (1:250), ACTA2 (1:400) and CD144 ((CDH5) 1:100). Alexa Fluor conjugated secondary antibodies (Thermo Fisher) were used at 1:300 in all cases.
For immunofluorescence staining of cryosections, samples were fixed in 4% paraformaldehyde (PFA) overnight at 4°C. Samples were washed in 1X PBS, then cryoprotected in sucrose and mounted in Optimal Cutting Temperature (OCT) compound. 8-10 μm cryosections were cut using a Leica CM1950 cryostat. Sections were blocked (1X PBS containing 0.1% Triton X-100 (PBX), 1% Bovine Serum Albumin (BSA) and 2% Normal Goat Serum (NGS)) for 1 hours at RT and then primary antibodies diluted in blocking buffer were incubated overnight. Following three 10-minute washes in PBX, secondary antibodies diluted in blocking buffer were incubated for 1 hours at RT. Slides were then washed three times (10 min) in PBX and where indicated, samples were counterstained with DAPI for visualization of cell nuclei before mounting with Vectashield® Antifade Mounting Medium (CA, USA).
For immunofluorescence staining of vibratome sections, samples were fixed in 4% PFA overnight at 4°C, washed in 1X PBS and then mounted in 5% low melting temperature agarose. 150-200 μm vibratome sections were cut using a Leica VT1000S. Tissue slices were incubated in blocking buffer (1X PBS containing 0.5% Triton X-100, 0.5% Tween 20, 1% BSA and 3% NGS) for 2 hours at RT and then incubated with primary antibodies diluted in incubation buffer (1X PBS containing 0.25% Triton X-100, 0.25% Tween 20, 0.5% BSA and 1.5% NGS) overnight at 4°C. After primary antibody incubation, tissues were washed 5 times in PBX and then incubated with Alexa Fluor conjugated secondary antibodies for 3 hours at room temperature. Tissues were then washed 5 times in PBX and mounted in Vectashield®.
For whole mount staining of the embryonic skin, tissues were fixed in 4% PFA for 2 hours at RT, washed in 1X PBS and then incubated in blocking solution (1X PBS containing 0.3% Triton X-100 and 3% milk) for 2 hours at RT. Tissues were then incubated with primary antibodies diluted in blocking solution overnight at 4°C. After primary antibody incubation, tissues were washed 5 times in PBX and then incubated with Alexa Fluor conjugated secondary antibodies for 3 hours at room temperature. Tissues were then washed 5 times in PBX and mounted in Vectashield®.
For whole mount imaging of the heart and lungs, samples were fixed in 4% PFA overnight at 4°C, washed in 1X PBS and then blocked (1X PBS containing 0.1% Triton X-100 (PBX), 1% Bovine Serum Albumin (BSA) and 2% Normal Goat Serum (NGS)) for 3 hours at RT. Samples were incubated overnight at 4°C with primary antibodies diluted in incubation buffer (1X PBS containing 0.25% Triton X-100, 0.25% Tween 20, 0.5% BSA and 1.5% NGS). After primary antibody incubation, tissues were washed 5 times in PBX and then incubated with Alexa Fluor conjugated secondary antibodies overnight at 4°C. Tissues were then washed 5 times in PBX and mounted in 2% low melting temperature agarose for imaging.
For whole mount staining of the meninges, tissues were fixed while still attached to the skull cap in 4% PFA for 4 hours at 4°C. Following dissection of the dura mater/arachnoid from the skull cap, tissues were washed in 1X PBS and then incubated in blocking solution (1X PBS containing 0.1% Triton X-100 (PBX), 1% Bovine Serum Albumin (BSA) and 2% Normal Goat Serum (NGS)) for 3 hours at RT. Tissues were then incubated with primary antibodies diluted in blocking solution overnight at 4°C. After primary antibody incubation, tissues were washed 5 times in PBX and then incubated with Alexa Fluor conjugated secondary antibodies for 3 hours at room temperature. Tissues were then washed 5 times in PBX and mounted on glass slides in Vectashield®. Imaging of immunofluorescence stained tissues was performed using a Zeiss LSM700 upright or LSM800 inverted (RRID:SCR_015963) confocal microscope. Whole mount imaging of freshly dissected embryos at E16.5 and adult hearts was performed using a Nikon SMZ25 stereomicroscope.
Acknowledgments
We thank Taija Mäkinen for Prox1fl mice (EUCOMM Consortium), Thomas Braun and Birgit Spitznagel for Pax3Cre, Pax7Cre, and Myf5Cre mice, Sigolène Meilhac for Mef2c-AHFCre mice, Ryota Matsuoka for Rosa26tdtomato mice, Sébastien Gauvrit for valuable support, and Thomas Braun, Sébastien Gauvrit, Ines Martinez-Corral, Michael Potente, Paul Riley, Sven Reischauer, and Shuichi Watanabe for discussion. This work was supported by an Excellence Cluster Cardiopulmonary System (ECCPS EXC147, DFG) grant (O.A.S.), the BHF Centre of Research Excellence, Oxford (RE/13/1/30181, O.A.S.), the Max Planck Society (D.Y.R.S.), and the European Research Council (ERC) (D.Y.R.S.).
Author Contributions
O.A.S. and D.Y.R.S. conceived the study, interpreted data, and wrote the paper. O.A.S. designed and performed experiments.
Declaration of Interests
The authors declare no competing interests.
Published: May 23, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.devcel.2019.04.034.
Contributor Information
Oliver A. Stone, Email: oliver.stone@dpag.ox.ac.uk.
Didier Y.R. Stainier, Email: didier.stainier@mpi-bn.mpg.de.
Supplemental Information
References
- Bellini C., Hennekam R.C. Clinical disorders of primary malfunctioning of the lymphatic system. Adv. Anat. Embryol. Cell Biol. 2014;214:187–204. doi: 10.1007/978-3-7091-1646-3_14. [DOI] [PubMed] [Google Scholar]
- Braun T., Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011;12:349–361. doi: 10.1038/nrm3118. [DOI] [PubMed] [Google Scholar]
- Brouillard P., Boon L., Vikkula M. Genetics of lymphatic anomalies. J. Clin. Invest. 2014;124:898–904. doi: 10.1172/JCI71614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M., Rigby P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell. 2014;28:225–238. doi: 10.1016/j.devcel.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Couly G., Coltey P., Eichmann A., Le Douarin N.M. The angiogenic potentials of the cephalic mesoderm and the origin of brain and head blood vessels. Mech. Dev. 1995;53:97–112. doi: 10.1016/0925-4773(95)00428-9. [DOI] [PubMed] [Google Scholar]
- Diogo R., Kelly R.G., Christiaen L., Levine M., Ziermann J.M., Molnar J.L., Noden D.M., Tzahor E. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature. 2015;520:466–473. doi: 10.1038/nature14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleka K.A., Gitler A.D., Zhang M., Zhou D.D., High F.A., Epstein J.A. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev. Biol. 2005;280:396–406. doi: 10.1016/j.ydbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- François M., Caprini A., Hosking B., Orsenigo F., Wilhelm D., Browne C., Paavonen K., Karnezis T., Shayan R., Downes M. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- Francois M., Harvey N.L., Hogan B.M. The transcriptional control of lymphatic vascular development. Physiol. 2011;26:146–155. doi: 10.1152/physiol.00053.2010. [DOI] [PubMed] [Google Scholar]
- Grada A.A., Phillips T.J. Lymphedema: pathophysiology and clinical manifestations. J. Am. Acad. Dermatol. 2017;77:1009–1020. doi: 10.1016/j.jaad.2017.03.022. [DOI] [PubMed] [Google Scholar]
- He L., Papoutsi M., Huang R., Tomarev S.I., Christ B., Kurz H., Wilting J. Three different fates of cells migrating from somites into the limb bud. Anat. Embryol. 2003;207:29–34. doi: 10.1007/s00429-003-0327-4. [DOI] [PubMed] [Google Scholar]
- Herbert S.P., Stainier D.Y. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubaud A., Pourquié O. Signalling dynamics in vertebrate segmentation. Nat. Rev. Mol. Cell Biol. 2014;15:709–721. doi: 10.1038/nrm3891. [DOI] [PubMed] [Google Scholar]
- Huntington G.S., McClure C.F.W. The anatomy and development of the jugular lymph sacs in the domestic cat (Felis domestica) Am. J. Anat. 1910;10:177–312. [Google Scholar]
- Hutcheson D.A., Zhao J., Merrell A., Haldar M., Kardon G. Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for beta-catenin. Genes Dev. 2009;23:997–1013. doi: 10.1101/gad.1769009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon G., Heanue T.A., Tabin C.J. Pax3 and Dach2 positive regulation in the developing somite. Dev. Dyn. 2002;224:350–355. doi: 10.1002/dvdy.10107. [DOI] [PubMed] [Google Scholar]
- Keller C., Hansen M.S., Coffin C.M., Capecchi M.R. Pax3:Fkhr interferes with embryonic Pax3 and Pax7 function: implications for alveolar rhabdomyosarcoma cell of origin. Genes Dev. 2004;18:2608–2613. doi: 10.1101/gad.1243904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R.G., Buckingham M.E., Moorman A.F. Heart fields and cardiac morphogenesis. Cold Spring Harb. Perspect. Med. 2014;4 doi: 10.1101/cshperspect.a015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L., Norman S., Vieira J.M., Masters M., Rohling M., Dubé K.N., Bollini S., Matsuzaki F., Carr C.A., Riley P.R. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature. 2015;522:62–67. doi: 10.1038/nature14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescroart F., Hamou W., Francou A., Théveniau-Ruissy M., Kelly R.G., Buckingham M. Clonal analysis reveals a common origin between nonsomite-derived neck muscles and heart myocardium. Proc. Natl. Acad. Sci. USA. 2015;112:1446–1451. doi: 10.1073/pnas.1424538112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Corral I., Ulvmar M.H., Stanczuk L., Tatin F., Kizhatil K., John S.W., Alitalo K., Ortega S., Makinen T. Nonvenous origin of dermal lymphatic vasculature. Circ. Res. 2015;116:1649–1654. doi: 10.1161/CIRCRESAHA.116.306170. [DOI] [PubMed] [Google Scholar]
- Mayeuf-Louchart A., Lagha M., Danckaert A., Rocancourt D., Relaix F., Vincent S.D., Buckingham M. Notch regulation of myogenic versus endothelial fates of cells that migrate from the somite to the limb. Proc. Natl. Acad. Sci. USA. 2014;111:8844–8849. doi: 10.1073/pnas.1407606111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A.I., Shirali A.S., Aragón R., Ma F., Hernandez G., Vaughn D.A., Mack J.J., Lim T.Y., Sunshine H., Zhao P. Endothelial regeneration of large vessels is a biphasic process driven by local cells with distinct proliferative capacities. Cell Stem Cell. 2018;23:210–225.e6. doi: 10.1016/j.stem.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzis V., Steinhauser S., Pakanavicius E., Gouti M., Stamataki D., Ivanovitch K., Watson T., Rayon T., Mousavy Gharavy S.N., Lovell-Badge R. Nervous system regionalization entails axial allocation before neural differentiation. Cell. 2018;175:1105–1118.e17. doi: 10.1016/j.cell.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer P.S., Rockson S.G. New developments in clinical aspects of lymphatic disease. J. Clin. Invest. 2014;124:915–921. doi: 10.1172/JCI71608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann C., Panáková D., Werdich A.A., Musso G., Burger A., Lawson K.L., Carr L.A., Nevis K.R., Sabeh M.K., Zhou Y. Chamber identity programs drive early functional partitioning of the heart. Nat. Commun. 2015;6:8146. doi: 10.1038/ncomms9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoike T., Markham D.W., Rossant J., Sato T.N. Evidence for novel fate of Flk1+ progenitor: contribution to muscle lineage. Genesis. 2003;35:153–159. doi: 10.1002/gene.10175. [DOI] [PubMed] [Google Scholar]
- Nguyen P.D., Hollway G.E., Sonntag C., Miles L.B., Hall T.E., Berger S., Fernandez K.J., Gurevich D.B., Cole N.J., Alaei S. Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature. 2014;512:314–318. doi: 10.1038/nature13678. [DOI] [PubMed] [Google Scholar]
- Ny A., Koch M., Schneider M., Neven E., Tong R.T., Maity S., Fischer C., Plaisance S., Lambrechts D., Héligon C. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat. Med. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- Pardanaud L., Luton D., Prigent M., Bourcheix L.M., Catala M., Dieterlen-Lievre F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development. 1996;122:1363–1371. doi: 10.1242/dev.122.5.1363. [DOI] [PubMed] [Google Scholar]
- Pichol-Thievend C., Betterman K.L., Liu X., Ma W., Skoczylas R., Lesieur E., Bos F.L., Schulte D., Schulte-Merker S., Hogan B.M. A blood capillary plexus-derived population of progenitor cells contributes to genesis of the dermal lymphatic vasculature during embryonic development. Development. 2018;145 doi: 10.1242/dev.160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plein A., Fantin A., Denti L., Pollard J.W., Ruhrberg C. Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature. 2018;562:223–228. doi: 10.1038/s41586-018-0552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M., Mäkinen T. Vascular heterogeneity and specialization in development and disease. Nat. Rev. Mol. Cell Biol. 2017;18:477–494. doi: 10.1038/nrm.2017.36. [DOI] [PubMed] [Google Scholar]
- Reischauer S., Stone O.A., Villasenor A., Chi N., Jin S.W., Martin M., Lee M.T., Fukuda N., Marass M., Witty A. Cloche is a bHLH-PAS transcription factor that drives haemato-vascular specification. Nature. 2016;535:294–298. doi: 10.1038/nature18614. [DOI] [PubMed] [Google Scholar]
- Sabin F.R. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat. 1902;1:367–389. [Google Scholar]
- Sambasivan R., Kuratani S., Tajbakhsh S. An eye on the head: the development and evolution of craniofacial muscles. Development. 2011;138:2401–2415. doi: 10.1242/dev.040972. [DOI] [PubMed] [Google Scholar]
- Schneider M., Othman-Hassan K., Christ B., Wilting J. Lymphangioblasts in the avian wing bud. Dev. Dyn. 1999;216:311–319. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<311::AID-DVDY1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Srinivasan R.S., Dillard M.E., Lagutin O.V., Lin F.J., Tsai S., Tsai M.J., Samokhvalov I.M., Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczuk L., Martinez-Corral I., Ulvmar M.H., Zhang Y., Laviña B., Fruttiger M., Adams R.H., Saur D., Betsholtz C., Ortega S. cKit lineage hemogenic endothelium-derived cells contribute to mesenteric lymphatic vessels. Cell Rep. 2015;10:1708–1721. doi: 10.1016/j.celrep.2015.02.026. [DOI] [PubMed] [Google Scholar]
- Tallquist M.D., Weismann K.E., Hellström M., Soriano P. Early myotome specification regulates PDGFA expression and axial skeleton development. Development. 2000;127:5059–5070. doi: 10.1242/dev.127.23.5059. [DOI] [PubMed] [Google Scholar]
- Ulvmar M.H., Mäkinen T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc. Res. 2016;111:310–321. doi: 10.1093/cvr/cvw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzi M.P., McCulley D.J., De Val S., Dodou E., Black B.L. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev. Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Wasteson P., Johansson B.R., Jukkola T., Breuer S., Akyürek L.M., Partanen J., Lindahl P. Developmental origin of smooth muscle cells in the descending aorta in mice. Development. 2008;135:1823–1832. doi: 10.1242/dev.020958. [DOI] [PubMed] [Google Scholar]
- Wigle J.T., Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Wilting J., Aref Y., Huang R., Tomarev S.I., Schweigerer L., Christ B., Valasek P., Papoutsi M. Dual origin of avian lymphatics. Dev. Biol. 2006;292:165–173. doi: 10.1016/j.ydbio.2005.12.043. [DOI] [PubMed] [Google Scholar]
- Wilting J., Brand-Saberi B., Huang R., Zhi Q., Köntges G., Ordahl C.P., Christ B. Angiogenic potential of the avian somite. Dev. Dyn. 1995;202:165–171. doi: 10.1002/aja.1002020208. [DOI] [PubMed] [Google Scholar]
- Wilting J., Papoutsi M., Schneider M., Christ B. The lymphatic endothelium of the avian wing is of somitic origin. Dev. Dyn. 2000;217:271–278. doi: 10.1002/(SICI)1097-0177(200003)217:3<271::AID-DVDY5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Yang Y., García-Verdugo J.M., Soriano-Navarro M., Srinivasan R.S., Scallan J.P., Singh M.K., Epstein J.A., Oliver G. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012;120:2340–2348. doi: 10.1182/blood-2012-05-428607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv K., Isogai S., Castranova D., Dye L., Hitomi J., Weinstein B.M. Live imaging of lymphatic development in the zebrafish. Nat. Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- You L.R., Lin F.J., Lee C.T., DeMayo F.J., Tsai M.J., Tsai S.Y. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.