Abstract
Extracorporeal life support (ECLS) encompasses a wide range of extracorporeal modalities that offer short- and intermediate-term mechanical support to the failing heart or lung. Apart from the daily use of cardiopulmonary bypass (CPB) in the operating room, there has been a resurgence of interest and utilization of veno-arterial and veno-venous extracorporeal membrane oxygenation (VA- and VV-ECMO, respectively) and extracorporeal carbon dioxide removal (ECCO2R) in recent years. This might be attributed to the advancement in technology, nonetheless the morbidity and mortality associated with the clinical application of this technology is still significant. The initiation of ECLS triggers a systemic inflammatory response, which involves the activation of the coagulation cascade, complement systems, endothelial cells, leukocytes, and platelets, thus potentially contributing to morbidity and mortality. This is due to the release of cytokines and other biomarkers of inflammation, which have been associated with multiorgan dysfunction. On the other hand, ECLS can be utilized as a therapy to halt the inflammatory response associated with critical illness and ICU therapeutic intervention, such as facilitating ultra-protective mechanical ventilation. In addition to addressing the impact on outcome of the relationship between inflammation and ECLS, two different but complementary pathophysiological perspectives will be developed in this review: ECLS as the cause of inflammation and ECLS as the treatment of inflammation. This framework may be useful in guiding the development of novel therapeutic strategies to improve the outcome of critical illness.
Keywords: Inflammation, Biomarkers, Cytokines, Extracorporeal life support, Extracorporeal membrane oxygenation, Extracorporeal carbon dioxide removal, Cardiopulmonary bypass
Background
Inflammation is a central facet in the complex pathophysiology of critical illness. Irrespective of cause, critical illness initiates the innate and adaptive immune systems, resulting in systemic inflammatory response syndrome (SIRS) [1–6]. Elevated levels of pro-inflammatory cytokines have been associated with mortality in trauma, complex surgical interventions, sepsis, adult respiratory distress syndrome (ARDS), and cardiogenic shock [7]. Additionally, anti-inflammatory response if unbalanced results in anergy and immunosuppression [8]. Furthermore, multiple organ failure has been postulated to be due to massive activation of inflammatory mediators by critical illness resulting in vascular endothelial damage, permeability edema, and impaired oxygen availability to mitochondria [9]. Following the inception of modern intensive care units (ICU), therapeutic interventions and life support strategies have led to significant reduction in inflammatory mediators and hence mortality [10–14].
Extracorporeal life support (ECLS) is a term than has been used interchangeably with extracorporeal membrane oxygenation (ECMO), but it encompasses all extracorporeal technologies, including cardiopulmonary bypass (CPB), ECMO in all its configurations, and extracorporeal carbon dioxide removal (ECCO2R). Since the success of CPB for short-term circulatory support in the 1950’s, enthusiasm has grown to translate this technology to intermediate and long-term support for critically ill patients [15]. The first report of the use of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) for respiratory failure was two decades later [16]. Although initial randomized clinical trials failed to demonstrate any clinical benefit of this technique [17, 18], with the advancement in technology and improvement in the safety profile, a resurgence of ECMO have been seen in the last decade with an exponential expansion in the number of ECMO centers worldwide. Moreover, improvement in outcomes has also been reported with survival of 57% to hospital discharge for patients with respiratory failure and 41% to hospital discharge for patients with cardiac failure [19].
Although lifesaving in many situations, complications of ECLS, whether mechanical, pump related, secondary to bleeding, or infection, are common and often contribute to morbidity and mortality [15, 19]. One of the relevant complications of ECLS is the associated inflammatory response. A rapid rise in pro-inflammatory cytokines following initiation of ECLS [20–22] is thought to be associated with an innate immune response [23], which if severe may lead to end-organ dysfunction and death [24, 25].
It is challenging to discern the extent of the inflammatory response that is solely due to ECLS or due to critical illness, underlying disease or ICU therapeutic interventions including complex surgical procedures and mechanical ventilation (MV) [26–30]. Furthermore, while the potential mechanical and inflammatory injury caused by other means of life support such as MV is well recognized within the critical care community [31], the importance of the inflammation associated with the application of ECLS is less understood.
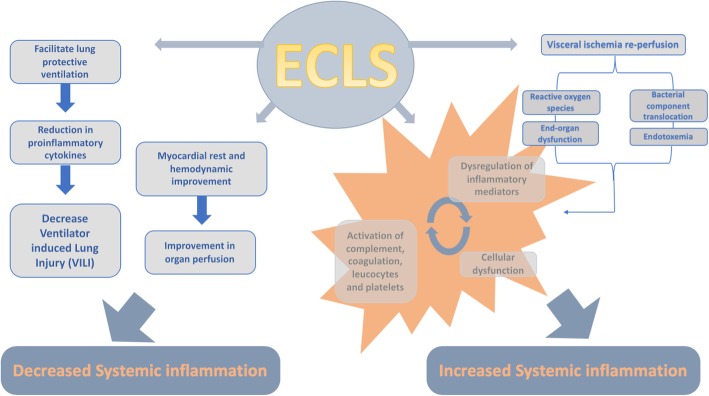
In addition to addressing the impact on outcome of the relationship between inflammation and ECLS, two different but complementary pathophysiological perspectives will be developed in this review: ECLS as the cause of inflammation and ECLS as the treatment of inflammation (Fig. 1). This framework may be useful in guiding the development of novel therapeutic strategies to improve the outcome of critical illness.
Fig. 1.
Pathophysiology ECLS and associated inflammatory response
ECLS as a cause of systemic inflammation
Upon exposure of blood to the extracorporeal circuit during ECLS an inflammatory response might be triggered that mimics SIRS [32]. This is mediated by both humoral and cellular activation pathways, which are fundamentally interdependent but not fully understood during ECLS [33]. Most of the investigations on this specific issue have been developed with the CBP system, and hence a comprehensive evaluation is limited by the lack of rigorous studies on this issue conducted on ECMO and ECCO2R. Additionally, a majority of the studies are conducted in neonates and pediatrics and used an older technology, with less advanced pumps, circuits, and biocompatible materials, pre-dating modern ECLS. Therefore, the findings can be only prudently extended to all the more modern ECLS configurations and uses.
Contact system, coagulation cascade, and complement
Following initiation of ECLS, the contact system becomes activated and subsequent byproducts of this activation promote coagulation and drive inflammation [33]. This activation process is rapid [34], resulting in neutrophil activation, release of nitric oxide and pro-inflammatory cytokines, as demonstrated during CPB [35, 36] and in neonatal ECMO [23]. The contact system activation triggers both intrinsic and extrinsic coagulation pathways, leading to clot formation and inflammation [33]. Notably, in simulated closed ECC, the expression of tissue factor (TF) by activated monocytes, or alternatively the TNF-α- and IL-6-induced release of soluble TF by endothelial cells, was evident without the need to be triggered by tissue injury and resulted in a 30-fold increase in thrombin formation [37, 38]. The complement system is also triggered upon initiation of ECLS [23, 39]. This mechanism is usually rapid with a peak in 1–2 h [23, 40–42] but is short lived and limited to 1–2 days following initiation of ECLS [40, 43]. This activation is mediated by C5a, C3a, C3b, and terminal complement complex and causes an increase in leukocyte recruitment, vascular permeability, and endothelial dysfunction [40, 43, 44].
Role of platelets
Platelets are a major mediator of inflammation and not just hemostasis during ECLS [45]. Platelet activation has been extensively studied during CPB [46]. CPB causes structural and biochemical changes in platelets including differential expression of membrane receptors and formation of platelet conjugates due to shear forces created by circulatory pumps [47]. Less is known on the mechanisms of platelet activation during ECMO and ECCO2R. In one of these investigations in neonates supported with VV-ECMO for respiratory failure, platelets were found to adhere to the fibrinogen absorbed by the circuit, resulting in a time-dependent platelet activation along with persistent and progressive platelet dysfunction leading to the release of pro-inflammatory cytokines and expression of TF [48, 49].
Role of the endothelium and leukocytes
Endothelial dysfunction in critical illness has been associated with poor outcomes [50] and plays also a major role in ECLS-induced inflammation. Alteration in endothelial cell gene expression occurs due to the effect of cytokines, complement, and reactive oxygen species, leading to pro-inflammatory mediators release and increased transmigration of leukocytes [48, 51]. The resulting neutrophil infiltration has been described to lead to ECLS-associated lung injury and end-organ damage [23–25, 52, 53]. Activation of neutrophils has been found in an experimental simulated ECC to be instantaneous [54], peaking within the first few hours of ECMO initiation and declining thereafter [55].
Bacterial translocation
Other potential inflammatory mechanisms studied during ECLS are gut barrier dysfunction, bacterial translocation, and endotoxins release. During CPB and ECMO, endotoxins can be released in response to translocation of bacteria from ischemic gut mucosa into the blood stream [56, 57]. Lipopolysaccharide is released by Gram-negative bacteria and induces macrophages to release TNF-α and endothelial cells to release IL-6 [58]. Endotoxins stimulate circulating monocytes to produce cytokines, such as TNF-α [59] and blood-borne TF [60], thereby activating the coagulation cascade. Additionally, thrombin generation promotes inflammation, leading to a vicious circle.
Human leukocyte antigen sensitization
Another interesting mechanism by which ECLS may promote inflammation is by triggering human leukocyte antigen (HLA) sensitization in subjects bridged to transplant with extracorporeal means of life support. HLA sensitization has been reported in pediatric patients supported with a ventricular assist device and ECMO while awaiting heart transplant [61, 62]. In addition, a recent report indicated that also patients bridged to lung transplant with ECMO might develop HLA sensitization [63]. However, the potential etiological mechanisms resulting into allosensitization during ECMO remain unclear and speculative.
CBP-specific inflammatory response
CPB involves unique features that further contribute to inflammation (Table 1). The clamping of the aorta during surgery inflicts an ischemia-reperfusion injury to both the heart and the lungs and results in a significant inflammatory reaction [64]. Moreover, the protamine administered at the end of CPB for heparin reversal results in protamine-heparin complexes that are known to exacerbate the inflammatory response via activation of the classical and lectin complement pathways [65]. Furthermore, hemodilution can be employed in CPB and could lead to increased neutrophil activation and therefore SIRS [66, 67]. Finally, surgical trauma and the presence of blood-air interface due to cardiotomy suctioning, venting of blood, and venous reservoirs, which are incorporated in the circuit contribute to the inflammatory response [68].
Table 1.
ECLS modalities
| CPB | VA-ECMO | VV-ECMO | ECCO2R | |
|---|---|---|---|---|
| Organ support | Cardiac and pulmonary | Cardiac and pulmonary | Pulmonary: oxygenation and ventilation | Pulmonary: ventilation |
| Duration | Minutes to hours | Days to weeks | Days to weeks | Days to weeks |
| Anticoagulation | Very high-dose heparin | Low-dose heparin | Low-dose or no heparin | Low-dose heparin |
| Reversal | Protamine | No | No | No |
| Air-blood interface | Yes | No | No | No |
CPB and postoperative pulmonary dysfunction
The impact of CPB on postoperative lung function has been debated. Traditional strategies of no MV during CPB might induce pulmonary dysfunction, due to development of micro-atelectasis, hydrostatic pulmonary edema, and ischemic lung injury secondary to reduction in bronchial artery flow [69]. Furthermore, ECLS-induced inflammation has been associated with pulmonary dysfunction. In adults undergoing CPB, IL-8 levels in the bronchoalveolar lavage were significantly correlated to arterial oxygenation and intrapulmonary shunt at the end of the surgery [70]. Moreover, the length of MV was longer in patients with an exaggerated inflammatory response to CPB [71]. Finally, postoperative concentration of IL-8 was higher in patients ventilated for more than 24 h in comparison to patients ventilated for less than 24 h [72].
Nonetheless, it has been suggested that post-CPB pulmonary dysfunction might be triggered by anesthesia or surgical technique. In patients with good ventricular function and without prior pulmonary diseases, coronary artery bypass on or off pump caused a similar degree of postoperative pulmonary dysfunction [73]. In addition, it has been speculated that the similar left atrium/right atrium ratio of leukocyte count in ventilated and non-ventilated patients during CPB reduces the possibility of the inflammatory response accounting for difference in the incidence of lung injury [74]
Clinical implications of ECLS-associated inflammatory response
Several studies demonstrated a considerable association between inflammation and outcome during ECLS in its different configurations. In neonates undergoing CPB, Interlukin 6 (IL-6) and IL-8 concentrations correlated with postoperative myocardial dysfunction [75], lactate concentrations, blood product administration, duration of MV, and ICU and hospital length of stay [76, 77]. Moreover, in adults post-cardiac surgery, increased IL-6 levels after CPB were predictive of infection in patients with impaired left ventricular function [78], and preoperative IL-8 concentrations correlated with prolonged postoperative MV [79]. In addition, one specific genetic polymorphism of IL-6 was associated with acute lung injury [80].
During veno-venous extracorporeal membrane oxygenation (VV-ECMO) for severe ARDS, IL-6, IL-8, and tumor necrosis factor-α (TNF-α) levels were associated with an increased risk of in-hospital mortality [25]. IL-6 levels were persistently increased in non-survivors among a mixed group of patients undergoing VV-ECMO and VA-ECMO [81]. Furthermore, higher levels of TNF-α have been correlated with mortality in neonates undergoing VV- or VA-ECMO [23, 52]. Of note, IL-6 was identified as a potentially useful prognostic marker for mortality during ECMO support [81], pulmonary dysfunction after CPB [82], and acute kidney injury after cardiac surgery, both in children [83] and in adults [84].
Greater release of IL-10 after CPB was associated with improved cardiac index and pulmonary gas exchange [85] and increased chance of survival following cardiogenic shock in adults supported with ECMO [86]. Additionally, IL-10 levels in ARDS patients correlated with severity of illness and predicted unsuccessful ECMO weaning and mortality [87].
Potential therapeutic interventions in ECLS-induced inflammation
A number of different strategies, including pharmacologic agents and non-pharmacologic interventions (i.e., innovative surgical techniques, ECLS circuit modifications, the conduction of anesthesia and ventilation), have been evaluated in experimental [88] and clinical trials [33, 89] with the aim of minimizing the impact of ECLS-related systemic inflammation on patients’ outcome in pediatric [89, 90] and adult patients [91]. However, the impact of these strategies on the post-ECLS clinical course has been conflicting.
Although the administration of steroids during pediatric cardiac surgery has been associated with the attenuation of CPB-induced inflammation [89], their impact on postoperative clinical outcome remains modest [92]. The administration of steroids was associated with a reduction in postoperative infection and duration of postoperative MV and length of stay, but no beneficial effects on mortality and organ complications in adult cardiac surgery patients have been reported [93, 94].
Preoperative statin therapy was associated with a reduction in post-CPB inflammation [95, 96] and an improvement in morbidity and mortality after cardiac surgery [97–99]. However, a recent meta-analysis of randomized control trials (RCTs) found no evidence of benefit for patients’ outcomes [100]. Patients with high preoperative IL-6 levels might be the best candidates for the preemptive administration of statins in cardiac surgery with CPB [101]. Other anti-inflammatory pharmacologic strategies, such as protease inhibitors (i.e., sivelestat, ulinastatin, aprotinin) [102–104] and milrinone [105], have been associated with improved postoperative clinical outcomes, although additional studies are needed to provide a better perspective regarding future applications.
Monoclonal antibodies have been studied as modulators of ECLS-induced inflammation. A novel inhibitory antibody against factor XIIa has been shown to reduced inflammation in ex vivo and animal models of ECMO [106]. Moreover, human monoclonal antibody directed at C5 significantly inhibited neutrophil activation in a model of simulated extracorporeal circuit (ECC) [107]. Mesenchymal stromal cells (MSC) therapy infusion in an animal model of CPB significantly reduced inflammatory cytokines within 3 h and subsequently reduced the harm associated with ischemia-reperfusion injury [108]. Promising results have also been shown with hemoadsorption during CPB [109] and ECMO [110]. However, all these therapeutic options remain experimental.
Many technical modifications of the CPB circuit and surgical procedures were implemented to minimize systemic inflammation secondary to the activation of blood components after contact with the CPB circuit and pulmonary dysfunction after ischemia-reperfusion injury [90, 91]. A minimized extracorporeal circulation system [111] and the circuit coating with poly-2-methoxyethyl acrylate [112] or heparin [113] have been associated with a decrease in the systemic inflammatory response, thus potentially improving organ function after cardiac surgery. By reducing the ischemic insult to the lungs and inflammatory activation, pulmonary perfusion during CPB may decrease systemic inflammatory response and have a protective effect on the lungs [114–117]. However, robust evidence for any beneficial effects is lacking according to a recent meta-analysis [118].
ECLS as a therapy for systemic inflammation
Despite the fact that different modalities of ECLS have been implicated in driving an intense inflammatory response, ECLS can also be employed to offset it. By replacing the function of the heart or the lung, ECLS may result into a direct reduction of inflammation due for instance to improved perfusion and gas exchange or may allow the reduction of the pro-inflammatory “stress” induced by other life support means, such as MV, with an indirect effect on treating systemic inflammation.
ARDS, VV-ECMO, and ECCO2R
In ARDS, pulmonary and systemic inflammation exacerbated by high MV settings, the so-called ventilator-induced lung injury, can be reduced by a lung-protective ventilation strategy [12], which has been demonstrated to increase patients’ survival [119]. In an interesting recent observational trial, the initiation of VV-ECMO support in mechanically ventilated patients for ARDS was associated with a remarkable decrease in cytokine levels, potentially explained by the achievement of “lung rest” with less alveolar stress and strain [25].
Recently, the hypothesis that the implementation of ultra-protective MV may allow the achievement of minimal alveolar stress and strain, thus further reducing pulmonary and systemic inflammation in ARDS, has been addressed in experimental and observational clinical studies utilizing different ECLS strategies [120–122]. The use of ECCO2R has been reported to significantly reduce ARDS patients’ inflammatory response [123, 124]. In an interesting proof-of-concept clinical study, patient with ARDS with high plateau airway pressure despite the delivery of protective MV with tidal volumes of 6 cc/kg of predicted body weight were treated with ECCO2R for 3 days in order to further decrease tidal volumes and alveolar distending pressures. ECCO2R allowed the tidal volume to be decreased to less than 4 cc/kg of predicted body weight with the consequent significant reduction of the plateau airway pressure, while maintaining normal pH and PaCO2. Reduction in the MV intensity resulted in the decrease of alveolar overdistension, as demonstrated by CT scan imaging, and in the significant decreases of the bronchoalveolar inflammatory cytokines IL-6, IL-8, IL-1b, and IL-1Ra [124]. These results were confirmed in a more recent randomized controlled trial that, comparing ultra-protective MV facilitated by ECCO2R to conventional lung-protective ventilation, resulted in significant reduction in IL-6 within 24 h of initiation of pumpless arterio-venous ECCO2R, but no effect on ventilator-free days or mortality [125]. However, rigorous clinical trials on this topic are needed before this approach can be recommended in clinical practice [126–128].
Mechanical ventilation during CPB
Although the impact of protective MV during CPB on cytokine levels, pulmonary function, and clinical outcomes is still controversial [129–131], most studies described its beneficial effect on post-CPB systemic inflammatory response [132–135] and lung function [74, 136], thereby potentially improving clinical outcomes [74]. For example, in adult patients undergoing CPB, IL-6 and IL-8 levels in the bronchoalveolar lavage fluid and plasma were higher with high tidal volume/low positive end-expiratory pressure than with low tidal volume/high positive end-expiratory pressure [132]. However, the interesting results of a pilot randomized controlled trial, comparing MV versus no MV during CPB, showed that the group treated with MV had less pulmonary edema and shorter overall duration of MV [74]. It has been proposed that this benefit derives from the partial preservation of bronchial arterial flow. Despite a recent meta-analysis of randomized controlled trials showing that ventilation during CPB may improve post-CPB oxygenation and gas exchange [137], the positive effects of the designated MV techniques are probably short-term and with a questionable impact on the clinical outcome [137, 138].
VA-ECMO and cardiogenic shock
Institution of ECLS has been associated with reduction inflammation in patients with cardiogenic shock. This was reported to result in hemodynamic improvement in patients with left ventricular assist device used for cardiogenic shock [139]. Furthermore, a significant reduction in the levels of IL-6 and IL-10 was reported in patients following institution of VA-ECMO for post-cardiotomy syndrome and myocarditis [53]. The resultant hemodynamic stability was theorized to lead to improved end-organ perfusion and contributed to recovery from multiple organ failure. Moreover, the use of heparin-coated biocompatible circuits was thought to minimize blood-material interaction and result in reduction of ECMO-induced systemic inflammation.
Conclusions
Critical illness-associated inflammatory process is complex. It can be secondary to acute illness or due to complex ICU therapeutic interventions. ECLS can induce an inflammatory process that has been associated with morbidity and mortality. On the other hand, it can offer a therapeutic benefit in facilitating lung and cardiac support, which might limit the determinants of inflammation. In the future, the development of progressively more advanced ECLS technology will certainly provide a safer means of advanced life support with potentially higher chances to demonstrate their therapeutic benefit.
Acknowledgements
Not applicable
Funding
This article did not receive sponsorship for publication.
Availability of data and materials
Not applicable.
About this supplement
This articles has been published as part of Intensive Care Medicine Experimental Volume 7 Supplement 1 2019: Proceedings from the Third International Symposium on Acute Pulmonary Injury and Translational Research (INSPIRES III). The full contents of the supplement are available at <https://icm-experimental.springeropen.com/articles/supplements/volume-7-supplement-1>
Abbreviations
- CPB
Cardiopulmonary bypass
- ECC
Extracorporeal circuit
- ECCO2R
Extracorporeal carbon dioxide removal
- ECLS
Extracorporeal life support
- ICU
Intensive care unit
- IL-10
Interlukin-10
- IL-6
Interlukin-6
- IL-8
Interleukin-8.
- MV
Mechanical ventilation
- SIRS
Systemic inflammatory response syndrome
- TF
Tissue factor
- TNF-α
Tumor necrosis factor-alpha
- VA-ECMO
Veno-arterial extracorporeal membrane oxygenation
- VV-ECMO
Veno-venous extracorporeal membrane oxygenation
Authors’ contributions
AA conceived the review, performed the literature review, drafted the first draft of the manuscript, and revised the manuscript. TP performed the literature review, drafted the manuscript, and revised the manuscript. LDS revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdulrahman Al-Fares, Email: abdulrahman.al.fares@mail.utoronto.ca.
Tommaso Pettenuzzo, Email: Tommaso.Pettenuzzo@uhn.ca.
Lorenzo Del Sorbo, Email: lorenzo.delsorbo@utoronto.ca.
References
- 1.Pierce A, Pittet JF. Inflammatory response to trauma: implications for coagulation and resuscitation. Curr Opin Anaesthesiol. 2014;27:246–252. doi: 10.1097/ACO.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosmann M, Ward PA. The inflammatory response in sepsis. Trends Immunol. 2013;34:129–136. doi: 10.1016/j.it.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 4.Roumen RM, Hendriks T, van der Ven-Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, van der Meer JW, Goris RJ. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218:769–776. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baughman RP, Gunther KL, Rashkin MC, Keeton DA, Pattishall EN. Changes in the inflammatory response of the lung during acute respiratory distress syndrome: prognostic indicators. Am J Respir Crit Care Med. 1996;154:76–81. doi: 10.1164/ajrccm.154.1.8680703. [DOI] [PubMed] [Google Scholar]
- 6.Headley AS, Tolley E, Meduri GU. Infections and the inflammatory response in acute respiratory distress syndrome. Chest. 1997;111:1306–1321. doi: 10.1378/chest.111.5.1306. [DOI] [PubMed] [Google Scholar]
- 7.Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J Intensive Care Med. 2011;26:73–87. doi: 10.1177/0885066610384188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonelli M. Sepsis and septic shock: pro-inflammatory or anti-inflammatory state? J Chemother. 1999;11:536–540. doi: 10.1179/joc.1999.11.6.536. [DOI] [PubMed] [Google Scholar]
- 9.Goris RJ, te Boekhorst TP, Nuytinck JK, Gimbrere JS. Multiple-organ failure. Generalized autodestructive inflammation? Arch Surg. 1985;120:1109–1115. doi: 10.1001/archsurg.1985.01390340007001. [DOI] [PubMed] [Google Scholar]
- 10.Baue AE, Chaudry IH. Prevention of multiple systems failure. Surg Clin North Am. 1980;60:1167–1178. doi: 10.1016/S0039-6109(16)42240-1. [DOI] [PubMed] [Google Scholar]
- 11.Goris RJ, Gimbrere JS, van Niekerk JL, Schoots FJ, Booy LH. Early osteosynthesis and prophylactic mechanical ventilation in the multitrauma patient. J Trauma. 1982;22:895–903. doi: 10.1097/00005373-198211000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 13.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP, Network NARDSCT Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.CCM.0000149854.61192.DC. [DOI] [PubMed] [Google Scholar]
- 14.Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson M, Umberger R. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 15.Fortenberry JDLR. The history and development of extracorporeal support. In: Brogan TVLL, Lorusso R, MacLaren G, Peek G, editors. Extracorporeal life support: the ELSO Red Book 5th ed. Ann Arbor: Extracorporeal Life Support Organization; 2017. pp. 1–15. [Google Scholar]
- 16.Hill JD, O'Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, Gerbode F. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286:629–634. doi: 10.1056/NEJM197203232861204. [DOI] [PubMed] [Google Scholar]
- 17.Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, Morris AH, Peirce EC, 2nd, Thomas AN, Proctor HJ, Drinker PA, Pratt PC, Bagniewski A, Miller RG., Jr Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–2196. doi: 10.1001/jama.1979.03300200023016. [DOI] [PubMed] [Google Scholar]
- 18.Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF, Jr, Weaver LK, Dean NC, Thomas F, East TD, Pace NL, Suchyta MR, Beck E, Bombino M, Sittig DF, Bohm S, Hoffmann B, Becks H, Butler S, Pearl J, Rasmusson B. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295–305. doi: 10.1164/ajrccm.149.2.8306022. [DOI] [PubMed] [Google Scholar]
- 19.Thiagarajan RR, Barbaro RP, Rycus PT, McMullan DM, Conrad SA, Fortenberry JD, Paden ML, centers Em Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63:60–67. doi: 10.1097/MAT.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Krawiec C, Patel S, Kunselman AR, Song J, Lei F, Baer LD, Undar A. Laboratory evaluation of hemolysis and systemic inflammatory response in neonatal nonpulsatile and pulsatile extracorporeal life support systems. Artif Organs. 2015;39:774–781. doi: 10.1111/aor.12466. [DOI] [PubMed] [Google Scholar]
- 21.Mc IRB, Timpa JG, Kurundkar AR, Holt DW, Kelly DR, Hartman YE, Neel ML, Karnatak RK, Schelonka RL, Anantharamaiah GM, Killingsworth CR, Maheshwari A. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab Invest. 2010;90:128–139. doi: 10.1038/labinvest.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirthler M, Simoni J, Dickson M. Elevated levels of endotoxin, oxygen-derived free radicals, and cytokines during extracorporeal membrane oxygenation. J Pediatr Surg. 1992;27:1199–1202. doi: 10.1016/0022-3468(92)90787-8. [DOI] [PubMed] [Google Scholar]
- 23.Plotz FB, van Oeveren W, Bartlett RH, Wildevuur CR. Blood activation during neonatal extracorporeal life support. J Thorac Cardiovasc Surg. 1993;105:823–832. [PubMed] [Google Scholar]
- 24.Liu CH, Kuo SW, Hsu LM, Huang SC, Wang CH, Tsai PR, Chen YS, Jou TS, Ko WJ. Peroxiredoxin 1 induces inflammatory cytokine response and predicts outcome of cardiogenic shock patients necessitating extracorporeal membrane oxygenation: an observational cohort study and translational approach. J Transl Med. 2016;14:114. doi: 10.1186/s12967-016-0869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burrell AJC, Lubnow M, Enger TB, Nanjayya VB, Philipp A, Malfertheiner MV, Lunz D, Bein T, Pellegrino VA, Muller T. The impact of venovenous extracorporeal membrane oxygenation on cytokine levels in patients with severe acute respiratory distress syndrome: a prospective, observational study. Crit Care Resusc. 2017;19:37–44. [PubMed] [Google Scholar]
- 26.Griffith DM, Lewis S, Rossi AG, Rennie J, Salisbury L, Merriweather JL, Templeton K, Walsh TS, Investigators R Systemic inflammation after critical illness: relationship with physical recovery and exploration of potential mechanisms. Thorax. 2016;71:820–829. doi: 10.1136/thoraxjnl-2015-208114. [DOI] [PubMed] [Google Scholar]
- 27.Wan S, Marchant A, DeSmet JM, Antoine M, Zhang H, Vachiery JL, Goldman M, Vincent JL, LeClerc JL. Human cytokine responses to cardiac transplantation and coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1996;111:469–477. doi: 10.1016/S0022-5223(96)70458-0. [DOI] [PubMed] [Google Scholar]
- 28.Kaneda H, Waddell TK, de Perrot M, Bai XH, Gutierrez C, Arenovich T, Chaparro C, Liu M, Keshavjee S. Pre-implantation multiple cytokine mRNA expression analysis of donor lung grafts predicts survival after lung transplantation in humans. Am J Transplant. 2006;6:544–551. doi: 10.1111/j.1600-6143.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 29.Mathur A, Baz M, Staples ED, Bonnell M, Speckman JM, Hess PJ, Jr, Klodell CT, Knauf DG, Moldawer LL, Beaver TM. Cytokine profile after lung transplantation: correlation with allograft injury. Ann Thorac Surg. 2006;81:1844–1849. doi: 10.1016/j.athoracsur.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 30.Langer F, Schramm R, Bauer M, Tscholl D, Kunihara T, Schafers HJ. Cytokine response to pulmonary thromboendarterectomy. Chest. 2004;126:135–141. doi: 10.1378/chest.126.1.135. [DOI] [PubMed] [Google Scholar]
- 31.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2014;370:980. doi: 10.1056/NEJMc1400293. [DOI] [PubMed] [Google Scholar]
- 32.Davies MG, Hagen PO. Systemic inflammatory response syndrome. Br J Surg. 1997;84:920–935. doi: 10.1002/bjs.1800840707. [DOI] [PubMed] [Google Scholar]
- 33.Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. 2016;20:387. doi: 10.1186/s13054-016-1570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wendel HP, Scheule AM, Eckstein FS, Ziemer G. Haemocompatibility of paediatric membrane oxygenators with heparin-coated surfaces. Perfusion. 1999;14:21–28. doi: 10.1177/026765919901400104. [DOI] [PubMed] [Google Scholar]
- 35.Wachtfogel YT, Hack CE, Nuijens JH, Kettner C, Reilly TM, Knabb RM, Bischoff R, Tschesche H, Wenzel H, Kucich U, et al. Selective kallikrein inhibitors alter human neutrophil elastase release during extracorporeal circulation. Am J Physiol. 1995;268:H1352–H1357. doi: 10.1152/ajpheart.1995.268.3.H1352. [DOI] [PubMed] [Google Scholar]
- 36.Cugno M, Nussberger J, Biglioli P, Giovagnoni MG, Gardinali M, Agostoni A. Cardiopulmonary bypass increases plasma bradykinin concentrations. Immunopharmacology. 1999;43:145–147. doi: 10.1016/S0162-3109(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 37.Morgan EN, Pohlman TH, Vocelka C, Farr A, Lindley G, Chandler W, Griscavage-Ennis JM, Verrier ED. Nuclear factor kappaB mediates a procoagulant response in monocytes during extracorporeal circulation. J Thorac Cardiovasc Surg. 2003;125:165–171. doi: 10.1067/mtc.2003.99. [DOI] [PubMed] [Google Scholar]
- 38.Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96:1233–1239. doi: 10.1161/01.RES.0000171805.24799.fa. [DOI] [PubMed] [Google Scholar]
- 39.Wehlin L, Vedin J, Vaage J, Lundahl J. Activation of complement and leukocyte receptors during on- and off pump coronary artery bypass surgery. Eur J Cardiothorac Surg. 2004;25:35–42. doi: 10.1016/S1010-7940(03)00652-3. [DOI] [PubMed] [Google Scholar]
- 40.Vallhonrat H, Swinford RD, Ingelfinger JR, Williams WW, Ryan DP, Tolkoff-Rubin N, Cosimi AB, Pascual M. Rapid activation of the alternative pathway of complement by extracorporeal membrane oxygenation. ASAIO J. 1999;45:113–114. doi: 10.1097/00002480-199901000-00025. [DOI] [PubMed] [Google Scholar]
- 41.Moen O, Fosse E, Braten J, Andersson C, Fagerhol MK, Venge P, Hogasen K, Mollnes TE. Roller and centrifugal pumps compared in vitro with regard to haemolysis, granulocyte and complement activation. Perfusion. 1994;9:109–117. doi: 10.1177/026765919400900205. [DOI] [PubMed] [Google Scholar]
- 42.Hocker JR, Wellhausen SR, Ward RA, Simpson PM, Cook LN. Effect of extracorporeal membrane oxygenation on leukocyte function in neonates. Artif Organs. 1991;15:23–28. doi: 10.1111/j.1525-1594.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 43.Gardinali M, Cicardi M, Frangi D, Bergamaschini L, Gallazzi M, Gattinoni L, Agostoni A. Studies of complement activation in ARDS patients treated by long-term extracorporeal CO2 removal. Int J Artif Organs. 1985;8:135–140. doi: 10.1177/039139888500800306. [DOI] [PubMed] [Google Scholar]
- 44.Chenoweth DE, Cooper SW, Hugli TE, Stewart RW, Blackstone EH, Kirklin JW. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981;304:497–503. doi: 10.1056/NEJM198102263040901. [DOI] [PubMed] [Google Scholar]
- 45.Gemmell CH, Ramirez SM, Yeo EL, Sefton MV. Platelet activation in whole blood by artificial surfaces: identification of platelet-derived microparticles and activated platelet binding to leukocytes as material-induced activation events. J Lab Clin Med. 1995;125:276–287. [PubMed] [Google Scholar]
- 46.Bick RL. Alterations of hemostasis associated with cardiopulmonary bypass: pathophysiology, prevention, diagnosis, and management. Semin Thromb Hemost. 1976;3:59–82. doi: 10.1055/s-0028-1086129. [DOI] [PubMed] [Google Scholar]
- 47.Weerasinghe A, Taylor KM. The platelet in cardiopulmonary bypass. Ann Thorac Surg. 1998;66:2145–2152. doi: 10.1016/S0003-4975(98)00749-8. [DOI] [PubMed] [Google Scholar]
- 48.Cheung PY, Sawicki G, Salas E, Etches PC, Schulz R, Radomski MW. The mechanisms of platelet dysfunction during extracorporeal membrane oxygenation in critically ill neonates. Crit Care Med. 2000;28:2584–2590. doi: 10.1097/00003246-200007000-00067. [DOI] [PubMed] [Google Scholar]
- 49.Maugeri N, Brambilla M, Camera M, Carbone A, Tremoli E, Donati MB, de Gaetano G, Cerletti C. Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J Thromb Haemost. 2006;4:1323–1330. doi: 10.1111/j.1538-7836.2006.01968.x. [DOI] [PubMed] [Google Scholar]
- 50.Duffy MJ, Mullan BA, Craig TR, Shyamsundar M, MacSweeney RE, Thompson G, Stevenson M, McAuley DF. Impaired endothelium-dependent vasodilatation is a novel predictor of mortality in intensive care. Crit Care Med. 2011;39:629–635. doi: 10.1097/CCM.0b013e318206bc4a. [DOI] [PubMed] [Google Scholar]
- 51.Boyle EM, Jr, Pohlman TH, Johnson MC, Verrier ED. Endothelial cell injury in cardiovascular surgery: the systemic inflammatory response. Ann Thorac Surg. 1997;63:277–284. doi: 10.1016/S0003-4975(96)01061-2. [DOI] [PubMed] [Google Scholar]
- 52.Fortenberry JD, Bhardwaj V, Niemer P, Cornish JD, Wright JA, Bland L. Neutrophil and cytokine activation with neonatal extracorporeal membrane oxygenation. J Pediatr. 1996;128:670–678. doi: 10.1016/S0022-3476(96)80133-8. [DOI] [PubMed] [Google Scholar]
- 53.Kawahito K, Misawa Y, Fuse K. Extracorporeal membrane oxygenation support and cytokines. Ann Thorac Surg. 1998;65:1192–1193. doi: 10.1016/S0003-4975(98)00043-5. [DOI] [PubMed] [Google Scholar]
- 54.Wachtfogel YT, Kucich U, Hack CE, Gluszko P, Niewiarowski S, Colman RW, Edmunds LH., Jr Aprotinin inhibits the contact, neutrophil, and platelet activation systems during simulated extracorporeal perfusion. J Thorac Cardiovasc Surg. 1993;106:1–9. [PubMed] [Google Scholar]
- 55.Graulich J, Walzog B, Marcinkowski M, Bauer K, Kossel H, Fuhrmann G, Buhrer C, Gaehtgens P, Versmold HT. Leukocyte and endothelial activation in a laboratory model of extracorporeal membrane oxygenation (ECMO) Pediatr Res. 2000;48:679–684. doi: 10.1203/00006450-200011000-00021. [DOI] [PubMed] [Google Scholar]
- 56.Riddington DW, Venkatesh B, Boivin CM, Bonser RS, Elliott TS, Marshall T, Mountford PJ, Bion JF. Intestinal permeability, gastric intramucosal pH, and systemic endotoxemia in patients undergoing cardiopulmonary bypass. JAMA. 1996;275:1007–1012. doi: 10.1001/jama.1996.03530370045029. [DOI] [PubMed] [Google Scholar]
- 57.Kurundkar AR, Killingsworth CR, McIlwain RB, Timpa JG, Hartman YE, He D, Karnatak RK, Neel ML, Clancy JP, Anantharamaiah GM, Maheshwari A. Extracorporeal membrane oxygenation causes loss of intestinal epithelial barrier in the newborn piglet. Pediatr Res. 2010;68:128–133. doi: 10.1203/PDR.0b013e3181e4c9f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jirik FR, Podor TJ, Hirano T, Kishimoto T, Loskutoff DJ, Carson DA, Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989;142:144–147. [PubMed] [Google Scholar]
- 59.Jansen NJ, van Oeveren W, Gu YJ, van Vliet MH, Eijsman L, Wildevuur CR. Endotoxin release and tumor necrosis factor formation during cardiopulmonary bypass. Ann Thorac Surg. 1992;54:744–747. doi: 10.1016/0003-4975(92)91021-Z. [DOI] [PubMed] [Google Scholar]
- 60.Ernofsson M, Thelin S, Siegbahn A. Monocyte tissue factor expression, cell activation, and thrombin formation during cardiopulmonary bypass: a clinical study. J Thorac Cardiovasc Surg. 1997;113:576–584. doi: 10.1016/S0022-5223(97)70373-8. [DOI] [PubMed] [Google Scholar]
- 61.Yang J, Schall C, Smith D, Kreuser L, Zamberlan M, King K, Gajarski R. HLA sensitization in pediatric pre-transplant cardiac patients supported by mechanical assist devices: the utility of Luminex. J Heart Lung Transplant. 2009;28:123–129. doi: 10.1016/j.healun.2008.11.908. [DOI] [PubMed] [Google Scholar]
- 62.Hong BJ, Delaney M, Guynes A, Warner P, McMullan DM, Kemna MS, Boucek RJ, Law YM. Human leukocyte antigen sensitization in pediatric patients exposed to mechanical circulatory support. ASAIO J. 2014;60:317–321. doi: 10.1097/MAT.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 63.Hayes D, Jr, Preston TJ, Kirkby S, Nicol KK. Human leukocyte antigen sensitization in lung transplant candidates supported by extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2013;188:627–628. doi: 10.1164/rccm.201303-0428LE. [DOI] [PubMed] [Google Scholar]
- 64.Warren OJ, Smith AJ, Alexiou C, Rogers PL, Jawad N, Vincent C, Darzi AW, Athanasiou T. The inflammatory response to cardiopulmonary bypass: part 1--mechanisms of pathogenesis. J Cardiothorac Vasc Anesth. 2009;23:223–231. doi: 10.1053/j.jvca.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 65.Bruins P, te Velthuis H, Eerenberg-Belmer AJ, Yazdanbakhsh AP, de Beaumont EM, Eijsman L, Trouwborst A, Hack CE. Heparin-protamine complexes and C-reactive protein induce activation of the classical complement pathway: studies in patients undergoing cardiac surgery and in vitro. Thromb Haemost. 2000;84:237–243. doi: 10.1055/s-0037-1614002. [DOI] [PubMed] [Google Scholar]
- 66.Gourlay T, Samartzis I, Taylor KM. The effect of haemodilution on blood-biomaterial contact-mediated CD11b expression on neutrophils: ex vivo studies. Perfusion. 2003;18:87–93. doi: 10.1191/0267659103pf648oa. [DOI] [PubMed] [Google Scholar]
- 67.DeFoe GR, Ross CS, Olmstead EM, Surgenor SD, Fillinger MP, Groom RC, Forest RJ, Pieroni JW, Warren CS, Bogosian ME, Krumholz CF, Clark C, Clough RA, Weldner PW, Lahey SJ, Leavitt BJ, Marrin CA, Charlesworth DC, Marshall P, O'Connor GT. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg. 2001;71:769–776. doi: 10.1016/S0003-4975(00)02393-6. [DOI] [PubMed] [Google Scholar]
- 68.Gabel J, Westerberg M, Bengtsson A, Jeppsson A. Cell salvage of cardiotomy suction blood improves the balance between pro- and anti-inflammatory cytokines after cardiac surgery. Eur J Cardiothorac Surg. 2013;44:506–511. doi: 10.1093/ejcts/ezt019. [DOI] [PubMed] [Google Scholar]
- 69.Asimakopoulos G, Smith PL, Ratnatunga CP, Taylor KM. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg. 1999;68:1107–1115. doi: 10.1016/S0003-4975(99)00781-X. [DOI] [PubMed] [Google Scholar]
- 70.Kotani N, Hashimoto H, Sessler DI, Muraoka M, Wang JS, O'Connor MF, Matsuki A. Neutrophil number and interleukin-8 and elastase concentrations in bronchoalveolar lavage fluid correlate with decreased arterial oxygenation after cardiopulmonary bypass. Anesth Analg. 2000;90:1046–1051. doi: 10.1097/00000539-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 71.Holmes JH, Connolly NC, Paull DL, Hill ME, Guyton SW, Ziegler SF, Hall RA. Magnitude of the inflammatory response to cardiopulmonary bypass and its relation to adverse clinical outcomes. Inflamm Res. 2002;51:579–586. doi: 10.1007/PL00012432. [DOI] [PubMed] [Google Scholar]
- 72.Rothenburger M, Soeparwata R, Deng MC, Berendes E, Schmid C, Tjan TD, Wilhelm MJ, Erren M, Bocker D, Scheld HH. The impact of anti-endotoxin core antibodies on endotoxin and cytokine release and ventilation time after cardiac surgery. J Am Coll Cardiol. 2001;38:124–130. doi: 10.1016/S0735-1097(01)01323-7. [DOI] [PubMed] [Google Scholar]
- 73.Cox CM, Ascione R, Cohen AM, Davies IM, Ryder IG, Angelini GD. Effect of cardiopulmonary bypass on pulmonary gas exchange: a prospective randomized study. Ann Thorac Surg. 2000;69:140–145. doi: 10.1016/S0003-4975(99)01196-0. [DOI] [PubMed] [Google Scholar]
- 74.John LC, Ervine IM. A study assessing the potential benefit of continued ventilation during cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2008;7:14–17. doi: 10.1510/icvts.2007.158451. [DOI] [PubMed] [Google Scholar]
- 75.Hovels-Gurich HH, Vazquez-Jimenez JF, Silvestri A, Schumacher K, Minkenberg R, Duchateau J, Messmer BJ, von Bernuth G, Seghaye MC. Production of proinflammatory cytokines and myocardial dysfunction after arterial switch operation in neonates with transposition of the great arteries. J Thorac Cardiovasc Surg. 2002;124:811–820. doi: 10.1067/mtc.2002.122308. [DOI] [PubMed] [Google Scholar]
- 76.Allan CK, Newburger JW, McGrath E, Elder J, Psoinos C, Laussen PC, del Nido PJ, Wypij D, FX MG., Jr The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesth Analg. 2010;111:1244–1251. doi: 10.1213/ANE.0b013e3181f333aa. [DOI] [PubMed] [Google Scholar]
- 77.Mahle WT, Matthews E, Kanter KR, Kogon BE, Hamrick SE, Strickland MJ. Inflammatory response after neonatal cardiac surgery and its relationship to clinical outcomes. Ann Thorac Surg. 2014;97:950–956. doi: 10.1016/j.athoracsur.2013.10.069. [DOI] [PubMed] [Google Scholar]
- 78.Sander M, von Heymann C, von Dossow V, Spaethe C, Konertz WF, Jain U, Spies CD. Increased interleukin-6 after cardiac surgery predicts infection. Anesth Analg. 2006;102:1623–1629. doi: 10.1213/01.ane.0000215998.21739.48. [DOI] [PubMed] [Google Scholar]
- 79.Rothenburger M, Soeparwata R, Deng MC, Schmid C, Berendes E, Tjan TD, Wilhelm MJ, Erren M, Bocker D, Scheld HH. Prediction of clinical outcome after cardiac surgery: the role of cytokines, endotoxin, and anti-endotoxin core antibodies. Shock. 2001;16(Suppl 1):44–50. doi: 10.1097/00024382-200116001-00009. [DOI] [PubMed] [Google Scholar]
- 80.Wang JF, Bian JJ, Wan XJ, Zhu KM, Sun ZZ, Lu AD. Association between inflammatory genetic polymorphism and acute lung injury after cardiac surgery with cardiopulmonary bypass. Med Sci Monit. 2010;16:CR260–CR265. [PubMed] [Google Scholar]
- 81.Risnes I, Wagner K, Ueland T, Mollnes T, Aukrust P, Svennevig J. Interleukin-6 may predict survival in extracorporeal membrane oxygenation treatment. Perfusion. 2008;23:173–178. doi: 10.1177/0267659108097882. [DOI] [PubMed] [Google Scholar]
- 82.Halter J, Steinberg J, Fink G, Lutz C, Picone A, Maybury R, Fedors N, DiRocco J, Lee HM, Nieman G. Evidence of systemic cytokine release in patients undergoing cardiopulmonary bypass. J Extra Corpor Technol. 2005;37:272–277. [PMC free article] [PubMed] [Google Scholar]
- 83.Dennen P, Altmann C, Kaufman J, Klein CL, Andres-Hernando A, Ahuja NH, Edelstein CL, Cadnapaphornchai MA, Keniston A, Faubel S. Urine interleukin-6 is an early biomarker of acute kidney injury in children undergoing cardiac surgery. Crit Care. 2010;14:R181. doi: 10.1186/cc9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Musleh GS, Datta SS, Yonan NN, Grotte GJ, Prendergast BA, Hasan RI, Deyrania AK. Association of IL6 and IL10 with renal dysfunction and the use of haemofiltration during cardiopulmonary bypass. Eur J Cardiothorac Surg. 2009;35:511–514. doi: 10.1016/j.ejcts.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 85.Giomarelli P, Scolletta S, Borrelli E, Biagioli B. Myocardial and lung injury after cardiopulmonary bypass: role of interleukin (IL)-10. Ann Thorac Surg. 2003;76:117–123. doi: 10.1016/S0003-4975(03)00194-2. [DOI] [PubMed] [Google Scholar]
- 86.Hong T-H, Hu F-C, Kuo S-W, Ko W-J, Chow L-P, Hsu L-M, Huang S-C, Yu S-L, Chen Y-S. Predicting outcome in patients under extracorporeal membrane oxygenation due to cardiogenic shock through dynamic change of lymphocytes and interleukins. IJC Metabolic & Endocrine. 2015;7:36–44. doi: 10.1016/j.ijcme.2014.11.001. [DOI] [Google Scholar]
- 87.Liu CH, Kuo SW, Ko WJ, Tsai PR, Wu SW, Lai CH, Wang CH, Chen YS, Chen PL, Liu TT, Huang SC, Jou TS. Early measurement of IL-10 predicts the outcomes of patients with acute respiratory distress syndrome receiving extracorporeal membrane oxygenation. Sci Rep. 2017;7:1021. doi: 10.1038/s41598-017-01225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liguori GR, Kanas AF, Moreira LF. Managing the inflammatory response after cardiopulmonary bypass: review of the studies in animal models. Rev Bras Cir Cardiovasc. 2014;29:93–102. doi: 10.5935/1678-9741.20140017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bronicki RA, Hall M. Cardiopulmonary bypass-induced inflammatory response: pathophysiology and treatment. Pediatr Crit Care Med. 2016;17:S272–S278. doi: 10.1097/PCC.0000000000000759. [DOI] [PubMed] [Google Scholar]
- 90.Durandy Y. Minimizing systemic inflammation during cardiopulmonary bypass in the pediatric population. Artif Organs. 2014;38:11–18. doi: 10.1111/aor.12195. [DOI] [PubMed] [Google Scholar]
- 91.Landis RC, Brown JR, Fitzgerald D, Likosky DS, Shore-Lesserson L, Baker RA, Hammon JW. Attenuating the systemic inflammatory response to adult cardiopulmonary pypass: a critical review of the evidence base. J Extra Corpor Technol. 2014;46:197–211. [PMC free article] [PubMed] [Google Scholar]
- 92.Scrascia G, Rotunno C, Guida P, Amorese L, Polieri D, Codazzi D, Paparella D. Perioperative steroids administration in pediatric cardiac surgery: a meta-analysis of randomized controlled trials*. Pediatr Crit Care Med. 2014;15:435–442. doi: 10.1097/PCC.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 93.Dieleman JM, van Paassen J, van Dijk D, Arbous MS, Kalkman CJ, Vandenbroucke JP, van der Heijden GJ, Dekkers OM (2011) Prophylactic corticosteroids for cardiopulmonary bypass in adults. Cochrane Database Syst Rev 11:CD005566. [DOI] [PubMed]
- 94.Dieleman JM, Nierich AP, Rosseel PM, van der Maaten JM, Hofland J, Diephuis JC, Schepp RM, Boer C, Moons KG, van Herwerden LA, Tijssen JG, Numan SC, Kalkman CJ, van Dijk D, Dexamethasone for Cardiac Surgery Study G Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA. 2012;308:1761–1767. doi: 10.1001/jama.2012.14144. [DOI] [PubMed] [Google Scholar]
- 95.Morgan C, Zappitelli M, Gill P. Statin prophylaxis and inflammatory mediators following cardiopulmonary bypass: a systematic review. Crit Care. 2009;13:R165. doi: 10.1186/cc8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ozguler IM, Burma O, Uysal A, Akbulut H. Rosuvastatin lowers systemic inflammatory response in coronary artery bypass graft accompanied by cardiopulmonary bypass surgery: a randomized controlled study. Clin Invest Med. 2015;38:E154–E163. doi: 10.25011/cim.v38i4.24260. [DOI] [PubMed] [Google Scholar]
- 97.Clark LL, Ikonomidis JS, Crawford FA, Jr, Crumbley A, 3rd, Kratz JM, Stroud MR, Woolson RF, Bruce JJ, Nicholas JS, Lackland DT, Zile MR, Spinale FG. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: an 8-year retrospective cohort study. J Thorac Cardiovasc Surg. 2006;131:679–685. doi: 10.1016/j.jtcvs.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 98.Collard CD, Body SC, Shernan SK, Wang S, Mangano DT, Multicenter Study of Perioperative Ischemia Research Group I, Ischemia R, Education Foundation I Preoperative statin therapy is associated with reduced cardiac mortality after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2006;132:392–400. doi: 10.1016/j.jtcvs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 99.Virani SS, Nambi V, Lee VV, Elayda M, Reul RM, Wilson JM, Ballantyne CM. Does preoperative statin therapy improve outcomes in patients undergoing isolated cardiac valve surgery? Am J Cardiol. 2008;102:1235–1239. doi: 10.1016/j.amjcard.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 100.Putzu A, Capelli B, Belletti A, Cassina T, Ferrari E, Gallo M, Casso G, Landoni G. Perioperative statin therapy in cardiac surgery: a meta-analysis of randomized controlled trials. Crit Care. 2016;20:395. doi: 10.1186/s13054-016-1560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martinez-Comendador J, Alvarez JR, Sierra J, Teijeira E, Adrio B. Preoperative statin therapy in cardiac surgery is more effective in patients who display preoperative activation of the inflammatory system. Tex Heart Inst J. 2013;40:42–49. [PMC free article] [PubMed] [Google Scholar]
- 102.Fujii M, Miyagi Y, Bessho R, Nitta T, Ochi M, Shimizu K. Effect of a neutrophil elastase inhibitor on acute lung injury after cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2010;10:859–862. doi: 10.1510/icvts.2009.225243. [DOI] [PubMed] [Google Scholar]
- 103.He S, Lin K, Ma R, Xu R, Xiao Y. Effect of the urinary tryptin inhibitor ulinastatin on cardiopulmonary bypass-related inflammatory response and clinical outcomes: a meta-analysis of randomized controlled trials. Clin Ther. 2015;37:643–653. doi: 10.1016/j.clinthera.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 104.Xu CE, Zou CW, Zhang MY, Guo L. Effects of high-dose ulinastatin on inflammatory response and pulmonary function in patients with type-A aortic dissection after cardiopulmonary bypass under deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth. 2013;27:479–484. doi: 10.1053/j.jvca.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 105.Gong M, Lin XZ, Lu GT, Zheng LJ. Preoperative inhalation of milrinone attenuates inflammation in patients undergoing cardiac surgery with cardiopulmonary bypass. Med Princ Pract. 2012;21:30–35. doi: 10.1159/000332411. [DOI] [PubMed] [Google Scholar]
- 106.Larsson M, Rayzman V, Nolte MW, Nickel KF, Bjorkqvist J, Jamsa A, Hardy MP, Fries M, Schmidbauer S, Hedenqvist P, Broome M, Pragst I, Dickneite G, Wilson MJ, Nash AD, Panousis C, Renne T. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014;6:222ra217. doi: 10.1126/scitranslmed.3006804. [DOI] [PubMed] [Google Scholar]
- 107.Rinder CS, Rinder HM, Smith BR, Fitch JC, Smith MJ, Tracey JB, Matis LA, Squinto SP, Rollins SA. Blockade of C5a and C5b-9 generation inhibits leukocyte and platelet activation during extracorporeal circulation. J Clin Invest. 1995;96:1564–1572. doi: 10.1172/JCI118195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qiang Y, Liang G, Yu L. Human amniotic mesenchymal stem cells alleviate lung injury induced by ischemia and reperfusion after cardiopulmonary bypass in dogs. Lab Invest. 2016;96:537–546. doi: 10.1038/labinvest.2016.37. [DOI] [PubMed] [Google Scholar]
- 109.Bernardi MH, Rinoesl H, Dragosits K, Ristl R, Hoffelner F, Opfermann P, Lamm C, Preissing F, Wiedemann D, Hiesmayr MJ, Spittler A. Effect of hemoadsorption during cardiopulmonary bypass surgery - a blinded, randomized, controlled pilot study using a novel adsorbent. Crit Care. 2016;20:96. doi: 10.1186/s13054-016-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bruenger F, Kizner L, Weile J, Morshuis M, Gummert JF. First successful combination of ECMO with cytokine removal therapy in cardiogenic septic shock: a case report. Int J Artif Organs. 2015;38:113–116. doi: 10.5301/ijao.5000382. [DOI] [PubMed] [Google Scholar]
- 111.Fromes Y, Gaillard D, Ponzio O, Chauffert M, Gerhardt MF, Deleuze P, Bical OM. Reduction of the inflammatory response following coronary bypass grafting with total minimal extracorporeal circulation. Eur J Cardiothorac Surg. 2002;22:527–533. doi: 10.1016/S1010-7940(02)00372-X. [DOI] [PubMed] [Google Scholar]
- 112.Ueyama K, Nishimura K, Nishina T, Nakamura T, Ikeda T, Komeda M. PMEA coating of pump circuit and oxygenator may attenuate the early systemic inflammatory response in cardiopulmonary bypass surgery. ASAIO J. 2004;50:369–372. doi: 10.1097/01.MAT.0000130679.55946.4D. [DOI] [PubMed] [Google Scholar]
- 113.Mahmood S, Bilal H, Zaman M, Tang A. Is a fully heparin-bonded cardiopulmonary bypass circuit superior to a standard cardiopulmonary bypass circuit? Interact Cardiovasc Thorac Surg. 2012;14:406–414. doi: 10.1093/icvts/ivr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richter JA, Meisner H, Tassani P, Barankay A, Dietrich W, Braun SL. Drew-Anderson technique attenuates systemic inflammatory response syndrome and improves respiratory function after coronary artery bypass grafting. Ann Thorac Surg. 2000;69:77–83. doi: 10.1016/S0003-4975(99)01131-5. [DOI] [PubMed] [Google Scholar]
- 115.Sievers HH, Freund-Kaas C, Eleftheriadis S, Fischer T, Kuppe H, Kraatz EG, Bechtel JF. Lung protection during total cardiopulmonary bypass by isolated lung perfusion: preliminary results of a novel perfusion strategy. Ann Thorac Surg. 2002;74:1167–1172. doi: 10.1016/S0003-4975(02)03853-5. [DOI] [PubMed] [Google Scholar]
- 116.Suzuki T, Ito T, Kashima I, Teruya K, Fukuda T. Continuous perfusion of pulmonary arteries during total cardiopulmonary bypass favorably affects levels of circulating adhesion molecules and lung function. J Thorac Cardiovasc Surg. 2001;122:242–248. doi: 10.1067/mtc.2001.114779. [DOI] [PubMed] [Google Scholar]
- 117.Kiessling AH, Guo FW, Gokdemir Y, Thudt M, Reyher C, Scherer M, Beiras-Fernandez A, Moritz A. The influence of selective pulmonary perfusion on the inflammatory response and clinical outcome of patients with chronic obstructive pulmonary disease undergoing cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2014;18:732–739. doi: 10.1093/icvts/ivu062. [DOI] [PubMed] [Google Scholar]
- 118.Buggeskov KB, Gronlykke L, Risom EC, Wei ML, Wetterslev J. Pulmonary artery perfusion versus no perfusion during cardiopulmonary bypass for open heart surgery in adults. Cochrane Database Syst Rev. 2018;2:CD011098. doi: 10.1002/14651858.CD011098.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Acute Respiratory Distress Syndrome N. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 120.Iglesias M, Jungebluth P, Petit C, Matute MP, Rovira I, Martinez E, Catalan M, Ramirez J, Macchiarini P. Extracorporeal lung membrane provides better lung protection than conventional treatment for severe postpneumonectomy noncardiogenic acute respiratory distress syndrome. J Thorac Cardiovasc Surg. 2008;135:1362–1371. doi: 10.1016/j.jtcvs.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 121.Grasso S, Stripoli T, Mazzone P, Pezzuto M, Lacitignola L, Centonze P, Guarracino A, Esposito C, Herrmann P, Quintel M, Trerotoli P, Bruno F, Crovace A, Staffieri F. Low respiratory rate plus minimally invasive extracorporeal Co2 removal decreases systemic and pulmonary inflammatory mediators in experimental Acute Respiratory Distress Syndrome. Crit Care Med. 2014;42:e451–e460. doi: 10.1097/CCM.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 122.Guldner A, Kiss T, Bluth T, Uhlig C, Braune A, Carvalho N, Quast T, Rentzsch I, Huhle R, Spieth P, Richter T, Saddy F, Rocco PR, Kasper M, Koch T, Pelosi P, de Abreu MG. Effects of ultraprotective ventilation, extracorporeal carbon dioxide removal, and spontaneous breathing on lung morphofunction and inflammation in experimental severe acute respiratory distress syndrome. Anesthesiology. 2015;122:631–646. doi: 10.1097/ALN.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 123.Iglesias M, Martinez E, Badia JR, Macchiarini P. Extrapulmonary ventilation for unresponsive severe acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg. 2008;85:237–244. doi: 10.1016/j.athoracsur.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 124.Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, Faggiano C, Quintel M, Gattinoni L, Ranieri VM. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111:826–835. doi: 10.1097/ALN.0b013e3181b764d2. [DOI] [PubMed] [Google Scholar]
- 125.Bein T, Weber-Carstens S, Goldmann A, Muller T, Staudinger T, Brederlau J, Muellenbach R, Dembinski R, Graf BM, Wewalka M, Philipp A, Wernecke KD, Lubnow M, Slutsky AS. Lower tidal volume strategy ( approximately 3 ml/kg) combined with extracorporeal CO2 removal versus 'conventional' protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39:847–856. doi: 10.1007/s00134-012-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fan E, Brodie D, Slutsky AS. Fifty Years of Research in ARDS. Mechanical ventilation during extracorporeal support for acute respiratory distress syndrome. For Now, a Necessary Evil. Am J Respir Crit Care Med. 2017;195:1137–1139. doi: 10.1164/rccm.201702-0292ED. [DOI] [PubMed] [Google Scholar]
- 127.Belfast Health and social care Trust (2016) Protective ventilation with venovenous lung assist in respiratory failure (REST) [http://clinicaltrials.gov/ct2/show/NCT02654327] (NLM Identifier: NCT02654327).
- 128.European Society of Intensive Care Medicine (2014) Strategy of ultraprotective lung ventilation with extracorporeal CO2 removal for new-onset moderate to severe ARDS (SUPERNOVA) [http://clinicaltrials.gov/ct2/show/NCT02282657] (NLM Identifier: NCT02282657).
- 129.Koner O, Celebi S, Balci H, Cetin G, Karaoglu K, Cakar N. Effects of protective and conventional mechanical ventilation on pulmonary function and systemic cytokine release after cardiopulmonary bypass. Intensive Care Med. 2004;30:620–626. doi: 10.1007/s00134-003-2104-5. [DOI] [PubMed] [Google Scholar]
- 130.Gagnon J, Laporta D, Beique F, Langlois Y, Morin JF. Clinical relevance of ventilation during cardiopulmonary bypass in the prevention of postoperative lung dysfunction. Perfusion. 2010;25:205–210. doi: 10.1177/0267659110373839. [DOI] [PubMed] [Google Scholar]
- 131.Durukan AB, Gurbuz HA, Salman N, Unal EU, Ucar HI, Yorgancioglu CE. Ventilation during cardiopulmonary bypass did not attenuate inflammatory response or affect postoperative outcomes. Cardiovasc J Afr. 2013;24:224–230. doi: 10.5830/CVJA-2013-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zupancich E, Paparella D, Turani F, Munch C, Rossi A, Massaccesi S, Ranieri VM. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: a randomized clinical trial. J Thorac Cardiovasc Surg. 2005;130:378–383. doi: 10.1016/j.jtcvs.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 133.Reis Miranda D, Gommers D, Struijs A, Dekker R, Mekel J, Feelders R, Lachmann B, Bogers AJ. Ventilation according to the open lung concept attenuates pulmonary inflammatory response in cardiac surgery. Eur J Cardiothorac Surg. 2005;28:889–895. doi: 10.1016/j.ejcts.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 134.Beer L, Szerafin T, Mitterbauer A, Debreceni T, Maros T, Dworschak M, Roth GA, Ankersmit HJ. Continued mechanical ventilation during coronary artery bypass graft operation attenuates the systemic immune response. Eur J Cardiothorac Surg. 2013;44:282–287. doi: 10.1093/ejcts/ezs659. [DOI] [PubMed] [Google Scholar]
- 135.Beer L, Szerafin T, Mitterbauer A, Debreceni T, Maros T, Dworschak M, Roth GA, Ankersmit HJ. Low tidal volume ventilation during cardiopulmonary bypass reduces postoperative chemokine serum concentrations. Thorac Cardiovasc Surg. 2014;62:677–682. doi: 10.1055/s-0034-1387824. [DOI] [PubMed] [Google Scholar]
- 136.Ng CS, Arifi AA, Wan S, Ho AM, Wan IY, Wong EM, Yim AP. Ventilation during cardiopulmonary bypass: impact on cytokine response and cardiopulmonary function. Ann Thorac Surg. 2008;85:154–162. doi: 10.1016/j.athoracsur.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 137.Chi D, Chen C, Shi Y, Wang W, Ma Y, Zhou R, Yu H, Liu B. Ventilation during cardiopulmonary bypass for prevention of respiratory insufficiency: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e6454. doi: 10.1097/MD.0000000000006454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schreiber JU, Lance MD, de Korte M, Artmann T, Aleksic I, Kranke P. The effect of different lung-protective strategies in patients during cardiopulmonary bypass: a meta-analysis and semiquantitative review of randomized trials. J Cardiothorac Vasc Anesth. 2012;26:448–454. doi: 10.1053/j.jvca.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 139.Goldstein DJ, Moazami N, Seldomridge JA, Laio H, Ashton RC, Jr, Naka Y, Pinsky DJ, Oz MC. Circulatory resuscitation with left ventricular assist device support reduces interleukins 6 and 8 levels. Ann Thorac Surg. 1997;63:971–974. doi: 10.1016/S0003-4975(96)01117-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.