Abstract
INTRODUCTION
Osteoporosis is the main cause of fractures among women after menopause. This study aimed to evaluate the efficacy and safety of denosumab compared to bisphosphonates in treating postmenopausal osteoporosis.
METHODS
Databases including PubMed and the Cochrane Central Register of Controlled Trials were systematically searched for randomised controlled trials (RCTs) that directly compared denosumab and bisphosphonates. RCTs that studied both denosumab and bisphosphonates in postmenopausal women with osteoporosis and had a Jadad score ≥ 3 were included.
RESULTS
Nine studies were eligible for inclusion. They were further categorised into six cohort groups. All studies had denosumab with oral bisphosphonates as the active comparator. Four out of six cohort studies showed significant improvements in bone strength (p < 0.001) at the distal radius, tibia, total hip, femoral neck, lumbar spine and trochanter at 12 months for patients on denosumab compared to the bisphosphonate group. Serum C-telopeptide of cross-linked collagen, a bone turnover marker, was consistently lower in the denosumab group in all studies. There were no significant differences in hypocalcaemia, atypical fractures, fragility fractures, osteonecrosis of the jaw, all infections (including fever or influenza-like symptoms), gastrointestinal side effects or dermatological conditions in all studies, except for one that did not document side effects.
CONCLUSION
Denosumab can be used both as a first-line agent and an alternative to bisphosphonate in the treatment of postmenopausal osteoporosis. There is currently insufficient data to show that denosumab is not inferior to bisphosphonates in fracture prevention.
Keywords: bisphosphonates, bone strength, denosumab, efficacy, postmenopausal osteoporosis
INTRODUCTION
Osteoporosis is a chronic and progressive condition characterised by decreased bone mass and microarchitectural (geometry) deterioration. The resultant bone fragility increases fracture risk. Women are more prone to osteoporosis, as they can lose up to 20% of their bone mass in the 5–7 years following menopause. After menopause, including surgical menopause resulting from bilateral oophorectomy, the fall in the production of oestrogen (the hormone that protects bones) leads to bone loss and increases the risk of fractures.
Over the last 30 years in Singapore, cases of hip fractures have increased fivefold in women aged ≥ 50 years and 1.5-fold in men of the same age group. The age-adjusted hip fractures rates among women aged > 50 years are about 450 out of 100,000.(1) In Singapore, the number of hip fractures per year is projected to increase from 1,300 in 1998 to 9,000 in 2050 because of the ageing population.(2) An analysis of patients who sustained osteoporotic hip fractures in Singapore found that they had a mortality of 20% at two years. Of the survivors, 20% became semi- or fully dependent, and 42% became less or non-ambulant. Only 8% were cared for by chronic healthcare facilities, suggesting that the main social and economic burden was borne by the families of those affected.(2)
Fracture risk is measured by bone strength, which is determined by factors such as bone geometry, porosity and mineral properties,(3) and has different methods of measurement. With technological improvements, our understanding of fracture risk at sites dominated by cortical bones has developed beyond bone density.(4,5)
Antiresorptive agents such as bisphosphonates, calcitonin, oestrogen and oestrogen agonists/antagonists, and the anabolic drug teriparatide are widely available for the treatment of osteoporosis. Bisphosphonates are commonly used but limited by adverse effects such as oesophagitis, gastritis, jaw osteonecrosis and atypical femoral fractures. Bisphosphonates are also contraindicated in patients with creatinine clearance ≤ 35 mL/min.
Denosumab, a novel antiresorptive agent, also inhibits osteoclast-mediated bone resorption but works through a different pathway from bisphosphonates. Denosumab(6) binds with high affinity and specificity to RANKL (receptor activator of nuclear factor-kappa B ligand), prevents it from binding with RANK (receptor activator of nuclear factor-kappa B) receptors on osteoclasts and osteoclast precursors, and hence inhibits the synthesis, activity and lifespan of existing osteoclasts. In turn, denosumab inhibits bone resorption and remodelling, as defined by increases in bone mineral density (BMD) and reduction in porosity(3) at all measured skeletal sites, and decreases in biochemical markers of bone turnover.(7) Denosumab can be the better choice between the two drugs, as it is better tolerated than bisphosphonates and easily administered through two annual injections, which may possibly increase the compliance rate. However, denosumab is slightly more costly than bisphosphonates, and has been hypothesised to increase risk for infection and malignancy.(8)
The different mechanisms by which bisphosphonates and denosumab inhibit bone resorption raise the question of how these two agents compare with respect to efficacy measurements and safety profile. Many randomised controlled trials (RCTs) are currently comparing denosumab or bisphosphonates to a placebo, to evaluate their efficacy. Six systematic reviews compared denosumab with bisphosphonates; two meta-analyses used vertebral fracture as the endpoint,(9,10) while two other reviews used differences in BMD changes(11) and fragility fractures(12) as endpoints in indirect head-to-head comparisons. Two other studies compared denosumab with only specific bisphosphonates (ibandronate and risedronate, or alendronate alone).(13,14)
We performed this systematic review of RCTs that have conducted head-to-head comparisons of denosumab and bisphosphonates to evaluate their efficacy and safety in treating postmenopausal osteoporosis.
METHODS
Databases including PubMed and the Cochrane Central Register of Controlled Trials (CENTRAL) were systematically searched for RCTs that directly compared denosumab and bisphosphonates (Fig. 1). As denosumab is a new drug in the market, a time limit was not set in the search. The following Medical Subject Headings terms were used: ‘denosumab‘ and ‘bisphosphonates‘, and the search was limited to RCT trials that matched the filter. A free text search was also carried out. Citations and reference lists of relevant articles were checked for additional studies. RCTs that studied both denosumab and bisphosphonates in postmenopausal women with osteoporosis and had a Jadad score ≥ 3 were included.
Fig. 1.
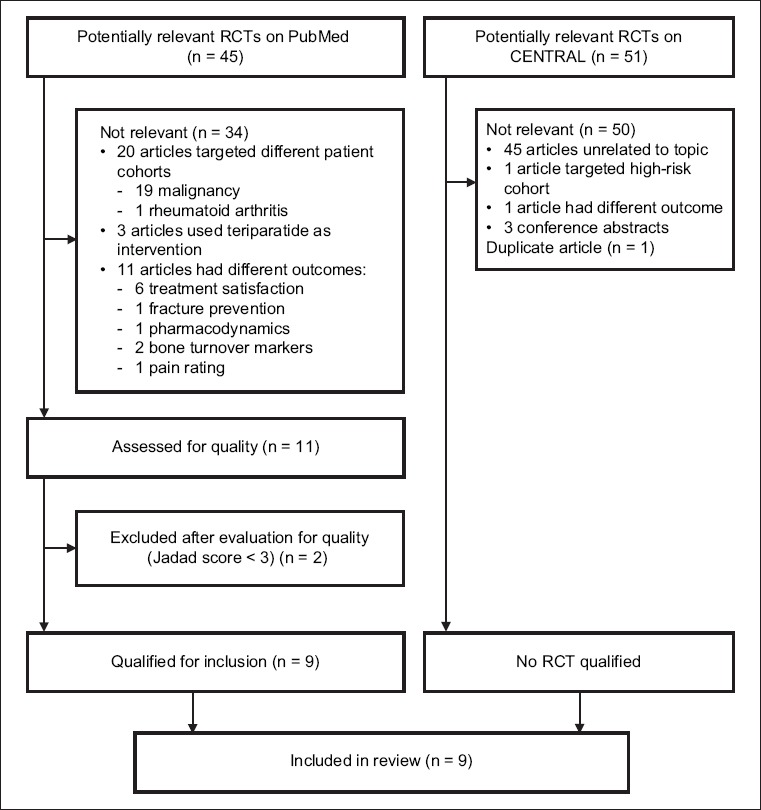
Flow diagram shows the literature screening process. CENTRAL: Cochrane Central Register of Controlled Trials; RCT: randomised controlled trial
The following data was extracted from each included study: (a) study design; (b) study quality based on Jadad score (those with a score < 3 were excluded); (c) study population (i.e. age group, prior bisphosphonate treatment and duration, location and 25-hydroxy vitamin D level); (d) intervention (i.e. dose of denosumab); (e) comparator (i.e. types and doses of bisphosphonates and placebo); (f) follow-up duration; (g) drop-out numbers; (h) primary outcome (i.e. changes in bone mineral density at the hip, lumbar spine, distal radius or distal tibia, and bone porosity and secondary outcome in terms of bone turnover markers and adverse effects); (i) results; and (j) adverse effects. These are summarised in Tables I and II.
Table I.
Adverse events in the selected studies.

Table II.
Summary of the randomised control trials.
RESULTS
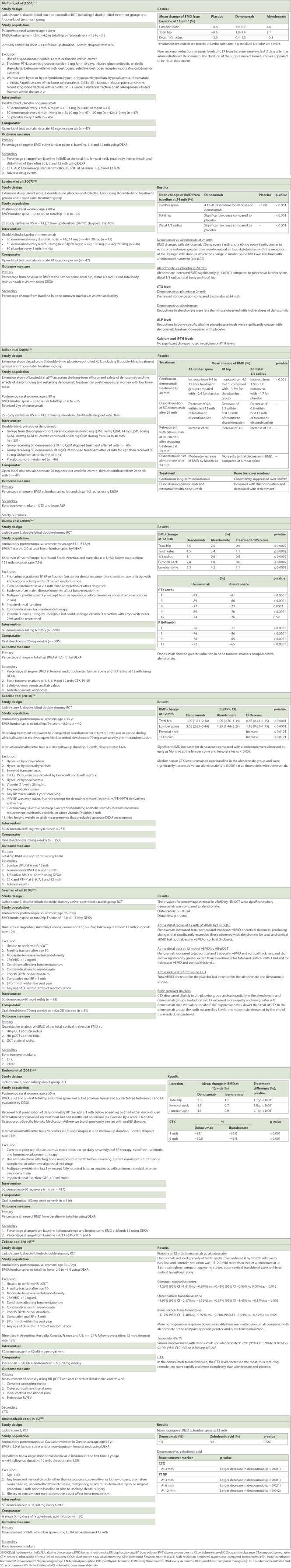
A PubMed search yielded 45 potential articles, of which only nine were relevant and of sufficient quality. A similar search on CENTRAL did not yield any articles that were relevant except for one duplicate study. Two studies with a Jadad score < 3 were excluded: the first study by Roux et al(15) was a post-hoc analysis with a sample population that was highly selective and not representative of the study population. In the other study by Beck et al,(16) the randomisation method was not explained, and withdrawals and dropouts were not fully accounted for. The Jadad score was used to assess the methodological quality of the nine selected papers,(17-25) which were all RCTs. Five studies had a Jadad score of 3,(17-19,22,25) one study had a score of 4(24) and three studies had a score of 5.(20,21,23) The nine studies were based on six different study cohorts: three articles(17-19) assessed outcomes in a single original cohort at different time points and two articles reported different modalities for bone strength measurement for the same study cohort.(23,24)
Among the nine selected RCTs, the study populations ranged from age 60.5 to 68.1 years. Time since menopause, reported for four study cohorts,(17,19-22) varied from 13.1 to 20.4 years. BMD scores, based on dual-energy X-ray absorptiometry (DEXA) results of the hip or lumbar spine, ranged from –2.0 to –4.0. Most of the study cohorts were multicentre and international, with populations from Argentina, Australia, Canada, France, Western Europe, and North and South America, except for those by Lewiecki et al, Miller et al and McClung et al, which involved the same group of participants from 29 study centres confined to the United States. The biggest trial, conducted by Brown et al,(20) comprised 1,189 participants from 86 sites in Western Europe, North and South America, and Australia. The smallest trial was conducted by Anastasilakis et al,(25) with only 64 participants.
Three studies included original patient cohorts who had previously used bisphosphonates.(21,22,25) Intravenous zoledronic acid treatment was given for one year in the study by Anastasilakis et al(25) in 2015 before the participants either received more intravenous zoledronic acid or denosumab. Recknor et al’s study in 2013 included patients who received at least one month of bisphosphonate before screening but had either discontinued bisphosphonate treatment or had insufficient adherence score < 6 on the Osteoporosis-Specific Morisky Medication Adherence Scale.(22) In Kendler et al’s 2010 study,(21) participants were required to have received 70 mg of alendronate-equivalent for at least six months, and all subjects received open-label, branded alendronate 70 mg once weekly for a one-month run-in period prior to randomisation. The other studies recruited patients who had no prior bisphosphonates use.
As their inclusion criteria, three cohort studies(21,22,24,25) required vitamin D level to be screened. Out of the three study cohorts, two(21,24,25) recruited participants with vitamin D level ≥12 ng/mL, while the other(22) recruited participants with vitamin D level > 20 ng/mL. The rest of the three study cohorts(17-19,22,25) did not use vitamin D level as selection criteria.
Eight studies had denosumab with oral bisphosphonates as the active comparator, while one study had intravenous bisphosphonate instead. Three study cohorts(17-19,23,24) included placebo controls. Doses of denosumab were kept at 60 mg subcutaneously every six months except for the extension trials,(17-19) which had varying doses of subcutaneous denosumab 6 mg, 14 mg or 30 mg every three months and 14 mg, 60 mg, 100 mg or 210 mg every six months. Alendronate 70 mg once weekly was the choice of oral bisphosphonate in all trials except for two studies, one by Recknor et al,(22) which used ibandronate 150 mg once per month, and the other by Anastasilakis et al,(25) which used an intravenous infusion of zoledronic acid 5 mg.
The nine studies had various primary outcomes, including percentage change in BMD at the hip(19-22) and lumbar spine(17-19) from baseline using DEXA; and change in total cortical and trabecular BMD at the distal radius and distal tibia(23) from baseline using high-resolution peripheral quantitative computed tomography (HR-pQCT). One study reported change in bone porosity(24) of the cortex and trabecular bone at the distal radius and tibia based on HR-pQCT findings. Measurements at the femoral neck, lumbar spine and lumbar radius were included as secondary outcome measures in different studies. All studies included serum bone turnover markers, serum C-telopeptide of cross-linked collagen (CTX) or procollagen type 1 N-terminal propeptide (P1NP), or both. Two studies(17,25) included calcium and parathyroid hormone levels as the secondary outcomes. The selected studies are outlined in Table II.
Except for two extension studies by Lewiecki et al and Miller et al,(18,19) which conducted 24 and 48 months of follow-up, respectively, the rest of the study cohorts followed up the patients for 12 months. Eight(17-23,25) out of nine papers measured BMD, while one study(24) measured porosity. Of the eight, six used alendronate as the comparator, one used ibandronate(22) and the other used intravenous zoledronic acid.(25) Bone strength was measured at the following sites – total hip, femoral neck, trochanter, lumbar spine, distal radius and distal tibia.
Three papers(17-19) that assessed outcomes at 12, 24 and 48 months for the same cohort had multiple arms with different doses of subcutaneous denosumab. In McClung et al’s(17) report in 2006, results at 12 months showed that the change in BMD from baseline at the lumbar spine was a 3.0%–6.7% increase for denosumab compared to a 4.6% increase for alendronate and a 0.8% decrease for the placebo. The corresponding changes at the total hip were a 1.9%–3.6% increase for denosumab, 2.1% increase for alendronate and -0.6% decrease for the placebo. At the distal one-third radius, there was a 0.4%–1.3% increase for denosumab, and a 0.5% decrease and 2.0% decrease in the alendronate and placebo groups, respectively. These authors did not report if the percentage change in BMD for denosumab was significantly different from that for alendronate.
As reported in 2007,(18) at 24 months, the denosumab group experienced similar bone density changes at all four skeletal sites compared to the alendronate group. Significant improvements in bone density (p < 0.001) from baseline were also reported with alendronate and denosumab at the lumbar spine when compared with a placebo. Although there was a significant increase in BMD with denosumab and alendronate use when compared to a placebo, the difference between the effects of denosumab and alendronate on BMD were not compared in this study. The same cohort was followed up over 48 months in a 2008 extension study,(19) in which alendronate was discontinued at 24 months. When denosumab was continued for 48 months, BMD increased from 9.4% to 11.8% at the lumbar spine compared to a -2.4% decrease for the placebo group (p < 0.001) and 4.0% to 6.1% at the hip compared to a -3.5% decrease for the placebo group (p < 0.001). Patients for whom alendronate was discontinued at 24 months had a decrease in BMD at the lumbar spine, total hip and distal radius. A similar RCT by Brown et al in 2009,(20) conducted on 1,189 patients, showed a statistically significant treatment difference in BMD at 12 months when denosumab was compared with alendronate, with a 0.9% increase at the total hip, 1.1% increase at the trochanter, 0.5% increase at the one-third radius, 0.6% increase at the femoral neck and 1.1% increase at the lumbar spine (p ≤ 0.0002 all sides). Kendler et al(21) demonstrated in 2010 that denosumab increased BMD significantly by 0.85% (p < 0.0001) and 1.18% (p < 0.0001) at the total hip and lumbar spine, respectively, compared to alendronate. Increase in BMD was also observed at the femoral neck and one-third radius at 12 months; however, it was not significant (p < 0.0121).
In the only study that used ibandronate as the comparator,(22) denosumab was significantly better than ibandronate at improving bone density. At 12 months, BMD gain at the total hip was 2.3% (95% confidence interval [CI] 2.0%–2.5%) in the denosumab group and 1.1% (95% CI 0.9%–1.4%) in the ibandronate group (p < 0.001). Corresponding BMD changes were 1.7% and 0.7% (p < 0.001) at the femoral neck, and 4.1% and 2.0% (p < 0.001) at the lumbar spine. Hence, a difference of 1.2% (p < 0.001) was noted at the total hip, 1.0% (p < 0.001) at the femoral neck and 2.1% (p < 0.001) at the lumbar spine.
Anastasilakis et al(25) conducted the only study that used intravenous bisphosphonate in the form of zoledronic acid. They found that there was no significant difference in BMD gain at the lumbosacral spine between the two treatment types, with a mean increase of 4.5% for the denosumab group and 4.4% for the zoledronic acid group (p = 0.560).
Porosity, as opposed to density, was measured in the study by Zebaze et al in 2014.(24) Denosumab was found to reduce porosity at six months and further reduce it at 12 months, a statistically significant 1.5-to-2.0-fold reduction compared to alendronate at all three cortical regions at the distal radius: compact-appearing cortex (p = 0.012), outer cortical transition zone (p = 0.003) and inner cortical transitional zone (p = 0.021). However, there was no significant difference between denosumab and alendronate in terms of trabecular bone volume/total volume.
In the 2010 study by Seeman et al,(23) the percentage increase in volumetric BMD values between the denosumab and alendronate groups, as found using HR-pQCT, was significantly different for total and cortical vBMD at the distal radius (p = 0.024) and distal tibia (p = 0.003), but not for trabecular vBMD and cortical thickness.
Serum CTX was analysed as one of the turnover markers in all studies. The greatest decrease in CTX level was seen with denosumab, as compared to bisphosphonates and a placebo, in all studies. Three studies(20,23,25) also analysed P1NP and showed a reduction in the denosumab group compared to the bisphosphonate group. In the trial conducted by Seeman et al,(23) P1NP suppression was slower than that of CTX in the denosumab group compared to the bisphosphonate group; the nadir occurred by three months and the suppression lessened by the end of the six-month dosing interval. Similar results were also seen with bone-specific alkaline phosphatase. However, calcium and intact parathyroid hormone levels did not show any significant changes.
All studies analysed the adverse effects of denosumab or compared it with a placebo or the bisphosphonate of choice, except for those by Zebaze et al(24) and Anastasilakis et al(25) (Table I). Among the antiresorptive agents, the adverse effects that were of interest were hypocalcaemia, atypical fractures, fragility fractures, osteonecrosis of the jaw, all infections (including fever or influenza-like symptoms), gastrointestinal side effects and dermatological conditions. In one study,(22) the incidence of serious adverse events, including gastrointestinal and respiratory disorders, were significantly higher (p < 0.05) in the denosumab group than the ibandronate group. In another, there was a significantly higher incidence of dyspepsia and osteoarthritis in the alendronate group compared to the denosumab group.(18) There were no significant differences in adverse events for the rest of the studies.
DISCUSSION
In this review, the efficacy and safety of denosumab was compared with that of other oral bisphosphonates in postmenopausal women with low bone density. In four out of six study cohorts,(20-25) denosumab was superior to alendronate and ibandronate in terms of efficacy. They also found that having subcutaneous injections of denosumab every six months leads to good bone mineralisation.
As shown by Zebaze et al,(24) denosumab causes less porosity than alendronate. Denosumab is more effective on cortical bone than trabecular bone. This is important as 80% of bone is cortical and 70% of all appendicular bone loss is cortical(26), occuring mainly by intracortical remodelling. In addition, 80% of fractures in women over 65 years of age are non-vertebral. Given the aforementioned factors, it stands to reason that denosumab is effective in reducing intracortical bone remodelling in women with postmenopausal osteoporosis.
Medication adherence has been shown to be better with denosumab than with bisphosphonates. A study by Kendler et al(27) reported greater treatment satisfaction when patients transitioned to denosumab as compared to switching to a monthly oral bisphosphonate. Another study by Palacios et al(28) showed that participants preferred denosumab to alendronate while on treatment and had more positive perceptions of it, which were also associated with better adherence. Thus, denosumab can be considered as a preferred choice for use in osteoporosis treatment among postmenopausal women.
Denosumab costs approximately SGD 800 per year for two injections, or nearly eight times more than a yearly supply of generic bisphosphonates. Besides the cost, the fear of needles may be a deterring factor as well. These are important considerations that need to be discussed with the patient to allow her to make an informed choice, and to individualise the treatment for postmenopausal osteoporosis. Nevertheless, for those with intolerance or contraindications to bisphosphonate, denosumab is an effective alternative. In terms of bone turnover markers, all studies consistently showed that serum CTX was lowered in the denosumab group. By reducing remodelling, denosumab decreases the microarchitectural deterioration and porosity that cause osteoporosis.
This study was interested in bone strength improvement as a clinical outcome, rather than the patient-centred outcome of fracture risk reduction. We did not consider fracture reduction as the primary outcome measure in patient care. Fracture reduction was compared in a study conducted by Nakamura et al in 2014(29) on Japanese postmenopausal women and men with osteoporosis. Two arms of the study were double-blinded to denosumab and placebo, while the third arm was an open-label alendronate group. Denosumab was shown to significantly reduce the risk of new or worsening vertebral fracture by 65.7%; the incidence of new or worsening vertebral fracture was 3.6% with denosumab and 10.3% with a placebo at 24 months (hazard ratio 0.343; 95% CI 0.194–0.606; p = 0.0001). Another similar study, the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) trial,(30) showed reduction in vertebral and non-vertebral fractures with the use of denosumab. However, these two trials did not directly compare denosumab and alendronate in fracture risk reduction. A recent review conducted by Benjamin et al(31) compared the efficacy of denosumab with that of bisphosphonates in postmenopausal osteoporosis. However, the inclusion criteria was not robust and some potentially significant articles were excluded. In comparison, this review selected more articles based on our inclusion criteria, a Jadad score of 3 and above.
Adverse events, based on incidence rates of malignancy and infections, were not significantly different between the denosumab-treated group and the bisphosphonate-treated group in the studies. There were no reports of osteonecrosis of the jaw, atypical femur fracture or hypocalcaemia following the use of denosumab, and it was not contraindicated in patients with renal impairment.
The studies analysed in this review had some limitations. The study on bone porosity(24) had missing data that was handled by leaving out a large number of patients, so that 41% of the participants were not analysed. However, the baseline characteristics of the three groups were similar. In addition, the follow-up rate was as low as 70% in some of the studies.(20) Better follow-up could have contributed to a better evaluation of the outcomes. Future studies can be conducted with various bisphosphonates such as risedronate (apart from alendronate, ibandronate and zoledronic acid), to study the use of bisphosphonates or denosumab in postmenopausal women in both improving bone strength and reducing fracture. The long-term safety profile of denosumab also needs further evaluation.
In conclusion, denosumab can be used both as a first-line agent and an alternative to bisphosphonate in the treatment of postmenopausal osteoporosis. There is currently insufficient data to show that denosumab is not inferior to bisphosphonates in fracture prevention.
REFERENCES
- 1.Koh LK, Saw SM, Lee JJ, Leong KH, Lee J National Working Committee on Osteoporosis. Hip fracture incidence rates in Singapore 1991-1998. Osteoporos Int. 2001;12:311–8. doi: 10.1007/s001980170121. [DOI] [PubMed] [Google Scholar]
- 2.International Osteoporosis Foundation. The Asian audit:epidemiology, costs and burden of osteoporosis in Asia. 2009. [Accessed May 3, 2016]. Available at: https://www.iofbonehealth.org/sites/default/files/PDFs/Audit%20Asia/Asian_regional_audit_2009.pdf .
- 3.Sornay-Rendu E, Boutroy S, Munoz F, Bouxsein ML. Cortical and trabecular architecture are altered in postmenopausal women with fractures. Osteoporos Int. 2009;20:1291–7. doi: 10.1007/s00198-009-1008-9. [DOI] [PubMed] [Google Scholar]
- 4.Dhainaut A, Hoff M, Syversen U, Haugeberg G. Cortical hand bone porosity and its association with distal radius fracture in middle aged and elderly women. PLoS One. 2013;8:e68405. doi: 10.1371/journal.pone.0068405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zebaze RM, Ghasem-Zadeh A, Bohte A, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women:a cross-sectional study. Lancet. 2010;375:1729–36. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 6.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 7.Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2008;93:2149–57. doi: 10.1210/jc.2007-2814. [DOI] [PubMed] [Google Scholar]
- 8.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 9.Messori A, Fadda V, Maratea D, Trippoli S, Marinai C. Anti-reabsorptive agents in women with osteoporosis:determining statistical equivalence according to evidence-based methods. J Endocrinol Invest. 2014;37:769–73. doi: 10.1007/s40618-014-0124-3. [DOI] [PubMed] [Google Scholar]
- 10.Migliore A, Broccoli S, Massafra U, Cassol M, Frediani B. Ranking antireabsorptive agents to prevent vertebral fractures in postmenopausal osteoporosis by mixed treatment comparison meta-analysis. Eur Rev Med Pharmacol Sci. 2013;17:658–67. [PubMed] [Google Scholar]
- 11.Mandema JW, Zheng J, Libanati C, Perez Ruixo JJ. Time course of bone mineral density changes with denosumab compared with other drugs in postmenopausal osteoporosis:a dose-response-based meta-analysis. J Clin Endocrinol Metab. 2014;99:3746–55. doi: 10.1210/jc.2013-3795. [DOI] [PubMed] [Google Scholar]
- 12.Murad MH, Drake MT, Mullan RJ, et al. Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures:a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2012;97:1871–80. doi: 10.1210/jc.2011-3060. [DOI] [PubMed] [Google Scholar]
- 13.Brown JP, Roux C, Ho PR, et al. Denosumab significantly increases bone mineral density and reduces bone turnover compared with monthly oral ibandronate and risedronate in postmenopausal women who remained at higher risk for fracture despite previous suboptimal treatment with an oral bisphosphonate. Osteoporos Int. 2014;25:1953–61. doi: 10.1007/s00198-014-2692-7. [DOI] [PubMed] [Google Scholar]
- 14.Lin T, Wang C, Cai XZ, et al. Comparison of clinical efficacy and safety between denosumab and alendronate in postmenopausal women with osteoporosis:a meta-analysis. Int J Clin Pract. 2012;66:399–408. doi: 10.1111/j.1742-1241.2011.02806.x. [DOI] [PubMed] [Google Scholar]
- 15.Roux C, Hofbauer LC, Ho PR, et al. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy:efficacy and safety results from a randomized open-label study. Bone. 2014;58:48–54. doi: 10.1016/j.bone.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Beck TJ, Lewiecki EM, Miller PD, et al. Effects of denosumab on the geometry of the proximal femur in postmenopausal women in comparison with alendronate. J Clin Densitom. 2008;11:351–9. doi: 10.1016/j.jocd.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 17.McClung MR, Lewiecki EM, Cohen SB, et al. AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–31. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 18.Lewiecki EM, Miller PD, McClung MR, et al. AMG 162 Bone Loss Study Group. Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res. 2007;22:1832–41. doi: 10.1359/jbmr.070809. [DOI] [PubMed] [Google Scholar]
- 19.Miller PD, Bolognese MA, Lewiecki EM, et al. Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy:a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–9. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass:a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009;24:153–61. doi: 10.1359/jbmr.0809010. [DOI] [PubMed] [Google Scholar]
- 21.Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010;25:72–81. doi: 10.1359/jbmr.090716. [DOI] [PubMed] [Google Scholar]
- 22.Recknor C, Czerwinski E, Bone HG, et al. Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy:a randomized open-label trial. Obstet Gynecol. 2013;121:1291–9. doi: 10.1097/AOG.0b013e318291718c. [DOI] [PubMed] [Google Scholar]
- 23.Seeman E, Delmas PD, Hanley DA, et al. Microarchitectural deterioration of cortical and trabecular bone:differing effects of denosumab and alendronate. J Bone Miner Res. 2010;25:1886–94. doi: 10.1002/jbmr.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zebaze RM, Libanati C, Austin M, et al. Differing effects of denosumab and alendronate on cortical and trabecular bone. Bone. 2014;59:173–9. doi: 10.1016/j.bone.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Anastasilakis AD, Polyzos SA, Gkiomisi A, et al. Denosumab versus zoledronic acid in patients previously treated with zoledronic acid. Osteoporos Int. 2015;26:2521–7. doi: 10.1007/s00198-015-3174-2. [DOI] [PubMed] [Google Scholar]
- 26.Kanis JA, Borgstrom F, Zethraeus N, Johnell O, Oden A, Jonsson B. Intervention thrsholds for osteoporosis in the UK. Bone 2005. 36:22–32. doi: 10.1016/j.bone.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Kendler DL, Macarios D, Lillestol MJ, et al. Influence of patient perceptions and preferences for osteoporosis medication on adherence behavior in the Denosumab Adherence Preference Satisfaction study. Menopause. 2014;21:25–32. doi: 10.1097/GME.0b013e31828f5e5d. [DOI] [PubMed] [Google Scholar]
- 28.Palacios S, Agodoa I, Bonnick S, et al. Treatment satisfaction in postmenopausal women suboptimally adherent to bisphosphonates who transitioned to denosumab compared with risedronate or ibandronate. J Clin Endocrinol Metab. 2015;100:E487–92. doi: 10.1210/jc.2014-3594. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Matsumoto T, Sugitomo T, et al. Clinical Trials Express:fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis:denosumab fracture intervention randomized placebo controlled trial (DIRECT) J Clin Endocrinol Metab. 2014;99:2599–607. doi: 10.1210/jc.2013-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papapoulos S, Chapurlat R, Libanati C, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis:results from the first two years of the FREEDOM extension. J Bone Miner Res. 2012;27:694–701. doi: 10.1002/jbmr.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamin B, Benjamin MA, Swe M, Sugathan S. Review on the comparison of effectiveness between denosumab and bisphosphonates in post-menopausal osteoporosis. Osteoporos Sarcopenia. 2016;2:77–81. doi: 10.1016/j.afos.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]