Abstract
This scientific commentary refers to ‘Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury’, by Agostinone et al. (doi:10.1093/brain/awy142).
This scientific commentary refers to ‘Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury’, by Agostinone et al. (doi:10.1093/brain/awy142).
Neuroscientists studying injuries and diseases of the CNS have largely focused on mechanisms of neuronal dysfunction or death and of axon degeneration; and reciprocally, on developing strategies to maintain cellular function and to promote axon regeneration (Fig. 1). We now know the pathways that lead to cell death or dysfunction in multiple models, and promising treatment strategies for neuroprotection and/or cellular replacement are under development. Because communication within the CNS occurs largely through axodendritic synapses, it is also essential to protect or restore the axons and dendrites of surviving neurons. Axons are commonly compromised in CNS injuries and diseases, and therapies that address physical or chemical barriers to growth, as well as neuron-intrinsic, glial, and inflammatory regulators of axon growth, have emerged in animal studies, with a few potential treatments having entered clinical trials. Dendrites are likewise affected by CNS injury and disease, and several CNS diseases are known to directly affect synaptic structures. Importantly, however, the structural degeneration of dendrites has been little studied, and their regeneration relatively neglected. In this issue of Brain, Agostinone and co-workers help to fill this gap by identifying an intracellular signalling pathway that underlies injury-induced dendritic retraction and growth in the mammalian visual system, and by demonstrating a relatively straightforward strategy to restore dendritic structure and circuitry (Agostinone et al., 2018).
Glossary
Dendritic complexity/morphology: In adult mammals, the shape and location of dendritic arbors can be used to identify neuronal subclasses (including RGCs). Dendritic morphology is commonly assessed using measurements of dendrite length, area, branching, and Sholl analysis, which involves counting the number of intersections between dendrites and concentric rings centred at the soma. Dendritic arbours shrink and lose complexity, or degenerate, in injury and disease. Along with dendritic morphology, circuit function requires the structural and functional integrity of synaptic connections.
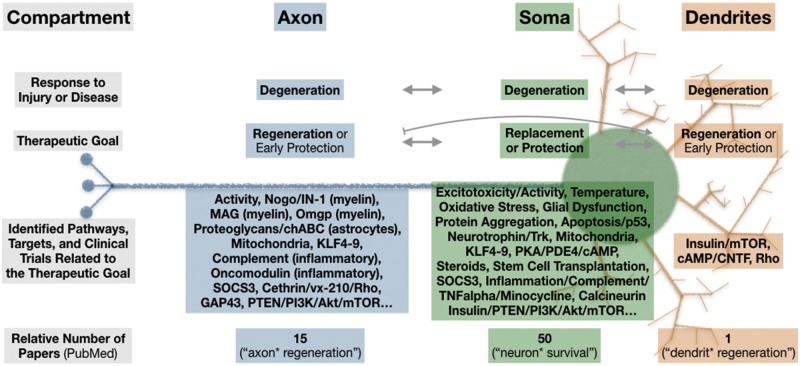
Figure 1.
Little is known about adult mammalian dendrite regeneration. Along with somata and axons, dendrites degenerate in CNS injuries (including optic nerve transection) and diseases (including glaucoma). Despite the obvious importance of dendrites to CNS function, and the interdependence (arrows) between neuronal compartments for both degeneration and regeneration/protection/replacement, much more research has been directed toward axon regeneration and soma replacement/protection. A simplified measure of this disparity is the relative number of publications found on PubMed (search terms in parentheses, asterisk represents wildcard), which reveals that for every one dendrite regeneration paper, there are ∼15 publications on axon regeneration and 50 for neuron survival. In this issue of Brain, Agostinone et al. identify a role for insulin/mTOR signalling in dendrite regeneration. Note common targets, including mTOR, as well as unique targets, and possible antagonism of dendrite growth on axon growth (bar-headed line). This simplified diagram does not include data from non-mammalian or developmental models and is not exhaustive. Axon and dendrite protection strategies likely require very early clinical intervention and are therefore noted but not emphasized here.
Using an in vivo model of traumatic nerve injury, this article provides evidence that the dendritic and somatic atrophy that occur in retinal ganglion cells (RGCs) after injury to the optic nerve can be reversed with insulin acting through the mTOR pathway. The rodent optic nerve injury model closely mimics clinical optic nerve trauma and is a useful model for clinical glaucoma. This model has also helped generate strategies to address other types of CNS injury. The visual system is particularly well suited to investigating neural responses to injury, with the dendrites, somata, and axons of RGCs occupying distinct zones in the retina (inner plexiform layer, retina ganglion cell layer, and nerve fibre layer) and optic nerve, which are relatively accessible CNS structures. Current consensus from optic nerve injury, other glaucoma models, and clinical data is that dendritic retraction is an early event in glaucomatous pathology, beginning before somatic loss, axon pathology, and overt functional changes. In agreement with this literature, Agostinone et al. demonstrate that RGC dendritic complexity is reduced by 3 days after optic nerve transection, as determined using four common histological measures of genetically-labelled dendritic trees: total dendritic length, dendritic field area, number of dendrite branches, and Sholl analysis.
To date, most studies of endogenous and treatment-induced dendritic regrowth have used invertebrate peripheral sensory neuron injury models. Recent experiments in Caenorhabditis elegans described spontaneous dendrite regeneration (Oren-Suissa et al., 2017), while experiments in Drosophila confirmed and extended these findings and have begun to elucidate the underlying mechanism, which appears to involve the PTEN/PI3K/Akt/mTOR pathway and bantam microRNA (Song et al., 2012) and to be activity-dependent (Thompson-Peer et al., 2016). Two recent, intriguing papers make use of in vivo two-photon microscopy to show dendritic growth in the brains of adult rodent models. Zhao et al. (2017) provide 3 h of time-lapse images of a single, normal, mostly morphologically stable dendrite in the mouse motor cortex. They contrast this to images of a single dendrite after nanosurgical ablation, showing a dynamic spontaneous morphological response, although the ultimate result is increased distance between the proximal and distal ends of the severed dendrite. These data hint at an exciting potential for regrowth while remaining in line with other literature suggesting that the summation of endogenous signals present during injury and disease results in dendritic arbour retraction in mammals. Paveliev et al. (2016) present images and quantification of spontaneous and treatment-induced dendritic growth in mouse somatosensory cortex after brain prick injury. However, because therapy with pleiotrophin began immediately after injury, it is difficult to distinguish protective from regenerative effects.
The experiments by Agostinone et al. address regeneration by showing that daily insulin treatment starting 3 days post-injury successfully reverses the loss of dendritic complexity, with multiple dendritic measures returning to normal. Importantly, the timing of insulin intervention to several days post-axotomy—after dendrite retraction has begun—allows the authors to conclude that insulin promotes regrowth of dendrites, as opposed to promoting dendritic stabilization or preventing dendritic loss after optic nerve transection. Given the characterization of dendritic pathology as very early events that occur before clinical presentation in many CNS injuries and diseases, and the resulting timing of intervention, dendritic regrowth is a more relevant therapeutic target than dendritic protection. Another rodent visual system study with similarly delayed treatment identified other dendritic growth strategies [blocking Rho GTPase; elevating ciliary neurotrophic factor (CNTF) plus cAMP], albeit in the presence of a peripheral nervous system graft, and found that while the agents used had effects, the resulting dendritic morphology was abnormal (Drummond et al., 2014). Interestingly, the outcome of the present study appears to be a restoration of normal morphology. This study’s elegant, simple design allows for a conceptual distinction between preservation and restoration, with consequences for treatment identification, characterization, and clinical administration, and should be considered for future studies on mammalian dendrite repair.
Agostinone and colleagues further show, using siRNA-mediated knock-down techniques, that the various insulin-mediated dendritic effects require two separate mTOR signalling complexes, mTORC1/Raptor and mTORC2/Rictor, and that pharmacological inhibition of all mTOR signalling fully blocks the dendritic growth effects of insulin. Yet while this paper appears to be alone in describing a partial mechanism for regrowth of dendrites in the mammalian CNS, the key proteins and pathway identified are not new to us. Insulin or insulin-like growth factor-1 (IGF1) have been reported to be neuroprotective in this and other rodent CNS injury models. Agostinone et al. confirm previous reports with data showing insulin-, and specifically, mTOR-mediated neuroprotection of RGCs at subacute time points (7 and 14 days post-injury), and extend the published time course for this model by reporting significant neuroprotection with reduced effect sizes at more chronic time points (28 and 42 days). These more chronic data are key to any treatment aimed at clinical translation, especially given that most neuroprotective effects in this model have been disappointingly transient, including those of mTOR as shown here, making these data impressive but imperfect. This reinforces the need for neuroprotective treatments (or combinations of treatments) with long-term effects, and the development of improved slow-release or repeated administration strategies.
The PTEN/PI3K/Akt/mTOR pathway mechanism, shown here to be required (mTOR) for insulin-induced dendritic regrowth, is shared not only with that for dendritic development, invertebrate dendritic regeneration, and neuroprotection, but also with a well-described mechanism for CNS axon regeneration (Park et al., 2008). The suggestion of a common pathway for soma protection, axon regeneration, and dendrite regeneration in multiple CNS injury models may have notable implications for clinical therapeutic manipulations of this pathway. The larger literature strongly suggests some interdependence of neuronal compartments for maintenance, degeneration and regrowth. Because intact somata, axons, and dendrites are all essential for neuronal communication and CNS function, a shared pathway is an exciting prospect and should be highlighted as a general target, meriting even more research in this area.
In contrast, other evidence points to an antagonism between dendrite growth and innervation versus axon growth or regeneration (Cull, 1974; Goldberg et al., 2002; Francis and Freeman, 2016). Such antagonism may or may not play a role in the current work by Agostinone et al., but axonal regeneration requires a different injury model and was not investigated here. However, this issue has clear implications for therapeutic development for any disease or injury with axonal pathology, including glaucoma. The present study provides limited evidence of insulin-mediated synapse restoration and improvement in retinal circuit function at 7 days post-injury, which may be indicative of local recovery, but the study did not address perception or behaviour, which would require regeneration of injured axons and which are critical issues for clinical therapies. Future studies will be required to determine the full effect on the larger system, perhaps using a glaucoma model in which, during a therapeutic window that needs to be defined, retinal axons are compromised but still present. In addition, if the potential inhibitory action of dendrites on axons is confirmed in future studies, careful attention to the timing of intervention may improve outcomes.
Although much remains to be explored, the translational potential of insulin treatment for glaucoma is exciting. This potential may even reach beyond glaucoma to other CNS injuries and diseases. Enthusiasm is based on the strong, partially sustained neuroprotection and neuro-restoration after injury, the current clinical use of insulin, the post-injury timing of administration, and the eye drop route of administration, which all seem favourable for translation. Moreover, prying open the figurative window to the vastly under-explored but crucial research topic of dendrite regeneration, and demonstrating the potential for treatment-induced dendrite regrowth, are important steps forward.
References
- Agostinone J, Alacron-Martinez L, Gemlin C, Yu WQ, Wong ROL, Di Polo A. Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain 2018; 141: 1963–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull RE. Role of nerve-muscle contact in maintaining synaptic connections. Exp Brain Res 1974; 20: 307–10. [DOI] [PubMed] [Google Scholar]
- Drummond ES, Rodger J, Penrose M, Robertson D, Harvey AR. Effects of intravitreal injection of a Rho-GTPase inhibitor (BA-210), or CNTF combined with an analogue of cAMP, on the dendritic morphology of regenerating retinal ganglion cells. Restor Neurol Neurosci 2014; 32: 391–402. [DOI] [PubMed] [Google Scholar]
- Francis MM, Freeman MR. Dendrites actively restrain axon outgrowth and regeneration. Proc Natl Acad Sci USA 2016; 113: 5465–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 2002; 296: 1860–4. [DOI] [PubMed] [Google Scholar]
- Oren-Suissa M, Gattegno T, Kravtsov V, Podbilewicz B. Extrinsic repair of injured dendrites as a paradigm for regeneration by fusion in Caenorhabditis elegans. Genetics 2017; 206: 215–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN mTOR pathway. Science 2008; 322: 963–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paveliev M, Fenrich KK, Kislin M, Kuja-Panula J, Kulesskiy E, Varjosalo M, et al. HB-GAM (pleiotrophin) reverses inhibition of neural regeneration by the CNS extracellular matrix. Sci Rep 2016; 6: 33916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Ori-McKenney KM, Zheng Y, Han C, Jan Y, Jan YN. Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes Dev 2012; 26: 1612–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Peer K, DeVault L, Li T, Jan LY, Jan YN. In vivo dendrite regeneration after injury is different from dendrite development. Genes Dev 2016; 30: 1776–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Chen S, Luo Y, Li J, Badea S, Ren C, et al. Time-lapse changes of in vivo injured neuronal substructures in the central nervous system after low energy two-photon nanosurgery. Neural Regen Res 2017; 12: 751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]