Linezolid is administered as a fixed dose to all patients despite evidence of increased exposure and myelosuppression in renal impairment. The objectives of these studies were to assess the risk of thrombocytopenia with standard-dose linezolid in renal impairment and to identify an alternate dosing strategy.
KEYWORDS: glomerular filtration rate, kidney, myelosuppression, oxazolidinones, pharmacokinetics, thrombocytopenia
ABSTRACT
Linezolid is administered as a fixed dose to all patients despite evidence of increased exposure and myelosuppression in renal impairment. The objectives of these studies were to assess the risk of thrombocytopenia with standard-dose linezolid in renal impairment and to identify an alternate dosing strategy. In study 1, data from adult patients receiving linezolid for ≥10 days were retrospectively reviewed to determine the frequency of thrombocytopenia in patients with and without renal impairment. Time-to-event analyses were performed using Cox proportional-hazards models. In study 2, population pharmacokinetic modeling was employed to build covariate-structured models using an independent data set of linezolid concentrations obtained during routine therapeutic drug monitoring (TDM). Monte Carlo simulations were performed to identify linezolid dosing regimens that maximized attainment of therapeutic trough concentrations (2 to 8 mg/liter) across various renal-function groups. Toxicity analysis (study 1) included 341 patients, 133 (39.0%) with renal impairment. Thrombocytopenia occurred more frequently among patients with renal impairment (42.9% versus 16.8%; P < 0.001), and renal impairment was independently associated with this toxicity in multivariable analysis (adjusted hazard ratio [aHR], 2.37; 95% confidence interval [CI], 1.52 to 3.68). Pharmacokinetic analyses (study 2) included 1,309 linezolid concentrations from 603 adult patients. Age, body surface area, and estimated glomerular filtration rate (eGFR) were identified as covariates of linezolid clearance. Linezolid dose reductions improved the probability of achieving optimal exposures in simulated patients with eGFR values of <60 ml/min. Thrombocytopenia occurs more frequently in patients with renal impairment receiving standard linezolid doses. Linezolid dose reduction and trough-based TDM are predicted to mitigate this treatment-limiting toxicity.
INTRODUCTION
Linezolid is an oxazolidinone antibiotic active against multidrug-resistant pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Mycobacterium tuberculosis (1, 2). In clinical trials, linezolid demonstrated good efficacy and safety when used to treat infections requiring <14 days of therapy; however, postmarketing studies soon identified thrombocytopenia with longer durations of treatment (3–5). This myelosuppression limits the utility of linezolid for complicated infections requiring >14 days of therapy.
Approximately 30% to 40% of the linezolid dose is eliminated unchanged in the urine, and renal function is a significant source of interpatient variability in linezolid clearance (CL) (2, 6–8). The use of a fixed dose in renal impairment is based on a single-dose pharmacokinetic (PK) study conducted in uninfected persons with renal impairment, which may not reflect linezolid PK after multiple dosing in infected patients (9). Indeed, reports soon emerged of increased thrombocytopenia in this population with multiple-dose administration (10, 11). This finding has since been replicated in multiple observational studies, most of which have been conducted in Asian populations (12–19).
A clear exposure-response relationship has been identified for thrombocytopenia among patients receiving linezolid (20). Linezolid trough concentrations above 7 to 8 mg/liter have been consistently associated with greater odds of developing thrombocytopenia (21–25). Furthermore, renal impairment has been identified as a significant risk factor for elevated linezolid trough concentrations in real-world clinical studies (26, 27). Thus, observational data indicate that patients with renal impairment are at a higher risk for both supratherapeutic linezolid exposure and toxicity, which suggests that dose modification, or at least more frequent monitoring, may be necessary in this patient population. However, existing toxicity studies are limited largely to Asian patient populations and do not employ time-to-event analyses despite the known impact of duration of therapy on thrombocytopenia risk. The objectives of this analysis were 2-fold: (i) to assess the relationship between renal impairment and thrombocytopenia in patients receiving linezolid and (ii) to identify the impact of patient renal function on linezolid CL using real-world data from routine therapeutic drug monitoring (TDM) in order to determine pragmatic dose modifications for patients with renal impairment.
RESULTS
Toxicity.
A total of 341 patients met the criteria for inclusion in study 1, 133 (39.0%) with and 208 (61.0%) without renal impairment. Aggregate demographic and clinical baseline characteristics of these patients are listed in Table 1 (study 1). Abbreviations and baseline characteristics stratified by estimated glomerular filtration rate (eGFR) are displayed in Table S2 in the supplemental material. Patients with renal impairment (eGFR < 60 ml/min/1.73 m2) were older (59 ± 15 versus 50 ± 17 years; P < 0.001), had a higher body mass index (32.4 ± 12.7 versus 28.9 ± 10.4 kg/m2; P = 0.005), and had a higher degree of comorbidity {Charlson comorbidity index, [median (25th percentile, 75th percentile)], 5 (3, 7) versus 3 (1, 7); P = 0.001}. Baseline laboratory values were more commonly abnormal in patients with renal impairment, including total bilirubin (1.0 ± 1.2 versus 0.7 ± 0.8 mg/dl; P < 0.001), platelet count (231 × 103 ± 109 × 103 versus 311 × 103 ± 167 × 103 cells/μl; P < 0.001), and hemoglobin (9.0 ± 1.7 versus 9.6 ± 1.9 g/dl; P < 0.001). The median (interquartile range [IQR]) duration of linezolid therapy was 21 (14, 32) days in patients with renal impairment and 19 (14, 33) days in patients without renal impairment (P = 0.89).
TABLE 1.
Baseline characteristics of patients included in study 1 and study 2
| Characteristic | Valueb |
|
|---|---|---|
| Study 1 | Study 2 | |
| Demographic | ||
| n | 341 | 603 |
| Age (yr) | 54 (17) | 62 (15) |
| Sex | ||
| Male | 199 (58.4%) | 409 (67.8%) |
| Female | 142 (41.6%) | 194 (32.2%) |
| Ht (cm) | 171 (11) | 171 (9) |
| Wt (kg) | 88 (31) | 76 (19) |
| Body surface area (m2) | 2.02 (0.36) | 1.89 (0.25) |
| Body mass index (kg/m2) | 30.3 (11.5) | 26.1 (6.1) |
| Clinical | ||
| Charlson comorbidity index | 4 (2, 7) | |
| Intensive care unit | 88 (66.2%) | 230 (38.1%) |
| eGFRa (ml/min/1.73 m2) | 78 (43) | 75 (35) |
| eGFR (ml/min) | 88 (49) | 81 (39) |
| Laboratory | ||
| Serum creatinine (mg/dl) | 1.46 (1.43) | 1.31 (1.23) |
| Total bilirubin (mg/dl) | 0.81 (0.99) | |
| Hemoglobin (g/dl) | 9.4 (1.9) | |
| Platelet count (103 cells/μl) | 279.5 (151.8) | |
The eGFR was calculated using the CKDEPI equation.
Data are presented as n (%), mean (standard deviation), or median (interquartile range). Study 1, toxicity; study 2, pharmacokinetics.
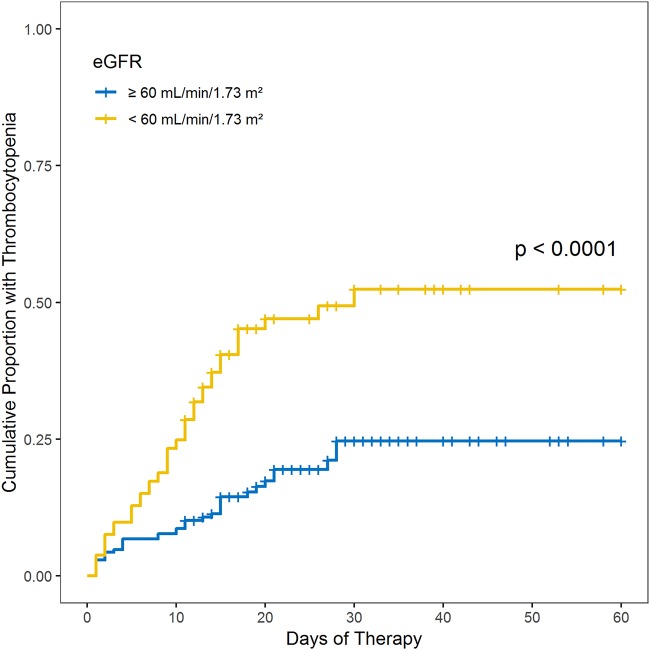
In total, 92 (27.0%) patients developed thrombocytopenia and 47 (13.8%) developed severe thrombocytopenia. Both thrombocytopenia (42.9% versus 16.8%; P < 0.001) and severe thrombocytopenia (19.5% versus 10.1%; P = 0.02) occurred more frequently among patients with renal impairment (eGFR < 60 ml/min/1.73 m2) than among those with normal renal function (eGFR ≥ 60 ml/min/1.73 m2). An unadjusted Kaplan-Meier plot of thrombocytopenia as a function of eGFR is displayed in Fig. 1. Renal impairment was associated with an increased risk of thrombocytopenia in univariable and multivariable Cox proportional-hazard model analyses (Table 2). A low platelet count and high total bilirubin at baseline were independently associated with thrombocytopenia. A sensitivity analysis was conducted using severe thrombocytopenia as the outcome of interest to evaluate whether the effect of renal impairment was limited to mild reductions in platelet count. In this analysis, renal impairment (adjusted hazard ratio [aHR], 1.85; 95% confidence interval [CI], 1.03 to 3.33) and low baseline platelet count (aHR, 1.89; 95% CI, 1.12 to 3.52) were also found to be independently associated with severe thrombocytopenia.
FIG 1.
Unadjusted Kaplan-Meier plot of thrombocytopenia among patients with and without renal impairment treated with linezolid in study 1. Thrombocytopenia was defined as a platelet count of <112.5 × 103 cells/μl (75% lower limit of normal) at any time during therapy for patients with platelet counts above the lower limit of normal (150 × 103 cells/μl) at baseline. For patients with platelet counts below the lower limit of normal at baseline (75 × 103 to 149 × 103 cells/μl), thrombocytopenia was defined as a 25% reduction from the baseline value.
TABLE 2.
Univariable and multivariable Cox proportional-hazards model analysis of thrombocytopenia among patients in study 1
| Variable | Univariable analysis |
Multivariable analysise |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | aHR | 95% CI | P value | |
| Renal impairmenta | 3.05 | 2.00–4.65 | <0.001 | 2.37 | 1.52–3.68 | 0.0001 |
| Age (yr) | 1.01 | 0.99–1.00 | 0.10 | |||
| Male | 0.78 | 0.52–1.18 | 0.20 | |||
| Caucasian | 0.70 | 0.44–1.14 | 0.10 | |||
| Ht (cm) | 0.99 | 0.97–1.00 | 0.09 | 0.99 | 0.97–1.00 | 0.13 |
| Wt (kg) | 1.00 | 0.99–1.00 | 0.70 | |||
| BMIb (kg/m2) | 1.00 | 0.98–1.02 | 0.90 | |||
| Charlson index | 1.08 | 1.03–1.13 | 0.001 | 1.04 | 0.99–1.10 | 0.12 |
| Low baseline platelet countc | 2.88 | 1.87–4.43 | <0.001 | 2.17 | 1.40–3.38 | 0.0006 |
| High baseline total bilirubind | 3.16 | 2.02–4.93 | <0.001 | 2.44 | 1.54–3.88 | 0.0002 |
eGFR, <60 ml/min/1.73 m2.
BMI, body mass index.
Platelet count, <150 × 103 cells/μl.
Total bilirubin, >1.2 mg/dl.
Boldface indicates statistically significant variables independently associated with thrombocytopenia.
Pharmacokinetics.
A total of 603 patients contributed 1,309 linezolid plasma concentrations for the independent PK analysis in study 2. Table 1 provides a summary of the demographic and clinical characteristics of the included patients (study 2). The labeled linezolid dosing regimen (600 mg every 12 h) was used initially in 98.2% of patients through either the oral (45.1%) or intravenous (54.9%) routes. The median (IQR) linezolid peak (Cmax) and trough (Cmin) concentrations were 15.06 (11.85, 20.49) and 5.71 (2.92, 9.84) mg/liter, respectively. Plots of linezolid concentrations versus time since last dose used in model development can be found in Fig. S1 and S2 in the supplemental material.
A 1-compartment model with linear elimination provided the best fit of the observed concentration-time data based on reduction in the objective function value (OFV) and visual inspection of diagnostic plots (see Fig. S3 in the supplemental material). Between-subject variability (BSV) could be estimated for both CL and volume (V), but not the absorption rate constant (Ka), in the base model. Parameter estimates from the base model are provided in Table S3 in the supplemental material. Covariate model building identified eGFR (in milliliters per minute), body surface area (BSA), and age as covariates of linezolid CL and BSA as a covariate of V (see Tables S4 and S5 in the supplemental material). These covariates were not strongly correlated with one another in the population (| r | ≤ 0.4). A table of parameter estimates (see Table S6 in the supplemental material) from the final covariate-structured model, as well as the population (see Fig. S4 and S7 in the supplemental material) and individual (see Fig. S5 and S6 in the supplemental material) level diagnostic plots, are provided in the supplemental material. The mean (standard deviation [SD]) individual empirical Bayesian estimates of linezolid CL and V were 4.95 (2.38) liters/h and 44.0 (11.2) liters, respectively, across all patients, with Ka estimated at 1.40 h−1 in the population. Patients with renal impairment (eGFR ≤ 60 ml/min) had significantly lower mean (SD) individual linezolid CL than those with normal renal function [3.80 (1.94) versus 5.47 (2.38) liters/h; P < 0.001].
The simulated probabilities of achieving a therapeutic Cmin (2 to 8 mg/liter) were 60%, 52%, and 33% with eGFR values of ≥90 ml/min, 60 to 89 ml/min, and <60 ml/min using a standard linezolid dose of 600 mg every 12 h (q12h) (Table 3). The low probability of attaining therapeutic Cmin values in patients with renal impairment is primarily due to an increase in the probability of attaining supratherapeutic Cmin values (>8 mg/liter) at an eGFR of <60 ml/min (53% to 65%) compared to an eGFR of ≥60 ml/min (19% to 40%) at this dosage. An empirical dose reduction to 300 mg every 12 h provided the best balance of safety and efficacy, achieving therapeutic (2 to 8 mg/liter) concentrations in approximately 65% of simulated patients with eGFR of <60 ml/min. An initial dose of 600 mg every 12 h remained optimal in simulated patients with eGFRs of ≥60 ml/min; however, an increased dose of 450 to 600 mg every 8 h may be needed to ensure at least 90% of patients achieve concentrations associated with efficacy (>2 mg/liter) at eGFR of ≥90 ml/min.
TABLE 3.
Simulated probability of attaining linezolid trough concentrations associated with efficacy and toxicity stratified by renal function in study 2
| Linezolid dosage regimen (mg) | Trough level (mg/liter) | % probability at an eGFR (ml/min) ofa: |
|||
|---|---|---|---|---|---|
| <30 | 30–59 | 60–89 | ≥90 | ||
| 600 q8h | >2 | 100 | 100 | 99 | 97 |
| >8 | 90 | 84 | 75 | 52 | |
| 2–8 | 10 | 16 | 24 | 45 | |
| 450 q8h | >2 | 100 | 99 | 98 | 93 |
| >8 | 80 | 71 | 60 | 35 | |
| 2–8 | 20 | 28 | 38 | 58 | |
| 600 q12h | >2 | 98 | 86 | 92 | 79 |
| >8 | 65 | 53 | 40 | 19 | |
| 2–8 | 33 | 33 | 52 | 60 | |
| 300g q8h | >2 | 99 | 97 | 94 | 83 |
| >8 | 58 | 47 | 36 | 15 | |
| 2–8 | 41 | 50 | 58 | 68 | |
| 450 q12h | >2 | 96 | 93 | 87 | 69 |
| >8 | 49 | 37 | 26 | 10 | |
| 2–8 | 47 | 56 | 61 | 59 | |
| 300 q12h | >2 | 90 | 83 | 74 | 50 |
| >8 | 26 | 18 | 10 | 3 | |
| 2–8 | 64 | 65 | 64 | 47 | |
| 600 q24h | >2 | 68 | 54 | 40 | 19 |
| >8 | 13 | 8 | 3 | 1 | |
| 2–8 | 55 | 46 | 37 | 18 | |
| 300 q24h | >2 | 38 | 26 | 16 | 5 |
| >8 | 2 | 1 | 0 | 0 | |
| 2–8 | 36 | 25 | 16 | 5 | |
The eGFR was calculated using the CKDEPI equation.
DISCUSSION
Linezolid is currently administered as a fixed dose of 600 mg every 12 h despite high between-subject variability in exposure, reduced CL in renal impairment, and a known association between high exposure and thrombocytopenia. The only labeled warning for increased linezolid toxicity is with prolonged therapy (>14 days), which limits its utility as an alternative for deep-seated infections (28, 29). Individualized dosing and monitoring of linezolid could potentially overcome this limitation of toxicity on the duration of therapy, especially in patients with renal impairment. This option may be especially cost-effective with oral linezolid through avoidance of complications associated with provision of outpatient parenteral therapy (OPAT) (30, 31).
Renal impairment and end-stage renal disease have been associated with 2- and 3- to 6-fold increases in the odds of developing thrombocytopenia, respectively, in patients receiving linezolid (10, 12, 13, 18, 19). In the present analysis, we demonstrate a 2-fold increase in the risk of thrombocytopenia with an eGFR of <60 ml/min/1.73 m2. A low platelet count and elevated total bilirubin were associated with a similar magnitude of increased thrombocytopenia risk, factors that were also identified in the only other study of linezolid myelosuppression in an American patient population (19).
It is likely that linezolid myelosuppression is exposure dependent and that increased linezolid exposure is the underlying reason for more frequent toxicity in renal impairment. Trough concentration thresholds of 6.3, 6.53, and 8.2 mg/liter have all been associated with 50% odds of thrombocytopenia in various studies by logistic regression (21, 23, 25). Additionally, a pharmacokinetic/toxicodynamic model identified a Cmin of 8.06 mg/liter as the threshold associated with a 50% decrease in platelet precursor cells (24). Other pharmacokinetic/toxicodynamic models have also identified inhibition of platelet formation, rather than immune-mediated or non-immune-mediated platelet destruction, as the most likely primary mechanism leading to thrombocytopenia (32, 33). A study by Tsuji and colleagues assessed both inhibition of platelet formation and stimulation of platelet elimination as explanatory mechanisms of linezolid-induced thrombocytopenia and estimated that thrombocytopenia occurred via an inhibitory mechanism in 97% of patients, using a mixture model (33). Importantly, these studies have also identified renal function to be an important determinant of thrombocytopenia risk (32, 33).
The single-dose PK of linezolid from 18 healthy volunteers with renal impairment enrolled in the phase 1 clinical trial suggested dose adjustment was not needed in this population (6, 7, 9). We opine that a multidose study should have been performed prior to regulatory approval of the agent to appropriately characterize the PK and toxicity potential of linezolid and its major metabolites, which accumulate significantly in renal impairment (9). Multiple postapproval population PK studies using data obtained from infected patients have consistently found renal function to be one of the most important predictors of linezolid CL (8, 23, 32–34). Furthermore, we have previously demonstrated an association between renal impairment (creatinine clearance, <40 ml/min) and a linezolid Cmin of >7 mg/liter (adjusted odds ratio [aOR], 3.05; 95% CI, 2.23 to 4.15), which is consistent with the findings by Morata and colleagues at the same eGFR threshold (aOR, 4.27; 95% CI, 1.36 to 13.43) (26, 27).
The population PK model identified in the present work is consistent with those previously reported in the literature (8, 23, 32–35). Between-subject variability in linezolid CL was approximately 50% following incorporation of patient-specific covariates, which aligns with values of 30% to 60% reported previously (33). Monte Carlo simulations determined that a reduced dose of 300 mg every 12 h is required to decrease the probability of achieving a potentially toxic Cmin of >8 mg/liter among patients with an eGFR of <60 ml/min. This dose reduction is identical to that identified in a PK analysis by Sasaki and colleagues for patients with creatinine clearance of <30 ml/min (32). Notably, the simulated probability of achieving a linezolid Cmin within the therapeutic range of 2 to 8 mg/liter was only 50% to 65% across dosing regimens and renal groups; therefore, renal-dose adjustments alone are unlikely to ensure adequate safety and efficacy of linezolid with prolonged therapy. Additionally, consideration of the severity of a patient’s illness and the MIC of the pathogen is also important with any empirical antibiotic dose reduction. There are known limitations to the assessment of renal function in critically ill patients, and subtherapeutic linezolid exposure has been documented in this population, even with standard empirical dosing (36, 37). Furthermore, while the therapeutic Cmin range of 2 to 8 mg/liter is acceptable for the majority of susceptible Gram-positive pathogens, isolates with MICs of >2 mg/liter may not achieve area under the concentration time curve (AUC)/MIC ratio or time above the MIC (T > MIC) targets at this threshold.
The use of TDM for patients receiving prolonged courses of linezolid can improve patient care and is essential to any intervention evaluating empirical dose reduction in patients with renal impairment (21, 38). We have previously found that TDM-guided dose modification facilitated resolution of thrombocytopenia and safe continuation of therapy in one-third of patients who developed toxicity on standard empirical doses (21). However, expanded use of TDM has the potential to increase total treatment cost due to the cost of the assay and interpretation, which may not be offset by a decrease in drug cost from dose reduction. We suggest that empirical dose reduction and TDM in patients with renal impairment are likely most cost-effective in infections requiring >14 days of therapy because of the delayed onset of myelosuppression in most patients and the cost of alternative treatments for these conditions (e.g., OPAT). Further work to prospectively validate our findings and assess the cost-effectiveness of this intervention is needed.
This study has limitations inherent to the retrospective design and potential for confounding clinical conditions that cannot be excluded. Notably, our data do not definitively link linezolid exposure and toxicity in patients with renal impairment. The separate associations demonstrated here between (i) renal impairment and myelosuppression and (ii) renal impairment and linezolid exposure are both subject to confounding by indications due to the potential effects of severe infection on the risk of myelosuppression and eliminating organ function. We used multivariable models to control for confounding patient and clinical factors; however, the potential for residual confounding exists. Furthermore, reliance on nominal times of administration and sample collection based on standards of care may have influenced the observed between-subject variation in the linezolid PK and led to model misspecification due to deviations from the sampling protocol in clinical practice. However, our model is consistent with previously published models, which increases confidence in the results. Prospective validation for off-label linezolid dosing regimens is a critical next step prior to widespread implementation. Given the high between-subject variability in linezolid PK, TDM is likely necessary to ensure optimal therapy across patient populations. In the interim, reappraisal of the linezolid label by regulatory agencies for a potential warning of increased myelosuppression in renal impairment should be advocated based on the existing body of evidence.
MATERIALS AND METHODS
Data sources.
Two independent sources of data were used in the analyses. Study 1 used clinical and laboratory data from patients treated with linezolid that were obtained retrospectively from a clinical database (DataDirect) maintained by the University of Michigan, Ann Arbor, MI, USA. Codified patient data, including demographics, diagnoses, laboratory measurements, medication administrations, and discharge prescriptions, were obtained from the database. Laboratory and medication data were associated with exact dates and times, reflecting barcode scanning of inpatient medications and laboratory collections and electronic prescribing of discharge prescriptions.
Study 2 used linezolid PK data obtained retrospectively from a database maintained at the Institute of Clinical Pharmacology, Santa Maria della Misericordia University Hospital of Udine, Udine, Italy, reflecting routine clinical TDM in patients treated at Santa Maria della Misericordia University Hospital of Udine. Linezolid TDM was available thrice weekly, with quantification of the plasma concentration performed using a validated high-performance liquid chromatography method with UV detection as described previously (39). The intra- and interday assay coefficients of variation were <10%, and the lower limit of quantification was 0.2 mg/liter. According to the protocol, linezolid Cmax is obtained 30 minutes after the end of a 1-h intravenous infusion and 2 h after oral dosing, with Cmin concentrations obtained 5 min prior to the next dose. The oral suspension is routinely used for dose adjustments, which allows greater flexibility in the dose than the tablet alone. The dates of linezolid treatment and TDM were indexed; however, exact times of medication administration and laboratory collection were not available. All doses were assumed to have been administered exactly as ordered (e.g., every 12 h), with concentrations obtained at the times specified by the protocol on the date of TDM. Demographic data, intensive care unit admission, and serum creatinine measurements were obtained, while other laboratory values were not consistently available in the database. An estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKDEPI) equation for both data sets (40). Data organization and visualization were performed using R (version 3.4.1) and R Studio (version 1.0.153) (41).
Patients.
The DataDirect database was queried to identify inpatient encounters occurring between 1 January 2007 and 31 August 2018 for study 1. Adult patients (≥18 years) treated with intravenous and/or oral linezolid were eligible for inclusion in toxicity analyses. Patients were excluded if they received <10 days of total linezolid therapy, defined as the sum of inpatient days and any additional days of therapy prescribed on discharge. Other reasons for exclusion included (i) a baseline absolute neutrophil count of <500 cells/μl, (ii) baseline total bilirubin at >5 times the upper limit of normal, (iii) baseline platelet count of <75 × 103 cells/μl, (iv) baseline hemoglobin of <6.8 g/dl for males or 6 g/dl for females, (v) missing baseline height/weight or laboratory data, and (vi) no repeat platelet count after day 10. Baseline was defined as the time of linezolid initiation.
For the PK analyses in study 2, adult patients (≥18 years old) treated with intravenous and/or oral linezolid at Santa Maria della Misericordia University Hospital of Udine with at least one linezolid plasma concentration measured were eligible for inclusion. Patients were excluded if renal replacement therapy was provided during linezolid treatment or if covariate data were missing or incomplete. Primary covariates of interest included demographics (age and sex), body size (height, weight, and BSA), serum creatinine, and intensive care unit admission.
Toxicity analyses.
The outcome of interest in study 1 was the frequency of thrombocytopenia among patients with and without renal impairment. Renal impairment was defined as an eGFR of <60 ml/min/1.73 m2 to reflect the threshold at which drug doses are generally adjusted and the cutoff for reporting exact values of eGFR by clinical laboratories. Thrombocytopenia was defined as a platelet count of <112.5 × 103 cells/μl (75% lower limit of normal) at any time during therapy for patients with platelet counts at or above the lower limit of normal (≥150 × 103 cells/μl) at baseline. For patients with low platelet counts at baseline (75 × 103 to 149 × 103 cells/μl), thrombocytopenia was defined as a 25% reduction from the baseline value. Severe thrombocytopenia was defined as a platelet count of <75 × 103 cells/μl for patients with a normal baseline and <50 × 103 cells/μl for those with low baseline platelet counts, respectively. Patients were classified as having the outcome of thrombocytopenia or severe thrombocytopenia at the first occurrence of a measured value below the threshold and censored thereafter.
Descriptive statistics were calculated using Student's t test for continuous data and a chi-squared or Fisher exact test for categorical data as appropriate. The frequency of thrombocytopenia between renal groups was assessed using Student's t test. The time to development of thrombocytopenia between patients with and without renal impairment was assessed visually using Kaplan-Meier curves and evaluated statistically using the log rank test. Multivariable Cox regression analysis was used to control for variables with the potential to confound the effect of renal impairment on thrombocytopenia. Variables with a univariable P value of <0.20 were eligible for inclusion in the final multivariable Cox regression model. The final multivariable model was selected using forward selection and backward elimination procedures based on Akaike’s information criterion (AIC). A sensitivity analysis was performed by repeating these analyses with severe thrombocytopenia, rather than thrombocytopenia, as the outcome of interest.
Pharmacokinetic analyses.
Population PK analyses were performed in study 2 using nonlinear mixed-effects modeling in NONMEM (version 7.4.3; Icon Development Solutions, Ellicott City, MD, USA) implemented using Pirana (version 2.9.6). The first-order conditional estimation method with interaction was used throughout the model development process. Diagnostic plots were created in R using R Studio.
One- and two-compartment structural models with linear, Michaelis-Menten, mixed linear–Michaelis-Menten, and capacity-limited elimination were considered based on inspection of the data and review of the literature (8, 35, 42). Continuous time, rather than time since last dose, was used as the independent variable. Fixed effects were parameterized in terms of CL and V. Between-subject variability of deviation from the typical values of CL and V (η) was modeled using an exponential function, and residual variability was assessed using additive, proportional, and additive plus proportional error models. The base model was selected based on the −2-log-unit likelihood OFV and visual inspection of diagnostic plots.
Exploratory analysis was performed prior to covariate modeling to examine the distribution of covariates in the population, as well as the correlation between covariates of interest. Following identification of the base model, relationships between individual empirical Bayesian estimates of PK parameters and patient covariates were examined visually. Continuous covariates were centered on their median values in the population, with covariate-parameter relationships described using linear, power, or exponential functional forms. Covariates were tested using a forward selection process with a threshold decrease in OFV of 3.84 (P < 0.05; 1 df) for retention in the model. Nonnested models were compared using AIC. Following identification of the full covariate model, a backward elimination procedure was applied with a threshold OFV increase of 10.83 (P < 0.001; 1 df) for covariate retention in the final model. Standard diagnostic plots were created to evaluate the final covariate-structured model, including population and individual observed-versus-predicted concentrations, conditional weighted and individual weighted residuals versus predicted concentration and time since last dose, and histograms of the individual η estimates. The precision of estimated parameters was assessed using the relative standard error in estimation obtained from the covariance step. A prediction-corrected visual predictive check was performed using the final covariate-structured model and a 90% prediction interval.
Monte Carlo simulations were performed using the final covariate-structured model to identify pragmatic potential linezolid dose adjustments for renal impairment. Simulations were performed in NONMEM with the covariate sets from individual patients included in model building serving as a template for 500 simulated subjects receiving intravenous dosing and 500 simulated subjects receiving oral dosing. Thus, the total number of simulated subjects was equal to 1,000 times the number of subjects used in model building. Total daily doses of 300 to 1,800 mg/day were evaluated in increments between 150 and 300 mg in order to reflect pragmatic dose adjustments using the available formulations. Target Cmin values of >2 mg/liter for efficacy and >8 mg/liter for toxicity were used to reflect current practice in centers with TDM (27). A linezolid Cmin of >8 mg/liter has been associated with ≥50% odds of thrombocytopenia or decrease in platelet precursor cells in prior analyses (21, 23–25). Linezolid activity is best described by the pharmacokinetic/pharmacodynamic parameter T > MIC or AUC/MIC; however, a surrogate Cmin value of >2 mg/liter is often used in practice and has been associated with >80% probability of bacterial eradication (20, 25). Probabilities of target attainment were calculated for each dosing level among simulated subjects with eGFR values of <30 ml/min, 30 to 59 ml/min, 60 to 89 ml/min, and ≥90 ml/min.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Twisha S. Patel for her contributions to the design of the retrospective observational toxicity analyses.
This work was supported by startup funds from the University of Michigan College of Pharmacy to M.P.P.
F.P. participated in a speaker bureau for Angelini, Basilea Pharmaceutica, Gilead, Hikma, Merck Sharp & Dohme, Nordic Pharma, Pfizer, and Sanofi Aventis and in an advisory board for Angelini, Basilea Pharmaceutica, Gilead, Merck Sharp & Dohme, Nordic Pharma, and Pfizer. M.P.P. reports grants from Merck, personal fees from Paratek, and personal fees from Shinogi, all outside the submitted work. We have no other actual or potential conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00605-19.
REFERENCES
- 1.Livermore DM. 2003. Linezolid in vitro: mechanism and antibacterial spectrum. J Antimicrob Chemother 51 Suppl 2:ii9–ii16. doi: 10.1093/jac/dkg249. [DOI] [PubMed] [Google Scholar]
- 2.Pfizer. 2018. Zyvox package insert. Pfizer, Philadelphia, PA.
- 3.Stevens DL, Smith LG, Bruss JB, McConnell-Martin MA, Duvall SE, Todd WM, Hafkin B. 2000. Randomized comparison of linezolid (PNU-100766) versus oxacillin-dicloxacillin for treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother 44:3408–3413. doi: 10.1128/aac.44.12.3408-3413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinstein E, Cammarata S, Oliphant T, Wunderink R, Linezolid Nosocomial Pneumonia Study G. 2001. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis 32:402–412. doi: 10.1086/318486. [DOI] [PubMed] [Google Scholar]
- 5.Gerson SL, Kaplan SL, Bruss JB, Le V, Arellano FM, Hafkin B, Kuter DJ. 2002. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother 46:2723–2726. doi: 10.1128/aac.46.8.2723-2726.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slatter JG, Stalker DJ, Feenstra KL, Welshman IR, Bruss JB, Sams JP, Johnson MG, Sanders PE, Hauer MJ, Fagerness PE, Stryd RP, Peng GW, Shobe EM. 2001. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14)C]linezolid to healthy human subjects. Drug Metab Dispos 29:1136–1145. [PubMed] [Google Scholar]
- 7.Stalker DJ, Jungbluth GL, Hopkins NK, Batts DH. 2003. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J Antimicrob Chemother 51:1239–1246. doi: 10.1093/jac/dkg180. [DOI] [PubMed] [Google Scholar]
- 8.Meagher AK, Forrest A, Rayner CR, Birmingham MC, Schentag JJ. 2003. Population pharmacokinetics of linezolid in patients treated in a compassionate-use program. Antimicrob Agents Chemother 47:548–553. doi: 10.1128/AAC.47.2.548-553.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brier ME, Stalker DJ, Aronoff GR, Batts DH, Ryan KK, O'Grady M, Hopkins NK, Jungbluth GL. 2003. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob Agents Chemother 47:2775–2780. doi: 10.1128/aac.47.9.2775-2780.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu VC, Wang YT, Wang CY, Tsai IJ, Wu KD, Hwang JJ, Hsueh PR. 2006. High frequency of linezolid-associated thrombocytopenia and anemia among patients with end-stage renal disease. Clin Infect Dis 42:66–72. doi: 10.1086/498509. [DOI] [PubMed] [Google Scholar]
- 11.Lin YH, Wu VC, Tsai IJ, Ho YL, Hwang JJ, Tsau YK, Wu CY, Wu KD, Hsueh PR. 2006. High frequency of linezolid-associated thrombocytopenia among patients with renal insufficiency. Int J Antimicrob Agents 28:345–351. doi: 10.1016/j.ijantimicag.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto K, Takeda Y, Takeshita A, Fukunaga N, Shigemi A, Yaji K, Shimodozono Y, Yamada K, Ikawa K, Morikawa N. 2009. Renal function as a predictor of linezolid-induced thrombocytopenia. Int J Antimicrob Agents 33:98–99. doi: 10.1016/j.ijantimicag.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi Y, Takesue Y, Nakajima K, Ichiki K, Tsuchida T, Tatsumi S, Ishihara M, Ikeuchi H, Uchino M. 2011. Risk factors associated with the development of thrombocytopenia in patients who received linezolid therapy. J Infect Chemother 17:382–387. doi: 10.1007/s10156-010-0182-1. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Guo DH, Cao X, Cai Y, Xu Y, Zhu M, Ma L. 2012. Risk factors for thrombocytopenia in adult Chinese patients receiving linezolid therapy. Curr Ther Res Clin Exp 73:195–206. doi: 10.1016/j.curtheres.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirano R, Sakamoto Y, Tachibana N, Ohnishi M. 2014. Retrospective analysis of the risk factors for linezolid-induced thrombocytopenia in adult Japanese patients. Int J Clin Pharm 36:795–799. doi: 10.1007/s11096-014-9961-6. [DOI] [PubMed] [Google Scholar]
- 16.Natsumoto B, Yokota K, Omata F, Furukawa K. 2014. Risk factors for linezolid-associated thrombocytopenia in adult patients. Infection 42:1007–1012. doi: 10.1007/s15010-014-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato H, Hamada Y, Hagihara M, Hirai J, Yamagishi Y, Matsuura K, Mikamo H. 2015. Bicytopenia, especially thrombocytopenia in hemodialysis and non-hemodialysis patients treated with linezolid therapy. J Infect Chemother 21:707–712. doi: 10.1016/j.jiac.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Hanai Y, Matsuo K, Ogawa M, Higashi A, Kimura I, Hirayama S, Kosugi T, Nishizawa K, Yoshio T. 2016. A retrospective study of the risk factors for linezolid-induced thrombocytopenia and anemia. J Infect Chemother 22:536–542. doi: 10.1016/j.jiac.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Rabon AD, Fisher JP, MacVane SH. 2019. Incidence and risk factors for development of thrombocytopenia in patients treated with linezolid for 7 days or greater. Ann Pharmacother 53:220–221. doi: 10.1177/1060028018807939. [DOI] [PubMed] [Google Scholar]
- 20.Cattaneo D, Alffenaar JW, Neely M. 2016. Drug monitoring and individual dose optimization of antimicrobial drugs: oxazolidinones. Expert Opin Drug Metab Toxicol 12:533–544. doi: 10.1517/17425255.2016.1166204. [DOI] [PubMed] [Google Scholar]
- 21.Pea F, Viale P, Cojutti P, Del Pin B, Zamparini E, Furlanut M. 2012. Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J Antimicrob Chemother 67:2034–2042. doi: 10.1093/jac/dks153. [DOI] [PubMed] [Google Scholar]
- 22.Cattaneo D, Orlando G, Cozzi V, Cordier L, Baldelli S, Merli S, Fucile S, Gulisano C, Rizzardini G, Clementi E. 2013. Linezolid plasma concentrations and occurrence of drug-related haematological toxicity in patients with gram-positive infections. Int J Antimicrob Agents 41:586–589. doi: 10.1016/j.ijantimicag.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto K, Shigemi A, Takeshita A, Watanabe E, Yokoyama Y, Ikawa K, Morikawa N, Takeda Y. 2014. Analysis of thrombocytopenic effects and population pharmacokinetics of linezolid: a dosage strategy according to the trough concentration target and renal function in adult patients. Int J Antimicrob Agents 44:242–247. doi: 10.1016/j.ijantimicag.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Boak LM, Rayner CR, Grayson ML, Paterson DL, Spelman D, Khumra S, Capitano B, Forrest A, Li J, Nation RL, Bulitta JB. 2014. Clinical population pharmacokinetics and toxicodynamics of linezolid. Antimicrob Agents Chemother 58:2334–2343. doi: 10.1128/AAC.01885-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong HY, Xie J, Chen LH, Wang TT, Zhao YR, Dong YL. 2014. Therapeutic drug monitoring and receiver operating characteristic curve prediction may reduce the development of linezolid-associated thrombocytopenia in critically ill patients. Eur J Clin Microbiol Infect Dis 33:1029–1035. doi: 10.1007/s10096-013-2041-3. [DOI] [PubMed] [Google Scholar]
- 26.Morata L, De la Calle C, Gómez-Cerquera JM, Manzanedo L, Casals G, Brunet M, Cobos-Trigueros N, Martínez JA, Mensa J, Soriano A. 2016. Risk factors associated with high linezolid trough plasma concentrations. Expert Opin Pharmacother 17:1183–1187. doi: 10.1080/14656566.2016.1182154. [DOI] [PubMed] [Google Scholar]
- 27.Pea F, Cojutti PG, Baraldo M. 2017. A 10-Year experience of therapeutic drug monitoring (TDM) of linezolid in a hospital-wide population of patients receiving conventional dosing: is there enough evidence for suggesting TDM in the majority of patients? Basic Clin Pharmacol Toxicol 121:303–308. doi: 10.1111/bcpt.12797. [DOI] [PubMed] [Google Scholar]
- 28.Spellberg B, Lipsky BA. 2012. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis 54:393–407. doi: 10.1093/cid/cir842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iversen K, Ihlemann N, Gill SU, Madsen T, Elming H, Jensen KT, Bruun NE, Hofsten DE, Fursted K, Christensen JJ, Schultz M, Klein CF, Fosboll EL, Rosenvinge F, Schonheyder HC, Kober L, Torp-Pedersen C, Helweg-Larsen J, Tonder N, Moser C, Bundgaard H. 2019. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 308:415–424. doi: 10.1056/NEJMoa1808312. [DOI] [PubMed] [Google Scholar]
- 30.Pulcini C, Couadau T, Bernard E, Lorthat-Jacob A, Bauer T, Cua E, Mondain V, Chichmanian RM, Dellamonica P, Roger PM. 2008. Adverse effects of parenteral antimicrobial therapy for chronic bone infections. Eur J Clin Microbiol Infect Dis 27:1227–1232. doi: 10.1007/s10096-008-0570-y. [DOI] [PubMed] [Google Scholar]
- 31.Shrestha NK, Shrestha J, Everett A, Carroll D, Gordon SM, Butler RS, Rehm SJ. 2016. Vascular access complications during outpatient parenteral antimicrobial therapy at home: a retrospective cohort study. J Antimicrob Chemother 71:506–512. doi: 10.1093/jac/dkv344. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki T, Takane H, Ogawa K, Isagawa S, Hirota T, Higuchi S, Horii T, Otsubo K, Ieiri I. 2011. Population pharmacokinetic and pharmacodynamic analysis of linezolid and a hematologic side effect, thrombocytopenia, in Japanese patients. Antimicrob Agents Chemother 55:1867–1873. doi: 10.1128/AAC.01185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuji Y, Holford NHG, Kasai H, Ogami C, Heo YA, Higashi Y, Mizoguchi A, To H, Yamamoto Y. 2017. Population pharmacokinetics and pharmacodynamics of linezolid-induced thrombocytopenia in hospitalized patients. Br J Clin Pharmacol 83:1758–1772. doi: 10.1111/bcp.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuji Y, Yukawa E, Hiraki Y, Matsumoto K, Mizoguchi A, Morita K, Kamimura H, Karube Y, To H. 2013. Population pharmacokinetic analysis of linezolid in low body weight patients with renal dysfunction. J Clin Pharmacol 53:967–973. doi: 10.1002/jcph.133. [DOI] [PubMed] [Google Scholar]
- 35.Abe S, Chiba K, Cirincione B, Grasela TH, Ito K, Suwa T. 2009. Population pharmacokinetic analysis of linezolid in patients with infectious disease: application to lower body weight and elderly patients. J Clin Pharmacol 49:1071–1078. doi: 10.1177/0091270009337947. [DOI] [PubMed] [Google Scholar]
- 36.Morata L, Cuesta M, Rojas JF, Rodriguez S, Brunet M, Casals G, Cobos N, Hernandez C, Martinez JA, Mensa J, Soriano A. 2013. Risk factors for a low linezolid trough plasma concentration in acute infections. Antimicrob Agents Chemother 57:1913–1917. doi: 10.1128/AAC.01694-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zoller M, Maier B, Hornuss C, Neugebauer C, Dobbeler G, Nagel D, Holdt LM, Bruegel M, Weig T, Grabein B, Frey L, Teupser D, Vogeser M, Zander J. 2014. Variability of linezolid concentrations after standard dosing in critically ill patients: a prospective observational study. Crit Care 18:R148. doi: 10.1186/cc13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pea F, Cojutti P, Dose L, Baraldo M. 2016. A 1 year retrospective audit of quality indicators of clinical pharmacological advice for personalized linezolid dosing: one stone for two birds? Br J Clin Pharmacol 81:341–348. doi: 10.1111/bcp.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng GW, Stryd RP, Murata S, Igarashi M, Chiba K, Aoyama H, Aoyama M, Zenki T, Ozawa N. 1999. Determination of linezolid in plasma by reversed-phase high-performance liquid chromatography. J Pharm Biomed Anal 20:65–73. doi: 10.1016/S0731-7085(98)00310-0. [DOI] [PubMed] [Google Scholar]
- 40.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Chronic Kidney Disease Epidemiology Collaboration. 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.R Core Team. 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- 42.Plock N, Buerger C, Joukhadar C, Kljucar S, Kloft C. 2007. Does linezolid inhibit its own metabolism? Population pharmacokinetics as a tool to explain the observed nonlinearity in both healthy volunteers and septic patients. Drug Metab Dispos 35:1816–1823. doi: 10.1124/dmd.106.013755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.