Due to the natural resistance of nontuberculous mycobacteria (NTM) against multiple antibiotics, treatment of infections caused by them is often long-course and less successful. The main objective of our study was the evaluation of in vitro susceptibility of 209 isolates consisting of different NTM species against bedaquiline and delamanid. Furthermore, reference strains of 33 rapidly growing mycobacterium (RGM) species and 19 slowly growing mycobacterium (SGM) species were also tested.
KEYWORDS: bedaquiline, delamanid, nontuberculous mycobacteria, susceptibility
ABSTRACT
Due to the natural resistance of nontuberculous mycobacteria (NTM) against multiple antibiotics, treatment of infections caused by them is often long-course and less successful. The main objective of our study was the evaluation of in vitro susceptibility of 209 isolates consisting of different NTM species against bedaquiline and delamanid. Furthermore, reference strains of 33 rapidly growing mycobacterium (RGM) species and 19 slowly growing mycobacterium (SGM) species were also tested. Bedaquiline exhibited strong in vitro activity against both reference strains and clinical isolates of different SGM species, as the majority of the strains demonstrated MICs far below 1 μg/ml. Bedaquiline (Bdq) also exhibited potent activity against the recruited RGM species. A total of 29 out of 33 reference RGM strains had MICs lower than 1 μg/ml. According to the MIC distributions, the tentative epidemiological cutoff (ECOFF) values, and the pharmacokinetic data, a uniform breakpoint of 2 μg/ml was temporarily proposed for NTM’s Bdq susceptibility testing. Although delamanid (Dlm) was not active against most of the tested reference strains and clinical isolates of RGM species, it exhibited highly variable antimicrobial activities against the 19 tested SGM species. Eleven species had MICs lower than 0.25 μg/ml, and 7 species had MICs greater than 32 μg/ml. Large numbers of M. kansasii (39/45) and M. gordonae (6/10) clinical isolates had MICs of ≤0.125 μg/ml. This study demonstrated that bedaquiline had potent activity against different NTM species in vitro, and delamanid had moderate activity against certain species of SGM. The data provided important insights on the possible clinical application of Bdq and Dlm to treat NTM infections.
INTRODUCTION
Nontuberculous mycobacteria (NTM) are a group of organisms within the genus Mycobacterium, except for the Mycobacter tuberculosis complex and Mycobacter leprae, which can cause tuberculosis-like diseases in the lung or other organs (1). During the past decades, the prevalence of lung infections caused by NTM has increased globally and has even surpassed tuberculosis (TB) in some countries; thus, it emerged as a new threat to human health (2–5). Regimens targeting the most prevalent NTM species have been recommended by some professional institutions but are usually weakly evidence based. Therefore, the treatment outcomes of NTM infections are often unsatisfactory with high chances of failure, relapse, and default (6–8). In addition, treatment options after failure of the first-line drug regimens are scarce, and the treatment with alternative drugs combinations are yet to be established. Hence, the continuous screening of new anti-NTM medications is necessary.
Bedaquiline (Bdq) and delamanid (Dlm) are two new anti-TB drugs that have recently been demonstrated to have strong efficacies against multiple drug-resistant tuberculosis (MDR-TB) members, both in vitro and in vivo. Based on the genotypic and phenotypic similarities within the genus Mycobacterium, those two drugs might also have bacterial inhibitory activities against different NTM species, although only limited data are available to justify this deduction. Bdq is an oral antimycobacterial agent belonging to a new class of drugs called diarylquinolines. It has low equivalent MICs for some NTM species (2, 9, 10), and a pilot study using a Bdq-containing regimen to treat a few patients infected with Mycobacterium avium complex (MAC) or Mycobacterium abscessus favored the potential clinical and microbiological activity of Bdq (11). Dlm, a new agent derived from the nitro-dihydro-imidazooxazole class of compounds that inhibits mycolic acid synthesis, has strong in vitro activity against M. tuberculosis, including MDR-TB strains (12–14). Limited studies have demonstrated that Dlm harbors strong inhibitory activities against clinical isolates of MAC (15). Understanding the activities of Dlm against different NTM species also remains limited.
To better understand the efficacy of Bdq and Dlm against different NTM species, we determined the MICs of 52 NTM reference strains and 209 NTM isolates collected in Beijing, China. In addition, we attempted to define the tentative epidemiological cutoff (ECOFF) values for the most prevalent NTM species, which is an important factor in setting up the breakpoint for a drug susceptibility testing. Furthermore, we investigated the potential molecular mechanism of Dlm resistance among different NTM species. The aim of our study was to facilitate drug selection for treating NTM infections, especially for the species with high prevalence.
RESULTS
MICs and ECOFFs of Bdq against NTM strains.
The MICs of the 52 reference strains against Bdq are presented in Table 1. Bdq demonstrated uniform strong antibacterial activity against almost all of the tested SGM species, with MICs far below 1 μg/ml, except Mycobacterium rhodesiae (MIC, 1 μg/ml), Mycobacterium xenopi (MIC, 2 μg/ml), and Mycobacterium celatum (MIC, >32 μg/ml). Furthermore, Bdq also exhibited very potent in vitro activity against the recruited RGM reference strains; 29 out of 33 RGM species had MICs of ≤1 μg/ml, and 18 of them had MICs of ≤0.25 μg/ml. Among the four leftover RGM species, one had MICs of 2 μg/ml, and the other three had MICs of >2 μg/ml.
TABLE 1.
MICs of Bdq and Dlm against reference strains of 33 RGM species and 19 SGM species
| Strain by Mycobacterium type | Species (strain) | MIC (μg/ml) by antimicrobial agent |
|
|---|---|---|---|
| Bdq | Dlm | ||
| RGM | |||
| ATCC 19977 | Mycobacterium abscessus | 0.5 | >32 |
| ATCC 27406 | Mycobacterium agri | 0.016 | 1 |
| ATCC 27280 | Mycobacterium aichiense | 0.031 | 8 |
| ATCC 23366 | Mycobacterium aurum | 0.25 | >32 |
| ATCC 33464 | Mycobacterium austroafricanum | 0.125 | >32 |
| ATCC 14472 | Mycobacterium chelonae | 2 | >32 |
| ATCC 19627 | Mycobacterium chitae | 1 | >32 |
| ATCC 27278 | Mycobacterium chubuense | 0.5 | >32 |
| DSM 44829 | Mycobacterium cosmeticum | 1 | >32 |
| ATCC 19340 | Mycobacterium diernhoferi | <0.008 | >32 |
| ATCC 35219 | Mycobacterium fallax | <0.008 | >32 |
| ATCC 35753 | Mycobacterium farcinogenes | <0.008 | >32 |
| ATCC 14474 | Mycobacterium flavescens | >32 | >32 |
| ATCC 6841 | Mycobacterium fortuitum | 0.5 | >32 |
| ATCC 27726 | Mycobacterium gadium | 1 | <0.125 |
| ATCC 43909 | Mycobacterium gilvum | 0.016 | >32 |
| ATCC BAA-955 | Mycobacterium goodii | 1 | >32 |
| DSM 44124 | Mycobacterium mucogenicum | 0.25 | >32 |
| ATCC 25795 | Mycobacterium neoaurum | 0.5 | >32 |
| ATCC 27023 | Mycobacterium obuense | <0.008 | 1 |
| ATCC 19686 | Mycobacterium parafortuitum | 1 | >32 |
| DSM 43271 | Mycobacterium peregrinum | 0.125 | >32 |
| ATCC 11758 | Mycobacterium phlei | 0.125 | >32 |
| ATCC 33776 | Mycobacterium porcinum | 0.5 | >32 |
| ATCC 35154 | Mycobacterium pulveris | 16 | <0.125 |
| ATCC 35796 | Mycobacterium senegalense | 0.25 | >32 |
| ATCC 700731 | Mycobacterium septicum | 0.016 | >32 |
| ATCC 25275 | Mycobacterium simiae | 0.016 | >32 |
| ATCC 33027 | Mycobacterium sphagni | 0.5 | >32 |
| ATCC 19420 | Mycobacterium smegmatis | 0.25 | >32 |
| ATCC 19527 | Mycobacterium thermoresistibile | 4 | >32 |
| ATCC 27282 | Mycobacterium tokaiense | 0.125 | >32 |
| ATCC 15483 | Mycobacterium vaccae | 0.25 | >32 |
| SGM | |||
| ATCC 25276 | Mycobacterium asiaticum | 0.031 | >32 |
| ATCC 25291 | Mycobacterium avium | <0.008 | 0.25 |
| DSM 44243 | Mycobacterium celatum | >32 | 2 |
| DSM 44622 | Mycobacterium chimaera | <0.008 | <0.125 |
| ATCC 15754 | Mycobacterium gastri | <0.008 | 0.25 |
| ATCC 14470 | Mycobacterium gordonae | <0.008 | <0.125 |
| ATCC 13950 | Mycobacterium intracellulare | <0.008 | >32 |
| ATCC 12478 | Mycobacterium kansasii | <0.008 | <0.125 |
| ATCC 29571 | Mycobacterium malmoense | 0.031 | <0.125 |
| ATCC 19422 | Mycobacterium microti | <0.008 | <0.125 |
| ATCC 19530 | Mycobacterium nonchromogenicum | 0.016 | >32 |
| DSM 44648 | Mycobacterium parascrofulaceum | 0.031 | 0.25 |
| ATCC 27024 | Mycobacterium rhodesiae | 1 | >32 |
| ATCC 19981 | Mycobacterium scrofulaceum | 0.016 | >32 |
| ATCC 35799 | Mycobacterium szulgai | 0.016 | <0.125 |
| ATCC 15755 | Mycobacterium terrae | <0.008 | >32 |
| ATCC 23292 | Mycobacterium triviale | 0.5 | >32 |
| ATCC 27294 | Mycobacterium tuberculosis (H37Rv) | 0.031 | <0.125 |
| ATCC 19250 | Mycobacterium xenopi | 2 | <0.125 |
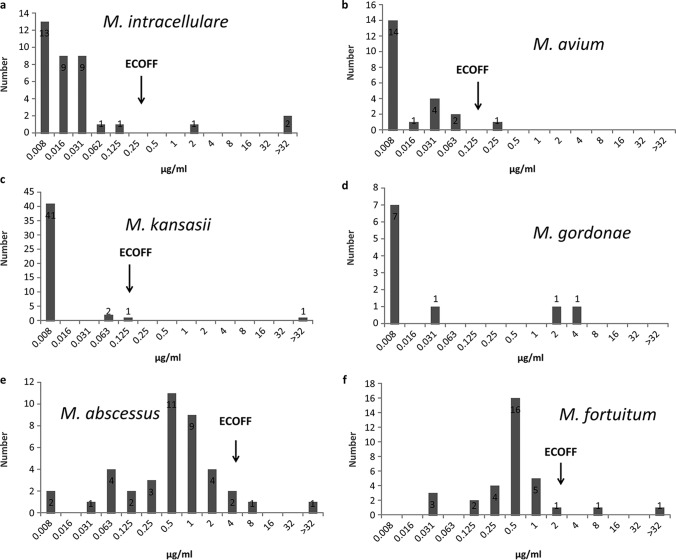
The MIC distributions of the most prevalent NTM species against Bdq are shown in Fig. 1. The susceptibility profile of the clinical isolates to Bdq was in concordance with that of the reference strains, i.e., strong antibacterial activity against the absolute majority isolates of SGM for all the included species. Similar activities were demonstrated against the included RGM isolates of different species but with relatively higher MIC values in contrast with SGM. Among the recruited SGM species, Bdq exhibited the strongest activity against Mycobacterium kansasii, with a tentative ECOFF at 0.125 μg/ml, whereas the overwhelming majority of the isolates had MICs below 0.008 μg/ml. Bdq also demonstrated strong activities against M. avium and M. intracellulare, with tentative ECOFFs at 0.125 μg/ml and 0.25 μg/ml, respectively. The activity of Bdq against Mycobacterium gordonae isolates was discrete. Eight isolates had MICs lower than 0.031 μg/ml, while another two had MICs of ≥2 μg/ml. Due to the limited number of isolates, the ECOFF of M. gordonae was not determined. Among the recruited RGM species, Bdq harbored good activities against M. abscessus and Mycobacterium fortuitum, with MIC90 values of 2 μg/ml and of 1 μg/ml, respectively. The ECOFF for M. abscessus and M. fortuitum were at 4 μg/ml and 2 μg/ml, respectively. The susceptibility testing outcomes for species with less than five isolates are shown in Table 2. Although there were small numbers for each species, Bdq presented the same inhibitory tendency against them.
FIG 1.
The MIC distributions of Bdq against the six most prevalent NTM species.
TABLE 2.
The MICs of Bdq and Dlm against clinically isolated species with less than 5 isolates
| Category | Species | No. of isolates | MIC(s) (μg/ml) by antimicrobial agent (no. of isolates)a |
|
|---|---|---|---|---|
| Bdq | Dlm | |||
| SGM | Mycobacterium arupense | 4 | <0.0078 (2), 0.0625, 4 | 0.5, >32 (3) |
| M. xenopi | 4 | 0.0313, 0.0016, 0.5, 1 | <0.125 (3), >32 | |
| M. szulgai | 3 | <0.0078 (3) | <0.125 (2), 0.5 | |
| Mycobacterium fuerth | 2 | <0.0078, 2 | >32 (2) | |
| M. parascrofulaceum | 1 | <0.0078 | 16 | |
| M. terrae | 1 | <0.0078 | >32 | |
| M. malmoense | 1 | <0.0078 | <0.125 | |
| RGM | M. chelonae | 3 | 0.0313, 0.0625, >32 | >32 (3) |
| Mycobacterium holsaticum | 1 | 1 | <0.125 | |
| Mycobacterium massiliense | 3 | 0.25 (2), 1 | >32 (3) | |
No. of isolates is 1 unless stated otherwise in parentheses.
MICs and ECOFF of Dlm against NTM strains.
Dlm exhibited highly variable antimicrobial activities against the 19 tested SGM species. Although, 11 species had MICs lower than 0.25 μg/ml, 7 species had MICs greater than 32 μg/ml, and 1 species had an MIC of 2 μg/ml. However, Dlm did not present evident inhibitory activity against most of the tested RGM species; 28 out of 33 tested RGM isolates had MICs greater than 32 μg/ml. Only four RGM species had MICs below 2 μg/ml, i.e., Mycobacterium gadium and Mycobacterium pulveris (MIC, <0.125 μg/ml) and Mycobacterium agri and Mycobacterium obuense (MIC, 1 μg/ml).
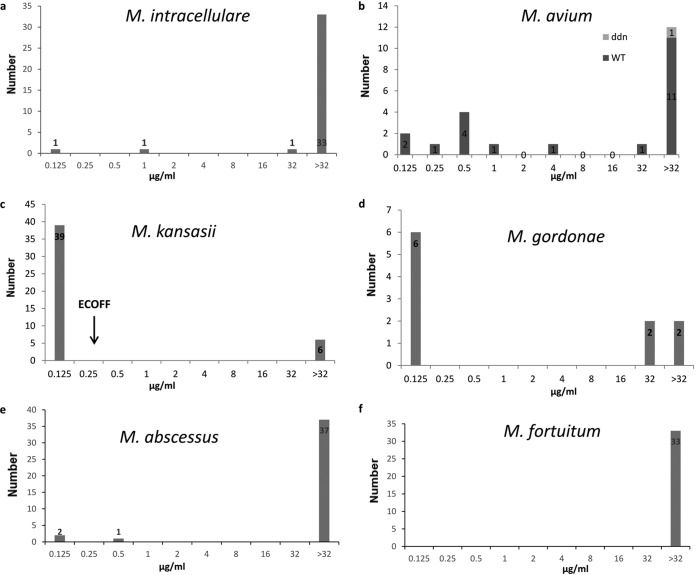
The MIC distributions of Dlm against the most prevalent NTM species are shown in Fig. 2. The susceptibility profiles of clinical isolates and reference strains against Dlm showed concordance, i.e., partial antibacterial activity against SGM, but little activity against RGM isolates. Among the six most frequently isolated SGM species, Dlm exhibited the strongest activity against M. kansasii with a tentative ECOFF at 0.25 μg/ml. The activity of Dlm against M. gordonae isolates was very different. While six isolates had MICs lower than 0.125 μg/ml, another four had MICs of ≥32 μg/ml. Among the 22 M. avium isolates, 7 had MICs of ≤0.25 μg/ml, while the remaining 15 had MICs of ≥0.5 μg/ml. Dlm did not present good inhibitory activity against M. intracellulare and M. abscessus, and the majority of the isolates were highly resistant to Dlm except a few isolates which had MICs of ≤1 μg/ml. Furthermore, Dlm harbored the least inhibitory activities against M. fortuitum, as all of the 33 isolates had MICs greater than 32 μg/ml. No ECOFF was determined for most of these species either due to a limited number of isolates or due to the weak activity of Dlm. In addition, the susceptibility testing outcomes for species with less than five isolates are presented in Table 2.
FIG 2.
The MIC distributions of Dlm against the six most prevalent NTM species.
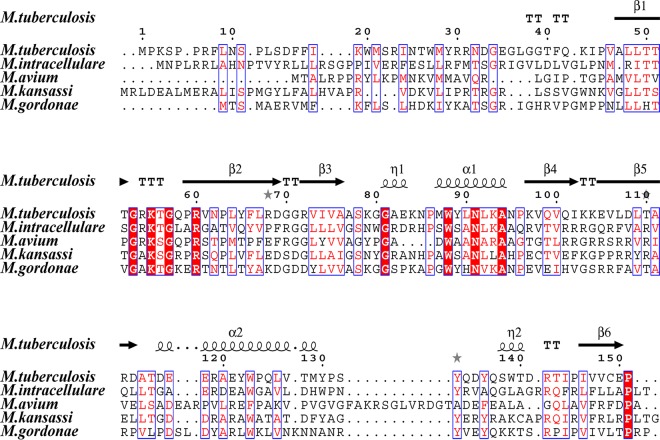
Alignment and mutation of Ddn homologues.
Multiple amino acid alignment for the Ddn homologues of different mycobacterial species and the topology of proteins are shown in Fig. 3. The interactions between ddn and F420 included extensive hydrogen-bond and hydrophobic interactions, and most of the key amino acid involved are highly conserved among TB and NTM species, as indicated by the sequence alignment which includes Gly53, Lys55, Arg60, Trp88, and Asn91 (Fig. 3). In order to determine the relationship between mutations in ddn homologous genes and Dlm resistance, the full-length ddn homologues of M. avium were sequenced. Among the 13 Dlm highly resistant M. avium isolates (MIC, ≥32 μg/ml), mutations within the Ddn homologues associated with Dlm resistance were identified, i.e., Thr79Arg and Ser86Cys which were detected in one M. avium isolate with MIC of >32 μg/ml. Furthermore, some synonymous mutations were also observed in drug resistance isolates, including Pro107Pro (CCC→CCT), Gly121Gly (GGG→GGA), Pro12Pro (CCG→CCC), and Arg73Arg (CGG→CGC). In addition, no mutation in ddn was detected in the remaining 9 isolates with MIC of ≤4 μg/ml.
FIG 3.
Sequence alignment outcome of the of Ddn homologue proteins. Alignment of the amino acid sequences of M. tuberculosis, M. intracellulare, M. avium, M. kansasii, and M. gordonae. The topology of the Rv3547 encoded protein of M. tuberculosis is shown at the top. Red boxes with white letters indicate a single, fully conserved residue. Blue frames indicate highly conserved residues. β-Strands are rendered as arrows.
DISCUSSION
The prevalence of NTM infection has been noticed to be increasing, which may reflect the increasing awareness of physicians that leads to more frequent diagnosis or the improved laboratory capabilities (6). Most of the NTM species demonstrate strong resistance to multiple antimicrobial agents (16), and, as a consequence, treatment of NTM infections and establishment of an effective regimen are generally challenging. Identifying drugs potent against NTM infections is urgently needed. Bdq and Dlm are highly efficient treatments of drug-resistant tuberculosis; however, only limited data are available on their antibacterial activity against NTM species either in vitro or in vivo. In this study, we first evaluate the efficacy of Dlm against NTM isolates collected from mainland China. Furthermore, we also evaluated the activity of Bdq and Dlm against 52 reference strains of different mycobacterium species to gain insights on their potential use for specific NTM species. As they are new drugs, well-recognized susceptibility testing methods for Bdq and Dlm have not been developed, and breakpoints to define drug resistance for them have never been discussed prudently (9, 10). Defining the ECOFF value based on data produced by multiple centers with a large number of strains is an important step to establish the breakpoints. The MIC data of different NTM species against Bdq and Dlm still remain scarce.
In this study, Bdq presented excellent inhibitory activity against the overwhelming majority of the recruited SGM reference strains and clinical isolates. A total of 16 out of 19 reference strains of SGM species exhibited MICs far below 1 μg/ml. Bdq also exhibited very good inhibitory activities against most of the tested clinical SGM isolates, especially the most prevalent and highly pathogenic species, such as M. kansasii and Mycobacterium avium complex (MAC), as previously described (10). Pang et al. also demonstrated that Bdq had good inhibitory activities against clinical strains of some SGM species isolated from China (9). All the tested NTM isolates in this study were collected in four hospitals located in mideastern China from 2011 to 2015, while the NTM isolates in our study were collected in Beijing Chest Hospital located in northern China from 2015 to 2016. Despite the differences in isolate collection time, location, and sample size, Bdq exhibited uniform bactericidal activity against SGM in these two studies. Notably, Bdq also exhibited good activity against the recruited RGM. A total of 29 out of 33 RGM species had MICs lower than 1 μg/ml, and most clinical isolates of different RGM species also had MICs lower than 1 μg/ml, including the most drug-resistant species, such as M. abscessus and M. fortuitum. RGM infection treatment is often very difficult because the bacteria can have more severe drug resistance patterns than that of SGM bacteria, and drugs with strong potency in vitro have not been reported yet. Although only a few studies report the clinical use of Bdq on NTM infection, based on our results, an extrapolation of its usage to treat infection caused by different SGM species is quite reasonable. However, further clinical trials are required to identify its real efficacy for patient care.
In contrast with Bdq, Dlm showed less strong antibacterial activity against either RGM or SGM. Almost all of the involved RGM reference strains and clinical isolates had very high MICs against Dlm, suggesting a low chance to be active for RGM infection treatment. Considering SGM, Dlm presented very different inhibitory activity against different species. Eleven of the 19 tested reference strains of different SGM species had MICs below 0.25 μg/ml, while 7 species had MICs of ≥32 μg/ml. For clinical isolates, Dlm exhibited good inhibitory activities against the majority isolates of M. kansasii and M. gordonae, the minority of M. avium, but almost no inhibitory activity against M. intracellulare. The high MIC variance within or between different NTM species highlighted the need for susceptibility testing before Dlm administration. A recent study with 20 isolates of MAC showed that all the tested M. intracellulare isolates had MICs below 0.4 μg/ml, which is not consistent with our results (15). Differences in the choice of susceptibility testing method might have caused this discordance, while different origins and the small number of isolates tested could also have incurred deviations. More studies from different teams and regions will help to evaluate the efficacies of Dlm objectively.
Due to the lack of a well-recognized method and breakpoint for Bdq susceptibility testing for M. tuberculosis and NTM, the breakpoint adopted in various studies was different (17, 18). Pang et al. adopted a 2-μg/ml breakpoint using the Microplate alamarBlue assay for RGM species and 1 μg/ml for SGM (18). Brown-Elliott et al. showed that all of the 103 tested MAC isolates had Bdq MICs of ≤0.03 μg/ml by broth microdilution using Mueller-Hinton (MH) broth (10). The tentative ECOFFs defined in our assay for M. kansasii, M. avium, and M. intracellulare were all ≤1 μg/ml in our study and were 4 μg/ml for M. abscessus and 2 μg/ml for M. fortuitum. A 2-h postdosage study verified that the drug was well absorbed, and the median Cmax of Bdq in 37 healthy volunteers was 3.39 μg/ml (19). According to these pharmacokinetic data, Bdq could theoretically inhibit the growth of most SGM NTM species and many RGM species in vivo, although clinical experience on Bdq for NTM patient care is rare. By combining the ECOFF and pharmacokinetic data, a uniform breakpoint at 2 μg/ml could be temporarily proposed for NTM’s Bdq susceptibility testing, including both RGM and SGM species. According to this breakpoint, the Bdq resistance rates of the six most prevalent species were 5.6%(2/36), 0%(0/22), 2.2%(1/45), 10.0%(1/10), 10.0%(4/40), and 6.1%(2/33) for M. intracellulare, M. avium, M. kansasii, M. gordonae, M. abscessus, and M. fortuitum, respectively. Further validation about this breakpoint is needed to justify its proposal.
No recommended breakpoint of NTM species against Dlm has been proposed previously. The tentative ECOFFs defined in our assay for M. kansasii was 0.125 μg/ml, and ECOFFs for other species were not proposed due to a small number of isolates or disappointing inhibitory activity. Dlm is administered at the dosage of 100 mg twice daily, i.e., at the lower limit of dosages with proven activity. With this dosage, the average 2-h postdosage plasma concentration was 0.39 μg/ml among healthy volunteers administered with Dlm alone (20). Based on the pharmacokinetic data and the MIC outcomes acquired in ours and other studies, we tentatively propose a breakpoint for the Dlm susceptibility testing at >0.25 μg/ml. According to this breakpoint, the Dlm resistant rates of the four most prevalent SGM species were 97.2% (35/36), 86.4%(19/22), 13.3%(6/45), and 40.0%(4/10) for M. intracellulare, M. avium, M. kansasii, and M. gordonae, respectively. Dlm may be usable for some SGM species infection treatment but not very promising for RGM infection treatment.
Dlm is intracellularly activated by F420-dependent mycobacterial nitroreductase encoded by the gene Rv3547 (ddn), and mutations in this gene could lead to Dlm resistance among clinical isolates of M. tuberculosis (21, 22). In contrast with other genes (fgd1, fbiA, fbiB, and fbiC) reported to be related with Dlm resistance, a mutation within ddn owned the highest frequency (29%) of Dlm resistance (21, 23). In this study, isolates of M. avium demonstrated high susceptibility variations within species against Dlm (Fig. 2). Thus, we analyzed ddn sequences of the probable susceptible isolates and probable resistant isolates. One M. avium isolate with an MIC of >32 μg/ml harbored a Thr79Arg mutation and Ser86Cys mutation simultaneously. According to the crystal structure of a Ddn protein of M. tuberculosis (PDB accession number 3R5L), Thr79 and Ser86 were located in β5, which interacted with β4 and forms the larger sheet at the base of the structure. This beta barrel-like structure is probably involved in binding hydrophobic Dlm; nevertheless, this molecular model-based speculation needs to be verified by more laboratory experiments in the future. Furthermore, a study found that a mutation located at Leu107 in β5 caused Dlm resistance in M. tuberculosis (21). Thus, mutations within the sheet are probably associated with Dlm resistance. In our study, only 1 out of 13 isolates of M. avium with MIC of ≥32 μg/ml had a ddn homologous gene mutation. Hence, ddn homologues might not be the only target for Dlm to explore its bacteriostatic activity.
Our study had several limitations. Most notably, the small sample size can cause bias. Thus, the proposed ECOFFs might change slightly with increasing sample size. Second, given that Bdq and Dlm had never been used before the strain collecting, all these strains were considered “wild type.” Further studies, including strains that had been exposed to the given drugs are needed for defining appropriate breakpoints of NTM DST. Furthermore, we only used the reference strain H37Rv (ATCC 27294) as a drug-susceptible control for the broth micro dilution method, which is recommended as a “pan-susceptible” control organism by Clinical and Laboratory Standards Institute (CLSI), whereas no resistant-control strain was recommended by CLSI and used in this study.
In conclusion, this study demonstrated that Bdq had high in vitro activities against the overwhelming majority of NTM species, including RGM and SGM. Dlm presented inhibitory activity against certain SGM species; however, the susceptibility variations within the same species highlight the requirement of a susceptibility screening before drug administration. According to the tentative ECOFF data in our assay and pharmacokinetic data from other reports, 2 μg/ml could be temporarily proposed as breakpoint for NTM susceptibility testing for Bdq. Our study provided important insights on clinical therapy of Bdq and Dlm for the treatment of NTM infections.
MATERIALS AND METHODS
Ethics statement.
As the study only concerned laboratory testing of mycobacteria without the direct involvement of human subjects, ethics approval was not sought.
Reference strains and clinical isolates.
All the mycobacterial reference strains stored in the Bio-bank in Beijing Chest Hospital (Beijing, China) were tested against Bdq and Dlm in vitro, including 33 rapidly growing mycobacterium (RGM) and 19 slowly growing mycobacterium (SGM) species. These reference strains were obtained either from the American Type Culture Collection (ATCC) or from the German Collection of Microorganisms (DSM). The species constitution of these reference strains are listed in Table 1. A total of 209 NTM clinical isolates, collected between 2015 and 2016 in Beijing Chest Hospital, were recruited, including 40 M. abscessus, 33 M. fortuitum, 36 M. intracellulare, 22 M. avium, 45 M. kansasii, and 10 M. gordonae isolates. The species constitution of the remaining 23 isolates is presented in Table 2. All the 209 NTM clinical strains were isolated from tuberculosis-suspected patients. The strains were classified as NTM preliminarily with p-nitrobenzoic acid-containing medium and then were differentiated into species level by 16S rRNA, hsp65, rpoB, and 16-23S rRNA internal transcribed spacer sequencing (9). All the isolates were stored at −80°C and subcultured on Lowenstein-Jensen (LJ) medium before performing the microplate alamarBlue assay (MABA).
MIC determination.
Bdq and Dlm were purchased from Liye-Pharmaceutical (Nanjing, China) and Shanghai Biochempartner Co., Ltd. (Shanghai, China), respectively. Both drugs were dissolved in dimethyl sulfoxide (DMSO). Stock solutions were aseptically prepared at concentrations of 2 mg/ml. The broth microdilution method was performed according to guidelines of CLSI (24). Cation-adjusted Mueller-Hinton broth (CAMHB) enriched with oleic acid-albumin-dextrose-catalase (OADC) was used for SGM, while CAMHB without OADC was used for RGM. The inoculum was prepared with fresh culture grown on Lowenstein-Jensen medium. The broth microdilution format was set up as 2-fold dilution, and the concentration for Bdq ranged from 0.0078 μg/ml to 32 μg/ml, while for Dlm the concentration ranged from 0.125 μg/ml to 32 μg/ml. Briefly, a bacterial suspension of 0.5 McFarland standard was prepared and then diluted and inoculated into a 96-well microtiter plate to achieve a final bacterial load at 105 CFUs per well. Plates were then incubated at 37°C for 7 days for SGM and 3 days for RGM, respectively. A 70-μl solution containing 20 μl alamarBlue and 50 μl Tween 80 (5%) was added to each well and incubated for 24 h at 37°C before assessing color development. A change from blue to pink or purple indicated bacterial growth (25). The MIC was defined as the lowest concentration of antibiotic that prevented a color change from blue to pink.
ECOFF determination.
For species with enough isolates and excellent inhibitory activity demonstrated by Bdq/Dlm, the ECOFF was determined according to the distribution profile of the MIC values. For unimodal MIC distribution profile, ECOFF was defined as the concentration that could inhibit >95% of the bacterial population; for bimodal MIC distribution profile, ECOFF was set between the two populations.
Ddn encoding gene sequencing and protein alignment.
The aerobic killing mechanism of Dlm involves the inhibition of cell wall mycolic acid biosynthesis. This reaction is catalyzed by deazaflavin (F420)-dependent nitroreductase (Ddn), which is encoded by Rv3547 in M. tuberculosis (12). Mutations in ddn, causing the inhibition of cell wall mycolic acid biosynthesis, result in DLM resistance (21, 26). Sequence analysis of the ddn gene of the M. avium isolates was performed. The primers used to sequence ddn in M. avium was as follows: forward, 5′-GATCATCACCGCAGCCGACA-3′; and reverse, 5′-TTCGACCGCCGCAATGAACG-3′. The ddn gene of the reference strains of the four most isolated species plus M. tuberculosis were also sequenced, and mutation was defined in contrast with the sequences of the reference strains. The amplification products were sequenced by Tsingke Company (Beijing, China). Multiple sequence alignment of the homologous proteins was performed using the Clustal Omega software. Structure-based multiple sequence alignment was performed with ESPript 3 based on the crystal structure of Rv3547 protein of M. tuberculosis from http://espript.ibcp.fr/ESPript/ESPript/.
ACKNOWLEDGMENTS
This study was supported by research funding from the Infectious Diseases Special Project, Ministry of Health of China (2017ZX10201301-004-002 and 2018ZX10302302-004-005), the Natural Science Fund of China (81672065), Beijing Natural Science Foundation grants (7174288 and 7182117), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201824), and Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20181602).
REFERENCES
- 1.Raju RM, Raju SM, Zhao Y, Rubin EJ. 2016. Leveraging advances in tuberculosis diagnosis and treatment to address nontuberculous mycobacterial disease. Emerg Infect Dis 22:365–369. doi: 10.3201/eid2203.151643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brode SK, Marchand-Austin A, Jamieson FB, Marras TK. 2017. Pulmonary versus nonpulmonary nontuberculous mycobacteria, Ontario, Canada. Emerg Infect Dis 23:1898–1901. doi: 10.3201/eid2311.170959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin C, Russell C, Soll B, Chow D, Bamrah S, Brostrom R, Kim W, Scott J, Bankowski MJ. 2018. Increasing prevalence of nontuberculous mycobacteria in respiratory specimens from US-affiliated Pacific Island jurisdictions. Emerg Infect Dis 24:485–491. doi: 10.3201/eid2403.171301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santin M, Barrabeig I, Malchair P, Gonzalez-Luquero L, Benitez MA, Sabria J, Palau-Benavent M, Cañete C, Lloret-Queraltó JA, Grijota-Camino MD, Dorca J, Alcaide F. 2018. Pulmonary infections with nontuberculous mycobacteria, Catalonia, Spain, 1994–2014. Emerg Infect Dis 24:1091–1094. doi: 10.3201/eid2406.172095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X, Liu P, Liu G, Zhao L, Hu Y, Wei G, Luo J, Huang H. 2016. The prevalence of non-tuberculous mycobacterial infections in mainland China: systematic review and meta-analysis. J Infect 73:558–567. doi: 10.1016/j.jinf.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 6.de Mello KG, Mello FC, Borga L, Rolla V, Duarte RS, Sampaio EP, Holland SM, Prevots DR, Dalcolmo MP. 2013. Clinical and therapeutic features of pulmonary nontuberculous mycobacterial disease, Rio de Janeiro, Brazil. Emerg Infect Dis 19:393–399. doi: 10.3201/eid1903.120735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Q, Chu H, Ye M, Zhang Z, Li B, Yang S, Ma W, Yu F. 2018. The clarithromycin susceptibility genotype affects the treatment outcome of patients with Mycobacterium abscessus lung disease. Antimicrob Agents Chemother 62:e02360-17. doi: 10.1128/AAC.02360-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu HB, Jiang RH, Li L. 2014. Treatment outcomes for Mycobacterium avium complex: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 33:347–358. doi: 10.1007/s10096-013-1962-1. [DOI] [PubMed] [Google Scholar]
- 9.Pang Y, Zheng H, Tan Y, Song Y, Zhao Y. 2017. In vitro activity of bedaquiline against nontuberculous mycobacteria in China. Antimicrob Agents Chemother 61:e02627. doi: 10.1128/aac.02627-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown-Elliott BA, Philley JV, Griffith DE, Thakkar F, Wallace RJ Jr. 2017. In vitro susceptibility testing of bedaquiline against Mycobacterium avium complex. Antimicrob Agents Chemother 61:e01798-16. doi: 10.1128/AAC.01798-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philley JV, Wallace RJ Jr., Benwill JL, Taskar V, Brown-Elliott BA, Thakkar F, Aksamit TR, Griffith DE. 2015. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 148:499–506. doi: 10.1378/chest.14-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, Ledwidge R, Dowd CS, Lee IY, Kim P, Zhang L, Kang S, Keller TH, Jiricek J, Barry CE III.. 2008. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 322:1392–1395. doi: 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, Gao M, Awad M, Park SK, Shim TS, Suh GY, Danilovits M, Ogata H, Kurve A, Chang J, Suzuki K, Tupasi T, Koh WJ, Seaworth B, Geiter LJ, Wells CD. 2012. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 366:2151–2160. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- 14.Skripconoka V, Danilovits M, Pehme L, Tomson T, Skenders G, Kummik T, Cirule A, Leimane V, Kurve A, Levina K, Geiter LJ, Manissero D, Wells CD. 2013. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J 41:1393–1400. doi: 10.1183/09031936.00125812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieger D, Schonfeld N, Vesenbeckh S, Bettermann G, Bauer TT, Russmann H, Mauch H. 2016. Is delamanid a potential agent in the treatment of diseases caused by Mycobacterium avium-intracellulare? Eur Respir J 48:1803–1804. doi: 10.1183/13993003.01420-2016. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Lian LL, Wan L, Zhang J, Zhao X, Jiang Y, Zhao LL, Liu H, Wan K. 2013. Antimicrobial susceptibility of standard strains of nontuberculous mycobacteria by microplate alamarBlue assay. PLoS One 8:e84065. doi: 10.1371/journal.pone.0084065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li G, Pang H, Guo Q, Huang M, Tan Y, Li C, Wei J, Xia Y, Jiang Y, Zhao X, Liu H, Zhao LL, Liu Z, Xu D, Wan K. 2017. Antimicrobial susceptibility and MIC distribution of 41 drugs against clinical isolates from China and reference strains of nontuberculous mycobacteria. Int J Antimicrob Agents 49:364–374. doi: 10.1016/j.ijantimicag.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Cowman S, Burns K, Benson S, Wilson R, Loebinger MR. 2016. The antimicrobial susceptibility of non-tuberculous mycobacteria. J Infect 72:324–331. doi: 10.1016/j.jinf.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Dooley KE, Park JG, Swindells S, Allen R, Haas DW, Cramer Y, Aweeka F, Wiggins I, Gupta A, Lizak P, Qasba S, van Heeswijk R, Flexner C. 2012. Safety, tolerability, and pharmacokinetic interactions of the antituberculous agent TMC207 (bedaquiline) with efavirenz in healthy volunteers: AIDS Clinical Trials Group Study A5267. J Acquir Immune Defic Syndr 59:455–462. doi: 10.1097/QAI.0b013e3182410503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallikaarjun S, Wells C, Petersen C, Paccaly A, Shoaf SE, Patil S, Geiter L. 2016. Delamanid coadministered with antiretroviral drugs or antituberculosis drugs shows no clinically relevant drug-drug interactions in healthy subjects. Antimicrob Agents Chemother 60:5976–5985. doi: 10.1128/AAC.00509-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujiwara M, Kawasaki M, Hariguchi N, Liu Y, Matsumoto M. 2018. Mechanisms of resistance to delamanid, a drug for Mycobacterium tuberculosis. Tuberculosis (Edinb) 108:186–194. doi: 10.1016/j.tube.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Schena E, Nedialkova L, Borroni E, Battaglia S, Cabibbe AM, Niemann S, Utpatel C, Merker M, Trovato A, Hofmann-Thiel S, Hoffmann H, Cirillo DM. 2016. Delamanid susceptibility testing of Mycobacterium tuberculosis using the resazurin microtitre assay and the BACTEC MGIT 960 system. J Antimicrob Chemother 71:1532–1539. doi: 10.1093/jac/dkw044. [DOI] [PubMed] [Google Scholar]
- 23.Haver HL, Chua A, Ghode P, Lakshminarayana SB, Singhal A, Mathema B, Wintjens R, Bifani P. 2015. Mutations in genes for the F420 biosynthetic pathway and a nitroreductase enzyme are the primary resistance determinants in spontaneous in vitro-selected PA-824-resistant mutants of Mycobacterium tuberculosis. Antimicrob Agents Chemother 59:5316–5323. doi: 10.1128/AAC.00308-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes; approved standard, 2nd ed. CLSI document M24-A2 CLSI, Wayne, PA. [PubMed] [Google Scholar]
- 25.Coeck N, de Jong BC, Diels M, de Rijk P, Ardizzoni E, Van Deun A, Rigouts L. 2016. Correlation of different phenotypic drug susceptibility testing methods for four fluoroquinolones in Mycobacterium tuberculosis. J Antimicrob Chemother 71:1233–1240. doi: 10.1093/jac/dkv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feuerriegel S, Koser CU, Bau D, Rusch-Gerdes S, Summers DK, Archer JA, Marti-Renom MA, Niemann S. 2011. Impact of Fgd1 and ddn diversity in Mycobacterium tuberculosis complex on in vitro susceptibility to PA-824. Antimicrob Agents Chemother 55:5718–5722. doi: 10.1128/AAC.05500-11. [DOI] [PMC free article] [PubMed] [Google Scholar]