BK polyomavirus (BKPyV)-associated nephropathy (BKPyVAN) is one of the major causes of kidney graft dysfunction, and there are no BKPyV-specific antiviral therapies available. BKPyV neutralizing antibodies (NAbs) play key roles in protecting against BKPyV replication and represent a potential therapeutic or preventive strategy. In this study, we evaluated NAb titers in intravenous immunoglobulin (i.v. Ig) preparations and in kidney transplant recipients (KTR) before and after i.v.
KEYWORDS: BK nephropathy, BK virus, intravenous immunoglobulin, kidney transplantation, viral infection
ABSTRACT
BK polyomavirus (BKPyV)-associated nephropathy (BKPyVAN) is one of the major causes of kidney graft dysfunction, and there are no BKPyV-specific antiviral therapies available. BKPyV neutralizing antibodies (NAbs) play key roles in protecting against BKPyV replication and represent a potential therapeutic or preventive strategy. In this study, we evaluated NAb titers in intravenous immunoglobulin (i.v. Ig) preparations and in kidney transplant recipients (KTR) before and after i.v. Ig administration. NAb titers directed against major BKPyV genotypes were measured using a BKPyV pseudovirion system. Thirty-three KTR receiving high (1 g/kg of body weight/day; n = 17) or low (0.4 g/kg/day; n = 16) i.v. Ig doses were included. Median NAb titers in i.v. Ig preparations ranged from 5.9 log10 50% inhibitory concentration (IC50) for genotype I to 4.1 log10 IC50 for genotype IV. A mean of 90% of patients (range, 88% to 100%) displaying low or negative BKPyV NAb titers against genotype I reached 4 log10 IC50 after the first i.v. Ig administration. This value was reached by a mean of 44% (range, 13% to 83%) and 19% (range, 0% to 38%) of patients against genotype II and genotype IV, respectively. The benefit of i.v. Ig administration persisted until the following course of treatment (day 22 ± 7 days) for genotypes I and II, and no cumulative effect was observed through the three doses. Our findings demonstrate that i.v. Ig administration results in a significant increase in BKPyV NAb titers in KTR. These in vitro and in vivo pharmacokinetic data provide the rationale for a proof-of-concept study investigating the efficacy of i.v. Ig for the prevention of BKPyV infection in KTR.
INTRODUCTION
BK polyomavirus (BKPyV)-associated nephropathy (BKPyVAN), one of the worrisome causes of kidney graft dysfunction, is a growing medical problem as the population of transplant recipients continues to increase (1, 2). BKPyVAN arises from the use of highly potent immunosuppressive drugs (3–5). Disruption of the balance between BKPyV replication and host immune control is generally viewed as a key element of viral pathogenesis (6). BKPyV replication occurs in 40% to 50% of kidney transplant recipients (KTR), followed by BKPyVAN in up to 10% of KTR (7). When nephropathy develops, it leads to graft dysfunction and graft loss in up to 50% of patients (7, 8).
At present, there are no available BKPyV-specific antiviral therapies. A range of therapeutic agents have been studied for the ability to treat polyomavirus infections, including leflunomide, quinolones, and cidofovir, with variable success (9). Limited treatment modalities exist, and the most widely accepted intervention is reduction of immunosuppressive therapy after confirmation or presumption of BKPyVAN (10, 11). Unfortunately, this approach is not always successful and increases the risk of acute rejection (5% to 25%) and donor-specific antibody (DSA) emergence (12, 13). Given the incomplete success of preemptive and supportive strategies for BKPyV-associated diseases, there is an urgent need to develop anti-BKPyV therapies. Human intravenous immunoglobulin (i.v. Ig) forms the basis of immunopreventive and immunotherapeutic strategies against many important human viral infections (14–18). Randhawa et al. demonstrated that commercial preparations of i.v. Ig (Privigen and Cytogam) contain potent neutralizing antibodies that are capable of neutralizing all major BKPyV genotypes in vitro (19). In a recent study, we showed that BKPyV neutralizing antibodies (NAbs) play a key role in protection against BKPyV replication in kidney transplant recipients (20). A NAb titer against the donor’s strain lower than 4 log10 50% inhibitory concentration (IC50) before transplantation was significantly associated with BKPyV replication after transplantation (hazard ratio [HR] = 1.88; 95% confidence interval [CI] = 1.06 to 3.45; P = 0.03). Taken together, these data suggest that i.v. Ig administration may help to prevent BKPyV replication in vivo. In this study, we evaluated the capacities of three commercial i.v. Ig preparations (Privigen, Clairyg, and Octagam) to increase BKPyV NAb titers in vivo in KTR treated with low (0.4 g/kg of body weight/day) or high (1 g/kg/day) i.v. Ig doses.
RESULTS
High titers of BKPyV genotype-specific NAbs in i.v. Ig preparations.
Forty-three commercial i.v. Ig batches were tested: Privigen, n = 25; Octagam, n = 9; and Clairyg, n = 9 (see Tables S1, S2, and S3 in the supplemental material). All the i.v. Ig preparations tested contained NAbs against the three major BKPyV genotypes. The NAb titers ranged from 5.1 to 6.7 log10 IC50 against genotype I, from 4.4 to 5.5 log10 IC50 against genotype II, and from 3.6 to 4.8 log10 IC50 against genotype IV. In agreement with the results of the Kruskal-Wallis tests (see Fig. S1A, B, and C in the supplemental material), Bayesian modeling of the posterior distributions of the NAb titers according to the BKPyV genotype and commercial preparation showed a 99% probability that the NAb titers were higher for genotype I than for genotype II or genotype IV (Fig. 1). Octagam showed lower BKPyV NAb titers against genotype I (−0.5 log10) than Clairyg or Privigen, with a probability of 96% (Fig. 1).
FIG 1.
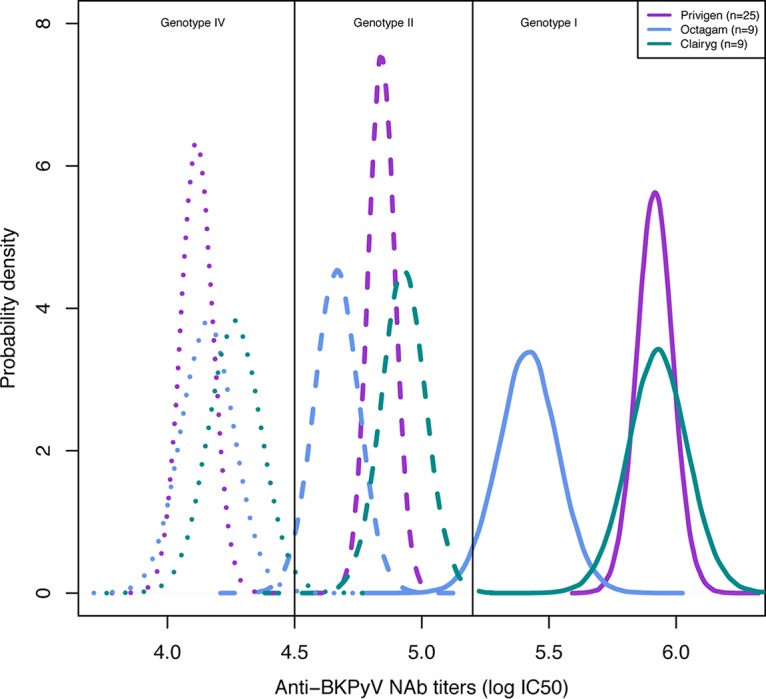
Posterior distributions of the BKPyV NAb titers according to the genotype and commercial preparation by Bayesian modeling. The density of Pr describes the continuous probability distribution of the BKPyV NAb titers according to the genotype and commercial preparation. The BKPyV NAb titer distribution values are depicted according to the BKPyV genotype (I, II, and IV) and for each commercial preparation (Privigen, Octagam, and Clairyg). There was no overlap between genotypes, reflecting higher titers for genotype I, followed by genotype II and genotype IV, for each commercial preparation. The highest anti-BKPyV genotype I NAb titers were obtained for Privigen and Clairyg.
All the batches were tested in triplicate, and variations within the same i.v. Ig batch were evaluated by calculating the mean intrabatch coefficient of variation (CV). All of the commercial preparations showed intrabatch CV lower than 10% for the tested BKPyV genotypes. Batch-to-batch variations within manufacturers were also evaluated (see Tables S1, S2, and S3) and showed CV lower than 10%.
The neutralizing effect of i.v. Ig (Privigen) was confirmed in a culture system of human renal proximal tubule epithelial cells (hRPTEC) infected by infectious BKPyV strains (genotype I, genotype II, or genotype IV). A final 1/100,000 dilution of i.v. Ig led to mean inhibition rates of 87% for genotypes I and II and 80% for genotype IV (see Fig. S2A and B in the supplemental material).
KTR sera post-i.v. Ig administration efficiently neutralize BKPyV genotypes.
Thirty-three KTR were enrolled in the study, including 17 treated with 1 g/kg/day i.v. Ig for 2 days every 3 weeks (±7 days) for antibody-mediated rejection (AMR group; n = 17) and 16 treated with 0.4 g/kg/day i.v. Ig for secondary immunodeficiency syndrome (SIDS group; n = 16) every 3 weeks (±7 days). Patients’ characteristics are described in Table 1 for both the AMR and the SIDS groups. Patients who received high doses of i.v. Ig for AMR or low doses for SIDS displayed low urinary protein excretion. The median proteinuria/creatinuria ratios were about 0.21 g/g (range, 0.07 to 0.81 g/g) and 0.45 g/g (range, 0.12 to 1.85 g/g) in AMR and SIDS patients, respectively. Only two patients in the SIDS group displayed proteinuria/creatinuria ratios above 1 g/g. IgG levels were available for most of the patients before i.v. Ig infusion, with a median of 6.4 g/liter (range, 2.2 to 11.5 g/liter). After i.v. Ig infusion, IgG levels were available mostly for SIDS patients, with a median of 8.9 g/liter (range, 7 to 11.7 g/liter). BKPyV DNA load monitoring was performed monthly for all patients for up to 12 months of follow-up, every 3 months during the second year after transplantation, and then every 6 months. During this period, no patient displayed BKPyV replication in the group receiving high doses of i.v. Ig. Among those receiving low doses of i.v. Ig, 3 out of 16 developed BKPyV viruria: 1 patient who was highly immunosuppressed developed transient viremia (56 days); 2 patients remained viruric. Among them, one patient harbored a BKPyV of genotype II, for which i.v. Ig is hardly efficient.
TABLE 1.
Patients’ characteristicsa
| Characteristic | Value for patients with i.v. Ig for: |
|
|---|---|---|
| AMR (n = 17) | SIDS (n = 16) | |
| Median age (yr) (range) | 42.2 (18.0–72.6) | 58.3 (17.7–69.5) |
| Male [n (%)] | 9 (52.9) | 12 (75) |
| 1st graft [n (%)] | 15 (88) | 16 (100) |
| Living donor [n (%)] | 2 (12) | 4 (25) |
| No. of HLA mismatches [mean (range)] | 3.4 (1–5) | 3.9 (0–6) |
| Delay (mo) between graft and first i.v. Ig injection [median (range)] | 19.1 (0.8–134.7) | 0.8 (0.7–1.6) |
Patient cohort, n = 33.
At day 0, NAb titers against each BKPyV genotype were similar between the AMR and the SIDS groups (P value = 0.16 for genotype I; P value = 0.14 for genotype II; P value = 0.08 for genotype IV; Mann-Whitney test, α = 5%). Overall, the percentages of patients displaying BKPyV NAb titers lower than 4 log10 IC50 were 64% (21/33) for genotype I and 91% (30/33) for genotype II and genotype IV. Among these patients, 9.5% (2/21) showed negative BKPyV NAb titers (<2.5 log10 IC50) against genotype I, 33% (10/30) against genotype II, and 53% (16/30) against genotype IV (see Tables S4, S5, and S6 in the supplemental material).
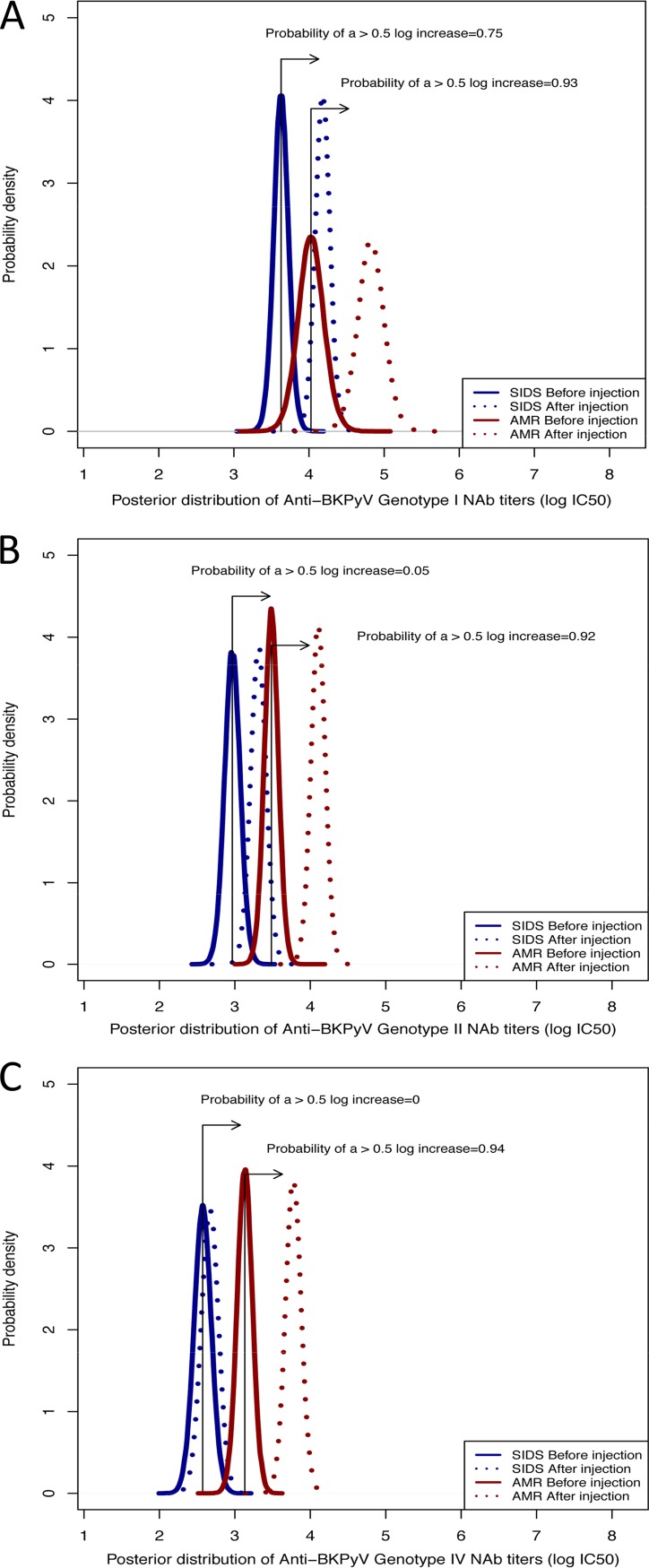
Analysis of NAb titers the day after the first injection (day 1) in the AMR group showed a median increase of +1.3 log10 IC50 (range, 0.5 to 1.8) for genotype I, +0.9 log10 IC50 (range, 0.2 to 2.4) for genotype II, and +1.1 log10 IC50 (range, 0.2 to 2.5) for genotype IV (see Tables S4, S5, and S6). In the SIDS group, the NAb titer increase was +1.3 log10 IC50 (range, 0.4 to 2.0) for genotype I, +0.9 log10 IC50 (range, 0 to 1.1) for genotype II, and +0.3 log10 IC50 (range, 0 to 1.0) for genotype IV (see Tables S4, S5, and S6). Subsequent to the first injection, 90% (19/21) of patients reached a NAb titer of at least 4 log10 IC50 against genotype I, including 100% of the patients in the AMR group and 88% in the SIDS group; 44% (12/27) against genotype II, including 83% (10/12) in the AMR group versus 13% (2/15) in the SIDS group; and 18.5% (5/27) against genotype IV, including 38% (5/13) in the AMR group and no patients (0/14) in the SIDS group. According to Bayesian modeling of the posterior distributions of the NAb titers before and after i.v. Ig injection, the observed increases were not relevantly different between the two groups for genotype I (probability of a 0.5 log10 increase [Pr], >0.75). However, this increase was higher in the AMR group than in the SIDS group for both genotype II (Pr = 0.92 versus 0.05, respectively) and genotype IV (Pr = 0.94 versus 0, respectively) (Fig. 2).
FIG 2.
Posterior distributions of the BKPyV NAb titers before and after i.v. Ig injection according to the genotype and type of patients by Bayesian modeling. The density of Pr describes the continuous probability distribution of BKPyV NAb titers before and after i.v. Ig injection according to the genotype and the patient group (AMR or SIDS). The BKPyV NAb titer distribution values are depicted according to the BKPyV genotype (I [A], II [B], and IV [C]) before and after i.v. Ig injection for both SIDS (blue) and AMR (red) patients. The observed NAb titer increases were not relevantly different between the two groups of patients for genotype I (probability of a 0.5 log10 increase [Pr], >0.75). However, the increase was greater in the AMR group than in the SIDS group for both genotype II (Pr = 0.92 versus 0.05, respectively) and genotype IV (Pr = 0.94 versus 0, respectively).
In both AMR and SIDS patients, BKPyV NAb titers against genotypes I and II remained stable over two i.v. Ig injections (Fig. 3). The decrease observed was lower than or equal to −0.5 log10 IC50 (for genotype I, the AMR median was −0.5 log10 IC50 and the SIDS median was −0.4 log10 IC50 [P value, 0.44]; for genotype II, the AMR median was −0.4 log10 IC50, and the SIDS median was −0.3 log10 IC50 [P value, 0.0925]; Mann-Whitney test, α = 5%).
FIG 3.
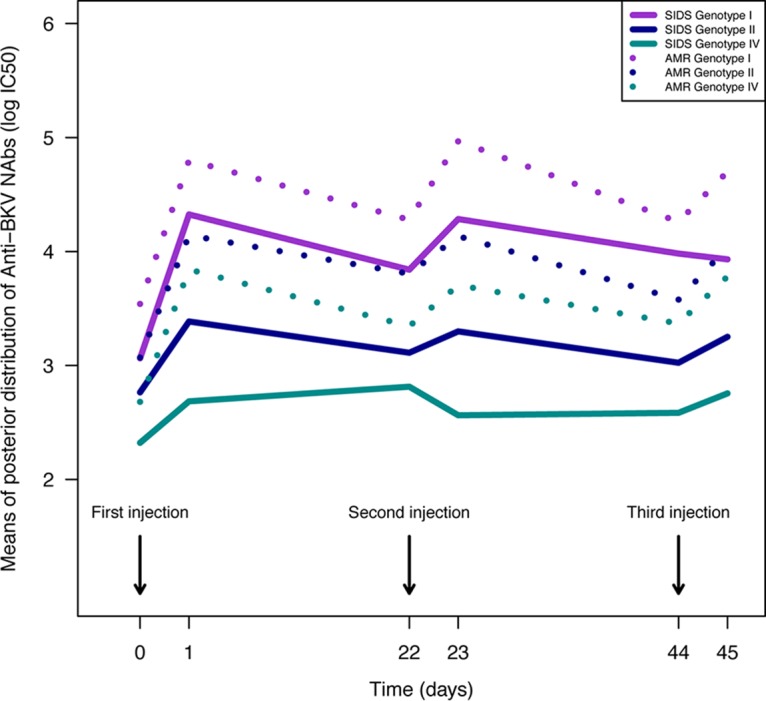
Dynamics of posterior means of NAb titers over time according to BKPyV genotype in patients receiving successive i.v. Ig administrations. The estimated means of BKPyV NAb titers are depicted according to the BKPyV genotype (I, II, and IV) for both SIDS and AMR patients. There was no cumulative effect of i.v. Ig administration for any genotype.
DISCUSSION
Human i.v. Ig is not only a source of passive immunity, but also a powerful immunomodulatory and anti-inflammatory agent. These molecules are therefore widely used in solid organ transplantation (i.e., for HLA and ABO blood group antibody desensitization, treatment of AMR, or treatment of SIDS) (21–25).
In this study, we showed that (i) i.v. Ig contained high NAb titers against the major BKPyV genotypes, (ii) NAb titers were similar between batches of the same commercial preparation but differed depending on the i.v. Ig supplier, and (iii) i.v. Ig administration increased BKPyV genotype-specific NAb titers in KTR.
The three i.v. Ig types tested contained high NAb titers against BKPyV genotypes I and II and only low titers against genotype IV. Since i.v. Ig preparations are derived from pooled human plasma from thousands of donors, this result may reflect the prevalence of BKPyV genotypes in the healthy population. This is true for BKPyV genotype I, which is known to be the most prevalent. However, NAb titers against BKPyV genotype II were higher than expected. Similar findings were reported by Pastrana et al. in healthy donors and by Randhawa et al. in i.v. Ig, where the genotype II seroprevalence was below that of genotype I but higher than that of genotype IV (19, 26, 27). In a longitudinal study, we also demonstrated that the genotype II seroprevalence in KTR was approximately 10-fold higher than the PCR-based prevalence (28). This difference cannot be explained by the PCR results because our PCR assay was able to detect and adequately quantify all of the BKPyV genotypes (29); the difference also cannot be explained by cross-reactivity because genotypes I and II have low sequence homology in the major NAb epitope, which is the VP1 BC loop. On the other hand, the difference suggests that genotype II may be prone to clearance from the genitourinary tract or have low reactivation potential; further research is warranted to address this observation.
NAb titers were similar between batches but were different between the commercial i.v. Ig preparations. Octagam showed lower NAb titers against genotype I (−0.5 log10) than Privigen and Clairyg. The impacts of the manufacturing techniques and donor characteristics on the antibody concentrations in the three commercial preparations remain to be determined.
The results of the in vivo experiments were consistent with the in vitro data. Administration of i.v. Ig either at low (0.4 g/kg/day) or high (1 g/kg/day) doses to KTR significantly increased the BKPyV NAb titers in KTR sera by up to 2.4 log10 units against genotype I, and only high doses led to NAb titer increases, up to 1 log10 unit, against genotype II and genotype IV. In KTR, we (20) and others (30) have shown that NAb titers increase after the onset of BKPyV replication and that an early and effective neutralizing response against the replicating strain (mainly of donor origin) prevents the development of viremia. We therefore defined a cutoff value of 4 log10 IC50 at transplantation as a protective NAb titer against BKPyV replication, supporting the potential benefit of administering NAbs as a preventive strategy against BKPyV infection (20). In the present study, 64% of patients displayed low or negative BKPyV NAb titers against genotype I before i.v. Ig administration, and 90% of these patients reached the value of 4 log10 IC50 after the first administration. This value was reached against genotype II in 83% of patients treated with high doses of i.v. Ig and in only 13% of patients treated with low i.v. Ig doses. As expected, only a few treated patients were able to reach the 4 log10 IC50 value against genotype IV. These results strongly suggested that i.v. Ig could be used at a dose of 0.4 g/kg/day to reach the cutoff value of 4 log10 IC50 for BKPyV NAbs against genotype I. Due to their paucity in i.v. Ig preparations, only high doses of i.v. Ig may induce protective NAb titers against genotypes II and IV.
The benefit of i.v. Ig administration persisted until the next injection (i.e. 22 days ± 7 days) in the two groups. This lasting effect of i.v. Ig administration for genotype I suggested that one injection per month over the first 3 months posttransplantation could restore humoral protection during this period—the time of highest risk for BKPyV reactivation—and beyond. The beneficial effect of i.v. Ig administration was not maintained for genotype II in the SIDS group and for genotype IV in both the SIDS and AMR groups.
Human i.v. Ig has been used as an empirical therapy against BKPyV infection; however, currently available studies are not easy to evaluate because of the absence of controlled arms. All such studies used i.v. Ig therapy after the occurrence of BKPyV viremia and/or BKPyVAN, associated or not with a concomitant treatment intervention (14–18). To our knowledge, this is the first time that i.v. Ig was considered to be a potential tool to prevent postgraft BKPyV infection in KTR.
In summary, our study has provided in vitro and in vivo pharmacokinetic data showing that i.v. Ig administration resulted in an increase in BKPyV NAb titers in kidney transplant recipients, reaching a protective threshold for at least two of the three major genotypes. The immunomodulatory effects of i.v. Ig may be beneficial and may provide an optimal balance between rejection and viral reactivation risks. The potential cost of this preventive strategy is estimated to be around €3,000 (for SIDS, in an adult weighing 70 kg). The cost of i.v. Ig therapy could vary greatly according to the country and is closer to $12,000 in the United States. However, these estimated costs should be considered in comparison to the cost generated by a patient hospitalization due to BKPyV disease complications (around €4,698 [$6,109] for 1 day of hospitalization) and/or the tremendous cost of graft loss and return to dialysis (31). Overall, although limited by the small sample size (164 samples from 33 patients) and the retrospective design of the study, our findings provide a rationale for a proof-of-concept study investigating the efficacy of i.v. Ig for the prevention of BKPyV infection in kidney transplant recipients.
MATERIALS AND METHODS
Study population.
Kidney transplant recipients who were BKPyV seronegative or displayed low NAb titers (<4 log10 IC50) against at least one BKPyV genotype and received i.v. Ig therapy were enrolled in this study. Every 3 weeks (±7 days), all the KTR received a dose of 1 g/kg/day of i.v. Ig over 2 days for AMR proved by allograft biopsy and/or detection of DSAs, or a dose of 0.4 g/kg/day for SIDS. Blood samples were collected in the AMR group before and 1 day after each i.v. Ig administration and in the SIDS group before and 1 to 7 days after each i.v. Ig administration. We assigned day 0, day 22, and day 44 as the days before i.v. Ig administration and day 1, day 23, and day 45 as the days after i.v. Ig administration. BKPyV replication was monitored using BKPyV quantitative real-time PCR (BK Virus R-Gene kit; bioMérieux, Marcy l’Etoile, France) in urine and blood samples collected at the time of transplantation, monthly for all patients for up to 12 months of follow-up, every 3 months during the second year after transplantation, and then every 6 months. To ensure that variations in BKPyV NAb titers were due only to i.v. Ig administration and not to recipient immune humoral responses upon viral reactivation (20), only non-replicative recipients were included in the study. Gamma globulin titers were tested in all KTR at 10 days, 3 months, and 1 year and yearly after transplantation. The research protocol was approved by the institutional review board of Strasbourg University Hospitals under reference number CPP-EST DC-2013-1990.
I.v. Ig preparations.
Three i.v. Ig preparations (human immunoglobulin derived from healthy human subjects) were tested: (i) Privigen, 100 mg/ml (CSL Behring GmbH, Marburg, Germany); (ii) Octagam, 100 mg/ml (Octapharma, Lachen, Switzerland); and (iii) Clairyg, 50 mg/ml (LFB-Biomédicaments, Les Ulis, France). Privigen was used in 30 patients (14 AMR and 16 SIDS), Octagam in 1 patient (AMR), and Clairyg in 2 patients (AMR).
Neutralization assay.
Neutralization assays were performed using a BKPyV pseudovirion system expressing the capsid proteins of BKPyV genotype I, II, or IV, as previously described (20, 26). The assay enabled the quantification of antibody titers that functionally neutralized the infectivity of the various BKPyV genotypes, each of which is known to be a distinct neutralization serotype (26). BKPyV genotype III was not represented in our study because genotype III represents less than 1% of BKPyV isolates in Europe and several studies have suggested that BKPyV genotypes II and III may belong to the same genotype (20, 32). The neutralization titer was defined as the sample dilution that yielded 50% inhibition of pseudovirion infectivity (IC50) and was expressed as the log10 of the IC50. Sera were considered nonneutralizing if the 1:100 dilution (2.0 log10) did not lead to a reduction of at least 50% of the luminometric signal relative to the control condition without serum or with the negative control (i.e., 50% neutralization of the reporter vector). A neutralization titer of 2.5 log10 IC50 was set as the threshold of antibody-mediated neutralization quantification. To minimize any variation, a single pseudovirion stock was produced for each genotype, aliquoted, and used for all experiments. The neutralizing effect of i.v. Ig (Privigen) on pseudovirions was confirmed in a culture system that used primary cells (hRPTEC), the infectious BKPyV strain Dunlop (genotype Ia), and patient-derived BKPyV strains (genotype I, genotype II, and genotype IV). The infectivity rates were measured by real-time PCR (Light Cycler 480; Roche Diagnostics GmbH, Mannheim, Germany), and the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene was used as a reference gene for normalization of the quantitative-PCR data. Each genotype was tested in duplicate, and two sets of experiments were performed.
Statistical analyses.
Statistical analyses were performed using GraphPad (San Diego, CA, USA) Prism 6 software. The distributions of continuous data were compared using nonparametric Mann-Whitney and Kruskal-Wallis tests when comparing groups of patients or i.v. Ig preparations, and Wilcoxon signed-rank tests were used for paired comparisons. The distribution of categorical variables was compared using chi-square or Fisher’s exact tests.
We used Bayesian methods (33–35) to estimate the posterior probability distribution of the BKPyV NAb titers (mixed linear models) with noninformative priors using two different models aimed at (i) comparing NAb titers against the different BKPyV genotypes in commercial preparations and (ii) assessing the dynamics of individuals’ NAb titers over time according to the i.v. Ig dosing and the baseline titer. The probability of a substantially increased NAb titer (i.e., a NAb titer of >4 log10 IC50) was calculated from the posterior distributions. Statistical interpretation of the Bayesian analysis was expressed as the probability (Pr) of observing a difference in the NAb titer greater than 0.5 log10 IC50 between two commercial preparations, two genotypes, two groups, or two time points. The 0.5 threshold corresponded to the standard deviation of the neutralization assay and was considered clinically relevant. All Bayesian analyses were performed with JAGS using the rjags package in R version 3.1.1.
Supplementary Material
ACKNOWLEDGMENTS
We thank Christopher B. Buck for expression plasmids for VP1, VP2, and VP3 and the National Cancer Institute Tumor Repository for 293TT cells.
This work was supported by grants from the Hôpitaux Universitaires de Strasbourg (Recherche Non-Interventionnelle [RNI] 2016, no. 6368), the Agence Nationale de la Recherche (ANR), Laboratoire d’Excellence TRANSPLANTEX (ANR-11-LABX-0070_TRANSPLANTEX), and Institut National de la Santé et de la Recherche Médicale (UMR_S 1109).
We have no conflicts of interest to disclose.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00393-19.
REFERENCES
- 1.Hirsch HH, Snydman DR. 2005. BK virus: opportunity makes a pathogen. Clin Infect Dis 41:354–360. doi: 10.1086/431488. [DOI] [PubMed] [Google Scholar]
- 2.Hariharan S. 2006. BK virus nephritis after renal transplantation. Kidney Int 69:655–662. doi: 10.1038/sj.ki.5000040. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J. 2002. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med 347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch HH, Randhawa P, AST Infectious Diseases Community of Practice. 2013. BK polyomavirus in solid organ transplantation. Am J Transplant 13(Suppl 4):179–188. doi: 10.1111/ajt.12110. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch HH, Vincenti F, Friman S, Tuncer M, Citterio F, Wiecek A, Scheuermann EH, Klinger M, Russ G, Pescovitz MD, Prestele H. 2013. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant 13:136–145. doi: 10.1111/j.1600-6143.2012.04320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borni-Duval C, Caillard S, Olagne J, Perrin P, Braun-Parvez L, Heibel F, Moulin B. 2013. Risk factors for BK virus infection in the era of therapeutic drug monitoring. Transplantation 95:1498–1505. doi: 10.1097/TP.0b013e3182921995. [DOI] [PubMed] [Google Scholar]
- 7.Kuypers DR. 2012. Management of polyomavirus-associated nephropathy in renal transplant recipients. Nat Rev Nephrol 8:390–402. doi: 10.1038/nrneph.2012.64. [DOI] [PubMed] [Google Scholar]
- 8.Wadei HM, Rule AD, Lewin M, Mahale AS, Khamash HA, Schwab TR, Gloor JM, Textor SC, Fidler ME, Lager DJ, Larson TS, Stegall MD, Cosio FG, Griffin MD. 2006. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN). Am J Transplant 6:1025–1032. doi: 10.1111/j.1600-6143.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 9.Barth H, Solis M, Lepiller Q, Sueur C, Soulier E, Caillard S, Stoll-Keller F, Fafi-Kremer S. 2017. 45 years after the discovery of human polyomaviruses BK and JC: time to speed up the understanding of associated diseases and treatment approaches. Crit Rev Microbiol 43:178–195. doi: 10.1080/1040841X.2016.1189873. [DOI] [PubMed] [Google Scholar]
- 10.Schaub S, Hirsch HH, Dickenmann M, Steiger J, Mihatsch MJ, Hopfer H, Mayr M. 2010. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant 10:2615–2623. doi: 10.1111/j.1600-6143.2010.03310.x. [DOI] [PubMed] [Google Scholar]
- 11.Menter T, Mayr M, Schaub S, Mihatsch MJ, Hirsch HH, Hopfer H. 2013. Pathology of resolving polyomavirus-associated nephropathy. Am J Transplant 13:1474–1483. doi: 10.1111/ajt.12218. [DOI] [PubMed] [Google Scholar]
- 12.Mayr M, Nickeleit V, Hirsch HH, Dickenmann M, Mihatsch MJ, Steiger J. 2001. Polyomavirus BK nephropathy in a kidney transplant recipient: critical issues of diagnosis and management. Am J Kidney Dis 38:E13. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch HH, Babel N, Comoli P, Friman V, Ginevri F, Jardine A, Lautenschlager I, Legendre C, Midtvedt K, Muñoz P, Randhawa P, Rinaldo CH, Wieszek A, ESCMID Study Group of Infection in Compromised Hosts. 2014. European perspective on human polyomavirus infection, replication and disease in solid organ transplantation. Clin Microbiol Infect 20(Suppl 7):74–88. doi: 10.1111/1469-0691.12538. [DOI] [PubMed] [Google Scholar]
- 14.Sener A, House AA, Jevnikar AM, Boudville N, McAlister VC, Muirhead N, Rehman F, Luke PP. 2006. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: one-year follow-up of renal allograft recipients. Transplantation 81:117–120. doi: 10.1097/01.tp.0000181096.14257.c2. [DOI] [PubMed] [Google Scholar]
- 15.Sharma AP, Moussa M, Casier S, Rehman F, Filler G, Grimmer J. 2009. Intravenous immunoglobulin as rescue therapy for BK virus nephropathy. Pediatr Transplant 13:123–129. doi: 10.1111/j.1399-3046.2008.00958.x. [DOI] [PubMed] [Google Scholar]
- 16.Anyaegbu EI, Almond PS, Milligan T, Allen WR, Gharaybeh S, Al-Akash SI. 2012. Intravenous immunoglobulin therapy in the treatment of BK viremia and nephropathy in pediatric renal transplant recipients. Pediatr Transplant 16:E19–E24. doi: 10.1111/j.1399-3046.2010.01384.x. [DOI] [PubMed] [Google Scholar]
- 17.Vu D, Shah T, Ansari J, Naraghi R, Min D. 2015. Efficacy of intravenous immunoglobulin in the treatment of persistent BK viremia and BK virus nephropathy in renal transplant recipients. Transplant Proc 47:394–398. doi: 10.1016/j.transproceed.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Kable K, Davies CD, OʼConnell PJ, Chapman JR, Nankivell BJ. 2017. Clearance of BK virus nephropathy by combination antiviral therapy with intravenous immunoglobulin. Transplant Direct 3:e142. doi: 10.1097/TXD.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randhawa P, Pastrana DV, Zeng G, Huang Y, Shapiro R, Sood P, Puttarajappa C, Berger M, Hariharan S, Buck CB. 2015. Commercially available immunoglobulins contain virus neutralizing antibodies against all major genotypes of polyomavirus BK. Am J Transplant 15:1014–1020. doi: 10.1111/ajt.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solis M, Velay A, Porcher R, Domingo-Calap P, Soulier E, Joly M, Meddeb M, Kack-Kack W, Moulin B, Bahram S, Stoll-Keller F, Barth H, Caillard S, Fafi-Kremer S. 2018. Neutralizing antibody-mediated response and risk of BK virus-associated nephropathy. J Am Soc Nephrol 29:326–334. doi: 10.1681/ASN.2017050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan SC, Tyan D, Czer L, Toyoda M. 1998. Immunomodulatory actions of intravenous actions of intravenous immunoglobulins (i.v. Ig): Potential applications in applications in solid organ transplant recipients. Pediatr Transplant 2:92–105. [PubMed] [Google Scholar]
- 22.Casadei DH, del C Rial M, Opelz G, Golberg JC, Argento JA, Greco G, Guardia OE, Haas E, Raimondi EH. 2001. A randomized and prospective study comparing treatment with high-dose intravenous immunoglobulin with monoclonal antibodies for rescue of kidney grafts with steroid-resistant rejection. Transplantation 71:53–58. doi: 10.1097/00007890-200101150-00009. [DOI] [PubMed] [Google Scholar]
- 23.Jordan SC, Tyan D, Stablein D, McIntosh M, Rose S, Vo A, Toyoda M, Davis C, Shapiro R, Adey D, Milliner D, Graff R, Steiner R, Ciancio G, Sahney S, Light J. 2004. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly-HLA sensitized adult patients with end stage renal disease: Report of the NIH IG02 trial. J Am Soc Nephrol 15:3256–3262. doi: 10.1097/01.ASN.0000145878.92906.9F. [DOI] [PubMed] [Google Scholar]
- 24.Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, Peng A, Villicana R, Jordan SC. 2008. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 359:242–251. doi: 10.1056/NEJMoa0707894. [DOI] [PubMed] [Google Scholar]
- 25.Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, El-Gamal Y, Harville TO, Hossny E, Mazer B, Nelson R, Secord E, Jordan SC, Stiehm ER, Vo AA, Ballow M. 2017. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol 139:S1–S46. doi: 10.1016/j.jaci.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Pastrana DV, Ray U, Magaldi TG, Schowalter RM, Çuburu N, Buck CB. 2013. BK polyomavirus genotypes represent distinct serotypes with distinct entry tropism. J Virol 87:10105–10113. doi: 10.1128/JVI.01189-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randhawa PS, Schonder K, Shapiro R, Farasati N, Huang Y. 2010. Polyomavirus BK neutralizing activity in human immunoglobulin preparations. Transplantation 89:1462–1465. doi: 10.1097/TP.0b013e3181daaaf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solis M, Meddeb M, Sueur C, Domingo-Calap P, Soulier E, Chabaud A, Perrin P, Moulin B, Bahram S, Stoll-Keller F, Caillard S, Barth H, Fafi-Kremer S, French BKV Study Group. 2015. Sequence variation in amplification target genes and standards influences interlaboratory comparison of BK virus DNA load measurement. J Clin Microbiol 53:3842–3852. doi: 10.1128/JCM.02145-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sueur C, Solis M, Meddeb M, Soulier E, Domingo-Calap P, Lepiller Q, Freitag R, Bahram S, Caillard S, Barth H, Stoll-Keller F, Fafi-Kremer S. 2014. Toward standardization of BK virus monitoring: evaluation of the BK virus R-gene kit for quantification of BK viral load in urine, whole-blood, and plasma specimens. J Clin Microbiol 52:4298–4304. doi: 10.1128/JCM.02031-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastrana DV, Brennan DC, Cuburu N, Storch GA, Viscidi RP, Randhawa PS, Buck CB. 2012. Neutralization serotyping of BK polyomavirus infection in kidney transplant recipients. PLoS Pathog 38: 1078–1084. doi: 10.1371/journal.ppat.1002650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilis L, Morisset S, Billaud G, Ducastelle-Leprêtre S, Labussière-Wallet H, Nicolini FE, Barraco F, Detrait M, Thomas X, Tedone N, Sobh M, Chidiac C, Ferry T, Salles G, Michallet M, Ader F, Lyon BK Virus Study Group. 2014. High burden of BK virus-associated hemorrhagic cystitis in patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 49:664–670. doi: 10.1038/bmt.2013.235. [DOI] [PubMed] [Google Scholar]
- 32.Luo C, Bueno M, Kant J, Randhawa P. 2008. Biologic diversity of polyomavirus BK genomic sequences: implications for molecular diagnostic laboratories. J Med Virol 80:1850–1857. doi: 10.1002/jmv.21281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wijeysundera DN, Austin PC, Hux JE, Beattie WS, Laupacis A. 2009. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J Clin Epidemiol 62:13–21. doi: 10.1016/j.jclinepi.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR. 1999. Methods in health service research. An introduction to Bayesian methods in health technology assessment. BMJ 319:508–512. doi: 10.1136/bmj.319.7208.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamina M, Tomlinson G, Guller U. 2009. Bayesian statistics in oncology. a guide for the clinical investigator. Cancer 115:5371–5381. doi: 10.1002/cncr.24628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.