Optimal treatment options remain unknown for infective endocarditis (IE) caused by penicillin-resistant (PEN-R) viridans group streptococcal (VGS) strains. The aims of this study were to report two cases of highly PEN-R VGS IE, perform a literature review, and evaluate various antibiotic combinations in vitro and in vivo.
KEYWORDS: animal models, combination therapy, high-level penicillin resistance, in vitro, infective endocarditis, viridans group streptococci
ABSTRACT
Optimal treatment options remain unknown for infective endocarditis (IE) caused by penicillin-resistant (PEN-R) viridans group streptococcal (VGS) strains. The aims of this study were to report two cases of highly PEN-R VGS IE, perform a literature review, and evaluate various antibiotic combinations in vitro and in vivo. The following combinations were tested by time-kill studies and in the rabbit experimental endocarditis (EE) model: PEN-gentamicin, ceftriaxone-gentamicin, vancomycin-gentamicin, daptomycin-gentamicin, and daptomycin-ampicillin. Case 1 was caused by Streptococcus parasanguinis (PEN MIC, 4 μg/ml) and was treated with vancomycin plus cardiac surgery. Case 2 was caused by Streptococcus mitis (PEN MIC, 8 μg/ml) and was treated with 4 weeks of vancomycin plus gentamicin, followed by 2 weeks of vancomycin alone. Both patients were alive and relapse-free after ≥6 months follow-up. For the in vitro studies, except for daptomycin-ampicillin, all combinations demonstrated both synergy and bactericidal activity against the S. parasanguinis isolate. Only PEN-gentamicin, daptomycin-gentamicin, and daptomycin-ampicillin demonstrated both synergy and bactericidal activity against the S. mitis strain. Both strains developed high-level daptomycin resistance (HLDR) during daptomycin in vitro passage. In the EE studies, PEN alone failed to clear S. mitis from vegetations, while ceftriaxone and vancomycin were significantly more effective (P < 0.001). The combination of gentamicin with PEN or vancomycin increased bacterial eradication compared to that with the respective monotherapies. In summary, two patients with highly PEN-R VGS IE were cured using vancomycin-based therapy. In vivo, regimens of gentamicin plus either β-lactams or vancomycin were more active than their respective monotherapies. Further clinical studies are needed to confirm the role of vancomycin-based regimens for highly PEN-R VGS IE. The emergence of HLDR among these strains warrants caution in the use of daptomycin therapy for VGS IE.
INTRODUCTION
Viridans group streptococci (VGS) are the second most common agent of infective endocarditis (IE) worldwide (1, 2) and remain the most common cause of IE in developing countries (3). Although the overall incidence of VGS IE has declined during the last 2 decades (4), these infections are challenging for clinicians, especially when the causative strains are antibiotic resistant.
The prevalence of VGS strains with reduced susceptibility to penicillin (PEN) and other antibiotics has been increasing in recent years (5). For example, the rates of “relative resistance” (MIC, ≥0.12 to <0.5 μg/ml) and resistance (MIC, ≥0.5 μg/ml) to PEN among VGS range from 5% to 20%. High-level PEN resistance (PEN-R; MIC, ≥4 μg/ml) in VGS is generally caused by modifications in PEN-binding proteins (6). Recent data suggest that the development of PEN-R among oral cavity VGS isolates is related to the excessive use of oral β-lactams (6–9).
As described above, the American Heart Association defines relative PEN resistance and “full” PEN-R in VGS based on PEN MICs of ≥0.12 to <0.5 μg/ml and ≥0.5 μg/ml, respectively (1). In contrast, the European Society of Cardiology defines intermediate PEN-R and full PEN-R in VGS by penicillin MICs of 0.25 to 2 μg/ml and ≥4.0 μg/ml, respectively (2). Both scientific groups and others, however, agree that the clinical experience in the treatment of IE caused by high-level PEN-R VGS is very limited, and optimal treatment remains problematic (1, 2, 10). For therapy of IE caused by such VGS, guidelines recommend regimens somewhat similar to those used for treating enterococcal IE (e.g., PEN or ceftriaxone plus gentamicin, or vancomycin alone) (1, 2).
Little published data exist regarding alternatives to vancomycin-based regimens for treating highly PEN-R VGS. In vitro, the lipoglycopeptides, telavancin, oritavancin, and dalbavancin all show good activity against VGS, with an overall MIC90 of ≤0.06 μg/ml (11–13) for susceptible VGS, as does dalbavancin for highly PEN-R strains (14). The daptomycin MIC90 was 1 μg/ml in our large collection of Streptococcus mitis, suggesting that this agent might be a plausible alternative for β-lactam-resistant VGS (15). However, high-level daptomycin resistance (HLDR) develops rapidly and frequently both in vitro and in vivo among the Streptococcus mitis-Streptococcus oralis VGS subgroup (15). Meanwhile, no significant clinical experience is available for lipoglycopeptides against highly PEN-R VGS. Ceftaroline has excellent in vitro activity against VGS strains (16), including highly PEN-R strains (17), but clinical experience is also lacking in this scenario.
In this study, we describe two cases of IE due to highly PEN-R VGS successfully treated with vancomycin-based therapy and delineate (i) their clinical treatment outcomes, and (ii) the in vitro and in vivo activities of different antibiotic combinations against such VGS strains, relevant to treating IE.
(Selected aspects of the data presented in this study were reported in part at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], 27 to 30 September 2006, San Francisco, CA, [abstract B-1818] [18]; at the 55th ICAAC/International Congress of Chemotherapy and Infection [ICC], San Diego, CA, 17 to 21 September 2015 [abstract C-656] [19]; and at the 13th International Symposium on Modern Concepts on Endocarditis and Cardiovascular Infections [ISCVID], Rio de Janeiro, Brazil, 4 to 6 June 2015 [abstract 28] [20].)
RESULTS
Case reports.
(i) Case 1. The case 1 patient was a 49-year-old man with a history of intravenous drug use who 6 months earlier had undergone aortic bioprosthetic valve replacement for VGS native valve IE. The patient now presented with low-grade fever, chills, lightheadedness, dizziness, and palpitations. He denied recent dental or other procedures, sick contacts, or ongoing substance abuse. His last intravenous drug use was more than 1 year previously. On admission, he was afebrile, had a heart rate of 40 beats/minute, and had normal blood pressure. There was a systolic ejection murmur noted but no peripheral stigmata of IE. Electrocardiogram revealed third-degree heart block. Multiple sets of blood cultures were positive for VGS, later identified to the species level as Streptococcus parasanguinis (SPAR-8497). Initial empirical treatment was started with vancomycin (dosage adjusted to achieve trough plasma concentrations of 15 to 20 μg/ml) plus ceftriaxone (2 g/24 h). Transesophageal echocardiography (TEE) on admission demonstrated a well-seated aortic valve bioprosthesis without vegetations. Although he remained stable hemodynamically, 3 days later when blood cultures were negative, a temporary pacemaker was placed.
Susceptibility testing of the VGS revealed high-level PEN-R (MIC, 4.0 μg/ml), as well as ceftriaxone resistance, but vancomycin susceptibility (Table 1). Treatment was continued with vancomycin alone. Repeat TEE done on hospital days 3 and 8 revealed a 1- by 0.5-cm mobile echodensity on the aortic valve consistent with a vegetation and an echolucent crescent-shaped area suspicious for a periannular abscess cavity. A positron emission tomography-computed tomography (PET-CT) scan performed on hospital day 11 revealed increased 18-FDG* (18F-FDG*) uptake around the prosthetic aortic valve. His aortic valve was replaced with a 23-mm St. Jude Trifecta valve on hospital day 14. No overt abscess cavity was observed; however, there was tissue destruction in the aortic valve root area thought to be consistent with early abscess formation. Valve and periannular tissue cultures were negative. Although his postoperative course was complicated by acute kidney injury and heart failure, he recovered and was discharged in stable condition to a rehabilitation facility on hospital day 27. He completed a 6-week course of vancomycin. He was alive and relapse-free at 1 year follow-up.
TABLE 1.
Broth microdilution method antibiotic susceptibilities of the two strains used in the in vitro studies
| Antibiotic | MIC (μg/ml) for: |
|
|---|---|---|
| SPAR-8497 | SMIT-351 | |
| Penicillin | 4 | 8 |
| Ampicillin | 16 | 16 |
| Ceftriaxone | 4 | 4 |
| Gentamicin | 2 | 8 |
| Daptomycin | 2 | 0.5 |
| Vancomycin | 0.25 | 0.5 |
(ii) Case 2. The case 2 patient was a 37-year-old man with a history of intravenous drug use. Seven months earlier, he underwent mitral and aortic valve replacements due to native valve IE caused by Abiotrophia defectiva. At this present admission, he described 2 weeks of fever that followed a dental cleaning procedure. On admission, there were no signs of cardiac failure, new murmurs, or peripheral embolization on physical examination. Vancomycin (to achieve ∼20 μg/ml trough serum levels) and gentamicin (240 mg/day in three divided doses) were started empirically. Blood cultures yielded S. mitis (SMIT-351; PEN MIC, 8 μg/ml). TEE showed a 7-mm vegetation on the mitral bioprosthesis; no prosthetic dysfunction was noted in either valve. Abdominal-pelvic CT scans revealed no visceral emboli. Blood cultures 72 h after the initiation of antibiotic treatment were negative.
The patient received 4 weeks of vancomycin plus gentamicin (stopped due to acute renal failure that resolved after discontinuation of gentamicin), followed by 2 weeks of vancomycin alone. He did not require cardiac surgery. Blood cultures remained negative at the 10-month follow-up. He developed progressive mitral valve dysfunction and heart failure 10 months after the episode of IE and died due to complications after cardiac surgery. Surgical pathology and necropsy revealed no evidence of active IE.
In vitro studies.
The antibiotic susceptibilities of the two strains used in the in vitro studies are displayed in Table 1. The PEN MIC of SPAR-8497 was 4 μg/ml; the MICs for ceftriaxone and vancomycin were 4 μg/ml and 0.25 μg/ml, respectively. For SMIT-351, the MICs for PEN, ceftriaxone, and vancomycin were 8, 4, and 0.5 μg/ml, respectively.
The results of time-kill studies are summarized in Table 2 and shown graphically in Fig. S1 and S2 in the supplemental material. Against SPAR-8497, all combinations were both bactericidal and synergistic, except for daptomycin plus ampicillin, which was synergistic but not bactericidal. Against SMIT-351, combinations of PEN or daptomycin with gentamicin and of daptomycin plus ampicillin were each synergistic and bactericidal. Ceftriaxone plus gentamicin was synergistic but not bactericidal, while the combinations of vancomycin plus gentamicin and daptomycin plus ceftriaxone were indifferent.
TABLE 2.
Qualitative results of time-killing studies of the two VGS strains obtained from blood cultures of IE cases 1 and 2a
| Antibiotic combination | Qualitative result for strain: |
|
|---|---|---|
| SPAR-8497 | SMIT-351 | |
| Penicillin + gentamicin | Synergy/bactericidal | Synergy/bactericidal |
| Ceftriaxone + gentamicin | Synergy/bactericidal | Synergy |
| Vancomycin + gentamicin | Synergy/bactericidal | Indifference |
| Daptomycin + gentamicin | Synergy/bactericidal | Synergy/bactericidal |
| Daptomycin + ampicillin | Synergy | Synergy/bactericidal |
| Daptomycin + ceftriaxone | Synergy/bactericidal | Indifference |
The inoculum was equal to 106 CFU/ml.
Of interest, we have previously shown the propensity of VGS to develop high-level daptomycin resistance in vitro and in vivo (15). In vitro susceptibility testing of organisms that remained viable after time-kill studies detected the emergence of high-level daptomycin resistance (MIC, ≥512 μg/ml) in both study strains after 24 h of exposure to daptomycin alone at inhibitory or subinhibitory concentrations (previously described for strain SMIT-351 [15]). Similarly, from the different daptomycin combination regimens tested in vitro, we detected two daptomycin-resistant isolates following daptomycin plus ampicillin exposures (daptomycin MICs, 48 μg/ml and 4 μg/ml).
In vivo studies.
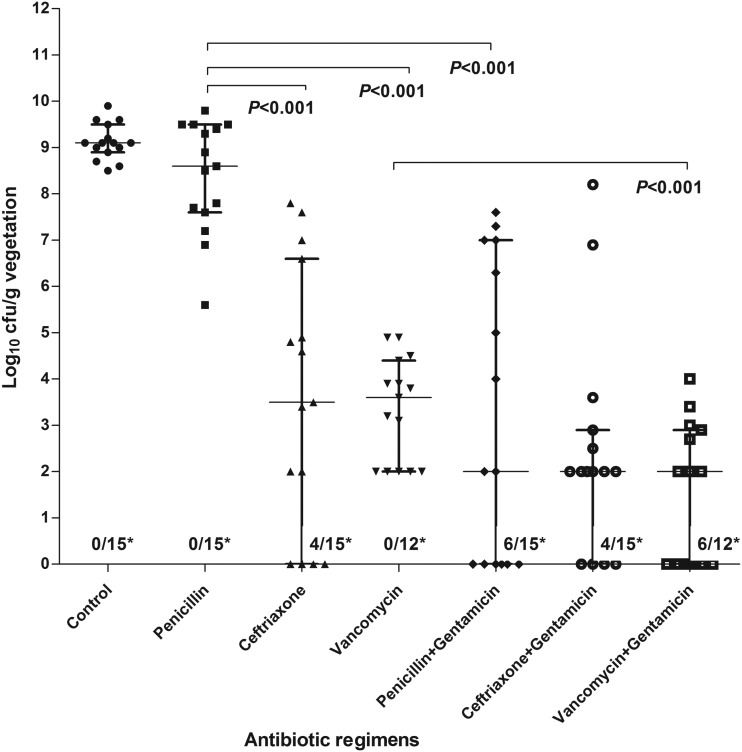
The activities of drugs in monotherapy or combination therapy against SMIT-351 (chosen for EE studies due its higher PEN MIC than that of SPAR-8497) are summarized in Fig. 1 and Table 3. Except for PEN monotherapy (P = 0.134), all antibiotics alone or in combination with gentamicin were more active than the control group in reducing intravegetation bacterial densities. Both ceftriaxone and vancomycin monotherapies were significantly more effective (P < 0.001) than the PEN regimen. Retest by Etest of organisms residual after vancomycin treatment did not detect any change in vancomycin MICs. The combined regimens of PEN plus gentamicin and vancomycin plus gentamicin were more active than PEN and vancomycin monotherapies, respectively, in increasing the proportion of sterile vegetations (P = 0.017 and 0.014, respectively) and reducing the density of bacteria within vegetations (P < 0.001 and <0.001, respectively). Regimens of ceftriaxone with or without gentamicin were equally effective in reducing or eradicating organisms from vegetations (P = 1.000 and 0.267). In a comparison of the three antibiotic combination therapies, we did not find any statistical significant difference among them (P > 0.05 for all comparisons). However, taking into consideration both the reductions in bacterial counts and sterilization of vegetations, vancomycin plus gentamicin was the most active regimen in this EE model. Of note, among the 15 animals treated with PEN-gentamicin, 7 had only modest reductions in bacterial counts per gram of vegetation (Fig. 1).
FIG 1.
Treatment of experimental endocarditis caused by the SMIT-351 strain. Densities of the SMIT-351 strain (log10 CFU per gram of vegetation) in the IE model without antibiotic treatment and the different antibiotic treatment arms. For each group, individual data for each rabbit are represented by a dot. The medians and interquartile ranges are represented by horizontal and vertical bars, respectively. *, number of rabbits with sterile vegetations/total number of rabbits.
TABLE 3.
Treatment of experimental endocarditis caused by SMIT-351 straina
| Antibiotic group | No. of sterile veg/total no. of veg (%) | CFU log10/g veg (median [IQR]) |
|---|---|---|
| Control | 0/15 (0) | 9.1 (9–9.6) |
| Penicillin | 0/15 (0)a | 8.6 (7.6–9.4)b,c,d |
| Ceftriaxone | 4/15 (27)e | 3.5 (0–6.6)b,f |
| Vancomycin | 0/12 (0)g | 3.4 (2–4)c,h |
| Penicillin + gentamicin | 6/15 (38)a | 2 (0–6.6)d |
| Ceftriaxone + gentamicin | 4/15 (27)e | 2 (1–2.7)f |
| Vancomycin + gentamicin | 6/12 (50)g | 1 (0–2.2)h |
Each letter shows the comparison between two antibiotic groups: a, P = 0.017; b, P < 0.001; c, P < 0.001; d, P < 0.001; e, P = 1.000; f, P = 0.267; g, P = 0.014; h, P < 0.001.
Literature review.
The initial search identified 32 articles, of which only 6 contained sufficient clinical data to meet our analytic requirements (21–26). In the secondary search, two additional publications were found; these yielded three relevant IE cases (27, 28). In total, 17 cases of IE caused by high-level PEN-R VGS were found. The clinical and therapeutic features of these 17 cases plus our two current cases are shown in Table 4. Antibiotic treatment was very heterogeneous. Of the 19 cases, 2 patients died during active therapy for IE (one treated with PEN plus streptomycin, and the other treated with vancomycin plus gentamicin), and 2 others died months after an apparent cure of IE. Of those cured, 5 were treated with PEN or ceftriaxone plus an aminoglycoside, 6 were treated with a β-lactam agent alone, 2 were treated with vancomycin alone, and 3 were treated with various combinations of vancomycin or ceftriaxone plus gentamicin. One patient was reportedly cured with gentamicin alone.
TABLE 4.
Clinical and therapeutic features of reported cases of infective endocarditis due to high resistance to PENa
| Patient no. by study (reference) | Yr diag-nosed | Species | PEN MIC (μg/ml) | Type of endocarditis | Treatment (duration)b | Outcome |
|---|---|---|---|---|---|---|
| Garrod and Waterworth (27) | ||||||
| 1 | 1962 | ND | 4.0 | Native | SM (7 days), PEN plus SM (10 days) | Cured |
| 2 | 1962 | ND | 8.0 | Native | PEN plus ERT, PEN plus SM (6 wk) | Cured |
| Doyle et al. (28) | ||||||
| 3 | 1967 | ND | 10.0 | Native | PEN plus SM plus probenecid (3 wk) | Cured |
| Knoll et al. (24) | ||||||
| 4 | 1978 | ND | 4.0 | Native | PEN G (3 wk), SM (2 wk) | Relapsed, died |
| 5 | 1987 | Streptococcus sanguinis | 4.0 | Prosthetic | PEN G (4 wk) | Cured |
| 6 | 1987 | S. sanguinis | 4.0 | Prosthetic | GM (4 wk) | Cured |
| Levitz (21) | ||||||
| 7 | 1999 | Streptococcus mitis | >4.0 | Prosthetic | VAN (4 wk) | Cured |
| Levy et al. (23) | ||||||
| 8 | 2001 | S. sanguinis | >4.0 | Native | VAN plus GM (16 days) | Died during admission |
| Sabella et al. (22) | ||||||
| 9 | 2001 | S. mitis | 4.0 | Native | VAN plus CEF (6 wk) plus GM (4 wk) | Cured |
| Nandhakumar et al. (25) | 2008 | |||||
| 10 | ND | Streptococcus oralis | 4 | Native | CEF, AMP (4 wk total) | Cured |
| 11 | ND | S. oralis | 4 | Native | AMP, CIPR (4 wk total) | Cured |
| 12 | ND | S. oralis | 16 | Native | CEF, AMP (4 wk total) | Cured |
| 13 | ND | S. oralis | 8 | Native | AMP, CEF (4 wk total) | Cured |
| 14 | ND | S. oralis | 8 | Native | PEN G (4 wk) | Cured |
| 15 | ND | S. oralis | 4 | Native | CEF plus GM (4 wk) | Cured |
| 16 | ND | S. oralis | 4 | Native | CEF plus GM (4 wk) | Cured |
| Fujitani et al. (26) | ||||||
| 17 | 2008 | Streptococcus parasanguinis | 4.0 | Native | VAN plus GM (10 days), CEF plus GM (7 days), VAN (6 wk) | Cured, but died of unknown cause 4 mo later |
| Present study | ||||||
| 18 | 2016 | S. parasanguinis | 4.0 | Prosthetic | VAN plus CEF (4 days), VAN (6 wk) | Cured |
| 19 | 2001 | S. mitis | 8.0 | Prosthetic | VAN plus GM (4 wk), VAN (2 wk) | Cured, but died 10 mo after IE admission |
High resistance was defined as an MIC of ≥4 μg/ml. ND, not determined.
SM, streptomycin; PEN, penicillin; ERT, erythromycin; GM, gentamicin; VAN, vancomycin; CEF, ceftriaxone; AMP, ampicillin; CIPR, ciprofloxacin.
DISCUSSION
Treatment guidelines for IE due to VGS that are “resistant to PEN” have been promulgated for more than 3 decades. Current guidelines are not evidence based but rather are based on consensus expert opinion and on a very limited number of case reports (1, 2). Consequently, additional clinical reports and experimental studies that help clarify this difficult therapeutic arena, particularly those addressing IE caused by highly PEN-R VGS, are likely to be valuable.
The current report is one of the first providing both experimental (in vitro and in vivo) and clinical data related to antibiotic treatment of IE caused by highly PEN-R VGS strains (PEN MICs, ≥4 μg/ml). As pointed out by Le and Bayer (29), data documenting a correlation between favorable clinical outcomes and synergy between β-lactams and aminoglycosides are relatively scarce.
Our investigations yielded several interesting observations. First, the combination of PEN plus gentamicin showed significant synergy and bactericidal activity in time-kill studies against both of our highly PEN-R strains. Additionally, this combination was heterogeneously effective against SMIT-351 (PEN MIC, 8 μg/ml) in the animal model, with marked reduction of bacterial densities in only 8 of the 15 animals. Fujitani et al. (26) similarly found in vitro synergy for this combination against a high-level-PEN-R S. parasanguinis strain causing IE. As we found with the SMIT-351 strain, Fujitani et al. also noted that ceftriaxone plus gentamicin had less activity against their isolate than did the combination of PEN plus gentamicin. These data provide some support for the treatment of IE caused by highly PEN-R VGS with a combination of a β-lactam plus gentamicin, as noted in our literature review (Table 4).
Second, interestingly, in the in vivo model of EE, regimens using gentamicin plus either PEN or vancomycin were more active than their respective monotherapies against highly PEN-R VGS IE. Using the same rabbit model of EE, Martínez et al. (30) tested different combinations of antibiotics against a strain of Streptococcus sanguinis, with a PEN MIC of 8 μg/ml. The combination of PEN plus gentamicin was not synergistic against this strain, while the most active combination was vancomycin plus gentamicin. These observations coupled with the limited impact of the penicillin-plus-gentamicin regimen in 7 of our animals with EE gave pause to routinely accepting PEN plus gentamicin as optimal therapy for IE caused by highly PEN-R VGS. Furthermore, although the combination of gentamicin with PEN, ceftriaxone, or vancomycin in vivo in the animal model yielded significantly greater antimicrobial activity than with their respective monotherapies, the overall activity of these combinations was relatively inferior to that of β-lactam monotherapy against fully PEN-susceptible VGS (31, 32).
Third, the in vitro and in vivo studies of our isolates and the successful treatment of our two patients add to data supporting the potential utility of vancomycin alone or in combination with gentamicin (potential nephrotoxicity not withstanding) in the treatment of IE caused by highly PEN-R VGS.
Finally, despite our current compelling observations and our review of the literature, available laboratory and clinical data remain too limited to draw firm and consensus conclusions regarding the clinical treatment of IE caused by highly PEN-R VGS. As noted in Table 4, including our cases, there are only 19 reported cases of this entity. Of these, 17 cases have been treated successfully. Six of them received a β-lactam–aminoglycoside combination, 2 were treated with a combination of vancomycin and aminoglycosides, 5 with a β-lactam monotherapy, 1 with vancomycin monotherapy, 1 with gentamicin monotherapy, 1 with vancomycin plus ceftriaxone, and 1 with ampicillin plus ciprofloxacin. Notably, of the 2 cases that failed to therapy, one was treated with β-lactam plus aminoglycoside combination, and the other with vancomycin plus aminoglycoside combination. Not only was there notable heterogeneity in the type of antibiotics used for treatment, but the length of therapy among these 17 cases was surprisingly short. Only 5 cases were treated for longer than 4 weeks, and among the other 12 cases, 4 patients were treated for less than 4 weeks.
Daptomycin is the only lipoglycopeptide with published in vivo data against VGS strains causing IE. However, following exposure to daptomycin in monotherapy, rapid development of HLDR occurs in at least 25% of S. mitis group strains in vitro; this phenomenon was replicated in vivo in EE (15). Of interest, recent investigations utilizing either the rabbit IE model or an ex vivo model featuring simulated endocardial vegetations suggest that daptomycin plus gentamicin (15) or daptomycin plus advanced-generation cephalosporins (ceftriaxone or ceftaroline) (17), respectively, can circumvent emergence of HLDR in VGS strains; however, clinical data for such combinations are lacking. We found that daptomycin plus ampicillin was synergistic in time-kill curves against both tested strains; however, HLDR was detected twice in SPAR-8497 residual after this exposure. This combination was bactericidal against the S. mitis strain. Daptomycin plus ampicillin has been shown to be synergistic, and bactericidal activity against enterococci (33) has prevented or reduced the emergence of daptomycin resistance in enterococci and Staphylococcus aureus (34, 35). Furthermore, this combination has been effective in the treatment of patients with Enterococcus faecalis endocarditis (36). Our results with the combination of daptomycin and ceftriaxone, ampicillin, or gentamicin in vitro suggest some potential for daptomycin combination regimens against PEN-R VGS endocarditis. However, the emergence of HLDR following the in vitro exposure of SPAR-8497 to daptomycin-ampicillin warrants caution and further investigation.
The current study has several limitations. Our clinical data come from only two patients, which precludes any generalization of outcomes. They do, however, add to the overall data regarding the treatment of IE caused by PEN-R VGS, especially using vancomycin-based regimens. Also, daptomycin was tested only in the in vitro studies. Moreover, only one of the two strains from clinical cases was included in the in vivo studies. Last, vancomycin dosing strategies in our experimental IE studies were not designed to achieve an area under the concentration-time curve over 24 h in steady state divided by the MIC (AUC/MIC) of >400 μg/ml, a target that has been correlated with favorable outcomes in S. aureus bacteremia clinically (37–39).
Our study contributes two additional cases of IE caused by highly PEN-R VGS which were cured with vancomycin-based regimens. It also explores alternative regimens that might be used to effectively treat IE caused by such organisms, and it underscores the potential vulnerability of daptomycin monotherapy and possibly daptomycin plus β-lactam combination therapy, namely, the emergence of HLDR in vitro in these settings. In vivo, in terms of the combined metric of reductions in bacterial density in vegetations plus vegetation sterilizations, regimens of gentamicin plus either PEN or vancomycin were more active than their respective monotherapies. We urge the performance of additional experimental and clinical studies to confirm the role of vancomycin-based regimens for highly PEN-R VGS IE.
MATERIALS AND METHODS
Design.
The study design consisted of clinical descriptions of two IE cases caused by highly PEN-R VGS, in vitro antibiotic susceptibility studies of the initial bloodstream isolates from each case, testing of optimal antibiotic efficacy with one of these isolates in the rabbit model of EE, and a literature review of previously reported clinical cases of highly PEN-R VGS IE and their antibiotic therapies.
Setting.
The study was conducted in two urban tertiary-care hospitals, with one in Spain (Barcelona) and one in the United States (Boston, MA).
Study microorganisms.
Two highly PEN-R VGS strains were isolated from blood cultures of two patients diagnosed with pulmonary valve (PV) IE, as follows: Streptococcus parasanguinis strain SPAR-8497, isolated from Beth Israel Deaconess Medical Center, Boston, MA; and Streptococcus mitis-S. oralis strain SMIT-351, isolated from Hospital Clínic-IDIBAPS, Barcelona, Spain.
The blood samples were obtained as a part of the routine clinical management of patients with IE in both institutions. These were processed routinely for identification and antimicrobial susceptibilities in the respective clinical microbiology laboratories. They were then considered “discarded specimens”; thus, obtaining an informed consent for use of these samples in our research laboratories was deemed unnecessary, according to national regulations.
In vitro studies.
(i) Identification. The species identification of the two bloodstream VGS isolates was carried out by a phenotypic method (API Rapid ID32 Strep system) supported by a molecular method (16S rRNA ribotyping).
(ii) Antibiotic susceptibility testing. MICs were tested by broth microdilution assays using Mueller-Hinton broth supplemented with 5% lysed horse blood (40). For daptomycin, broth was supplemented with 50 mg/liter calcium chloride, according to the CLSI recommendations (41). Streptococcus pneumoniae ATCC 49619 was used as a quality control strain.
(iii) Time-kill studies. Time-kill study (TKS) methodology was used to test the activity of single versus combined antibiotic regimens against both strains, according to previously described criteria (42). Mueller-Hinton broth medium supplemented with horse blood was used to determine the TK curves, as previously described (15, 43). A final inoculum between 5 × 105 and 1 × 106 CFU/ml was used. Concentrations between 0.5 and 1 times the MICs of the study strains were chosen for synergy testing.
Definitions.
Synergy was defined as at least a 2-log10 decrease in the number of CFU per milliliter between the combination regimens compared to that with the most active single agent after 24 h. To qualify, the number of surviving organisms in the presence of the combination had to be at least 2 log10 CFU/ml less than the starting inoculum. At least one of the drugs in a combination of interest had to be present in a concentration that did not significantly affect the growth curve of the test organism when used alone (43). Bactericidal activity for antibiotics alone or in combination was defined a reduction from the initial inoculum of ≥3 log10 in CFU at 24 h. Indifference was defined when a reduction in CFU counts of ≤2 log10 was achieved with the antibiotic combination versus the most active single agent.
In vivo studies.
(i) Animal model. Female New Zealand White rabbits (body weight, ∼2.5 kg) provided by San Bernardo Farms (Pamplona, Spain) were used. Housing took place in the animal facilities of the University of Barcelona, School of Medicine, which are equipped with a high-efficiency particulate air filter in an automatic air exchange system, as well as circadian light cycles. The rabbits were fed ad libitum. The Committee of Animals Ethics of the University of Barcelona approved all animal experimentation in this study.
(ii) Human-like pharmacokinetic studies. All antibiotics were administered by using a computer-controlled infusion pump system designed to reproduce human-equivalent serum pharmacokinetic (PK) in rabbits after an intravenous (i.v.) infusion. Drug doses were chosen to simulate the human-like PK profiles of PEN (4 million units/4 h), vancomycin (1 g/12 h), daptomycin (6 mg/kg of body weight every 24 h), ampicillin (2 g/4 h), ceftriaxone (2 g/24 h), and gentamicin (240 mg/24 h).
(iii) Experimental aortic valve IE model. EE was induced according to the method described by Garrison and Freedman (44). Briefly, a catheter was inserted through the right carotid artery into the left ventricle of anesthetized rabbits; a separate catheter used for antibiotic administration was placed into the inferior vena cava through the jugular vein. The infusion pump delivered 2 ml/h of 0.9% saline solution until the beginning of antimicrobial administration. Twenty-four hours later, each animal was inoculated via the marginal ear vein with strain SMIT-351 (1 ml of 1 × 108 CFU/ml). This inoculum has been shown to be greater than or equal to the 95% infective dose (ID95) for this strain in the EE model (15). Before the initiation of antimicrobial therapy, 1 ml of blood was obtained to confirm bacteremia (i.e., successful induction of EE). Antibiotic treatments were then started at 24 h of challenge, and animals were treated for 48 h. After the completion of treatment, an additional 6 drug half-lives were allowed to elapse before the animals were sacrificed to limit antibiotic carryover effects (animals were anesthetized and humanely euthanized by an i.v. bolus of pentobarbital, as per institutional and national guidelines). Aortic valve vegetations were obtained, weighed, and homogenized in 2 ml of saline solution, and quantitative and qualitative cultures were then performed.
(iv) Treatment groups. The infected rabbits were separated into the different treatment arms simulating human PK, as follows: monotherapy regimens were PEN, ceftriaxone, or vancomycin, and combination regimens were PEN plus gentamicin, ceftriaxone plus gentamicin, and vancomycin plus gentamicin. The results of daptomycin monotherapy and combined with gentamicin against SMIT-351 have been previously published and were not repeated (15).
(v) Analysis of endocardial vegetations. The CFU counts recovered from vegetations were expressed as the median log10 CFU per gram of vegetation (veg). Individual counts were assigned a value of 2 log10 CFU/g veg if there was no growth on the quantitative plates, but qualitative cultures from the homogenates incubated for 7 additional days were positive. The result was assigned a value of zero if both the qualitative and quantitative cultures were negative. Representative isolates recovered from vegetations from all treatment groups were stored, and their MICs were retested for the study drugs to detect the potential emergence of in vivo antibiotic resistance.
Statistical analysis.
Results were expressed as the median and the interquartile range (IQR) of the number of log10 CFU/g veg. The Mann-Whitney nonparametric test was used to compare the log10 CFU/g of valve tissue values among the different treatment groups. Fisher’s exact test was used to compare treatment groups for their rates of vegetation sterilization.
Review of the literature.
We searched PubMed to identify peer-reviewed reports of cases of IE due to highly PEN-R VGS published from January 1960 to July 2017 in all languages. The search criteria included the following terms: “viridans streptococci,” “endocarditis,” “PEN resistance,” “high-level PEN resistance”-“endocarditis,” “streptococci,” and “non-susceptible viridans streptococci”). We included only articles reporting both the clinical and antibiotic treatment data for adult patients with definite endocarditis (by modified Duke Criteria [45]) caused by VGS with a PEN MIC of ≥4 μg/ml and with at least 6 months follow-up. In a secondary strategy, cases from articles, books, or conference communications that fulfilled the above-mentioned criteria were also included.
Ethics approval.
Information for the patient admitted to the Hospital Clínic of Barcelona was included in the database of the International Collaboration on Endocarditis, coordinated by the Duke Clinical Research Institute (NC, USA), for which the institutional review board (IRB) of the Hospital Clínic de Barcelona provided approval. Regarding the other patient, the Committee on Clinical Investigations (the institutional review board) of the Beth Israel Deaconess Medical Center waived the need for committee approval and patient consent for publication based on the use of a discarded clinical specimen and deidentification of patient-related data. This article does not contain any individual person’s data that might lead to the identification of the two patients included in the study as the two the IRB confirmed.
Supplementary Material
ACKNOWLEDGMENTS
The data sets generated and/or analyzed during the current study are not publicly available due to confidentiality reasons related to ongoing research but are available from the corresponding author upon reasonable request.
J.M.M. has received consulting honoraria and/or research grants from AbbVie, Angelini, Bristol-Myers Squibb, Contrafect, Medtronic, Merck, Novartis, Gilead Sciences, and ViiV. F.M. has received consulting honoraria from Novartis and Janssen-Cilag. A.S.B. received research grants from Astellas, ContraFect, Intron, Theravance, and Trellis. A.W.K. receives honoraria for serving on data safety committee monitoring studies sponsored by Pfizer and Merck. All other authors declare no conflicts of interest.
This work was supported by the Ministerio de Sanidad y Consumo of Spain (grant FIS 02/0322, Instituto de Salud Carlos III, Madrid, Spain), a medical school grant from Cubist Pharmaceuticals, Inc. (Lexington, MA), the Spanish Network for Research in Infectious Diseases (grant REIPI RD06/0008), and by the Fundación Máximo Soriano Jiménez (Barcelona, Spain). J.M.P. received a “Rio Hortega” research grant (CM14/00135, 2015 to 2016) from the Instituto de Salud Carlos III and the Ministerio de Economia and Competitividad, Madrid (Spain). J.M.M. received a personal 80:20 research grant from the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain, during 2017 to 2019. The European Regional Development Fund (ERDF) “A way to build Europe” also provided funding. A.S.B. was supported in part by a grant from the National Institutes of Health, (S)-NIAID R01-AI130056. None of the funding bodies had any role in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
Author contributions were as follows: J.M.P., R.N., A.S.B., J.M.M., and A.W.K. conceived and designed the study; J.M.P., R.N., C.G.-D.-L.-M., C.F., J.A., M.A., and J.G.-G. collected the data; J.M.P., R.N., C.G.-D.-L.-M., A.S.B., J.M.M., and A.W.K. analyzed the data; J.M.P., R.N., C.G.-D.-L.-M., A.S.B., J.M.M., and A.W.K. drafted the manuscript; and C.F., J.A., M.A., J.G.-G., E.Q., F.M., A.M., and A.W.K. critically revised the manuscript. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work.
There are no specific acknowledgments related to personal assistance, endorsement, or approval of the views reflected in the article beyond the role of the authors.
The members of the Hospital Clínic Endocarditis Study Group, Hospital Clínic-IDIBAPS, University of Barcelona School of Medicine, Barcelona, Spain, are Jose M. Miró, Juan Ambrosioni, Juan M. Pericàs, Adrian Téllez, Marta Hernandez-Meneses, Delia Garcia-Pares, and Asunción Moreno (Infectious Diseases Service); Cristina Garcia de la Maria and Javier Garcia-González (Experimental Endocarditis Laboratory); Manel Almela, Climent Casals, Francesc Marco, and Jordi Vila (Microbiology Service); Eduard Quintana, Elena Sandoval, Juan C. Paré, Carlos Falces, Daniel Pereda, Ramon Cartañá, Salvador Ninot, Manel Azqueta, Marta Sitges, Barbara Vidal, José L. Pomar, Manuel Castella, José M. Tolosana, and José Ortiz (Cardiovascular Institute); Guillermina Fita and Irene Rovira (Anesthesiology Department); David Fuster (Nuclear Medicine Service); Jose Ramírez (Pathology Department); Mercè Brunet (Toxicology Service); Dolors Soy (Pharmacy Service); Pedro Castro (Intensive Care Unit); and Jaume Llopis (Department of Statistics, Faculty of Biology, University of Barcelona).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00516-19.
Contributor Information
Jose M. Miró, Infectious Diseases Service
Juan Ambrosioni, Infectious Diseases Service.
Juan M. Pericàs, Infectious Diseases Service
Adrian Téllez, Infectious Diseases Service.
Marta Hernandez-Meneses, Infectious Diseases Service.
Delia Garcia-Pares, Infectious Diseases Service.
Asunción Moreno, Infectious Diseases Service.
Cristina Garcia de la Maria, Experimental Endocarditis Laboratory.
Javier Garcia-González, Experimental Endocarditis Laboratory.
Manel Almela, Microbiology Service.
Climent Casals, Microbiology Service.
Francesc Marco, Microbiology Service.
Jordi Vila, Microbiology Service.
Eduard Quintana, Cardiovascular Institute.
Elena Sandoval, Cardiovascular Institute.
Juan C. Paré, Cardiovascular Institute
Carlos Falces, Cardiovascular Institute.
Daniel Pereda, Cardiovascular Institute.
Ramon Cartañá, Cardiovascular Institute.
Salvador Ninot, Cardiovascular Institute.
Manel Azqueta, Cardiovascular Institute.
Marta Sitges, Cardiovascular Institute.
Barbara Vidal, Cardiovascular Institute.
José L. Pomar, Cardiovascular Institute
Manuel Castella, Cardiovascular Institute.
José M. Tolosana, Cardiovascular Institute
José Ortiz, Cardiovascular Institute.
Guillermina Fita, Anesthesiology Department.
Irene Rovira, Anesthesiology Department.
David Fuster, Nuclear Medicine Service.
Jose Ramírez, Pathology Department.
Mercè Brunet, Toxicology Service.
Dolors Soy, Pharmacy Service.
Pedro Castro, Intensive Care Unit.
Jaume Llopis, Department of Statistics, Faculty of Biology, University of Barcelona.
Collaborators: Jose M. Miró, Juan Ambrosioni, Juan M. Pericàs, Adrian Téllez, Marta Hernandez-Meneses, Delia Garcia-Pares, Asunción Moreno, Cristina Garcia de la Maria, Javier Garcia-González, Manel Almela, Climent Casals, Francesc Marco, Jordi Vila, Eduard Quintana, Elena Sandoval, Juan C. Paré, Carlos Falces, Daniel Pereda, Ramon Cartañá, Salvador Ninot, Manel Azqueta, Marta Sitges, Barbara Vidal, José L. Pomar, Manuel Castella, José M. Tolosana, José Ortiz, Guillermina Fita, Irene Rovira, David Fuster, Jose Ramírez, Mercè Brunet, Dolors Soy, Pedro Castro, and Jaume Llopis
REFERENCES
- 1.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O’Gara P, Taubert KA, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 2.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL, ESC Scientific Document Group. 2015. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 3.Ambrosioni J, Hernandez-Meneses M, Téllez A, Pericàs JM, Falces C, Tolosana JM, Vidal B, Almela M, Quintana E, Llopis J, Moreno A, Miro JM, Hospital Clinic Infective Endocarditis Investigators. 2017. The changing epidemiology of infective endocarditis in the twenty-first century. Curr Infect Dis Rep 19:21. doi: 10.1007/s11908-017-0574-9. [DOI] [PubMed] [Google Scholar]
- 4.Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falcó V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH, International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doern G, Ferraro M, Brueggemann A, Ruoff KL. 1996. Emergence of high rates of antimicrobial resistance among viridans group streptococci in the United States. Antimicrob Agents Chemother 40:891–894. doi: 10.1128/AAC.40.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farber BF, Eliopoulos GM, Ward JI, Ruoff KL, Syriopoulou V, Moellering RC Jr. 1983. Multiply resistant viridans streptococci: susceptibility to beta-lactam antibiotics and comparison of PEN-binding protein patterns. Antimicrob Agents Chemother 24:702–705. doi: 10.1128/AAC.24.5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryskier A. 2002. Viridans group streptococci: a reservoir of resistant bacteria in oral cavities. Clin Microbiol Infect 8:65–69. doi: 10.1046/j.1198-743x.2001.00398.x. [DOI] [PubMed] [Google Scholar]
- 8.Parrillo JE, Borst GC, Mazur MH, Iannini P, Klempner MS, Moellering RC, Anderson SE. 1979. Endocarditis due to resistant viridans streptococci during oral PEN chemoprophylaxis. N Engl J Med 300:296–300. doi: 10.1056/NEJM197902083000608. [DOI] [PubMed] [Google Scholar]
- 9.Westling K, Julander I, Ljungman P, Jalal S, Nord CE, Wretlind B. 2006. Viridans group streptococci in blood culture isolates in a Swedish university hospital: antibiotic susceptibility and identification of erythromycin resistance genes. Int J Antimicrob Agents 28:292–296. doi: 10.1016/j.ijantimicag.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 10.Karchmer AW. 1981. Issues in the treatment of endocarditis caused by viridans streptococci, p 31–59. In Bisno AL. (ed), Treatment of infective endocarditis. Grune and Stratton, New York, NY. [Google Scholar]
- 11.Mendes RE, Sader HS, Farrell DJ, Jones RN. 2012. Worldwide appraisal and update (2010) of telavancin activity tested against a collection of Gram-positive clinical pathogens from five continents. Antimicrob Agents Chemother 56:3999–4004. doi: 10.1128/AAC.00011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Garrote F, Cercenado E, Alcalá L, Bouza E. 1998. In vitro activity of the new glycopeptide LY333328 against multiply resistant gram-positive clinical isolates. Antimicrob Agents Chemother 42:2452–2455. doi: 10.1128/AAC.42.9.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones RN, Sader HS, Flamm RK. 2013. Update of dalbavancin spectrum and potency in the USA: report from the SENTRY Antimicrobial Surveillance Program (2011). Diagn Microbiol Infect Dis 75:304–307. doi: 10.1016/j.diagmicrobio.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Huband MD, Castanheira M, Farrell DJ, Flamm RK, Jones RN, Sader HS, Mendes RE. 2016. In vitro activity of dalbavancin against multidrug-resistant Staphylococcus aureus and streptococci from patients with documented infections in Europe and surrounding regions (2011–2013). Int J Antimicrob Agents 47:495–499. doi: 10.1016/j.ijantimicag.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 15.García-de-la-Mària C, Pericas JM, Del Río A, Castañeda X, Vila-Farrés X, Armero Y, Espinal PA, Cervera C, Soy D, Falces C, Ninot S, Almela M, Mestres CA, Gatell JM, Vila J, Moreno A, Marco F, Miró JM, Hospital Clinic Experimental Endocarditis Study Group. 2013. Early in vitro and in vivo development of high-level daptomycin resistance is common in mitis group streptococci after exposure to daptomycin. Antimicrob Agents Chemother 57:2319–2325. doi: 10.1128/AAC.01921-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sader HS, Rhomberg PR, Castanheira M, Farrell DJ, Flamm RK, Mendes RE, Jones RN. 2016. Ceftaroline activity tested against viridans group streptococci from US hospitals. Diagn Microbiol Infect Dis 84:232–235. doi: 10.1016/j.diagmicrobio.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Yim J, Smith JR, Singh NB, Rice S, Stamper K, Garcia de la Maria C, Bayer AS, Mishra NN, Miró JM, Tran TT, Arias CA, Sullam P, Rybak MJ. 2017. Evaluation of daptomycin combinations with cephalosporins or gentamicin against Streptococcus mitis group strains in an in vitro model of simulated endocardial vegetations (SEVs). J Antimicrob Chemother 72:2290–2296. doi: 10.1093/jac/dkx130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miro JM, Armero Y, Garcia De La Maria C, Amat E, Almela M, del Rio A, Moreno A, Mestres CA, Jimenez de Anta MT, Gatell JM, Marco F, the Hospital Endocarditis Study Group . 2006. Treatment (Rx) of experimental endocarditis (EE) due to penicillin-resistant viridans-group streptococci (VGS), abstr B-1818. 46th Intersci Conf Antimicrob Agents Chemother (ICAAC), 27 to 30 September 2006, San Francisco, CA. [Google Scholar]
- 19.Pericàs JM, Nathavitharana R, Garcia-de-la-Mària C, Falces C, Almela M, Armero Y, Moreno A, Marco F, Miró JM, Karchmer AW. 2015. Endocarditis due to penicillin highly-resistant Viridans group streptococci, abstr C-656. 55th ICAAC/Int Cong Chemother Infect (ICC), 17 to 21 September 2015, San Diego, CA. [Google Scholar]
- 20.Pericàs JM, Nathavitharana R, Garcia-de-la-Mària C, Falces C, Almela M, Armero Y, Moreno A, Marco F, Miró JM, Karchmer AW. 2015. Treatment of endocarditis due to penicillin highly-resistant Viridans group, streptococci, abstr 28. 13th International Symposium on Modern Concepts on Endocarditis and Cardiovascular Infections (ISCVID), 4 to 6 June 2015, Rio de Janeiro, Brazil. [Google Scholar]
- 21.Levitz RE. 1999. Prosthetic-valve endocarditis caused by penicillin-resistant Streptococcus mitis. N Engl J Med 340:1843–1844. doi: 10.1056/NEJM199906103402319. [DOI] [PubMed] [Google Scholar]
- 22.Sabella C, Murphy D, Drummond-Webb J. 2001. Endocarditis due to Streptococcus mitis with high-level resistance to penicillin and cefotaxime. JAMA 285:2195. doi: 10.1001/jama.285.17.2195. [DOI] [PubMed] [Google Scholar]
- 23.Levy CS, Kogulan P, Gill VJ, Croxton MB, Kane JG, Lucey DR. 2001. Endocarditis caused by penicillin-resistant viridans streptococci: 2 cases and controversies in therapy. Clin Infect Dis 33:577–579. doi: 10.1086/321910. [DOI] [PubMed] [Google Scholar]
- 24.Knoll B, Tleyjeh IM, Steckelberg JM, Wilson WR, Baddour LM. 2007. Infective endocarditis due to penicillin-resistant viridans group streptococci. Clin Infect Dis 44:1585–1592. doi: 10.1086/518174. [DOI] [PubMed] [Google Scholar]
- 25.Nandhakumar B, Senthilkumar S, Menon T, Shanmugasundaram S. 2008. Penicillin-resistant viridans group streptococci from blood cultures of infective endocarditis patients in South India. Int J Antimicrob Agents 32:543–544. doi: 10.1016/j.ijantimicag.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Fujitani S, Rowlinson MC, George WL. 2008. Penicillin G-resistant viridans group streptococcal endocarditis and interpretation of the American Heart Association’s Guidelines for the Treatment of Infective Endocarditis. Clin Infect Dis 46:1064–1066. doi: 10.1086/529199. [DOI] [PubMed] [Google Scholar]
- 27.Garrod LP, Waterworth PM. 1962. The risks of dental extraction during PEN treatment. Br Heart J 24:39–46. doi: 10.1136/hrt.24.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doyle EF, Spagnuolo M, Taranta A, Kuttner AG, Markowitz M. 1967. The risk of bacterial endocarditis during antirheumatic prophylaxis. JAMA 201:129–134. [PubMed] [Google Scholar]
- 29.Le T, Bayer AS. 2003. Combination antibiotic therapy for infective endocarditis. Clin Infect Dis 36:615–621. doi: 10.1086/367661. [DOI] [PubMed] [Google Scholar]
- 30.Martínez F, Martín-Luengo F, García A, Valdés M. 1995. Treatment with various antibiotics of experimental endocarditis caused by penicillin-resistant Streptococcus sanguis. Eur Heart J 16:687–691. doi: 10.1093/oxfordjournals.eurheartj.a060974. [DOI] [PubMed] [Google Scholar]
- 31.Wilson WR, Zak O, Sande MA. 1985. Penicillin therapy for treatment of experimental endocarditis caused by viridans streptococci in animals. J Infect Dis 151:1028–1033. doi: 10.1093/infdis/151.6.1028. [DOI] [PubMed] [Google Scholar]
- 32.Enzler MJ, Rouse MS, Henry NK, Geraci JE, Wilson WR. 1987. In vitro and in vivo studies of streptomycin-resistant, penicillin-susceptible streptococci from patients with infective endocarditis. J Infect Dis 155:954–958. doi: 10.1093/infdis/155.5.954. [DOI] [PubMed] [Google Scholar]
- 33.Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, Nizet V, Yeaman MR, Moise PA. 2012. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 56:838–844. doi: 10.1128/AAC.05551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Entenza JM, Giddey M, Vouillamoz J, Moreillon P. 2010. In vitro prevention of the emergence of daptomycin resistance in Staphylococcus aureus and enterococci following combination with amoxicillin/clavulanic acid or ampicillin. Int J Antimicrob Agents 35:451–456. doi: 10.1016/j.ijantimicag.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Mehta S, Singh C, Plata KB, Chanda PK, Paul A, Riosa S, Rosato RR, Rosato AE. 2012. β-Lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother 56:6192–6200. doi: 10.1128/AAC.01525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sierra-Hoffman M, Iznaola O, Goodwin M, Mohr J. 2012. Combination therapy with ampicillin and daptomycin for treatment of Enterococcus faecalis endocarditis. Antimicrob Agents Chemother 56:6064. doi: 10.1128/AAC.01760-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kullar R, Davis SL, Levine DP, Rybak MJ. 2011. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis 52:975–981. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 38.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MVN, Anderson TL, Roberts SA, Warren SJC, Gao W, Howden BP, Johnson PDR. 2013. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 57:1654–1663. doi: 10.1128/AAC.01485-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung Y, Song KH, Cho J, Kim HS, Kim NH, Kim TS, Choe PG, Chung JY, Park WB, Bang JH, Kim ES, Park KU, Park SW, Kim HB, Kim NJ, Oh MD. 2014. Area under the concentration-time curve to minimum inhibitory concentration ratio as a predictor of vancomycin treatment outcome in methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents 43:179–183. doi: 10.1016/j.ijantimicag.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 40.Isenberg HD. 2004. Clinical microbiology procedures handbook. ASM Press, Washington, DC. [Google Scholar]
- 41.Clinical and Laboratory Standards Institute (CLSI). 2007. Performance standards for antimicrobial testing; 17th informational supplement. CLSI document M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 42.Pericàs JM, García-de-la-Mària C, Brunet M, Armero Y, García-González J, Casals G, Almela M, Quintana E, Falces C, Ninot S, Fuster D, Llopis J, Marco F, Moreno A, Miró JM, Hospital Clinic Endocarditis Study Group. 2017. Early development of daptomycin nonsusceptibilty in high-level aminoglycoside resistant Enterococcus faecalis in vitro predicts the efficacy of the combination of high-dose daptomycin plus ampicillin in the rabbit model of experimental endocarditis. J Antimicrob Chemother 72:1714–1722. doi: 10.1093/jac/dkx016. [DOI] [PubMed] [Google Scholar]
- 43.Lorian V. (ed). 1991. Antibiotics in laboratory medicine, 3rd ed, p 432–492. Williams and Wilkins, Baltimore, MD. [Google Scholar]
- 44.Garrison PK, Freedman LR. 1970. Experimental endocarditis I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J Biol Med 42:394–410. [PMC free article] [PubMed] [Google Scholar]
- 45.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.