A careful management of antimicrobials is essential in the critically ill with acute kidney injury, especially if renal replacement therapy is required. Acute kidney injury may lead per se to clinically significant modifications of drugs’ pharmacokinetic parameters, and the need for renal replacement therapy represents a further variable that should be considered to avoid inappropriate antimicrobial therapy.
KEYWORDS: CRRT, acute kidney injury, antimicrobials, continuous renal replacement therapy, convection, diffusion, extracorporeal clearance, pharmacodynamics, pharmacokinetics, renal replacement therapy
ABSTRACT
A careful management of antimicrobials is essential in the critically ill with acute kidney injury, especially if renal replacement therapy is required. Acute kidney injury may lead per se to clinically significant modifications of drugs’ pharmacokinetic parameters, and the need for renal replacement therapy represents a further variable that should be considered to avoid inappropriate antimicrobial therapy. The most important pharmacokinetic parameters, useful to determine the significance of extracorporeal removal of a given drug, are molecular weight, protein binding, and distribution volume. In many cases, the extracorporeal removal of antimicrobials can be relevant, with a consistent risk of underdosing-related treatment failure and/or potential onset of bacterial resistance. It should also be taken into account that renal replacement therapies are often not standardized in critically ill patients, and their impact on plasma drug concentrations may substantially vary in relation to membrane characteristics, treatment modality, and delivered dialysis dose. Thus, in this clinical scenario, the knowledge of the pharmacokinetic and pharmacodynamic properties of different antimicrobial classes is crucial to tailor maintenance dose and/or time interval according to clinical needs. Finally, especially for antimicrobials known for a tight therapeutic range, therapeutic drug monitoring is strongly suggested to guide dosing adjustment in complex clinical settings, such as septic patients with acute kidney injury undergoing renal replacement therapy.
INTRODUCTION
Acute kidney injury (AKI) is frequently associated with infective complications, especially in the intensive care unit (ICU), and sepsis-related AKI is characterized by exceedingly high mortality risk (1–3). In this setting, renal replacement therapy (RRT), frequently as continuous RRT (CRRT) (4), is often required. Most of the ICU patients undergoing RRT for AKI are treated with antimicrobials, and an appropriate drug dosing adjustment is essential to avoid overdosing-related toxicity as well as underdosing-related treatment failure and/or potential onset of bacterial resistance (5–7). In this clinical scenario, antimicrobial treatment should be tailored by adjusting the single doses and/or by modifying the time interval (8). Indeed, sepsis-related AKI often develops in the context of multiple organ dysfunction syndrome (MODS) and leads to relevant modifications of several pharmacokinetic (PK) parameters. Moreover, fluid overload, commonly observed in critically ill patients (9), may significantly affect the volume of distribution (V) of several drugs (6). In this context, the start of RRT adds further complexity related to the additional extracorporeal clearance (CLEC) of many antimicrobials. In this regard, the knowledge of the main principles regulating transport of solutes across dialysis membranes may allow the overcoming of this issue throughout the assessment of RRT effects on an antimicrobial’s concentration in blood and may guide drug dosing adjustments (5). Unfortunately, PK studies on drug dosing in AKI patients are not available for every antibiotic (6, 10).
The present review is aimed at summarizing the PK and pharmacodynamic (PD) principles guiding drug dosing adjustment during RRT and also providing practical indications on the use of the more frequently adopted antimicrobials on the basis of the most recent literature findings.
OVERVIEW OF THE MAIN GENERAL PRINCIPLES OF PHARMACOKINETICS
The most important factors able to affect drug PK during RRT are volume of distribution (V), protein binding, and molecular weight (MW); the knowledge of these parameters, along with total body clearance (CLTB), allows determination of the significance of extracorporeal removal of a given drug.
The volume of distribution corresponds to the ratio of the amount of drug in the body at a given time and plasma concentration at that time (11). In other terms, it represents the theoretical volume necessary to contain the total amount of an administered drug at the same concentration measured in plasma (12), and it should be regarded as a proportionality factor between a plasma concentration and the corresponding amount of drug in the whole body (11). As snapshot plasma drug concentrations may vary according to the state of drug disposition (i.e., just after intravenous [i.v.] administration, during the distribution phase, during the terminal phase of drug disposition, or at equilibrium), the proportionality ratio between the amount of drug in the body and the plasma concentration will change; thus, several V will be obtained in different situations (11). In clinical practice, V at equilibrium (Vss), obtained when plasma concentrations are measured under steady-state conditions (i.e., during continuous i.v. drug infusion or multiple-drug administration once steady-state plasma concentrations have been achieved), represents the most appropriate V to compute a “loading dose” (11). In this regard, it should be underlined that V of several antibiotics (e.g., aminoglycosides, β-lactams, and vancomycin) can increase up to 100% in critically ill patients compared with healthy volunteers (13–16). As a consequence, the risk to give an insufficient loading dose is very high in the critically ill, and multiple doses may be required to achieve a sufficient antibiotic exposure. Thus, a more aggressive use of loading doses of specific antimicrobials should be considered to get rapid therapeutic levels in septic patients (17).
Clearance (CL) is defined as the volume of plasma from which a solute is completely removed per unit of time (7, 18); CL is a proportionality factor, expressed by the ratio of elimination rate (by all routes) to plasma drug concentration (CL = rate of elimination/concentration) (18). In clinical practice, CL represents the PK parameter used to compute drug maintenance dose (11, 18).
The total body clearance (CLTB) refers to the sum of all the CL processes (metabolism and excretion) occurring in the different organs for a given drug (i.e., liver, kidney, gastrointestinal mucosa, lung, and skin) (12). In addition, once RRT is started, the contribution of the extracorporeal clearance (CLEC) to the CLTB has to be taken into account. The renal or CLEC of a drug is commonly considered significant when it is estimated as being higher than 25% of CLTB (12). Extracorporeal fractional CL (EC fractional CL) quantifies the contribution of the CLEC to the CLTB (7). Drugs characterized by tubular reabsorption (i.e., fluconazole) may show an CLEC unexpectedly higher than the drug CL observed in normal subjects (19, 20).
A large V (≥2 liters/kg) indicates a prevalent distribution in the extravascular compartment and/or a significant tissue binding with a considerable imbalance between the plasma drug concentration and total drug amount distributed in the whole body. If a drug has a large V, the amount detectable in plasma is much lower than the amount present in the other compartments (21). When V is small (<1 liter/kg), it may be supposed that the drug mainly stays in the intravascular compartment; as a consequence, a small V is generally associated with a clinically significant removal of the drug by RRT. Conversely, drugs with a V of ≥2 liters/kg undergo a negligible removal. Indeed, the rapid redistribution of the drug from other compartments counteracts a significant reduction of drug plasma levels despite an apparently adequate instantaneous CLEC (22). On these grounds, intermittent hemodialysis (IHD) leads to a temporary reduction of plasma levels of drugs with large V, which is followed by a posttreatment “rebound,” while during CRRT, an equilibrium removal/redistribution is commonly established (22).
Only the free (i.e., unbound) fraction (f) of a drug is susceptible to removal through RRT. The sieving coefficient (SC), the ratio of ultrafiltrate to plasma solute concentration, significantly correlates with the f of the drug in convective RRT modalities (20). Alteration of drug protein binding due to different mechanisms or clinical conditions (e.g., uremic toxins, blood pH, bilirubin, competition with other drugs, and modification of the drug/protein molar ratio) can explain the possible discrepancies between expected and measured SC (20).
With the exception of few drugs, the molecular weight of most commonly used antimicrobials is lower than 1,000 Da and plays a key role, especially in diffusive RRT modalities, as the diffusion coefficient of a molecule is inversely proportional to MW. Indeed, while in convective modalities SC is generally comparable to the f also for drugs with an MW around 1,000 to 1,500 Da (e.g., vancomycin), in diffusive techniques the ratio of dialysate to plasma solute concentration (saturation coefficient [SA]) is more strictly dependent on MW and tends to decrease progressively as MW increases (SA < SC; diffusive CL < convective CL) (23). However, also dialyzer membrane characteristics may play a key role. Specifically, with “low-flux” membranes (ultrafiltration coefficient, i.e., concentration in the ultrafiltrate [CUf] of <12 ml/mm Hg h−1), the CL of drugs with an MW of >1,000 Da could be clinically irrelevant, or at least not comparable to that of low-MW molecules. Conversely, the more widely adopted “high-flux” membranes (CUf of >12 ml/mm Hg h−1) are characterized by high porosity (cutoff around 20,000 Da) with significant removal of drugs with an MW of >1,000 Da also in the diffusive RRT modality (22). Although specific data are not yet available, this could be particularly true for the high-cutoff (HCO) membranes and for the recently introduced high-retention-onset (HRO) membranes (24). Therefore, when performing RRT with highly permeable membranes, one should bear in mind that also diffusive CL of high-MW drugs could be not negligible and could reduce the drug plasma concentration below the therapeutic target.
Finally, the interaction between the drug and dialysis membrane electric charges is described by the Gibbs-Donnan effect: anionic proteins (albumin) on the blood side of the membrane tend to retain cationic molecules (e.g., aminoglycosides and levofloxacin) while facilitating the transport of anionic drugs (e.g., some cephalosporins) (12, 25). However, the clinical significance of this interaction appears scarcely relevant (12).
MECHANISMS OF DRUG REMOVAL DURING RRT
The contribution of RRT to CLTB may vary according to dialysis prescription (e.g., RRT modality or dialysis dose). In addition to PK characteristics of the different drugs, the physical mechanisms of solute removal across the membrane may have different impacts on the CL of a specific drug (Table 1).
TABLE 1.
The main factors determining antimicrobial removal during renal replacement therapies and/or influencing the CLTBa
| Critically ill patient characteristic or PK changes | Drug-related factor(s) | RRT-related factor(s) |
|---|---|---|
| Residual renal function | Protein binding | RRT modality (IHD, PIRRT, CRRT) |
| Changes of nonrenal CL | V | Physical mechanisms of solute removal (convection, diffusion, adsorption) |
| Variations of V | MW | Dialysis dose, pre- or postdilution |
| Interference with other drugs | Molecular size and structure | Membrane characteristics |
| Blood pH | Hydrosolubility | Treatment duration (downtime, dialyzer running time) |
| Hypoalbuminemia | Electric charge | Vascular access recirculation |
CLTB, total body clearance; RRT, renal replacement therapy; IHD, intermittent hemodialysis; PIRRT, prolonged intermittent renal replacement therapy; CRRT, continuous renal replacement therapy; V, distribution volume; MW, molecular weight.
Hemofiltration is based uniquely upon convective transport of solutes. The removal of molecules by convection depends on the characteristics of the filter membranes; in particular, cutoff can be considered the limit of MW above which the SC becomes negligible (SC < 0.1) (24).
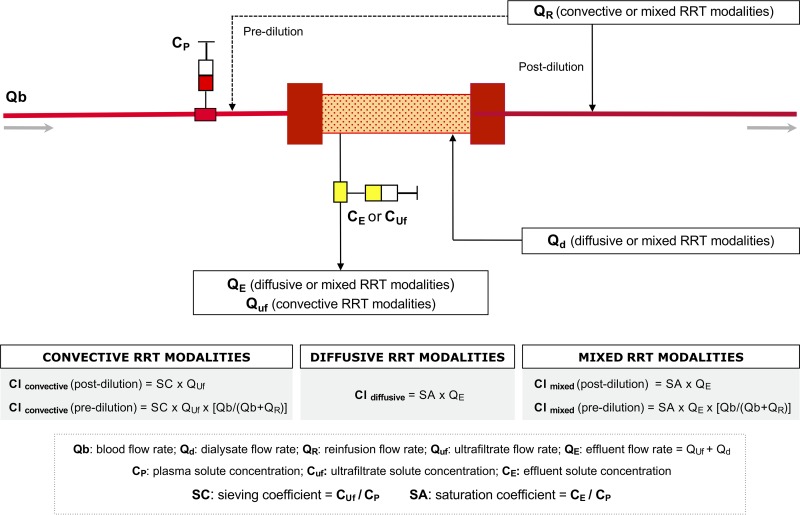
Taking into account the known or measured SC, in convective modalities the CLEC can be estimated in relation to the ultrafiltration rate (QUf) (21) and calculated as follows (Fig. 1): CLconvective (ml/min) = SC × QUf (ml/min). If SC is unknown, for drugs with an MW below the membrane cutoff, it can be assumed that the entire f of the drug will cross the membrane and SC can be approximated to f; in this case, the formula CLconvective (ml/min) = f × QUf (ml/min) can be adopted, where f (1 – protein-bound fraction) can be derived by the protein binding information reported in the main pharmacologic tables (8). In fact, despite a high variability of drug protein binding in critically ill patients, it has been demonstrated that SC in continuous venovenous hemofiltration (CVVH) is significantly related to the known f in most of the tested drugs (20). Moreover, significant differences in convective CL of urea, creatinine, gentamicin, and vancomycin emerged not only in relation to different QUf and different membranes but also in relation to hemofilter running time (26).
FIG 1.
Proposed methods to calculate solutes’ extracorporeal clearance (CLEC) with different renal replacement therapy (RRT) modalities.
In diffusive RRT modalities, the solute removal depends on diffusion coefficient, membrane surface, concentration gradient and membrane thickness. The diffusion coefficient is inversely related to solute MW and is affected by several physical parameters. Unlike convective transport, the estimation of CL in diffusive or mixed RRT modalities is more complex due to the greater variability of SA compared with SC (21). As mentioned above, SA represents the ratio between the concentration of a specific solute measured in the effluent (spent dialysate or dialysate + ultrafiltrate) and the concentration of the solute in the blood entering the filter (21). It can change considerably with the variation of different parameters, such as MW, blood-to-dialysate flow ratio, and membrane characteristics (21). Therefore, SA values, obtained by direct measurements or by literature data, are needed to calculate the CLEC of drugs during diffusive or mixed CRRT modalities according to the formula (Fig. 1) CLdiffusive or CLdiffusive/convective (ml/min) = SA × QE (ml/min), where QE (ml/min) = effluent flow rate (spent dialysate or dialysate + ultrafiltrate).
In continuous venovenous hemodialysis (CVVHD) and continuous venovenous hemodiafiltration (CVVHDF) (effluent rate ≪ blood flow rate) with “high-flux” membranes, the SA value for drugs with MW <500 Da is generally superimposable to the f; thus, the diffusive or diffusive/convective CL can be calculated using f and QE (21, 22): CLdiffusive or CLdiffusive/convective (ml/min) = f × QE (ml/min). However, as previously discussed, SA tends to decrease with increasing MW and/or dialysate/blood flow ratio (SA < SC; diffusive CLdiffusive < CLconvective) (27). In addition, one should take into account the possible decline of solute CRRT CL in relation to circuit running time (28). Alternatively, regardless of mechanisms of transport across dialysis membranes, the CLEC of a given solute can be calculated on the basis of the plasma extraction rate (E), which is CL (ml/min) = E × QB (ml/min) × (1 – hematocrit), where E = 1 − venous outlet solute concn/arterial inlet solute concn and QB (ml/min) = blood flow rate.
Finally, adsorption represents a further potential mechanism of drug removal during RRT; its relevance has been reported as extremely variable among different membranes, but its clinical significance is still unclear (5).
DRUG REMOVAL IN DIFFERENT RRT MODALITIES
The water-soluble antibiotics (e.g., β-lactams and aminoglycosides) are mainly eliminated by the kidney, are poorly transported across cell membranes, and display low V. Because of these characteristics, they are removed efficiently by RRT with a consequent need for careful dose adjustments (29). Conversely, lipophilic antibiotics (e.g., macrolides, tetracyclines, and linezolid) are easily transported across cellular membranes and are generally characterized by a large V; they usually have a predominant hepatic elimination, with a few exceptions, such as quinolones, which show a variable fraction of renal elimination. Consequently, extracorporeal removal of lipophilic antibiotics is often negligible, and dose adjustments are rarely required (29).
Although based on the same general principles, each RRT modality has specific characteristics and may impact drug removal differently; as a consequence, drug dosing adjustments should be accordingly tailored.
CRRT.
CRRT modalities provide a relatively constant CL of drugs when performed under optimal operative conditions, while IHD and prolonged intermittent renal replacement therapy (PIRRT) are characterized by two different PK phases (intradialytic and interdialytic). As discussed above, CLEC of a given drug can be roughly estimated on the basis of its PK parameters, physical mechanisms of transport across dialysis membranes, and prescribed dialysis dose (30). Alternatively, the amount of drug removal during RRT can be directly measured by different methods, among which the most widely adopted is based on the product of the dialysate flow rate and the average dialysate drug concentration over a given collection time (“dialysate recovery method”) (31). However, the estimation of CRRT contribution to CLTB could be more complex in relation to either variables related to dialysis per se (e.g., modality, dose, filter running time, downtime) or variables related to the rapidly evolving clinical setting in patients with AKI and MODS (e.g., modifications of residual renal CL and nonrenal CL, V changes, and protein binding variability) (30) (Table 1).
IHD and PIRRT.
For dialyzable drugs, intermittent RRT modalities (IHD and PIRRT) are characterized by two distinctive PK phases (on-RRT and off-RRT), with an intradialytic elimination rate much faster than the interdialytic one. As a consequence, the half-life (t1/2) of a given drug in the intradialytic phase will be significantly shorter than that observed in the interdialytic phase (32). In addition, although the whole CLEC of a dialyzable drug during a 24-h CRRT session is usually comparable to or even higher than that observed during a 4-h IHD or 8-h PIRRT session, the instantaneous CLEC is markedly higher during intermittent RRT modalities. Therefore, since the extent of drug removal during RRT is strictly related to plasma drug concentration, the time interval between drug administration and the start of an IHD or PIRRT session is crucial in determining the amount of drug removed during the treatment. Indeed, the start of the IHD or PIRRT session too close to drug administration (i.e., during the distribution phase) could have a much higher impact on drug removal—sometimes also for molecules with relatively low dialyzability (33, 34). Thus, different timing of antibiotic administration could lead to profound differences in the area under the curve (AUC) of the drug plasma concentration, with a large effect on the attainment of PD targets, especially for time-dependent antibiotics (35). On this basis, IHD or PIRRT sessions ideally should be started at the end of a dosing interval; moreover, due to possible extensive removal, several antibiotics could require a supplemental dose at the end of dialysis (34).
GENERAL PRINCIPLES OF ANTIMICROBIAL DOSE ADJUSTMENT DURING RRT
In order to maximize efficacy and reduce the risk of toxicity, antibiotic prescription and drug dose adjustments should be focused to meet the most appropriate PK/PD target, namely, the percentage of time above the MIC (%T>MIC) or its multiple, optimal peak concentration (Cmax), the ratio between Cmax and MIC (Cmax/MIC), and the ratio between 24-h AUC and MIC (AUC24/MIC) (5, 36). Some antibiotic classes (i.e., aminoglycosides and quinolones) are characterized by the postantibiotic effect (PAE), represented by the prolonged suppression of bacterial growth in the absence of a detectable concentration of the drug (5, 22, 36); the extent and duration of PAE may be variable, with different impacts on drug dosing strategy. In relation to the mechanism of action and specific antibiotic characteristics, the bactericidal effect is time dependent (has a need for concentrations steadily above the MIC or MIC multiple) or concentration dependent (effect correlated to Cmax/MIC or other PK/PD targets) (22, 36) (Table 2). For the sake of simplicity, for time-dependent antibiotics, the modification of the single doses is usually appropriate, without any variation of administration time interval. Conversely, for concentration-dependent antibiotics, the modulation of dosing interval without changes of single doses appears the most appropriate strategy. As a general rule, the loading dose of a drug is strictly dependent on V and does not generally require any adjustment, even in patients with severe AKI. Unlike the loading dose, maintenance doses are dictated by the drug CLTB (5). The usual method to calculate the subsequent doses of a given drug is based on the knowledge of the PD target levels and the trough plasma concentration (therapeutic drug monitoring [TDM]). The difference between the target and the trough concentration allows calculation of the dose (D) to deliver D (mg) = (target concn − trough concn) (mg/liter) × V (liters/kg) × body wt (kg).
TABLE 2.
Pharmacokinetic and pharmacodynamic parameters of the main antimicrobials adopted in ICUsa
| Antimicrobial | MW (Da) | f (%) | V (liters/kg) | SC (SA) | Elimination route | t1/2 (h) | Renal excretion (%) | RRT removal (EC fractional CL, %) | PK/PD target |
|---|---|---|---|---|---|---|---|---|---|
| Aminoglycosides | |||||||||
| Amikacin | 586 | >95 | 0.22–0.5 | 0.95 | R | 2 | 95 | Y (95) | Cmax/MIC = 8–10 |
| Gentamicin | 478 | >95 | 0.36 | 0.81 | R | 1.5–4 | 95 | Y (90) | Cmax/MIC = 8–10 |
| Tobramycin | 467 | 90–100 | 0.26 | 0.9 | R | 2–3 | 93 | NA | Cmax/MIC = 8–10 |
| β-Lactams | |||||||||
| Amoxicillin/clavulanate | 365/199 | 82/75 | 0.36/0.21 | 0.71/1 | R/H | 1–1.4/1 | >50/25–40 | NA | %T>MIC |
| Ampicillin/sulbactam | 581/255 | 85/62 | 0.29/0.25 | 0.69/− | R/R | 1.2/1 | 90/75–80 | NA | %T>MIC |
| Piperacillin/tazobactam | 518/300 | 70/78 | 0.24/0.40 | 0.80 | R/R | 1/1 | 75–90/65 | Y (40/60) | %T>MIC |
| Oxacillin | 401 | 6–10 | 0.4 | 0.02 | R | 0.5–0.7 | 90 | N | %T>MIC |
| Cefazolin | 454 | 20 | 0.19 | NA | R | 2 | 70–80 | NA | %T>MIC |
| Cefotetan | 575 | 22 | 0.14 | NA | R | 3–4.6 | 50–80 | NA | %T>MIC |
| Cefoxitin | 427 | 35–21 | 0.23 | 0.64 | R | 1 | 85 | NA | %T>MIC |
| Cefuroxime | 424 | 50–67 | 0.19 | 0.57 | R | 1.5 | 66–100 | NA | %T>MIC |
| Cefepime | 481 | 84 | 0.3 | 0.86 (0.78) | R | 1.7–2.3 | 85 | Y (40–59) | %T>MIC |
| Cefotaxime | 455 | 50–70 | 0.28 | 0.62 | R and H | 1.5 | 60 | NA | %T>MIC |
| Ceftaroline | 684 | 80 | 0.29 | NA | R | 2.7 | 88 | NA | %T>MIC |
| Ceftazidime | 547 | 90 | 0.28–0.40 | 0.90 (0.81) | R | 1.6–1.9 | 60–85 | Y (57) | %T>MIC |
| Ceftazidime/avibactam | 547/265 | 90/92 | 0.28/0.31 | 0.90/0.93 | R/R | 2.8/2.7 | 90/97 | Y (57/54) | %T>MIC |
| Ceftizoxime | 383 | 70 | 0.34 | 0.63 | R | 1.7 | 99 | NA | %T>MIC |
| Ceftolozane/tazobactam | 666/300 | 80/78 | 0.19/0.40 | NA | R/R | 3.1/1 | 95/80 | Y (83/36) | %T>MIC |
| Ceftriaxone | 554 | 10 | 0.1–0.2 | 0.15 | R | 5–9 | 30–65 | N | %T>MIC |
| Ceftobiprole | 534 | 85 | 0.25 | NA | R | 3.3 | 90 | NA | %T>MIC |
| Doripenem | 420 | 92 | 0.24 | 0.67 (0.76) | R | 1 | 70 | Y (32) | %T>MIC |
| Ertapenem | 475 | 20–40 | 0.12 | 0.21 | R | 4 | 90 | NA | %T>MIC |
| Imipenem/cilastatin | 317/380 | 80/56 | 0.22–0.24 | 1/0.75 | R/R | 1/− | 20–70/60 | Y (25–32) | %T>MIC |
| Meropenem | 383 | 98 | 0.35 | >0.90 (>0.90) | R | 1 | 70 | Y (40) | %T>MIC |
| Meropenem/vaborbactam | 383/297 | 98/77 | 0.28/0.25 | 1/0.78 | R/R | 1/1.7 | 70/75–95 | NA | %T>MIC |
| Glyco-, glycolipo-, and lipopeptides | |||||||||
| Dalbavancin | 1,817 | 2–7 | 0.11 | <0.1 (<0.1) | R and H | 147–258 | 50 | N | AUC24/MIC |
| Daptomycin | 1,620 | 20 | 0.1–0.13 | 0.2 (0.15) | R | 8–9 | 78 | Y (50) | AUC24/MIC |
| Oritavancin | 1,793 | 15 | 1.25 | NA | R | 245 | 90 | N | AUC24/MIC |
| Telavancin | 1,755 | 10 | 0.13 | NA | R | 8 | 99 | N | AUC24/MIC |
| Teicoplanin | 1,885 | 10–40 | 0.5–1.2 | 0.15 | R | 4–11 | 40–60 | V (10–32) | AUC24/MIC |
| Vancomycin | 1,448 | 50–90 | 0.47–1.1 | 0.70 | R | 4–11 | 90–100 | Y (60) | AUC24/MIC ≥ 400 |
| Glycylcycline | |||||||||
| Tigecycline | 585 | 11–29 | 0.12 | NA | H | 42 | 33 | N | AUC24/MIC |
| Lincosamides | |||||||||
| Clindamycin | 425 | 5–15 | 1.1 | 0.49 | H | 2.4 | 10 | N | AUC24/MIC |
| Macrolides | |||||||||
| Azithromycin | 749 | 50–93 | 0.47 | NA | H | 68 | 12 | N | AUC24/MIC |
| Monobactam | |||||||||
| Aztreonam | 435 | 44 | 0.18 | NA | R | 2 | 99 | NA | %T>MIC |
| Nitroimidazoles | |||||||||
| Metronidazole | 171 | 80 | 0.6–0.85 | 0.84 | R | 6–14 | 60–80 | NA | AUC24/MIC |
| Oxazolidinones | |||||||||
| Linezolid | 338 | 70 | 0.5–0.8 | 0.77–0.81 | H | 4.8–5.4 | 30 | V (20) | AUC24/MIC > 100 |
| Tedizolid | 370 | 10–30 | 0.95–1.14 | NA | H | 12 | 18 | N | AUC24/MIC |
| Polymyxins | |||||||||
| Colistin | 1,155 | 59–74 | 0.3–0.4 | NA (0.4–0.6) | R | 14 | 99 | Y (43–59) | AUC24/MIC |
| Quinolones | |||||||||
| Ciprofloxacin | 331 | 60–80 | 2.5 | 0.89 | R and H | 4.1 | 50–70 | V (15–26) | AUC24/MIC |
| Levofloxacin | 361 | 60–75 | 1.1–1.5 | 0.96 | R | 6–8 | 67–87 | Y (30–50) | AUC24/MIC |
| Moxifloxacin | 401 | 50 | 1.7–3.5 | 0.84 | H | 12–15 | 15–20 | N (10–15) | AUC24/MIC |
| Rifamycins | |||||||||
| Rifampin | 823 | 20 | 0.65 | NA | H and R | 1.5–5 | 30 | N (2) | AUC24/MIC |
| Tetracyclines | |||||||||
| Doxycycline | 444 | 7 | 0.75–1.91 | NA | H and R | 18 | 23–40 | N | AUC24/MIC |
| Minocycline | 457 | 24 | 1.14–1.62 | NA | H | 16 | 20 | N | AUC24/MIC |
| Antifungal agents | |||||||||
| Amphotericin B | 926 | 10 | 4 | 0.35 | U | 180–360 | 5–10 | N (12) | Cmax/MIC |
| Fluconazole | 306 | 88 | 0.7 | 0.96 (0.88) | R | 20–40 | 80 | Y (87) | AUC24/MIC |
| Isavuconazole | 437 | <1 | 6.42 | NA | H and R | 130 | 50 | N | AUC24/MIC |
| Itraconazole | 706 | <1 | 0.14 | NA | H | 16–25 | <1 | N | AUC24/MIC |
| Posaconazole | 700 | <1 | 3.22–4.21 | NA | H | 20–66 | 17 | N | AUC24/MIC |
| Voriconazole | 349 | 40 | 4.6 | NA | H | 12 | <2 | N | AUC24/MIC |
| Anidulafungin | 1,140 | <1 | 0.4–0.7 | 0 | H | 26.5 | <1 | N | AUC24/MIC |
| Caspofungin | 1,093 | 3 | 0.11 | NA | H | 9–11 | 1 | N | AUC24/MIC |
| Micafungin | 1,270 | <1 | 0.39 | 0 | H | 14–17 | Negligible | N | AUC24/MIC |
MW, molecular weight; f, free fraction; V, distribution volume; SC, sieving coefficient; SA, saturation coefficient; t1/2, half-life; H, hepatic; R, renal; U, unknown; RRT, renal replacement therapy; EC fractional CL, extracorporeal fractional clearance; NA, not available; Y, yes (CLEC/CLTB ratio of ≥25%); N, no (CLEC/CLTB ratio of <25%); V, variable; PK/PD, pharmacokinetic/pharmacodynamic target; AUC24/MIC, ratio between 24-h area under the curve (AUC) and MIC (specified when available); Cmax/MIC, ratio between peak serum concentration and MIC (specified when available); %T>MIC, percentage of time above the MIC or MIC multiple.
For drugs characterized by a first-order kinetic (most of the antibiotics), the plasma concentration at “steady state” (Css) is equal to the average of the “peak” and “trough” concentrations. Assuming that Css matches mean plasma levels, the concentration in the ultrafiltrate (CUf), or in the effluent (CE), will be equivalent to the product of Css × f, and the amount removed will be obtained by the product of CUf × QUf, or CE × QE (7). For example, considering that SC is generally equivalent to the f, the amount of drug to deliver during CVVH will be calculated as follows: drug removal (mg) = Css (mg/liter) × SC × ultrafiltrate vol (liters) in the dosing interval.
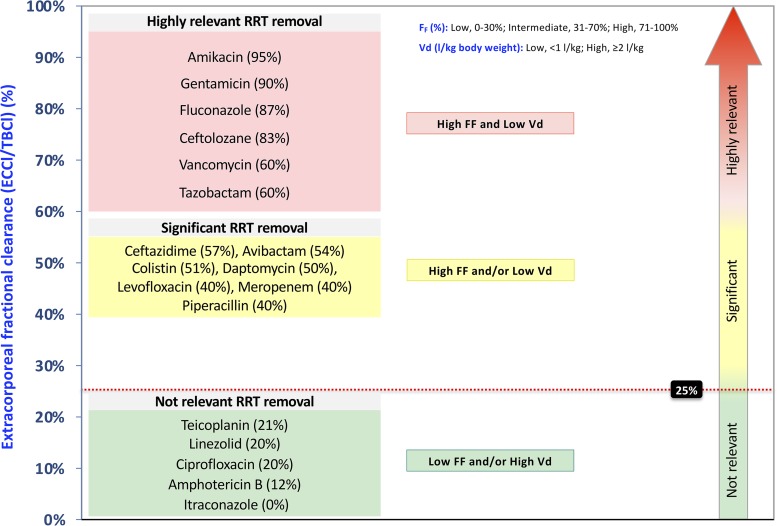
In diffusive or mixed modalities (CVVHD and CVVHDF), SA and effluent volume will be used instead of SC and ultrafiltrate volume, respectively. In IHD or PIRRT, considering the rapid variation of drug concentrations in blood during the treatment, the “dialysate recovery method” could be applied by using a micropump-based collection system, which allows continuous sampling of the total dialysate for the measurement of antibiotic removal (33). However, when applicable, TDM is useful for further corrections if the dose adjustment does not guarantee optimal plasma concentrations, especially for drugs characterized by a narrow therapeutic range (37). The proposed methods to calculate CLEC with different dialysis modalities are shown in Fig. 1. Although not easily applicable in clinical practice, these methods are useful in comprehending the principles at the basis of drug removal (27, 38). The clinical impact of drug removal depends on EC fractional CL. The values of EC fractional CL for different antibiotics are reported in Fig. 2. However, it should be underlined that the EC fractional CL threshold of 25% represents a simplification aimed at roughly evaluating the need for drug dosing adjustment in patients undergoing RRT and that also other factors, such as the susceptibility of the pathogen, drug safety profile, and sepsis severity, are crucial to guide an optimal antimicrobial dosing in complex clinical settings.
FIG 2.
The extracorporeal fractional clearance (CLEC/CLTB [percentage]) of a given antimicrobial during renal replacement therapy derives from the ratio of extracorporeal clearance (CLEC) to total body clearance (CLTB) (shown here as “ECCl/TBCl”) and indicates the relative contribution of CLEC to CLTB. As displayed above, the variability of V and f (here “Vd” and “FF”) results in a substantially different impact of CRRT on CLTB of antimicrobials, while an MW up to 1,000 to 1,500 Da (e.g., vancomycin or colistin) does not represent per se a limit to extracorporeal removal across the membranes commonly used in CRRT. MW, molecular weight; V, distribution volume.
A simplified approach to drug dose adjustment during CRRT relies on the assumption that CRRTs are associated with a relatively steady CLEC over the time (39). Therefore, CRRT modalities, unlike IHD and PIRRT, allow for simplified drug dose adjustments based on the “total creatinine CL” (residual renal CL + CLEC) (39). Indeed, the extracorporeal creatinine CL can be measured or easily estimated in relation to the prescribed dialysis dose, while the residual renal CL can be directly measured by urine collection during a given time interval or assumed as null in anuric patients. When the value of “total creatinine CL” is obtained, the dose or interval adjustment could be roughly performed by consulting guidelines on drug prescription in renal failure (8, 40–42). However, this extreme simplification of drug dosing adjustment during CRRT presents several limits (e.g., it does not take into account modifications of nonrenal clearance in AKI) and could lead to drug underdosing in some cases.
In order to provide practical indications for the treatment of patients undergoing RRT, the most commonly adopted dosing strategies for the different antimicrobials, derived from the recent literature, are reported in Table 3.
TABLE 3.
Intravenous antimicrobial dosing in critically ill patients with acute kidney injurya
| Antimicrobial | Conventional IHD | PIRRT | CRRT |
|---|---|---|---|
| Aminoglycosides | |||
| Amikacin | 7.5 mg/kg q48h + 3.75 mg/kg after dialysis | No data | 7.5 mg/kg q24h |
| Gentamicin | 1.7–2 mg/kg q48h + 0.85–1 mg/kg after dialysis | 6 mg/kg q48h 1 h before PIRRT session | 1.7–2 mg/kg q24h |
| Tobramycin | 1.7–2 mg/kg q48h + 0.85–1 mg/kg after dialysis | 6 mg/kg q48h 1 h before PIRRT session | 1.7–2 mg/kg q24h |
| β-Lactams | |||
| Amoxicillin/clavulanate | 0.5/0.1 g q24h + 0.5/0.1 after dialysis | No data | No data |
| Ampicillin/sulbactam | 1.5 g q12h (after dialysis on dialysis day) | No data | 1.5–3 g q6h–q8h |
| Piperacillin/tazobactam | 2.25 g q8h–q12h + 0.75 g after dialysis | 2.25–3.375 g q6h–q8h or continuous infusion | 2.25–3.375 g q6h–q8h or continuous infusion |
| Oxacillin | No adjustment | No adjustment | No adjustment |
| Cefazolin | 0.5–1 g q24h or 1–2 g q48h–q72h + 0.5–1 g after dialysis | No data | 1 g q8h or 2 g q12h |
| Cefotetan | 1–2 g q48h + 1 g after dialysis | No data | 0.75 g q12h |
| Cefoxitin | 2 g q24h–q48h + 1 g after dialysis | No data | 2 g q8h–q12h |
| Cefuroxime | 0.75–1.5 g q24h (after dialysis on dialysis day) | No data | 0.75–1.5 g q8h–q12h |
| Cefepime | 0.5–1 g q24h + 1 g after dialysis | 1 g q6h | 1 g q8h or 2 g q12h |
| Cefotaxime | 2 g q24h + 1 g after dialysis | No data | 1–2 g q12h–q24h |
| Ceftaroline | 0.2–0.4 g q12h (after dialysis on dialysis day) | No data | 0.4 to 0.6 g q12h |
| Ceftazidime | 2 g q24h–q48h + 1 g after dialysis | 2 g q12h | 1g q8h or 2 g q12h or continuous infusion |
| Ceftazidime/avibactam | 0.94 g q48h (after dialysis on dialysis day) | No data | 1.25 g q8h |
| Ceftizoxime | 2 g q24h + 1 g after dialysis | No data | 2 g q12h–q24h |
| Ceftolozane/tazobactam | 0.15 g q8h (after dialysis on dialysis day) | No data | No data |
| Ceftriaxone | No adjustment | No adjustment | No adjustment |
| Ceftobiprole | 0.25 g q24h (after dialysis on dialysis day) | No data | No data |
| Doripenem | 0.25 g q12h–q24h | 0.5 g q8h | 0.5–1 g q8h–q12h |
| Ertapenem | 0.5 g q24h + 0.15 g after dialysis (if dose is given within 6 h pre-IHD) | 1 g q24h | 0.5–1 g q24h |
| Imipenem/cilastatin | 0.25 g q12h (after dialysis on dialysis day) | 0.5 g q6h | 0.25–0.5 g q6h–q8h |
| Meropenem | 0.5–1 g q24h (after dialysis on dialysis day) | 0.5 g q8h | 0.75 g q8h or 1.5 g q12h or continuous infusion |
| Meropenem/vaborbactam | 0.5/0.5 g q12h | No data | No data |
| Glyco-, glycolipo-, and lipopeptides | |||
| Dalbavancin | No adjustment | No data | No adjustment |
| Daptomycin | 4–6 mg/kg q48h (after dialysis on dialysis day) + 50% additional dose after IHD session preceding 72-h interdialytic period | 6 mg/kg q24h | 6 mg/kg q24h |
| Oritavancin | No adjustment | No adjustment | No adjustment |
| Telavancin | No data | No data | No data |
| Teicoplanin | 6–12 mg/kg q72h | No data | 6–12 mg/kg q48h |
| Vancomycin | 7.5–15 mg/kg q48h–q72h (after dialysis on dialysis day) | 15 mg/kg (after PIRRT session) | 10–15 mg/kg q24h–q48h or continuous infusion |
| Glycylcycline | |||
| Tigecycline | No adjustment | No adjustment | No adjustment |
| Lincosamides | |||
| Clindamycin | No adjustment | No adjustment | No adjustment |
| Macrolides | |||
| Azithromycin | No adjustment | No adjustment | No adjustment |
| Monobactam | |||
| Aztreonam | 0.5 g q8h + 0.25 g after dialysis | No data | 1 g q8h or 2 g q12h |
| Nitroimidazoles | |||
| Metronidazole | 7.5 mg/kg q12h (after dialysis on dialysis day) | No data | 7.5 mg/kg q6h |
| Oxazolidinones | |||
| Linezolid | 600 mg q12h (after dialysis on dialysis day) | 600 mg q12h (after PIRRT session) | No adjustment |
| Tedizolid | No adjustment | No adjustment | No adjustment |
| Polymyxins | |||
| Colistin | 1.5 million IU q12h + 1.5 million IU after dialysis | 3.0 million IU q8h | 4.5 million IU q12h or 3.0 million IU q8h |
| Quinolones | |||
| Ciprofloxacin | 0.4 g q24h (after dialysis on dialysis day) | No data | 0.2–0.4 g q12h |
| Levofloxacin | 0.5 g q48h (after dialysis on dialysis day) | No data | 0.25 g q24h or 0.5 g q48h |
| Moxifloxacin | No adjustment | No adjustment | No adjustment |
| Rifamycins | |||
| Rifampin | No adjustment | No adjustment | No adjustment |
| Tetracyclines | |||
| Doxycycline | No adjustment | No adjustment | No adjustment |
| Minocycline | No adjustment | No adjustment | No adjustment |
| Antifungal agents | |||
| Amphotericin B | No adjustment | No adjustment | No adjustment |
| Fluconazole | 0.1–0.4 g q24h (after dialysis on dialysis day) | 0.4 g q12h | 0.4–1.2 g q24h |
| Isavuconazole | No adjustment | No adjustment | No adjustment |
| Itraconazole | 0.1 g q12h–q24h (oral route); i.v. contraindicated in patients with eGFR <30 ml/min (risk of cyclodextrin vehicle accumulation) | No data | 0.1–0.2 g q12h (oral route); i.v. route contraindicated in patients with eGFR of <30 ml/min (risk of cyclodextrin vehicle accumulation) |
| Posaconazole | No adjustment | No adjustment | No adjustment |
| Voriconazole | No data, but oral route preferred to avoid accumulation of SBECDb vehicle | No data, but oral route preferred to avoid accumulation of SBECD vehicle | 4 mg/kg q12h, oral route (although removal of SBECD with CRRT has been recently reported) |
| Anidulafungin | No adjustment | No adjustment | No adjustment |
| Caspofungin | No adjustment | No adjustment | No adjustment |
| Micafungin | No adjustment | No adjustment | No adjustment |
As a general rule, the “loading dose” of a drug, not reported in the table, is strictly dependent on V and does not require any adjustment in patients with renal failure, including those undergoing RRT for AKI. Consistent variations of V can occur in critically ill patients, thus influencing the effect of loading and maintenance doses on antibiotic plasma concentrations. Maintenance doses reported in the table derive from references included in the review and from the main guides for antimicrobial therapy. Whenever possible, maintenance doses should be guided by therapeutic drug monitoring (TDM), especially for drugs with a narrow therapeutic range.
SBECD, sulfobutylether-β-cyclodextrin.
AMINOGLYCOSIDES
These antibiotics have a concentration-dependent antibacterial efficacy, which is maximal in the presence of an elevated Cmax/MIC (5). Moreover, a higher Cmax is generally followed by a longer duration of the PAE. The drug administration in a single daily dose appears more effective and at the same time is associated with a lower toxicity (43). In patients with AKI, the most appropriate dosing adjustment strategy is to prolong the time interval between doses while maintaining the single doses unchanged (43). Aminoglycosides are characterized by a small V and an elevated f with SC/SA around 0.9. Therefore, the CLEC is relatively high with both convective and diffusive RRT modalities (44, 45). In the specific setting of IHD or PIRRT, the comparison of different gentamicin dosing regimens showed that the administration over 30 to 60 min just before dialysis session with an extended interval may be a valid strategy (45, 46). Thus, predialysis administration of a full dose of aminoglycosides allows for higher target Cmax with maximal therapeutic effect, while enhancing CLEC to minimize toxicity; this strategy appears to be more effective, and possibly less toxic, than the conventional postdialysis dosing regimen (45, 46). Otherwise, during CRRT, a higher than usual first dose of amikacin, while reaching therapeutic Cmax, was associated with a prolonged concentration above the threshold of renal toxicity. Thus, the therapeutic benefit of high-dose aminoglycoside therapy in septic patients receiving CRRT should be balanced with its potential side effects (44). In this context, after estimating CLTB on the basis of previously described main principles, the t1/2 of a given aminoglycoside during CRRT could be obtained in order to calculate the time to reach target trough concentration; this time will be considered the appropriate time interval for the subsequent doses aimed at maintaining the optimal PD target (5). However, considering the narrow therapeutic index of aminoglycosides, TDM is strongly suggested for adequate and safe dose adjustments independently of the RRT modality adopted (22).
β-LACTAMS
To reach maximal efficacy, the plasma concentration of β-lactams needs to remain above the MIC as long as possible in the dose interval (22, 47). Indeed, the clinical effectiveness of these time-dependent antibiotics appears closely related to the percentage of time with a free drug plasma concentration higher than pathogen’s MIC (%T>MIC): the higher this percentage, the higher the effectiveness (48). Additionally, the maximum bactericidal effect is observed when the β-lactam Css is more than 4 times the pathogen’s MIC (48).
To ensure the attainment of the appropriate PK/PD targets, as CRRT provides a steady additional CL, the dose interval should be shortened without changing the single doses, and continuous infusion has been also proposed (47, 49). Indeed, in 7 patients undergoing CVVHDF, after a 2 g loading dose, target serum concentrations were optimally maintained by the continuous infusion of ceftazidime (3 g/day) (47). More recently, after a 4.5 g loading dose, 500 mg/h continuous infusion of piperacillin-tazobactam was reported to ensure optimal drug exposure for less-susceptible pathogens throughout the time treatment (%T>MIC = 100%) (49). The continuous-infusion strategy has been proposed for some β-lactams also in PIRRT (50, 51). For instance, in a prospective study in ICU patients receiving sustained low-efficiency dialysis (SLED), a population PK model showed that piperacillin-tazobactam at a 9 g/day continuous infusion was appropriate (%T>MIC = 100%) for susceptible organisms with a MIC of ≤32 mg/liter (51). Among time-dependent antibiotics, carbapenems have a significant RRT removal (22, 52–54). The intermittent administration of meropenem during CVVHDF was associated with subtherapeutic concentrations during the time interval, while continuous infusion (2 g/24 h) allowed the PK/PD target to be reached (%T>MIC = 100%) (55).
As for the use of combined β-lactams and β-lactamase inhibitors (56), although the optimal dosing strategy of ceftazidime/avibactam and other newly introduced antimicrobials in combination has not yet been fully investigated in different RRT modalities, preliminary data in CVVH showed a significant EC fractional CL of ceftazidime/avibactam (57).
In order to meet the appropriate PK/PD target of time-dependent antibiotics during CRRT, PK principles may help to calculate maintenance doses: for example, it has been proposed that the sum of CLEC plus nonrenal CL and residual renal CL could be used to estimate CLTB and calculate the maintenance infusion rate of a given antibiotic (5). This approach could be useful, as the CRRT dose is often not standardized and different CRRT intensities may impact consistently %T>MIC (35), especially if high-volume CRRT modalities are adopted (58).
Although β-lactams are not characterized by a tight therapeutic range, TDM remains a useful tool for guiding drug dosing in complex clinical settings. Recently, the use of TDM was reported to identify the need for β-lactam dose changes in 35% of critically ill patients undergoing CRRT: in particular, 24% of TDM sample values prompted a decrease in the prescribed dose, while a dose increase was required in the remaining 11% (59).
GLYCO-, GLYCOLIPO-, AND LIPOPEPTIDES
Vancomycin has a time-dependent bactericidal effect (36), and the PK/PD target that best correlates to its efficacy is the AUC24/MIC (60). Current vancomycin therapeutic guidelines indicate that targeting a trough concentration of 15 to 20 mg/liter would attain an AUC24/MIC of ≥400 in most patients with normal kidney function if the MIC is ≤1 mg/liter (60, 61). For pathogens with a vancomycin MIC of ≥2 mg/liter, this PK/PD target is not achievable with conventional dosing (60, 61). Despite the relatively high MW, the use of “high-flux” membranes is generally associated with a relevant EC fractional CL in different RRT modalities (61–63). However, along with a high variability related to the dialysis dose and the selected membrane, a lower vancomycin CL has been reported in diffusive compared to convective modalities (62). Thus, given the narrow therapeutic range of vancomycin, an accurate TDM is strongly needed. During CRRT, a continuous vancomycin infusion has been suggested to avoid large fluctuations of plasma concentration (64). In patients receiving PIRRT, a high variability of vancomycin PK parameters has been reported; to meet the PK/PD target, a weight-based loading dose (15 to 25 mg/kg), followed by a maintenance regimen after the PIRRT session, preferably guided by TDM, has been suggested (61, 65).
Teicoplanin is characterized by a high MW, a low V, and a highly variable f, especially in patients with hypoalbuminemia (29). Reported values of SC in CVVH are around 0.15, but the variability of PK parameters could lead to significant variations of EC fractional CL (29, 66, 67).
Daptomycin is a lipopeptide characterized by concentration-dependent activity, high protein binding, and low V; despite a low f, the CLEC in CVVHD was reported as relatively high compared to nonrenal CL, resulting in a significant EC fractional CL (around 50%) (68). Although the suggested maintenance dose may vary according to dialysis intensity and modality, a daily dose ranging from 8 to 12 mg/kg has been recently suggested to avoid underdosing (69, 70). Otherwise, in patients receiving thrice-weekly IHD, administration of daptomycin (4 to 6 mg/kg) thrice weekly after each IHD session appears to be safe and effective, with a 50% increased dose (from 6 to 9 mg/kg) being suggested at the end of IHD session preceding the long interdialytic period (71).
Dalbavancin, a second-generation lipoglycopeptide, is characterized by a high protein binding and a low V. As expected, in vitro models showed a very low CLEC (SA and SC of <0.1) (72).
OXAZOLIDINONES
Linezolid is a synthetic antibiotic with a peculiar activity on multiresistant strains of Gram-positive bacteria (i.e., Staphylococcus aureus and Enterococcus) characterized by time-dependent killing. Although hepatic metabolism prevails, around 30% of the dose undergoes renal excretion. Notwithstanding, in a prospective study aimed at evaluating single-dose PK of linezolid in patients undergoing different RRT modalities for oliguric AKI, an RRT-related reduction of plasma drug concentration to subtherapeutic levels was reported in most of the patients (31). In particular, the drug t1/2 was lower than 4 to 6 h in all patients treated with IHD or SLED, suggesting the administration of the drug at the end of dialysis as the most appropriate therapeutic schedule (31). A subsequent pilot study, including 5 critically ill patients undergoing IHD, evaluated linezolid population PK on and off dialysis after multiple doses (600 mg every 12 h [q12h]). Compared to linezolid administration not preceding IHD (“without HD”), drug trough levels were significantly lower if linezolid was administered prior to a dialysis session (“with HD”), providing further evidence that hemodialysis reduces linezolid concentrations (33). On this basis, although linezolid administration at the end of dialysis remains the most appropriate schedule, the authors speculated that, at least in specific circumstances (high body weight and dialysis sessions early after linezolid infusion), a supplemental dose after HD or a higher loading dose at the start of HD could allow the steady state to be achieved earlier (33). Subtherapeutic plasma levels of linezolid have been reported elsewhere during SLED, suggesting the need for higher doses or the adoption of TDM to achieve effective antimicrobial therapy (73). Despite a relatively high SC, a linezolid EC fractional CL around 20% has been reported during CRRT, suggesting no need for relevant modifications of the usual dose (74). However, a wide variability in linezolid PK/PD parameters has been reported in septic patients undergoing CRRT, conceivably related to the patients’ critical conditions and/or organ dysfunctions (75, 76). Therefore, particular attention should be paid to linezolid therapy in order to avoid antibiotic underdosing in specific clinical settings (75, 77) and/or in case of infections due to bacteria with a higher MIC (>2 mg/liter) (78).
Tedizolid is a novel oxazolidinone drug primarily excreted by the liver, active against certain linezolid-resistant pathogens and characterized by a longer t1/2 than linezolid. Tedizolid does not require dosing adjustment in patients with renal dysfunction (79). In vitro CVVH/CVVHD models showed that EC fractional CL was modest, suggesting no need for dose adjustment (80).
POLYMYXINS
The polymyxins, antibiotics characterized by a high risk of nephrotoxicity and neurotoxicity, have been reintroduced in recent years as a treatment option against Gram-negative multiresistant microorganisms (36). Colistin (polymyxin E) is a multicomponent lipopeptide containing colistin A and B. The drug is commercially available as its prodrug, colistimethate sodium (CMS [MW of 1,743 Da]), which is hydrolyzed spontaneously in plasma to colistin (20% to 30% of the CMS dose) (81). Bactericidal activity of colistin is mainly concentration dependent, with a PAE at higher concentrations; however, colistin is classified as both a concentration- and time-dependent antibiotic, and AUC24/MIC represents the most reliable predictor of antibacterial activity. One million IU of CMS corresponds to 80 mg and equals 30 mg of colistin base activity. In critically ill patients, colistin is characterized by a variably protein binding and a low V (81). Both CMS and colistin undergo renal excretion, although a variable amount of nonrenal elimination and tubular reabsorption may also play a role (29, 82). Information about colistin PK during RRT vary in relation to the different modalities adopted in the ICU. Colistin CLEC by CRRT may become a relevant component of the CLTB; indeed, a significant lowering of colistin levels during CVVHDF was observed with similar CLEC for CMS and colistin (11.2 and 11.9 ml/min, respectively) (83). Colistin EC fractional CL was reported as 43% to 59% during CVVHDF, and a CMS dose ranging from 150 mg q18h to 75 mg q8h was inappropriately low when actual colistin concentrations (1.4 to 1.7 mg/liter) were compared with the theoretical Css (>2.5mg/liter) needed to maintain an AUC24/MIC of >60 (84). Thus, based on these findings, also confirmed elsewhere (82, 85), a CMS maintenance dose similar to or higher than that used in patients with preserved kidney function is needed to achieve adequate colistin Css during CRRT (83, 86–88). A further study reported relatively high SA during CVVHDF (0.42 for colistin A and 0.48 for colistin B) with a high extent of colistin removal, suggesting the dose should not be reduced (89). In patients with septic shock and AKI undergoing coupled plasma filtration-adsorption/CVVHDF or hemoperfusion with polymyxin B fiber cartridges, colistin removal was high, especially in the case of coupled plasma filtration-adsorption/CVVHDF; however, the short duration of hemoperfusion probably resulted in very little impact on total-body colistin content (90). Regarding PK of colistin during IHD, a case report analyzed the variations in colistin plasma levels in 2 patients with pneumonia from multidrug-resistant Gram-negative bacteria and AKI undergoing 4-h IHD; 2 million IU q12h has been suggested as the most appropriate dose in patients undergoing daily IHD (91). In a case series of 8 ICU patients with AKI receiving daily 4-h IHD, CMS nonrenal CL was found to be greater than expected, but colistin exposure was in any case 3-fold higher than that in ICU patients with preserved renal function—probably due to a greater fraction of CMS being converted into colistin (92). In this setting, a CMS dose of 1.5 million IU q12h with a supplemental 1.5-million-IU dose given after the IHD session (i.e., total of 3 million IU for non-IHD days and 4.5 million IU for the IHD day) was appropriate to maintain a colistin plasma concentration around 3 to 4 mg/liter. This strategy allowed a high probability of target attainment for bacteria with a MIC of ≤1.5 mg/liter (free AUC/MIC of >15, nonpulmonary infections) and 0.5 mg/liter (free AUC/MIC of >50, pulmonary infections) (92).
A recent study provided a detailed description of the disposition of CMS and colistin during IHD in patients with end-stage renal disease (ESRD) (34). A single CMS dose was administered over 30 min, and the IHD session was started 1.5 to 5.5 h after the CMS infusion. CMS recovered in the dialysate was around 30% of the administered dose. Therefore, the authors suggested that in patients with ESRD, IHD should be done at the conclusion of a dosing interval to minimize CMS removal before its conversion to colistin (34). Moreover, CMS clearance estimated by dialysis from transdialyzer extraction was 30% greater than that derived from the amount in dialysate, which indicates adsorption by the membrane (34).
Few data are currently available to guide standardized dose recommendations during PIRRT. A single case report has evaluated single- and multiple-dose PK of colistin in a critically ill patient undergoing extended daily dialysis (EDD) (93). After a 6-million-IU loading dose, a maintenance dose of 3 million IU q8h was given. Depending on the blood and dialysate flow rates, the ECCL of colistin ranged between 54 and 71 ml/min. Colistin concentrations were appropriate, suggesting that in patients undergoing EDD, the dose of 9 million IU q24h did not lead to drug accumulation (93).
In any case, independent of the RRT modality adopted, a more extensive use of TDM may be envisaged to optimize colistin dosing and reduce its toxicity.
QUINOLONES
The antibacterial activity of quinolones is concentration dependent, and the AUC24/MIC is the PK/PD parameter most predictive of their efficacy (36). Ciprofloxacin removal during CVVH/CVVHDF is variably reported with a relatively low EC fractional CL (94, 95). Levofloxacin, characterized by a lower V compared to ciprofloxacin and moxifloxacin, appears to have a higher EC removal (94, 96, 97). Conversely, the EC fractional CL of moxifloxacin, a quinolone with prevalent hepatic metabolism, accounted for less than 10%, barely affecting the PK of the drug in CVVHDF (98). Regarding PIRRT, in 10 ICU patients receiving single-dose moxifloxacin and undergoing SLED, 30% of moxifloxacin was removed by a single RRT session in addition to the nonrenal CL (99). Although the moxifloxacin t1/2 during SLED was shorter than that off-SLED, only a small amount of moxifloxacin was recovered from the dialysate, suggesting a quota of drug adsorption (99). In the same study, in 5 patients receiving single-dose levofloxacin, the t1/2 during SLED was markedly shorter than that off-SLED, with an EC fractional CL around 50%. Adsorption of levofloxacin in vitro has been described during CVVH with the use of polyacrylonitrile (PAN) hemofilters; the process appeared concentration dependent and reversible, suggesting that adsorption is unlikely to affect levofloxacin PK significantly in vivo (100).
ANTIFUNGAL AGENTS
Based on their PK properties, most of the antifungal agents are characterized by a negligible RRT removal with an EC fractional CL largely below 25% (101–105). In particular, concentration-time profiles of amphotericin B lipid complex were very similar on- and off-CVVH, suggesting that a standard dose can be administered during CRRT (101). A negligible CLEC during CRRT has been reported also for the echinocandin caspofungin, licensed for the treatment of invasive candidiasis and for salvage therapy of invasive aspergillosis; thus, no caspofungin dosing adjustment appears necessary for patients undergoing CRRT (102, 103).
Voriconazole, a triazole antifungal agent, is currently recommended as the first-line therapy for patients with invasive Aspergillus infection and can also be used to treat patients with other life-threatening systemic mycoses (104). Intravenous administration of voriconazole is not recommended in patients with an estimated glomerular filtration rate (eGFR) of <50 ml/min to avoid potentially toxic accumulation of sulfobutylether-β-cyclodextrin (SBECD), a vehicle for the intravenous formulation (104). In a prospective PK study conducted in 10 critically ill patients undergoing CVVH, voriconazole ECCL was not clinically significant, while SBECD was effectively removed at a rate similar to QUf. On this basis, the standard dose of intravenous (i.v.) voriconazole can be prescribed in patients undergoing CVVH without significant risk of SBECD accumulation (104).
The PK profile of fluconazole, an antifungal drug active on different Candida strains and characterized by a wide therapeutic range, represents one of the few exceptions among antifungal agents, being characterized by a low V and a high f (8). Its renal excretion prevails, and the drug is subject to tubular reabsorption (22). In a 24-h CRRT session, 70% of the fluconazole dose is roughly removed (19). During CRRT, the CLEC is similar to or higher than the drug CL observed in subjects with normal renal function (19, 20). This finding may appear surprising but can be explained by a partial tubular reabsorption of the drug after glomerular filtration in patients with normal renal function (19, 20). Therefore, in patients undergoing CRRT, a daily dose of 800 to 1,200 mg has been suggested with the aim of achieving a fungicidal drug concentration (106, 107).
CONCLUSIONS
In the critically ill, AKI may lead to clinically significant modifications of antibacterial PK parameters. Generally, a prevalent renal excretion of a given drug predicts a clinically relevant RRT removal, and drug dosing adjustment is commonly required if the EC fractional CL is higher than 25%. In septic ICU patients with AKI, a careful management of antimicrobials is essential, especially in clinical settings requiring high-intensity RRT. Indeed, the RRT removal of antibacterial drugs can be higher than expected, with a consistent risk of underdosing if the CLEC is underestimated and/or drug dose adjustment aimed at the attainment of PK/PD targets is not properly applied.
The most important PK parameters, useful to determine the significance of EC removal of a given drug, are MW, protein binding, and V; yet, for drugs with a high V, whole-body drug removal by RRT may be negligible despite a low MW and a low protein binding. Furthermore, the time interval between the antibiotic dose administration and the start of RRT also has a crucial impact in patients undergoing PIRRT or IHD, as starting RRT too closely to drug administration may result in inappropriately high drug removal. Conversely, greater antibiotic removal could be useful to reduce toxicity in the case of some concentration-dependent antimicrobials (i.e., aminoglycosides).
Finally, it should be highlighted that any attempt to simplify antimicrobial dosing adjustment may be challenged in clinical practice by the high intrapatient and interpatient PK variability that characterizes critically ill patients. Thus, especially for antimicrobials known for a tight therapeutic range, TDM appears essential and is strongly suggested to guide drug dosing adjustment in a multifaceted and rapidly evolving clinical setting such as ICU septic patients undergoing RRT for AKI.
ACKNOWLEDGMENT
The authors declare that they have no relevant financial interests.
REFERENCES
- 1.Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, Bagshaw SM, Glassford NJ, Lankadeva Y, Vaara ST, Schneider A. 2017. Acute kidney injury in sepsis. Intensive Care Med 43:816–828. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- 2.Mehta RL, Bouchard J, Soroko SB, Ikizler TA, Paganini EP, Chertow GM, Himmelfarb J, Program to Improve Care in Acute Renal Disease (PICARD) Study Group. 2011. Sepsis as a cause and consequence of acute kidney injury: Program to Improve Care in Acute Renal Disease. Intensive Care Med 37:241–248. doi: 10.1007/s00134-010-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fani F, Regolisti G, Delsante M, Cantaluppi V, Castellano G, Gesualdo L, Villa G, Fiaccadori E. 2018. Recent advances in the pathogenetic mechanisms of sepsis-associated acute kidney injury. J Nephrol 31:351–359. doi: 10.1007/s40620-017-0452-4. [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. 2012. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int S2:1–138. [Google Scholar]
- 5.Choi G, Gomersall CD, Tian Q, Joynt GM, Freebairn R, Lipman J. 2009. Principles of antibacterial dosing in continuous renal replacement therapy. Crit Care Med 37:2268–2282. doi: 10.1097/CCM.0b013e3181aab3d0. [DOI] [PubMed] [Google Scholar]
- 6.Eyler RF, Mueller BA. 2011. Antibiotic dosing in critically ill patients with acute kidney injury. Nat Rev Nephrol 7:226–235. doi: 10.1038/nrneph.2011.12. [DOI] [PubMed] [Google Scholar]
- 7.Morabito S, Pistolesi V, Maggiore U, Fiaccadori E, Pierucci A. 2012. Pharmacokinetics of antibiotics in continuous renal replacement therapies (CRRT). G Ital Nefrol 29:425–444. [PubMed] [Google Scholar]
- 8.Aronoff GR, Bennett WM, Berns JS, Brier ME, Kasbekar N, Mueller BA, Pasko DA, Smoyer WE. 2007. Drug prescribing in renal failure: dosing guidelines for adults and children, 5th ed American College of Physicians, Philadelphia, PA. [Google Scholar]
- 9.Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. 2010. Fluid balance and acute kidney injury. Nat Rev Nephrol 6:107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 10.Connor MJ Jr, Salem C, Bauer SR, Hofmann CL, Groszek J, Butler R, Rehm SJ, Fissell WH. 2011. Therapeutic drug monitoring of piperacillin-tazobactam using spent dialysate effluent in patients receiving continuous venovenous hemodialysis. Antimicrob Agents Chemother 55:557–560. doi: 10.1128/AAC.00548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toutain PL, Bousquet-Mélou A. 2004. Volumes of distribution. J Vet Pharmacol Ther 27:441–453. doi: 10.1111/j.1365-2885.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- 12.Bugge JF. 2001. Pharmacokinetics and drug dosing adjustments during continuous venovenous hemofiltration or hemodiafiltration in critically ill patients. Acta Anaesthesiol Scand 45:929–934. doi: 10.1034/j.1399-6576.2001.450802.x. [DOI] [PubMed] [Google Scholar]
- 13.Gonçalves-Pereira J, Póvoa P. 2011. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of b-lactams. Crit Care 15:R206. doi: 10.1186/cc10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang GJ, Tang JJ, Lin BS, Kong CW, Lee TY. 1999. Factors affecting gentamicin pharmacokinetics in septic patients. Acta Anaesthesiol Scand 43:726–730. doi: 10.1034/j.1399-6576.1999.430707.x. [DOI] [PubMed] [Google Scholar]
- 15.del Mar Fernández de Gatta Garcia M, Revilla N, Calvo MV, Domínguez-Gil A, Sánchez Navarro A. 2007. Pharmacokinetic/pharmacodynamics analysis of vancomycin in ICU patients. Intensive Care Med 33:279–285. doi: 10.1007/s00134-006-0470-5. [DOI] [PubMed] [Google Scholar]
- 16.Taccone FS, Laterre P-F, Dugernier T, Spapen H, Delattre I, Witebolle X, De Backer D, Layeux B, Wallemacq P, Vincent J-L, Jacobs F. 2010. Insufficient B-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care 14:R126. doi: 10.1186/cc9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamal JA, Economou CJ, Lipman J, Roberts JA. 2012. Improving antibiotic dosing in special situations in the ICU: burns, renal replacement therapy and extracorporeal membrane oxygenation. Curr Opin Crit Care 18:460–471. doi: 10.1097/MCC.0b013e32835685ad. [DOI] [PubMed] [Google Scholar]
- 18.Toutain PL, Bousquet-Mélou A. 2004. Plasma clearance. J Vet Pharmacol Ther 27:415–425. doi: 10.1111/j.1365-2885.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- 19.Muhl E, Martens T, Iven H, Rob P, Bruch HP. 2000. Influence of continuous veno-venous haemodiafiltration and continuous veno-venous haemofiltration on the pharmacokinetics of fluconazole. Eur J Clin Pharmacol 56:671–678. doi: 10.1007/s002280000216. [DOI] [PubMed] [Google Scholar]
- 20.Bouman CS, van Kan HJ, Koopmans RP, Korevaar JC, Schultz MJ, Vroom MB. 2006. Discrepancies between observed and predicted continuous venovenous hemofiltration removal of antimicrobial agents in critically ill patients and the effects on dosing. Intensive Care Med 32:2013–2019. doi: 10.1007/s00134-006-0397-x. [DOI] [PubMed] [Google Scholar]
- 21.Mueller BA, Pasko DA, Sowinski KM. 2003. Higher renal replacement therapy dose delivery influences on drug therapy. Artif Organs 27:808–814. doi: 10.1046/j.1525-1594.2003.07283.x. [DOI] [PubMed] [Google Scholar]
- 22.Bugge JF. 2004. Influence of renal replacement therapy on pharmacokinetics in critically ill patients. Best Pract Res Clin Anaesthesiol 18:175–187. doi: 10.1016/j.bpa.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Vincent HH, Vos MC, Akçahuseyin E, Goessens WH, van Duyl WA, Schalekamp MA. 1993. Drug clearance by continuous haemodiafiltration (CAVHD). Analysis of sieving coefficients and mass transfer coefficients of diffusion. Blood Purif 11:99–107. doi: 10.1159/000170103. [DOI] [PubMed] [Google Scholar]
- 24.Ronco C. 2017. The rise of expanded hemodialysis. Blood Purif 44:I–VIII. doi: 10.1159/000476012. [DOI] [PubMed] [Google Scholar]
- 25.Choi G, Gomersall CD, Tian Q, Joynt GM, Li AM, Lipman J. 2010. Principles of antibacterial dosing in continuous renal replacement therapy. Blood Purif 30:195–212. doi: 10.1159/000321488. [DOI] [PubMed] [Google Scholar]
- 26.Pasko DA, Churchwell MD, Salama NN, Mueller BA. 2011. Longitudinal hemodiafilter performance in modeled continuous renal replacement therapy. Blood Purif 32:82–88. doi: 10.1159/000324191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Böhler J, Donauer J, Keller F. 1999. Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int Suppl 72:S24–S28. doi: 10.1046/j.1523-1755.1999.07202.x. [DOI] [PubMed] [Google Scholar]
- 28.Macedo E, Claure-Del Granado R, Mehta RL. 2011. Effluent volume and dialysis dose in CRRT: time for reappraisal. Nat Rev Nephrol 8:57–60. doi: 10.1038/nrneph.2011.172. [DOI] [PubMed] [Google Scholar]
- 29.Pea F, Viale P, Pavan F, Furlanut M. 2007. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin Pharmacokinet 46:997–1038. doi: 10.2165/00003088-200746120-00003. [DOI] [PubMed] [Google Scholar]
- 30.Churchwell MD, Mueller BA. 2009. Drug dosing during continuous renal replacement therapy. Semin Dial 22:185–188. doi: 10.1111/j.1525-139X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 31.Fiaccadori E, Maggiore U, Rotelli C, Giacosa R, Parenti E, Picetti E, Sagripanti S, Manini P, Andreoli R, Cabassi A. 2004. Removal of linezolid by conventional intermittent hemodialysis, sustained low-efficiency dialysis, or continuous venovenous hemofiltration in patients with acute renal failure. Crit Care Med 32:2437–2442. doi: 10.1097/01.CCM.0000147687.06808.92. [DOI] [PubMed] [Google Scholar]
- 32.Ariano RE, Fine A, Sitar DS, Rexrode S, Zelenitsky SA. 2005. Adequacy of a vancomycin dosing regimen in patients receiving high-flux hemodialysis. Am J Kidney Dis 46:681–687. doi: 10.1053/j.ajkd.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Fiaccadori E, Maggiore U, Rotelli C, Giacosa R, Parenti E, Picetti E, Manini P, Andreoli R, Cabassi A. 2006. Does haemodialysis significantly affect serum linezolid concentrations in critically ill patients with renal failure? A pilot investigation. Nephrol Dial Transplant 21:1402–1406. doi: 10.1093/ndt/gfl048. [DOI] [PubMed] [Google Scholar]
- 34.Jitmuang A, Nation RL, Koomanachai P, Chen G, Lee HJ, Wasuwattakul S, Sritippayawan S, Li J, Thamlikitkul V, Landersdorfer CB. 2015. Extracorporeal clearance of colistin methanesulphonate and formed colistin in end-stage renal disease patients receiving intermittent haemodialysis: implications for dosing. J Antimicrob Chemother 70:1804–1811. doi: 10.1093/jac/dkv031. [DOI] [PubMed] [Google Scholar]
- 35.Scoville BA, Mueller BA. 2013. Medication dosing in critically ill patients with acute kidney injury treated with renal replacement therapy. Am J Kidney Dis 61:490–500. doi: 10.1053/j.ajkd.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 36.Trotman RL, Williamson JC, Shoemaker DM, Salzer WL. 2005. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin Infect Dis 41:1159–1166. doi: 10.1086/444500. [DOI] [PubMed] [Google Scholar]
- 37.Begg EJ, Barclay ML, Kirkpatrick CM. 2001. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol 52(Suppl 1):35S–43S. doi: 10.1046/j.1365-2125.2001.0520s1035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joos B, Schmidli M, Keusch G. 1996. Pharmacokinetics of antimicrobial agents in anuric patients during continuous venovenous haemofiltration. Nephrol Dial Transplant 11:1582–1585. doi: 10.1093/ndt/11.8.1582. [DOI] [PubMed] [Google Scholar]
- 39.Keller F, Böhler J, Czock D, Zellner D, Mertz AK. 1999. Individualized drug dosage in patients treated with continuous hemofiltration. Kidney Int Suppl 72:S29–S31. doi: 10.1046/j.1523-1755.1999.07210.x. [DOI] [PubMed] [Google Scholar]
- 40.Burdette SD, Trotman R, Cmar J. 2012. Mobile infectious disease references: from the bedside to the beach. Clin Infect Dis 55:114–125. doi: 10.1093/cid/cis261. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. 2018. The Sanford guide to antimicrobial therapy 2018, 48th ed Antimicrobial Therapy, Inc, Sperryville, VA. [Google Scholar]
- 42.Bartlett JG, Auwaerter PG, Pham P. 2018. The Johns Hopkins ABX guide 2018. Johns Hopkins Medicine, Unbound Medicine, Inc, Charlottesville, VA. [Google Scholar]
- 43.Zhanel GG, Ariano RE. 1992. Once daily aminoglycoside dosing: maintained efficacy with reduced nephrotoxicity? Ren Fail 14:1–9. doi: 10.3109/08860229209039110. [DOI] [PubMed] [Google Scholar]
- 44.Taccone FS, de Backer D, Laterre PF, Spapen H, Dugernier T, Delattre I, Wallemacq P, Vincent JL, Jacobs F. 2011. Pharmacokinetics of a loading dose of amikacin in septic patients undergoing continuous renal replacement therapy. Int J Antimicrob Agents 37:531–535. doi: 10.1016/j.ijantimicag.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 45.Roberts JA, Field J, Visser A, Whitbread R, Tallot M, Lipman J, Kirkpatrick CM. 2010. Using population pharmacokinetics to determine gentamicin dosing during extended daily diafiltration in critically ill patients with acute kidney injury. Antimicrob Agents Chemother 54:3635–3640. doi: 10.1128/AAC.00222-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sowinski KM, Magner SJ, Lucksiri A, Scott MK, Hamburger RJ, Mueller BA. 2008. Influence of hemodialysis on gentamicin pharmacokinetics, removal during hemodialysis, and recommended dosing. Clin J Am Soc Nephrol 3:355–361. doi: 10.2215/CJN.02920707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mariat C, Venet C, Jehl F, Mwewa S, Lazarevic V, Diconne E, Fonsale N, Carricajo A, Guyomarc'h S, Vermesch R, Aubert G, Bidault R, Bertrand JC, Zeni F. 2006. Continuous infusion of ceftazidime in critically ill patients undergoing continuous venovenous haemodiafiltration: pharmacokinetic evaluation and dose recommendation. Crit Care 10:R26. doi: 10.1186/cc3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME. 2018. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis 18:108–120. doi: 10.1016/S1473-3099(17)30615-1. [DOI] [PubMed] [Google Scholar]
- 49.Roger C, Cotta MO, Muller L, Wallis SC, Lipman J, Lefrant JY, Roberts JA. 2017. Impact of renal replacement modalities on the clearance of piperacillin-tazobactam administered via continuous infusion in critically ill patients. Int J Antimicrob Agents 50:227–231. doi: 10.1016/j.ijantimicag.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Jang SM, Gharibian KN, Lewis SJ, Fissell WH, Tolwani AJ, Mueller BA. 2018. A Monte Carlo simulation approach for beta-lactam dosing in critically ill patients receiving prolonged intermittent renal replacement therapy. J Clin Pharmacol 58:1254–1265. doi: 10.1002/jcph.1137. [DOI] [PubMed] [Google Scholar]
- 51.Kanji S, Roberts JA, Xie J, Alobaid A, Zelenitsky S, Hiremath S, Zhang G, Watpool I, Porteous R, Patel R. 2018. Piperacillin population pharmacokinetics in critically ill adults during sustained low-efficiency dialysis. Ann Pharmacother 52:965–973. doi: 10.1177/1060028018773771. [DOI] [PubMed] [Google Scholar]
- 52.Giles LJ, Jennings AC, Thomson AH, Creed G, Beale RJ, McLuckie A. 2000. Pharmacokinetics of meropenem in intensive care units receiving continuous veno-venous hemofiltration or hemodiafiltration. Crit Care Med 28:632–637. doi: 10.1097/00003246-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Burkhardt O, Hafer C, Langhoff A, Kaever V, Kumar V, Welte T, Haller H, Fliser D, Kielstein JT. 2009. Pharmacokinetics of ertapenem in critically ill patients with acute renal failure undergoing extended daily dialysis. Nephrol Dial Transplant 24:267–271. doi: 10.1093/ndt/gfn472. [DOI] [PubMed] [Google Scholar]
- 54.Deshpande P, Chen J, Gofran A, Murea M, Golestaneh L. 2010. Meropenem removal in critically ill patients undergoing sustained low-efficiency dialysis (SLED). Nephrol Dial Transplant 25:2632–2636. doi: 10.1093/ndt/gfq090. [DOI] [PubMed] [Google Scholar]
- 55.Langgartner J, Vasold A, Glück T, Reng M, Kees F. 2008. Pharmacokinetics of meropenem during intermittent and continuous intravenous application in patients treated by continuous renal replacement therapy. Intensive Care Med 34:1091–1096. doi: 10.1007/s00134-008-1034-7. [DOI] [PubMed] [Google Scholar]
- 56.Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagacé-Wiens PRS, Denisuik A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. 2013. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs 73:159–177. doi: 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 57.Wenzler E, Bunnell KL, Bleasdale SC, Benken S, Danziger LH, Rodvold KA. 2017. Pharmacokinetics and dialytic clearance of ceftazidime-avibactam in a critically ill patient on continuous venovenous hemofiltration. Antimicrob Agents Chemother 61:e00464-17. doi: 10.1128/AAC.00464-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bilgrami I, Roberts JA, Wallis SC, Thomas J, Davis J, Fowler S, Goldrick PB, Lipman J. 2010. Meropenem dosing in critically ill patients with sepsis receiving high-volume continuous venovenous hemofiltration. Antimicrob Agents Chemother 54:2974–2978. doi: 10.1128/AAC.01582-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Economou CJP, Wong G, McWhinney B, Ungerer JPJ, Lipman J, Roberts JA. 2017. Impact of β-lactam antibiotic therapeutic drug monitoring on dose adjustments in critically ill patients undergoing continuous renal replacement therapy. Int J Antimicrob Agents 49:589–594. doi: 10.1016/j.ijantimicag.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 60.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC Jr, Craig WA, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adults: summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 29:1275–1279. doi: 10.1592/phco.29.11.1275. [DOI] [PubMed] [Google Scholar]
- 61.Lewis SJ, Mueller BA. 2018. Development of a vancomycin dosing approach for critically ill patients receiving hybrid hemodialysis using Monte Carlo simulation. SAGE Open Med 6:2050312118773257. doi: 10.1177/2050312118773257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joy MS, Matzke GR, Frye RF, Palevsky PM. 1998. Determinants of vancomycin clearance by continuous venovenous hemofiltration and continuous venovenous hemodialysis. Am J Kidney Dis 31:1019–1027. doi: 10.1053/ajkd.1998.v31.pm9631848. [DOI] [PubMed] [Google Scholar]
- 63.DelDot ME, Lipman J, Tett SE. 2004. Vancomycin pharmacokinetics in critically ill patients receiving continuous hemodiafiltration. Br J Clin Pharmacol 58:259–268. doi: 10.1111/j.1365-2125.2004.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beumier M, Roberts JA, Kabtouri H, Hites M, Cotton F, Wolff F, Lipman J, Jacobs F, Vincent JL, Taccone FS. 2013. A new regimen for continuous infusion of vancomycin during continuous renal replacement therapy. J Antimicrob Chemother 68:2859–2865. doi: 10.1093/jac/dkt261. [DOI] [PubMed] [Google Scholar]
- 65.Economou CJP, Kielstein JT, Czock D, Xie J, Field J, Richards B, Tallott M, Visser A, Koenig C, Hafer C, Schmidt JJ, Lipman J, Roberts JA. 2018. Population pharmacokinetics of vancomycin in critically ill patients receiving prolonged intermittent renal replacement therapy. Int J Antimicrob Agents 52:151–157. doi: 10.1016/j.ijantimicag.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 66.Bellmann R, Falkensammer G, Seger C, Weiler S, Kountchev J, Joannidis M. 2010. Teicoplanin pharmacokinetics in critically ill patients on continuous veno-venous hemofiltration. Int J Clin Pharmacol Ther 48:243–249. [PubMed] [Google Scholar]
- 67.Yagasaki K, Gando S, Matsuda N, Kameue T, Ishitani T, Hirano T, Iseki K. 2003. Pharmacokinetics of teicoplanin in critically ill patients undergoing continuous hemodiafiltration. Intensive Care Med 29:2094–2095. doi: 10.1007/s00134-003-1914-9. [DOI] [PubMed] [Google Scholar]
- 68.Vilay AM, Grio M, Depestel DD, Sowinski KM, Gao L, Heung M, Salama NN, Mueller BA. 2011. Daptomycin pharmacokinetics in critically ill patients receiving continuous venovenous hemodialysis. Crit Care Med 39:19–25. doi: 10.1097/CCM.0b013e3181fa36fb. [DOI] [PubMed] [Google Scholar]
- 69.Xu X, Khadzhynov D, Peters H, Chaves RL, Hamed K, Levi M, Corti N. 2017. Population pharmacokinetics of daptomycin in adult patients undergoing continuous renal replacement therapy. Br J Clin Pharmacol 83:498–509. doi: 10.1111/bcp.13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soraluce A, Asín-Prieto E, Rodríguez-Gascón A, Barrasa H, Maynar J, Carcelero E, Soy D, Isla A. 2018. Population pharmacokinetics of daptomycin in critically ill patients. Int J Antimicrob Agents 52:158–165. doi: 10.1016/j.ijantimicag.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Haselden M, Leach M, Bohm N. 2013. Daptomycin dosing strategies in patients receiving thrice-weekly intermittent hemodialysis. Ann Pharmacother 47:1342–1347. doi: 10.1177/1060028013503110. [DOI] [PubMed] [Google Scholar]
- 72.Vilay AM, Shah KH, Churchwell MD, Patel JH, DePestel DD, Mueller BA. 2010. Modeled dalbavancin transmembrane clearance during intermittent and continuous renal replacement therapies. Blood Purif 30:37–43. doi: 10.1159/000316685. [DOI] [PubMed] [Google Scholar]
- 73.Swoboda S, Ober MC, Lichtenstern C, Saleh S, Schwenger V, Sonntag HG, Haefeli WE, Hempel G, Hoppe-Tichy T, Weigand MA. 2010. Pharmacokinetics of linezolid in septic patients with and without extended dialysis. Eur J Clin Pharmacol 66:291–298. doi: 10.1007/s00228-009-0766-9. [DOI] [PubMed] [Google Scholar]
- 74.Meyer B, Kornek GV, Nikfardjam M, Karth GD, Heinz G, Locker GJ, Jaeger W, Thalhammer F. 2005. Multiple-dose pharmacokinetics of linezolid during continuous venovenous hemofiltration. J Antimicrob Chemother 56:172–179. doi: 10.1093/jac/dki133. [DOI] [PubMed] [Google Scholar]
- 75.Villa G, Di Maggio P, De Gaudio AR, Novelli A, Antoniotti R, Fiaccadori E, Adembri C. 2016. Effects of continuous renal replacement therapy on linezolid pharmacokinetic/pharmacodynamics: a systematic review. Crit Care 20:374. doi: 10.1186/s13054-016-1551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roger C, Muller L, Wallis SC, Louart B, Saissi G, Lipman J, Lefrant JY, Roberts JA. 2016. Population pharmacokinetics of linezolid in critically ill patients on renal replacement therapy: comparison of equal doses in continuous venovenous haemofiltration and continuous venovenous haemodiafiltration. J Antimicrob Chemother 71:464–470. doi: 10.1093/jac/dkv349. [DOI] [PubMed] [Google Scholar]
- 77.Villa G, Cassetta MI, Tofani L, Valente S, Chelazzi C, Falsini S, De Gaudio AR, Novelli A, Ronco C, Adembri C. 2015. Linezolid extracorporeal removal during haemodialysis with high cut-off membrane in critically ill patients. Int J Antimicrob Agents 46:465–468. doi: 10.1016/j.ijantimicag.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 78.Carcelero E, Soy D, Guerrero L, Poch E, Fernandez J, Castro P, Ribas J. 2012. Linezolid pharmacokinetics in patients with acute renal failure undergoing continuous venovenous hemodiafiltration. J Clin Pharmacol 52:1430–1435. doi: 10.1177/0091270011417717. [DOI] [PubMed] [Google Scholar]
- 79.Zhanel GG, Love R, Adam H, Golden A, Zelenitsky S, Schweizer F, Gorityala B, Lagacé-Wiens PR, Rubinstein E, Walkty A, Gin AS, Gilmour M, Hoban DJ, Lynch JP III, Karlowsky JA. 2015. Tedizolid: a novel oxazolidinone with potent activity against multidrug-resistant Gram-positive pathogens. Drugs 75:253–270. doi: 10.1007/s40265-015-0352-7. [DOI] [PubMed] [Google Scholar]
- 80.Lewis SJ, Switaj LA, Mueller BA. 2015. Tedizolid adsorption and transmembrane clearance during in vitro continuous renal replacement therapy. Blood Purif 40:66–71. doi: 10.1159/000430904. [DOI] [PubMed] [Google Scholar]
- 81.Fiaccadori E, Antonucci E, Morabito S, d'Avolio A, Maggiore U, Regolisti G. 2016. Colistin use in patients with reduced kidney function. Am J Kidney Dis 68:296–306. doi: 10.1053/j.ajkd.2016.03.421. [DOI] [PubMed] [Google Scholar]
- 82.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J, Rayner CR, Nation RL, Deans R, Boots R, Widdecombe N, Douglas A, Lipman J. 2005. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 49:4814–4815. doi: 10.1128/AAC.49.11.4814-4815.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Markou N, Fousteri M, Markantonis SL, Zidianakis B, Hroni D, Boutzouka E, Baltopoulos G. 2012. Colistin pharmacokinetics in intensive care unit patients on continuous venovenous haemodiafiltration: an observational study. J Antimicrob Chemother 67:2459–2462. doi: 10.1093/jac/dks257. [DOI] [PubMed] [Google Scholar]
- 85.Karvanen M, Plachouras D, Friberg LE, Paramythiotou E, Papadomichelakis E, Karaiskos I, Tsangaris I, Armaganidis A, Cars O, Giamarellou H. 2013. Colistin methanesulfonate and colistin pharmacokinetics in critically ill patients receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 57:668–671. doi: 10.1128/AAC.00985-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Menna P, Salvatorelli E, Mattei A, Cappiello D, Minotti G, Carassiti M. 2018. Modified colistin regimen for critically ill patients with acute renal impairment and continuous renal replacement therapy. Chemotherapy 63:35–38. doi: 10.1159/000484974. [DOI] [PubMed] [Google Scholar]
- 87.Karaiskos I, Friberg LE, Galani L, Ioannidis K, Katsouda E, Athanassa Z, Paskalis H, Giamarellou H. 2016. Challenge for higher colistin dosage in critically ill patients receiving continuous venovenous haemodiafiltration. Int J Antimicrob Agents 48:337–341. doi: 10.1016/j.ijantimicag.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 88.Nation RL, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Forrest A, Paterson DL, Li J, Silveira FP. 2017. Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis 64:565–571. doi: 10.1093/cid/ciw839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leporati M, Bua RO, Mariano F, Carignano P, Stella M, Biancone L, Vincenti M. 2014. Determination by LC-MS/MS of colistins A and B in plasma and ultrafiltrate from critically ill patients undergoing continuous venovenous hemodiafiltration. Ther Drug Monit 36:182–191. doi: 10.1097/FTD.0b013e3182a8997c. [DOI] [PubMed] [Google Scholar]
- 90.Mariano F, Leporati M, Carignano P, Stella M, Vincenti M, Biancone L. 2015. Efficient removal of colistin A and B in critically ill patients undergoing CVVHDF and sorbent technologies. J Nephrol 28:623–631. doi: 10.1007/s40620-014-0143-3. [DOI] [PubMed] [Google Scholar]
- 91.Marchand S, Frat JP, Petitpas F, Lemaître F, Gobin P, Robert R, Mimoz O, Couet W. 2010. Removal of colistin during intermittent haemodialysis in two critically ill patients. J Antimicrob Chemother 65:1836–1837. doi: 10.1093/jac/dkq185. [DOI] [PubMed] [Google Scholar]
- 92.Jacobs M, Grégoire N, Mégarbane B, Gobin P, Balayn D, Marchand S, Mimoz O, Couet W. 2016. Population pharmacokinetics of colistin methanesulfonate and colistin in critically ill patients with acute renal failure requiring intermittent hemodialysis. Antimicrob Agents Chemother 60:1788–1793. doi: 10.1128/AAC.01868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strunk AK, Schmidt JJ, Baroke E, Bode-Böger SM, Martens-Lobenhoffer J, Welte T, Kielstein JT. 2014. Single- and multiple-dose pharmacokinetics and total removal of colistin in a patient with acute kidney injury undergoing extended daily dialysis. J Antimicrob Chemother 69:2008–2010. doi: 10.1093/jac/dku075. [DOI] [PubMed] [Google Scholar]
- 94.Malone RS, Fish DN, Abraham E, Teitelbaum I. 2001. Pharmacokinetics of levofloxacin and ciprofloxacin during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother 45:2949–2954. doi: 10.1128/AAC.45.10.2949-2954.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wallis SC, Mullany DV, Lipman J, Rickard CM, Daley PJ. 2001. Pharmacokinetics of ciprofloxacin in ICU patients on continuous veno-venous haemodiafiltration. Intensive Care Med 27:665–672. doi: 10.1007/s001340100857. [DOI] [PubMed] [Google Scholar]
- 96.Guenter SG, Iven H, Boos C, Bruch HP, Muhl E. 2002. Pharmacokinetics of levofloxacin during continuous venovenous hemodiafiltration and continuous venovenous hemofiltration in critically ill patients. Pharmacotherapy 22:175–183. doi: 10.1592/phco.22.3.175.33546. [DOI] [PubMed] [Google Scholar]
- 97.Hansen E, Bucher M, Jakob W, Lemberger P, Kees F. 2001. Pharmacokinetics of levofloxacin during continuous veno-venous hemofiltration. Intensive Care Med 27:371–375. doi: 10.1007/s001340000836. [DOI] [PubMed] [Google Scholar]
- 98.Fuhrmann V, Schenk P, Jaeger W, Ahmed S, Thalhammer F. 2004. Pharmacokinetics of moxifloxacin in patients undergoing continuous venovenous haemodiafiltration. J Antimicrob Chemother 54:780–784. doi: 10.1093/jac/dkh421. [DOI] [PubMed] [Google Scholar]
- 99.Czock D, Hüsig-Linde C, Langhoff A, Schöpke T, Hafer C, de Groot K, Swoboda S, Kuse E, Haller H, Fliser D, Keller F, Kielstein JT. 2006. Pharmacokinetics of moxifloxacin and levofloxacin in intensive care unit patients who have acute renal failure and undergo extended daily dialysis. Clin J Am Soc Nephrol 1:1263–1268. doi: 10.2215/CJN.01840506. [DOI] [PubMed] [Google Scholar]
- 100.Choi G, Gomersall CD, Lipman J, Wong A, Joynt GM, Leung P, Ramsay SJ, Ho OM. 2004. The effect of adsorption, filter material and point of dilution on antibiotic elimination by haemofiltration: an in vitro study of levofloxacin. Int J Antimicrob Agents 24:468–472. doi: 10.1016/S0924-8579(04)00242-0. [DOI] [PubMed] [Google Scholar]
- 101.Bellmann R, Egger P, Djanani A, Wiedermann CJ. 2004. Pharmacokinetics of amphotericin B lipid complex in critically ill patients on continuous veno-venous haemofiltration. Int J Antimicrob Agents 23:80–83. doi: 10.1016/j.ijantimicag.2003.05.014. [DOI] [PubMed] [Google Scholar]
- 102.Roger C, Wallis SC, Muller L, Saissi G, Lipman J, Brüggemann RJ, Lefrant JY, Roberts JA. 2017. Caspofungin population pharmacokinetics in critically ill patients undergoing continuous veno-venous haemofiltration or haemodiafiltration. Clin Pharmacokinet 56:1057–1068. doi: 10.1007/s40262-016-0495-z. [DOI] [PubMed] [Google Scholar]
- 103.Weiler S, Seger C, Pfisterer H, Stienecke E, Stippler F, Welte R, Joannidis M, Griesmacher A, Bellmann R. 2013. Pharmacokinetics of caspofungin in critically ill patients on continuous renal replacement therapy. Antimicrob Agents Chemother 57:4053–4057. doi: 10.1128/AAC.00335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kiser TH, Fish DN, Aquilante CL, Rower JE, Wempe MF, MacLaren R, Teitelbaum I. 2015. Evaluation of sulfobutylether-β-cyclodextrin (SBECD) accumulation and voriconazole pharmacokinetics in critically ill patients undergoing continuous renal replacement therapy. Crit Care 19:32. doi: 10.1186/s13054-015-0753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jang SM, Hough G, Mueller BA. 2018. Ex vivo rezafungin adsorption and clearance during continuous renal replacement therapy. Blood Purif 46:214–219. doi: 10.1159/000489212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bergner R, Hoffmann M, Riedel KD, Mikus G, Henrich DM, Haefeli WE, Uppenkamp M, Walter-Sack I. 2006. Fluconazole dosing in continuous veno-venous haemofiltration (CVVHF): need for a high daily dose of 800 mg. Nephrol Dial Transplant 21:1019–1023. doi: 10.1093/ndt/gfi284. [DOI] [PubMed] [Google Scholar]
- 107.Yagasaki K, Gando S, Matsuda N, Kameue T, Ishitani T, Hirano T, Iseki K. 2003. Pharmacokinetics and the most suitable dosing regimen of fluconazole in critically ill patients receiving continuous hemodiafiltration. Intensive Care Med 29:1844–1848. doi: 10.1007/s00134-003-1980-z. [DOI] [PubMed] [Google Scholar]