The β-lactams imipenem and cefoxitin are used for the treatment of Mycobacterium abscessus lung infections. Here, we show that these cell wall synthesis inhibitors trigger a lethal bacterial ATP burst by increasing oxidative phosphorylation. Cotreatment of M. abscessus with the antimycobacterial ATP synthase inhibitor bedaquiline suppresses this ATP burst and eliminates the bactericidal activity of β-lactams.
KEYWORDS: Mycobacterium abscessus, bedaquiline, β-lactam, drug-drug interaction
ABSTRACT
The β-lactams imipenem and cefoxitin are used for the treatment of Mycobacterium abscessus lung infections. Here, we show that these cell wall synthesis inhibitors trigger a lethal bacterial ATP burst by increasing oxidative phosphorylation. Cotreatment of M. abscessus with the antimycobacterial ATP synthase inhibitor bedaquiline suppresses this ATP burst and eliminates the bactericidal activity of β-lactams. Thus, the addition of bedaquiline to β-lactam-containing regimes may negatively affect treatment outcome.
TEXT
Cell wall synthesis inhibitors such as isoniazid cause a burst in intracellular ATP in Mycobacterium tuberculosis (1, 2). This increase in ATP concentration is suppressed by the ATP synthase inhibitor bedaquiline (BDQ) and protonophore uncouplers of oxidative phosphorylation. This suggests that antimycobacterial cell wall synthesis inhibitors trigger a mycobacterial cell envelope stress response resulting in increased oxidative phosphorylation and, thus, elevated ATP levels (2). Surprisingly, treatment of tubercle bacilli with inhibitors of oxidative phosphorylation suppressed the bactericidal effect of isoniazid, suggesting that the isoniazid-induced ATP burst contributes to cell death mediated by the drug (1, 2).
Lung infections caused by Mycobacterium abscessus, a rapidly growing mycobacterium, are extremely difficult to eradicate (3). Antituberculosis drugs, including isoniazid, are not active against this opportunistic nontuberculous mycobacterial (NTM) pathogen. The macrolides clarithromycin and azithromycin are the main drugs used in combination with parenteral medications (the aminoglycoside amikacin and a β-lactam). The macrolides are the only oral agents reliably active in vitro against M. abscessus (4). The β-lactams used for M. abscessus treatment are imipenem and cefoxitin (4). Recent reports suggest that treating with two β-lactam drugs may have benefit (5–7). Furthermore, the new β-lactamase inhibitors relebactam and vaborbactam (8) as well as avibactam (6) were shown to significantly improve in vitro activity of various marketed β-lactams (8). These findings suggest β-lactam-containing regimens may be increasingly applied in the future. BDQ, developed as an antituberculosis drug, was shown to be active against M. abscessus (9, 10) and is added in cases of treatment failures (11).
β-Lactams inhibit peptidoglycan synthesis and, thus, interfere with cell wall synthesis. It is not known whether β-lactams, similar to isoniazid in M. tuberculosis, cause an ATP burst in M. abscessus and, if they do, whether this contributes to the bactericidal activity of the drugs. If this is the case, adding BDQ to β-lactam-containing regimens for M. abscessus treatment may be counterproductive.
To determine whether β-lactams cause an increase in intrabacterial ATP, exponentially growing cultures of a sequenced clinical strain of M. abscessus (12) were treated with imipenem and cefoxitin for 4 h (about one generation time). ATP contents of cultures were then measured using Promega’s BacTiter-Glo assay as described previously (2). By keeping treatment time short and drug concentrations low (1× MIC), the effects of drug treatment on bacterial viability were minimized. Figure 1 shows that the two β-lactams indeed caused a burst in ATP. To determine whether the ATP increase is specific for these cell wall synthesis inhibitors, we treated M. abscessus cultures with clarithromycin, amikacin, linezolid, tigecycline, rifabutin, moxifloxacin, clofazimine, or bedaquiline. In contrast to the β-lactams, inhibitors of protein, RNA, DNA, or ATP synthesis did not increase ATP levels, suggesting that this effect is specific for agents interfering with cell wall synthesis.
FIG 1.
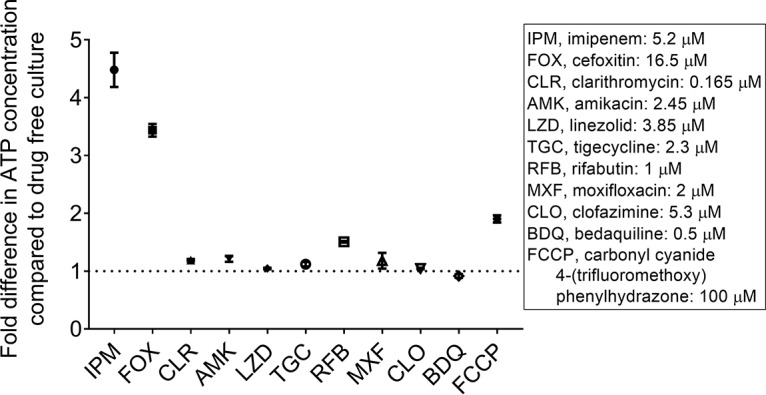
Effect of treatment of M. abscessus cultures with various drugs on bacterial ATP content. Growing cultures were treated for 4 h with inhibitors of peptidoglycan synthesis (imipenem, cefoxitin), inhibitors of protein (clarithromycin, amikacin, linezolid, tigecycline), RNA (rifabutin), DNA synthesis (moxifloxacin), clofazimine, or inhibitors of oxidative phosphorylation—bedaquiline, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone—at MICs. ATP content of the cultures was measured and normalized for growth based on each culture’s optical density at 600 nm (OD600). On the y axis, the fold difference of the ATP level of drug-treated culture compared to the ATP level of drug-free control culture is shown. MIC values are displayed in the right box, together with abbreviation of the drug names. Each experiment was performed at least three times independently. Mean values and standard deviations are shown. Note that whereas non-β-lactam drugs did not affect ATP content of M. abscessus cultures, the protonophore FCCP appears to cause a weak increase under our test conditions. The reason for this effect remains to be determined.
To determine whether inhibitors of oxidative phosphorylation suppress the ATP burst observed in cultures treated with β-lactams, bacteria were cotreated with a β-lactam and subinhibitory concentrations of either BDQ or an uncoupler of oxidative phosphorylation, the protonophore carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) (13). Both oxidative phosphorylation inhibitors suppressed the ATP burst triggered by the β-lactams when coadministered (Fig. 2). Together, this suggests that M. abscessus harbors, similar to M. tuberculosis, a cell envelope stress response in which cell wall synthesis inhibitors trigger increased oxidative phosphorylation.
FIG 2.

Effect of cotreatment of M. abscessus cultures with β-lactams and inhibitors of oxidative phosphorylation on bacterial ATP content. Growing cultures were treated for 4 h with (A) increasing concentrations of BDQ from 0 μM up to the drug’s MIC (0.5 μM) either without β-lactam added (control, orange bars), together with IPM at MIC (5.2 μM), or with FOX at MIC (16.5 μM). Green bars indicate β-lactam alone. Blue bars indicate β-lactam in combination with increasing BDQ concentrations. (B) Same experiment as in panel A with FCCP replacing BDQ. Cultures were treated with increasing concentrations of FCCP from 0 μM up to the protonophore’s MIC (100 μM) either without β-lactam added, together with IPM at MIC, or with FOX at MIC. For MICs and drug abbreviations, see Fig. 1. The ATP content of cultures after drug treatment was measured and normalized for growth. Each experiment was performed at least three times independently. Asterisks represent the significance determined by the Student’s t test comparing the ATP content of the cotreated culture to the ATP content of the β-lactam-only-treated culture as follows: *, <0.05; **; <0.01. ATP content of drug-free (DF) and drug-free plus vehicle (V) cultures. Drug-free indicates that nothing was added to cultures. Vehicle cultures indicate that the same volume of vehicle (dimethyl sulfoxide [DMSO])—without drug—was added that was added to drug cultures.
To determine whether suppression of the ATP burst by BDQ affects the bactericidal activity of β-lactams, the effect of β-lactam–BDQ cotreatment on the viability of M. abscessus cultures was determined. To achieve substantial killing by the β-lactams, the treatment time was extended from 4 to 48 h and the concentrations were increased from 1× to 3 to 5× MIC (which is still in the pharmacologically achievable range [14, 15]). Figure 3 shows that the β-lactams alone exerted bactericidal activity. Addition of BDQ to β-lactam-containing cultures reduced the bactericidal activity of the β-lactams in a dose-dependent manner (Fig. 3A). The same effect was observed for cotreatment of cultures with the protonophore FCCP (Fig. 3B). These results suggest that suppression of the β-lactam-induced ATP burst reduces the bactericidal effect of these drugs.
FIG 3.
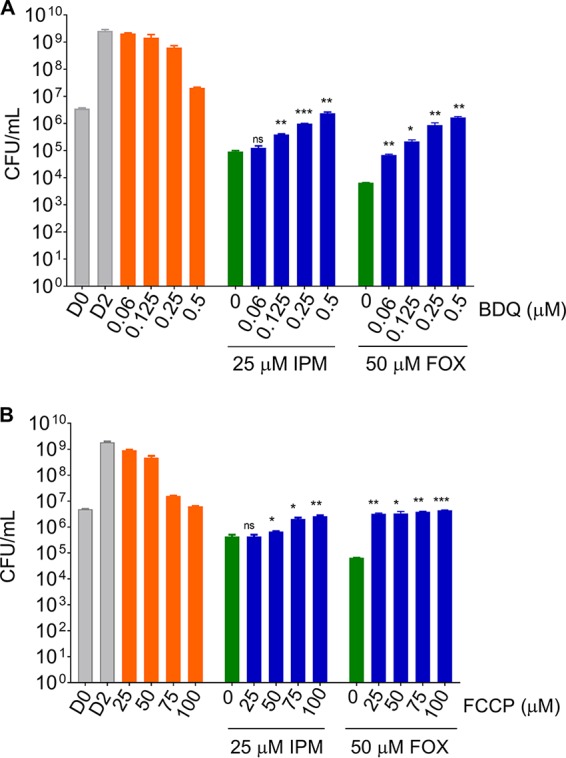
Effect of cotreatment of M. abscessus cultures with β-lactams and inhibitors of oxidative phosphorylation on bacterial viability. Growing cultures were treated for 48 h with (A) increasing concentrations of BDQ from 0 μM up to the drug’s MIC (0.5 μM) either without β-lactam added (control, orange bars), together with IPM at 5× MIC (25 μM), or with FOX at 3× MIC (50 μM). Green bars indicate β-lactam alone. Blue bars indicate β-lactam in combination with increasing BDQ concentrations. (B) Same experiment as in panel A with FCCP replacing BDQ. Cultures were treated with increasing concentrations of FCCP from 0 μM up to the protonophore’s MIC (100 μM) either without β-lactam added, together with IPM at 5× MIC, or with FOX at 3× MIC. For MICs and drug abbreviations, see Fig. 1. Viability of cultures was measured by CFU enumeration on agar. Each experiment was performed at least three times independently. Asterisks represent the significance determined by the Student’s t test comparing the recovered bacteria from the cotreated culture to the bacteria recovered from the β-lactam-only-treated culture as follows: *, <0.05; **, <0.01; ***, <0.001. D0 and D2, CFU of drug-free control cultures at the beginning and end of the experiment.
We show here that BDQ antagonizes the bactericidal activity of β-lactams in M. abscessus. Drug-drug potency interaction studies in vitro are typically carried out using growth assays, thus addressing the question of whether drugs antagonize in terms of growth inhibition as opposed to a bactericidal effect. To determine whether the observed antagonistic effect of BDQ on the bactericidal activity of β-lactams extends to growth inhibition, we carried out checkerboard analyses and measured turbidity as a readout for growth as described (16, 17) (see Fig. S1 in the supplemental material). These experiments revealed that BDQ–β-lactam combinations are additive, i.e., not antagonistic, in growth inhibition assays. Thus, there is a disconnect between drug-drug potency interaction regarding growth inhibition and bactericidal activity, and the antagonistic effect of BDQ on the bactericidal activity of β-lactams would have been missed if only standard checkerboard analyses had been carried out.
In conclusion, we show that imipenem and cefoxitin trigger an ATP burst in M. abscessus by increasing oxidative phosphorylation. Treatment of M. abscessus with BDQ or a protonophore suppressed the ATP burst and, importantly, eliminated the bactericidal activity of the β-lactams. Imipenem or cefoxitin are often used in regimens to treat lung disease caused by this NTM pathogen (3). Our in vitro data suggest that the addition of BDQ to β-lactam-containing regimens may negatively affect treatment outcome. Murine studies are warranted to test for antagonism of β-lactam–bedaquiline combinations in vivo to provide evidence that our finding may be of clinical relevance.
Our results suggest that M. abscessus, similar to M. tuberculosis (2), harbors a cell envelope stress response in which inhibition of cell wall synthesis triggers increased oxidative phosphorylation resulting in a burst of ATP. This ATP surge appears to contribute to the bactericidal activity across different classes of antimycobacterial cell wall synthesis inhibitors, including isoniazid in the case of M. tuberculosis and β-lactams in the case of M. abscessus. It is interesting to note that our findings support a new concept in understanding antibiotic death physiology suggested recently by Collins and colleagues. The authors propose that drug-induced changes in ATP homeostasis critically drive lethal metabolic alterations (18).
Our study focused on the interaction of β-lactams and BDQ in M. abscessus, not M. tuberculosis. It is important to note that β-lactams (meropenem or imipenem-cilastatin along with amoxicillin-clavulanate) are used for the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis, often together with BDQ (19). Furthermore, BDQ is increasingly considered in novel regimens for the treatment of drug-susceptible tuberculosis (20). Whether BDQ also antagonizes the activity of β-lactams in M. tuberculosis has not been determined. However, it was shown that meropenem (as well as the two mechanistically different cell wall inhibitors, ethambutol and the benzothiazinone BTZ043) triggered an ATP burst in tubercle bacilli similar to isoniazid (1). Considering the clinical practice of coadministering β-lactams and BDQ, an antagonistic effect would have far-reaching implications for therapy of drug-resistant tuberculosis. Thus, in vitro and in vivo studies are urgently required to address a possible antagonism of β-lactams and BDQ in M. tuberculosis.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI132374.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
M.L. carried out the experiments. M.L. and T.D. wrote the manuscript.
We declare that we have no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00827-19.
REFERENCES
- 1.Zeng S, Soetaert K, Ravon F, Vandeput M, Bald D, Kauffmann J-M, Mathys V, Wattiez R, Fontaine V. 2019. Isoniazid bactericidal activity involves electron transport chain perturbation. Antimicrob Agents Chemother 63:e01841-18. doi: 10.1128/AAC.01841-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shetty A, Dick T. 2018. Mycobacterial cell wall synthesis inhibitors cause lethal ATP burst. Front Microbiol 9:1898. doi: 10.3389/fmicb.2018.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu ML, Aziz DB, Dartois V, Dick T. 2018. NTM drug discovery: status, gaps and the way forward. Drug Discov Today 23:1502–1519. doi: 10.1016/j.drudis.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 5.Pandey R, Chen L, Manca C, Jenkins S, Glaser L, Vinnard C, Stone G, Lee J, Mathema B, Nuermberger EL, Bonomo RA, Kreiswirth BN. 2019. Dual beta-lactam combinations highly active against Mycobacterium abscessus complex in vitro. mBio 10:e02895-18. doi: 10.1128/mBio.02895-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Story-Roller E, Maggioncalda EC, Lamichhane G. 2019. Select beta-lactam combinations exhibit synergy against Mycobacterium abscessus in vitro. Antimicrob Agents Chemother 63:e02613-18. doi: 10.1128/AAC.02613-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Story-Roller E, Maggioncalda EC, Lamichhane G. 2019. Synergistic efficacy of β-lactam combinations against Mycobacterium abscessus pulmonary infection in mice. Antimicrob Agents Chemother 63:e00614-19. doi: 10.1128/AAC.00614-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaushik A, Ammerman NC, Lee J, Martins O, Kreiswirth BN, Lamichhane G, Parrish NM, Nuermberger EL. 2019. In vitro activity of the new beta-lactamase inhibitors relebactam and vaborbactam in combination with beta-lactams against Mycobacterium abscessus complex clinical isolates. Antimicrob Agents Chemother 63:e02623-18. doi: 10.1128/AAC.02623-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown-Elliott BA, Wallace RJ Jr. 2019. In vitro susceptibility testing of bedaquiline against Mycobacterium abscessus complex. Antimicrob Agents Chemother 63:e01919-18. doi: 10.1128/AAC.01919-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupont C, Viljoen A, Thomas S, Roquet-Banères F, Herrmann J-L, Pethe K, Kremer L. 2017. Bedaquiline inhibits the ATP synthase in Mycobacterium abscessus and is effective in infected zebrafish. Antimicrob Agents Chemother 61:e01225-17. doi: 10.1128/AAC.01225-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philley JV, Wallace RJ Jr, Benwill JL, Taskar V, Brown-Elliott BA, Thakkar F, Aksamit TR, Griffith DE. 2015. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 148:499–506. doi: 10.1378/chest.14-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yee M, Klinzing D, Wei JR, Gengenbacher M, Rubin EJ, Dick T. 2017. Draft genome sequence of Mycobacterium abscessus bamboo. Genome Announc 5:e00388-17. doi: 10.1128/genomeA.00388-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benz R, McLaughlin S. 1983. The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophys J 41:381–398. doi: 10.1016/S0006-3495(83)84449-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carver PL, Nightingale CH, Quintiliani R. 1989. Pharmacokinetics and pharmacodynamics of total and unbound cefoxitin and cefotetan in healthy volunteers. J Antimicrob Chemother 23:99–106. doi: 10.1093/jac/23.1.99. [DOI] [PubMed] [Google Scholar]
- 15.Sakka SG, Glauner AK, Bulitta JB, Kinzig-Schippers M, Pfister W, Drusano GL, Sorgel F. 2007. Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem-cilastatin in critically ill patients in a randomized, controlled trial. Antimicrob Agents Chemother 51:3304–3310. doi: 10.1128/AAC.01318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aziz DB, Teo JWP, Dartois V, Dick T. 2018. Teicoplanin-tigecycline combination shows synergy against Mycobacterium abscessus. Front Microbiol 9:932. doi: 10.3389/fmicb.2018.00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh MH, Yu CM, Yu VL, Chow JW. 1993. Synergy assessed by checkerboard. A critical analysis. Diagn Microbiol Infect Dis 16:343–349. doi: 10.1016/0732-8893(93)90087-N. [DOI] [PubMed] [Google Scholar]
- 18.Yang JH, Wright SN, Hamblin M, McCloskey D, Alcantar MA, Schrubbers L, Lopatkin AJ, Satish S, Nili A, Palsson BO, Walker GC, Collins JJ. 2019. A white-box machine learning approach for revealing antibiotic mechanisms of action. Cell 177:1649–1661. doi: 10.1016/j.cell.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. 2014. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. World Health Organization, Geneva, Switzerland: https://www.who.int/tb/publications/pmdt_companionhandbook/en/. [PubMed] [Google Scholar]
- 20.Libardo MDJ, Boshoff HIM, Barry CE III. 2018. The present state of the tuberculosis drug development pipeline. Curr Opin Pharmacol 42:81–94. doi: 10.1016/j.coph.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


