Emtricitabine (FTC) is a first-line antiviral drug recommended for the treatment of AIDS during pregnancy. We hypothesized that transporters located in the placenta contribute to FTC transfer across the blood-placenta barrier. BeWo cells, cell models with stable or transient expression of transporter genes, primary human trophoblast cells (PHTCs), and small interfering RNAs (siRNAs) were applied to demonstrate which transporters were involved.
KEYWORDS: emtricitabine, placenta, transporters, BeWo
ABSTRACT
Emtricitabine (FTC) is a first-line antiviral drug recommended for the treatment of AIDS during pregnancy. We hypothesized that transporters located in the placenta contribute to FTC transfer across the blood-placenta barrier. BeWo cells, cell models with stable or transient expression of transporter genes, primary human trophoblast cells (PHTCs), and small interfering RNAs (siRNAs) were applied to demonstrate which transporters were involved. FTC accumulation in BeWo cells was reduced markedly by inhibitors of equilibrative nucleoside transporters (ENTs), concentrative nucleoside transporters (CNTs), organic cation transporters (OCTs), and organic cation/carnitine transporter 1 (OCTN1) and increased by inhibitors of breast cancer resistance protein (BCRP) and multidrug resistance-associated proteins (MRPs). ENT1, CNT1, OCTN1, MRP1/2/3, and BCRP, but not ENT2, CNT3, OCTN2, or multidrug resistance protein 1 (MDR1), were found to transport FTC. FTC accumulation in PHTCs was decreased significantly by inhibitors of ENTs and OCTN1. These results suggest that ENT1, CNT1, and OCTN1 probably contribute to FTC uptake from maternal circulation to trophoblasts and that ENT1, CNT1, and MRP1 are likely involved in FTC transport between trophoblasts and fetal blood, whereas BCRP and MRP1/2/3 facilitate FTC transport from trophoblasts to maternal circulation. Coexistence of tenofovir or efavirenz with FTC in the cell medium did not influence FTC accumulation in BeWo cells or PHTCs.
INTRODUCTION
AIDS is a global epidemic, and among carriers of AIDS, approximately half are women. Specifically, there were ∼18 million women HIV carriers in 2016, and most of them were of child-bearing age (1). Antiretroviral therapy for HIV-positive pregnant women is used widely to prevent mother-to-child transmission and is also a primary treatment for maternal HIV infection.
Emtricitabine (FTC) is a nucleoside reverse transcriptase inhibitor. It was approved by the U.S. Food and Drug Administration in 2003. Currently, it is a widely used antiretroviral drug recommended by the WHO for use in pregnant woman for the prevention of mother-to-child transmission. FTC is a highly polar cytidine analog with low pKa (2.65) and logP (approximately −0.43), which indicates that FTC is highly hydrophilic for cell membranes. Scholars have reported that FTC penetrates the placenta, with a transport ratio between umbilical cord plasma and maternal plasma of 0.8 to 1.5 (2–6). We speculated that drug transporters might have an indispensable role in FTC transfer across the placenta.
Organic cation transporter 3 (OCT3) and organic anion transporter 4 (OAT4) are highly expressed in the placenta. OCT3 and OAT4 are localized on the basal sides of trophoblasts in the placenta (7, 8) and help to transport substrates from the fetus to the placenta. Organic cation/carnitine transporter 1 (OCTN1) and OCTN2 are expressed in the apical membranes of trophoblasts, which may mediate the uptake of substrates from maternal blood into trophoblasts (9, 10). Equilibrative nucleoside transporters (ENTs) and concentrative nucleoside transporters (CNTs) are also expressed in the placenta. ENT1 and ENT2 are expressed in both the apical and basal membranes of the placenta (11–13), but the sensitivities of ENT1 and ENT2 to nitrobenzylthioinosine (NBTI) and dipyridamole differ (14, 15). ENT1 can be suppressed by NBTI at ≤1 μM, whereas ENT2 is unaffected by NBTI at ≤1 μM but can be inhibited by NBTI at 100 μM (14, 16–18). CNTs have been detected at the mRNA level in full-term human placentas (19). Breast cancer resistance protein (BCRP), multidrug resistance-associated proteins 1, 2, and 3 (MRP1/2/3), and multidrug resistance protein 1 (MDR1) are expressed on the apical sides of trophoblasts, and MRP1 is also expressed on the basal sides of trophoblasts (20). Scholars have reported that FTC is a substrate of MRP1 and multidrug and toxin extrusion proteins 1 (MATE1) (21, 22), and our previous data showed that ENTs are involved in FTC transport in BeWo cells (23). Multiple transporters of the solute carrier family (SLC) and ATP-binding cassette (ABC) families mentioned above are expressed on syncytiotrophoblasts and probably contribute to FTC permeation across the placental barrier; however, scant information is available about which transporters are involved in transplacental transport of FTC.
To prevent mother-to-child transmission efficiently, combination antiretroviral therapy involving use of a drug that can be transported in large amounts from maternal membranes to the fetus for preexposure prophylaxis has been recommended by the WHO (24). Hence, in 2016, FTC-tenofovir disoproxil fumarate-efavirenz (EFV) was recommended as a first-line combination antiviral therapy during pregnancy. Highly active antiretroviral therapy is intended to achieve synergism between the compounds and reduce the likelihood of the development of drug resistance (25), but combination drug therapy often results in unexpected changes in drug levels within tissues that can lead to possible drug toxicity or subtherapy (26). However, whether coadministered tenofovir (TFV) and/or EFV influences FTC transfer across the placenta has not been clarified.
One aim of the present study was to explore which transporters contribute to FTC transfer across the placenta using BeWo cells (a human choriocarcinoma cell line), cells stably or transiently transfected with transporters, and primary human trophoblast cells (PHTCs). The other aim was to predict whether TFV and/or EFV would influence FTC transfer across the placenta by comparing FTC accumulation in BeWo cells and PHTCs in the absence or presence of TFV and/or EFV. Our results will provide information to understand FTC application in pregnant women.
RESULTS
Multiple transporters contribute to FTC accumulation in BeWo cells.
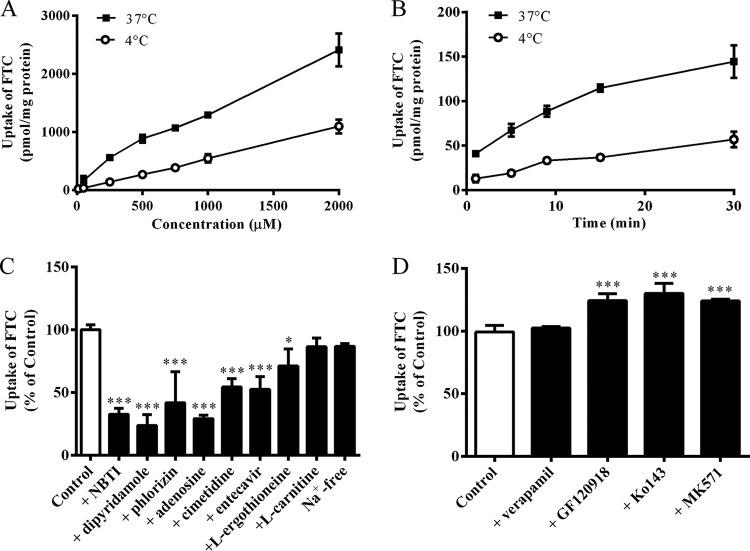
To study if transporters contribute to transmembrane movement of FTC, concentration- and time-dependent experiments at 4°C and 37°C were carried out. As shown in Fig. 1A and B, FTC accumulation in BeWo cells at 37°C was significantly higher than that at 4°C, which suggested that transporters mediated FTC transfer into BeWo cells. Furthermore, accumulation of FTC (10 μM, 3 min) in BeWo cells was inhibited remarkably by 1 μM NBTI (an inhibitor of ENT1; P < 0.001), 50 μM dipyridamole (an inhibitor of ENTs; P < 0.001), 200 μM phlorizin (an inhibitor of CNTs; P < 0.001), 200 μM adenosine (a substrate/inhibitor of ENTs and CNTs; P < 0.001), 250 μM cimetidine (an inhibitor of OCTs and OCTN1; P < 0.001), 100 μM entecavir (a substrate of ENT1; P < 0.001), and 100 μM l-ergothioneine (a substrate of OCTN1; P < 0.05) but not by 100 μM l-carnitine (a substrate/inhibitor of OCTN2) or Na+-free buffer (Fig. 1C). In addition, BCRP inhibitors (10 μM GF120918 and 5 μM Ko143, P < 0.001) and an MRP inhibitor (50 μM MK571, P < 0.001) increased FTC accumulation significantly in BeWo cells, whereas 100 μM verapamil (an inhibitor of MDR1) did not show any effect on FTC accumulation (Fig. 1D). These results suggested that ENTs, CNTs, OCTs, OCTN1, BCRP, and MRPs are probably involved in FTC accumulation in BeWo cells.
FIG 1.
(A) FTC accumulation in BeWo cells at ≤2,000 μM for 3 min at 4°C or 37°C. (B) Time-dependent accumulation of FTC (10 μM) in BeWo cells at 4°C or 37°C. (C) Effects of specific inhibitors of ENTs or CNTs (1 μM NBTI/50 μM dipyridamole and 200 μM phlorizin), general inhibitor of nucleoside transporters (200 μM adenosine), OCTs/OCTN1 inhibitor (250 μM cimetidine), a substrate of ENT1 reported previously (100 μM entecavir), a substrate of OCTN1 (100 μM l-ergothioneine), a substrate/inhibitor of OCTN2 (100 μM l-carnitine), and Na+-free buffer (Na+ was replaced by N-methyl-d-glucamine) on the accumulation of FTC (10 μM, 3 min) in BeWo cells, compared with the control (10 μM FTC). *, P < 0.05; ***, P < 0.001. (D) Effects of ABC transporter inhibitors of MDR1 (100 μM verapamil), BCRP (10 μM GF120918 and 5 μM Ko143), and MRPs (50 μM MK571) on the accumulation of FTC in BeWo cells. ***, P < 0.001.
ENT1 is involved in the transport of FTC whereas ENT2 is not.
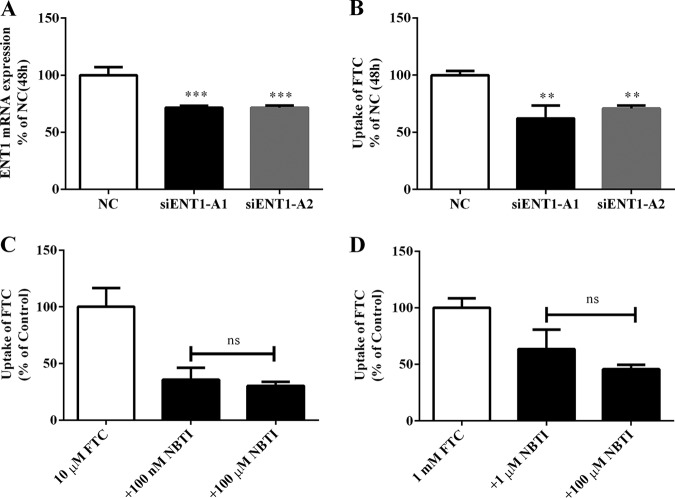
A short interfering RNA (siRNA) assay was applied to ascertain further the role of ENT1 in the placental transfer of FTC. As shown in Fig. 2A and B, compared with the BeWo cells transfected with negative-control (NC) siRNA, partial silencing of ENT1 by siRNAs resulted in significant decreases in the expression of ENT1 mRNA (P < 0.001) and FTC accumulation (P < 0.01). Human ENT1 and ENT2 proteins have 46% homologous amino acid sequences and transport some similar substrates, but these two proteins have different sensitivities to NBTI. ENT1 was inhibited specifically by a low concentration (≤1 μM) of NBTI, whereas ENT2 was inhibited by a higher concentration (≥100 μM) of NBTI. These results showed that, regardless of the FTC concentration, inhibition of FTC accumulation by NBTI at low and high concentrations was not significantly different. These results implied that ENT1 is involved in FTC transport but that ENT2 is not.
FIG 2.
(A) Influence of siRNA-ENT1 on expression of ENT1 mRNA compared with the NC siRNA. ***, P < 0.001. (B) Influence of siRNA-ENT1 on FTC accumulation in BeWo cells compared with NC. **, P < 0.01. (C and D) Effects of different concentrations of NBTI on FTC accumulation in BeWo cells. ns, P > 0.05.
FTC is confirmed to be a substrate of CNT1 but not CNT3.
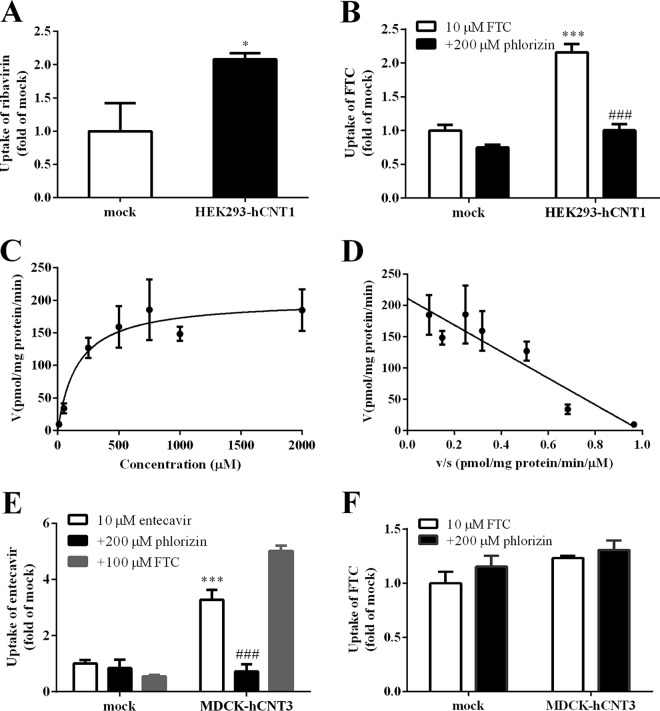
FTC accumulation in BeWo cells was strongly inhibited by adenosine and phlorizin (Fig. 1C), which suggested that CNTs are involved in FTC transport. To ascertain further the contribution of CNT1/3 to FTC transport, FTC uptake was measured in HEK293 cells or MDCK cells transiently transfected with human CNT1 (hCNT1) and hCNT3, respectively. Protein function in HEK293-hCNT1 or MDCK-hCNT3 cells was validated by comparing the accumulation of ribavirin (a substrate of CNT1; P < 0.05) (Fig. 3A) or entecavir (a substrate of CNT3), respectively, in transfected cells with that in mock cells (Fig. 3E). Accumulation of FTC (10 μM, 3 min) in HEK293-hCNT1 was 2.1-fold that observed in mock cells (P < 0.001), which was reduced significantly by 200 μM phlorizin (P < 0.001) (Fig. 3B), and the uptake mediated by CNT1 followed Michaelis-Menten kinetics with Km and Vmax values of 162 ± 54.1 μM and 201 ± 15.2 pmol/mg protein/min, respectively (Fig. 3C and D). FTC accumulation in MDCK-hCNT3 was not significantly different from that in mock cells. The results mentioned above implied that FTC is a substrate of CNT1 but not CNT3 (Fig. 3F).
FIG 3.
(A) Ribavirin accumulation in mock and HEK293-hCNT1 cells compared with accumulation in mock cells. *, P < 0.05. (B) FTC accumulation in mock and HEK293-hCNT1 cells in the absence or presence of phlorizin (CNT inhibitor). ***, P < 0.001 versus mock cells; ###, P < 0.001 versus no inhibitor. (C) Concentration-dependent profiles of FTC uptake in HEK293-hCNT1. (D) HEK293-hCNT1-mediated uptake was analyzed by means of the Eadie-Hofstee plot. (E) Entecavir accumulation in mock and MDCK-hCNT3 cells in the absence or presence of phlorizin (CNT inhibitor) and FTC. ***, P < 0.001 versus mock cells; ###, P < 0.001 versus no inhibitor. (F) FTC accumulation in mock and MDCK-hCNT3 cells.
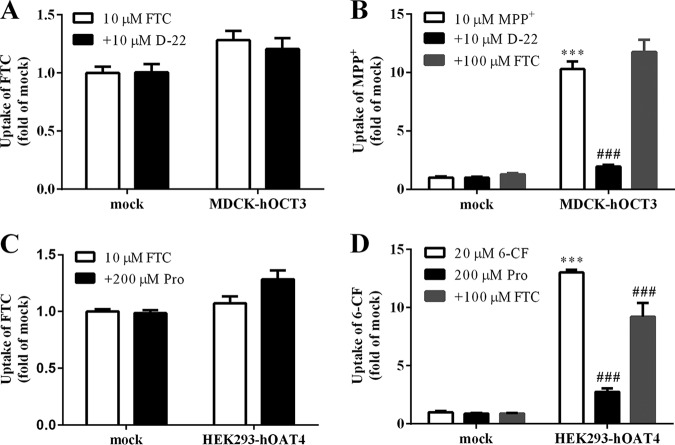
FTC is not a substrate of OCT3 or OAT4.
OCT3 is expressed highly in the human placenta but it is rarely expressed in BeWo cells. Hence, we applied MDCK-hOCT3 cells to test whether OCT3 would transport FTC. The result showed that FTC was neither a substrate nor inhibitor of OCT3 (Fig. 4A and B). We also investigated if OAT4 participated in FTC transport, because OAT4 is highly expressed in the placenta. FTC accumulation in HEK293-hOAT4 cells was not different from that in mock cells. However, 100 μM FTC significantly inhibited the accumulation of 6-carboxylfluorescein (6-CF; 20 μM, 2 min) in HEK293-hOAT4 cells (P < 0.001), which implied that FTC is not a substrate but instead an inhibitor of OAT4 (Fig. 4C and D).
FIG 4.
(A) FTC accumulation in mock and MDCK-hOCT3 cells. (B) Accumulation of probe substrate in mock and MDCK-hOCT3 cells in the absence or presence of FTC and D-22 (an inhibitor of OCT3). ***, P < 0.001 versus mock cells; ###, P < 0.001 versus no inhibitor. (C) FTC accumulation in mock and HEK293-hOAT4 cells. (D) Accumulation of probe substrate in mock and HEK293-hOAT4 cells in the absence or presence of FTC and probenecid (Pro, an inhibitor of OAT4). ***, P < 0.001 versus mock cells; ###, P < 0.001 versus no inhibitor.
FTC is a substrate of OCTN1 but not of OCTN2.
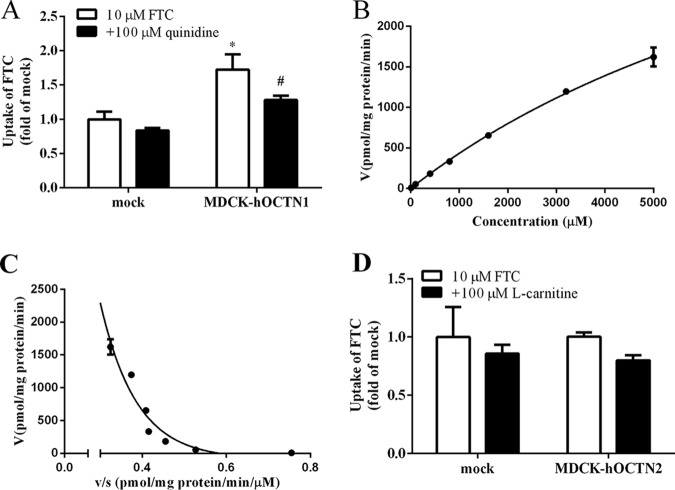
OCTN1 and OCTN2 are expressed in the placenta, and so we investigated if FTC was a substrate of OCTN1 or OCTN2 using stably transfected cells. FTC accumulation in MDCK-hOCTN1 cells was 1.7-fold that observed in mock cells (Fig. 5A), which was reduced significantly by 100 μM quinidine (P < 0.05), and the uptake kinetics of FTC by OCTN1 displayed atypical dynamics under Eadie-Hofstee analysis. At low concentrations (10 to 400 μM), the Km and Vmax values were 489 ± 119 μM and 0.364 ± 0.0709 nmol/mg protein/min, respectively, whereas at high concentrations (800 to 5,000 μM), the Km and Vmax values were 13.3 ± 1.18 mM and 6.01 ± 0.450 nmol/mg protein/min, respectively (Fig. 5B and C). FTC accumulation in MDCK-hOCTN2 cells was not markedly different from that in mock cells, and the OCTN2 inhibitor l-carnitine (100 μM) also did not inhibit FTC accumulation (Fig. 5D). Therefore, FTC was a substrate of OCTN1 but not OCTN2.
FIG 5.
(A) FTC accumulation in mock and MDCK-hOCTN1 cells in the absence or presence of quinidine.*, P < 0.05 versus mock cells; #, P < 0.05 versus no inhibitor. (B) Concentration-dependent profiles of FTC uptake in MDCK-hOCTN1. (C) MDCK-hOCTN1-mediated uptake was analyzed by means of the Eadie-Hofstee plot. (D) FTC accumulation in mock cells and MDCK-hOCTN2 cells in the absence or presence of l-carnitine (an inhibitor of OCTN2).
FTC is a substrate of BCRP, MRP2, and MRP3 but not MDR1.
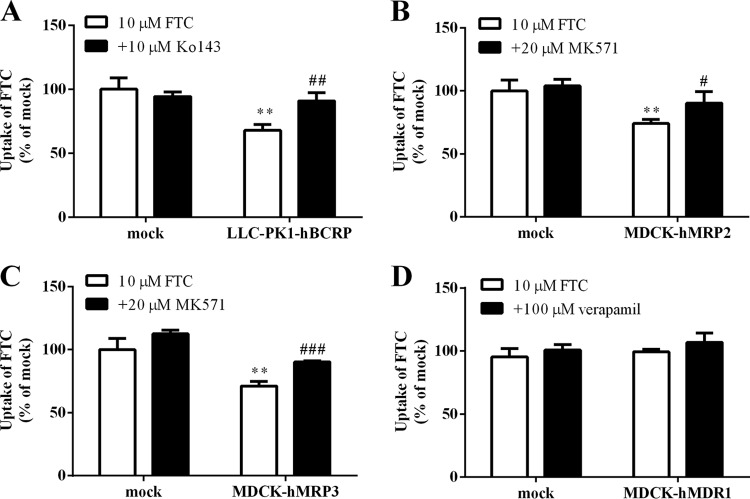
Based on the results of the inhibition study with efflux transporters inhibitors in BeWo cells, we further investigated if FTC was a substrate of efflux transporters by using transgenic cells. As shown in Fig. 6A, accumulation of FTC (10 μM, 60 min) in Lilly Laboratories cell-porcine kidney 1 (LLC-PK1)-hBCRP cells was 68% of that observed in mock cells (P < 0.01), whereas 10 μM Ko143 (an inhibitor of BCRP) increased FTC accumulation markedly (P < 0.01). Moreover, FTC accumulation in MDCK-hMRP2 and MDCK-hMRP3 cells was 26% and 24% lower than in mock cells, respectively (Fig. 6B and C), and 20 μM MK571 (an inhibitor of MRPs) increased FTC accumulation. However, FTC accumulation in MDCK-hMDR1 and mock cells was not significantly different (Fig. 6D). The results described above further confirmed that FTC was a substrate of BCRP, MRP2, and MRP3 but not MDR1.
FIG 6.
(A) FTC accumulation in LLC-PK1 and LLC-PK1-hBCRP cells. (B) FTC accumulation in mock and MDCK-hMRP2 cells. (C) FTC accumulation in mock and MDCK-hMRP3 cells. (D) FTC accumulation in mock and MDCK-hMDR1 cells. Cells were incubated with FTC in the absence or presence of inhibitors for 60 min. **, P < 0.05 versus mock cells; #, P < 0.05; ##, P < 0.01; and ###, P < 0.001 versus no inhibitor.
FTC accumulation in PHTCs.
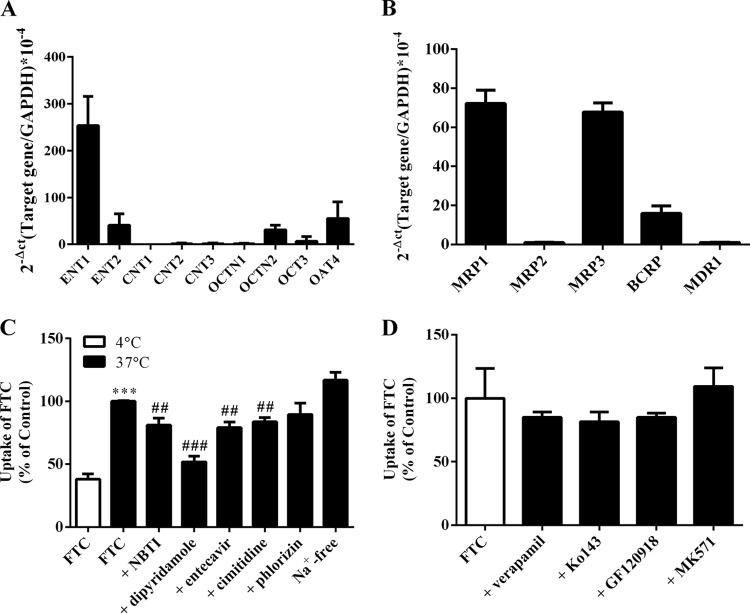
FTC accumulation in PHTCs was studied to investigate further if transporters were involved in the placental transport of FTC. mRNA expression of relevant transporters in PHTCs are depicted in Fig. 7A and B. As shown in Fig. 7C, FTC accumulation at 37°C was obviously higher than that at 4°C, suggesting that transporters mediated the transfer of FTC in PHTCs. Cellular accumulation of FTC (20 μM) at 37°C was reduced significantly in the presence of inhibitors of ENTs (NBTI, 100 μM, P < 0.01; dipyridamole, 50 μM, P < 0.001), ENT1 (entecavir, 400 μM, P < 0.01), and OCTs or OCTN1 (cimetidine, 500 μM, P < 0.01) but not in Na+-free buffer or a CNT inhibitor (phlorizin, 500 μM). Unexpectedly, the inhibitors of BCRP (Ko143, 5 μM; GF120918, 10 μM) and MRPs (MK571, 50 μM) did not increase FTC accumulation in PHTCs (Fig. 7D), although we demonstrated that FTC was a substrate of BCRP, MRP2, and MRP3. In agreement with the results in BeWo cells and MDCK-hMDR1 cells, the MDR1 inhibitor verapamil (100 μM) also did not increase FTC accumulation in PHTCs.
FIG 7.
mRNA expression of SLC (A) and ABC (B) transporters in PHTCs. Effect of inhibitors of SLC (C) and ABC (D) transporters on FTC accumulation in PHTCs. ***, P < 0.001 versus 4°C; ##, P < 0.01; ###, P < 0.001 versus without an inhibitor at 37°C.
Influence of combined antiretroviral drugs on FTC accumulation in BeWo cells and PHTCs.
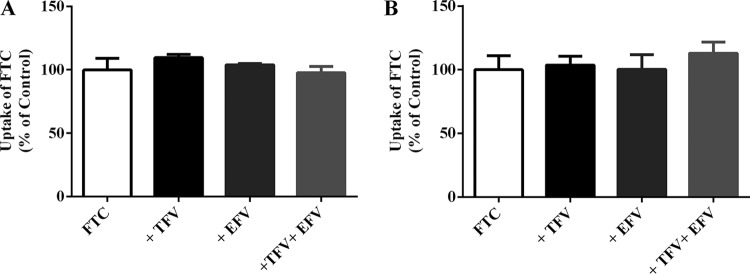
FTC-tenofovir disoproxil fumarate (an oral prodrug of TFV)-EFV is recommended as a first-line combination antiviral therapy during pregnancy. Hence, we explored if coexistence of TFV and/or EFV affected FTC accumulation in BeWo cells and PHTCs. As shown in Fig. 8A and B, accumulation of FTC (10 μM) was not altered significantly in the presence of TFV (20 μM), EFV (20 μM), or TFV together with EFV. These results revealed that TFV and/or EFV did not influence placental transfer of FTC.
FIG 8.
FTC accumulation (10 μM) in BeWo cells (A) and PHTCs (B) in the absence or presence of TFV (20 μM), EFV (20 μM), or TFV (20 μM) together with EFV (20 μM).
DISCUSSION
We elucidated the contribution of transporters in the transplacental transport of FTC using BeWo cells, cells stably or transiently transfected with transporters, and PHTCs. The SLC transporters ENT1, CNT1, and OCTN1 and the ABC transporters BCRP and MRP1/2/3 probably contributed to FTC transfer across the placenta.
ENTs are widely expressed in various tissues, and ENTs contribute to the transport of endogenous nucleosides and various anticancer and antiviral nucleoside drugs, such as adenosine, gemcitabine, ribavirin, and abacavir (27–30). Our data revealed that NBTI and dipyridamole (two ENT-specific inhibitors) strongly inhibited FTC uptake in BeWo cells and PHTCs and that NBTI inhibited FTC accumulation in JEG-3 cells (see Fig. S1 in the supplemental material), which suggests that ENTs take part in FTC accumulation in BeWo cells, PHTCs, and JEG-3 cells. Owing to the selectivity of ENT1 and ENT2 to NBTI, we used siRNA to demonstrate that ENT1 contributed to the transport of FTC whereas ENT2 did not in BeWo cells. Thus, we deduced that ENT1, but not ENT2, contributed to FTC transport in the placenta. However, Karbanova et al. (6), reported that ENTs do not play a vital role in transfer of FTC across the placenta based on the observation that NBTI did not significantly inhibit FTC accumulation, results that are inconsistent with our data. We speculated that the discrepancy in the results might be attributed to the difference in the expression levels of transporters in experimental models. Additionally, studies reported a decade ago showed that FTC crosses the placenta by simple diffusion, since the fetal/maternal ratio of FTC was near 1 (31). A drug with a fetal/maternal ratio near 1 usually means it is mainly transferred through the placenta by passive diffusion, but transporters may also be involved. A previous study found that lamivudine crosses the placenta by simple diffusion (32), but Ceckova et al. suggested that MATE1 is responsible for lamivudine transport (33).
CNT1 recognizes pyrimidine nucleosides, CNT2 recognizes purine nucleosides, and CNT3 recognizes purine and pyrimidine nucleosides (34). FTC is a cytidine analog, so we speculated that FTC is probably a substrate of CNT1 or CNT3. Two inhibitors of CNTs, phlorizin and adenosine, remarkably decreased FTC accumulation in BeWo cells and JEG-3 cells (Fig. S1), and so we deduced that CNT1 and/or CNT3 likely participated in FTC transport in BeWo cells and JEG-3 cells. Furthermore, our results demonstrated that FTC was a substrate of CNT1 but not CNT3 by using cells transiently transfected with a transporter, and the results revealed that CNT1, but not CNT3, contributed to FTC uptake in BeWo cells or JEG-3 cells. However, phlorizin reduced FTC accumulation in PHTCs to some degree without reaching statistical significance, which was probably due to a decrease in CNT1 expression during the isolation and cultivation of cells. We found that CNT1 mRNA expression was higher in human full-term placentas than in first-trimester placentas (see Fig. S2), suggesting that CNT1 has an important role in full-term pregnancy. We also detected CNT1/2/3 mRNAs in full-term human placentas. Staud et al. reported that CNT1 is expressed at the apical membrane and basal membrane (13), whereas Errasti-Murugarren et al. demonstrated that CNT1 is the only type of CNT expressed functionally in human syncytiotrophoblasts (11). In addition, Yao et al. reported that CNT1 transports adenosine and uridine with an apparent Km of 26 μM (35); our data showed that CNT1 transported FTC with an apparent Km of 162 μM, which suggested that FTC is a low-affinity substrate of CNT1.
OCT3 and OAT4 are highly expressed in the fetal-facing membrane of human syncytiotrophoblasts (7, 36) but have low expression in BeWo cells. Thus, it is important to discover whether OCT3 and OAT4 contribute to FTC transfer across the placenta. We demonstrated that FTC is not a substrate of OCT3 or OAT4 by using cells stably transfected with transporters, even though it has been reported that FTC is an inhibitor of OCTs (37). Therefore, neither OCT3 nor OAT4 was involved in FTC transport across the placenta.
OCTN1 and OCTN2 are expressed in the maternal-facing membranes of human syncytiotrophoblasts (9, 10), where OCTN2 mediates most maternal-fetal carnitine transport in humans (38, 39). We used transporter-transfected cells to demonstrate that FTC is a substrate of OCTN1 but not OCTN2, and the OCTN1 inhibitor cimetidine markedly inhibited FTC accumulation in BeWo cells and PHTCs, which further confirmed that FTC was a substrate of OCTN1. However, our data showed that OCTN1 transported FTC with a high apparent Km (low concentration, 489 μM; high concentration, 13.3 mM), and we found that the level of OCTN1 mRNA in human placentas is low (data not shown); therefore, OCTN1 plays a minor role in FTC transport in the placenta.
Additionally, peptide transporter 2 (PEPT2) and organic anion transporting polypeptides (OATPs) are also expressed in human placenta (26, 40–42); however, the inhibitors of PEPT2 (Glysar, 100 μM) and OATPs (rifamycin SV, 100 μM) did not reduce the accumulation of FTC in BeWo cells (data not shown), which showed that PEPT2 and OATPs might be not involved in FTC transport.
The efflux transporters MDR1, BCRP, and MRP1/2/3 are highly expressed in human placenta (13, 20, 43, 44) to pump their substrates from syncytiotrophoblasts. We revealed that the BCRP inhibitors GF120918 and Ko143 and MRP inhibitor MK571, but not verapamil, increased FTC accumulation in BeWo cells. It has been reported that FTC is a substrate of MRP1 by using peripheral blood mononuclear cells (22), and we also found that FTC was a substrate of MRP1 using MDCK-hMRP1 cells (see Fig. S3). Furthermore, we demonstrated, for the first time, that FTC is a substrate of MRP2/3 and BCRP by using MDCK-hMRP2/3 and LLC-PK1-hBCRP cells and that FTC is not a substrate of MDR1. Therefore, we deduced that MRP1/2/3 and BCRP, but not MDR1, contributed to the efflux of FTC from BeWo cells. Unexpectedly, the inhibitors of BCRP and MRPs did not significantly increase FTC accumulation in PHTCs, which did not match with the results in BeWo cells. The reasons for these inconsistent results were not clarified, but we speculate that it might be due to the lower expression of efflux transporters in PHTCs than in BeWo cells.
FTC is often used in combination with other antiviral drugs, such as tenofovir disoproxil fumarate and EFV, and a previous study proved that dual combination (tenofovir disoproxil fumarate and EFV) did not change the pharmacokinetic parameters of both agents (45). However, the distribution of drugs in different tissues depends on various transporters, and whether triple combination would influence FTC transplacental transport was not clear. Thus, we investigated FTC uptake in BeWo cells and PHTCs in the presence of TFV and/or EFV. Our data revealed that TFV and/or EFV did not influence FTC accumulation in BeWo cells or in PHTCs; therefore, we reasoned that TFV and/or EFV might not affect FTC transfer across the placenta. However, Bousquet et al. revealed that TFV and EFV increase FTC accumulation in peripheral blood mononuclear cells (25). These different results might have been due to differences in expression of transporters in BeWo cells, PHTCs, and peripheral blood mononuclear cells.
BeWo cells consist mostly of cytotrophoblasts and few syncytialized cells, whereas the fetal-maternal barrier is mainly constituted of syncytialized trophoblasts. Meanwhile, the expression of drug transporters in BeWo cells does not exactly match that in human placenta tissue (46). The transporter expression profile of PHTCs is more similar to that of the human placenta barrier; PHTCs may be more suitable than BeWo cells to study transport mechanisms of xenobiotics. However, during the isolation and cultivation of PHTCs, the expression levels of transporters might be decreased or even lost, and PHTCs cannot proliferate and cannot form tightly connected monolayers. Besides, our previous study showed that mRNA expression levels of OCTN1/2, OCT3, and OAT4 are different between BeWo cells and PHTCs (47). Since cell models have their own limitations, to obtain more reliable results, transporter-transfected cell models, choriocarcinoma cell lines, and PHTCs were together applied in our study to better clarify placenta transfer of FTC. An ex vivo dually perfused human placenta model and even in vivo studies will be used in our further studies.
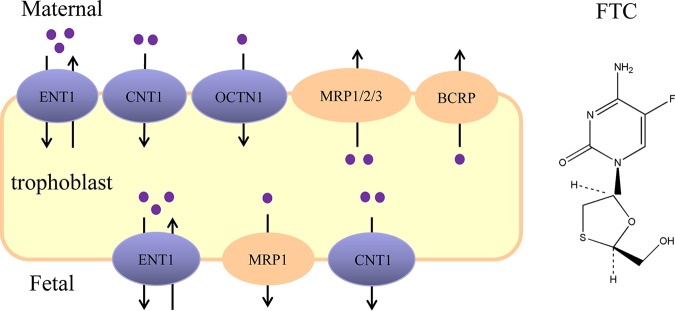
In conclusion, our comprehensive study of the placental transfer of FTC demonstrated that ENT1, CNT1, OCTN1, BCRP, and MRP1/2/3 are involved in FTC transport. Considering the location of transporters in syncytiotrophoblasts, we predicted that (i) ENT1, CNT1, and OCTN1 contribute to FTC uptake from the maternal circulation to trophoblasts; (ii) ENT1, CNT1, and MRP1 help FTC move from trophoblasts to fetal circulation; and (iii) BCRP and MRP1/2/3 are involved in the efflux of FTC from trophoblasts to the maternal circulation (Fig. 9). Coexistence of TFV and/or EFV did not affect FTC transfer across the placenta. Our results could offer insight into the cellular mechanisms of FTC transfer across the placenta.
FIG 9.
The predicted placental transfer of FTC (schematic). ENT1, CNT1, and OCTN1 probably contribute to uptake of FTC from maternal circulation to trophoblasts, whereas BCRP and MRP1/2/3 facilitate FTC transport from trophoblasts to maternal circulation. ENT1, CNT1, and MRP1 are likely involved in FTC transport between trophoblasts, the placenta, and fetal blood.
MATERIALS AND METHODS
Chemicals.
Fetal bovine serum (FBS) was purchased from ScienCell Research Laboratories (Carlsbad, CA, USA). Trypsin was obtained from Invitrogen (Carlsbad, CA, USA). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Biological Industries (Beijing, China). Kaighn’s modification of Ham’s F-12 medium (F-12K) and M199 medium were obtained from Jinuo (Hangzhou, China). GF120918, MK571, Ko143, NBTI, 1-methyl-4-phenylpyridinium iodide (MPP+), decynium-22 (D-22), Gly-Sar, 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate (CDCFDA), calcein-AM, Hoechst 33342, 6-carboxylfluorescein (6-CF), and rhodamine 123 were obtained from Sigma-Aldrich (St. Louis, MO). FTC was purchased from TCI Chemical Industries (Shanghai, China). l-Carnitine-(methyl-d3) (d3-l-Car) was obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). l-Carnitine, l-ergothioneine, probenecid (Pro), dipyridamole, cimetidine, adenosine, phlorizin, and rifamycin SV were obtained from Aladdin (Shanghai, China). Verapamil hydrochloride was purchased from the National Institutes for Food and Drug Control (Beijing, China). Entecavir was provided by Dalian Meilun Biotechnology Co., Ltd. (Dalian, China). The bicinchoninic acid (BCA) protein assay kit was from Beyotime Biotechnology (Shanghai, China). Sodium dodecyl sulfate (SDS) was purchased from Amresco (Solon, OH, USA). Percoll was obtained from GE Healthcare Bio-Sciences (Uppsala, Sweden). Acetonitrile was purchased from Tedia (Cincinnati, OH, USA). Other chemicals or solvents were of the highest grade available commercially.
The blank vector (pEnter), hCNT1 (SLC28A1) expression plasmid, hCNT3 (SLC28A3) expression plasmid, and hOAT4 (SLC22A11) expression plasmid were obtained from ViGene Biosciences (Shandong, China). Small interfering RNA (siRNA)-hENT1 and the negative-control (NC) siRNA were obtained from GenePharma (Shanghai, China).
Cell culture and transfection.
BeWo cells were kindly provided by Ximei Wu (School of Medicine, Zhejiang University, Hangzhou, China) and cultured in F-12K supplemented with 15% FBS and 1% penicillin-streptomycin. Human embryonic kidney 293 (HEK293) cells were kindly provided by Feng Han (College of Pharmaceutical Sciences, Zhejiang University). Madin-Darby canine kidney (MDCK) cells stably transfected with full-length hOCTN1 cDNA (MDCK-hOCTN1), hOCTN2 cDNA (MDCK-hOCTN2), hOCT3 cDNA (MDCK-hOCT3), and hMDR1 cDNA (MDCK-hMDR1) as well as Lilly Laboratories cell-porcine kidney 1 (LLC-PK1) cells stably expressing BCRP (LLC-PK1-hBCRP) were established by our research team (48–50). MDCK-hMRP2 cells were kindly provided by Piet Borst (Netherlands Cancer Institute, Amsterdam, the Netherlands). MDCK-hMRP3 cells were kindly provided by Min Huang (School of Pharmaceutical Sciences, Sun Yat-sen University [Guangzhou, China]). MDCK and HEK293 cells were cultured in DMEM with 10% FBS and 1% penicillin-streptomycin. LLC-PK1 cells were grown in M199 containing 6% FBS and 1% penicillin-streptomycin. All cells were incubated in a humidified air-CO2 incubator (5% [vol/vol]) at 37°C.
MDCK and HEK293 cells were seeded at an appropriate density in 24-well plates. On day 2 when they had reached 70% to 90% confluence, MDCK cells were transiently transfected with hCNT3 (MDCK-hCNT3) using Lipofectamine 3000 reagent (Invitrogen) according to the manufacturer’s protocol. HEK293 cells were transiently transfected with hOAT4 (HEK293-hOAT4) and hCNT1 (HEK293-hCNT1) using Lipofectamine 2000 reagent (Invitrogen) when they had reached 90% confluence.
Silencing of ENT1 expression.
BeWo cells were transfected with ENT1 siRNAs (siENT1-A1 and siENT1-A2) or NC siRNA using jetPRIME (Polyplus, Illkirch, France) according to the manufacturer’s protocol. Briefly, BeWo cells were seeded in 12-well plates at 105/well. On day 2, when cells had reached 50% confluence, they were treated with 55 pmol of siRNA for each group. Then, 48 h later, the mRNA level of ENT1 and FTC accumulation in BeWo cells were measured. siRNA sequences are listed in Table S1 in the supplemental material.
Quantitative real-time PCR.
Total RNAs were extracted from BeWo cells, PHTCs, and placenta tissues (placentas of pregnancy complications and viral infection were excluded) using an RNAsimple Total RNA kit (Tiangen Biotech, Beijing, China). Then, cDNAs were synthesized using a PrimeScript RT Reagent kit (TaKaRa Biotech, Kusatsu, Japan). Quantitative real-time PCR (RT-qPCR) was carried out on a StepOne plus Real-Time PCR system using SYBR Premix Ex Taq II (TaKaRa Biotech). Expression of the target mRNAs was normalized to that of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primer pairs used were previously reported (23), and CNTs primers are listed in Table S2.
Cellular accumulation.
MDCK-hOCT3, MDCK-hOCTN1/2, LLC-PK1-hBCRP, MDCK-hMDR1, MDCK-hMRP1/2/3, and the respective mock cells were seeded in 24-well plates at 2 × 105/well. Accumulation studies were carried out on day 3 according to a method created by our research team (47–50). The activity of the transgenic cells mentioned above was tested by using classical substrates: MPP+ (10 μM) for hOCT3, l-ergothioneine (3 μM) for hOCTN1, d3-l-Car (3 μM) for hOCTN2, Hoechst 33342 (10 μM) for hBCRP, rhodamine 123 (5 μM) for hMDR1, calcein-AM (10 μM) for hMRP1/2, and CDCFDA (15 μM) for hMRP3.
To measure accumulation in the cells mentioned above, the medium was removed and cells were washed twice and preincubated with prewarmed Hanks’ balanced salt solution (HBSS; pH 7.4) for 10 min or 30 min at 37°C. Accumulation was initiated by adding HBSS containing FTC or the classical substrates of the studied transporters with/without inhibitors of influx/efflux transporters at 37°C for 3 min (for hOCT3 and hOCTN1/2) or 60 min (for hBCRP, hMDR1, and hMRP1/2/3). Accumulation was terminated by removing the incubation solution and then adding ice-cold phosphate-buffered saline (PBS) instantly to wash cells twice. Finally, cells were lysed with 100 μl of 0.1% SDS and stored at −80°C until analyses. Measurement of FTC accumulation in BeWo cells at 4°C or 37°C was similar to that for the cells mentioned above.
To ascertain if FTC was a substrate of hOAT4 and hCNT1/3, cellular uptake of FTC was measured using the method described for the cells mentioned above except that the buffer was replaced with 2-(N-morpholino)ethanesulfonic acid (MES; pH 6.0) or Krebs-Ringer-Henseleit (KRH; pH 7.4). The activity of the transgenic cell models was verified using the probe substrate of 6-CF (20 μM, 2 min), ribavirin (1 mM, 3 min), and entecavir (10 μM, 3 min).
The intracellular concentrations of FTC, MPP+, d3-l-Car, and l-ergothioneine from cell lysates were tested using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The intracellular level of rhodamine 123 (excitation wavelength [λex], 485 nm; emission wavelength [λem], 535 nm), calcein-AM (λex, 490 nm; λem, 515 nm), Hoechst 33342 (λex, 355 nm; λem, 460 nm), CDCFDA (λex, 504 nm; λem, 529 nm), or 6-CF (λex, 490 nm; λem, 525 nm) was determined by a microplate reader (Spectra Max M2; Molecular Devices, Silicon Valley, CA, USA). Accumulation was normalized to the total protein content in lysates with a BCA protein assay kit.
FTC accumulation in PHTCs.
PHTCs were isolated from human placenta uncomplicated and without viral infection delivered at full term (38 to 40 weeks) using a method reported previously with some modifications (23, 51). In brief, aliquots of villous tissue were cut randomly from the maternal side of the placenta and rinsed thrice with saline containing 1% penicillin-streptomycin and PBS containing 1% penicillin-streptomycin. Then, the tissue was digested with 0.07% trypsin and 0.2 mg/ml of DNase I in 200 ml of DMEM high glucose (25 mM glucose) (DMEM-H-G). The mixture was incubated in a shaking water bath (37°C) for digestion five times successively at 30, 40, 15, 15, and 15 min each. The third, fourth, and fifth digestions were used to collect pellets after centrifugation at 1,340 × g for 10 min at room temperature. The precipitate was resuspended with 5 ml of DMEM-H-G, which was purified using a 5% to 65% Percoll gradient at step increments of 5% and centrifuged at 1,340 × g for 15 min at room temperature. The middle layer containing cytotrophoblasts was removed carefully to a new tube after centrifugation, washed once with DMEM-H-G, and resuspended instantly in medium containing 15% FBS and 1% penicillin-streptomycin. Finally, cytotrophoblasts were seeded in 12-well plates at 1.5 × 106/well, and the accumulation assay was carried out after 72 h of incubation.
LC-MS/MS.
The concentrations of entecavir, MPP+, l-ergothioneine, and d3-l-Car in cell samples were measured with an LC-MS system (1290/6460; Agilent Technologies, Santa Clara, CA, USA) with a triple quadrupole mass spectrometer (Agilent Technologies) using a method described previously (23, 48, 52). For FTC determination, 80 μl of the cell lysate was mixed with 160 μl of acetonitrile containing the internal standard (5 ng/ml of MPP+) for 5 min. Then, the mixture was centrifuged at 7,000 × g for 15 min at room temperature, and the supernatant was analyzed by LC-MS/MS. Isocratic chromatographic separation was undertaken on an X-Bridge BEH HILIC column (2.5 μm, 2.1 mm by 50 mm; Waters, Milford, MA, USA) at 30°C with gradient elution (0 to 1.0 min, 80% of B; 1.0 to 1.6 min, 80% to 70% of B; 1.6 to 2.5 min, 70% to 80% of B; 2.5 to 4.0 min, 80% of B) at 0.2 ml/min, where mobile phases A and B were 0.1% formic acid in 20 mM ammonium acetate-water and 0.1% formic acid in acetonitrile, respectively. MS was conducted using an electrospray ionization source in positive ion mode. Quantification was obtained using multiple reaction monitoring mode at m/z transitions of 248.2 > 129.9 for FTC and 170 > 128 for MPP+. The fragmentor voltage was set at 50 V and 100 V, and the collision energy was 2 V and 30 V for FTC and MPP+, respectively.
Statistical analyses.
Data are the means ± standard deviations from at least two independent experiments in triplicates (n = 3). The Michaelis-Menten constants Km and Vmax were calculated using Prism 6.0 (Graph Pad, San Diego, CA, USA) by fitting the data to the Michaelis-Menten equation V = Vmax [S]/(Km + [S]), where V is the uptake velocity and [S] is the substrate concentration. One-way analysis of variance (ANOVA) followed by Dunnett’s multiple-comparison test was used to evaluate the statistical differences among different groups and unpaired Student’s t test was used to compare two groups; P values of <0.05 were considered significant.
Ethics statement.
The protocol for studies on primary human trophoblast cells was approved by the Ethics Committee of Women’s Hospital, School of Medicine, Zhejiang University. All pregnant women provided written informed consent to participate in our study.
Supplementary Material
ACKNOWLEDGMENTS
We thank Haihong Hu (Institute of Drug Metabolism and Pharmaceutical Analysis, Zhejiang University) for managing the instruments.
This work was supported by the National Natural Science Foundation of China (81873838, 81802630) and Zhejiang Province Natural Science Foundation of China (LY17H310003).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00199-19.
REFERENCES
- 1.UNAIDS. 2017. UNAIDS data. http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf.
- 2.Stek AM, Best BM, Luo W, Capparelli E, Burchett S, Hu C, Li H, Read JS, Jennings A, Barr E, Smith E, Rossi SS, Mirochnick M. 2012. Effect of pregnancy on emtricitabine pharmacokinetics. HIV Med 13:226–235. doi: 10.1111/j.1468-1293.2011.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rimawi BH, Johnson E, Rajakumar A, Tao S, Jiang Y, Gillespie S, Schinazi RF, Mirochnick M, Badell ML, Chakraborty R. 2017. Pharmacokinetics and placental transfer of elvitegravir, dolutegravir, and other antiretrovirals during pregnancy. Antimicrob Agents Chemother 61:e02213-16. doi: 10.1128/AAC.02213-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Else LJ, Taylor S, Back DJ, Khoo SH. 2011. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the fetal compartment (placenta and amniotic fluid). Antivir Ther 16:1139–1147. doi: 10.3851/IMP1918. [DOI] [PubMed] [Google Scholar]
- 5.Hirt D, Urien S, Rey E, Arrive E, Ekouevi DK, Coffie P, Leang SK, Lalsab S, Avit D, Nerrienet E, McIntyre J, Blanche S, Dabis F, Treluyer JM. 2009. Population pharmacokinetics of emtricitabine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Antimicrob Agents Chemother 53:1067–1073. doi: 10.1128/AAC.00860-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karbanova S, Cerveny L, Ceckova M, Ptackova Z, Jiraskova L, Greenwood S, Staud F. 2017. Role of nucleoside transporters in transplacental pharmacokinetics of nucleoside reverse transcriptase inhibitors zidovudine and emtricitabine. Placenta 60:86–92. doi: 10.1016/j.placenta.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Sata R, Ohtani H, Tsujimoto M, Murakami H, Koyabu N, Nakamura T, Uchiumi T, Kuwano M, Nagata H, Tsukimori K, Nakano H, Sawada Y. 2005. Functional analysis of organic cation transporter 3 expressed in human placenta. J Pharmacol Exp Ther 315:888–895. doi: 10.1124/jpet.105.086827. [DOI] [PubMed] [Google Scholar]
- 8.Ugele B, Bahn A, Rex-Haffner M. 2008. Functional differences in steroid sulfate uptake of organic anion transporter 4 (OAT4) and organic anion transporting polypeptide 2B1 (OATP2B1) in human placenta. J Steroid Biochem Mol Biol 111:1–6. doi: 10.1016/j.jsbmb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. 1998. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem 273:20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, George RL, Huang W, Wang H, Conway SJ, Leibach FH, Ganapathy V. 2000. Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. Biochim Biophys Acta 1466:315–327. doi: 10.1016/S0005-2736(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 11.Errasti-Murugarren E, Diaz P, Godoy V, Riquelme G, Pastor-Anglada M. 2011. Expression and distribution of nucleoside transporter proteins in the human syncytiotrophoblast. Mol Pharmacol 80:809–817. doi: 10.1124/mol.111.071837. [DOI] [PubMed] [Google Scholar]
- 12.Govindarajan R, Bakken AH, Hudkins KL, Lai Y, Casado FJ, Pastor-Anglada M, Tse CM, Hayashi J, Unadkat JD. 2007. In situ hybridization and immunolocalization of concentrative and equilibrative nucleoside transporters in the human intestine, liver, kidneys, and placenta. Am J Physiol Regul Integr Comp Physiol 293:R1809–R1822. doi: 10.1152/ajpregu.00293.2007. [DOI] [PubMed] [Google Scholar]
- 13.Staud F, Ceckova M. 2015. Regulation of drug transporter expression and function in the placenta. Expert Opin Drug Metab Toxicol 11:533–555. doi: 10.1517/17425255.2015.1005073. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths M, Beaumont N, Yao SY, Sundaram M, Boumah CE, Davies A, Kwong FY, Coe I, Cass CE, Young JD, Baldwin SA. 1997. Cloning of a human nucleoside transporter implicated in the cellular uptake of adenosine and chemotherapeutic drugs. Nat Med 3:89–93. doi: 10.1038/nm0197-89. [DOI] [PubMed] [Google Scholar]
- 15.Yao SY, Ng AM, Vickers MF, Sundaram M, Cass CE, Baldwin SA, Young JD. 2002. Functional and molecular characterization of nucleobase transport by recombinant human and rat equilibrative nucleoside transporters 1 and 2 chimeric constructs reveal a role for the ENT2 helix 5-6 region in nucleobase translocation. J Biol Chem 277:24938–24948. doi: 10.1074/jbc.M200966200. [DOI] [PubMed] [Google Scholar]
- 16.Takano M, Kimura E, Suzuki S, Nagai J, Yumoto R. 2010. Human erythrocyte nucleoside transporter ENT1 functions at ice-cold temperatures. Drug Metab Pharmacokinet 25:351–360. doi: 10.2133/dmpk.DMPK-09-RG-099. [DOI] [PubMed] [Google Scholar]
- 17.Yao SY, Ng AM, Cass CE, Baldwin SA, Young JD. 2011. Nucleobase transport by human equilibrative nucleoside transporter 1 (hENT1). J Biol Chem 286:32552–32562. doi: 10.1074/jbc.M111.236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abd-Elfattah AS, Ding M, Jessen ME, Wechsler AS. 2012. On-pump inhibition of es-ENT1 nucleoside transporter and adenosine deaminase during aortic crossclamping entraps intracellular adenosine and protects against reperfusion injury: role of adenosine A1 receptor. J Thorac Cardiovasc Surg 144:243–249. doi: 10.1016/j.jtcvs.2011.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto T, Kuniki K, Takekuma Y, Hirano T, Iseki K, Sugawara M. 2007. Ribavirin uptake by cultured human choriocarcinoma (BeWo) cells and Xenopus laevis oocytes expressing recombinant plasma membrane human nucleoside transporters. Eur J Pharmacol 557:1–8. doi: 10.1016/j.ejphar.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 20.Afrouzian M, Al-Lahham R, Patrikeeva S, Xu M, Fokina V, Fischer WG, Abdel-Rahman SZ, Costantine M, Ahmed MS, Nanovskaya T. 2018. Role of the efflux transporters BCRP and MRP1 in human placental bio-disposition of pravastatin. Biochem Pharmacol 156:467–478. doi: 10.1016/j.bcp.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reznicek J, Ceckova M, Cerveny L, Muller F, Staud F. 2017. Emtricitabine is a substrate of MATE1 but not of OCT1, OCT2, P-gp, BCRP or MRP2 transporters. Xenobiotica 47:77–85. doi: 10.3109/00498254.2016.1158886. [DOI] [PubMed] [Google Scholar]
- 22.Bousquet L, Pruvost A, Didier N, Farinotti R, Mabondzo A. 2008. Emtricitabine: inhibitor and substrate of multidrug resistance associated protein. Eur J Pharm Sci 35:247–256. doi: 10.1016/j.ejps.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Ma Z, Yang X, Jiang T, Bai M, Zheng C, Zeng S, Sun D, Jiang H. 2017. Multiple SLC and ABC transporters contribute to the placental transfer of entecavir. Drug Metab Dispos 45:269–278. doi: 10.1124/dmd.116.073304. [DOI] [PubMed] [Google Scholar]
- 24.AIDSinfo. 2017. Panel on treatment of HIV-infected pregnant women and prevention of perinatal transmission. 2017. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. https://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf.
- 25.Bousquet L, Pruvost A, Guyot AC, Farinotti R, Mabondzo A. 2009. Combination of tenofovir and emtricitabine plus efavirenz: in vitro modulation of ABC transporter and intracellular drug accumulation. Antimicrob Agents Chemother 53:896–902. doi: 10.1128/AAC.00733-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kis O, Robillard K, Chan GN, Bendayan R. 2010. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci 31:22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Choi MK, Kim MH, Maeng HJ, Song IS. 2015. Contribution of CNT1 and ENT1 to ribavirin uptake in human hepatocytes. Arch Pharm Res 38:904–913. doi: 10.1007/s12272-014-0437-y. [DOI] [PubMed] [Google Scholar]
- 28.Cerveny L, Ptackova Z, Ceckova M, Karahoda R, Karbanova S, Jiraskova L, Greenwood SL, Glazier JD, Staud F. 2018. Equilibrative nucleoside transporter 1 (ENT1, SLC29A1) facilitates transfer of the antiretroviral drug abacavir across the placenta. Drug Metab Dispos 46:1817–1826. doi: 10.1124/dmd.118.083329. [DOI] [PubMed] [Google Scholar]
- 29.Mackey JR, Yao SY, Smith KM, Karpinski E, Baldwin SA, Cass CE, Young JD. 1999. Gemcitabine transport in Xenopus oocytes expressing recombinant plasma membrane mammalian nucleoside transporters. J Natl Cancer Inst 91:1876–1881. doi: 10.1093/jnci/91.21.1876. [DOI] [PubMed] [Google Scholar]
- 30.Molina-Arcas M, Casado FJ, Pastor-Anglada M. 2009. Nucleoside transporter proteins. Curr Vasc Pharmacol 7:426–434. doi: 10.2174/157016109789043892. [DOI] [PubMed] [Google Scholar]
- 31.Mirochnick M, Capparelli E. 2004. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 43:1071–1087. doi: 10.2165/00003088-200443150-00002. [DOI] [PubMed] [Google Scholar]
- 32.Mandelbrot L, Peytavin G, Firtion G, Farinotti R. 2001. Maternal-fetal transfer and amniotic fluid accumulation of lamivudine in human immunodeficiency virus-infected pregnant women. Am J Obstet Gynecol 184:153–158. doi: 10.1067/mob.2001.108344. [DOI] [PubMed] [Google Scholar]
- 33.Ceckova M, Reznicek J, Ptackova Z, Cerveny L, Muller F, Kacerovsky M, Fromm MF, Glazier JD, Staud F. 2016. Role of ABC and solute carrier transporters in the placental transport of lamivudine. Antimicrob Agents Chemother 60:5563–5572. doi: 10.1128/AAC.00648-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cano-Soldado P, Pastor-Anglada M. 2012. Transporters that translocate nucleosides and structural similar drugs: structural requirements for substrate recognition. Med Res Rev 32:428–457. doi: 10.1002/med.20221. [DOI] [PubMed] [Google Scholar]
- 35.Yao SY, Ng AM, Ritzel MW, Gati WP, Cass CE, Young JD. 1996. Transport of adenosine by recombinant purine- and pyrimidine-selective sodium/nucleoside cotransporters from rat jejunum expressed in Xenopus laevis oocytes. Mol Pharmacol 50:1529–1535. [PubMed] [Google Scholar]
- 36.Tomi M, Eguchi H, Ozaki M, Tawara T, Nishimura S, Higuchi K, Maruyama T, Nishimura T, Nakashima E. 2015. Role of OAT4 in uptake of estriol precursor 16alpha-hydroxydehydroepiandrosterone sulfate into human placental syncytiotrophoblasts from fetus. Endocrinology 156:2704–2712. doi: 10.1210/en.2015-1130. [DOI] [PubMed] [Google Scholar]
- 37.Minuesa G, Volk C, Molina-Arcas M, Gorboulev V, Erkizia I, Arndt P, Clotet B, Pastor-Anglada M, Koepsell H, Martinez-Picado J. 2009. Transport of lamivudine [(-)-beta-l-2′,3′-dideoxy-3′-thiacytidine] and high-affinity interaction of nucleoside reverse transcriptase inhibitors with human organic cation transporters 1, 2, and 3. J Pharmacol Exp Ther 329:252–261. doi: 10.1124/jpet.108.146225. [DOI] [PubMed] [Google Scholar]
- 38.Lahjouji K, Elimrani I, Lafond J, Leduc L, Qureshi IA, Mitchell GA. 2004. l-Carnitine transport in human placental brush-border membranes is mediated by the sodium-dependent organic cation transporter OCTN2. Am J Physiol Cell Physiol 287:C263–C269. doi: 10.1152/ajpcell.00333.2003. [DOI] [PubMed] [Google Scholar]
- 39.Bai M, Zeng Q, Chen Y, Chen M, Li P, Ma Z, Sun D, Zhou H, Zheng C, Zeng S, Jiang H Jr. 2019. Maternal plasma l-carnitine reduction during pregnancy is mainly attributed to OCTN2 mediated placental uptake and does not result in maternal hepatic fatty acid beta-oxidation decline. Drug Metab Dispos 47:582–591. doi: 10.1124/dmd.119.086439. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Yan Z, Dong M, Zhu X, Wang H, Wang Z. 2012. Alteration in placental expression of bile acids transporters OATP1A2, OATP1B1, OATP1B3 in intrahepatic cholestasis of pregnancy. Arch Gynecol Obstet 285:1535–1540. doi: 10.1007/s00404-011-2183-4. [DOI] [PubMed] [Google Scholar]
- 41.Berveiller P, Degrelle SA, Segond N, Cohen H, Evain-Brion D, Gil S. 2015. Drug transporter expression during in vitro differentiation of first-trimester and term human villous trophoblasts. Placenta 36:93–96. doi: 10.1016/j.placenta.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Botka CW, Wittig TW, Graul RC, Nielsen CU, Higaka K, Amidon GL, Sadee W. 2000. Human proton/oligopeptide transporter (POT) genes: identification of putative human genes using bioinformatics. AAPS PharmSci 2:E16. doi: 10.1208/ps020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evseenko D, Paxton JW, Keelan JA. 2006. Active transport across the human placenta: impact on drug efficacy and toxicity. Expert Opin Drug Metab Toxicol 2:51–69. doi: 10.1517/17425255.2.1.51. [DOI] [PubMed] [Google Scholar]
- 44.Vahakangas K, Myllynen P. 2009. Drug transporters in the human blood-placental barrier. Br J Pharmacol 158:665–678. doi: 10.1111/j.1476-5381.2009.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramanathan S, Shen G, Cheng A, Kearney BP. 2007. Pharmacokinetics of emtricitabine, tenofovir, and GS-9137 following coadministration of emtricitabine/tenofovir disoproxil fumarate and ritonavir-boosted GS-9137. J Acquir Immune Defic Syndr 45:274–279. doi: 10.1097/QAI.0b013e318050d88c. [DOI] [PubMed] [Google Scholar]
- 46.Prouillac C, Lecoeur S. 2010. The role of the placenta in fetal exposure to xenobiotics: importance of membrane transporters and human models for transfer studies. Drug Metab Dispos 38:1623–1635. doi: 10.1124/dmd.110.033571. [DOI] [PubMed] [Google Scholar]
- 47.Bai M, Ma Z, Sun D, Zheng C, Weng Y, Yang X, Jiang T, Jiang H. 2017. Multiple drug transporters mediate the placental transport of sulpiride. Arch Toxicol 91:3873–3884. doi: 10.1007/s00204-017-2008-8. [DOI] [PubMed] [Google Scholar]
- 48.Wang W, Bai M, Jiang T, Li C, Li P, Zhou H, Wang Z, Li L, Jiang H. 2019. Clozapine-induced reduction of l-carnitine reabsorption via inhibition/down-regulation of renal carnitine/organic cation transporter 2 contributes to liver lipid metabolic disorder in mice. Toxicol Appl Pharmacol 363:47–56. doi: 10.1016/j.taap.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Sun S, Wang K, Lei H, Li L, Tu M, Zeng S, Zhou H, Jiang H. 2014. Inhibition of organic cation transporter 2 and 3 may be involved in the mechanism of the antidepressant-like action of berberine. Prog Neuropsychopharmacol Biol Psychiatry 49:1–6. doi: 10.1016/j.pnpbp.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Sun S, Chen Z, Li L, Sun D, Tian Y, Pan H, Bi H, Huang M, Zeng S, Jiang H. 2012. The two enantiomers of tetrahydropalmatine are inhibitors of P-gp, but not inhibitors of MRP1 or BCRP. Xenobiotica 42:1197–1205. doi: 10.3109/00498254.2012.702247. [DOI] [PubMed] [Google Scholar]
- 51.Li JN, Ge YC, Yang Z, Guo CM, Duan T, Myatt L, Guan H, Yang K, Sun K. 2011. The Sp1 transcription factor is crucial for the expression of 11beta-hydroxysteroid dehydrogenase type 2 in human placental trophoblasts. J Clin Endocrinol Metab 96:E899–E907. doi: 10.1210/jc.2010-2852. [DOI] [PubMed] [Google Scholar]
- 52.Yang X, Ma Z, Zhou S, Weng Y, Lei H, Zeng S, Li L, Jiang H. 2016. Multiple drug transporters are involved in renal secretion of entecavir. Antimicrob Agents Chemother 60:6260–6270. doi: 10.1128/AAC.00986-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.