Echinocandins (caspofungin, micafungin, anidulafungin), targeting β-1,3-glucan synthesis of the cell wall, represent one of the three currently available antifungal drug classes for the treatment of invasive fungal infections. Despite their limited antifungal activity against Aspergillus spp., echinocandins are considered an alternative option for the treatment of invasive aspergillosis (IA).
KEYWORDS: Aspergillus, anidulafungin, calcineurin, caspofungin, heat shock protein 90, ibrexafungerp, micafungin, paradoxical effect, rezafungin
ABSTRACT
Echinocandins (caspofungin, micafungin, anidulafungin), targeting β-1,3-glucan synthesis of the cell wall, represent one of the three currently available antifungal drug classes for the treatment of invasive fungal infections. Despite their limited antifungal activity against Aspergillus spp., echinocandins are considered an alternative option for the treatment of invasive aspergillosis (IA). This drug class exhibits several advantages, such as excellent tolerability and its potential for synergistic interactions with some other antifungals. The objective of this review is to discuss the in vitro and clinical efficacy of echinocandins against Aspergillus spp., considering the complex interactions between the drug, the mold, and the host. The antifungal effect of echinocandins is not limited to direct inhibition of hyphal growth but also induces an immunomodulatory effect on the host’s response. Moreover, Aspergillus spp. have developed important adaptive mechanisms of tolerance to survive and overcome the action of echinocandins, such as paradoxical growth at increased concentrations. This stress response can be abolished by several compounds that potentiate the activity of echinocandins, such as drugs targeting the heat shock protein 90 (Hsp90)-calcineurin axis, opening perspectives for adjuvant therapies. Finally, the present and future places of echinocandins as prophylaxis, monotherapy, or combination therapy of IA are discussed in view of the emergence of pan-azole resistance among Aspergillus fumigatus isolates, the occurrence of breakthrough IA, and the advent of new long-lasting echinocandins (rezafungin) or other β-1,3-glucan synthase inhibitors (ibrexafungerp).
INTRODUCTION
Molds of the genus Aspergillus (particularly, Aspergillus fumigatus) are the causal agents of invasive aspergillosis (IA), a life-threatening infection affecting immunocompromised hosts, such as hematological cancer or transplant patients. The current antifungal armamentarium for the treatment of IA is limited to three antifungal drug classes. Fungicidal drugs, such as the triazoles (e.g., voriconazole, posaconazole, or isavuconazole) and the polyenes (amphotericin B formulations) represent the first-choice treatments, whereas the fungistatic echinocandins (caspofungin, anidulafungin, and micafungin) represent an alternative and are only marginally used as monotherapy (1, 2). However, use of echinocandins is gaining interest because of the emergence of acquired azole resistance in A. fumigatus isolates and the limitations related to drug interactions and/or toxicity with azoles and amphotericin B. The aim of this review is to discuss the role of the echinocandins in the treatment of IA, from the mechanistic point of view of drug-pathogen-host interactions to clinical application and perspectives.
ECHINOCANDINS AGAINST ASPERGILLUS
Mechanisms of action.
The echinocandin drugs are lipopeptides derived from fungal secondary metabolites. This antifungal class currently consists of three commercially available drugs, i.e., caspofungin, micafungin, and anidulafungin; and a novel molecule with a prolonged half-life, rezafungin (CD101), is currently in phase 3 evaluation. All echinocandins are available for parenteral (intravenous) administration only. Their antifungal activity relies on inhibition of the biosynthesis of β-1,3-glucan, one of the major polysaccharides of the cell wall in ascomycetous fungi, by targeting β-1,3-glucan synthase (encoded by fks1) in a noncompetitive way (3). β-1,3-Glucan is an important structural component of the cell wall, which plays an essential role in protection from the environment, containment of osmotic pressure, morphogenesis of hyphae, and invasive properties in host tissues (4). In addition to its structural role, β-1,3-glucan is an important trigger of the innate immune system, which is recognized by the Dectin-1 receptor at the surface of host immune cells (5). The concept that the antifungal effect of echinocandins may also result from an immunopharmacological effect emerged recently. Lamaris et al. (6) showed that caspofungin exposure was associated with a concentration-dependent increase in β-1,3-glucan exposure in the cell wall of A. fumigatus isolates. This effect peaked at a caspofungin concentration of 0.06 μg/ml, with a subsequent decline in β-1,3-glucan exposure at higher concentrations. Preexposure of A. fumigatus isolates to caspofungin 0.06 μg/ml also resulted in increased hyphal damage induced by polymorphonuclear neutrophils (PMNs) in vitro and increased expression of Dectin-1 by PMNs, which supports the role of echinocandins in triggering the host’s immune response against the mold (6). Moretti et al. (7) observed different host response patterns in terms of PMN recruitment and cytokine production in experimental models of IA with escalating doses of caspofungin. Experiments with knockout mice for different innate immune receptors suggested modulatory roles of Dectin-1, TLR-2, TLR-4, and TLR-9 on the net activity of caspofungin.
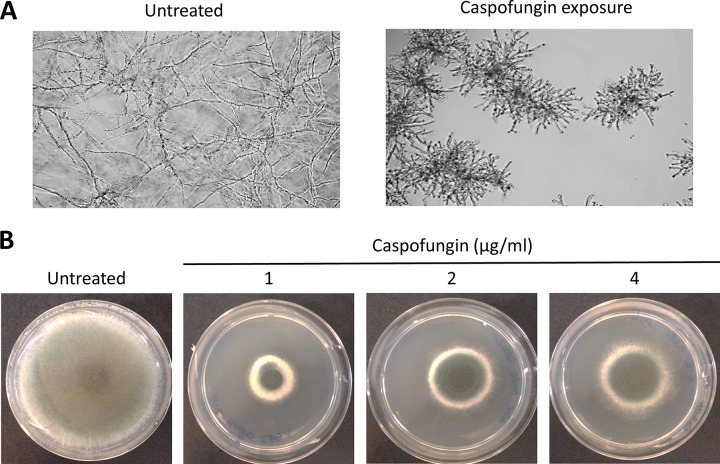
Whereas echinocandins are fungicidal against the most relevant pathogenic yeasts, such as Candida spp., they are fungistatic against Aspergillus spp. and some other pathogenic filamentous fungi. In vitro, echinocandins induce a hyphal growth arrest with turgescence and blunting of hyperbranched hyphae (Fig. 1A). This effect is usually observed at low concentrations (<0.03 μg/ml) for most Aspergillus spp., thus defining a minimal effective concentration (MEC), instead of an actual MIC.
FIG 1.
Tolerance of Aspergillus fumigatus to caspofungin. (A) Microscopic observation of A. fumigatus isolates after 24 h growth at 37°C in liquid RPMI 1640 medium in the absence of drug and in the presence of caspofungin 1 μg/ml. (B) Visualization of the paradoxical effect of caspofungin on A. fumigatus isolates. Pictures were taken after 5 days of growth at 37°C on glucose minimal medium agar plates supplemented with caspofungin at increasing concentrations (0, 1, 2, and 4 μg/ml).
Tolerance.
The ability of the fungus to maintain residual growth above the threshold of inhibition of the drug is referred to as tolerance. This is an epigenetic phenomenon, which, contrary to resistance, is not the result of acquired mutations but of mechanisms of stress response. Another expression of the ability of Aspergillus spp. to generate compensatory mechanisms of adaptation to the stress induced by echinocandins is the paradoxical effect (PE) (8). This phenomenon refers to decreased activity of the drug and recuperation of fungal growth at increasing concentrations above a certain threshold (Fig. 1B). It is comparable to the Eagle effect that was initially described for antibacterial drugs. The PE was first observed in 1988 in Candida spp. yeasts with the experimental echinocandin drug cilofungin and described in more detail by Stevens et al. (9, 10) with caspofungin. Subsequently, the phenomenon was reported for different Aspergillus species (11, 12).
The PE results from the activation of intracellular signaling pathways, which leads to cell wall remodeling with increases in the chitin content to compensate for the loss of β-1,3-glucan. It is species, strain, and drug specific (13). A PE can be observed in ∼60% to 80% of A. fumigatus clinical isolates, occurring mainly with caspofungin, whereas this phenotype is usually absent with micafungin and anidulafungin or occurs only at higher concentrations (11, 12, 14). The PE has also been reported among isolates of Aspergillus flavus, Aspergillus terreus, and Aspergillus niger (11, 15, 16).
The mechanisms behind this phenomenon were first studied in yeasts, revealing roles for protein kinase C, the high osmolarity glycerol response, and the calcineurin pathway (17–19). In A. fumigatus isolates, the PE can be suppressed by targeting different steps of the calcineurin pathway (14). The initial trigger of the PE consists of the entry of calcium (Ca2+) and increase in intracellular Ca2+, which binds to calmodulin and activates calcineurin by phosphorylation at its serine-proline-rich region concomitantly with increased expression of calmodulin and calcineurin (20, 21). Ca2+ deprivation, which can be achieved by Ca2+ chelators (BAPTA) or Ca2+ channel blockers (verapamil), results in abolition of the PE in A. fumigatus isolates (21). Interestingly, caspofungin exposure at PE concentrations resulted in a more important increase in cytosolic Ca2+ compared to that with micafungin, which does not induce PE, illustrating the drug specificity of the PE (21).
Activated calcineurin then dephosphorylates the transcription factor CrzA that moves to the nucleus and binds to specific promoter regions (calcineurin-dependent reporter elements) of the chitin synthase-encoding genes (chsA, chsC, chsG, and csmB) (22). Both calcineurin and CrzA are necessary to increase expression of chitin synthases and chitin content of the cell wall after caspofungin exposure (14, 22, 23). The cnaA and crzA deletion mutants also exhibit decreased β-1,3-glucan in the cell wall and lack the PE in response to caspofungin (23).
Heat shock proteins 90 (Hsp90) and 70 (Hsp70) are essential molecular chaperones that are supposed to control the calcineurin pathway in this response. A certain level of Hsp90 is required to generate the PE, which can be abolished by substitution of the native hsp90 promoter, by Hsp90-inhibitory drugs (geldanamycin), or by compromising Hsp90 function with acetylation-mimetic mutations (K27A) or lysine deacetylase inhibitors (trichostatin A) (24, 25). Affecting the interaction between the chaperones Hsp90 and Hsp70 by mutation of the Hsp70 EELD C-terminal domain also results in suppression of the PE (26). Deletion of the Hsp90-Hsp70 organizing protein (Hop, corresponding to StiA in A. fumigatus) results in hypersensitivity to caspofungin (26). Thus, Hsp90 and Hsp70 seem to act in concert to control the calcineurin pathway in caspofungin stress response. However, there may be other downstream effectors in the complex network of Hsp90. Very recently, the mitochondrial respiratory chain was shown to play a role in the PE, and activation of mitochondria in response to caspofungin was dependent on Hsp90 (27).
Analyses of the intracellular trafficking of the different actors of the Hsp90-calcineurin pathway showed that calcineurin and calmodulin localized at the hyphal tips and septa and that Hsp90 moves from the cytosol to the cell wall and sites of septa formation in the presence of caspofungin (27–30). Physical interaction between Hsp90 and calcineurin has been demonstrated in yeasts (31). However, the network of interactions of these stress proteins at the sites of cell wall regeneration remains to be elucidated.
In addition to the calcineurin pathway, a role of the cell wall integrity (CWI) pathway was recently highlighted. Deletions of the CWI MAPK gene mpkA and its downstream transcription factor rlmA resulted in loss of the PE (22). However, this pathway does not seem to play a role in PE via overexpression of chitin synthases. On the contrary, mpkA had a repressive effect on these genes but was also shown to positively impact the expression of other cell wall components, such as β-1,3-glucan or α-1,3-glucans (22, 32). Indeed, Loiko and Wagener (33) demonstrated that the key adaptive mechanism in PE does not seem to be related to the increased expression of chitin synthases but rather to the recovery of β-1,3-glucan synthase activity. Synthesis of β-1,3-glucan takes place at the cell membrane by the β-1,3-glucan synthase complex, consisting of a catalytic subunit, Fks1, and a regulatory subunit, RhoI. The crucial role of this complex in the PE was also supported by a recent study showing distinct localization patterns at low (non-PE) and high (PE) caspofungin concentrations (34). Under caspofungin exposure, Fks1 moves from the hyphal tips to vacuoles. However, continuous exposure to high caspofungin concentrations (4 μg/ml) will induce relocalization of Fks1 to the tips along with the phenotypic appearance of PE, which does not occur at lower concentrations. RhoI remains at the hyphal tips, where it is essential for Fks1 activation and the PE. Indeed, farnesol, which mislocalizes RhoI, abolishes the PE in A. fumigatus isolates (34).
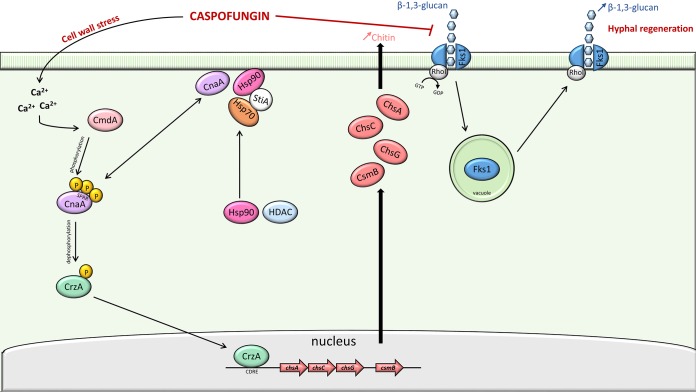
Taken together, these results suggest that tolerance to caspofungin in A. fumigatus isolates represents a complex and dynamic process occurring in two phases. Whereas calcineurin-dependent overexpression of chitin synthase seems to be essential for initial adaptation and survival in response to caspofungin stress, the PE, representing a delayed adaptive phenomenon, relies on the restoration of β-1,3-glucan synthase activity (Fig. 2).
FIG 2.
Schematic representation of the caspofungin paradoxical effect in Aspergillus fumigatus. The cell wall stress induced by caspofungin results in increased intracellular calcium (Ca2+). Calmodulin (CmdA) binds Ca2+ and activates the calcineurin α-catalytic subunit (CnaA) by phosphorylation at the serine-proline-rich region. CnaA dephosphorylates transcription factor CrzA, which moves to the nucleus and binds to specific promoter motifs (calcineurin-dependent reporter elements [CDRE]) to induce expression of the chitin synthase genes (chsA, chsC, chsG, and csmB). On caspofungin exposure, the heat shock protein 90 (Hsp90) shifts from the cytosol to the cell wall, where it possibly interacts directly with calcineurin or other client proteins. Hsp90 function relies on its interaction with Hsp70 and the Hsp90-Hsp70 organizing protein StiA. Histone deacetylases (HDAC) are also important for Hsp90 function. During the early phase of caspofungin exposure, the β-1,3-glucan synthase (FksA) in complex with the GTPase RhoI, is inhibited by caspofungin and moves into vacuoles. After prolonged exposure at high caspofungin concentration, FksA relocalizes to the cell wall and recovers its β-1,3-glucan synthesis activity.
The clinical relevance of the PE is unclear. Note that the first description of this phenomenon in Aspergillus derived from in vivo observations in animal models (35–37). In a murine model of IA with escalating doses of caspofungin, Wiederhold et al. (37) initially observed a dose-dependent effect, with optimal efficacy on the reduction of pulmonary fungal burden with doses of 1 mg/kg but a significant loss of efficacy at 4 mg/kg. Interestingly, this transitional margin between the optimal and decreased efficacy observed in vitro and in murine models corresponded to the clinical range of therapeutic doses and trough plasma concentrations of caspofungin (38). However, the paradoxical increase in fungal burden observed in vivo does not necessarily correlate with the in vitro paradoxical growth. Moretti et al. (7) observed that the proinflammatory effect of caspofungin at higher doses was still present with Aspergillus strains lacking the in vitro PE. This study suggests that the expression of different pattern recognition receptors at the surface of immune cells varies according to different caspofungin concentrations and influences the in vivo activity of caspofungin, with Dectin-1, TLR-2, and TLR-9 playing roles in the increased fungal burden and proinflammatory effect at higher doses (7).
Acquired resistance.
Besides tolerance, which is an inherent ability of wild-type A. fumigatus isolates to adapt to echinocandin stress, resistance to echinocandins can be acquired by mutations in the β-1,3-glucan synthase-encoding gene (fks1). Although this mechanism of resistance has been well described in Candida spp. with specific mutations occurring in known hot spot regions of fks1 (39), it seems to be uncommon in Aspergillus spp. The potential of A. fumigatus to develop such resistance has been demonstrated by the generation of laboratory strains with an S678Y or S678P mutation in fks1 (corresponding to the S645 site of Candida albicans) associated with phenotypic resistance to all three echinocandins (40, 41). To date, only one clinical strain harboring an fks1 mutation (F675S) has been identified in a patient with chronic pulmonary aspergillosis with substantially elevated echinocandin MICs compared with the wild type after prolonged micafungin therapy (42). The loss of fitness resulting from these fks1 mutations may be the reason it rarely occurs naturally in Aspergillus spp. (43).
ECHINOCANDINS IN CLINICAL PRACTICE
Susceptibility testing and interpretation.
Testing of Aspergillus spp. susceptibility to echinocandins is not routinely recommended and is actually of little utility for clinical management. Because of the fungistatic activity of echinocandins, the threshold of activity is expressed as an MEC. Echinocandins are active in vitro against most Aspergillus spp. (44, 45). The activity of echinocandins is conserved against azole-resistant A. fumigatus isolates, including those harboring cyp51A mutations (46). Of the three echinocandins, micafungin and anidulafungin are the most active in vitro, with MECs usually one or two dilutions lower than that of caspofungin (44). The novel long-acting echinocandin rezafungin (CD101) demonstrated acceptable in vitro activity against Aspergillus spp., including azole-resistant A. fumigatus and cryptic species (47, 48).
Most studies of large collections of Aspergillus isolates showed a narrow range of MEC distribution for echinocandins (44, 49, 50). However, the epidemiological cutoff values (ECVs) obtained in these studies differ. Whereas Pfaller et al. (44, 49) reported an MEC of ≤0.06 μg/ml for >99% of isolates for all three echinocandins, higher caspofungin ECVs were reported in another study (0.25 to 1 μg/ml) (50). Reader-dependent variability in the appreciation of MEC cutoff may explain these variations, although adhesion to the strict definition of MEC (i.e., transition to compact rounded colonies) was found to have good reproducibility (51). Because of the lack of correlation between MEC and outcomes, both the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) abstain from any recommendation of interpretation for echinocandins against Aspergillus spp. (52, 53). The occurrence of mutations in hot spot regions of the fks1 gene is the only known mechanism of echinocandin resistance in Aspergillus spp. and appears to be a rare event (40–42). Substantially higher MECs (at least 20-fold) are expected in this setting. In general, antifungal susceptibility to echinocandins should not be routinely tested because of the lack of an established correlation between MECs and clinical response.
Efficacy of echinocandins in animal models of IA.
Several animal models of IA demonstrated the efficacy of echinocandins. Caspofungin monotherapy at different dosages (1 and 2.5 mg/kg daily) was associated with significantly improved survival and significant reduction of fungal burden in most tissues compared with untreated groups in a guinea pig model of IA (54). Micafungin showed comparable results in murine models, with a 50% effective dose of 0.25 to 0.5 mg/kg (55, 56). Micafungin seemed to exhibit a dose-dependent effect on survival, whereas caspofungin was associated with higher fungal burden in tissues at higher doses than at lower doses, without significant impact on survival (37, 54, 55), which supports some role of the PE with the latter drug. In comparative studies with other antifungal classes, the success rate of echinocandins for the treatment of pulmonary IA tended to be lower than that of voriconazole but comparable to that of amphotericin B (54, 55). In animal models, echinocandins achieved good penetration in most tissues, with the exception of the brain and eye because of their large size and amphipathic properties (57).
Echinocandins for the treatment of IA.
Randomized clinical trials comparing the efficacy of echinocandins versus other antifungal drugs for the treatment of IA are lacking. One trial compared the efficacy of caspofungin versus amphotericin B for the empirical antifungal treatment of persistent neutropenic fever (58). The success rates using a composite endpoint were similar. Caspofungin exhibited higher success rates among patients with fungal infections at baseline, but the small number of IA cases (12 in both arms) did not allow for drawing conclusions. Three prospective noncomparative phase 2 studies assessed the efficacy of caspofungin as first-line treatment of proven or probable IA in patients with hematological malignancies and reported success rates (complete or partial response at the end of therapy) of 30% to 55% (59–61). The other studies reporting data about the efficacy of caspofungin for the treatment of IA consisted mainly of retrospective analyses or prospective observational registries. According to a recent review, the overall success rate (complete or partial response after pooling of all cases from individual studies) was 54% and 47% for caspofungin as first-line or second-line/salvage therapy, respectively (62). A wide range of success rates was observed between studies (27% to 92% and 28% to 71% for first- and second-line treatment, respectively) (59). These results may be influenced by multiple factors, including the type of population and underlying diseases, the diagnostic work-up procedure and timing of diagnosis, the timing of the assessment of therapeutic response, and the timing of the switch from first-line antifungal therapy to second-line caspofungin. Most important, few of these studies provided a direct comparison with other antifungals. Only one study reported a significantly higher IA-associated mortality rate for caspofungin than for voriconazole (63). The efficacy of micafungin was also assessed in a few studies with a limited number of cases and comparable results (30% to 50% success rate) (64, 65). Data about anidulafungin monotherapy of IA are scarce. Although echinocandins may be recommended as alternative therapy of pulmonary IA, they should not be used for cerebral aspergillosis because of their poor penetration of the hematoencephalic barrier (1, 2). Data about their efficacy for other extrapulmonary IAs are limited.
Table 1 shows several situations for which echinocandins may be considered first-line or salvage therapy of IA.
TABLE 1.
Possible role and indications for echinocandins in the treatment of invasive aspergillosis
| Indication | Aima | Situation | Level of evidence (Ref.) |
|---|---|---|---|
| First-line treatment (monotherapy) | To treat IA when no alternative regimen (or potential risks outweighing benefits for other regimens) | Relative contraindications to azoles (underlying liver disease, drug-drug interactions, prolonged QT interval); relative contraindications to AMB (underlying kidney disease, nephrotoxic comedications) | Noncomparative prospective or retrospective studies (overall success rate, 30–90%) (62) |
| Second-line treatment (monotherapy) | To treat IA when first-line antifungals have failed or need to be discontinued | Toxicity of triazoles (hepatic test disturbances, visual/neurological side effects); toxicity of AMB (acute renal failure); failure of previous antifungal regimens | Noncomparative prospective or retrospective studies (overall success rate, 30–70%) (62) |
| In combination with triazoles or AMB | To obtain synergistic interactions (triazoles, AMB) | Severe and/or disseminated IA, galactomannan-positive IA; in case of failure of previous regimen or breakthrough IA; for IA due to azole-resistant A. fumigatus | One randomized controlled trial (trends, benefit limited to subgroup analyses) (81); expert opinion; murine models (75, 77) |
| To palliate potential PK/PD defect until first-line drug achieves appropriate serum level (triazoles) | In severe and/or disseminated IA | Expert opinion | |
| To palliate potential inefficacy of first-line drug (triazoles) | For empirical treatment, if suspicion or high local prevalence of azole-resistant A. fumigatus; breakthrough IA | Expert opinion (82) | |
| To obtain synergistic interactions on biofilms (triazoles, AMB) | For Aspergillus endocarditis or osteomyelitis with presence of prosthetic material | In vitro studies (79) |
AMB, amphotericin B; IA, invasive aspergillosis; PK/PD, pharmacokinetic/pharmacodynamic.
Echinocandins in combination therapy of IA.
In vitro and in vivo interactions of echinocandins with other antifungal drugs, particularly amphotericin B and triazoles, against Aspergillus spp. have shown various results. Importantly, the type of growth medium was found to influence the in vitro interactions and serum attenuated the synergistic effect, suggesting that these observations may not necessarily have the same significance in vivo (66). Moreover, differences in drug exposure were found to influence these interactions (67). Both synergistic and indifferent interactions have been described for combinations of echinocandins and amphotericin B in vitro and in murine models of IA, which were strain dependent and possibly drug dependent (66, 68–70). Similar observations were reported for the interactions between echinocandins and triazoles with the presence of synergism for most but not all isolates (54, 66, 71, 72). The combination of anidulafungin and voriconazole also demonstrated synergism against azole-resistant A. fumigatus isolates (73, 74). However, the synergism was decreased among isolates harboring mutations in the tandem repeats of cyp51A (i.e., the most frequently observed) compared with wild-type isolates or those harboring other types of mutations (74). These observations are supported by a murine model of pulmonary IA in which the combination of voriconazole and anidulafungin was synergistic against an azole-susceptible A. fumigatus isolate but only additive against the azole-resistant isolate harboring the TR34/L98H mutation, with higher doses required for the latter (75). The combination of posaconazole and caspofungin was synergistic in most cases (76). Interestingly, the synergism was also present against azole-resistant A. fumigatus isolates and was more pronounced for those harboring the tandem repeat of the promoter region or the M220 mutation in cyp51A compared with other mutations of resistance. This positive interaction was confirmed in vivo in a murine model of pulmonary IA demonstrating improved survival among mice infected with azole-resistant A. fumigatus strains and treated with posaconazole plus caspofungin compared with monotherapies (77). Interaction of echinocandins with the new mold-active triazole isavuconazole against Aspergillus spp. was described as indifferent for most cases in one study (78).
One study suggested that both voriconazole and amphotericin B may interact synergistically with caspofungin on Aspergillus biofilms (79).
Assessment of the efficacy of antifungal drug combinations in clinical practice is difficult due to multiple potential confounding factors. The combination of liposomal amphotericin B (standard dosage) and caspofungin was found to be superior to liposomal amphotericin B alone (high dosage) in a small pilot study (80). A large randomized double-blind placebo-controlled trial failed to demonstrate the superiority of the combination therapy of voriconazole and anidulafungin over voriconazole alone for the treatment of IA for the primary outcome of 6-week mortality, despite a trend in favor of the combination (81). However, a post hoc analysis in the subgroup of patients with IA diagnosis relying on positive galactomannan showed a significant benefit of the combination. The reason the combination therapy demonstrated superiority over voriconazole monotherapy only in the restricted population of galactomannan-positive IA is unclear. One possible explanation proposed by the authors is that this subgroup represents a more homogeneous population with fewer potential confounding factors for the outcome analysis.
Clinical experience is lacking to assess the efficacy of the triazole-echinocandin combination for the treatment of IA due to azole-resistant A. fumigatus, but expert opinions support the use of this combination as an alternative to liposomal amphotericin B monotherapy for the empirical treatment of IA in areas with a high prevalence of azole resistance (i.e., >10%) or in cases of documented azole resistance (82).
Echinocandins may also be combined initially with triazoles with the goal to rapidly achieve therapeutic levels in severe IA cases, considering that steady state for azoles is only reached after 5 to 7 days of therapy. Albeit theoretical and not supported by evidence, this approach is recommended by some experts.
Overall, evidence for a clear benefit of the combination of echinocandins with other antifungal drugs is limited but sufficient to recommend its use in particular situations, such as severe and/or disseminated IA, salvage therapy, or treatment of suspected or documented azole-resistant A. fumigatus (Table 1).
On the laboratory side, several compounds with modest antifungal activity per se demonstrated their ability to potentiate the activity of echinocandins, particularly caspofungin. Targeting the Hsp90-calcineurin axis can be achieved by different methods and results in hypersensitivity to caspofungin and abolition of the PE at higher concentrations. However, because this intracellular pathway is highly conserved in eukaryotes, use of these compounds in humans is limited by a lack of specificity and toxicity. Calcineurin inhibitors, such as tacrolimus (FK506) or cyclosporine, are strong immunosuppressive drugs that favor the occurrence and progression of IA. The discovery of a key serine-proline-rich region specific to the fungal calcineurin and essential for its function presents perspectives for the development of novel fungal-specific calcineurin inhibitors (83). Targeting Hsp90 is a difficult challenge because of the highly conserved structure of this chaperone among eukaryotes. Several Hsp90 inhibitors have passed the different stages of clinical development for an application in cancer therapy (84). However, the antifungal activity of these compounds, e.g., geldanamycin, is limited, and their positive interaction with echinocandins is observed only at toxic concentrations (85). A more promising approach consists of targeting the histone deacetylases (HDACs) to indirectly cripple Hsp90 function. Trichostatin A demonstrated in vitro synergistic interaction with caspofungin against Aspergillus spp. at concentrations that were well tolerated in mice (25, 85, 86). Novel HDAC inhibitors that are more stable are currently being contemplated for anticancer therapy, and their antifungal activity should be investigated. Finally, there may be an interest in investigating the role of some Ca2+ channel inhibitors used for the treatment of hypertension (e.g., verapamil), which can potentiate caspofungin activity and inhibit the PE (21).
Echinocandins for IA prophylaxis.
Because of the hepatotoxicity of azole compounds and their multiple drug interactions, echinocandins are increasingly considered for use in prophylaxis of invasive fungal infections. However, data to demonstrate their efficacy in this setting are lacking. Breakthrough invasive fungal infections (consisting mainly of IA) are usually observed at a frequency of 5% to 7% during echinocandin prophylaxis, but a wide range of occurrence (1% to 28%) has been reported in the literature (87). This is usually higher than the rate reported during mold-active azole prophylaxis. Indeed, one study found that echinocandin prophylaxis was an independent risk factor of breakthrough invasive fungal infections compared with voriconazole or posaconazole prophylaxis during chemotherapy of acute leukemia (88). The role of echinocandin prophylaxis for the prevention of IA seems to be limited to situations in which a mold-active azole is contraindicated.
Other β-1,3-glucan synthase inhibitors.
Ibrexafungerp (previously referred to as SCY-078 or MK-3118) is a semisynthetic β-1,3-glucan synthase inhibitor derived from the natural product enfumafungin (isolated from the fungus Hormomema spp.) and belonging to the triterpenoids (structurally different from the echinocandins). Ibrexafungerp has the advantage of bioavailability by the oral route and an antifungal spectrum similar to that of echinocandins with fungistatic activity against Aspergillus spp. In vitro testing showed MECs ranging from 0.06 to 0.25 μg/ml for the most relevant pathogenic Aspergillus spp., including azole-resistant A. fumigatus (89, 90). Because ibrexafungerp targets a different region of the β-glucan synthase than echinocandins, its activity is not affected by the common hot spot mutations described in Candida spp. and A. fumigatus isolates (91, 92). Ibrexafungerp demonstrated synergistic in vitro activity in combination with mold-active azoles (voriconazole, isavuconazole) or amphotericin B against azole-susceptible A. fumigatus (90), and a phase 2 clinical trial that will test the efficacy of the ibrexafungerp-voriconazole combination versus voriconazole monotherapy for the treatment of IA is forthcoming.
CONCLUSIONS AND PERSPECTIVES
Of the three antifungal drug classes, echinocandins are the least active against Aspergillus spp. However, echinocandins also have incontestable advantages, such as their quasi-absence of related toxicity and lack of drug-drug interactions, which represent frequent limitations for triazoles and amphotericin B. A new niche for echinocandins may arise from increasing reports of azole-resistant Aspergillus spp. This perspective should, however, be moderated with the warning that increased echinocandin use has also been associated with changes in the epidemiology of candidemia and emergence of echinocandin resistance among C. albicans and C. glabrata isolates (93, 94).
Recent advances in drug development increase the spectrum of this antifungal drug class, with the advent of a long-lasting echinocandin (rezafungin). Moreover, novel types of β-glucan synthase inhibitors are under development, such as ibrexafungerp, a compound structurally different from echinocandins but with comparable activity against Aspergillus spp. and availability for oral administration (47, 89). With the frequent need of prolonged antifungal therapy for IA, these new formulations may represent an alternative in the future. The widespread use of mold-active azole prophylaxis also raises the challenge of the therapeutic management of breakthrough IA, for which echinocandins may be considered in combination with other antifungals. Echinocandins display positive interactions with the two other existing antifungal drug classes and some experimental drugs. Moreover, their antifungal effect is not limited to their fungistatic activity against Aspergillus hyphae but also involves an important immunomodulatory effect on the host immune response, which plays a determinant role in the outcome of IA. Therefore, the story of echinocandins illustrates the complex and dynamic interactions between the antifungal drug, the fungus, and the host immunity during the course of invasive mycosis, which is a fascinating topic. Further studies should focus on better defining the role of the different echinocandins when used alone or in combination in the treatment of IA. An alarming observation is that despite the extended spectrum of marketed antifungal drugs in the last decade, we still have only three drug classes. Moreover, resistance in Aspergillus isolates is usually observed across all drugs within a class. Therefore, drug combinations may become a cornerstone to combat IA with more-resistant Aspergillus spp. in the future, and echinocandins appear as the optimal candidates to act synergistically with other antifungals.
ACKNOWLEDGMENTS
We declare that we had no direct source of funding for this work.
F.L. has been a member of advisory boards for MSD, Gilead, and Basilea.
F.L.’s laboratory receives financial support from the Swiss National Science Foundation (SNSF Ambizione-Score, grant number PZ00P3 161140) and the Santos-Suarez Foundation.
REFERENCES
- 1.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Flörl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M, Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Brüggemann RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A, Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux J-P, Garbino J, Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ, Lange C, Lehrnbecher T, Löffler J, Lortholary O, Maertens J, Marchetti O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M, Sanguinetti M, Sheppard DC, Sinkó J, Skiada A, Vehreschild MJGT, Viscoli C, Cornely OA. 2018. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 24(Suppl 1):e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. 2016. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilar-Zapata D, Petraitiene R, Petraitis V. 2015. Echinocandins: the expanding antifungal armamentarium. Clin Infect Dis 61 Suppl 6:S604–S611. doi: 10.1093/cid/civ814. [DOI] [PubMed] [Google Scholar]
- 4.Gow NAR, Latge JP, Munro CA. 2017. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr 5. doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown GD. 2006. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol 6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 6.Lamaris GA, Lewis RE, Chamilos G, May GS, Safdar A, Walsh TJ, Raad II, Kontoyiannis DP. 2008. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J Infect Dis 198:186–192. doi: 10.1086/589305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moretti S, Bozza S, D'Angelo C, Casagrande A, Della Fazia MA, Pitzurra L, Romani L, Aversa F. 2012. Role of innate immune receptors in paradoxical caspofungin activity in vivo in preclinical aspergillosis. Antimicrob Agents Chemother 56:4268–4276. doi: 10.1128/AAC.05198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinbach WJ, Lamoth F, Juvvadi PR. 2015. Potential microbiological effects of higher dosing of echinocandins. Clin Infect Dis 61(Suppl 6):S669–S677. doi: 10.1093/cid/civ725. [DOI] [PubMed] [Google Scholar]
- 9.Hall GS, Myles C, Pratt KJ, Washington JA. 1988. Cilofungin (LY121019), an antifungal agent with specific activity against Candida albicans and Candida tropicalis. Antimicrob Agents Chemother 32:1331–1335. doi: 10.1128/aac.32.9.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens DA, Espiritu M, Parmar R. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob Agents Chemother 48:3407–3411. doi: 10.1128/AAC.48.9.3407-3411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antachopoulos C, Meletiadis J, Sein T, Roilides E, Walsh TJ. 2007. Concentration-dependent effects of caspofungin on the metabolic activity of Aspergillus species. Antimicrob Agents Chemother 51:881–887. doi: 10.1128/AAC.01160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antachopoulos C, Meletiadis J, Sein T, Roilides E, Walsh TJ. 2008. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrob Agents Chemother 52:321–328. doi: 10.1128/AAC.00699-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamilos G, Lewis RE, Albert N, Kontoyiannis DP. 2007. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differences. Antimicrob Agents Chemother 51:2257–2259. doi: 10.1128/AAC.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortwendel JR, Juvvadi PR, Perfect BZ, Rogg LE, Perfect JR, Steinbach WJ. 2010. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob Agents Chemother 54:1555–1563. doi: 10.1128/AAC.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Wan Z, Liu W, Li R. 2015. Identification and susceptibility of Aspergillus section Nigri in China: prevalence of species and paradoxical growth in response to echinocandins. J Clin Microbiol 53:702–705. doi: 10.1128/JCM.03233-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadrich I, Neji S, Makni F, Ayadi A, Elloumi M, Ranque S. 2014. Trailing or paradoxical growth of Aspergillus flavus exposed to caspofungin is independent of genotype. J Med Microbiol 63:1584–1589. doi: 10.1099/jmm.0.076000-0. [DOI] [PubMed] [Google Scholar]
- 17.Munro CA, Selvaggini S, de Bruijn I, Walker L, Lenardon MD, Gerssen B, Milne S, Brown AJ, Gow NA. 2007. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol Microbiol 63:1399–1413. doi: 10.1111/j.1365-2958.2007.05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker LA, Munro CA, de Bruijn I, Lenardon MD, McKinnon A, Gow NA. 2008. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog 4:e1000040. doi: 10.1371/journal.ppat.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiederhold NP, Kontoyiannis DP, Prince RA, Lewis RE. 2005. Attenuation of the activity of caspofungin at high concentrations against Candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob Agents Chemother 49:5146–5148. doi: 10.1128/AAC.49.12.5146-5148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juvvadi PR, Lamoth F, Steinbach WJ. 2014. Calcineurin as a multifunctional regulator: unraveling novel functions in fungal stress responses, hyphal growth, drug resistance, and pathogenesis. Fungal Biol Rev 28:56–69. doi: 10.1016/j.fbr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juvvadi PR, Munoz A, Lamoth F, Soderblom EJ, Moseley MA, Read ND, Steinbach WJ. 2015. Calcium-mediated induction of paradoxical growth following caspofungin treatment is associated with calcineurin activation and phosphorylation in Aspergillus fumigatus. Antimicrob Agents Chemother 59:4946–4955. doi: 10.1128/AAC.00263-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ries LNA, Rocha MC, de Castro PA, Silva-Rocha R, Silva RN, Freitas FZ, de Assis LJ, Bertolini MC, Malavazi I, Goldman GH. 2017. The Aspergillus fumigatus CrzA transcription factor activates chitin synthase gene expression during the caspofungin paradoxical effect. mBio 8:e00705-17. doi: 10.1128/mBio.00705-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortwendel JR, Juvvadi PR, Pinchai N, Perfect BZ, Alspaugh JA, Perfect JR, Steinbach WJ. 2009. Differential effects of inhibiting chitin and 1,3-β-d-glucan synthesis in Ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob Agents Chemother 53:476–482. doi: 10.1128/AAC.01154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamoth F, Juvvadi PR, Gehrke C, Asfaw YG, Steinbach WJ. 2014. Transcriptional activation of heat shock protein 90 mediated via a proximal promoter region as trigger of caspofungin resistance in Aspergillus fumigatus. J Infect Dis 209:473–481. doi: 10.1093/infdis/jit530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamoth F, Juvvadi PR, Soderblom EJ, Moseley MA, Asfaw YG, Steinbach WJ. 2014. Identification of a key lysine residue in heat shock protein 90 required for azole and echinocandin resistance in Aspergillus fumigatus. Antimicrob Agents Chemother 58:1889–1896. doi: 10.1128/AAC.02286-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamoth F, Juvvadi PR, Soderblom EJ, Moseley MA, Steinbach WJ. 2015. Hsp70 and the cochaperone StiA (Hop) orchestrate Hsp90-mediated caspofungin tolerance in Aspergillus fumigatus. Antimicrob Agents Chemother 59:4727–4733. doi: 10.1128/AAC.00946-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aruanno M, Bachmann D, Sanglard D, Lamoth F. 2019. Link between heat shock protein 90 (Hsp90) and the mitochondrial respiratory chain in the caspofungin stress response of Aspergillus fumigatus. Antimicrob Agents Chemother doi: 10.1128/AAC.00208-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juvvadi PR, Fortwendel JR, Rogg LE, Burns KA, Randell SH, Steinbach WJ. 2011. Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus. Mol Microbiol 82:1235–1259. doi: 10.1111/j.1365-2958.2011.07886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juvvadi PR, Lamoth F, Steinbach WJ. 2014. Calcineurin-mediated regulation of hyphal growth, septation, and virulence in Aspergillus fumigatus. Mycopathologia 178:341–348. doi: 10.1007/s11046-014-9794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamoth F, Juvvadi PR, Fortwendel JR, Steinbach WJ. 2012. Heat shock protein 90 is required for conidiation and cell wall integrity in Aspergillus fumigatus. Eukaryot Cell 11:1324–1332. doi: 10.1128/EC.00032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imai J, Yahara I. 2000. Role of HSP90 in salt stress tolerance via stabilization and regulation of calcineurin. Mol Cell Biol 20:9262–9270. doi: 10.1128/mcb.20.24.9262-9270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocha MC, Fabri JH, Franco de Godoy K, Alves de Castro P, Hori JI, Ferreira da Cunha A, Arentshorst M, Ram AF, van den Hondel CA, Goldman GH, Malavazi I. 2016. Aspergillus fumigatus MADS-box transcription factor rlmA is required for regulation of the cell wall integrity and virulence. G3 (Bethesda) 6:2983–3002. doi: 10.1534/g3.116.031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loiko V, Wagener J. 2017. The paradoxical effect of echinocandins in Aspergillus fumigatus relies on recovery of the β-1, 3-glucan synthase Fks1. Antimicrob Agents Chemother 61:e01690-16. doi: 10.1128/AAC.01690-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno-Velasquez SD, Seidel C, Juvvadi PR, Steinbach WJ, Read ND. 2017. Caspofungin-mediated growth inhibition and paradoxical growth in Aspergillus fumigatus involve fungicidal hyphal tip lysis coupled with regenerative intrahyphal growth and dynamic changes in β-1,3-glucan synthase localization. Antimicrob Agents Chemother 61:e00710-17. doi: 10.1128/AAC.00710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petraitiene R, Petraitis V, Groll AH, Sein T, Schaufele RL, Francesconi A, Bacher J, Avila NA, Walsh TJ. 2002. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob Agents Chemother 46:12–23. doi: 10.1128/aac.46.1.12-23.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis RE, Albert ND, Kontoyiannis DP. 2008. Comparison of the dose-dependent activity and paradoxical effect of caspofungin and micafungin in a neutropenic murine model of invasive pulmonary aspergillosis. J Antimicrob Chemother 61:1140–1144. doi: 10.1093/jac/dkn069. [DOI] [PubMed] [Google Scholar]
- 37.Wiederhold NP, Kontoyiannis DP, Chi J, Prince RA, Tam VH, Lewis RE. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J Infect Dis 190:1464–1471. doi: 10.1086/424465. [DOI] [PubMed] [Google Scholar]
- 38.Wurthwein G, Cornely OA, Trame MN, Vehreschild JJ, Vehreschild MJ, Farowski F, Muller C, Boos J, Hempel G, Hallek M, Groll AH. 2013. Population pharmacokinetics of escalating doses of caspofungin in a phase II study of patients with invasive aspergillosis. Antimicrob Agents Chemother 57:1664–1671. doi: 10.1128/AAC.01912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope WW. 2013. Breakpoints for antifungal agents: an update from EUCAST focussing on echinocandins against Candida spp. and triazoles against Aspergillus spp. Drug Resist Updat 16:81–95. doi: 10.1016/j.drup.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Gardiner RE, Souteropoulos P, Park S, Perlin DS. 2005. Characterization of Aspergillus fumigatus mutants with reduced susceptibility to caspofungin. Med Mycol 43:299–305. doi: 10.1080/13693780400029023. [DOI] [PubMed] [Google Scholar]
- 41.Rocha EM, Garcia-Effron G, Park S, Perlin DS. 2007. A Ser678Pro substitution in Fks1p confers resistance to echinocandin drugs in Aspergillus fumigatus. Antimicrob Agents Chemother 51:4174–4176. doi: 10.1128/AAC.00917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jimenez-Ortigosa C, Moore C, Denning DW, Perlin DS. 2017. Emergence of echinocandin resistance due to a point mutation in the fks1 gene of aspergillus fumigatus in a patient with chronic pulmonary aspergillosis. Antimicrob Agents Chemother 61:e01277-17. doi: 10.1128/AAC.01277-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis RE, Liao G, Hou J, Prince RA, Kontoyiannis DP. 2011. Comparative in vivo dose-dependent activity of caspofungin and anidulafungin against echinocandin-susceptible and -resistant Aspergillus fumigatus. J Antimicrob Chemother 66:1324–1331. doi: 10.1093/jac/dkr142. [DOI] [PubMed] [Google Scholar]
- 44.Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2009. In vitro susceptibility of clinical isolates of Aspergillus spp. to anidulafungin, caspofungin, and micafungin: a head-to-head comparison using the CLSI M38-A2 broth microdilution method. J Clin Microbiol 47:3323–3325. doi: 10.1128/JCM.01155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lockhart SR, Zimbeck AJ, Baddley JW, Marr KA, Andes DR, Walsh TJ, Kauffman CA, Kontoyiannis DP, Ito JI, Pappas PG, Chiller T. 2011. In vitro echinocandin susceptibility of Aspergillus isolates from patients enrolled in the Transplant-Associated Infection Surveillance Network. Antimicrob Agents Chemother 55:3944–3946. doi: 10.1128/AAC.00428-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Ingen J, van der Lee HA, Rijs TA, Zoll J, Leenstra T, Melchers WJ, Verweij PE. 2015. Azole, polyene and echinocandin MIC distributions for wild-type, TR34/L98H and TR46/Y121F/T289A Aspergillus fumigatus isolates in the Netherlands. J Antimicrob Chemother 70:178–181. doi: 10.1093/jac/dku364. [DOI] [PubMed] [Google Scholar]
- 47.Wiederhold NP, Locke JB, Daruwala P, Bartizal K. 2018. Rezafungin (CD101) demonstrates potent in vitro activity against Aspergillus, including azole-resistant Aspergillus fumigatus isolates and cryptic species. J Antimicrob Chemother 73:3063–3067. doi: 10.1093/jac/dky280. [DOI] [PubMed] [Google Scholar]
- 48.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. 2016. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J Antimicrob Chemother 71:2868–2873. doi: 10.1093/jac/dkw214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2010. Wild-type minimum effective concentration distributions and epidemiologic cutoff values for caspofungin and Aspergillus spp. as determined by Clinical and Laboratory Standards Institute broth microdilution methods. Diagn Microbiol Infect Dis 67:56–60. doi: 10.1016/j.diagmicrobio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Espinel-Ingroff A, Fothergill A, Fuller J, Johnson E, Pelaez T, Turnidge J. 2011. Wild-type MIC distributions and epidemiological cutoff values for caspofungin and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob Agents Chemother 55:2855–2859. doi: 10.1128/AAC.01730-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Odds FC, Motyl M, Andrade R, Bille J, Canton E, Cuenca-Estrella M, Davidson A, Durussel C, Ellis D, Foraker E, Fothergill AW, Ghannoum MA, Giacobbe RA, Gobernado M, Handke R, Laverdiere M, Lee-Yang W, Merz WG, Ostrosky-Zeichner L, Peman J, Perea S, Perfect JR, Pfaller MA, Proia L, Rex JH, Rinaldi MG, Rodriguez-Tudela JL, Schell WA, Shields C, Sutton DA, Verweij PE, Warnock DW. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J Clin Microbiol 42:3475–3482. doi: 10.1128/JCM.42.8.3475-3482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arendrup MC, Meletiadis J, Mouton JW, Lagrou K, Hamal P, Guinea J, the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2017. EUCAST definitive document E.DEF 9.3.1: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_9_3_1_Mould_testing__definitive.pdf.
- 53.Clinical and Laboratory Standards Institute. 2017. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi—3rd ed CLSI document M38. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 54.Kirkpatrick WR, Perea S, Coco BJ, Patterson TF. 2002. Efficacy of caspofungin alone and in combination with voriconazole in a guinea pig model of invasive aspergillosis. Antimicrob Agents Chemother 46:2564–2568. doi: 10.1128/AAC.46.8.2564-2568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsumoto S, Wakai Y, Nakai T, Hatano K, Ushitani T, Ikeda F, Tawara S, Goto T, Matsumoto F, Kuwahara S. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of pulmonary aspergillosis. Antimicrob Agents Chemother 44:619–621. doi: 10.1128/aac.44.3.619-621.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ikeda F, Wakai Y, Matsumoto S, Maki K, Watabe E, Tawara S, Goto T, Watanabe Y, Matsumoto F, Kuwahara S. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob Agents Chemother 44:614–618. doi: 10.1128/aac.44.3.614-618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Felton T, Troke PF, Hope WW. 2014. Tissue penetration of antifungal agents. Clin Microbiol Rev 27:68–88. doi: 10.1128/CMR.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walsh TJ, Teppler H, Donowitz GR, Maertens JA, Baden LR, Dmoszynska A, Cornely OA, Bourque MR, Lupinacci RJ, Sable CA, dePauw BE. 2004. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med 351:1391–1402. doi: 10.1056/NEJMoa040446. [DOI] [PubMed] [Google Scholar]
- 59.Viscoli C, Herbrecht R, Akan H, Baila L, Sonet A, Gallamini A, Giagounidis A, Marchetti O, Martino R, Meert L, Paesmans M, Ameye L, Shivaprakash M, Ullmann AJ, Maertens J, Infectious Disease Group of the EORTC. 2009. An EORTC Phase II study of caspofungin as first-line therapy of invasive aspergillosis in haematological patients. J Antimicrob Chemother 64:1274–1281. doi: 10.1093/jac/dkp355. [DOI] [PubMed] [Google Scholar]
- 60.Herbrecht R, Maertens J, Baila L, Aoun M, Heinz W, Martino R, Schwartz S, Ullmann AJ, Meert L, Paesmans M, Marchetti O, Akan H, Ameye L, Shivaprakash M, Viscoli C. 2010. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: an European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant 45:1227–1233. doi: 10.1038/bmt.2009.334. [DOI] [PubMed] [Google Scholar]
- 61.Cornely OA, Vehreschild JJ, Vehreschild MJ, Wurthwein G, Arenz D, Schwartz S, Heussel CP, Silling G, Mahne M, Franklin J, Harnischmacher U, Wilkens A, Farowski F, Karthaus M, Lehrnbecher T, Ullmann AJ, Hallek M, Groll AH. 2011. Phase II dose escalation study of caspofungin for invasive aspergillosis. Antimicrob Agents Chemother 55:5798–5803. doi: 10.1128/AAC.05134-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heinz WJ, Buchheidt D, Ullmann AJ. 2016. Clinical evidence for caspofungin monotherapy in the first-line and salvage therapy of invasive Aspergillus infections. Mycoses 59:480–493. doi: 10.1111/myc.12477. [DOI] [PubMed] [Google Scholar]
- 63.Raad II, Zakhem AE, Helou GE, Jiang Y, Kontoyiannis DP, Hachem R. 2015. Clinical experience of the use of voriconazole, caspofungin or the combination in primary and salvage therapy of invasive aspergillosis in haematological malignancies. Int J Antimicrob Agents 45:283–288. doi: 10.1016/j.ijantimicag.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Denning DW, Marr KA, Lau WM, Facklam DP, Ratanatharathorn V, Becker C, Ullmann AJ, Seibel NL, Flynn PM, van Burik JA, Buell DN, Patterson TF. 2006. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect 53:337–349. doi: 10.1016/j.jinf.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kontoyiannis DP, Ratanatharathorn V, Young JA, Raymond J, Laverdiere M, Denning DW, Patterson TF, Facklam D, Kovanda L, Arnold L, Lau W, Buell D, Marr KA. 2009. Micafungin alone or in combination with other systemic antifungal therapies in hematopoietic stem cell transplant recipients with invasive aspergillosis. Transpl Infect Dis 11:89–93. doi: 10.1111/j.1399-3062.2008.00349.x. [DOI] [PubMed] [Google Scholar]
- 66.Elefanti A, Mouton JW, Verweij PE, Tsakris A, Zerva L, Meletiadis J. 2013. Amphotericin B- and voriconazole-echinocandin combinations against Aspergillus spp.: effect of serum on inhibitory and fungicidal interactions. Antimicrob Agents Chemother 57:4656–4663. doi: 10.1128/AAC.00597-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siopi M, Siafakas N, Vourli S, Mouton JW, Zerva L, Meletiadis J. 2016. Dose optimization of voriconazole/anidulafungin combination against Aspergillus fumigatus using an in vitro pharmacokinetic/pharmacodynamic model and response surface analysis: clinical implications for azole-resistant aspergillosis. J Antimicrob Chemother 71:3135–3147. doi: 10.1093/jac/dkw276. [DOI] [PubMed] [Google Scholar]
- 68.Spreghini E, Orlando F, Santinelli A, Pisa E, Loretelli C, Manso E, Milici ME, Scalise G, Barchiesi F. 2009. Anidulafungin in combination with amphotericin B against Aspergillus fumigatus. Antimicrob Agents Chemother 53:4035–4039. doi: 10.1128/AAC.00659-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sionov E, Mendlovic S, Segal E. 2006. Efficacy of amphotericin B or amphotericin B-intralipid in combination with caspofungin against experimental aspergillosis. J Infect 53:131–139. doi: 10.1016/j.jinf.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 70.Nishi I, Sunada A, Toyokawa M, Asari S, Iwatani Y. 2011. Evaluation of amphotericin B and micafungin combination against clinical isolates of Aspergillus species. J Chemother 23:102–106. doi: 10.1179/joc.2011.23.2.102. [DOI] [PubMed] [Google Scholar]
- 71.Perea S, Gonzalez G, Fothergill AW, Kirkpatrick WR, Rinaldi MG, Patterson TF. 2002. In vitro interaction of caspofungin acetate with voriconazole against clinical isolates of Aspergillus spp. Antimicrob Agents Chemother 46:3039–3041. doi: 10.1128/aac.46.9.3039-3041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manavathu EK, Alangaden GJ, Chandrasekar PH. 2003. Differential activity of triazoles in two-drug combinations with the echinocandin caspofungin against Aspergillus fumigatus. J Antimicrob Chemother 51:1423–1425. doi: 10.1093/jac/dkg242. [DOI] [PubMed] [Google Scholar]
- 73.Krishnan-Natesan S, Wu W, Chandrasekar PH. 2012. In vitro efficacy of the combination of voriconazole and anidulafungin against voriconazole-resistant cyp51A mutants of Aspergillus fumigatus. Diagn Microbiol Infect Dis 73:135–137. doi: 10.1016/j.diagmicrobio.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Seyedmousavi S, Meletiadis J, Melchers WJ, Rijs AJ, Mouton JW, Verweij PE. 2013. In vitro interaction of voriconazole and anidulafungin against triazole-resistant Aspergillus fumigatus. Antimicrob Agents Chemother 57:796–803. doi: 10.1128/AAC.00980-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seyedmousavi S, Bruggemann RJ, Melchers WJ, Rijs AJ, Verweij PE, Mouton JW. 2013. Efficacy and pharmacodynamics of voriconazole combined with anidulafungin in azole-resistant invasive aspergillosis. J Antimicrob Chemother 68:385–393. doi: 10.1093/jac/dks402. [DOI] [PubMed] [Google Scholar]
- 76.Mavridou E, Meletiadis J, Rijs A, Mouton JW, Verweij PE. 2015. The strength of synergistic interaction between posaconazole and caspofungin depends on the underlying azole resistance mechanism of Aspergillus fumigatus. Antimicrob Agents Chemother 59:1738–1744. doi: 10.1128/AAC.04469-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lepak AJ, Marchillo K, VanHecker J, Andes DR. 2013. Impact of in vivo triazole and echinocandin combination therapy for invasive pulmonary aspergillosis: enhanced efficacy against Cyp51 mutant isolates. Antimicrob Agents Chemother 57:5438–5447. doi: 10.1128/AAC.00833-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raffetin A, Courbin V, Jullien V, Dannaoui E. 2018. In vitro combination of isavuconazole with echinocandins against azole-susceptible and -resistant Aspergillus spp. Antimicrob Agents Chemother 62:e01382-17. doi: 10.1128/AAC.01382-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu W, Li L, Sun Y, Chen W, Wan Z, Li R, Liu W. 2012. Interaction of the echinocandin caspofungin with amphotericin B or voriconazole against Aspergillus biofilms in vitro. Antimicrob Agents Chemother 56:6414–6416. doi: 10.1128/AAC.00687-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caillot D, Thiebaut A, Herbrecht R, de Botton S, Pigneux A, Bernard F, Larche J, Monchecourt F, Alfandari S, Mahi L. 2007. Liposomal amphotericin B in combination with caspofungin for invasive aspergillosis in patients with hematologic malignancies: a randomized pilot study (Combistrat trial). Cancer 110:2740–2746. doi: 10.1002/cncr.23109. [DOI] [PubMed] [Google Scholar]
- 81.Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, Heinz WJ, Jagannatha S, Koh LP, Kontoyiannis DP, Lee DG, Nucci M, Pappas PG, Slavin MA, Queiroz-Telles F, Selleslag D, Walsh TJ, Wingard JR, Maertens JA. 2015. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med 162:81–89. doi: 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]
- 82.Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Bruggemann RJ, Chowdhary A, Cornely OA, Denning DW, Groll AH, Izumikawa K, Kullberg BJ, Lagrou K, Maertens J, Meis JF, Newton P, Page I, Seyedmousavi S, Sheppard DC, Viscoli C, Warris A, Donnelly JP. 2015. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 21-22:30–40. doi: 10.1016/j.drup.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 83.Juvvadi PR, Gehrke C, Fortwendel JR, Lamoth F, Soderblom EJ, Cook EC, Hast MA, Asfaw YG, Moseley MA, Creamer TP, Steinbach WJ. 2013. Phosphorylation of calcineurin at a novel serine-proline rich region orchestrates hyphal growth and virulence in Aspergillus fumigatus. PLoS Pathog 9:e1003564. doi: 10.1371/journal.ppat.1003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jhaveri K, Ochiana SO, Dunphy MP, Gerecitano JF, Corben AD, Peter RI, Janjigian YY, Gomes-DaGama EM, Koren J 3rd, Modi S, Chiosis G. 2014. Heat shock protein 90 inhibitors in the treatment of cancer: current status and future directions. Expert Opin Investig Drugs 23:611–628. doi: 10.1517/13543784.2014.902442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lamoth F, Alexander BD, Juvvadi PR, Steinbach WJ. 2015. Antifungal activity of compounds targeting the Hsp90-calcineurin pathway against various mould species. J Antimicrob Chemother 70:1408–1411. doi: 10.1093/jac/dku549. [DOI] [PubMed] [Google Scholar]
- 86.Lamoth F, Juvvadi PR, Steinbach WJ. 2015. Histone deacetylase inhibition as an alternative strategy against invasive aspergillosis. Front Microbiol 6:96. doi: 10.3389/fmicb.2015.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lionakis MS, Lewis RE, Kontoyiannis DP. 2018. Breakthrough invasive mold infections in the hematology patient: current concepts and future directions. Clin Infect Dis 67:1621–1630. doi: 10.1093/cid/ciy473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gomes MZ, Jiang Y, Mulanovich VE, Lewis RE, Kontoyiannis DP. 2014. Effectiveness of primary anti-Aspergillus prophylaxis during remission induction chemotherapy of acute myeloid leukemia. Antimicrob Agents Chemother 58:2775–2780. doi: 10.1128/AAC.01527-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pfaller MA, Messer SA, Motyl MR, Jones RN, Castanheira M. 2013. In vitro activity of a new oral glucan synthase inhibitor (MK-3118) tested against Aspergillus spp. by CLSI and EUCAST broth microdilution methods. Antimicrob Agents Chemother 57:1065–1068. doi: 10.1128/AAC.01588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghannoum M, Long L, Larkin EL, Isham N, Sherif R, Borroto-Esoda K, Barat S, Angulo D. 2018. Evaluation of the antifungal activity of the novel oral glucan synthase inhibitor SCY-078, singly and in combination, for the treatment of invasive aspergillosis. Antimicrob Agents Chemother 62:e00244-18. doi: 10.1128/AAC.00244-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jimenez-Ortigosa C, Paderu P, Motyl MR, Perlin DS. 2014. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida species and Aspergillus species isolates. Antimicrob Agents Chemother 58:1248–1251. doi: 10.1128/AAC.02145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walker SS, Xu Y, Triantafyllou I, Waldman MF, Mendrick C, Brown N, Mann P, Chau A, Patel R, Bauman N, Norris C, Antonacci B, Gurnani M, Cacciapuoti A, McNicholas PM, Wainhaus S, Herr RJ, Kuang R, Aslanian RG, Ting PC, Black TA. 2011. Discovery of a novel class of orally active antifungal beta-1,3-d-glucan synthase inhibitors. Antimicrob Agents Chemother 55:5099–5106. doi: 10.1128/AAC.00432-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bailly S, Maubon D, Fournier P, Pelloux H, Schwebel C, Chapuis C, Foroni L, Cornet M, Timsit JF. 2016. Impact of antifungal prescription on relative distribution and susceptibility of Candida spp. – trends over 10 years. J Infect 72:103–111. doi: 10.1016/j.jinf.2015.09.041. [DOI] [PubMed] [Google Scholar]