Solithromycin (CEM-101) is a novel fluoroketolide antimicrobial agent with activity against typical and atypical pathogens associated with community-acquired bacterial pneumonia. Using a neutropenic murine lung infection model, the objectives of this study were to identify the pharmacokinetic/pharmacodynamic (PK/PD) index most closely associated with efficacy and the magnitude of such indices associated with solithromycin efficacy against Streptococcus pneumoniae.
KEYWORDS: Streptococcus pneumoniae, fluoroketolide, murine lung infection model, pharmacokinetics/pharmacodynamics, solithromycin
ABSTRACT
Solithromycin (CEM-101) is a novel fluoroketolide antimicrobial agent with activity against typical and atypical pathogens associated with community-acquired bacterial pneumonia. Using a neutropenic murine lung infection model, the objectives of this study were to identify the pharmacokinetic/pharmacodynamic (PK/PD) index most closely associated with efficacy and the magnitude of such indices associated with solithromycin efficacy against Streptococcus pneumoniae. Plasma and epithelial lining fluid (ELF) samples for pharmacokinetics (PK) were collected serially over 24 hours from healthy mice administered single doses of solithromycin (0.625 to 40 mg/kg). Neutropenic CD-1 mice infected with 108 CFUs of one of five S. pneumoniae isolates were administered solithromycin (0.156 to 160 mg/kg/day) via oral gavage. Doses were administered in a fractionated manner for mice infected with one isolate, while mice infected with the remaining four isolates received solithromycin as either a regimen every 6 hours or every 12 hours. A three-compartment model best described solithromycin PK in the plasma and ELF (r2 = 0.935 and 0.831, respectively). The ratio of total-drug ELF to free-drug plasma area under the concentration-time curve (AUC) from time 0 to 24 hours was 2.7. Free-drug plasma and total-drug ELF AUC to minimum inhibitory concentration ratios (AUC/MIC ratios) were most predictive of efficacy (r2 = 0.851 and 0.850, respectively). The magnitude of free-drug plasma/total-drug ELF AUC/MIC ratios associated with net bacterial stasis and a 1- and 2-log10 CFU reduction from baseline was 1.65/1.26, 6.31/15.1, and 12.8/59.8, respectively. These data provided dose selection support for solithromycin for clinical trials in patients with community-acquired bacterial pneumonia.
TEXT
Solithromycin, also known as CEM-101, is a novel fluoroketolide antimicrobial agent with activity against typical (e.g., Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis) and atypical (e.g., Chlamydophila pneumoniae, Mycoplasma pneumoniae, and Legionella pneumophilia) pathogens associated with community-acquired bacterial pneumonia (CABP) (1–4). S. pneumoniae is of particular concern, as this pathogen is the leading cause of CABP (5, 6). Based on the data from the United States and collected globally between 2010 and 2016, the prevalence of penicillin-nonsusceptible pneumococcal isolates, as defined by the Clinical and Laboratory Standards Institute (CLSI) oral breakpoint, has been reported to range from 33.0% to 41.3% (1, 7–9). Additionally, resistance to many cephalosporins, macrolides, and trimethoprim-sulfamethoxazole is common among S. pneumoniae isolates (1, 7–9).
Given the demonstrated in vitro activity of solithromycin against S. pneumoniae and the above-described pathogens that are commonly associated with CABP (1–4), this agent could be a potential treatment for patients with CABP (10, 11). Animal infection models that utilize a dose-fractionation design represent a valuable tool for use to support dose selection for clinical trials. Through such studies, one can identify which pharmacokinetic/pharmacodynamic (PK/PD) index is best associated with efficacy and the magnitude of the index necessary for efficacy. PK/PD indices include ratios of the area under the concentration-time curve (AUC) or the maximum drug concentration (Cmax) to the MIC of the antimicrobial agent to the pathogen (AUC/MIC and Cmax/MIC ratios, respectively) or the percentage of time during a dosing interval that drug concentration exceeds the MIC of the antimicrobial agent to the pathogen (%T>MIC) (12). This animal-derived information can then be integrated with human pharmacokinetic (PK) data to support dose and dosing interval decisions for further clinical study (13, 14).
As described herein, a neutropenic murine lung infection model was used to determine the PK/PD indices in plasma and epithelial lining fluid (ELF) of the lung most closely associated with efficacy and the magnitude of such indices associated with the efficacy of solithromycin against S. pneumoniae.
RESULTS
In vitro susceptibility testing.
The solithromycin MIC values for the S. pneumoniae isolates ATCC 10813, CDC 1329, CDC 1396, CDC 1020, and CDC 673 were 0.06, 0.125, 0.06, 0.06, and 0.03 μg/ml, respectively.
Murine pharmacokinetic analysis.
The final PK model was best described by a three-compartment model consisting of a central, peripheral, and lung compartment. The elimination of solithromycin was modeled as a parallel first-order and a capacity-limited function. Drug absorption was described by a first-order absorption process with a fitted lag-time and a capacity-limited first-pass effect. The parameter estimates and associated standard error of the mean (%SEM) for these estimates based on the above-described PK model are shown in Table 1. Due to the nonlinearity of the absorption and elimination of solithromycin in mice, the parameters associated with the saturable first-pass effect and the clearance of solithromycin were modeled as fixed effects and, as a result, assumed no by-dose differences. The remainder of the PK parameters were modeled as random effects and, as a result, allowed for apparent differences among dosing cohorts.
TABLE 1.
Murine PK parameter estimates and associated standard error of the mean for solithromycina
| Parameter | Mean PK parameter estimate |
Interdose variability (%CV) |
||
|---|---|---|---|---|
| Final estimate | %SEM | Final estimate | %SEM | |
| Vm/f (mg/kg/h) | 0.248 | 11.5 | Fixed effect | |
| Km (mg/liter) | 0.0563 | 33.3 | Fixed effect | |
| Vc/f (liter/kg) | 2.91 | 13.9 | 6.01 | 222 |
| ka (h−1) | 1.08 | 35.1 | 76.7 | 60.2 |
| CLd/f (liter/kg/h) | 0.633 | 29.6 | 16.6 | 121 |
| Vp/f (liter/kg) | 1.67 | 22.0 | 36.7 | 83.2 |
| CL/f (liter/kg/h) | 0.699 | 6.99 | Fixed effect | |
| TLag (h) | 0.652 | 17.1 | 33.1 | 94.5 |
| FVmax (mg/kg/h) | 0.0785 | 25.7 | Fixed effect | |
| Fkm (mg/kg) | 0.0153 | 42.6 | Fixed effect | |
| kcl (h−1) | 0.805 | 30.3 | Fixed effect | |
| klc (h−1) | 3.63 | 19.8 | Fixed effect | |
| SDslp plasma | 0.338 | 9.27 | ||
| SDint plasma | 0.0005 | Fixed | ||
| SDslp ELF | 0.583 | 23.4 | ||
| SDint ELF | 0.005 | Fixed | ||
Vm/f is the maximum velocity of clearance by the saturable pathway; Km is the concentration at which this clearance is half-maximal; Vc/f is the volume of the central compartment; ka is first-order rate constant of absorption; Cld/f is the distributional clearance between the Vc/f and Vp/f; Vp/f is the volume of the peripheral compartment; CL/f is the linear clearance; Tlag is the fitted lag time; FVmax is the maximum velocity of oral first pass effect; Fkm is the amount of drug at which there is half-maximal oral first pass effect; kcl is the rate constant of drug transfer from the central compartment to the lung compartment; klc is the rate constant of drug transfer from the lung compartment to the central compartment; SDslp and SDint are the residual error SD model slope and intercept, respectively; %SEM, standard error of the mean.
The plasma PK data were well described by the PK model and provided a relatively unbiased fit to the data. As evidenced by an r2 of 0.935, there was good agreement between the observed and fitted solithromycin plasma concentration data with the line of best fit similar to the line of identity. The ELF data were also well characterized by the PK model (r2 of 0.831). The relationships between the fitted and observed solithromycin free-drug plasma and total-drug ELF concentration data are shown in Fig. 1.
FIG 1.
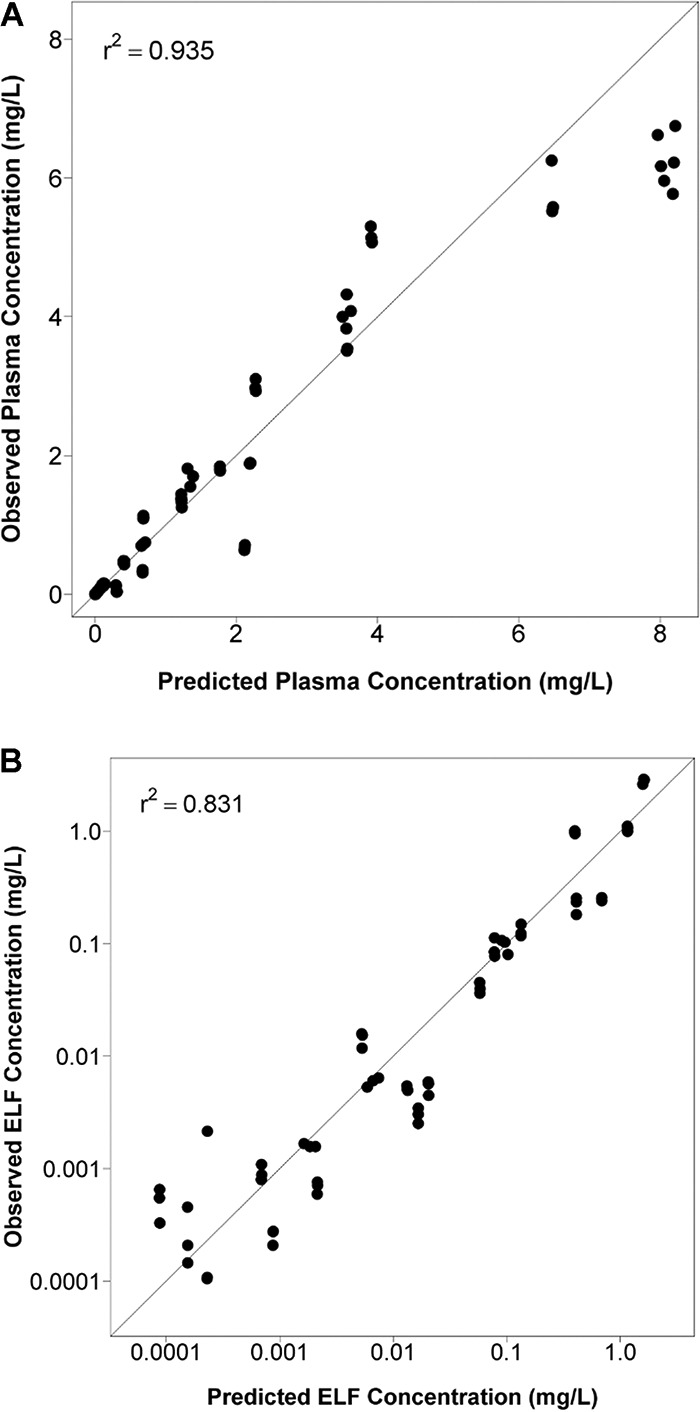
Relationship between the fitted and observed solithromycin total-drug plasma (A) and ELF (B) concentrations. Each symbol represents the concentration from each mouse, and the solid line represents the line of identity.
Pharmacokinetic/pharmacodynamic analysis.
To illustrate the relative plasma and ELF exposures expected for the range of total doses administered over 24 hours in the neutropenic lung infection model studies based on the murine PK model parameter estimates described in Table 1, simulated total-drug plasma, free-drug plasma, and total-drug ELF AUC values over 24 hours for total doses ranging from 0.156 to 160 mg/kg were generated and are shown in Table 2. Solithromycin total-drug ELF to total- and free-drug plasma AUC ratios were 0.22 and 2.7, respectively.
TABLE 2.
Simulated free-drug plasma and total-drug ELF AUC values for solithromycin dosing regimens administered to infected mice as a q24h regimen
| Dose (mg/kg) | Total-drug plasma AUCa (mg · h/liter) | Free-drug plasma AUCa (mg · h/liter) | Total-drug ELF AUCa ,b ,c (mg · h/liter) |
|---|---|---|---|
| 0.156d | 0.0131 | 0.00107 | 0.00290 |
| 0.625 | 0.100 | 0.00823 | 0.0222 |
| 2.5 | 0.884 | 0.0725 | 0.196 |
| 10 | 7.29 | 0.598 | 1.61 |
| 40 | 43.1 | 3.53 | 9.50 |
| 160 | 192 | 15.8 | 42.3 |
AUC values were determined over 24 hours.
The ratio of the total-drug ELF AUC to total-drug plasma AUC is 0.22.
The ratio of the total-drug ELF AUC to free-drug plasma AUC is 2.7.
A total dose of 0.156 was administered to infected mice in the neutropenic murine lung infection model studies as a q6h and q12h regimen rather than as a q24h regimen. q6h, q12h, and q24h represent regimens of every 6, 12, and 24 hours, respectively.
As described in the Materials and Methods, 2 hours after inoculation and prior to the administration of solithromycin, mice (3 mice/isolate) infected with one of the five S. pneumoniae isolates evaluated were sacrificed. Bacterial burdens of 6.80, 7.07, 6.45, 7.56, and 6.91 log10 CFUs were determined for S. pneumoniae ATCC 10813, CDC 1329, CDC 1396, CDC 1020, and CDC 673, respectively. These values were considered to be the baseline log10 CFU for the respective isolate in the PK/PD analysis.
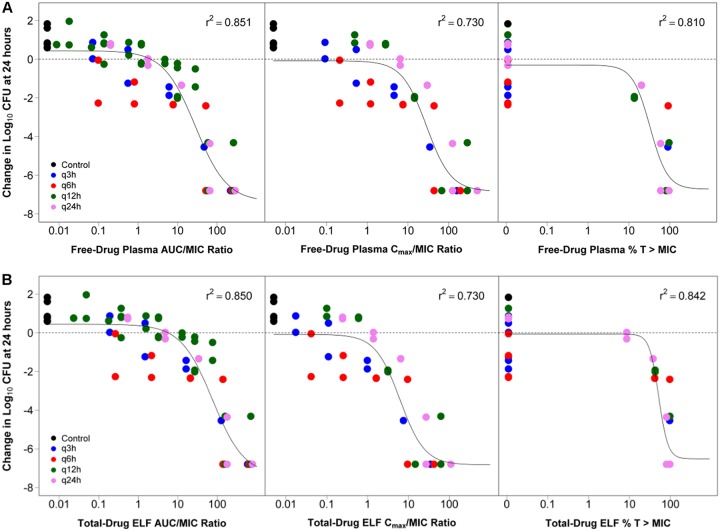
The relationship between the change in log10 CFU from baseline at 24 hours and free-drug plasma or total-drug ELF AUC/MIC and Cmax/MIC ratios and %T>MIC were well described for S. pneumoniae ATCC 10813 by the PK/PD models constructed using data for each matrix. The fitted relationships between the PK/PD indices for the free-drug plasma and total-drug ELF and change in log10 CFU from baseline at the 24-hour time point are shown in Fig. 2. The coefficients of determination (r2) ranged from 0.730 to 0.851 and 0.730 to 0.850 for relationships with PK/PD indices based on free-drug plasma and total-drug ELF exposures, respectively. Of the three PK/PD indices, free-drug plasma and total-drug ELF AUC/MIC ratio were most predictive of efficacy, with r2 of 0.851 and 0.850, respectively.
FIG 2.
Relationships between change in log10 CFU from baseline at 24 hours for S. pneumoniae ATCC 10813 and each of free-drug plasma (A) and total-drug ELF (B) PK/PD indices. Each symbol represents the change in log10 CFU from baseline in the lungs of mice at 24 hours. The horizontal line represents the bacterial burden at the start of therapy. q3h, q6h, q12h, and q24h represent regimens of every 3, 6, 12, and 24 hours, respectively.
Because the untreated control for mice infected with S. pneumoniae CDC 1020 and CDC 673 had a lower magnitude of bacterial burden in the lungs at the end of 24 hours compared to baseline, those isolates were considered unreliable and were excluded from the pooled PK/PD analysis. The relationships between the free-drug plasma or total-drug ELF AUC/MIC ratio and change in log10 CFU from baseline at 24 hours for S. pneumoniae, using data from mice infected with S. pneumoniae ATCC 10813, CDC 1396, and CDC 1329, are shown in Fig. 3. Parameter estimates and the associated percent standard errors (%SE) for the Hill-type models describing the relationship between the free-drug plasma or total-drug ELF AUC/MIC ratio and change in log10 CFU from baseline for the 24-hour time point for S. pneumoniae are provided in Table 3.
FIG 3.
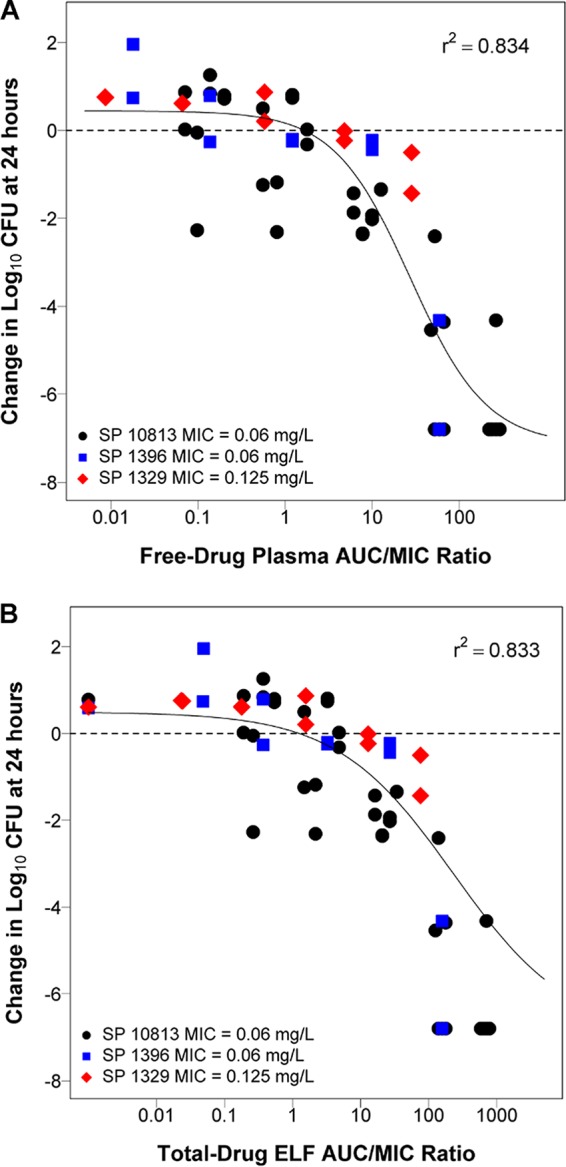
Relationship between the free-drug plasma (A) and total-drug ELF (B) AUC/MIC ratio and the change in log10 CFU from baseline at 24 hours based on pooled data for S. pneumoniae ATCC 10813, CDC 1396, and CDC 1329. Each symbol represents the change in log10 CFU from baseline in the lungs of mice at 24 hours. The horizontal line represents the bacterial burden at the start of therapy.
TABLE 3.
Parameter estimates and associated percent standard errors for the Hill-type models describing the relationship between the free-drug plasma or total-drug ELF AUC/MIC ratio and change in log10 CFU from baseline for the 24 hour time point for S. pneumoniaea
| Parameter | Parameter estimates and associated percent standard errors (%SE) |
|
|---|---|---|
| Free-drug plasma | Total-drug ELF | |
| Econ | 0.444 (0.337) | 0.496 (0.324) |
| Emax | 7.59 (0.669) | 7.45 (0.638) |
| EC50 | 0.991 (6.97) | 0.507 (5.72) |
| Hill | 27.2 (12.3) | 232 (9.58) |
| r2 | 0.834 | 0.833 |
Based on data pooled from mice infected with S. pneumoniae ATCC 10813, CDC 1329, and CDC 1396. Econ is the change in log10 CFU at 24 hours from baseline when no drug is administered; Emax is the maximum change in log10 CFU from baseline at 24 hours relative to Econ; EC50 is the AUC/MIC ratio at which there is half-maximal effect; Hill is the Hill coefficient; %SE is % standard error.
The magnitude of free-drug plasma and total-drug ELF AUC/MIC ratios associated with net bacterial stasis, and a 1- and 2-log10 CFU reduction from baseline, based on the Hill-type models developed using pooled data shown in Table 3, were computed. Free-drug plasma AUC/MIC ratio targets (% standard error [%SE]) associated with net bacterial stasis and a 1- and 2-log10 CFU reduction from baseline were 1.65 (37.9), 6.31 (22.9), and 12.8 (14.4), respectively. Total-drug ELF AUC/MIC ratio targets (%SE) associated with net bacterial stasis and a 1- and 2-log10 CFU reduction from baseline were 1.26 (51.1), 15.1 (28.9), and 59.8 (16.0), respectively. These data along with free-drug plasma and total-drug ELF AUC/MIC ratio targets associated with each of these endpoints for the individual isolates are shown in Table S1 and Table S2 in the supplemental material, respectively.
Discussion.
Using a neutropenic murine lung infection model, the objectives of the studies carried out to evaluate the PK/PD of solithromycin against S. pneumoniae were 2-fold. The first objective was to use data from dose-fractionation studies to identify the free-drug plasma and total-drug ELF PK/PD index most closely associated with solithromycin efficacy against S. pneumoniae. The second objective was to use data from dose-ranging studies to determine the magnitude of these indices associated with various levels of bacterial reduction for a panel of S. pneumoniae isolates.
Regardless of whether drug exposure was measured in plasma or in ELF, the AUC/MIC ratio was identified as the PK/PD index that best predicted solithromycin efficacy against S. pneumoniae. While investigators have measured drug exposure in plasma and ELF in separate cohorts of mice or modeled PK in plasma and ELF separately (15), it is more optimal to determine drug exposure simultaneously in murine plasma and ELF and comodel these data (16–18). The advantage of such an approach, as described herein, is the ability to estimate intercompartmental transfer rate constants and improve the precision of the PK parameter estimates. In addition, this approach will allow the ability to account for interspecies differences in the extent and rate of drug penetration when translating data from mice to humans for the purpose of selecting clinical dose regimens for further study (16).
In these analyses, solithromycin free-drug plasma and total-drug ELF AUC/MIC ratio targets of 1.65 and 1.26, respectively, were associated with net bacterial stasis. The magnitude of the free-drug plasma AUC/MIC ratio identified for net bacterial stasis for solithromycin based on data from a neutropenic murine lung infection model is much different than that identified for telithromycin based on a neutropenic murine thigh infection model. The PK/PD for telithromycin, the only ketolide that has been approved to date by the United States Food and Drug Administration (19), was evaluated against S. pneumoniae in a neutropenic murine thigh infection model (20). While free-drug serum AUC/MIC ratio was identified as the PK/PD index most closely associated with telithromycin efficacy, the magnitude of this index associated with net bacterial stasis is approximately 100 (20). This differs substantially from the free-drug plasma AUC/MIC ratio of 1.65 for solithromycin associated with net bacterial stasis based on a neutropenic murine lung infection model described herein. One reason for the discrepancy between the magnitude of the free-drug AUC/MIC ratio associated with same endpoint for each ketolide may be due to the differences in infection site (i.e., thigh compared to lung). As a result of differences in the site of infection, data based on a neutropenic murine thigh infection model may not accurately reflect drug accumulation in infected pulmonary tissue, and as a result, the PK/PD index associated with a particular level of effect may be over- or underestimated.
However, the magnitude of free-drug plasma AUC/MIC ratio for solithromycin associated with net bacterial stasis identified based on the neutropenic murine lung infection model (1.65) is similar to the magnitude of free-drug serum AUC/MIC ratio associated with an increased probability of microbiological eradication of 1.18 identified by Lodise et al. in telithromycin-treated patients with CABP (19, 21). The total-drug ELF AUC/MIC targets for solithromycin efficacy based on the neutropenic murine lung infection model cannot be compared with clinical data from the study by Lodise et al., as telithromycin concentrations in ELF were not measured in that study.
As stated above, in addition to considering the animal infection model that most accurately reflects effect site drug accumulation, it is also important when translating data from mice to humans to understand species-specific differences in both the extent and rate of drug penetration to effect site. For example, the penetration of solithromycin into human ELF, as measured by the ratio of total-drug ELF AUC to free-drug plasma AUC, is approximately 53 (22). In contrast, the penetration of solithromycin into rodent ELF, as measured by total-drug ELF AUC to free-drug plasma AUC, was 2.7. Similar findings were reported for telithromycin. The ratio of total-drug ELF AUC to free-drug serum AUC has been reported to range from 21.5 to 30.8 for telithromycin based on human PK data (23), whereas telithromycin penetration into rodent ELF (using the ratio of total-drug ELF AUC to free-drug plasma AUC) has been shown to be approximately 8.6 (24, 25). Based on these data, the penetration of solithromycin and telithromycin into human lung ELF may be approximately 20-fold and 2.5- to 3.6-fold higher, respectively, than that observed in rodents.
The above-described data underscore the importance of evaluating PK/PD relationships for efficacy using an appropriate infection model in order to make the most optimal inferences about dosing regimens for a given indication. For respiratory infections, these data also highlight the importance of evaluating the rate and extent of pulmonary penetration in both animal models and humans and, when possible, using PK/PD indices based on effect site exposures to support dose regimen decisions.
The information presented herein represents one critical input to support solithromycin dosing regimen decisions for the evaluation of dosing regimens that were chosen for clinical trials conducted in patients with CABP (10, 11). Other important inputs include PK data obtained from healthy subjects, including subjects from whom drug concentrations were measured in plasma and ELF, and in vitro surveillance data for solithromycin against S. pneumoniae. Using Monte Carlo simulation, the above-described nonclinical PK/PD relationships were integrated with population PK parameter estimates from healthy subject data and, in the context of the S. pneumoniae solithromycin MIC distribution data, were used to help discriminate among potential dosing regimens for clinical study (35, 36). This approach has been recommended by regulatory bodies (26, 27) and has been used successfully to identify clinically useful dosing regimens for anti-infective agents (13, 14).
In conclusion, in this study we identified the AUC/MIC ratio as the PK/PD index best predictive of solithromycin efficacy against S. pneumoniae, regardless of whether drug exposure was measured in plasma or in ELF. This PK/PD index is the same as that identified for other ketolide antimicrobial agents (20). These data were useful to support solithromycin dosing regimen decisions for clinical trials in patients with CABP that were undertaken (10, 11) and clinical trials in patients for the treatment of CABP, chronic respiratory diseases, and acute bronchitis and acute otitis media, laryngopharyngitis, and tonsillitis that are part of an ongoing development program in Japan.
MATERIALS AND METHODS
Bacteria, media, and study drug.
Five macrolide-resistant S. pneumoniae isolates (ATCC 10813, CDC 1329, CDC 1326, CDC 1020, and CDC 673) were evaluated in this study. All organisms were grown and quantified on sheep blood agar plates (Remel, Milwaukee, WI). The lower limit of bacterial quantification was 100 CFUs. Solithromycin was supplied by Cempra Pharmaceuticals, Inc. Chapel Hill, NC.
In vitro susceptibility studies.
The MIC values for all S. pneumoniae isolates were determined using standard microdilution techniques in accordance with the methods of the Clinical and Laboratory Standards Institute (CLSI) (28, 29).
Murine pharmacokinetic studies.
Groups of three healthy CD-1 mice per dose and time point were administered a single 0.625, 2.5, 10, or 40 mg/kg of body weight dose of solithromycin via oral gavage. Blood samples for plasma extraction were collected via cardiac puncture at 1, 2, 6, 9, 12, and 24 hours after solithromycin dose administration, with three mice sacrificed at each time point. ELF samples were also collected in the same mice by bronchoalveolar lavage at the same time points. The plasma and ELF samples were assayed for solithromycin using high-performance liquid chromatography/mass spectrometry with a lower limit of quantification of 10 ng/ml or 0.1 ng/ml for solithromycin in plasma and ELF. The percent coefficient of variation (%CV) of the assay was <7.67% and <13.0% for solithromycin in plasma and ELF, respectively. Plasma and ELF urea concentrations were also measured and used to correct the measured ELF solithromycin concentrations using the method described by Rennard, et al. (30).
Murine pharmacokinetic analysis.
Plasma and ELF pharmacokinetic (PK) data from the mice were fit by candidate PK models using Monte Carlo parametric expectation maximization as implemented in S-ADAPT 1.56 (31). Plasma and ELF PK data from the mice were pooled by dosing cohort, and the data were weighted using the inverse of the estimated measurement variance. Residual variability was described using an additive plus proportional residual error model. Model discrimination was by the comparison of objective function for nested models or Akaike’s information criterion (32) for either nested or nonnested models.
Murine lung infection model studies.
The animals were treated in accordance with the American Association for Accreditation of Laboratory Animal Care. The animal studies were also approved by the Animal Research Committee of the William S. Middleton Memorial Veteran Affairs Hospital.
Female CD-1 mice were rendered neutropenic (<100 neutrophils/mm3) via 2 intraperitoneal injections of cyclophosphamide for 4 days (150 mg/kg) and 1 day (100 mg/kg) prior to bacterial infection. S. pneumoniae in log-phase growth was prepared by growing freshly plated bacteria overnight to an absorbance of 0.3 at 580 nm (Spectronic 88, Bausch & Lomb, Inc., Rochester, NY). Cultures were centrifuged at 10,000 × g for 20 minutes and washed twice in 0.9% saline before being resuspended in saline. Diffuse pneumonia was induced in the neutropenic mice by intranasal instillation of 50 μl of a 108 CFU/ml inoculum of S. pneumoniae (33).
Two hours after infection, mice infected with S. pneumoniae ATCC 10813 were administered total solithromycin doses of 0.625, 2.5, 10, 40, or 160 mg/kg/day via oral gavage as an every 3-, 6-, 12-, or 24-hour regimen (q3h, q6h, q12h, and q24h, respectively) over a 24-hour period. In addition to these dose-fractionation studies, dose-ranging studies were carried out in which mice infected with S. pneumoniae CDC 1329 and CDC 1396 received solithromycin doses of 0.156, 0.625, 2.5, 10, or 40 mg/kg/day via oral gavage as a q12h regimen. Mice infected with S. pneumoniae CDC 1020 and CDC 673 received solithromycin doses of 0.156, 0.625, 2.5, 10, or 40 mg/kg/day via oral gavage as a q6h regimen.
Infected mice were sacrificed immediately prior to or 24 hours after the first dose of solithromycin, and the bacterial burden in each animal was determined (3 mice per isolate/dose/time point). Untreated mice (3 mice per isolate/time point) served as negative controls. Lungs were harvested and homogenized, and the homogenate was then serially diluted and plated on agar. The bacterial burden was determined in log10 CFU/lung.
Murine pharmacokinetic/pharmacodynamic analysis.
Using a point estimate of 91.8% for the protein binding of solithromycin in mice (data on file, Cempra Pharmaceuticals, Inc.) and the PK model derived from healthy mice, the free-drug plasma and total-drug ELF concentration versus time profiles for the solithromycin dosing regimens administered to the infected mice were simulated using S-ADAPT 1.56 (31). The simulations were then used to determine the AUC, Cmax, and %T>MIC from time 0 to 24 hours. The AUC was determined by numeric integration of the simulated murine concentration-time profiles, and Cmax was determined to be the highest concentration of the fitted simulated PK profile.
The relationships between the free-drug plasma or total-drug ELF AUC/MIC ratio, Cmax/MIC ratio, and %T>MIC and change in log10 CFU were evaluated for S. pneumoniae ATCC 10813 using Hill-type models implemented in S-ADAPT 1.56 (31). Relationships between change in log10 CFU from baseline at 24 hours and both the free-drug plasma or total-drug ELF PK-PD indices most associated with efficacy based on the dose-fractionation study data were also evaluated using dose-fractionation and dose-ranging study data pooled from mice infected with one of the five isolates. The log10 CFU data were weighted using the inverse of the estimated measurement variance. The residual variability was described using an additive residual error model. Log10 CFU values observed to be less than the lower limit of detection (2 log10 CFU) were noted as such and were fit using the Beal M3 method (34).
Using the final parameter estimates from the Hill-type models for the free-drug plasma and total-drug ELF PK-PD indices most associated with efficacy, the magnitude of such PK-PD indices associated with net bacterial stasis and a 1- and 2-log10 CFU net reduction from baseline were identified.
Supplementary Material
ACKNOWLEDGMENTS
This study was sponsored by Cempra Pharmaceuticals, Inc., Chapel Hill, NC, USA.
The authors acknowledge MicroConstants, Inc., for conducting the drug concentration determinations of solithromycin in mouse ELF and plasma and Sudie Rowshan and Thorsten Degenhardt of Cempra Pharmaceuticals, Inc., for coordinating various aspects of these evaluations. We thank Kara Keedy for her review of the manuscript.
O.O.O. and A.F. were employees of the Institute for Clinical Pharmacodynamics, Inc., at the time the research was conducted. O.O.O. is currently an employee of the United States Food and Drug Administration and A.F. was an employee of the University of North Carolina. S.M.B. and P.G.A. are employees of the Institute for Clinical Pharmacodynamics, Inc. The Institute for Clinical Pharmacodynamics, Inc., receives research funding from Achaogen Inc., Actelion Pharmaceuticals, Actavis Generics, AiCuris GmbH, Arsanis, Inc., Amplyx, Basilea Pharmaceutica, B. Braun Medical, Inc., Boston Pharmaceuticals, Cellceutix Corporation, Cempra Pharmaceuticals, Cidara Therapeutics, Inc., Contrafect Corporation, Debiopharm International SA, Emergent, Entasis Therapeutics, Geom Therapeutics, Inc., GlaxoSmithKline, Horizon, Insmed, Inc., Iterum Therapeutics Limited, Kalyra Pharmaceuticals, Matinas, The Medicines Company, Meiji Seika Pharma Co., Ltd., Melinta Therapeutics, Menarini Ricerche S.p.A., Merck Sharpe & Dohme., Nabriva Therapeutics, Naeja RGM Pharmaceuticals, Inc., Nexcida Therapeutics, Inc., Northern Antibiotics, Nosopharm, Novartis International, NuCana Biomed, Paratek Pharmaceuticals, Pernix Therapeutics, Polyphor, Ltd., Polypid, Ltd., Prothena Corporation, Roche Bioscience, ScPharmaceuticals, Scynexis, Shionogi, Inc., Sofinnova Ventures, Inc., Spero Therapeutics, Takeda Pharma, Theravance Biopharma Pharmaceutica, Tetraphase Pharmaceuticals, Turing Pharmaceuticals, VenatoRx, Vical, Wockhardt, Ltd., and Zavante Therapeutics. In addition, P.G.A. is a consultant for Duke University.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02606-18.
REFERENCES
- 1.Farrell DJ, Flamm RK, Sader HS, Jones RN. 2016. Results from the Solithromycin International Surveillance Program (2014). Antimicrob Agents Chemother 60:3662–3668. doi: 10.1128/AAC.00185-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallegol J, Fernandes P, Melano RG, Guyard C. 2014. Antimicrobial activity of solithromycin against clinical isolates of Legionella pneumophila serogroup 1. Antimicrob Agents Chemother 58:909–915. doi: 10.1128/AAC.01639-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roblin PM, Kohlhoff SA, Parker C, Hammerschlag MR. 2009. In vitro activity of CEM 101, a new fluoroketolide antibiotic, against Chlamydia trachamatis and Chlamydia (Chlamydophila) pneumonia. Antimicrob Agents Chemother 54:1358–1359. doi: 10.1128/AAC.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waites KB, Crabb DM, Duffy LB. 2009. Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob Agents Chemother 53:2139–2141. doi: 10.1128/AAC.00090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.File TM, Marrie TJ. 2010. Burden of community-acquired pneumonia in North American adults. Postgrad Med 122:130–141. doi: 10.3810/pgm.2010.03.2130. [DOI] [PubMed] [Google Scholar]
- 6.Welte T, Torres A, Nathwani D. 2012. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 67:71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 7.Flamm RK, Rhomberg PR, Huband MD, Farrell DJ. 2016. In vitro activity of delafloxacin tested against isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother 60:6381–6385. doi: 10.1128/AAC.00941-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flamm RK, Steenbergen JN, Huband MD, Rhomberg PR, Sader HS. Activity of omadacycline when tested against respiratory pathogens Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus isolated during 2016 from medical centers in the USA, abstr 5989. 2017. Abstr 113th Annual Conference of the American Thoracic Society, Washington, DC. [Google Scholar]
- 9.Pfaller MA, Mendes RE, Duncan LR, Flamm RK, Sader HS. 2018. In vitro activities of ceftaroline and comparators against Streptococcus pneumoniae isolates from U.S. hospitals: results from seven years of the AWARE Surveillance Program (2010 to 2016). Antimicrob Agents Chemother 62:e01555-17. doi: 10.1128/AAC.01555-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrera CM, Mykietiuk A, Metev H, Nitu MF, Karimjee N, Doreski PA, Mitha I, Tanaseanu CM, McDermott Molina J, Antonovsky Y, Van Rensburg DJ, Rowe BH, Flores-Figueroa J, Rewerska B, Clark K, Keedy K, Sheets A, Scott D, Horwith G, Das AF, Jamieson B, Fernandes P, Oldach D. 2016. Efficacy and safety of oral solithromycin versus oral moxifloxacin for treatment of community-acquired bacterial pneumonia: a global, double-blind, multicentre, randomised, active-controlled, non-inferiority trial. Lancet Infect Dis 16:421–430. doi: 10.1016/S1473-3099(16)00017-7. [DOI] [PubMed] [Google Scholar]
- 11.File TM, Rewerska B, Vucinić-Mihailovic V, Gonong JRV, Das AF, Keedy K, Taylor D, Sheets A, Fernandes P, Oldach D, Jamieson BD. 2016. SOLITAIRE-IV: a randomized, double-blind, multicenter study comparing the efficacy and safety of intravenous-to-oral solithromycin to intravenous-to-oral moxifloxacin for treatment of community-acquired bacterial pneumonia. Clin Infect Dis 63:1007–1016. doi: 10.1093/cid/ciw490. [DOI] [PubMed] [Google Scholar]
- 12.Craig WA. 2007. Pharmacodynamics of antimicrobials: general concepts and applications In Nightingale CH, Ambrose PG, Drusano GL, and Murakawa T. (ed), Antimicrobial Pharmacodynamics in Theory and Clinical Practice, 2nd ed Informa Healthcare Inc, New York, NY. [Google Scholar]
- 13.Bhavnani SM, Hammel JP, Cirincione BB, Wikler MA, Ambrose PG. 2005. Use of pharmacokinetic-pharmacodynamic target attainment analyses to support phase 2 and 3 dosing strategies for doripenem. Antimicrob Agents Chemother 49:3944–3947. doi: 10.1128/AAC.49.9.3944-3947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drusano GL, Preston SL, Hardalo C, Hare R, Banfield C, Andes D, Vesga O, Craig WA. 2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob Agents Chemother 45:13–22. doi: 10.1128/AAC.45.1.13-22.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koomanachai P, Crandon JL, Banevicius MA, Peng L, Nicolau DP. 2009. Pharmacodynamic profile of tigecycline against methicillin-resistant Staphylococcus aureus in an experimental pneumonia model. Antimicrob Agents Chemother 53:5060–5063. doi: 10.1128/AAC.00985-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhavnani SM, Rubino CM, Forrest A, Lehoux D, Okusanya OO, Drusano GL, Rodvold KA, Craig WA, Ambrose PG, Parr TJ Jr. 2007. Use of PK/PD principles to guide clinical drug development for oritavancin, abstr A-51. Abstr 47th Intersci Conf Antimicrob Agents Chemother, Chicago, IL. [Google Scholar]
- 17.Bulik CC, Okusanya OO, Lakota EA, Forrest A, Bhavnani SM, Hoover JL, Andes DR, Ambrose PG. 2017. Pharmacokinetic-pharmacodynamic evaluation of gepotidacin against Gram-positive organisms using data from murine infection models. Antimicrob Agents Chemother 61:e00115-16. doi: 10.1128/AAC.02404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y-W, Zhou Q, Onufrak NJ, Wirth V, Chen K, Wang J, Forrest A, Chan H-K, Li J. 2017. Aerosolized polymyxin B for treatment of respiratory tract infections: determination of pharmacokinetic-pharmacodynamic indices for aerosolized polymyxin B against Pseudomonas aeruginosa in a mouse lung infection model. Antimicrob Agents Chemother 61:e00211-17. doi: 10.1128/AAC.00211-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanofi-Aventis, U.S., LLC. 2015. Ketek (telithromycin) package insert. Bridgewater, NJ. [Google Scholar]
- 20.Craig WA, Kiem S, Andes DR. 2002. Free-drug 24-hr AUC/MIC is the PK/PD target that correlates with in vivo efficacy of macrolides, azilides, ketolides and clindamycin, abstr A-1264. Abstr 42nd Intersci Conf Antimicrob Agents Chemother, San Diego, CA. [Google Scholar]
- 21.Lodise TP, Preston S, Bhargava V, Bryskier A, Nusrat R, Chapel S, Rangaraju M, Drusano GL. 2005. Pharmacodynamics of an 800-mg dose of telithromycin in patients with community-acquired pneumonia caused by extracellular pathogens. Diagn Microbiol Infect Dis 52:45–52. doi: 10.1016/j.diagmicrobio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Rodvold KA, Gotfried MH, Still JG, Clark K, Fernandes P. 2012. Comparison of plasma, epithelial lining fluid, and alveolar macrophage concentrations of solithromycin (CEM-101) in healthy adult subjects. Antimicrob Agents Chemother 56:5076–5081. doi: 10.1128/AAC.00766-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiem S, Schentag JJ. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother 52:24–36. doi: 10.1128/AAC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, Lee MG. 2007. Dose-dependent pharmacokinetics of telithromycin after intravenous and oral administration to rats: Contribution of intestinal first-pass effect to low bioavailability. J Pharm Pharmaceut Sci 10:37–50. [PubMed] [Google Scholar]
- 25.Togami K, Chono S, Seki T, Morimoto K. 2009. Distribution characteristics of telithromycin, a novel ketolide antimicrobial agent applied for treatment of respiratory infection, in lung epithelial lining fluid and alveolar macrophages. Drug Metab Pharmacokinet 24:411–417. doi: 10.2133/dmpk.24.411. [DOI] [PubMed] [Google Scholar]
- 26.United States Department of Health and Human Services, Food and Drug Administration. 2014. Guidance for Industry. Community-acquired bacterial pneumonia: Developing drugs for treatment. Draft guidance. United States Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 27.European Medicines Agency, Committee for Medicinal Products for Human Use. 2016. Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. European Medicines Agency, Amsterdam, Netherlands. [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, approved standard 8th ed CLSI document M07-A8 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2019. Performance standards for Antimicrobial Susceptibility Testing, 29th ed CLSI document M100 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 30.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 31.Bauer RJ. 2008. S-ADAPT/MCPEM user’s guide: software for pharmacokinetic, pharmacodynamic and population data analysis, version 1.56. Berkley, CA. [Google Scholar]
- 32.Akaike H. 1979. A Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika 66:237–243. doi: 10.2307/2335654. [DOI] [Google Scholar]
- 33.Andes D, Craig WA. 2006. Pharmacodynamics of a new streptogramin, XRP 2868, in murine thigh and lung infection models. Antimicrob Agents Chemother 50:243–249. doi: 10.1128/AAC.50.1.243-249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28:481–504. doi: 10.1023/A:1012299115260. [DOI] [PubMed] [Google Scholar]
- 35.Okusanya OO, Bhavnani SM, Forrest A, Fernandes P, Ambrose PG. Pharmacokinetic-pharmacodynamic target attainment analysis supporting CEM-101 phase 2 dose selection, abstr A1-692. Abstr 50th Intersci Conf Antimicrob Agents Chemother, Boston, MA. [Google Scholar]
- 36.Okusanya OO, Bhavnani SM, Forrest A, Bulik CC, Oldach D, Fernandes P, Ambrose PG. Population pharmacokinetic and pharmacokinetic-pharmacodynamic target attainment analyses for solithromycin to support intravenous dose selection in patients with community-acquired bacterial pneumonia, abstr A-1269. Abstr 52nd Intersci Conf Antimicrob Agents Chemother, San Francisco, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.