Surgical site infections (SSIs) are commonly caused by Staphylococcus aureus. We report that a combination of three monoclonal antibodies (MEDI6389) that neutralize S. aureus alpha-toxin, clumping factor A, and four leukocidins (LukSF, LukED, HlgAB, and HlgCB) plus vancomycin had enhanced efficacy compared with control antibody plus vancomycin in two mouse models of S. aureus SSI.
KEYWORDS: ClfA, clumping factor A, MRSA, Staphylococcus aureus, alpha-toxin, hla, leukocidin, surgical
ABSTRACT
Surgical site infections (SSIs) are commonly caused by Staphylococcus aureus. We report that a combination of three monoclonal antibodies (MEDI6389) that neutralize S. aureus alpha-toxin, clumping factor A, and four leukocidins (LukSF, LukED, HlgAB, and HlgCB) plus vancomycin had enhanced efficacy compared with control antibody plus vancomycin in two mouse models of S. aureus SSI. Therefore, monoclonal antibody-based neutralization of multiple S. aureus virulence factors may provide an adjunctive perioperative approach to combat S. aureus SSIs.
INTRODUCTION
Despite advances in aseptic surgery, decolonization efforts, and perioperative antibiotics (1–3), Staphylococcus aureus remains the most common cause of surgical site infections (SSIs), with half complicated by methicillin-resistant S. aureus (MRSA) (4). Vancomycin (Vanc) is recommended for perioperative coverage in MRSA-colonized patients or in populations with a high prevalence of MRSA (5). Because antibiotic stewardship programs aim to reduce antibiotic usage to prevent other infections (e.g., Clostridium difficile) and select for resistant bacteria, there is an unmet need for nonantibiotic coverage against SSIs (6).
S. aureus produces virulence factors involved in SSI and biofilm formation, which inhibit antibiotic penetration and immune clearance (7–9). Notably, inhibition of alpha-toxin (AT, also known as hla) and/or clumping factor A (ClfA) by antibody-based vaccines or monoclonal antibodies (MAbs) was efficacious in preclinical models of S. aureus skin/wound and implant-associated infections (10–12). The combined anti-AT and anti-ClfA MAbs were significantly more efficacious than the individual MAbs in mouse models of S. aureus bacteremia and hematogenous implant infection (12–14). Additionally, S. aureus produces leukocidins, including Panton-Valentine leukocidin (encoded by LukSF-PV genes), LukAB, LukED, and gamma-hemolysin (comprised of HlgAB and HlgCB), which promote inflammation and tissue damage and might contribute to SSIs (15, 16). Herein, we evaluated MEDI6389, a combination of three human MAbs (anti-AT [MEDI4893*], anti-ClfA [SAR114], and anti-LukF/LukD/HlgB cross-reactive MAb [SAN481]), which neutralize AT, ClfA, LukSF, LukED, HlgAB, and HlgCB, as adjunctive perioperative coverage in two models of SSI.
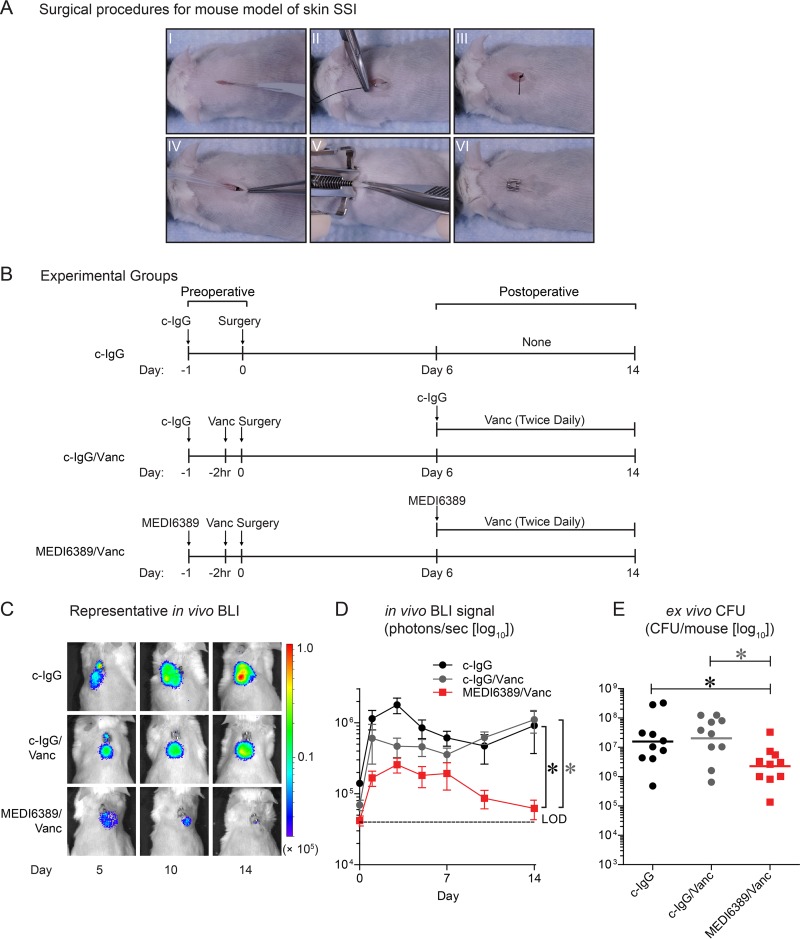
An S. aureus skin SSI model (17–19) was modified, whereby diabetic 10-week-old TallyHo/JngJ mice (blood glucose, >300 mg/dl) were anesthetized (2% isoflurane) and placed on a 37°C pad, and their backs were shaved and prepped with three betadine and 70% alcohol scrubs. A midline longitudinal 1-cm full-thickness skin incision was made (11-blade scalpel) (Fig. 1A, I). A 4-0 braided-silk suture with a C-3 needle was passed through the undersurface of the skin distal to the incision (Fig. 1A, II), tied twice, and cut, leaving a 1-cm tail (Fig. 1A, III). The suture was placed into the surgical site, and an inoculum of MRSA (strain USA300 LAC::lux [20]; 1 × 105 CFU in 5 μL phosphate-buffered saline [PBS]) was pipetted onto the suture (Fig. 1A, IV) before closure with a wound clip (Fig. 1A, V and VI).
FIG 1.
Enhanced efficacy of MEDI6389 in a skin SSI model. The mouse model of skin SSI with MRSA strain USA300 LAC::lux was performed in diabetic TallyHo/JngJ mice (n = 10/group). (A) Surgical procedures: full-thickness incision (I), placement of a silk suture on the undersurface of the skin (II), 1-cm suture tail (III), inoculum of MRSA pipetted onto the suture in the surgical site (IV), closure with a wound clip (V, VI). (B) Timeline of preoperative and postoperative administration in the experimental groups: c-IgG (isotype control MAb), c-IgG/Vanc, and MEDI6389/Vanc. (C) Representative in vivo BLI signals on a color scale overlaid on a gray-scale photograph of the mice. (D) Mean total flux (photons/s) ± SEM (logarithmic scale). *, P < 0.05 (2-way analysis of variance [ANOVA]). LOD, limit of detection. (E) Ex vivo CFU (horizontal bars, geometric mean) isolated from skin biopsies performed on euthanized mice on day 14 (logarithmic scale). *, P < 0.05, Kruskal-Wallis test with 2-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli to correct for multiple comparisons (29).
To determine the perioperative efficacy of adjunctive MEDI6389 plus Vanc, three experimental groups (n = 10 mice/group) were evaluated with preoperative and postoperative dosing (beginning on day 6 to account for the human MAb half-life in mice [see Fig. S1 in the supplemental material]): (i) preoperative c-IgG (isotype control MAb, anti-HIV gp120 [R347] [21]) (15 mg/kg i.p. [intraperitoneal] on day −1) and no postoperative administration on day 6 (none); (ii) c-IgG/Vanc: preoperative (c-IgG plus Vanc [110 mg/kg i.v., human exposure equivalent dose (22)] at −2 h) and postoperative (c-IgG on day 6 plus Vanc [110 mg/kg subcutaneous (s.c.) twice daily on days 6 to 14]); and (iii) MEDI6389/Vanc preoperative (MEDI6389 [5 mg/kg of each MAb i.p. on day −1] plus Vanc [110 mg/kg i.v. at −2 h]) and postoperative (MEDI6389 [once on day 6] plus Vanc [110 mg/kg s.c. twice daily on days 6 to14]) (Fig. 1B). MEDI6389/Vanc had decreased in vivo bioluminescence imaging (BLI) signals throughout the 14-day experiment compared with c-IgG or c-IgG/Vanc (P < 0.05) (Fig. 1C and D). On day 14, a 10-mm punch biopsy was performed, skin tissue was homogenized, and ex vivo CFUs were enumerated, as previously described (23). MEDI6389/Vanc had significantly decreased ex vivo CFUs compared with c-IgG or c-IgG/Vanc (P < 0.05) (Fig. 1E).
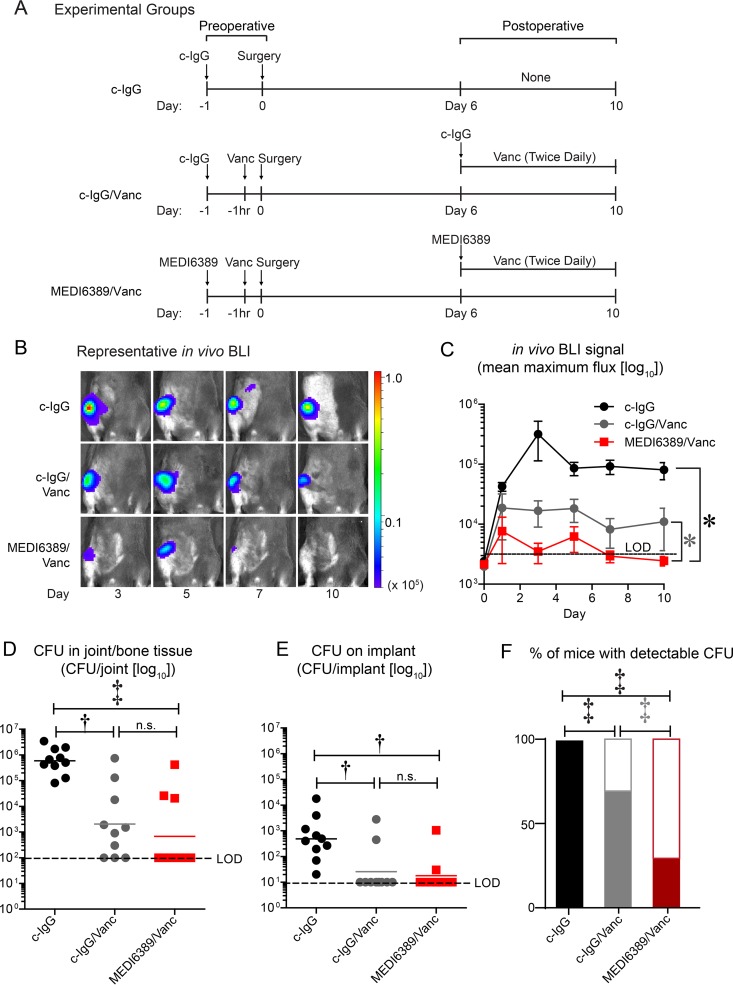
Next, perioperative efficacy of adjunctive MEDI6389 plus Vanc was evaluated in an orthopedic SSI model (24–26). Briefly, 8-week-old C57BL/6 anesthetized mice (2% isoflurane) underwent a medial parapatellar arthrotomy, and an orthopedic-grade titanium Kirschner wire (0.6 mm diameter, 9 mm length) was surgically placed into the intramedullary femoral canal with the distal end protruding into the knee joint. An inoculum of MRSA (strain SAP231 derived from parental strain NRS384 [27]; 1 × 103 CFU in 2 μl PBS) was pipetted onto the distal implant before reducing the patellar complex to midline and closure with absorbable sutures. The same experimental groups as in Fig. 1B were used except that preoperative Vanc was given at −1 h and the experimental duration was 10 days (Fig. 2A). MEDI6389/Vanc had decreased in vivo BLI signals compared with c-IgG or c-IgG/Vanc (P < 0.05) (Fig. 2B and C). On day 10, bone/joint tissue was homogenized, implants were sonicated, and ex vivo CFUs were enumerated, as previously described (26). MEDI6389/Vanc significantly decreased ex vivo CFUs from the bone/joint tissue (P < 0.001) and implants (P < 0.01) compared with c-IgG; there were no significant differences compared with c-IgG/Vanc (Fig. 2D and E). However, MEDI6389/Vanc had a significant decrease in the percentage of mice that had detectable ex vivo CFUs compared with c-IgG/Vanc (P < 0.001) (Fig. 2F).
FIG 2.
Enhanced efficacy of MEDI6389 in an orthopedic SSI model. The mouse model of orthopedic SSI with MRSA strain SAP231 was performed in C57BL/6 mice (n = 10/group). (A) Timeline of preoperative and postoperative administration in the experimental groups: c-IgG (isotype control MAb), c-IgG/Vanc, and MEDI6389/Vanc. (B) Representative in vivo BLI signals on a color scale overlaid on a gray-scale photograph of the mice. (C) Mean maximum flux (photons/s/cm2/steradian) ± SEM (logarithmic scale). LOD, limit of detection. *, P < 0.05 (2-way ANOVA). (D and E) Ex vivo CFU (horizontal bars, geometric mean) isolated from joint/bone tissue (D) and implants (E) from euthanized mice on day 10 (logarithmic scale). †, P < 0.01; ‡, P < 0.001, Kruskal-Wallis test with 2-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli to correct for multiple comparisons (29). n.s., not significant. (F) Percentage of mice with (filled) or without (open) ex vivo CFUs detected from tissue (D) or implant (E) specimens. ‡, P < 0.0001, Fisher’s exact test.
The study had some limitations. First, given the proof-of-concept efficacy for perioperative MEDI6389, the individual MAbs were not evaluated separately, but the anti-AT and anti-ClfA MAb combination had enhanced efficacy compared with the individual MAbs through complementary mechanisms (toxin neutralization, opsonophagocytic killing, and inhibition of biofilm formation and agglutination) (12–14). Second, additional efficacy of SAN481 was not observed in the orthopedic SSI model (see Fig. S2 in the supplemental material), which was likely due to the low sensitivity of mice to leukocidins (relative to humans and rabbits) (15, 16) or to limited leukocidin production or virulence in the SSI models. Importantly, SAN481 was not detrimental and might be beneficial against human SSIs. Third, the timing of preoperative Vanc was different in the skin (−2 h) and orthopedic (−1 h) SSI models because the −1-h-preoperative Vanc alone in the skin SSI model resulted in low initial in vivo BLI signals and CFUs in some mice (see Fig. S3 in the supplemental material), which prohibited studying the additional efficacy of MEDI6389. Because the half-life of Vanc is 0.97 h in mice (28), the beginning Vanc levels in the skin SSI model were likely half of those in the orthopedic SSI model. Finally, two different USA300 MRSA strains were used in the skin and orthopedic SSI models that produced lower (USA300 LAC::lux [20]) and higher (SAP231 [27]) bioluminescent signals, respectively, for optimal in vivo BLI. Nonetheless, MEDI6389 demonstrated enhanced efficacy against both strains. The data in this study suggest that neutralization of S. aureus virulence factors may provide adjunctive perioperative coverage against SSIs.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a sponsored research agreement from AstraZeneca through a Johns Hopkins-MedImmune Academic Industry Partnership.
L.S.M. has received grant support from AstraZeneca for the work reported in the manuscript and other grant support from Pfizer, Boehringer Ingelheim, Regeneron Pharmaceuticals, and Moderna Therapeutics. L.S.M. is also a shareholder of Noveome Biotherapeutics and is on the scientific advisory board of Integrated Biotherapeutics. These companies are developing therapeutics and vaccines against S. aureus and other pathogens. L.S.M. is also supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR069502 and R01AR073665) from the U.S. National Institutes of Health. T.S.C., C.T., and B.R.S. are employed by AstraZeneca and may hold AstraZeneca stock.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00346-19.
REFERENCES
- 1.Allegranzi B, Bischoff P, de Jonge S, Kubilay NZ, Zayed B, Gomes SM, Abbas M, Atema JJ, Gans S, van Rijen M, Boermeester MA, Egger M, Kluytmans J, Pittet D, Solomkin JS, WHO Guidelines Development Group. 2016. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 16:e276–e287. doi: 10.1016/S1473-3099(16)30398-X. [DOI] [PubMed] [Google Scholar]
- 2.Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, de Jonge S, de Vries F, Gomes SM, Gans S, Wallert ED, Wu X, Abbas M, Boermeester MA, Dellinger EP, Egger M, Gastmeier P, Guirao X, Ren J, Pittet D, Solomkin JS, WHO Guidelines Development Group. 2016. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 16:e288–e303. doi: 10.1016/S1473-3099(16)30402-9. [DOI] [PubMed] [Google Scholar]
- 3.Berrios-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, Reinke CE, Morgan S, Solomkin JS, Mazuski JE, Dellinger EP, Itani KMF, Berbari EF, Segreti J, Parvizi J, Blanchard J, Allen G, Kluytmans J, Donlan R, Schecter WP, Health Care Infection Control Practices Advisory Committee. 2017. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 152:784–791. doi: 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 4.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol 37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford T, Rodvold KA, Solomkin JS. 2012. Vancomycin for surgical prophylaxis? Clin Infect Dis 54:1474–1479. doi: 10.1093/cid/cis027. [DOI] [PubMed] [Google Scholar]
- 6.Sartelli M, Duane TM, Catena F, Tessier JM, Coccolini F, Kao LS, De Simone B, Labricciosa FM, May AK, Ansaloni L, Mazuski JE. 2016. Antimicrobial stewardship: a call to action for surgeons. Surg Infect (Larchmt) 17:625–631. doi: 10.1089/sur.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 8.Darouiche RO. 2004. Treatment of infections associated with surgical implants. N Engl J Med 350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 9.Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 10.Adhikari RP, Thompson CD, Aman MJ, Lee JC. 2016. Protective efficacy of a novel alpha hemolysin subunit vaccine (AT62) against Staphylococcus aureus skin and soft tissue infections. Vaccine 34:6402–6407. doi: 10.1016/j.vaccine.2016.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arrecubieta C, Matsunaga I, Asai T, Naka Y, Deng MC, Lowy FD. 2008. Vaccination with clumping factor A and fibronectin binding protein A to prevent Staphylococcus aureus infection of an aortic patch in mice. J Infect Dis 198:571–575. doi: 10.1086/590210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Cheng LI, Helfer DR, Ashbaugh AG, Miller RJ, Tzomides AJ, Thompson JM, Ortines RV, Tsai AS, Liu H, Dillen CA, Archer NK, Cohen TS, Tkaczyk C, Stover CK, Sellman BR, Miller LS. 2017. Mouse model of hematogenous implant-related Staphylococcus aureus biofilm infection reveals therapeutic targets. Proc Natl Acad Sci U S A 114:E5094–E5102. doi: 10.1073/pnas.1703427114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tkaczyk C, Hamilton MM, Sadowska A, Shi Y, Chang CS, Chowdhury P, Buonapane R, Xiao X, Warrener P, Mediavilla J, Kreiswirth B, Suzich J, Stover CK, Sellman BR. 2016. Targeting alpha toxin and ClfA with a multimechanistic monoclonal-antibody-based approach for prophylaxis of serious Staphylococcus aureus disease. mBio 7:e00528-16. doi: 10.1128/mBio.00528-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tkaczyk C, Kasturirangan S, Minola A, Jones-Nelson O, Gunter V, Shi YY, Rosenthal K, Aleti V, Semenova E, Warrener P, Tabor D, Stover CK, Corti D, Rainey G, Sellman BR. 2017. Multimechanistic monoclonal antibodies (MAbs) targeting Staphylococcus aureus alpha-toxin and clumping factor A: activity and efficacy comparisons of a MAb combination and an engineered bispecific antibody approach. Antimicrob Agents Chemother 61:e00629-17. doi: 10.1128/AAC.00629-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonzo F 3rd, Torres VJ. 2014. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol Mol Biol Rev 78:199–230. doi: 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spaan AN, van Strijp JAG, Torres VJ. 2017. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol 15:435–447. doi: 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gisby J, Bryant J. 2000. Efficacy of a new cream formulation of mupirocin: comparison with oral and topical agents in experimental skin infections. Antimicrob Agents Chemother 44:255–260. doi: 10.1128/aac.44.2.255-260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McRipley RJ, Whitney RR. 1976. Characterization and quantitation of experimental surgical-wound infections used to evaluate topical antibacterial agents. Antimicrob Agents Chemother 10:38–44. doi: 10.1128/aac.10.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rittenhouse S, Singley C, Hoover J, Page R, Payne D. 2006. Use of the surgical wound infection model to determine the efficacious dosing regimen of retapamulin, a novel topical antibiotic. Antimicrob Agents Chemother 50:3886–3888. doi: 10.1128/AAC.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tkaczyk C, Hua L, Varkey R, Shi Y, Dettinger L, Woods R, Barnes A, MacGill RS, Wilson S, Chowdhury P, Stover CK, Sellman BR. 2012. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin Vaccine Immunol 19:377–385. doi: 10.1128/CVI.05589-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegde SS, Reyes N, Skinner R, Difuntorum S. 2007. Efficacy of telavancin in a murine model of pneumonia induced by methicillin-susceptible Staphylococcus aureus. J Antimicrob Chemother 61:169–172. doi: 10.1093/jac/dkm417. [DOI] [PubMed] [Google Scholar]
- 23.Ortines RV, Liu H, Cheng LI, Cohen TS, Lawlor H, Gami A, Wang Y, Dillen CA, Archer NK, Miller RJ, Ashbaugh AG, Pinsker BL, Marchitto MC, Tkaczyk C, Stover CK, Sellman BR, Miller LS. 2018. Neutralizing alpha-toxin accelerates healing of Staphylococcus aureus-infected wounds in nondiabetic and diabetic mice. Antimicrob Agents Chemother 62:e02288-17. doi: 10.1128/AAC.02288-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickett JE, Thompson JM, Sadowska A, Tkaczyk C, Sellman BR, Minola A, Corti D, Lanzavecchia A, Miller LS, Thorek DL. 2018. Molecularly specific detection of bacterial lipoteichoic acid for diagnosis of prosthetic joint infection of the bone. Bone Res 6:13. doi: 10.1038/s41413-018-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero Pastrana F, Thompson JM, Heuker M, Hoekstra H, Dillen CA, Ortines RV, Ashbaugh AG, Pickett JE, Linssen MD, Bernthal NM, Francis KP, Buist G, van Oosten M, van Dam GM, Thorek DLJ, Miller LS, van Dijl JM. 2018. Noninvasive optical and nuclear imaging of Staphylococcus-specific infection with a human monoclonal antibody-based probe. Virulence 9:262–272. doi: 10.1080/21505594.2017.1403004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson JM, Saini V, Ashbaugh AG, Miller RJ, Ordonez AA, Ortines RV, Wang Y, Sterling RS, Jain SK, Miller LS. 2017. Oral-only linezolid-rifampin is highly effective compared with other antibiotics for periprosthetic joint infection: study of a mouse model. J Bone Joint Surg Am 99:656–665. doi: 10.2106/JBJS.16.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plaut RD, Mocca CP, Prabhakara R, Merkel TJ, Stibitz S. 2013. Stably luminescent Staphylococcus aureus clinical strains for use in bioluminescent imaging. PLoS One 8:e59232. doi: 10.1371/journal.pone.0059232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyes N, Skinner R, Benton BM, Krause KM, Shelton J, Obedencio GP, Hegde SS. 2006. Efficacy of telavancin in a murine model of bacteraemia induced by methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 58:462–465. doi: 10.1093/jac/dkl222. [DOI] [PubMed] [Google Scholar]
- 29.Benjamini Y, Krieger AM, Yekutieli D. 2006. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93:491–507. doi: 10.1093/biomet/93.3.491. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.