The Partners Demonstration Project was a prospective, open-label, implementation science-driven study of preexposure prophylaxis (PrEP) among heterosexual HIV serodiscordant couples in Kenya and Uganda. Adherence data were collected using the Medication Event Monitoring System (MEMS), and time of sexual activity was collected using the mobile phone short message service (SMS). Two plasma samples were collected at a single study visit.
KEYWORDS: PrEP, population pharmacokinetics, preexposure prophylaxis, tenofovir
ABSTRACT
The Partners Demonstration Project was a prospective, open-label, implementation science-driven study of preexposure prophylaxis (PrEP) among heterosexual HIV serodiscordant couples in Kenya and Uganda. Adherence data were collected using the Medication Event Monitoring System (MEMS), and time of sexual activity was collected using the mobile phone short message service (SMS). Two plasma samples were collected at a single study visit. We integrated adherence, pharmacokinetics, and SMS data using a population pharmacokinetic (PopPK) model to simulate tenofovir plasma concentrations from PrEP at the time of sexual activity. In the first stage of this analysis, we used data from the current study to update a prior PopPK model of tenofovir (TFV) developed with data from the Partners PrEP Study (a phase III clinical trial). The second stage involved simulating plasma concentrations at the time of sexual activity using empirical Bayes estimates (EBEs) derived from the final model. In addition, EBEs from a previously published parent metabolite model of TFV (MTN-001, an open-label 3-way crossover study in healthy women) was used to simulate tenofovir diphosphate (TFV-DP) concentrations. We estimated percent PrEP “coverage” as the number of reported sexual events during which simulated concentrations were above an a priori threshold concentrations associated with a high degree of protection from HIV infection: plasma TFV of >40 ng/ml and peripheral blood mononuclear cell (PBMC) TFV-DP concentration of >36 fmol/million cells. The levels of coverage were 72% for TFV and 81% for TFV-DP. These levels are consistent with a high degree of protection against HIV acquisition in this study of a pragmatic delivery model for antiretroviral-based HIV prevention.
INTRODUCTION
Tenofovir disoproxil fumarate (TDF) in combination with emtricitabine (FTC) was approved by the U.S. Food and Drug Administration in 2012 for the indication of preexposure prophylaxis (PrEP) of HIV in high-risk adults (1). Several clinical trials provided evidence for the efficacy of regimens including daily TDF-FTC for PrEP with a relative risk reduction ranging from 44 to 75% (2–4). Building on this evidence base, several demonstration projects (∼70 globally) are at different stages of completion that are aimed at local implementation of PrEP using TDF-FTC (5).
Medication adherence has been a major determinant of PrEP success. Efficacy of TDF-based PrEP was highly correlated with adherence to the medication (6, 7). The dose-response relationship, as interpreted by combining several PrEP clinical trials, demonstrated that higher concentrations of tenofovir (TFV) provided increased PrEP efficacy and that variability in drug concentrations was best attributed to adherence (8). A clinical trial simulation demonstrated that adherence patterns play a major role in PrEP protection and explained the divergent efficacy outcomes in various randomized clinical trials of PrEP (9). Moreover, because PrEP is a preventative medication, adherence should align with risk for HIV infection to provide optimal effectiveness—a concept called prevention-effective adherence (10). Thus, in addition to simply taking a pill, a complex interplay of medication adherence patterns, sexual activity patterns, and other risk behaviors such as unprotected sex may influence PrEP success in an individual (10).
TDF is a prodrug of TFV, which gets rapidly hydrolyzed by plasma esterases to form TFV, which enters the peripheral blood mononuclear cells (PBMC), where it is doubly phosphorylated to form the active moiety, tenofovir diphosphate (TFV-DP). The plasma half-life of TFV and intracellular half-life of TFV-DP are ∼17 and ∼60 h, respectively (11). “Forgiveness” of a drug regimen to adherence means that a drug continues to provide therapeutic benefits even if adherence is suboptimal (12).
The present analysis includes detailed adherence data collected through electronic monitoring, self-reported sexual activity data, and plasma TFV drug concentrations. Our objective is to update prior population pharmacokinetic (PopPK) model and interpret the plasma TFV concentration-based PrEP coverage by integrating adherence data, sexual activity (i.e., HIV risk), and pharmacokinetic data in a PopPK model framework. We estimated percent PrEP “coverage” as the number of reported sexual events during which simulated concentrations of TFV from PrEP were above an a priori threshold concentrations associated with a high degree of protection from HIV infection.
RESULTS
Summary of the final data used for modeling and simulation.
A total of 565 participants (404 from the Partners PrEP Study and 161 from the Partners Demonstration Project) were included in the present analysis. A total of 1,592 TFV plasma concentration data points were used in the model development. A summary of demographic characteristics is shown in Table 1. A mean of 47 (standard deviation [SD], 29) mobile phone short message service (SMS) surveys were completed per participant over a mean of 4.6 (SD, 2.2) 14-day reporting windows, reflecting a mean of 9.8 (SD, 6.0) months of study participation.
TABLE 1.
Participants’ demographic data
| Demographic variable | Value for: |
|
|---|---|---|
| The Partners PrEP Study | The Partners Demonstration Project | |
| No. of participants | 404 | 161 |
| No. of TFV concentrations | 1,276 | 316 |
| Sex, no. | ||
| Male | 224 | 114 |
| Female | 180 | 47 |
| Creatinine clearance, ml/min (mean ± SD) | 106 ± 31 | 121 ± 28 |
Population pharmacokinetic model.
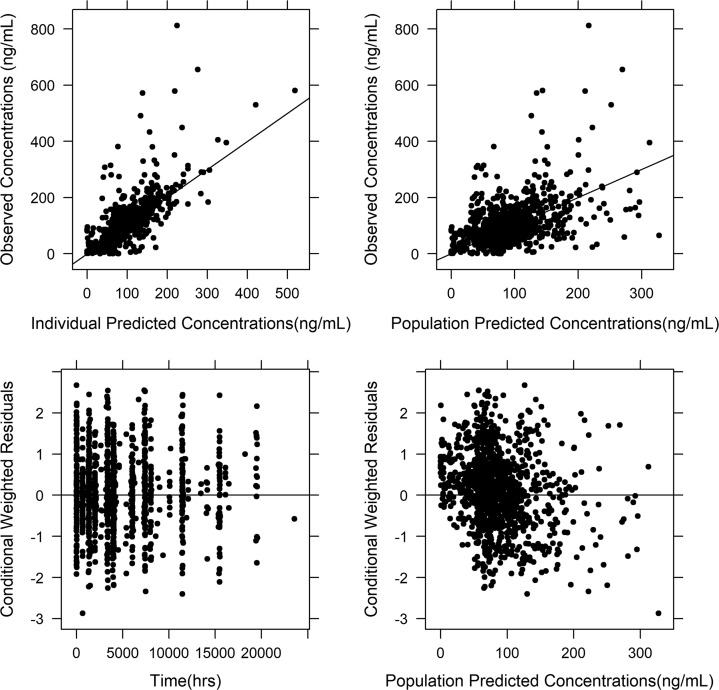
The updated model was similar to our previous model (13). The model was a two-compartment model with first-order absorption and elimination with absorption lag time (13). The structural model is shown in Fig. 1. The parameter estimates and their relative standard errors (RSEs) in the final model are shown in Table 2. Diagnostic plots of the updated model are shown in Fig. 2, which did not show any major bias.
FIG 1.
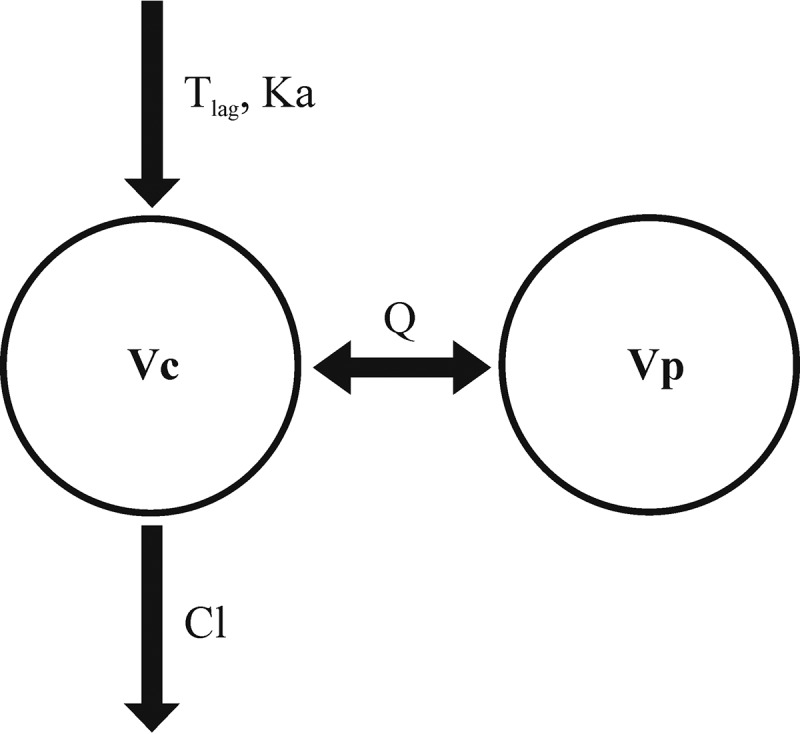
Two-compartment PopPK model of tenofovir with first-order absorption and lag time (Tlag). CL, Vc, Vp, and Q, clearance, volume of central compartment, volume of peripheral compartment, and intercompartmental clearance, respectively.
TABLE 2.
Population pharmacokinetic parameters of tenofovir from final modela
| Parameter | Estimate (RSE%) | Bootstrap median (95% CI) |
|---|---|---|
| ΘCL (liters/h) | 47.8 (2.5) | 47 (43.4–49.7) |
| ΘVC (liters) | 214 (16) | 211 (179–381) |
| ΘCRCL | 0.15 (32) | 0.16 (0.15–0.18) |
| ΘKa (liters/h) | 1.7 (15) | 1.5 (0.85–2.02) |
| ΘQ (liters/h) | 300 (21) | 289 (245–485) |
| ΘVP (liters) | 512 (7) | 490 (374–557) |
| ΘALAG1 (h) | 0.69 (15) | 0.69 (0.5–0.8) |
| IIV on CL | 22.6 (15) | 22.4 (16.3–26.5) |
| IIV on Vc | 76 (22) | 81.7 (51.8–88.8) |
| IIV on Ka | 79 (12) | 80 (45–89) |
| IIV on F1 | 10 (fixed) | 10 (fixed) |
| IIV on additive (ng/ml) | 150 (14) | 127 (87–160) |
| Residual variability | ||
| Additive error 1 (ng/ml) | 19 (13) | 15.8 (4.7–16.3) |
| Additive error 2 (ng/ml) | 0.14 (27) | 0.16 (0.15–0.18) |
| Proportional error (% CV) | 21 (7) | 21 (19.5–27.9) |
ΘCL, clearance; ΘVC, volume of central compartment; ΘCRCL, effect of CLCR on clearance; ΘKa, absorption rate constant; ΘALAG1, absorption lag time; IIV, interindividual variability; F1, bioavailability fraction in the model; CV, coefficient of variation. Additive error 1 is the additive error for the Partners PrEP Study; additive error 2 is the additive error for the Partners Demonstration Project.
FIG 2.
Basic goodness-of-fit plots for the developed population pharmacokinetic model. The graph at the top left represents individual predicted and observed concentrations, and the graph at the top right represents population predicted concentrations and observed concentrations. The graph at the bottom left represents time versus conditional weighted residuals, and the graph at the bottom right represents population predicted concentrations versus conditional weighted residuals. None of these plots showed any major bias in the model, suggesting that the developed model is acceptable.
Model qualification.
Updated parameter estimates of the final model were comparable to those in the prior report (13). The median value of all parameters generated by bootstrap generally fell within the 95% confidence interval (range, 2.5th to 97.5th percentiles). The bootstrap results are shown in Table 2. A visual predictive check of the final model showed good agreement between observed concentrations and the 90% prediction intervals (range, 5th and 95th percentiles). A plot of the visual predictive check is shown in Fig. 3.
FIG 3.
Visual predictive check. The plot illustrates observed concentrations and prediction intervals of VPC. Dashed lines show the 90% prediction interval. The solid line represents median of the predictions. The dots represent the observed concentrations.
Simulation of TFV and TFV-DP concentrations at the time of potential HIV exposure and assessment of PrEP coverage.
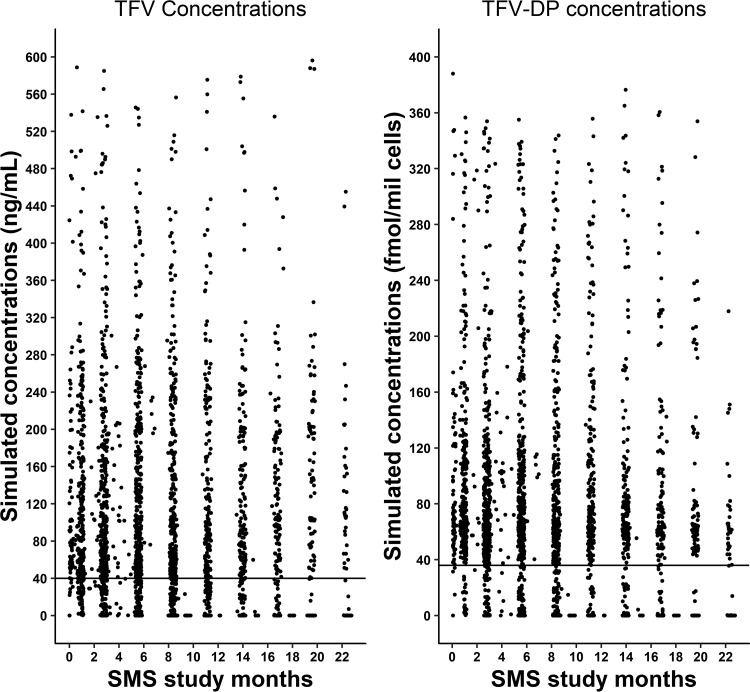
A total of 2,692 time points of sexual activity within the serodiscordant couples as indicated were reported in the SMS surveys during the study. Simulations using the PopPK model and Medication Event Monitoring System (MEMS) events were performed to predict the concentrations of TFV and TFV-DP at these time points. The results showed that 72% of all SMS-reported sexual activity times were above the a priori threshold value (>40 ng/ml) for TFV, and 81% of the reports were above the a priori threshold value for TFV-DP. The predicted percent coverage for the 2 days prior to the time of the SMS reports was similar to that for the day of the SMS survey for both TFV and TFV-DP. The predicted percentage coverage based on plasma TFV concentrations was higher (73%) for visit periods in the high-risk category than for the very-low-risk category (54%). TFV-DP had higher coverage (82%) in the high-risk group than did TFV. The percentages for coverage with TFV and TFV-DP are shown in Table 3, and the simulated TFV and TFV-DP concentrations during the SMS study windows are shown in Fig. 4. When these simulated concentrations were evaluated for longitudinal trends during the entire study period (i.e., 24 months), it was observed that the coverage of TFV was 85% during the initial SMS survey period, which gradually dropped to 53% during the last SMS survey period.
TABLE 3.
Coverage based on HIV risk acquisition category for TFV and TFV-DPa
| Risk of HIV acquisition | No. of SMS reports | % of reports above threshold (no./total) |
|
|---|---|---|---|
| TFV | TFV-DP | ||
| High | 640 | 73 (467/640) | 82 (523/640) |
| Low | 1,835 | 74 (1,351/1,835) | 84 (1,531/1,835) |
| Very low | 217 | 54 (117/217) | 60 (130/217) |
| Total | 2,692 | 72 (1,935/2,692) | 81 (2,184/2,692) |
Shown is the number of instances of SMS reports which were above the a priori threshold for TFV and TFV-DP concentrations. Further, the SMS reports were classified as high, low, and very low risk based on the risk stratification of the corresponding periods. A period is classified as high risk if the participant reported <100% condom use and the HIV-infected partner was not on ART for at least 6 months. A period is considered low risk if the partner was on ART for 6 months or more and participant reported 100% condom use. A period is considered very low risk if the participants reported no sex with the study partner.
FIG 4.
Simulated TFV and TFV-DP concentrations during SMS study periods. The plots show the TFV and TFV-DP concentrations above and below a priori threshold values (represented as solid lines parallel to the x axis) during SMS study windows. The thresholds were 40 ng/ml for TFV and 36 fmol/million cells for TFV-DP.
Effect of adherence and renal clearance on observing BQL concentrations.
The relationship between presence or absence of below-quantification-level (BQL) concentrations to normalized nontherapeutic time (NTT) and creatinine clearance (CLCR) is shown in Fig. 5. Participants with BQL concentrations had significantly higher (P < 0.001) median normalized NTT values than participants without any BQL samples. There was no difference observed in CLCR between these two groups. This finding indicates that nonadherence is the likely reason for observing BQL concentrations in the Partners Demonstration Project participants.
FIG 5.
Correlation of BQL versus normalized NTT and CLCR. The graph on the left shows the correlation between BQL and normalized NTT. The plot showed that the median normalized NTT value in the group with BQL concentrations (1) was significantly higher (P < 0.001) than for the group without BQL concentrations (0). The graph on the right shows the relation between BQL concentration and CLCR. The plot showed that difference in median values of CLCR is not significant (P = 0.316) between the group with BQL concentrations (1) and the group without BQL concentrations (0).
DISCUSSION
This analysis is the first to integrate data from adherence, pharmacokinetics, and time of sexual activity in a pharmacometric model framework to predict TFV/TFV-DP concentrations to interpret the PrEP coverage. Overall, the coverage of TFV was 72%, indicating that the vast majority of reported times of any sexual activity were covered by PrEP. The degree of coverage is consistent with the low HIV incidence (0.2 per 100 person years) reported in the Partners Demonstration Project (14). The percent coverage of TFV was higher (73% to 74%) during visit periods categorized as high and low risk than for very-low-risk periods (54%). The primary analysis of the Partners Demonstration Project reported that 88%, 83%, and 62% of the participant-visits achieved sufficient adherence, defined as ≥4 doses per week in high-risk, low-risk, and very-low-risk categories, respectively (14). These numbers were reduced to 75%, 69%, and 49% when the definition of sufficient adherence was defined as ≥6 doses per week (14). Notably, in both analyses, when the participants perceived a higher level of risk, they might have had better adherence than the periods in which they perceived a lower risk of HIV acquisition from their partners. One of the limitations in the previous analysis, however, was lack of consideration of tenofovir concentrations and the pharmacokinetic variability. The current analysis addressed that issue by accounting for between-subject and within-subject variability in the PopPK parameters, and coverage is calculated based on an individual’s concentrations at the time of sexual activity reports, and thus these estimates are more conservative.
The target a priori threshold concentrations of TFV in plasma and TFV-DP in PBMC for protection from HIV infection were based on prior analyses reported in clinical trials of PrEP (15, 16). Similar concentrations were seen with previously reported steady-state trough concentrations of TFV (31.2 to 64.4 ng/ml) in intensively sampled pharmacokinetic studies in which TDF doses of 300 mg were administered for 7 days and in the 5-week HPTN 066 study employing directly observed daily dosing (median trough concentration [Ctau], 52.2 ng/ml) (17, 18). In a meta-analysis of typical efficacy estimates from PrEP trials to derive the dose-response curve, the 90% effective concentration (EC90) associated with HIV protection was 105 to 110 ng/ml (9). However, EC90 was representative of the average concentration (Cave) rather than a steady-state trough in this meta-analysis, and the authors acknowledged that a time component should be included in a pharmacometric model framework for better interpretation. A recent comprehensive metapharmacometric analysis of data from the iPrEx, VOICE, and Partners PrEP trials proposed a target steady-state trough TFV level of 45.4 ng/ml (95% confidence interval, 30.8 to 59.9 ng/ml), which was associated with a 90% decrease in HIV-1 infection and agrees well with prior reports (19). The steady-state TFV trough concentrations in the range of 49 to 55.6 ng/ml in the serum were associated with TFV-DP concentrations of 29 to 38.5 fmol/106 cells of PBMC after 300-mg doses of TDF for 5 weeks (16). Thus, our target concentration thresholds were well in agreement with various previous reports. However, the targets used for plasma TFV and TFV-DP in our analysis are conservative, and thus, our coverage estimates are conservative as well. The threshold concentrations used in our analysis for TFV (40 ng/ml) and TFV-DP (36 fmol/million cells) are higher than the optimized cutoff concentrations with >90% sensitivity for TFV (35.5 ng/ml) and TFV-DP (16.8 fmol/million cells) as seen in the once-daily-dosing arm of HPTN 066 (16). In a sensitivity analysis conducted using TFV and TFV-DP optimized cutoff concentrations from HPTN 066 as thresholds, the coverage increased to 75% using TFV and 86% using TFV-DP in this population.
Several concentrations and dosing records were deemed implausible with the documented MEMS records and unlikely to add value to our efforts to update the prior PopPK model. The initial models including such observations led to highly biased residual plots which were deemed unacceptable. Therefore, these concentrations were dropped from the current analysis. Data from such representative participants are presented here. Consider a participant who had a regular dosing schedule as per the MEMS record, but the TFV level was below the lower limit of quantitation (LLOQ; 0.31 ng/ml) at 14 h postdose. This scenario might be because the participant may have opened the MEMS container without dosing in order to appear more adherent than he/she truly was. In a study employing directly observed therapy (DOT) to establish PK benchmarks, the steady-state trough was above the LLOQ of 0.31 ng/ml even with only 1 tablet/week of 300 mg of TDF (16). Thus, it is very unlikely that the MEMS dosing record in this patient represented dose-taking events. In another participant, the TFV level was 321 ng/ml at 76 h postdose (per MEMS), which is unlikely given that such a high concentration is a typical maximum concentration (Cmax) for TFV. For another participant, there was no dosing record for 3 months, but the observed TFV concentration was 48.9 ng/ml, which is close to the typical trough value associated with daily dosing. In another participant with regular adherence as per MEMS record, the observed TFV concentration was 2.24 ng/ml after 14.5 h, which is considered too low for the time postdose and would be reached at least 4 or more days following the last dose. Pharmacokinetic variability of TFV is moderate, as shown in a study with a DOT approach with intersubject and intrasubject variability of only 33% and 17%, respectively (16). A similar approach of dropping unreliable MEMS dosing records and associated concentrations was recommended in a population pharmacokinetic analysis of atazanavir (20). A systematic simulation study evaluated various approaches to handle nonadherence (dose omissions) in the PopPK analysis. Identifying and excluding nonadherent patients led to a reduction in parameter bias in that study. The parameter bias of the PopPK model reduced with the stepwise increase in removal of nonadherent participants until a majority of them (up to 50%) were dropped from the analysis (21).
Our final model parameter estimates agree well with prior models and trials that have explicitly addressed the issue of potential dosing errors due to nonadherence (22–24). A notable difference was that the clearance parameter in this analysis was 22% lower (48 liters/h versus 61 liters/h) than in our prior report using only the Partners PrEP Study data (13). This difference could be due to the additional adherence correction in the overall data set by Gibiansky’s method (using bioavailability correction) implemented in modeling (21). We observed similar effects on parameters in a sensitivity analysis during the development of our prior model. Excluding the BQL data in that analysis in our prior modeling effort resulted in a mean clearance estimate of 53.7 to 54.2 liters/h. Several of the BQL samples in the Partners PrEP Study were within 24 h of time postdose calculated from MEMS records. Those records were potentially dosing errors; however, given the study design of one PK sample per study visit, we could not implement the relative bioavailability correction. In addition, the current estimate agrees well with the historical data (phase 1 to 2) using rich PK sampling and urine collection reporting clearance between 32 and 57 liters/h (18).
In the current analysis, when the coverage was assessed across various SMS study windows from the enrollment until the completion of follow-up, a longitudinal trend of decline in coverage was observed. This observation was in line with the findings from the primary analysis regarding covariates that influence adherence (14). Follow-up of more than 6 months of participation in the study was negatively associated with an average adherence of ≥4 doses/week (relative risk, 0.94; P = 0.003). The partner living with HIV should have been on antiretroviral therapy (ART) long enough for viral suppression at that point, so adherence may have declined just prior to discontinuation. However, there may have been concerns about outside sexual partners or poor ART adherence of partners, leading some to continue PrEP regimen. At the individual level, some participants had concentrations of TFV below the a priori threshold at all SMS survey periods, although this was observed more during the latter part of the study.
There were several limitations to the current study. The individual pharmacokinetic parameter estimates had relatively high shrinkage given that we only had two samples per participant. Shrinkage is a phenomenon in which subject-specific estimates tend to shrink toward an appropriate population mean due to lack of information at the individual level (25, 26). Shrinkage can adversely affect the usefulness of the model for estimating individual parameters. We thus recommend using more informative sampling in PrEP clinical studies to improve the efficiency of modeling efforts. In addition to measurement error with MEMS and PK levels, SMS surveys may have also suffered from errors introduced by recollection or social desirability bias. Musinguzi et al. reported that SMS reports have a poor correlation to other objective measures of adherence, suggesting this as a possibility (27). To account for the possible uncertainty in SMS reports due to potential recollection bias on sexual activity times, we simulated the individual concentrations 24 h and 48 h prior in addition to reported SMS times. These simulated concentrations and predicted percentage coverage prior to the actual reported times were similar to the time of report, ruling out any fluctuations in coverage in this time window. The criterion of a TFV concentration of >40 ng/ml may make a participant look adherent even if a single dose is taken prior to the sexual reported time. However, the above-described sensitivity analysis shows that such participant scenarios are rare, given the same levels of coverage observed 24 and 48 h prior to reports of sexual activity. In contrast, TFV-DP concentrations are less dependent on daily fluctuations in adherence. Our analysis excluded several observed BQL and non-BQL values based on pharmacokinetic sensibility between the dosing records and associated concentrations. In case of participants with BQL concentrations, there was a correlation observed to nonadherence pattern (NTT) but not to creatinine clearance, implying cases of nonadherence. These participants also did not have dosing records prior to the sampling day for a considerable length of time. These cases likely represent participants with the lowest level of adherence that could result in downward bias of the coverage estimates. In the case of participants excluded with non-BQL concentrations, we may not be able to generalize their adherence status, as the time postdose was not deemed reasonable. Thus, our coverage may not represent the participants with the lowest level of adherence in the study. It is also important to recognize that there is potential for elevated adherence (“white coat adherence”) induced by the study procedures such as SMS surveys and clinic visits compared to that of the nonstudy population. The measured plasma concentration values and the time of sample collection are the most objective data points in this study. In order to put a context to a given drug concentration, we need to know the time postdose of that concentration, which comes from the information on the most recent dose taken prior to that concentration. Any uncertainty in the dosing information (adherence data) makes the conclusions based on concentration less reliable. However, our current analysis framework provides an efficient way to integrate and interpret various sources of data necessary to improve individual patient outcomes. If methods could be developed to avoid potential biases in adherence and SMS survey data, it might be possible to achieve the full benefits of therapeutic drug monitoring in the clinical setting with the implementation of this framework.
Conclusion.
This analysis estimated the plasma concentrations of tenofovir likely present at the time of potential HIV exposure among individuals participating in the Partners Demonstration Project to interpret PrEP coverage. Predicted TFV concentrations were above the a priori threshold in 72% of instances and TFV-DP concentrations in 82% of instances of potential HIV risk. The estimated drug concentrations of TFV and TFV-DP are adequate for protection in the majority of PrEP users who are generally adherent to medication in a near-real-world context. It is consistent with the low level of observed HIV transmission in that study and bodes well for the rollout of PrEP globally.
MATERIALS AND METHODS
Study design.
The Partners Demonstration Project was a prospective, open-label, implementation science-driven study of PrEP among heterosexual HIV serodiscordant couples in Kenya and Uganda. For full details on the study procedures, please refer to the primary publications on the results of the study (14, 28, 29). Briefly, serodiscordant couples were recruited for the study and followed for up to 24 months. The HIV-uninfected partner of the couple was offered PrEP until the partner living with HIV could take antiretroviral therapy (ART) for at least 6 months (i.e., the time anticipated for viral suppression). Medication was provided in a MEMS container (AARDEX Group, Switzerland), which records a time-and-date stamp for each container opening as a proxy for medication ingestion.
Within this project, a substudy assessed PrEP adherence and sexual behavior with mobile phone-based short message service (SMS) surveys (30). Participants consisted of a convenience sample who owned mobile phones and were able to charge them reliably. Substudy participants were sent SMS surveys for 7 days before and 7 days after each study visit during PrEP use. These SMS surveys were sent at participant-chosen times and were available to be responded to within 23 h after delivery. The surveys were initiated with an authentication step of entering a 4-digit password by participants. These surveys consisted of 7 questions, and participants were incentivized with US $0.50 of airtime for each completed survey. Additionally, during one of the routinely scheduled follow-up visits, two plasma samples were collected from each substudy participant at least 2 h apart. Participants knew these samples would be collected, but they were not informed as to the specific visit at which they would be collected to prevent “white coat” dosing (i.e., adherence behavior linked to known monitoring) (31). TFV levels were estimated by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) as reported elsewhere (32). The lower limit of quantitation (LLOQ) was 0.31 ng/ml.
Data set preparation.
The data set for modeling was prepared by integrating MEMS data on adherence, TFV concentration data, and SMS data on date and time of reported sexual activity. Careful inspection of the data set revealed issues in concordance between adherence data and plasma concentration data points in the Partners Demonstration Project Data; data cleaning was therefore conducted based on pharmacokinetic sensibility. Specifically, a total of 136 concentrations representing 71 participants were dropped from the data set as a result of a data-cleaning exercise, including 70 below-quantification-level (BQL) concentrations and 66 non-BQL concentrations. The dropped non-BQL concentrations were either higher or lower than what is possible given the associated MEMS records. These types of data, where MEMS data and TFV concentrations are incongruent and biologically implausible, suggest a likely type 3 process error in which either the dosing event was not adequately captured by the MEMS device (e.g., doses were taken out of the bottle at one time but not ingested until much later) or an error in sample collection and management was made prior to assessment of concentration, both of which are noninformative regarding the underlying process we were trying to understand. These data would not add to the accuracy of the model and were therefore dropped from analysis. Additionally, all 70 BQL data were excluded. Of these, 58 had at least 2 weeks or longer of missing doses recorded by MEMS prior to blood sample collection day. Inclusion of these data would not add value to our efforts to update the prior PopPK model. For the remaining 12 BQL data, the MEMS records indicated daily dose taking before the sample collection with a resulting time postdose within 24 to 36 h, which was considered unreasonable. These MEMS data for these participants may not have reflected actual dosing events; rather, the participants might have opened the MEMS container without actually taking medications.
Population pharmacokinetic modeling and simulation.
The PopPK analysis was conducted using NONMEM (version 7.3), with the gfortran compiler interfaced with Perl-speaks-NONMEM (PsN) (33). Data formatting and plotting were carried out using software package R (version 3.4.2) (34).
Our approach was to update the PopPK model previously reported by our group (13) in the first stage using the Partners PrEP Study data together with the new data from the present Partners Demonstration Project. In the second stage, simulations of plasma concentrations were conducted at the time of sexual activity reported by the SMS survey using individual pharmacokinetic parameters estimated from the updated PopPK model. Our prior PopPK model was a two-compartment model parameterized with first order absorption rate constant (Ka) and absorption lag time (Alag), clearance (CL), central and peripheral volumes (Vc and Vp), and intercompartmental clearance (Q). Creatinine clearance was included as covariate on CL. Exponential error was used to model between-subject variability (BSV) on parameters. Residual error was modeled as combined additive and proportional error model. In the current analysis, we made two changes to the prior model. The first change was the inclusion of separate residual error for modeling data from the Partners Demonstration Project to account for the heterogeneity due to differences in trial design. Second, a random effect on the bioavailability parameter (F1) was included (Gibiansky’s correction) to model the Partners Demonstration Project data for adjusting potential errors in the dosing records (21).
Model evaluation.
A nonparametric bootstrap of 500 iterations was performed and a 95% confidence interval of the population parameters was estimated (35). A visual predictive check (VPC) was performed with 1,000 simulations using the prediction correction option. Prediction correction was performed by normalizing the observed and simulated plasma concentrations in a bin with typical population predictions (36). The observed TFV plasma concentrations, as well as the 5th, 50th, and 95th percentiles (90% prediction intervals), were plotted against the corresponding percentiles for the simulated values. The agreement in distribution between observed and predicted concentrations was then evaluated.
Simulation of TFV plasma concentration at the time of potential HIV exposure and assessment of PrEP coverage.
Participant study periods between clinic visits were categorized as high, low, and very low risk for HIV acquisition as described previously (14). SMS reports were also assigned to various risk categories corresponding to the periods in which they were received. Specifically, the risk of HIV acquisition in a period between visits was considered high if the couple reported sex with <100% condom use prior to 6 months’ duration of ART use by the infected study partner. The risk was considered low if the couple reported sex but did not meet the definition of high risk (i.e., they had 100% reported condom use and/or 6 months of ART use), and risk was considered very low if no sex was reported (regardless of ART use). The risk was not considered zero for the participants because sex could have potentially occurred outside the study partner (37).
TFV concentrations were simulated using empirical Bayesian estimates (EBEs) from the updated PopPK model and MEMS-based time of TDF dosing events. TFV concentrations were simulated for each participant at SMS-reported sexual activity times, as well as for 2 days prior to sexual activity as a reasonable means to account for potential uncertainty in SMS-reported sexual activity times. Concentration thresholds were derived indirectly based primarily on prior studies showing highest protection with daily dosing and, secondarily, concentrations typical of daily dosing. A TFV concentration of 40 ng/ml was considered an a priori threshold for protection against HIV transmission based on lower 95% confidence bounds of steady-state trough concentrations form several early treatment studies with TDF-containing regimens. Further, Donnell et al. reported the estimated protective effect of PrEP based on plasma TFV steady-state concentrations of >40 ng/ml as 88% for individuals receiving TDF alone and 91% for individuals receiving FTC/TDF in the Partners PrEP trial (15). This value was between the median (52 ng/ml) and the more inclusive 90% sensitivity threshold (∼35.5 ng/ml) observed in a study (HPTN 066) which employed direct observation of dosing procedure to establish adherence benchmarks (16). The percentage of time points with SMS-reported sexual activity above this threshold was calculated for high-, low-, and very-low-risk study visit periods. PrEP coverage of a reported sexual event was calculated as the percentage of such events associated with a simulated plasma TFV concentration of >40 ng/ml.
Simulation of TFV-DP levels in PBMC at the time of potential HIV exposure and assessment of PrEP coverage.
TFV-DP, an active metabolite of TFV, was also of interest because of its longer half-life compared to that of TFV. Concentrations of TFV-DP in peripheral blood mononuclear cells (PBMC) were simulated using the EBEs generated based on the model reported by Burns et al. (22). The a priori threshold for protection was 36 fmol/million cells for TFV-DP (16). The percentage of time points with SMS-reported sexual activity above this threshold was then calculated within high-, low-, and very-low-risk study visit periods. PrEP coverage of a reported sexual events was calculated as the percentage of such events associated with a simulated PBMC TFV-DP concentration of >36 fmol/million cells.
Effects of adherence and renal clearance on observing BQL samples.
A subanalysis was carried out to understand the potential differences between measures of adherence and renal clearance in study participants in whom concentrations were BQL versus participants in whom no concentrations were BQL. Nontherapeutic time (NTT; in days) is a global adherence pattern indicator calculated as the sum of cumulative days of sequentially missed doses calculated from MEMS data and was used as a measure of adherence (17). NTT was normalized by the individual participant’s duration of study participation for comparison of participants with different durations of study participation. Creatinine clearance calculated using Cockroft-Gault method was used as a measure of renal clearance. A t test was used, and a P value of <0.05 was set a priori to conclude statistical significance.
ACKNOWLEDGMENTS
We thank the couples who participated in this study for their motivation and dedication and the referral partners, community advisory groups, institutions, and communities that supported this work.
Partners Demonstration Project Team Coordinating Center (University of Washington) and collaborating investigators (Harvard Medical School, Johns Hopkins University, Massachusetts General Hospital): Jared Baeten (protocol chair), Connie Celum (protocol co-chair), Renee Heffron (project director), Deborah Donnell (statistician), Ruanne Barnabas, Jessica Haberer, Harald Haugen, Craig Hendrix, Lara Kidoguchi, Mark Marzinke, Susan Morrison, Jennifer Morton, Norma Ware, and Monique Wyatt.
Partners Demonstration Project Team project sites: Kabwohe Clinical Research Centre (Kabwohe, Uganda), Stephen Asiimwe and Edna Tindimwebwa; Makerere University (Kampala, Uganda), Elly Katabira and Nulu Bulya; Kenya Medical Research Institute (Kisumu, Kenya), Elizabeth Bukusi and Josephine Odoyo; Kenya Medical Research Institute, University of Washington (Thika, Kenya), Nelly Rwamba Mugo and Kenneth Ngure.
Partners Demonstration Project Team data management: DF/Net Research.
The Partners Demonstration Project was funded by the Bill and Melinda Gates Foundation (OPP1056051), the National Institute of Mental Health of the U.S. National Institutes of Health (R01MH095507 and R01MH098744), and the U.S. Agency for International Development (AID-OAA-A-12-00023); the study was also supported by the University of Washington/Fred Hutch Center for AIDS Research (P30 AI027757) and the Johns Hopkins Center for AIDS Research (P30 AI094189), supported by NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK of the National Institutes of Health.
This work is made possible by the generous support of the American people through USAID; the contents are the responsibility of the authors and do not necessarily reflect the views of USAID, NIH, or the United States government.
PrEP medication was donated by Gilead Sciences.
Contributor Information
Stephen Asiimwe, Kabwohe Clinical Research Centre (Kabwohe, Uganda).
Edna Tindimwebwa, Kabwohe Clinical Research Centre (Kabwohe, Uganda).
Elly Katabira, Makerere University (Kampala, Uganda).
Nulu Bulya, Makerere University (Kampala, Uganda).
Elizabeth Bukusi, Kenya Medical Research Institute (Kisumu, Kenya).
Josephine Odoyo, Kenya Medical Research Institute (Kisumu, Kenya).
Nelly Rwamba Mugo, Kenya Medical Research Institute, University of Washington (Thika, Kenya).
Kenneth Ngure, Kenya Medical Research Institute, University of Washington (Thika, Kenya).
Collaborators: Jared Baeten, Connie Celum, Renee Heffron, Deborah Donnell, Ruanne Barnabas, Jessica Haberer, Harald Haugen, Craig Hendrix, Lara Kidoguchi, Mark Marzinke, Susan Morrison, Jennifer Morton, Norma Ware, Monique Wyatt, Stephen Asiimwe, Edna Tindimwebwa, Elly Katabira, Nulu Bulya, Elizabeth Bukusi, Josephine Odoyo, Nelly Rwamba Mugo, and Kenneth Ngure
REFERENCES
- 1.US Department of Health and Human Services. 2012. FDA approves first drug for reducing the risk of sexually acquired HIV infection. https://aidsinfo.nih.gov/news/1254/fda-approves-first-drug-for-reducing-the-risk-of-sexually-acquired-hiv-infection.
- 2.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT. 2012. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Advocacy for HIV Prevention. 2017. Ongoing and planned PrEP demonstration and implementation studies. https://www.avac.org/resource/ongoing-and-planned-prep-demonstration-and-implementation-studies. Accessed 7 May 2018.
- 6.Vrijens B, Urquhart J. 2014. Methods for measuring, enhancing, and accounting for medication adherence in clinical trials. Clin Pharmacol Ther 95:617–626. doi: 10.1038/clpt.2014.59. [DOI] [PubMed] [Google Scholar]
- 7.Amico KR, Stirratt MJ. 2014. Adherence to preexposure prophylaxis: current, emerging, and anticipated bases of evidence. Clin Infect Dis 59(Suppl 1):S55–S60. doi: 10.1093/cid/ciu266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haberer JE, Bangsberg DR, Baeten JM, Curran K, Koechlin F, Amico KR, Anderson P, Mugo N, Venter F, Goicochea P, Caceres C, O’Reilly K. 2015. Defining success with HIV pre-exposure prophylaxis: a prevention-effective adherence paradigm. AIDS 29:1277–1285. doi: 10.1097/QAD.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrix CW. 2013. Exploring concentration response in HIV pre-exposure prophylaxis to optimize clinical care and trial design. Cell 155:515–518. doi: 10.1016/j.cell.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrov DT, Masse BR, Donnell D. 2016. PrEP adherence patterns strongly affect individual HIV risk and observed efficacy in randomized clinical trials. J Acquir Immune Defic Syndr 72:444–451. doi: 10.1097/QAI.0000000000000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kearney BP, Flaherty JF, Shah J. 2004. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 43:595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 12.Shuter J. 2008. Forgiveness of non-adherence to HIV-1 antiretroviral therapy. J Antimicrob Chemother 61:769–773. doi: 10.1093/jac/dkn020. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y, Goti V, Chaturvedula A, Haberer JE, Fossler MJ, Sale ME, Bangsberg D, Baeten JM, Celum CL, Hendrix CW. 2016. Population pharmacokinetics of tenofovir in HIV-1-uninfected members of serodiscordant couples and effect of dose reporting methods. Antimicrob Agents Chemother 60:5379–5386. doi: 10.1128/AAC.00559-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haberer JE, Kidoguchi L, Heffron R, Mugo N, Bukusi E, Katabira E, Asiimwe S, Thomas KK, Celum C, Baeten JM. 2017. Alignment of adherence and risk for HIV acquisition in a demonstration project of pre-exposure prophylaxis among HIV serodiscordant couples in Kenya and Uganda: a prospective analysis of prevention-effective adherence. J Int AIDS Soc 20:21842. doi: 10.7448/IAS.20.1.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, Mujugira A, Mugo N, Ndase P, Hendrix C, Celum C. 2014. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 66:340–348. doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrix CW, Andrade A, Bumpus NN, Kashuba AD, Marzinke MA, Moore A, Anderson PL, Bushman LR, Fuchs EJ, Wiggins I, Radebaugh C, Prince HA, Bakshi RP, Wang R, Richardson P, Shieh E, McKinstry L, Li X, Donnell D, Elharrar V, Mayer KH, Patterson KB. 2016. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 32:32–43. doi: 10.1089/AID.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girard P, Blaschke TF, Kastrissios H, Sheiner LB. 1998. A Markov mixed effect regression model for drug compliance. Stat Med 17:2313–2333. doi:. [DOI] [PubMed] [Google Scholar]
- 18.Barditch-Crovo P, Deeks SG, Collier A, Safrin S, Coakley DF, Miller M, Kearney BP, Coleman RL, Lamy PD, Kahn JO, McGowan I, Lietman PS. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother 45:2733–2739. doi: 10.1128/AAC.45.10.2733-2739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katarina V, Craig WH, Robert MG, Connie LC, Jared B, Jeanne M, Peter LA, David G, Radojka MS. 2018. Tenofovir HIV-1 plasma prophylactic concentration: iPrEx, VOICE, PARTNERS meta-analysis, abstr. Conference on Retroviruses and Opportunistic Infections, Boston, MA. [Google Scholar]
- 20.Savic RM, Barrail-Tran A, Duval X, Nembot G, Panhard X, Descamps D, Verstuyft C, Vrijens B, Taburet AM, Goujard C, Mentré F, ANRS 134–COPHAR 3 Study Group. 2012. Effect of adherence as measured by MEMS, ritonavir boosting, and CYP3A5 genotype on atazanavir pharmacokinetics in treatment-naive HIV-infected patients. Clin Pharmacol Ther 92:575–583. doi: 10.1038/clpt.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibiansky L, Gibiansky E, Cosson V, Frey N, Stark FS. 2014. Methods to detect non-compliance and reduce its impact on population PK parameter estimates. J Pharmacokinet Pharmacodyn 41:279–289. doi: 10.1007/s10928-014-9364-2. [DOI] [PubMed] [Google Scholar]
- 22.Burns RN, Hendrix CW, Chaturvedula A. 2015. Population pharmacokinetics of tenofovir and tenofovir-diphosphate in healthy women. J Clin Pharmacol 55:62. doi: 10.1002/jcph.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaturvedula A, Fossler MJ, Hendrix CW. 2014. Estimation of tenofovir’s population pharmacokinetic parameters without reliable dosing histories and application to tracing dosing history using simulation strategies. J Clin Pharmacol 54:150–160. doi: 10.1002/jcph.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baheti G, Kiser JJ, Havens PL, Fletcher CV. 2011. Plasma and intracellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrob Agents Chemother 55:5294–5299. doi: 10.1128/AAC.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savic RM, Karlsson MO. 2009. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. Aaps J 11:558–569. doi: 10.1208/s12248-009-9133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu XS, Yuan M, Karlsson MO, Dunne A, Nandy P, Vermeulen A. 2012. Shrinkage in nonlinear mixed-effects population models: quantification, influencing factors, and impact. AAPS J 14:927–936. doi: 10.1208/s12248-012-9407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musinguzi N, Muwonge T, Ngure K, Katabira E, Mugo N, Burns BFO, Baeten JM, Heffron R, Haberer JE, Partners Mobile Adherence to PrEP (PMAP) Team. 2018. Comparison of short messaging service self-reported adherence with other adherence measures in a demonstration project of HIV preexposure prophylaxis in Kenya and Uganda. AIDS 32:2237–2245. doi: 10.1097/QAD.0000000000001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heffron R, Ngure K, Odoyo J, Bulya N, Tindimwebwa E, Hong T, Kidoguchi L, Donnell D, Mugo NR, Bukusi EA, Katabira E, Asiimwe S, Morton J, Morrison S, Haugen H, Mujugira A, Haberer JE, Ware NC, Wyatt MA, Marzinke MA, Frenkel LM, Celum C, Baeten JM, Partners Demonstration Project Team. 2018. Pre-exposure prophylaxis for HIV-negative persons with partners living with HIV: uptake, use, and effectiveness in an open-label demonstration project in East Africa [version 2; referees: 2 approved]. Gates Open Res 1:3. doi: 10.12688/gatesopenres.12752.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baeten J, Heffron R, Kidoguchi L, Mugo NR, Katabira E, Bukusi EA, Asiimwe S, Haberer JE, Morton J, Ngure K, Bulya N, Odoyo J, Tindimwebwa E, Hendrix C, Marzinke MA, Ware NC, Wyatt MA, Morrison S, Haugen H, Mujugira A, Donnell D, Celum C, Partners Demonstration Project Team. 2016. Integrated delivery of antiretroviral treatment and pre-exposure prophylaxis to HIV-1-serodiscordant couples: a prospective implementation study in Kenya and Uganda. PLoS Med 13:e1002099. doi: 10.1371/journal.pmed.1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haberer JE, Ngure K, Muwonge T, Mugo N, Katabira E, Heffron R, Musinguzi N, Bangsberg DR, Celum C, Baeten JM, Partners Mobile Adherence to PrEP (PMAP) Team. 2017. Brief report: context matters: PrEP adherence is associated with sexual behavior among HIV serodiscordant couples in East Africa. J Acquir Immune Defic Syndr 76:488–492. doi: 10.1097/QAI.0000000000001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinstein AR. 1990. On white-coat effects and the electronic monitoring of compliance. Arch Intern Med 150:1377–1378. doi: 10.1001/archinte.1990.00390190043003. [DOI] [PubMed] [Google Scholar]
- 32.Keller MJ, Madan RP, Torres NM, Fazzari MJ, Cho S, Kalyoussef S, Shust G, Mesquita PM, Louissaint N, Chen J, Cohen HW, Diament EC, Lee AC, Soto-Torres L, Hendrix CW, Herold BC. 2011. A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS One 6:e16475. doi: 10.1371/journal.pone.0016475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindbom L, Ribbing J, Jonsson EN. 2004. Perl-speaks-NONMEM (PsN)–a Perl module for NONMEM related programming. Comput Methods Programs Biomed 75:85–94. doi: 10.1016/j.cmpb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 34.R Core Team. 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- 35.Parke J, Holford NH, Charles BG. 1999. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed 59:19–29. doi: 10.1016/S0169-2607(98)00098-4. [DOI] [PubMed] [Google Scholar]
- 36.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eshleman SH, Wilson EA, Zhang XC, Ou SS, Piwowar-Manning E, Eron JJ, McCauley M, Gamble T, Gallant JE, Hosseinipour MC, Kumarasamy N, Hakim JG, Kalonga B, Pilotto JH, Grinsztejn B, Godbole SV, Chotirosniramit N, Santos BR, Shava E, Mills LA, Panchia R, Mwelase N, Mayer KH, Chen YQ, Cohen MS, Fogel JM. 2017. Virologic outcomes in early antiretroviral treatment: HPTN 052. HIV Clin Trials 18:100–109. doi: 10.1080/15284336.2017.1311056. [DOI] [PMC free article] [PubMed] [Google Scholar]