Pseudomonas asiatica is a recently proposed species of the genus Pseudomonas. This study describes eight isolates of carbapenem-resistant P. asiatica harboring blaNDM-1 and blaVIM-2, genes encoding metallo-β-lactamase (MBL). These isolates were obtained from urine samples of patients hospitalized in Myanmar. These isolates were resistant to carbapenems but susceptible to colistin.
KEYWORDS: NDM-1 metallo-β-lactamase, Pseudomonas asiatica, VIM-2 metallo-β-lactamase
ABSTRACT
Pseudomonas asiatica is a recently proposed species of the genus Pseudomonas. This study describes eight isolates of carbapenem-resistant P. asiatica harboring blaNDM-1 and blaVIM-2, genes encoding metallo-β-lactamase (MBL). These isolates were obtained from urine samples of patients hospitalized in Myanmar. These isolates were resistant to carbapenems but susceptible to colistin. All eight isolates were positive for a carbapenemase inactivation method, CIMTrisII, and seven were positive on an immunochromatographic assay for NDM-type MBL. One isolate was highly resistant to aminoglycosides. Whole-genome sequencing showed that seven isolates harbored blaNDM-1 and one harbored blaVIM-2, with these genes located on the chromosome. One isolate harbored blaNDM-1 and rmtC, a gene encoding 16S rRNA methylase. Five types of genomic environments surrounding blaNDM-1 and blaVIM-2 were detected in these eight isolates, with four isolates having the same type. These data indicate that P. asiatica isolates harboring genes encoding carbapenemases, including blaNDM-1 and blaVIM-2, are spreading in medical settings in Myanmar.
INTRODUCTION
The emergence and spread of metallo-β-lactamase (MBL)-producing isolates of Pseudomonas species have become a serious problem in medical settings worldwide (1). MBLs, such as NDM-1 and VIM-2, confer resistance to all β-lactams, except monobactams, and are characterized by their efficient hydrolysis of carbapenems (2, 3).
Pseudomonas asiatica is a recently proposed species belonging to the Pseudomonas putida group and is located close to P. putida and Pseudomonas monteilii (4). P. asiatica isolates were obtained from patients hospitalized in Japan and Myanmar and thought to be a human pathogen (4).
The present study describes eight isolates of carbapenem-resistant P. asiatica producing NDM-1 and VIM-2 MBLs obtained from patients in Myanmar.
RESULTS
Bacterial surveillance.
During the surveillance, 152 isolates of multidrug-resistant (MDR) Pseudomonas species were obtained. Of them, 14 were identified as P. putida using Vitek 2. Bacterial identification analysis based on average nucleotide identity (ANI) and digital DNA-DNA hybridization (dDDH) values revealed that, of these 14 MDR isolates, eight were P. asiatica species, one was a P. monteilii species, one was a Pseudomonas mendocina species, one was a Stenotrophomonas maltophilia species, and three were unidentified Pseudomonas species. The eight MDR P. asiatica isolates were obtained from eight individual patients, including four patients admitted to hospital A (in the center of Yangon), one admitted to hospital B (in the northern part of Yangon), and three admitted to hospital C (Mandalay, located 700 km north of Yangon). Seven strains were isolated from urine samples and one from a urine sample containing urinary stones. Of the eight patients, five were men and three were women, ranging in age from 27 to 77 years (mean ± standard deviation [SD] age, 58.8 ± 14.0 years) (see Table S1 in the supplemental material). Other clinical information was not obtained.
Drug susceptibility testing and carbapenemase production.
All eight P. asiatica isolates tested were resistant to ampicillin-sulbactam, aztreonam, cefepime, ceftazidime, ciprofloxacin, imipenem, levofloxacin, and meropenem, with MICs of >16 μg/ml, but were susceptible to colistin, with MICs of ≤1 μg/ml (Table 1). Of these isolates, one, MY34, was highly resistant to all four aminoglycosides tested with MICs of >2,048 μg/ml, whereas the remaining seven isolates were resistant to one to three of four aminoglycosides tested (Table 1). All eight isolates were positive for the carbapenemase inactivation method, CIMTrisII. Seven were positive for an NDM immunochromatographic assay, but one, MY660, was negative.
TABLE 1.
Drug susceptibility profiles and drug-resistance genes of clinical isolates of P. asiatica
| Strain no. | Antimicrobial susceptibility (MIC in μg/ml)a
|
Genes or mutations associated with drug resistance |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABK | AMK | ATM | CAZ | CIP | CST | FEP | GEN | IPM | LVX | MEM | SAM | TOB | β-lactamase(s) | 16S rRNA methylase | Aminoglycoside acetyl-, adenylyl-, phospho-, and nucleotidyl-transferase | Mutation in DNA gyrase |
||
| GyrA | ParC | |||||||||||||||||
| MY34 | >2,048 | >2,048 | 2,048 | >2,048 | 1,024 | 1 | 2,048 | >2,048 | 32 | 512 | 1,024 | >2,048 | >2,048 | blaNDM-1, blaCTX-M-15, blaVEB-1, blaOXA-10, blaOXA-101 | rmtC | aac(6')-II, aadA1, aadB, aph(3′)-VI, aph(3′')-Ib, aph(6)-Id, ant(2'')-Ia | T83I | S87W |
| MY371 | 2 | 64 | 64 | >2,048 | 512 | 1 | 2,048 | 4 | 512 | 1,024 | >2,048 | >2,048 | 64 | blaNDM-1 | aph(3′')-Ib, aph(6)-Id, ant(4')-IIb | T83I | S87W | |
| MY545 | 2 | 128 | 128 | >2,048 | 512 | 0.25 | >2,048 | 4 | 256 | 1,024 | >2,048 | >2,048 | 64 | blaNDM-1 | aph(3′')-Ib, aph(6)-Id, ant(4')-IIb | T83I | S87W | |
| MY569 | 2 | 16 | 512 | >2,048 | 64 | 1 | 2,048 | 256 | 256 | 128 | 2,048 | >2,048 | 2 | blaNDM-1, blaPME-1 | aac(3)-IIa, aph(3′)-VI, aph(3′')-Ib, aph(6)-Id | T83I | S87L | |
| MY601 | 0.5 | 16 | 1,024 | >2,048 | 512 | 0.5 | >2,048 | 256 | 512 | 256 | 1,024 | >2,048 | 8 | blaNDM-1, blaCTX-M-15, blaTEM-1 | aph(3′')-Ib, aph(6)-Id, aac(3)-IId, ant(2'')-Ia, ant(4')-IIb | T83I | S87L | |
| MY660 | 8 | 32 | 64 | 32 | 256 | 1 | 32 | >2,048 | 64 | 256 | 64 | 1,024 | 128 | blaVIM-2 | aac(3)-Id, aac(6')-Il, aadA2, aadB, aph(3′')-Ib, aph(6)-Id | T83I | S87L | |
| MY680 | 2 | 32 | 64 | >2,048 | 1,024 | 1 | 2,048 | 256 | 512 | 2,048 | 2,048 | >2,048 | 32 | blaNDM-1 | aadA15, aadB, aph(3′)-Ia, aph(3′)-IIa, aph(3′')-Ib, aph(6)-Id, ant(4')-IIb | T83I | S87L | |
| MY756 | 2 | 256 | 256 | >2,048 | 256 | 1 | 512 | 256 | 256 | 256 | 1,024 | >2,048 | 512 | blaNDM-1, blaPME-1 | ant(4')-IIb | T83I | S87L | |
CAZ, ceftazidime; FEP, cefepime; ATM, aztreonam; IPM, imipenem; MEM, meropenem; SAM, ampicillin-sulbactam; AMK, amikacin; ABK, arbekacin; GEN, gentamicin; CIP, ciprofloxacin; LVX, levofloxacin; CST, colistin; TOB, tobramycin.
Detection of antimicrobial resistance genes.
Eight types of β-lactamase-encoding genes were detected in the eight P. asiatica isolates (Table 1). Of these, two genes encoded MBL, whereas the remaining six genes encoded extended-spectrum β-lactamase (ESBL). The ESBL-encoding genes were detected in four of the eight isolates (Table 1), whereas a gene encoding 16S rRNA methylase, rmtC, was detected in one isolate, MY34. These isolates contained 16 genes encoding aminoglycoside modification enzymes (Table 1). All isolates had two point mutations encoding amino acid substitutions associated with quinolone resistance in P. putida (T83I in GyrA and S87W in ParC) (5, 6) and in Pseudomonas aeruginosa (T83I in GyrA and S87L in ParC) (7) (Table 1).
Genetic environments of blaNDM-1 and blaVIM-2.
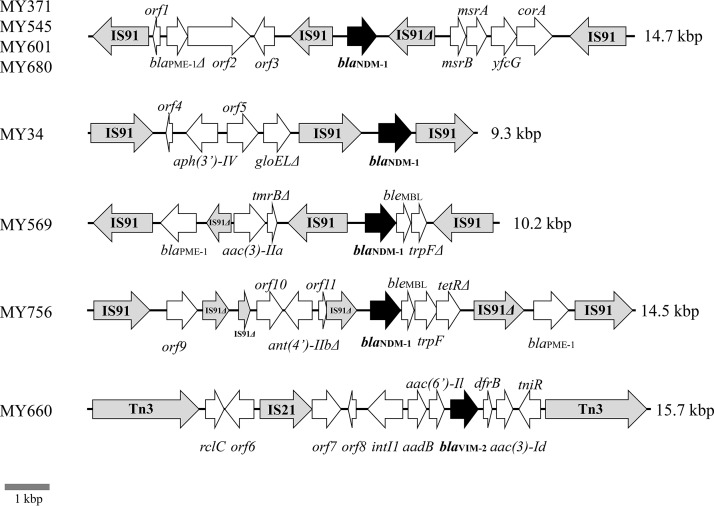
The genomic environments surrounding blaNDM-1 are shown in Fig. 1. Of the eight isolates, four, MY371, MY545, MY601, and MY680, had nearly identical genomic environments surrounding blaNDM-1, with the 14.7-kbp sequences having >99.6% sequence similarity with each other (GenBank accession numbers LC459616, LC460196, LC460198, and LC460200) (Fig. 1). The genomic environments of MY34 (GenBank accession number LC459615), MY569 (GenBank accession number LC460197), and MY756 (GenBank accession number LC460201) differed from each other (Fig. 1). The genomic environments surrounding blaVIM-2 of MY660 (GenBank accession number LC460199) are shown in Fig. 1 and contained a unique structure of class 1 integron with 5′-conserved segments (CS) but not 3′-CS (containing the qacEΔ1, sul1, and orf5), two of which class 1 integrons frequently harbored (8).
FIG 1.
Genomic environments of blaNDM-1 and blaVIM-2 in P. asiatica clinical isolates. Genes are represented as arrows, which indicate their transcription orientations and relative lengths. MBL genes, tnp genes, and truncated genes are shown as black arrows, gray arrows, and Δ, respectively. orf1, orf3, orf8, orf10, and orf11 are genes encoding hypothetical proteins; orf2 is a gene encoding an ABC transporter ATP-binding protein; orf4 is a gene encoding a mobile element protein; orf5 is a gene encoding a NADH dehydrogenase; orf6 is a gene encoding an AraC family transcriptional regulator; orf7 is a gene encoding an AAA family ATPase; and orf9 is a gene encoding a mechanosensitive ion channel protein.
Location of blaNDM-1 and blaVIM-2 in their genomes.
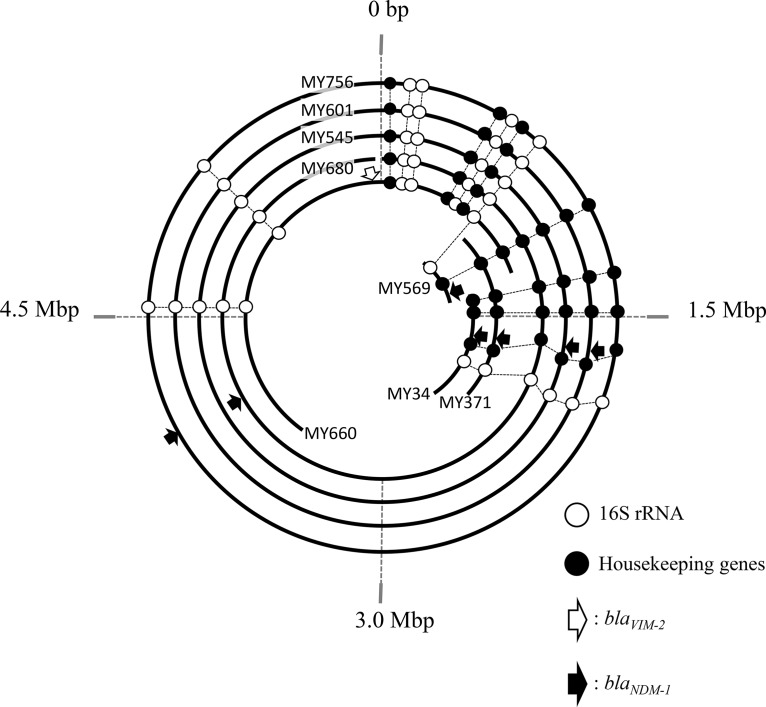
To determine the locations of the MBL-encoding genes of all eight isolates, the DNA sequences of short reads obtained from MiSeq and of long reads from MinION were assembled. In all isolates, blaNDM-1 and blaVIM-2 were detected on a single contig harboring the 16S rRNA genes and the other seven housekeeping genes, indicating that both blaNDM-1 and blaVIM-2 were located on the chromosomes of these isolates (Fig. 2). Contigs from three isolates, MY545, MY601, and MY756, were circularized and assembled as complete chromosomal sequences. However, those from the remaining five were not circularized (Fig. 2). Genetic maps were constructed of these contigs, based on distances between dnaA and other genes, including 16S rRNA genes and housekeeping genes (Fig. 2). Because three contigs, from MY34, MY371, and MY569, did not contain dnaA, the genetic map was constructed by comparisons with those of the circular contigs based on distances between 16S rRNA and each housekeeping gene (Fig. 2). The blaNDM-1 genes were located at ≈1.4 Mbp in MY569; ≈1.7 Mbp in MY34, MY371, MY545, and MY601; and ≈4.0 Mbp in MY680 and MY756. The blaVIM-2 gene in MY660 was located at −64,552 bp of dnaA (Fig. 2).
FIG 2.
Circular visualization of Pseudomonas asiatica contigs for location of MBL genes. The circles and curve lines represent chromosomal DNA sequences of the following P. asiatica isolates from the innermost curved or circularized line: MY569 (742,245 bp; accession number SWEI00000000), MY34 (1,297,202 bp; SWEL00000000), MY371 (1,475,821 bp; SWEK00000000), MY660 (4,005,680 bp; SWEG00000000), MY680 (6,101,876 bp; SWEF00000000), MY545 (5,914,934 bp; SWEJ00000000), MY601 (6,139,917 bp; SWEH00000000), and MY756 (6,110,371 bp; SWEE00000000), respectively. The start point at 0 bp was dnaA. The 16S rRNA genes are shown in white circles, housekeeping genes (gyrB, rpoD, rpoB, rpoN, rpoS, gyrA, and fabD) are shown in black circles, blaVIM-2 is a white arrow, and blaNDM-1 is represented by black arrows.
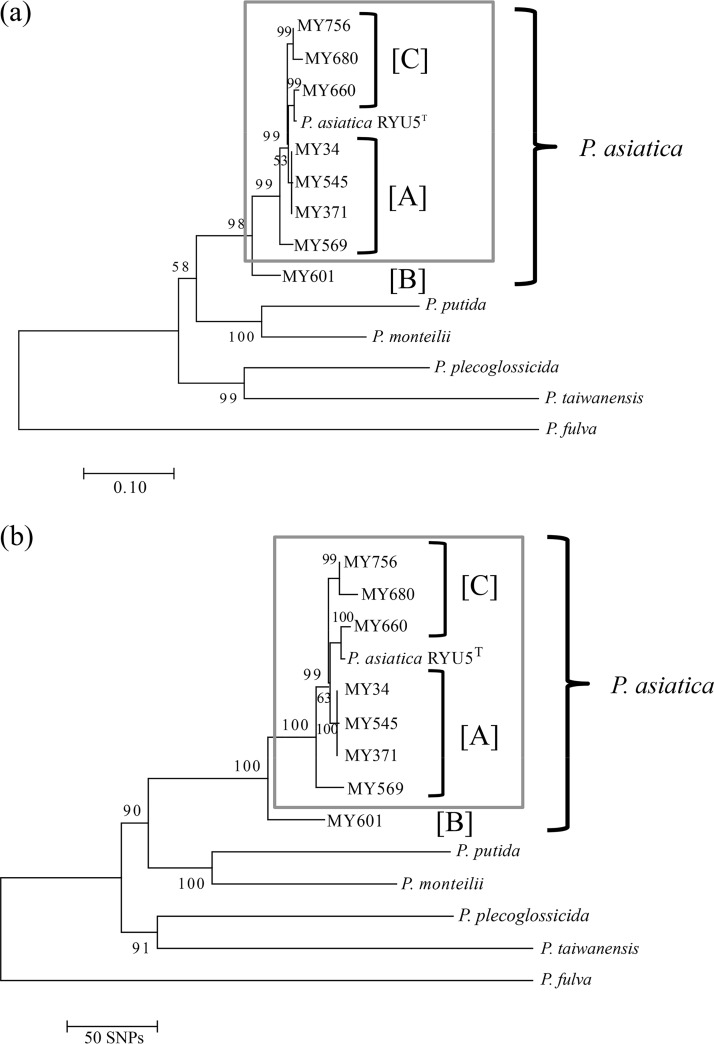
Phylogenetic analysis.
Maximum-likelihood phylogenetic and neighbor-joining phylogenetic trees were constructed using core genome single nucleotide polymorphisms (SNPs) of the eight isolates and the seven type strains belonging to the P. putida group (Fig. 3). The P. asiatica isolates were divided into two groups, with isolates belonging to each group obtained from two hospitals (A and C) and one hospital (B) (Fig. 3).
FIG 3.
Phylogenetic trees for the eight clinical isolates and the type strain of P. asiatica. The trees were constructed using maximum-likelihood (a) and neighbor-joining (b) phylogenetic analysis based on core genome SNPs.
DISCUSSION
P. asiatica is a likely pathogen in humans and causes nosocomial infections. We have detected a total of 10 clinical isolates of P. asiatica to date, including type strains in Japan and Myanmar (4), and an additional seven strains from clinical isolates in Myanmar. All of the isolates were obtained from hospitalized patients (4) (Table 1). The isolates obtained from hospitals A and C were closely related to each other; nonetheless, the location of hospital A (Yangon) is different from that of hospital C (Mandalay), and these isolates were also closely related to the type strain, which was originally isolated in Japan (4). P. asiatica isolates may be clonally spreading in Asian countries.
The blaNDM-1 and blaVIM-2 genes were located on the chromosome of all P. asiatica isolates (Fig. 2), suggesting that foreign genes tend to be inserted into the chromosome. In comparison, the blaVIM-1 and blaVIM-2 genes were located on the chromosome and/or a plasmid in carbapenem-resistant P. putida isolates (9), and blaVIM-2 was located on a plasmid in clinical isolates of carbapenem-resistant P. monteilii (10).
This is the first report of rmtC detected in an isolate belonging to the P. putida group as well as in an isolate of P. asiatica. The rmtC gene was located on the chromosome, as it was present in a contig harboring a housekeeping gene, nth (endonuclease III) (data not shown). The rmtC gene was first reported in clinical isolates of Proteus mirabilis in Japan and then reported in MDR isolates of Enterobacteriaceae (11). More recently, rmtC was found in clinical isolates of pan-aminoglycoside-resistant P. aeruginosa in India (12, 13). The rmtD3 gene, obtained from a clinical isolate of P. aeruginosa in Myanmar, was found to encode a new variant of 16S rRNA methylase (14). Taken together, these findings suggest that P. asiatica may be a reservoir of rmtC.
Five types of genomic environments were found to surround blaNDM-1 and blaVIM-2 in the P. asiatica clinical isolates from Myanmar, indicating the spread in hospitals of carbapenem-resistant P. asiatica isolates with several types of genomic environments. Four strains, MY371, MY545, MY601, and MY680, had the same genomic environment but were isolated at three hospitals located in different areas, indicating that P. asiatica with this genomic environment had spread among hospitals in Myanmar. The partial sequence of this genomic environment was >97% identical with that of P. aeruginosa N15-01092 from the United States (accession number CP012901; 4,093,958 to 4,098,533) (15), although sequences upstream and downstream of the partial sequence were unique.
The strategy of using MinION with MiSeq has become a standard protocol for the genetic analysis of bacteria to determine whether drug-resistant genes are located on the chromosome or plasmids. Long-read sequencing by MinION revealed the longer genomic environmental structures surrounding antimicrobial-resistant genes (16, 17).
To our knowledge, this is the first report to describe the molecular epidemiology of MDR P. asiatica clinical isolates. These bacteria possess several genes associated with drug resistance located on the chromosome. Further surveillance of P. asiatica is necessary in other Asian countries as well as in Myanmar.
MATERIALS AND METHODS
Surveillance.
A prospective surveillance study on MDR Gram-negative pathogens was performed from December 2016 to March 2018 in medical settings in Myanmar. A total of 543 carbapenem- and/or aminoglycoside-resistant Gram-negative isolates were obtained from patients in 22 hospitals and one public health laboratory. MDR strains are defined as strains showing no susceptibility to at least one agent in more than three antimicrobial categories, as described (18).
Drug susceptibility testing.
The MICs of antibiotics were determined using the microdilution method according to the guidelines of CLSI (M100-S25) (19).
Detection of carbapenemases.
Carbapenemase production was detected with the CIMTrisII carbapenem inactivation method (Kohjin Bio, Saitama, Japan) (20), and NDM-type MBL production was detected using a KBM LineCheck NDM immunochromatographic assay (Kojin Bio) (21).
Whole-genome sequencing.
The whole genomes of P. asiatica isolates were sequenced using MiSeq (Illumina, San Diego, CA) and MinION (Oxford Nanopore Technologies, Oxford, UK) according to the manufacturers’ instructions. Quality trimming and filtering of the obtained sequence reads generated by MiSeq were performed using CLC Genomics Workbench v11 (CLC bio, Aarthus, Denmark). MinION data were basecalled by Albacore v2.3.1 (Oxford Nanopore Technologies) and adapters trimmed by Porechop v0.2.3 (https://github.com/rrwick/Porechop). The long reads generated by MinION were assembled using Canu v1.7.1 (22) and polished with the short reads generated by MiSeq using Pilon v1.22 (23).
Bacterial species identification based on whole-genome sequences.
The species of the 14 isolates, identified as P. putida with Vitek 2 (bioMérieux, Marcy l’Étoile, France), were reidentified using ANI (24, 25) and dDDH (26) based on comparisons of their whole-genome sequences with the sequences of 17 type strains belonging to the P. putida group as described (4). The cutoff values of ANI and dDDH between these isolates and the type strains were 95% and 70%, respectively.
Detection of drug resistance genes.
The assembled genome sequences were searched for genes associated with drug resistance, including genes encoding β-lactamases, 16S rRNA methyltransferases, and aminoglycoside-acetyl/adenyltransferase using the ABRicate program (https://github.com/tseemann/abricate) and databases of the National Center for Biotechnology Information (NCBI), ResFinder, and the Comprehensive Antibiotic Resistance Database (CARD). The amino acid sequences of GyrA and ParC in each isolate were compared with those of P. putida KT2440 (5), a reference strain. The type strain of P. asiatica was resistant to ciprofloxacin and levofloxacin; therefore, P. putida, which is a closed species to P. asiatica and sensitive to these antibiotics, was used as a reference for quinolone-sensitive strains.
Phylogenic analysis.
Phylogenetic analysis was performed using kSNP3 v3.1 software with a k-mer length of 31 (27). Maximum-likelihood phylogenetic and neighbor-joining phylogenetic trees were estimated based on the core genome single nucleotide polymorphisms (SNPs) among contigs of the eight P. asiatica isolates and its type strain RYU5. Trees were visualized using FigTree v1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/).
Ethics approval.
The study protocol was approved by the Ministry of Health and Sports in the Republic of the Union of Myanmar (letter number Ethical Committee 2016), by the ethics committee of Juntendo University (number 809), and by the Biosafety Committee, Juntendo University (approval numbers BSL2/29-1). Allowed information about patients included age, gender, and sample tissues.
Data availability.
The genome sequence data generated by MiSeq and MinION were deposited in the DNA Data Bank of Japan (DDBJ) Sequence Read Archive under accession numbers DRA007940 and DRA007946. The data of assembled and annotated nucleotide sequences were deposited in the GenBank database with the accession numbers SWEE00000000 to SWEL00000000, LC459615, LC459616, and LC460196 to LC460201.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Japan Society for the Promotion of Science (grant number 18K07120 and 19K16652), the Research Program on Emerging and Re-emerging Infectious Diseases from the Japan Agency for Medical Research and Development (grant number 19fk0108061h0302), and the JU Research Fund (Keiko Yamazaki). S.W. received the endowed chair from Asahi Group Holdings, Ltd.
We declare no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00475-19.
REFERENCES
- 1.Hong DJ, Bae IK, Jang IH, Jeong SH, Kang HK, Lee K. 2015. Epidemiology and characteristics of metallo-beta-lactamase producing Pseudomonas aeruginosa. Infect Chemother 47:81–97. doi: 10.3947/ic.2015.47.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dortet L, Poirel L, Nordmann P. 2014. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed Res Int 2014:249856. doi: 10.1155/2014/249856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush K. 2001. New beta-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin Infect Dis 32:1085–1089. doi: 10.1086/319610. [DOI] [PubMed] [Google Scholar]
- 4.Tohya M, Watanabe S, Teramoto K, Uechi K, Tada T, Kuwahara-Arai K, Kinjo T, Maeda S, Nakasone I, Zaw NN, Mya M, Zan KH, Tin HH, Fujita J, Kirikae T. 2019. Pseudomonas asiatica sp. nov., isolated from hospitalized patients in Japan and Myanmar. Int J Syst Evol Microbiol 69:1361–1368. doi: 10.1099/ijsem.0.003316. [DOI] [PubMed] [Google Scholar]
- 5.Kumita W, Saito R, Sato K, Ode T, Moriya K, Koike K, Chida T, Okamura N. 2009. Molecular characterizations of carbapenem and ciprofloxacin resistance in clinical isolates of Pseudomonas putida. J Infect Chemother 15:6–12. doi: 10.1007/s10156-008-0661-9. [DOI] [PubMed] [Google Scholar]
- 6.Horii T, Muramatsu H, Iinuma Y. 2005. Mechanisms of resistance to fluoroquinolones and carbapenems in Pseudomonas putida. J Antimicrob Chemother 56:643–647. doi: 10.1093/jac/dki254. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto M, Shigemura K, Shirakawa T, Nakano Y, Miyake H, Tanaka K, Kinoshita S, Arakawa S, Kawabata M, Fujisawa M. 2012. Mutations in the gyrA and parC genes and in vitro activities of fluoroquinolones in 114 clinical isolates of Pseudomonas aeruginosa derived from urinary tract infections and their rapid detection by denaturing high-performance liquid chromatography. Int J Antimicrob Agents 40:440–444. doi: 10.1016/j.ijantimicag.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Deng Y, Bao X, Ji L, Chen L, Liu J, Miao J, Chen D, Bian H, Li Y, Yu G. 2015. Resistance integrons: class 1, 2 and 3 integrons. Ann Clin Microbiol Antimicrob 14:45. doi: 10.1186/s12941-015-0100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juan C, Zamorano L, Mena A, Alberti S, Perez JL, Oliver A. 2010. Metallo-beta-lactamase-producing Pseudomonas putida as a reservoir of multidrug resistance elements that can be transferred to successful Pseudomonas aeruginosa clones. J Antimicrob Chemother 65:474–478. doi: 10.1093/jac/dkp491. [DOI] [PubMed] [Google Scholar]
- 10.Ocampo-Sosa AA, Guzmán-Gómez LP, Fernández-Martínez M, Román E, Rodríguez C, Marco F, Vila J, Martínez-Martínez L. 2015. Isolation of VIM-2-producing Pseudomonas monteilii clinical strains disseminated in a tertiary hospital in northern Spain. Antimicrob Agents Chemother 59:1334–1336. doi: 10.1128/AAC.04639-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi Y, Wachino J-I, Arakawa Y. 2016. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect Dis Clin North Am 30:523–537. doi: 10.1016/j.idc.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahman M, Prasad KN, Pathak A, Pati BK, Singh A, Ovejero CM, Ahmad S, Gonzalez-Zorn B. 2015. RmtC and RmtF 16S rRNA methyltransferase in NDM-1-Producing Pseudomonas aeruginosa. Emerg Infect Dis 21:2059–2062. doi: 10.3201/eid2111.150271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohanam L, Menon T. 2017. Emergence of rmtC and rmtF 16S rRNA methyltransferase in clinical isolates of Pseudomonas aeruginosa. Indian J Med Microbiol 35:282–285. doi: 10.4103/ijmm.IJMM_16_231. [DOI] [PubMed] [Google Scholar]
- 14.Tada T, Shimada K, Mya S, Zan KN, Kuwahara K, Kirikae T, Tin HH. 2017. A new variant of 16S rRNA methylase, RmtD3, in a clinical isolate of Pseudomonas aeruginosa in Myanmar. Antimicrob Agents Chemother 62:e01806-17. doi: 10.1128/AAC.01806-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mataseje LF, Peirano G, Church DL, Conly J, Mulvey M, Pitout JD. 2016. Colistin-nonsusceptible Pseudomonas aeruginosa sequence type 654 with blaNDM-1 arrives in North America. Antimicrob Agents Chemother 60:1794–1800. doi: 10.1128/AAC.02591-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Liu L, McNally A, Zong Z. 2018. Coexistence of two blaNDM-5 genes on an IncF plasmid as revealed by nanopore sequencing. Antimicrob Agents Chemother 62:e00110-18. doi: 10.1128/AAC.00110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, Chen K, Chan EWC, Chen S. 2018. Resolution of dynamic MDR structures among the plasmidome of Salmonella using MinION single-molecule, long-read sequencing. J Antimicrob Chemother 73:2691–2695. doi: 10.1093/jac/dky243. [DOI] [PubMed] [Google Scholar]
- 18.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing; 28rd informational supplement. M100-S28. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Uechi K, Tada T, Kuwahara-Arai K, Sekiguchi JI, Yanagisawa I, Tome T, Nakasone I, Maeda S, Mya S, Zan KN, Tin HH, Kirikae T, Fujita J. 2019. An improved carbapenem inactivation method, CIMTrisII, for carbapenemase production by Gram-negative pathogens. J Med Microbiol 68:124–131. doi: 10.1099/jmm.0.000888. [DOI] [PubMed] [Google Scholar]
- 21.Tada T, Sekiguchi J, Arai-Kuwahara K, Mizutani N, Yanagisawa I, Hishinuma T, Zan KN, Mya S, Tin HH, Kirikae T. Assessment of a newly developed immunochromatographic assay for NDM-type metallo-β-lactamase producing Gram-negative pathogens in Myanmar. BMC Infectious Diseases, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. 2017. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res 27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, Earl AM. 2014. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konstantinidis KT, Tiedje JM. 2005. Genomic insights that advance the species definition for prokaryotes. Proc Natl Acad Sci U S A 102:2567–2572. doi: 10.1073/pnas.0409727102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon SH, Ha SM, Lim J, Kwon S, Chun J. 2017. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 110:1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 26.Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M. 2013. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner SN, Slezak T, Hall BG. 2015. kSNP3.0: SNP detection and phylogenetic analysis of genomes without genome alignment or reference genome. Bioinformatics 31:2877–2878. doi: 10.1093/bioinformatics/btv271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome sequence data generated by MiSeq and MinION were deposited in the DNA Data Bank of Japan (DDBJ) Sequence Read Archive under accession numbers DRA007940 and DRA007946. The data of assembled and annotated nucleotide sequences were deposited in the GenBank database with the accession numbers SWEE00000000 to SWEL00000000, LC459615, LC459616, and LC460196 to LC460201.