The presence and molecular characteristics of carbapenemase-producing Enterobacteriaceae (CPE) among meat products in China were investigated. A total of 110 carbapenem-resistant Enterobacteriaceae (CRE) isolates, including 94 Escherichia coli and 10 Klebsiella pneumoniae isolates, were identified from 105 of 794 (13.2%) samples. The positive rates markedly increased from 2016 (9.4%) to 2018 (22.2%). Only blaNDM genes were detected; 79.1% of blaNDM genes were carried by IncX3 plasmids.
KEYWORDS: China, Escherichia coli, blaNDM, food, plasmid
ABSTRACT
The presence and molecular characteristics of carbapenemase-producing Enterobacteriaceae (CPE) among meat products in China were investigated. A total of 110 carbapenem-resistant Enterobacteriaceae (CRE) isolates, including 94 Escherichia coli and 10 Klebsiella pneumoniae isolates, were identified from 105 of 794 (13.2%) samples. The positive rates markedly increased from 2016 (9.4%) to 2018 (22.2%). Only blaNDM genes were detected; 79.1% of blaNDM genes were carried by IncX3 plasmids. Routine monitoring of carbapenemase-producing Enterobacteriaceae in the animal food supply is highly recommended.
TEXT
In the last decades, there has been a rapid increase of carbapenem-resistant Enterobacteriaceae (CRE) along with the threat of limited treatment options, which are mainly attributed to carbapenemase enzymes, especially, Klebsiella pneumoniae carbapenemase (KPC) and New Delhi metallo-β-lactamase (NDM) (1, 2). Before 2014, the prevalence of CRE remained low in animals and the environment, and the existence of carbapenemase producers in food products was unknown (1, 3). However, in the last few years, CRE have gradually appeared in animals and food (4, 5). In China, multiple studies have demonstrated the increasing occurrence of CRE, mainly, NDM-producing Enterobacteriaceae, in livestock, pets, and the environment. However, little is known about their prevalence or molecular characteristics in meat products (4, 6, 7). In this study, the prevalence and molecular epidemiological features of CRE in retail meat from food markets in Guangzhou were evaluated.
Between 2016 and 2018, 794 fresh retail meat samples (512 pork, 222 chicken, and 60 beef samples) were collected twice a year from farmer’s markets and supermarkets in 7 districts of Guangzhou, China (Table 1; see also Fig. S1 in the supplemental material). Carbapenem-resistant Enterobacteriaceae isolates were selected by MacConkey agar containing 1 μg/ml imipenem. Colonies with different morphologies were selected from each positive sample of carbapenemase genes (blaNDM, blaKPC, blaIMP, blaVIM, and blaOXA-48-like) (8, 9), and only different species, identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) or 16S rRNA sequencing, were retained. In total, 110 nonduplicate carbapenemase-producing Enterobacteriaceae isolates were recovered from 105 (13.2%) samples (64 pork, 38 chicken, and 3 beef samples) (Table 1), with results showing that 110 isolates harbored 98 blaNDM-5, 11 blaNDM-1, and 1 blaNDM-7 gene, which was in accordance with studies on farm animals in China (4, 6, 7). The carriage rates of CRE in chicken samples (17.1% [38/222]) were higher than those in pork (12.5% [64/512]) and beef (5.0% [3/60]) samples. According to the annual isolation of pork and chicken samples, we found that the prevalence of CRE among chicken and pork samples increased significantly (P < 0.05) from 2016 to 2018 (9.4% [34/360] in 2016, 11.1% [15/135] in 2017, and 22.2% [53/239] in 2018). The high frequency of CRE in retail meat (13.2% [105/794]) and the increased prevalence in pork and chicken were worrying. Between 2013 and 2017, CRE was reported in pigs, chickens, and cows in China, with various detection rates (6.5% to 61.0%) (6, 7). Additionally, CRE were also detected in chickens and pigs at slaughterhouses (4). Data from the China Antimicrobial Surveillance Network (CHINET) revealed that the rate of carbapenem resistance in K. pneumoniae increased from 9.3% in 2011 to 20.9% in 2017, whereas in Escherichia coli, the rate was maintained at 1.0% to 1.9% over this period (10). Thus, it seemed that CRE detected in retail meat samples might mainly be attributable to contamination from livestock during slaughter but not from human activity and hospital waste.
TABLE 1.
Prevalence of carbapenemase-producing Enterobacteriaceae isolates by origin
| Year(s) | Sample type | No. of samples | No. of carbapenemase-producing isolates | No. and type of carbapenemase gene(s) |
|---|---|---|---|---|
| 2016 | Pork | 285 | 27 (23 E. coli, 3 K. pneumoniae, 1 E. aerogenes) | 26 blaNDM-5, 1 blaNDM-1 |
| Chicken | 75 | 7 E. coli | 7 blaNDM-5 | |
| Beef | 36 | 3 (1 E. coli, 2 K. pneumoniae) | 3 blaNDM-5 | |
| Total | 396 | 37 (31 E. coli, 5 K. pneumoniae, 1 E. aerogenes) | 36 blaNDM-5, 1 blaNDM-1 | |
| 2017 | Pork | 78 | 8 E. coli | 8 blaNDM-5 |
| Chicken | 57 | 7 E. coli | 6 blaNDM-5, 1 blaNDM-1 | |
| Beef | 24 | 0 | 0 | |
| Total | 159 | 15 E. coli | 14 blaNDM-5, 1 blaNDM-1 | |
| 2018 | Pork | 149 | 30 (24 E. coli, 4 K. pneumoniae, 1 E. aerogenes, 1 Proteus mirabilis) | 25 blaNDM-5, 5 blaNDM-1 |
| Chicken | 90 | 28 (24 E. coli, 1 K. pneumoniae, 3 P. mirabilis) | 23 blaNDM-5, 4 blaNDM-1, 1 blaNDM-7 | |
| Total | 239 | 58 (48 E. coli, 5 K. pneumoniae, 1 E. aerogenes, 4 P. mirabilis) | 48 blaNDM-5, 9 blaNDM-1, 1 blaNDM-7 | |
| 2016–2018 | Pork | 512 | 65 (55 E. coli, 7 K. pneumoniae, 2 E. aerogenes, 1 P. mirabilis) | 59 blaNDM-5, 6 blaNDM-1 |
| Chicken | 222 | 42 (38 E. coli, 1 K. pneumoniae, 3 P. mirabilis) | 36 blaNDM-5, 5 blaNDM-1, 1 blaNDM-7 | |
| Beef | 60 | 3 (1 E. coli, 2 K. pneumoniae) | 3 blaNDM-5 | |
| Total | 794 | 110 (94 E. coli, 10 K. pneumoniae, 2 E. aerogenes, 4 P. mirabilis) | 98 blaNDM-5, 11 blaNDM-1, 1 blaNDM-7 |
According to the CLSI guidelines (11) and epidemiological cutoff (ECOFF) values or clinical breakpoints of EUCAST (http://www.eucast.org), all 110 isolates showed resistance to ampicillin, cefotaxime, ceftazidime, and cefoxitin (see Fig. S2). Resistance genes, including mcr-1 to mcr-8, rmtB, fosA3, floR, and blaCTX-M, were screened by PCR and sequencing, as described previously (see Table S1). Twenty-one (19.1%) isolates coproduced CTX-M enzymes, and 94 (85.5%) carried the floR gene (see Table S2). In addition, 20, 16, and 3 isolates showed resistance to colistin, fosfomycin, and amikacin, respectively, while 16, 14, and 3 were positive for mcr-1, fosA3, and rmtB, respectively; no other mcr genes were detected. Interestingly, the mcr-1 positive rates of the 110 isolates decreased significantly (P < 0.05) from 2016 (24.3% [9/37]) to 2017 (13.3% [2/15]) and to 2018 (8.6% [5/58]). Furthermore, 103 isolates were sensitive to tigecycline (Table S2).
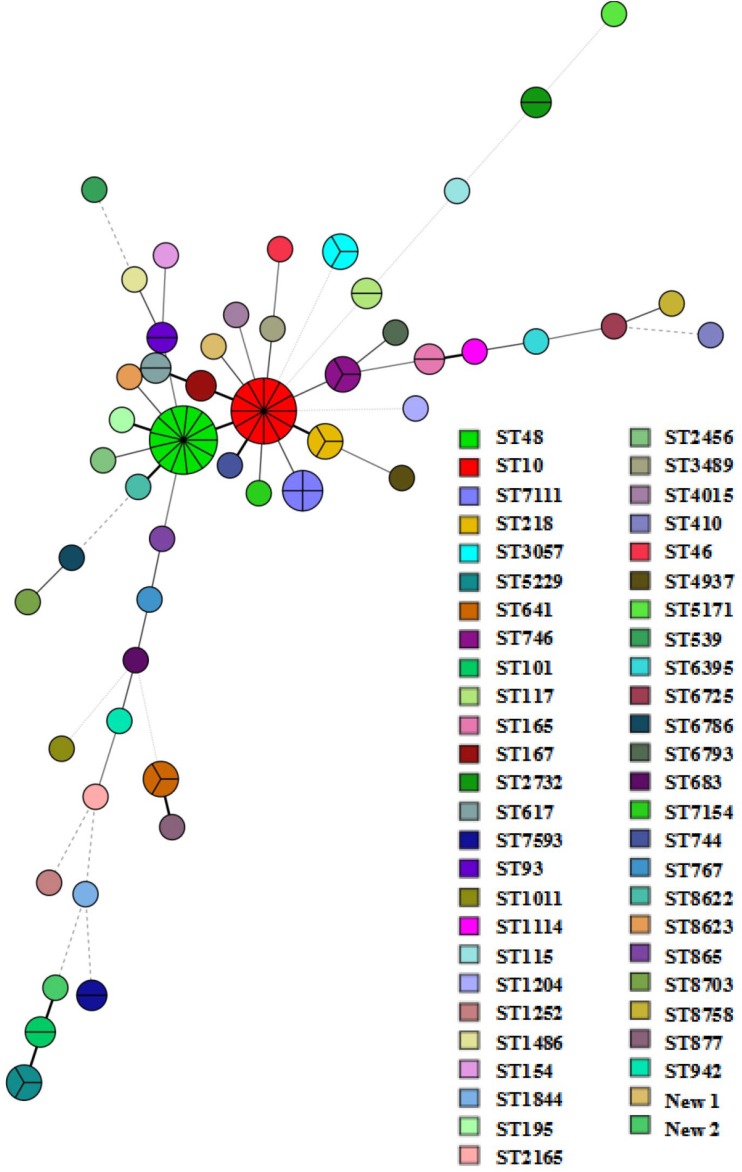
The 94 NDM-producing E. coli isolates belong to 51 distinct sequence types (STs). ST10 (n = 12) and ST48 (n = 13) were the most prevalent STs, followed by ST7111 (n = 4) (Fig. 1 and Table S2). A previous report pointed out the dominance of blaNDM-positive E. coli ST48 clones in one pig farm in China (7). ST10, ST48, ST117, ST2732, ST5229, ST746, ST119, and ST93 (Table S2) were detected in multiple years. Pulsed-field gel electrophoresis (PFGE) analysis was performed on the same ST clone. In total, 10 and 8 different PFGE patterns were observed among 12 ST10 strains and 13 ST48 strains, respectively (see Fig. S3a and b). Among the 10 K. pneumoniae isolates carrying blaNDM, 8 STs were detected, with ST1 (n = 3) being dominant, and had indistinguishable PFGE patterns (Fig. S3c). Thus, nonclonal dissemination played a vital role in the spread of NDM-producing Enterobacteriaceae strains from retail meat in China despite the occasional clonal spread. Most of the characterized STs were different from those of human sources in China. Yet, there were clones shared by isolates from humans and retail meat, such as E. coli ST10, ST167, ST410, ST641, ST744, and ST5229 and K. pneumoniae ST1, ST11, ST35, and ST45 (12–14).
FIG 1.
Minimum spanning tree based on multilocus sequence typing of the 95 blaNDM-positive E. coli isolates. Each ST is displayed as a circle, and the size of the circle is proportional to the number of the strains belonging to each type.
Conjugation and transformation experiments were performed on 52 NDM producers collected in 2016 to 2017. All 52 blaNDM plasmids were successfully transferred to recipients (E. coli C600str or DH5α) by conjugation or transformation. PCR-based replicon typing was performed on transconjugants/transformants as previously described (15–17). The replicon types included IncX3 (n = 50), F2:A−:B− (n = 1), and untypeable (n = 1) (Table 1). For the 58 NDM-producing isolates collected in 2018, S1-PFGE and hybridization analyses confirmed that blaNDM genes from 37 isolates were located on ∼50-kb plasmids and the others were located on 60- to 270-kb plasmids or chromosome. The ∼50-kb plasmids carrying blaNDM were confirmed to be IncX3 type by using primers covering both the backbone of IncX3 plasmid and blaNDM genes. Totally, among the 110 NDM producers, 87 (79.1%) blaNDM genes were carried by IncX3 plasmids (Table S2), which was consistent with previous reports that the IncX3 plasmid was a major vector driving the transmission of blaNDM (7, 12, 13, 18). IncX3 plasmid was first associated with the dissemination of blaNDM-1 among clinical CRE strains (19). Nowadays, it has disseminated in 23 countries, mostly in Asian countries, and was harbored by diverse Enterobacteriaceae species (see Fig. S5) (13). The wide distribution of blaNDM-carrying IncX3 plasmids might be explained by their ability to transfer among different bacterial species at a wide range of temperatures (30°C to 37°C) (13). However, further studies are needed to investigate the underlying mechanism and driving force.
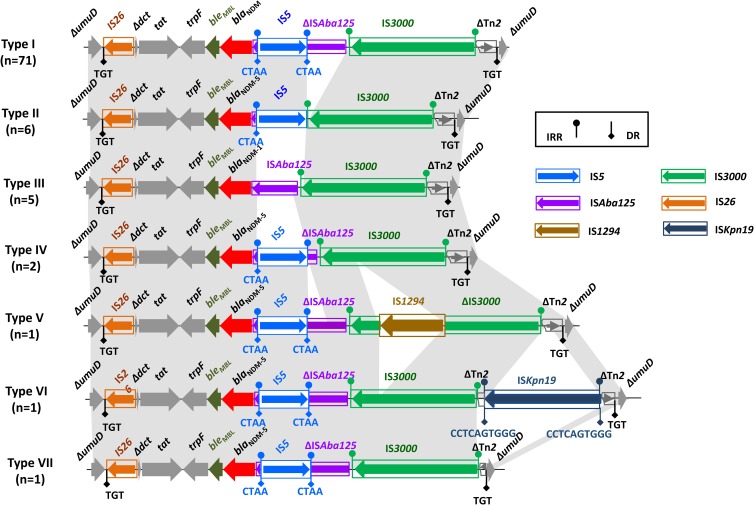
Genomic DNA of 22 blaNDM-positive isolates was extracted and subjected to sequencing using an Illumina HiSeq 2500 platform. To obtain the complete sequence of 9 IncX3 plasmids and analyze genetic backgrounds of other blaNDM gens carried by IncX3, we designed customized primers (see Table S3) based on plasmid pNDM5_IncX3 (GenBank accession number KU761328.1), a 46,161-bp IncX3 plasmid from K. pneumoniae (20). BLAST analysis indicated that 9 IncX3 plasmids carrying blaNDM-5 were highly similar (≥99%) to pNDM5_IncX3. Of note, the sequences of pHNYX638 (MK033577) showed 100% similarity and query coverage with pNDM5_IncX3 (see Fig. S4). pHNHZB05, pHNYX658, pHNHZ11, pHNYX644, pHNHZ18, and pHNYX667 were almost identical to pNDM5_IncX3, with only 1 to 5 nucleotide differences in plasmid backbones (pHNHZ18) or mobile modules. A total of 7 genetic contexts (type I to type VII) were found in 87 blaNDM-carrying IncX3 plasmids, which were due to insertions, truncation, and/or deletions of mobile elements (Fig. 2; Table S2). Type I was the most common structure in this study (81.6% [71/87]) and the GenBank database, encoded by IncX3 plasmids from Enterobacteriaceae of human, pig, and chicken sources in China and other countries (Fig. 2; Table S2 and Fig. S5). Type III was found in 3 blaNDM-1-carrying K. pneumoniae and Enterobacter aerogenes isolates and was identical to pCRENT-193_2 (CP024814) from Enterobacteriaceae of an inpatient in South Korea. Types II, IV, V, VI, and VII were all novel genetic structures found in this study and were unique in the GenBank database.
FIG 2.
Genetic contexts of blaNDM in IncX3 plasmids. Regions of ≥99.0% nucleotide sequence identity are shaded gray.
A dramatic increase of CRE in retail meat samples was observed, driven by the prevailing blaNDM-5-carrying IncX3 plasmid. The CRE and NDM-producing plasmids in retail meat might originate from livestock and endanger public health via direct contact, the food chain, and the environment. It is likely that blaNDM-5-carrying IncX3 might further spread via international travel and trade, as observed in extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae (21). From the one health perspective, more extensive surveillance and development of multisectoral strategies to control its dissemination are urgently needed.
Statistical analysis.
To compare the prevalence of CRE and mcr-1, a χ2 test was performed using SPSS version 23.0. A P value of ≤0.05 was considered statistically significant.
Accession number(s).
The complete IncX3 plasmid sequences carrying blaNDM-5 obtained in this study have been deposited in the GenBank database under the following accession numbers: MK033578, MK033584, MK033580, MK033583, MK033581, MK033579, MK033577, and MK088486.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the National Natural Science Foundation of China (numbers 31625026, 81661138002, and 81772238).
We thank Liming Zheng (College of Veterinary Medicine, South China Agricultural University) for statistical analysis.
We have no conflicts to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00573-19.
REFERENCES
- 1.Madec JY, Haenni M, Nordmann P, Poirel L. 2017. Extended-spectrum β-lactamase/AmpC- and carbapenemase-producing Enterobacteriaceae in animals: a threat for humans? Clin Microbiol Infect 23:826–833. doi: 10.1016/j.cmi.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerra B, Fischer J, Helmuth R. 2014. An emerging public health problem: acquired carbapenemase-producing microorganisms are present in food-producing animals, their environment, companion animals and wild birds. Vet Microbiol 171:290–297. doi: 10.1016/j.vetmic.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Köck R, Daniels-Haardt I, Becker K, Mellmann A, Friedrich AW, Mevius D, Schwarz S, Jurke A. 2018. Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: a systematic review. Clin Microbiol Infect 24:1241–1250. doi: 10.1016/j.cmi.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Yao X, Luo J, Lv L, Zeng Z, Liu JH. 2018. Emergence of Escherichia coli coproducing NDM-1 and KPC-2 carbapenemases from a retail vegetable, China. J Antimicrob Chemother 73:252–254. doi: 10.1093/jac/dkx335. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Zhang R, Li J, Wu Z, Yin W, Schwarz S, Tyrrell JM, Zheng Y, Wang S, Shen Z, Liu Z, Liu J, Lei L, Li M, Zhang Q, Wu C, Zhang Q, Wu Y, Walsh TR, Shen J. 2017. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat Microbiol 2:16260. doi: 10.1038/nmicrobiol.2016.260. [DOI] [PubMed] [Google Scholar]
- 7.Kong LH, Lei CW, Ma SZ, Jiang W, Liu BH, Wang YX, Guan R, Men S, Yuan QW, Cheng GY, Zhou WC, Wang HN. 2017. Various sequence types of Escherichia coli isolates coharboring blaNDM-5 and mcr-1 genes from a commercial swine farm in China. Antimicrob Agents Chemother 61:e02167-16. doi: 10.1128/AAC.02167-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother 65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 9.Campos JC, da Silva MJF, dos Santos PRN, Barros EM, Pereira Mde O, Seco BMS, Magagnin CM, Leiroz LK, de Oliveira TGM, de Faria-Júnior C, Cerdeira LT, Barth AL, Sampaio SCF, Zavascki AP, Poirel L, Sampaio JLM. 2015. Characterization of Tn3000, a transposon responsible for blaNDM-1 dissemination among Enterobacteriaceae in Brazil, Nepal, Morocco, and India. Antimicrob Agents Chemother 59:7387–7395. doi: 10.1128/AAC.01458-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu F, Zhu D, Wang F, Wang M. 2018. Current status and trends of antibacterial resistance in China. Clin Infect Dis 67:S128–S134. doi: 10.1093/cid/ciy657. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing, 27th informational supplement. CLSI document M100-S27. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Zhang R, Liu L, Zhou H, Chan EW, Li J, Fang Y, Li Y, Liao K, Chen S. 2017. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine 19:98–106. doi: 10.1016/j.ebiom.2017.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Tong MK, Chow KH, Cheng VC, Tse CW, Wu AK, Lai RW, Luk WK, Tsang DN, Ho PL. 2018. Occurrence of highly conjugative IncX3 epidemic plasmid carrying blaNDM in Enterobacteriaceae isolates in geographically widespread areas. Front Microbiol 9:2272. doi: 10.3389/fmicb.2018.02272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Wang X, Wang J, Ouyang P, Jin C, Wang R, Zhang Y, Jin L, Chen H, Wang Z, Zhang F, Cao B, Xie L, Liao K, Gu B, Yang C, Liu Z, Ma X, Jin L, Zhang X, Man S, Li W, Pei F, Xu X, Jin Y, Ji P, Wang H. 2018. Phenotypic and genotypic characterization of carbapenem-resistant Enterobacteriaceae: data from a longitudinal large-scale CRE study in China (2012–2016). Clin Infect Dis 67(Suppl 2):S196–S205. doi: 10.1093/cid/ciy660. [DOI] [PubMed] [Google Scholar]
- 15.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Johnson TJ, Bielak EM, Fortini D, Hansen LH, Hasman H, Debroy C, Nolan LK, Carattoli A. 2012. Expansion of the IncX plasmid family for improved identification and typing of novel plasmids in drug-resistant Enterobacteriaceae. Plasmid 68:43–50. doi: 10.1016/j.plasmid.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Villa L, García-Fernández A, Fortini D, Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother 65:2518–2529. doi: 10.1093/jac/dkq347. [DOI] [PubMed] [Google Scholar]
- 18.Shen ZQ, Hu YY, Sun QL, Hu FP, Zhou HW, Shu LB, Ma TF, Shen YB, Wang Y, Li J, Walsh TR, Zhang R, Wang SL. 2018. Emerging carriage of NDM-5 and MCR-1 in Escherichia coli from healthy people in multiple regions in China: a cross sectional observational study. EClinicalMedicine 6:11–12. doi: 10.1016/j.eclinm.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho PL, Li Z, Lo WU, Cheung YY, Lin CH, Sham PC, Cheng VC, Ng TK, Que TL, Chow KH. 2012. Identification and characterization of a novel incompatibility group X3 plasmid carrying blaNDM-1 in Enterobacteriaceae isolates with epidemiological links to multiple geographical areas in China. Emerg Microbes Infect 1:1–6. doi: 10.1038/emi.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li A, Yang Y, Miao M, Chavda KD, Mediavilla JR, Xie X, Feng P, Tang YW, Kreiswirth BN, Chen L, Du H. 2016. Complete sequences of mcr-1-harboring plasmids from extended-spectrum-β-lactamase- and carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:4351–4354. doi: 10.1128/AAC.00550-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doi Y, Iovleva A, Bonomo RA. 2017. The ecology of extended-spectrum β-lactamases (ESBLs) in the developed world. J Travel Med 24(Suppl 1):S44–S51. doi: 10.1093/jtm/taw102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.