Abstract
Carbon black (CB), a material consisting of finely divided particles, can be obtained by the partial combustion of heavy petroleum feedstock. The commercial preparation of CB nanoparticles require sophisticated equipment, chemical pre-treatment, and combination of complex separation and purification techniques. CB nanoparticles can also be recovered from scrubbed rubber, but yields are modest and the process is technically complex. Here, we report the development of a simple and inexpensive method for the preparation of CB nanoparticles from waste tires. Under optimal conditions, the yield of recovered CB nanoparticles (∼22 nm) was of approximately 81%; the nanomaterial presents good thermal stability and conductivity, and forms chain-like agglomerates; chemical composition analysis and solubility tests indicates that it is partly oxidized (C, 84.9%; S, 10.21%; O, 4.9%). The product was fully characterized by FTIR, Raman, TGA, BET, SEM and TEM. This preparation method could become a viable alternative to reduce the large amount of waste tires and decreasing their negative environmental impact, producing good quality CB nanoparticles useful for batteries, sensors, electronic devices, catalysis, pigments, concrete, and plastics, among many other applications.
Keywords: Nanotechnology, Oxidized carbon black, Rubber, Tires, Recyclable
1. Introduction
Plastics pollution is currently one of the most important environmental problems in the world. Over 320 million tons of plastics are produced every year. Nearly 1 billion tires are disposed each year, which adds to the estimated 4 billion unwanted waste tires already existing in landfills and stockpiles. More than 2.5 million tons of scrap tires are disposed every year, only in North America, becoming a growing environmental problem [1]. Disposal of used tires is a global problem; more than 50% are discarded without any treatment. By the year 2030, the number of waste tires would reach 1.2 billion tires yearly [2]. They constitute a problem due to their large volume, durability (more than 100 years for partial degradation), and may possess a strong environmental impact as some components or degradation products are toxic compounds (carbon monoxide, PAHs, heavy metals, among others) [3]. About 47% of a tire is rubber, 22% is carbon black (CB), 17% metals, 6% textiles and the rest other additives (ZnO, sulfur, clays and other compounds). Nearly a 74–76% of a tire are carbon-based materials [4]. Rubber from tires can be grinded into crumbs and recycled for use in numerous consumer products, or transformed by thermal or chemical processing into petrochemicals, fuels or other useful products [5]. Considering all this, recycling of waste plastics in new applications is highly desirable and has a beneficial impact on the environment and the economy. Some other alternatives for the reuse of waste tires include their use as a fuel in cement kilns [6], to produce carbon black [7,8], in asphalt pavements [9] or as aggregates in concrete [2,3,10].
Carbon black (CB) consists essentially of finely divided, spherical particles of carbon produced by incomplete combustion of carbonaceous fuels, both liquid and gaseous, that are chemically bonded forming agglomerate chains via weak Van der Waals interactions [11]. CB may have applications in areas such as antibody delivery [12] catalyst [13], supercapacitors [14], capacitors [15] UV stabilizers [16], pigments [17], concrete additive [18], paper additive [19], plastic additives [20], coatings [21] and structural reinforcement [22]. CB has been produced from different sources such as lignite [11,23], hydrocarbons [24], carbonaceous industrial waste [22,25], rice husks [26], tyres [7,27] and biomass [28]. Additionally, CB has been explored as a new source for the preparation of carbon nanomaterials [25]. Several factors influence CB preparation: the composition of the hydrocarbon feedstock; the temperature of synthesis; the conditions for thermo-oxidative treatment; the level of interaction of the oxidant with the medium, among others [24]. Temperature and processing time are two of the most important factors that affect yield and chemical nature of the product of thermal treatment. Thermal treatment at low temperatures (300–450 °C) usually favor the formation of carbon black particles, while medium temperatures (450–600 °C) favors the formation of liquid products (oils). Higher temperatures (>600 °C) trend to generate mainly gaseous products [29]. These factors affect the properties (size, composition, agglomeration) of the product. In the literature, there are many reports on CB synthesis such as: pyrolysis [7,24], hydrolysis [23,26], carbonization [26], AC thermal plasma [27] and vapor thermolysis [22]. Yields are variable and depend on several conditions such as carbon source or temperature; yields in the range from 35 to 70% are common from the oil furnace process, but from recycled waste tires yields are in the range from 36-50% and depend on temperature and heating time [29,30]. These techniques present some disadvantages, for example, the CB produced by the pyrolysis process is expensive and the quality of the product is inferior to that produced from petroleum products [2]. In addition, several other processing steps such as hydrolysis, carbonization and pyrolysis are required to, at the end, obtaining CB. Finally, these techniques require the use of sophisticated equipment, expensive gas mixtures and chemical pre-treatment.
CB has a high surface-to-volume ratio due to its small size (usually, below 50 nm). Normally, the carbon content of CB is >98%, but oxygen it is always presents in a small percent, due to the presence of numerous oxygen-containing functional groups on the surface of the nanoparticles. Through chemical oxidation a material known as oxidized carbon black (OCB). Methods for the preparation of OCB nanoparticles, usually from graphite, graphene [31] or by direct oxidation of CB nanoparticles in piranha solution (3:1, v/v, H2SO4:H2O2) [29], usually have low yields (from 8 to 25%), and produce other side products such as carbon nanotubes, graphene oxide sheets, among others. OCB nanoparticles have found applications as catalysts for water and alcohol oxidation reactions [32] and for antibody delivery across cell membranes [12]. CB is an alternative nanomaterial widely consumed in rubber products such as automotive tires due to its structural reinforcing properties, but it has been used as support for catalysts and the preparation of conductive inks [33].
Motivated by the need of contributing to the reduction of this type of waste and its transformation into a technologically valued product, in this paper a simple, inexpensive, one-step and high-yield process to obtain CB nanoparticles from waste tires is reported; the partially oxidized CB nanoparticles are water soluble, conductive, mesoporous, thermally stable and present moderate polidispersity.
2. Experimental
2.1. Chemicals
Small pieces of crumb rubber, of approximately 6 mm in size, were obtained from a waste tire (Hankook Ventus V2 brand), previously cleaned thoroughly with water, soup and acetone, and dried at room temperature. Two square pieces of the tire from the inner and outer sections with approximate dimensions of 10 × 10 cm were cut and grinded in small fragments using a rotary abrasive metal tool (automotive lime, Snap-on brand) to scrub the surface. The grinding of the tire was carried out carefully without reaching the reinforcing thread of the tire (metallic wire), which was carefully removed.
2.2. CB nanoparticles preparation
100 g of grinded waste tires were poured into a lab-made cylindrical stainless steel reactor (20 × 10 cm), which has a hermetic head cap that can be closed with 4 cap screws. The reactor containing the rubber was left inside a high-temperature furnace (Thermolyne 1400), pre-heated until it reached a temperature of 1000 °C. After heating during 4 hours, the furnace was turned off and left it to reach room temperature before removing the reactor. After opening the reactor, a black solid was recovered and grinded using an agate mortar. The grinded black solid was washed with water, filtered and dried under vacuum at room temperature. No further processing of the material was performed, and characterization was made on the dried product.
2.3. Sample characterization
Sample characterization was performed using several analytical techniques. The sample morphology and microstructure was characterized with an high-resolution scanning electron microscope (HRSEM) MAIA 3 (TESCAN, Czech Republic) equipped with an Bruker Quantax 200 energy dispersive spectroscopy (EDS) detector. Samples were mounted on top of an aluminum pin with a double conductive graphite tape, and analyzed using a 15 keV electron beam. Transmission Electron Microscope (TEM) images were obtained on a JEOL J-2100 electron microscope at an acceleration voltage of 200 kV. TEM samples were prepared by depositing one drop a dilute suspension of the sample in water on a carbon-coated copper grid and allowing the solvent to evaporate at room temperature. Fourier Transform Infrared Spectroscopy (FTIR) analysis were made using a FTIR Agilent Cary 630 spectrometer in the transmission range of 400–4000 cm−1 with an ATR detector and finely grinded samples. Thermogravimetric Analysis (TGA) was determined from room temperature to 800 °C, with a heating rate of 10 °C min−1 in nitrogen atmosphere with a flow speed of 90 mL min−1, using a Netzsch Regulus STA 2500 (TGA/DTA) thermal analyzer. Hydrodynamic diameters were determined by dynamic light scattering (DLS) using a Malvern Zetasizer NanoZS model MAL1166028 instrument equipped with a 4 Mw He–Ne solid-state laser operating at 633 nm. CB nanoparticles were dispersed in water and sonicated for 5 min to obtain a well-defined, non-flocculated starting state. Specific surface area of the CB samples was measured by nitrogen adsorption at liquid nitrogen temperature (77 K) using an ASAP Model 2020 Physisorption Analyzer and calculated using the Brunauer-Emmett-Teller (BET) isotherm method. Before the analysis, the samples were heated at 300 °C for 24 h at N2 flow in vacuum for degassing. Raman spectra was obtained with a micro ID Raman spectrometer (Ocean Optics, Florida, USA) equipped with a 785 nm laser (max power 71 mW). Samples were deposited on silicon substrates and the spectra was calibrated against the single-crystal Si peak at 520 cm−1 (spectral resolution of about 12 cm−1). Each spectrum was summed from 6 accumulations of 3 seconds each.
3. Results and discussion
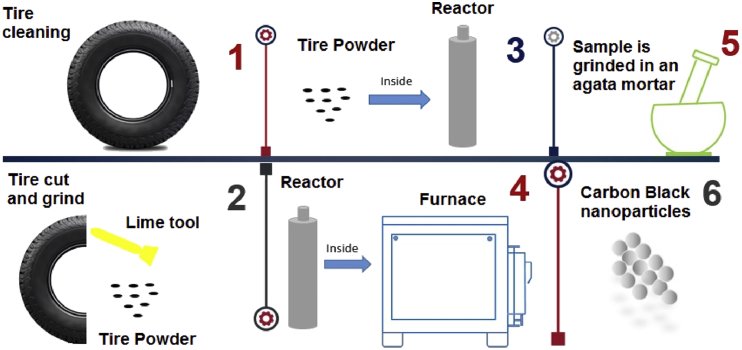
The preparation of carbon black (CB) nanoparticles followed the preparative process showed in Fig. 1, as described in the Experimental section.
Fig. 1.
Schematic representation of the carbon black nanoparticles preparative process steps.
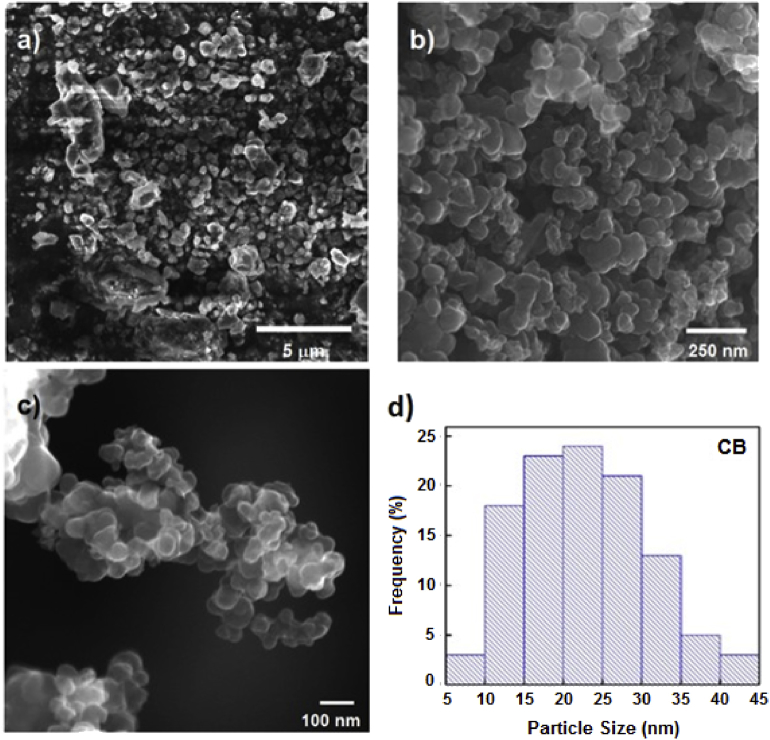
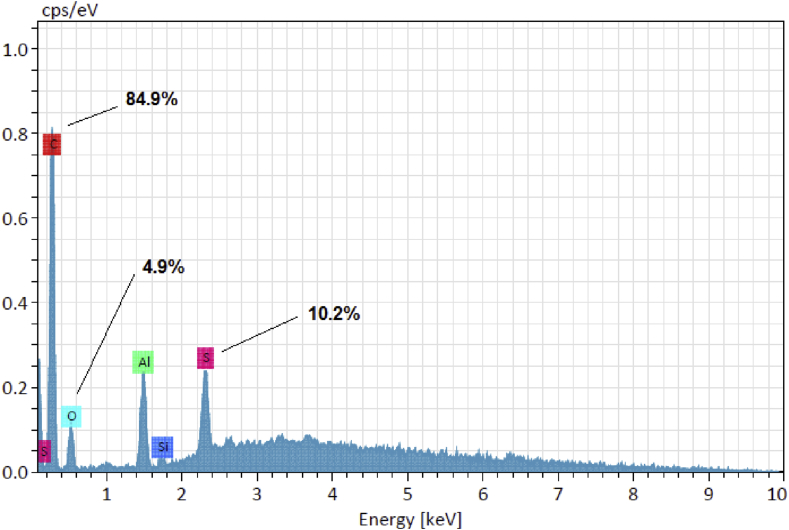
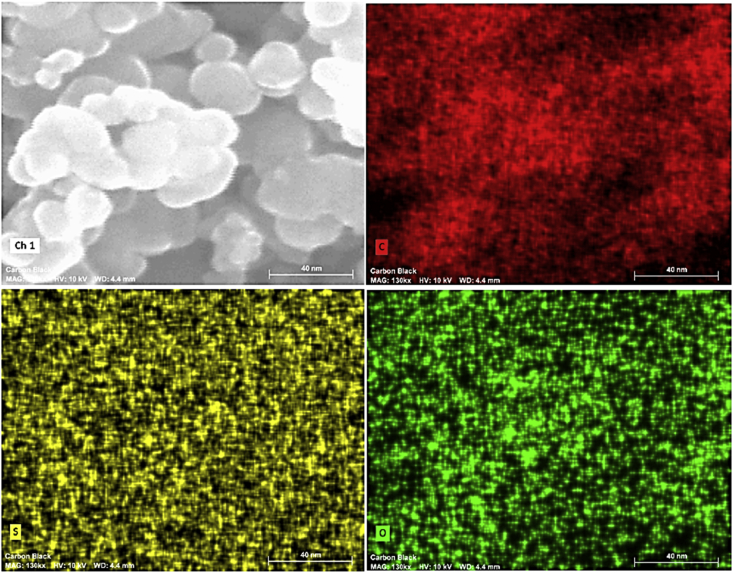
It has been previously reported that CB nanoparticles recovered from waste tires are structurally close to those obtained from hydrocarbon pyrolysis, with some small differences. Recovered CB nanoparticles presents a more diverse chemical composition, strongly related with the variety of additives present in the tires and the carbon source used for its synthesis. After the thermal decomposition of 100 g of scrapped waste tires, which do not contain any metallic reinforcement material as it was removed before treatment, into our lab-made stainless steel cylindrical reactor at a temperature of 1000 °C during 4 h, a total of 81 g (81% yield) of a solid, light, black powder was obtained. Acid-basic chemical pre-treatment of waste tires is another alternative for the elimination of inorganic additives such as silica and metals, among others [34]. For this work, a temperature of 1000 °C was selected for thermal processing of waste tire scraps as it was found optimal for gasification of the carbon precursors present in the tires and its transformation into carbon black nanoparticles, with yields larger than the usual amount of CB particles normally charged as fillers into tires. This result is in agreement with those reported in a recently published study where pyrolytic residue (PR) from waste tires was transformed into pyrolytic carbon black (PCB) with similar yields, but after chemical and ultrasonic treatment [35]. The mechanism of formation of CB nanoparticles may be explained by the vaporization of carbon precursors contained in the waste tire, including original CB particles added as fillers, textiles and other carbon-based additives, and nucleation of vaporized carbon onto CB primary particles, following a mechanism close to that reported for soot formation [25]. A small amount of the powdered sample was mounted on an aluminum pin and characterized by HRSEM and EDS. SEM analysis of the product (Fig. 2) clearly showed the formation of small, nearly monodispersed, carbon spheres with an average particle size of 22 nm (Fig. 2d). EDS analysis (Fig. 3) indicate that the as-obtained CB nanoparticles are chemically composed by 98.3% carbon, and 1.7% sulfur and trace amounts of oxygen (inset, Fig. 3). No electron charging on the surface of the samples was observed when analyzed even at voltages up to 15 keV and magnifications in the order of 300,000 times were achieved; all the later suggest that the as prepared CB nanoparticles are conductive. Spin-coating deposition of a 9–12 layers thin film of CB on a Si substrate and measurement of its resistivity gave values in the range from 1.2 to 1.6 kΩ (for comparison, the unmodified Si substrate had values in the range from 5 to 100 kΩ). These results are in agreement with others reported in the literature for CB and OCB nanoparticles [12,19,36,37]. Organically bound sulfur is a common impurity that can be found in CB nanoparticles, because of rubber vulcanization. However, thermal desulfurization usually occurs when the solid is heated at high temperatures between 500 and 1450 °C, forming volatile sulfur oxides, SOx, As sulfur may still be present in the voids of the CB mesoporous structure, an elemental mapping was obtained (Fig. 4), which clearly show that although carbon is homogenously distributed on the particles, sulfur and oxygen are located randomly through the CB surface, suggesting they are occupying specific spots (oxygen) or empty spaces in the mesoporous particle. Sulfur containing CB may be of interest for the development of new cathode materials for rechargeable batteries [38].
Fig. 2.
SEM images of: a) Grinded rubber before thermal treatment; b) CB obtained after thermal treatment; c) high magnification of CB agglomerates; d) histogram showing particle size distribution of as obtained CB NPs.
Fig. 3.
EDS analysis of CB nanoparticles obtained from waste tires. Normalized mass percent is indicated for C, S and O.
Fig. 4.
Elemental mapping analysis of CB nanoparticles obtained from thermal treatment of scrub rubber from waste tires.
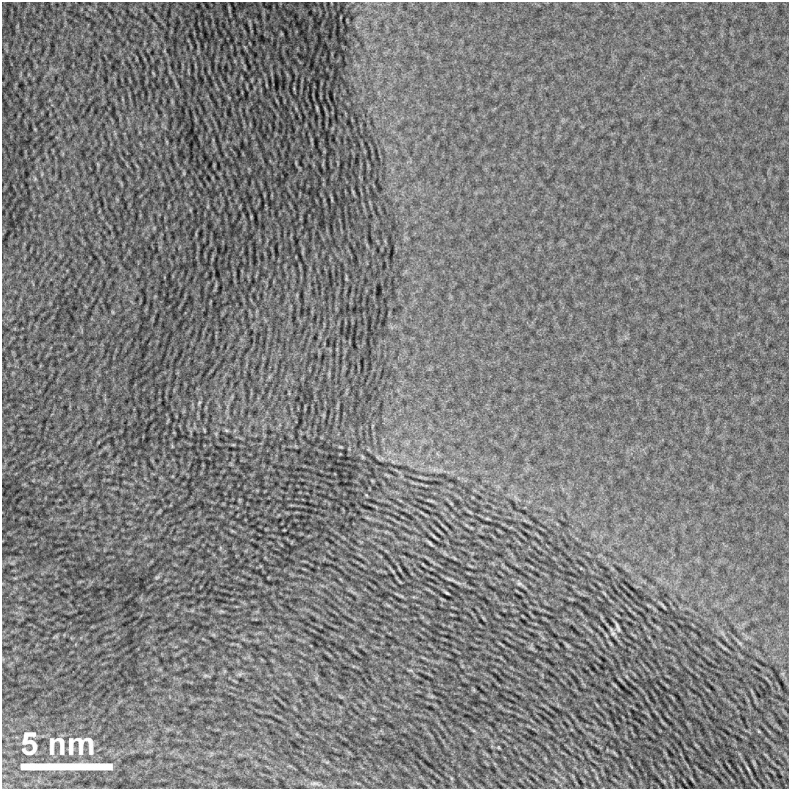
Fig. 5 shows a HRTEM image of the as prepared CB nanoparticles. A highly disorder multilayer graphitic structure, typical of other previously reported CB particles was found. It has been reported that the highly defective multi-shell soot particles (such as CB) could be used for the preparation of carbon nano-onions, and nano- and submicron-diamonds, as well as superhard carbons and Mackay crystals at very low cost [25]. The distance between the graphitic layers is 0.335 nm, which is nearly to the distance between two graphitic planes (0.334 nm).
Fig. 5.
HRTEM image of the CB nanoparticles, showing the characteristic multi-layered, highly disordered graphitic carbon nanostructure.
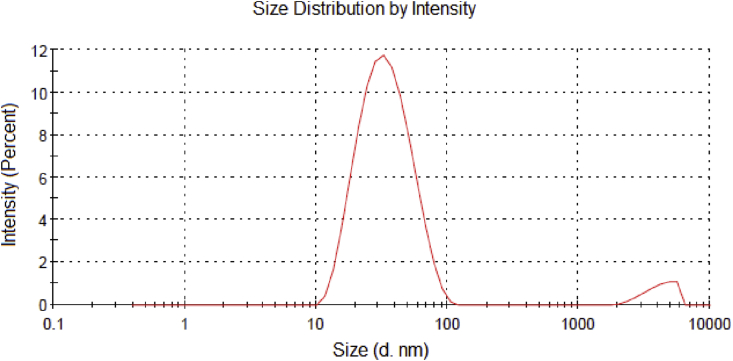
The particle size distribution of CB nanoparticles in water was investigated using DLS. While it has been reported that CB nanoparticles do not disperse in water, partially oxidized CB (OCB) nanoparticles are capable of forming a stable black colloidal suspension when dispersed in water. The particle size distribution curve is shown in Fig. 6. The data indicates that the CB nanoparticles has an average size of 36.03 nm, with a polydispersity index (PDI) value of 0.273, indicating that is moderately polydisperse, that means, the particle size distribution of CB nanoparticles is not uniform. This is an expected result, as individual CB nanoparticles trend to agglomerate forming long chain-like structures, with fractal-like shapes, creating a relatively large size dispersion. The CB nanoparticles have acid character due to their solubility in water, which may indicate the presence of oxygen containing (-OH, COOH) chemical functions on the surface. An experimental measurement of the pH of the water suspension of CB nanoparticles confirms that acidic character (pH ∼ 5).
Fig. 6.
DLS analysis of an ethanolic suspension of CB nanoparticles.
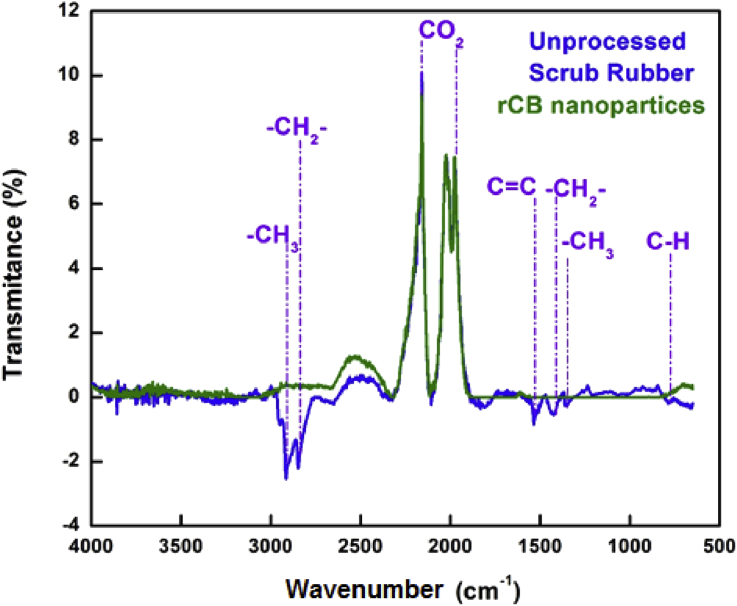
The comparative FTIR spectra of unprocessed scrub rubber and as-prepared CB nanoparticles is shown in Fig. 7. Unprocessed scrub rubber exhibits stretching vibrations corresponding to –CH3 at 2918 cm−1, plus a symmetric vibration corresponding to –CH2 at 2836 cm−1, a band approximately at 1521 cm−1 for a C=C stretching vibration, and three more bands at 1420, 1338 and 781 cm−1 corresponding to vibrations of CH2, CH3 and C–H groups, respectively. These vibrations are in agreement with what has been reported in the literature [39, 40, 41]. In comparison, the FTIR spectrum of CB nanoparticles obtained by thermal carbonization of scrub rubber presents only the typical band associated to the C–H vibration near to 781 cm−1 and no other vibrational band was detected in the spectrum, indicating the carbonaceous nature of this sample. The strong band at 2169 cm-1 corresponds to the typical asymmetrical stretching vibrations of CO2 [42]. This band is present mainly due to the porous nature of the sample that easily adsorbs molecules present in the environment. However, in the regions associated to O–H stretching asymmetric vibrations, only a very weak band from 3000-3200 cm−1 was observed and no evidence of C–O or C=O stretching vibrational bands was found, suggesting that the oxygen containing species may be restricted to a few –OH groups on the surface of the nanoparticles, which is in agreement with the low oxygen content determined by EDS analysis.
Fig. 7.
FTIR analyses of unprocessed scrub rubber from waste tires (blue) and CB nanoparticles as-obtained from the thermal treatment of scrub rubber (green).
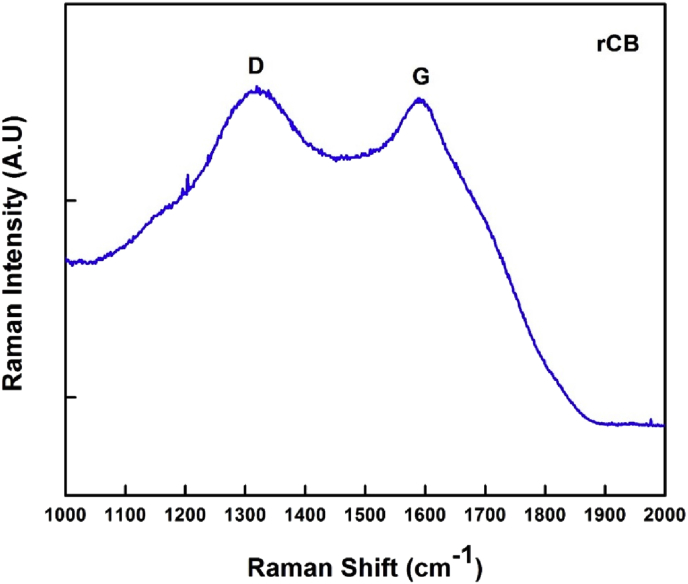
The Raman spectra acquired from as-prepared CB samples is plotted in Fig. 8. The spectra exhibits two broad and strongly overlapping peaks at intensity maxima of 1596 cm-1 (G band) and 1322 cm-1 (D band). The value for the G band indicates that CB nanoparticles are graphitized, as graphite corresponding G band appears around 1582 cm-1 [43]. The calculated ID/IG ratio was of 1.21, which indicates that the graphitized material contains numerous defects and a high proportion of sp3 hybridization. The D peak corresponds to disordered graphite. CB is usually a highly defective carbon form, and therefore overlapped D bands should be present corresponding to amorphous carbon, hydrocarbon or aliphatic moieties connected to the graphitic structural units [44].
Fig. 8.
Raman spectra of CB nanoparticles as-obtained from the thermal treatment of scrub rubber.
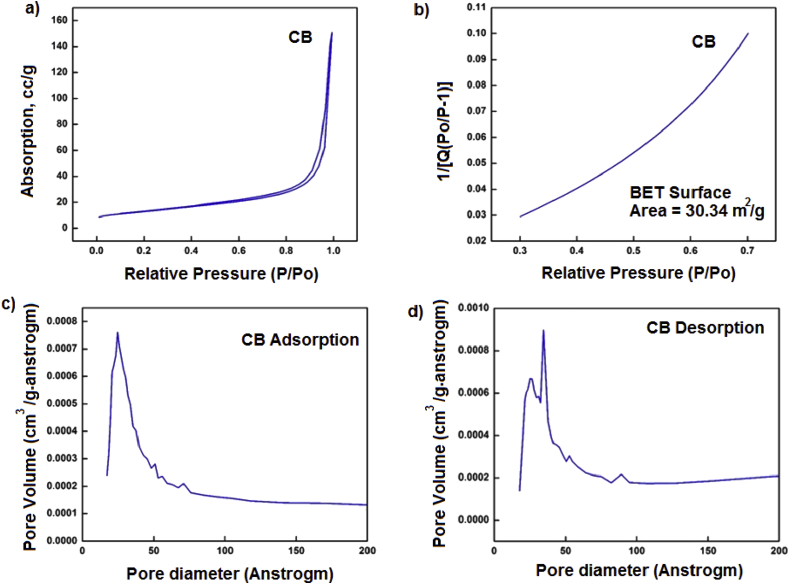
The mesoporous nature of the CB nanoparticles was studied by N2 adsorption and desorption measurements, while specific surface area of the nanoparticles was determined by the BET method, as presented in Fig. 9. Fig. 9a shows adsorption-desorption isotherm of CB. This material presents a typical reversible III type isotherm, which could be attributed to N2 adsorption-desorption in the mesoporous carbon nanostructure. The calculated specific surface area of the CB nanoparticles is approximately 30 m2/g (Fig. 9b). The low surface area value, in comparison to other CB nanoparticles, strongly suggest that part of the voids in the mesoporous structure may be occluded by impurities such as elemental sulfur, as it was suggested by the elemental mapping analysis. The Barret-Joyner-Halenda (BJH) model for determination of pore size distribution (Fig. 9c and d) of mesopores was applied, which is based on the assumption of capillary condensation of N2 in the pores. The CB nanoparticles have a wide pore size distribution, which can be attributed to the mesoporous structure and the effect of large-chain agglomeration. The average calculated pore size was of 30 Å.
Fig. 9.
Surface Measurements of carbon black nanoparticles: isotherm (a), surface area (b), adsorption diameter pore (c), and desorption diameter pore (d).
Several carbon mesoporous materials obtained from several waste sources have been explored in the past as adsorbents. Activated charcoal, mesoporous carbon (CMK3), porous carbon, carbon nanotubes, fullerenes or carbon black obtained from different sources (waste tires, biomass, hydrocarbons) under different processing methods (microwave irradiation, chemical decomposition, CVD, pyrolysis, gasification, combustion) and activation processes (oxidation, acid or basic treatment, physical modification) have been reported as good adsorbants toward several common substances (phenol, dyes, wastewater, heavy metals and pesticides) by several authors [45,46,47,48,49]. Table 1 compares the surface areas of some selected carbon-based adsorbents obtained from several sources and with different methods with that of the CB nanoparticles obtained in this work.
Table 1.
Comparison of different carbon-based adsorbents and CB nanoparticles as prepared in this work.
| Adsorbent | Source Waste | Method | Chemical Treatment - Activation Agent |
BET Surface Area (m2/g) | Reference |
|---|---|---|---|---|---|
| Activaded Charcoal | Tire | Pyrolisis | Air Nitrogen Nitrogen and Stream |
38–110 | [50] |
| Activated Carbon | Polyethylene | Pyrolisis | Sulfuric Acid Water Vapor |
513 | [51] |
| Carbon Black | Carbonaceous | AC termal plasma | Plasma Gas (N2, Ar, He, CO, H2) | 20–53 | [27] |
| Carbon Black | Waste tires | Thermal decomposition | None | 30 | This work |
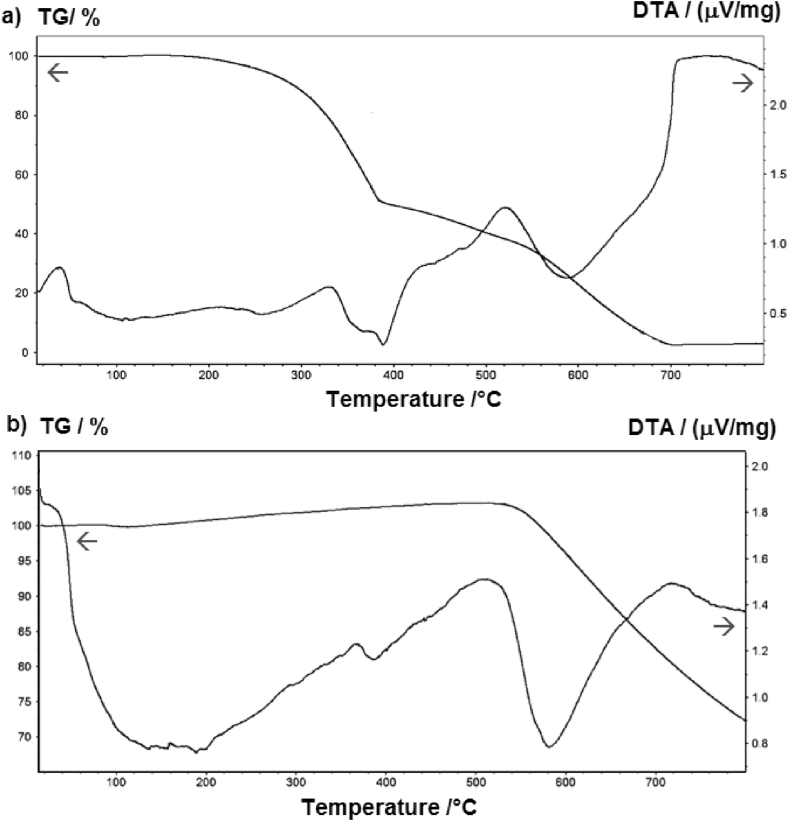
The thermal stability of the prepared CB nanoparticles was evaluated by TGA-DTA analysis under a nitrogen atmosphere. Fig. 10a shows the thermogram for unprocessed scrub rubber. Two stages were identified, the first starting at 199 °C where rubber stars to decompose, and some moisture, residual solvents and oils are lost as volatiles. That stage ends at a temperature of 383 °C, as an endothermic process, maybe associated with total gasification of rubber, accompanied by a significant loss of weight (nearly 50% of the total mass). In the last step, complete thermal degradation of the material occurs from there up to 684 °C, associated with the total decomposition of carbon fillers and other carbon additives until complete carbonization. In the other hand, Fig. 10b shows the thermogram corresponding to the decomposition of the CB nanoparticles; no thermal processes associated with residual rubber or carbon additives were detected from beginning and up to 500 °C. The continuous loss of mass starting at 520 °C seems to continue at a constant rate after 800 °C, suggesting that some organic moieties may be being decomposed and volatilized, with a partial weight loss of around 30% at the end of the temperature cycle. This thermal behavior is very similar to that reported by Zhang and coworkers in 2018 for CB particles prepared also from waste tires under pyrolytic conditions [35]. The fact that, in contrast with the rubber used as starting material, that only a 30% of the mass has been lost when temperature reached the limit of 800 °C, indicates that the as produced CB nanoparticles have larger thermal stability.
Fig. 10.
Curves of thermogravimetric and differential thermal analyses (TGA/DTA): (a) Rubber from scrap tires and (b) CB nanoparticles.
4. Conclusions
As carbon black usually constitutes around 20–35% of the mass of scrap rubber of tires, the high yield of recuperation of carbon black (CB) nanoparticles in this work must be a consequence of thermal transformation of rubber, and other carbon additives into pyrolysis residues that, under the high temperature processing (1,000 °C) and self-pressurized conditions produces mainly CB nanoparticles in large yield. While traditional methods for CB recovery from different sources are not higher than 26–30%, the method here presented for the preparation of very small (∼22 nm) CB nanoparticles has yields higher than 80%; to our knowledge, this is among the highest yield reported for the preparation of CB nanoparticles from waste tires and it is also a very good yield when compared to other conventional methods of preparation of CB nanoparticles starting from several carbon precursors. Only other recent report, with close yields, is available in the scientific literature [35]. HRSEM, EDS, FTIR, Raman, surface measurements, DLS and TGA analysis, indicates that the methodology here presented produces partly oxidized, water soluble, highly conductive CB nanoparticles with average size of 23 nm, with nearly spherical morphology, moderate PDI and high thermal stability. As waste tires are becoming a serious and growing environmental hazard, their transformation into a potentially useful technological material may open the door for alternative ways to dispose and use this type of products. CB nanoparticles may find applications as components for the development of batteries [52], flexible electronic devices [19], mechanical reinforcement additive [53,54], sensors [55], and fluorescent displays [56], among other uses. Current work on the exploration of practical application of CB nanoparticles from waste tires is being performed at our research group.
Declarations
Author contribution statement
Miguel Mendez-Rojas: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yesmin Panecatl: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ruben Gomez-Hernandez: Performed the experiments.
Funding statement
This work was supported by CONACYT INFR-2014/02-23053 and the Office of Graduate Studies and Research (UDLAP). This work was supported by CARBOMEX - Nanomaterials Research & Production.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
HRTEM images were obtained at Texas Christian University by Roberto González-Rodriguez, to whom we are deeply thankful.
Contributor Information
Yesmin Panecatl-Bernal, Email: panecatlbernal@gmail.com.
Miguel Ángel Méndez-Rojas, Email: miguela.mendez@udlap.mx.
References
- 1.Williams P.T., Besler S., Taylor D.T. The pyrolysis of scrap automotive tyres: the influence of temperature and heating rate on product composition. Fuel. 1990;69:481–486. [Google Scholar]
- 2.Thomas B.S., Gupta R.C., Panicker V.J. Recycling of waste tire rubber as aggregate in concrete: durability-related performance. J. Clean. Prod. 2016;112:504–513. [Google Scholar]
- 3.Liu H., Wang X., Jiao Y., Sha T. Experimental investigation of the mechanical and durability properties of crumb rubber concrete. Materials. 2016;9:172. doi: 10.3390/ma9030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans A., Evans R. 2006. Creating Markets for Recycled Resources. The Composition of a Tire: Typical Components; p. 5p. [Google Scholar]
- 5.Eastman A.L. American Chemical Society; Orlando FL: 1993. 144th Meeting of the Rubber Division. October 26. paper 5. [Google Scholar]
- 6.Nakomcic-Smaragdakis B., Cepic Z., Senk N., Doric J., Radovanovic L.J. Use of scrap tires in cement production and their impact on nitrogen and sulfur oxides emissions. Energy Sources, Part A Recovery, Util. Environ. Eff. 2016;38:485–493. [Google Scholar]
- 7.Martínez J.D., Murillo R., García T. Production of carbon black from the waste tires pyrolysis. Boletín del Grupo Español del Carbón. 2013;30:10–14. [Google Scholar]
- 8.Umi F.M.A., Ishak J., Nizar I.K., Kamaruddin H., Ridwan F.M. Physicochemical properties of pyrolytic carbon black from waste tires. Key Eng. Mater. 2014;594–595:178–182. [Google Scholar]
- 9.Bulei C., Todor M.P., Heput T., Kiss I. Directions for material recovery of used tires and their use in the production of new products intended for the industry of civil construction and pavements. IOP Conf. Ser. Mater. Sci. Eng. 2018;294 [Google Scholar]
- 10.Senin M.S., Sahidan S., Leman A.S., Othman N., Shamsuddin S., W Ibrahim M.H. The durability of concrete containing recycled tyres as a partial replacement of fine aggregate. IOP Conf. Ser. Mater. Sci. Eng. 2017;271 [Google Scholar]
- 11.Rice R.L., Lynch R.E., Berber J.S. Carbon black preparation from low-temperature lignite pitch. Rubber World. 1969;162:199–206. [Google Scholar]
- 12.Amornwachirabodee K., Tantimekin N., Pan-In P., Palaga T., Pienpinijtcham P., Pipattanaboon C. Oxidized carbon black: preparation, characterization and application in antibody delivery across cell membrane. Sci. Rep. 2018;8:2489. doi: 10.1038/s41598-018-20650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen X., Chen X., Tian N., Gong J., Liu J., Rummeli M.H. Nanosized carbon black combined with Ni2O3 as “universal” catalysts for synergistically catalyzing carbonization of polyolefin wastes to synthesize carbon nanotubes and application for supercapacitors. Environ. Sci. Technol. 2014;48:4048–4055. doi: 10.1021/es404646e. [DOI] [PubMed] [Google Scholar]
- 14.Ren X., An J., Yan S., Gao L., Xu S., Wang X. Assembly of Mn3O4/carbon black composite and its supercapacitor application. Int. J. Electrochem. Sci. 2016;11:5080–5089. [Google Scholar]
- 15.Balducci A A., Schutter C. Carbon blacks as active materials for electrochemical double layer capacitors. Boletín del Grupo Español del Carbon. 2015;37:1–5. [Google Scholar]
- 16.Wang X., Song K., Ou R. Effects of carbon black and titanium dioxide on ultraviolet weathering of wood flour-HDE/lumber composites using multi-phase Co-extrusion technology. BioResources. 2017;12:6173–6186. [Google Scholar]
- 17.Coccato A., Jehlicka J., Moens L., Vandenabeele P. Raman spectroscopy for the investigation of carbon-based black pigments. J. Raman Spectrosc. 2015;46:1003–1015. [Google Scholar]
- 18.Padma B., Pandeeswari K. Experimental investigation on the properties of concrete with carbon black and PET. Int. J. Adv. Res. 2016;4:1082–1088. [Google Scholar]
- 19.Santhiago M., Correa C.C., Bernardes J.S., Pereira M.P., Oliveira L.J.M., Strauss M. Flexible and foldable fully-printed carbon black conductive nanostructures on paper for high-performance electronic, electrochemical, and wearable devices. ACS Appl. Mater. Interfaces. 2017;9:24365–24372. doi: 10.1021/acsami.7b06598. [DOI] [PubMed] [Google Scholar]
- 20.Zielinski T., Kijenski J. Plasma carbon black-the new active additive for plastics. Compos. Appl. Sci. Manuf. 2005;36:467–471. [Google Scholar]
- 21.Enríquez E., Fernández J.F., De la Rubia M.A. Highly conductive coatings of carbon black/silica composites obtained by a sol-gel process. Carbon. 2012;50:4409–4417. [Google Scholar]
- 22.Moulin L., Da Silva S., Bounaceur A., Herblot M., Soudais Y. Assessment of recovered carbon black obtained by waste tires steam water thermolysis: an industrial application. Waste Biomass Valor. 2017;8:2757–2770. [Google Scholar]
- 23.Snowdon M.R., Mohanty A.K., Misra M. A study of carbonized lignin as an alternative to carbon black. ACS Sustain. Chem. Eng. 2014;2:1257–1263. [Google Scholar]
- 24.Razd’yakonova G.I., Surovikin V.F. The synthesis of different forms of electrically conductive carbon black by the thermo-oxidative pyrolysis of hydrocarbons. Phenomelogical and mathematical models of the synthesis process. Int. Polym. Sci. Technol. 2013;40:T/37–T/42. [Google Scholar]
- 25.Ozawa M., Osawa E. Carbon blacks as the source materials for carbon nanotechnology. In: Dai L., editor. Carbon Nanotechnology, Chapt. 6. Elsevier; Dordrecht: 2006. pp. 127–151. [Google Scholar]
- 26.Wang L., Wang X., Zou B., Ma X., Qu Y., Rong C. Preparation of carbon black form rice husk by hydrolysis, carbonization and pyrolysis. Bioresour. Technol. 2011;102:8220–8224. doi: 10.1016/j.biortech.2011.05.079. [DOI] [PubMed] [Google Scholar]
- 27.Fabry F., Fulcheri L. 6th International Conference on Engineering for Waste and Biomass Valorization Waste Engineering. 2016. Synthesis of carbon blacks and fullerene from carbonaceous wastes by 3-phase AC thermal plasma. May 23-26 Albi, France. [Google Scholar]
- 28.Klupfel L., Keiluweit M., Kleber M., Sander M. Redox properties of plant biomass-derived black carbon (biochar) Environ. Sci. Technol. 2014;48:5601–5611. doi: 10.1021/es500906d. [DOI] [PubMed] [Google Scholar]
- 29.Parthasarahty P., Choi H.S., Park H.C., Hwang J.G., Yoo H.S., Lee B.K., Upadhyay M. Influenece of process conditions on product yeld of waste tyre pyrolysis- A review. Korean J. Chem. Eng. 2016;33:2268–2286. [Google Scholar]
- 30.Piskorz J., Majerski P., Radlein D., Wik T., Scott D.S. Recovery of carbon black from scrap rubber. Energy Fuels. 1999;13:544–551. [Google Scholar]
- 31.Aravachukeat S., Palaga T., Wanichwecharungruang P. Clusters of carbon nanospheres derived from graphene oxide. ACS Appl. Mater. Interfaces. 2012;4:6808–6815. doi: 10.1021/am3019959. [DOI] [PubMed] [Google Scholar]
- 32.Suryanto B.H.R., Zhao C. Surface-oxidized carbon black as a catalyst for the water oxidation and alcohol oxidation reactions. Chem. Commun. 2016;52:6439. doi: 10.1039/c6cc01319h. [DOI] [PubMed] [Google Scholar]
- 33.Noked M., Soffer A., Aurbach D.J. The electrochemistry of activated carbonaceous materials: past, present, and future. J. Solid State Electrochem. 2011;15:1563–1578. [Google Scholar]
- 34.Shah J., Jan M.R., Mabood F., Shahid M. Conversion of waste tires into carbon black and their utilization as adsorbent. J. Chin. Chem. Soc. 2006;53:1085–1089. [Google Scholar]
- 35.Zhang X., Li H., Cao Q., Jin L., Wang F. Upgrading pyrolytic residue from waste tires to commercial carbon black. Waste Manag. Res. 2018;36:436–444. doi: 10.1177/0734242X18764292. [DOI] [PubMed] [Google Scholar]
- 36.Zappielo C.D., Nanicuacua D.M., Dos Santos W.N.L., F Da Silva D.L., Dall’Antonia H.D., De Oliveira F.M. Solid phase extraction to on-line preconcentrate trace cadmium using chemically modified nano-carbon black with 3-mercaptopropyltrimethoxysilane. J. Braz. Chem. Soc. 2016;27:1715–1726. [Google Scholar]
- 37.Acocella M.R., Corcione C.E., Giuri A., Maggio M., Guerra G., Maffezzoli A. Catalytic activity of oxidized carbon black and Graphene oxide for the crosslinking of epoxy resins. Polymers. 2017;9:133. doi: 10.3390/polym9040133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niu J., Kushima A., Li M., Wang Z., Li W., Wang C. Scalable synthesis of a sulfur nanosponge cathode for a lithium-sulfur battery with improved cyclability. J. Mater. Chem. 2014;2:19788. [Google Scholar]
- 39.Rolere S., Liengprayoon S., Vayse L., Sainte-Beuve J., Bonfils F. Investigating natural rubber composition with Fourier Transform Infrared (FT-IR) spectroscopy: a raid and non-destructive method to determine both protein and lipid contents simultaneously. Polym. Test. 2015;43:83–93. [Google Scholar]
- 40.Hossain K.M.Z., Chowdhury S. Grating of n-butyl acrylate with natural rubber latex fim by gamma radiation: a reaction mechanism. Daffodil Int. Univ. J. Sci. Technol. 2010;5:81–88. [Google Scholar]
- 41.Zieba-Palus J. The usefulness of infrared spectroscopy in examinations of adhesive tapes for forensic purposes. Forensic Sci. Criminol. 2017;2:1–9. [Google Scholar]
- 42.Gerakines P.A., Schutte W.A., Greenberg J.M., Van Dishoeck E.F. The infrared band strengths of H2O, CO and CO2 in laboratory simulations of astrophysical ice mixtures. Astron. Astrophys. 1995;296:810–818. [Google Scholar]
- 43.Ferrari A.C. Raman spectroscopy of graphene and graphite: disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007;143:47–57. [Google Scholar]
- 44.Bokobza L., Bruneel J.L., Couzi M. Raman spectra of carbon-based materials (from graphite to carbon black) and of some silicone composites. Chimia. 2015;1:77–94. [Google Scholar]
- 45.Gupta V.K., Nayak A., Agarwal S., Tyagi I. Potential of activated carbon from waste rubber tire for the adsorption of phenolics: effect of pre-treatment conditions. J. Colloid Interface Sci. 2013;417:420–430. doi: 10.1016/j.jcis.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 46.Mohammadi N., Khani H., Gupta V.K., Amereh E., Agarwal S. Adsorption process of methyl orange dye onto mesoporous carbon material-kinetic and thermodynamic studies. J. Colloid Interface Sci. 2011;362:457–462. doi: 10.1016/j.jcis.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 47.Gupta V.K., Saleh T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene-An overview. Environ. Sci. Pollut. Res. 2013;20:2828–2843. doi: 10.1007/s11356-013-1524-1. [DOI] [PubMed] [Google Scholar]
- 48.Saleh T.A., Gupta V.K. Processing methods, characteristics and adsorption behavior of tires derived carbons : a review. Adv. Colloid Interface Sci. 2014;211:93–101. doi: 10.1016/j.cis.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Gupta V.K., Nayak A., Agarwal S. Bioadsorbents for remediation of heavy metals: current status and their future prospects. Environ. Eng. Res. 2015;20:001–018. [Google Scholar]
- 50.Jha V.K., Subedi K. Preparation of activated charcoal adsorbent from waste tire. J. Nepal Chem. Soc. 2011;27:19–25. [Google Scholar]
- 51.Stoycheva I.G., Tsyntsarki B.G., Petrova B.N., Budinova T.K., Petrov N.V. New carbon adsorbent from polymer waste for effective removal of mercury from water. Desalination Water Treat. 2015;57:15435–15444. [Google Scholar]
- 52.Xiao W., Sun Q., Liu J., Xiao B., Glans P.A., Li J., Li R., Guo J., Yang W., Sham T.K., Sun X. Utilizing the full capacity of carbon black as anode for Na-ion batteries via solvent co-intercalation. Nano Research. 2017;10:4378–4387. [Google Scholar]
- 53.Carbas R.J.C., Silva L.F.M., Andrés L.F.S. The mechanical response of a structural epoxy adhesive reinforced with carbon black nanoparticles. Microsc. Microanal. 2018;25:1–5. doi: 10.1017/S1431927618015106. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava S.K., Mishra Y.K. Nanocarbon reinforced rubber nanocomposites: detailed insights about mechanical, dynamical mechanical properties, Payne, and Mullin effects. Nanomaterials. 2018;8:945. doi: 10.3390/nano8110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paczosa-Bator B., Cabaj L., Piech R., Skupien K. Potentiometric sensors with carbon blac supporting platinum nanoparticles. Anal. Chem. 2013;85:10255–10261. doi: 10.1021/ac402885y. [DOI] [PubMed] [Google Scholar]
- 56.Lim S.X., Wong K.L., Zhang Z., Castro-Neto A.H., Sow C.H. Polychromic carbon black: laser galvanized multicolour fluorescence display. Nano Res. 2019;12:733–740. [Google Scholar]