Abstract
Keywords: European Heart Rhythm Association, Heart Rhythm Society, Asia Pacific Heart Rhythm Society, Latin American Heart Rhythm Society, Cognitive, Arrythmias, Dementia
Table of Contents
Introduction 1400
Evidence review 1400
Relationships with industry and other conflicts 1400a
Decline of cognitive function: terminology and epidemiology 1400a
Terminology: cognitive decline, mild cognitive impairment, and dementia 1400a
Epidemiology of dementia 1400a
Methods for assessment of cognitive function 1400b
Role of imaging 1400c
Atrial fibrillation and cognitive function 1400c
Atrial fibrillation, overt stroke, and cognitive function 1400c
Atrial fibrillation, silent stroke, and cognitive function 1400e
Atrial fibrillation and cognitive function in the absence of stroke 1400g
Assessment of cognitive function in atrial fibrillation patients in clinical practice 1400g
Prevention of cognitive dysfunction in atrial fibrillation patients 1400h
Other arrhythmias and cognitive dysfunction 1400j
Cognitive dysfunction in patients with regular supraventricular tachycardias 1400j
Cognitive impairment after cardiac arrest 1400j
Cardiac implantable electronic devices and cognitive dysfunction 1400k
Catheter ablation 1400k
Implications for electrophysiological procedures and cognitive function 1400l
Current knowledge gaps, future directions, and areas for research 1400m
Recommendations 1400m
Introduction
This expert consensus statement of the European Heart Rhythm Association (EHRA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS) summarizes the consensus of the international writing group and is based on a thorough review of the medical literature regarding cognitive function in arrhythmias. The document is intended to describe the impact of different types of arrhythmias on cognitive function, to highlight possible risk markers for cognitive decline and to formulate implications for clinical practice regarding follow-up methods, prevention and treatment strategies. Our objective is to raise awareness of cognitive function among physicians treating patients with arrhythmias and to provide them with practical proposals that may lead to improvement of patient care in this regard.
This document reviews terminology and the epidemiology of cognitive dysfunction, methods for assessment of cognitive function and the role of imaging. Recent studies have suggested possible associations between cognitive decline and atrial fibrillation (AF). We review the reported literature on AF and cognitive function, including the scenarios of AF with overt stroke, silent stroke, or no stroke, and then make recommendations for assessment of cognitive function and prevention of cognitive decline in patients with AF in clinical practice. The document also reviews the association of other arrhythmias and cognitive dysfunction, including settings such as post-cardiac arrest, cardiac implantable devices, such as implantable cardioverter-defibrillators (ICDs) and pacemakers, or ablation procedures. Implications for electrophysiological procedures and cognitive function are discussed. Long QT syndrome and cognitive function is not addressed in the document. For quick reference, sub-chapters are followed by a short section on consensus recommendations. The document concludes with a summary of consensus statements, current knowledge gaps, and future directions of research.
Evidence review
Members of the Task Force were asked to perform a detailed literature review, weigh the strength of evidence for or against a particular treatment or procedure, and include estimates of expected health outcomes for which data exist. Patient-specific modifiers, co-morbidities, and issues of patient preference that might influence the choice of particular tests or therapies are considered, as are frequency of follow-up and cost-effectiveness. In controversial areas, or with regard to issues without evidence other than usual clinical practice, a consensus was achieved by agreement of the expert panel after thorough deliberations. This document was prepared by the Task Force with representation from EHRA, HRS, APHRS, and LAHRS. The document was peer-reviewed by official external reviewers representing EHRA, HRS, APHRS, and LAHRS.
Consensus statements are evidence-based and derived primarily from published data or determined through consensus opinion if data are not available. Current systems of ranking level of evidence are becoming complicated in a way that their practical utility might be compromised.1 In contrast to guidelines, we opted for an easier and user-friendly system of ranking using ‘coloured hearts’ that should allow physicians to easily assess the current status of the evidence and consequent guidance (Table 1). This EHRA grading of consensus statements does not have separate definitions of the level of evidence. This categorization, used for consensus statements, must not be considered as directly similar to that used for official society guideline recommendations, which apply a classification (Class I–III) and level of evidence (A, B, and C) to recommendations used in official guidelines.
Table 1.
Scientific rationale of recommendations*
| Definitions related to a treatment or procedure | Consensus statement instruction | Symbol |
|---|---|---|
| Scientific evidence that a treatment or procedure is beneficial and effective. Requires at least one randomized trial, or is supported by strong observational evidence and authors’ consensus (as indicated by an asterisk). | ‘Should do this’ |  |
| General agreement and/or scientific evidence favour the usefulness/efficacy of a treatment or procedure. May be supported by randomized trials based on a small number of patients or which is not widely applicable. | ‘May do this’ |  |
| Scientific evidence or general agreement not to use or recommend a treatment or procedure. | ‘Do not do this’ |  |
This categorization for our consensus document should not be considered as being directly similar to that used for official society guideline recommendations which apply a classification (I–III) and level of evidence (A, B, and C) to recommendations.
Thus, a green heart indicates a ‘should do this’ consensus statement or indicated treatment or procedure that is based on at least one randomized trial, or is supported by strong observational evidence that it is beneficial and effective. A yellow heart indicates general agreement and/or scientific evidence favouring a ‘may do this’ statement or the usefulness/efficacy of a treatment or procedure. A ‘yellow heart’ symbol may be supported by randomized trials based on a small number of patients or which is not widely applicable. Treatment strategies for which there is scientific evidence of potential harm and should not be used (‘do not do this’) are indicated by a red heart.
Finally, this is a consensus document that includes evidence and expert opinions from several countries. The pharmacological and non-pharmacological antiarrhythmic approaches discussed may, therefore, include drugs that do not have the approval of governmental regulatory agencies in all countries.
Relationships with industry and other conflicts
All members of the writing group, as well as reviewers, have disclosed any potential conflict of interest in detail and is available in Supplementary material online.
All recommendations were voted upon by the writing committee independently and reached ≥80% consensus for inclusion in recommendations tables. Each partner society officially reviewed the document and all reviewer comments were addressed. The final document and recommendations were approved by each partner society.
Decline of cognitive function: terminology and epidemiology
Terminology: cognitive decline, mild cognitive impairment, and dementia
Cognitive decline that is greater than expected from normal aging can be ascertained from changes in standardized cognitive test scores over time. Examples of standardized cognitive tests that evaluate different cognitive domains include Delayed Word Recall test (short-term memory),2 Digit Symbol Substitution test (executive function and processing speed),3 and Word Fluency test (executive function and expressive language).4
Mild cognitive impairment is an intermediate stage between the expected cognitive decline of normal aging and the more serious abnormality of dementia. Mild cognitive impairment is characterized by declines in cognitive function and objective long-term cognitive deficit that does not affect activities of daily living.5
Dementia is defined as deficits in ≥2 cognitive domains that represent a decline from previous level of functioning and that are sufficiently severe to affect activities of daily living. Both mild cognitive impairment and dementia can be further classified into subtypes.6 Mild cognitive impairment can be sub-typed into four groups (based on the scheme adopted by the National Institute on Aging Alzheimer’s Disease Centers Program for the Uniform Data Set) as amnestic or non-amnestic, single or multiple domain.5 Dementia can be classified into aetiologic diagnoses: Alzheimer’s disease, vascular dementia, Lewy body dementia, frontotemporal dementia, and other dementias.6
Epidemiology of dementia
A recent systematic review provided some insights into the contemporary (1980–2009) prevalence of dementia in individuals aged ≥60 years in 21 Global Burden of Disease regions: age-standardized prevalence for those aged ≥60 years varied in a narrow band (5–7% in most world regions), with a higher prevalence in Latin America (8.5%), and a lower prevalence in the four sub-Saharan African regions (2–4%).7 Approximately 35.6 million people lived with dementia worldwide in 2010, with numbers expected to almost double every 20 years, to 65.7 million in 2030 and 115.4 million in 2050.7 In 2010, 58% of all people with dementia lived in countries with low or middle incomes, with this proportion anticipated to rise to 63% in 2030 and 71% in 2050.7 Thus, dementia is a burgeoning global public health problem that prompts an urgent and more comprehensive understanding of its risk factors with the aim to discover novel prevention strategies.
The burden of dementia is rapidly increasing owing to the aging of the population. Other than advancing age, risk factors for dementia, particularly vascular dementia, have been extensively studied from an epidemiological perspective. Broadly, they can be classified as dementia due to non-modifiable risk factors, lifestyle factors, physiological risk factors, or clinical cardiovascular or cerebrovascular disease. Selected risk factors are shown in Table 2 and include many of the risk factors included in stroke risk scores in AF.
Table 2.
Selected risk factors for dementia
| Comments | |
|---|---|
| Non-modifiable risk factors | |
| Demographic factors | |
| Age | Dementia prevalence increases exponentially with age8 |
| Sex | Dementia prevalence greater in women than men7 |
| Ethnicity | VaD risk greater in blacks than whites9 |
| Genetic factors | Genetic alterations may affect cognitive function, e.g. apolipoprotein E ε4 allele and ABCA7 are associated with increased risk of AD; C9ORF72, MAPT, GRN gene mutations associated with frontotemporal dementia; rs12007229 is associated with VaD10 |
| Lifestyle factors | |
| Education | Lower education is associated with higher VaD risk11 |
| Physical activity | Increased physical activity is associated with lower risk of general dementia, Alzheimer’s dementia, and VaD risk, which was attenuated with further adjustment for baseline cognitive, psychosocial, and vascular factors. Review reported that seven out of eight studies found an association between increased physical activity and lower risk of cognitive decline12 |
| Body mass index | U-shaped association between body mass index and dementia, with dementia risk higher in individuals who were obese or underweight13 |
| Smoking | Meta-analysis reported that current smokers have higher risk of cognitive decline and dementia over follow-up, than non-smokers or former smokers14 |
| Social support and networks | Compared with small social networks, larger social networks were associated with a lower risk of incident dementia over time.15 |
| Cardiovascular risk factors | |
| Blood pressure | Higher mid-life blood pressure was associated with higher dementia risk16 and cognitive decline17 |
| Blood glucose | Diabetes was associated with increased dementia risk18 and cognitive decline19 |
| Lipids | Higher total serum cholesterol was associated with higher VaD and AD risk20,21 |
| Clinical cardiovascular or cerebrovascular disease | |
| Stroke | Stroke is associated with increased dementia risk22,23 |
| AF | AF is associated with increased dementia risk24,25 |
| Vascular/peripheral arterial disease | Carotid arterial disease is associated with incident dementia risk and cognitive decline26,27 Lower ankle brachial index is associated with increased dementia risk28 |
| Sleep apnoea | Sleep-disordered breathing is associated with an increased risk of cognitive impairment and a small worsening in executive function.29 |
ABCA7, ATP-binding cassette transporter A7; AD, Alzheimer’s disease; AF, atrial fibrillation; C9ORF72, chromosome 9 open reading frame 72; GRN, granulin; MAPT, microtubule-associated protein tau; VaD, vascular dementia.
Methods for assessment of cognitive function
Impairments of cognitive function often can be subtle and insidious, presenting as missed appointments, mislaying objects, or minor problems at work or home, which are often attributed to stress, age, or pressure of work. Any difference in appearance, behaviour or functioning reported by the patient or the family should alert the physician to the need for a formal assessment. The aim of this assessment is to examine higher cortical functions (attention, orientation, memory, language, praxis, and executive function) from patient narrative, collateral information from families, clinical examination, and standardized tests of cognitive function.30 For assessment of cognitive impairment, a combination of tools and methods are used (Table 3).
Table 3.
Assessment of cognitive impairment
| Suspect | Patient history, appearance, changes in behaviour |
| Confirm | Collateral history from family |
| Examine | Full medical examination, brief screening assessment |
| Investigate | Renal/liver/respiratory/thyroid compromise, B12, folate; syphilis serology (in high-risk patients) |
| Exclude | Depression, neurological/psychiatric disease, medication/drug use |
| Measure | Psychometric testing using validated battery |
| Image | Multimodal MRI (T1, T2, T2*, DWI) for brain changes |
| Establish | Diagnosis based on clinical + psychometric + imaging |
DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging.
During the assessment, particular attention needs to be paid to aspects such as vagueness with dates and events, repetition, inappropriate, or fixed ideas. A collateral account from a caregiver can provide clarification of symptoms and their duration. Specific areas requiring attention include features of depression, neurological or psychiatric diseases, drug/medication use, uncorrected visual and hearing problems, infections, cardiac/respiratory/renal failure, or fast AF, all of which potentially affect cognitive function. Investigations include complete blood count, blood glucose, creatinine, electrolytes, calcium, liver and thyroid function tests, serum folate, and B12 levels. Syphilis serology should be checked in high-risk patients. Magnetic resonance imaging can be helpful to estimate cerebrovascular and degenerative disease load and exclude tumours or normal pressure hydrocephalus.
A list of cognitive assessment tools is provided in Table 4. Several tools are available for cognitive assessment, but there is no consensus on a preferred approach. The choice of tool should vary with the purpose of testing and other factors, such as availability, familiarity, and feasibility.31 Common assessment tools are the two-step general practitioner assessment of cognition (GPCOG) and the Informant Questionnaire for Cognitive Decline in the Elderly (IQCODE), both of which have been validated in large populations.32–34 Standardized assessment tools are not diagnostic instruments and results need to be interpreted in the context of all available evidence.
Table 4.
Comparison of commonly used brief cognitive assessment tools and a list of more complex cognitive assessments
| Cognitive domains assessed |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive assessment tool | Number of items | Average completion time in elderly (≥65 years) patients, minutes | Equipment required | Memory |
Visuospatial/ constructional praxis | Frontal/ executive | Orientation | Attention/ calculation | Language | Informant component | Range of scoresa | ||
| Semantic | STM | Remote | Cut-off indicating cognitive impairmentb | ||||||||||
| AMT435 | 4 | 1 | Verbal | − | − | + | − | − | + | − | − | − | 0–4a |
| CDT36 | 3 | 2 | Pen and paper | + | − | − | ++ | + | − | + | − | − | 0–3a |
| SIS37 | 6 | 2 | Verbal | − | + | − | − | − | + | − | − | − | |
| Mini-Cog39 | 6 | 3 | Pen and paper | + | + | − | ++ | + | − | + | − | − |
|
| AMT35 | 10 | 3 | Verbal | + | + | + | − | − | ++ | ++ | − | − |
|
| MIS40 | 4 | 4 | Verbal | − | + | − | − | − | − | − | − | − |
|
| 6CIT41 | 6 | 5 | Verbal | − | ++ | − | − | − | ++ | ++ | − | − |
|
| GPCOG42 | 9 | 5–6 | Pen and paper | + | ++ | − | ++ | + | + | + | − | + |
|
| MMSE43,c | 30 | 8 | Pen, paper, and watch | − | + | − | + | − | +++ | ++ | ++ | − |
|
| MOCA44 | 30 | 10 | Pen and paper | − | ++ | − | +++ | ++ | +++ | +++ | +++ | − |
|
| OCS45 | 10 tasks | 15–20 | Pen and paper | + | +++ | ++ | +++ | +++ | +++ | +++ | +++ | − | −1 to 111a |
| ACE46 | 100 | 20 | Pen, paper, watch and specific pictures | ++ | +++ | ++ | +++ | ++ | +++ | ++ | +++ | − | <87b |
| More complex and extended cognitive examinationsd | |||||||||||||
| 3MS47: extension of MMSE including verbal fluency and further memory testing; overall score 0–100; score <78 for those aged ≥65 years | |||||||||||||
| CAMCOG48: 80 min, structured history taking from patient and informant, structured examination and mental state assessment | |||||||||||||
| CASI49: questions form MMS and 3MS; scored 0–100 takes 15–20 min to complete | |||||||||||||
| IQCODE50: 16-item informant questionnaire comparing patient cognition now to 10-years ago; each rated on five-point Likert scale | |||||||||||||
Adapted from Woodford and George.51
−, not specifically tested; +, minimal assessment; ++, moderate assessment; +++, relatively extensive assessment; 3MS, Modified Mini Mental Status Examination; 6CIT, 6-item Cognitive Impairment Test; ACE, Addenbrooke’s Cognitive Examination; AMT, Abbreviated Mental Test; CAMCOG, Cambridge Cognitive Examination; CASI, Cognitive Abilities Screening Instrument; CDT, Clock-Drawing Test; GPCOG, general practitioner assessment of cognition; InQ, Informant Questionnaire; IQCODE, Informant Questionnaire for Cognitive Decline in the Elderly; MIS, Memory Impairment Screen; MMSE, Mini Mental State Examination; MOCA, Montreal Cognitive Assessment; OCS, Oxford Cognitive Screen; SIS, Six-Item Screener; STM, short-term memory.
Range of scores.
Cut-off indicating cognitive impairment.
Standardized MMSE is also available.
Not an exhaustive list.
Role of imaging
Brain imaging studies can identify vascular disease as a cause of dementia. In an autopsy study of patients with dementia, pathologic diagnoses implicated vascular disease in about 25% of subjects, half of whom had pure vascular disease.52 The three main causes of vascular cognitive impairment are large vessel strokes, small vessel disease (SVD), and micro-haemorrhages. The preferred imaging modality, magnetic resonance imaging (MRI), has high specificity and sensitivity for detecting these changes and is an important adjunct to clinical and psychometric assessments. However, imaging findings need to be interpreted in the clinical context because of uncertain correlation with symptoms or psychometric test performance.53
Structural imaging is undertaken using T1- and T2-weighted spin echo sequences to identify infarcts and macro-haemorrhages, T2*-weighted gradient echo sequences for micro-haemorrhages, fluid-attenuated inversion recovery imaging for incomplete infarcts and leukoaraiosis and diffusion-weighted imaging (DWI) for visualising the integrity of functional network fibre tracts not captured by other imaging techniques. Magnetic resonance imaging provides several markers of micro- and macrostructural organization that are sensitive to change, related to clinical endpoints and has the potential to predict cognitive trajectories in individual patients.53
Magnetic resonance imaging signs that predict potential cognitive impairments include (i) large or bilateral infarcts due to large vessel disease; (ii) strategic infarcts secondary to embolization in regions as hippocampus, dominant thalamus, medial temporal, and deep frontal; (iii) lacunes, white matter hyperintensities (leukoaraiosis) and haemorrhages associated with SVD; and (iv) lobar micro-haemorrhages representative of amyloid angiopathies. In addition, although global cerebral atrophy and/or medial-temporal lobe atrophy may suggest an element of Alzheimer’s disease (mixed cognitive impairment), subcortical infarcts, per se, may trigger progressive focal thinning and grey matter atrophy in connected temporal and frontal cortical areas.31
Imaging of cerebral blood flow using arterial spin labelling, metabolic imaging with proton magnetic resonance spectroscopy and dynamic contrast-enhanced MRI can help estimate the extent of injury, vessel permeability, and inflammation. Although these can differentiate between dementias and separate pathological changes from those due to aging, they remain research techniques with limited clinical application.
Positron emission tomography scans have also been used to assess brain metabolic function, inflammation, amyloid or tau protein, which may be helpful in differentiating some types of dementia.53 An overview of commonly used imaging modalities in cognitive impairment is provided in Table 5.
Table 5.
Commonly used brain imaging modalities in cognitive impairment
| Modality | Use |
|---|---|
| CT | Large infarcts/haemorrhage, established small vessel disease, other pathologies, limited application |
| MRI | Imaging of choice for assessment of cognitive impairment54 |
| T1 and T2 MRI | Highly sensitive to old and new infarcts, estimation of white matter disease load, other pathologies (e.g. malignancies, cerebral oedema) |
| T2* MRI | Blood and blood products (e.g. haemorrhages), micro-haemorrhages, haemosiderin deposition, amyloid angiopathies |
| DWI MRI | Extremely sensitive to early ischaemic changes (recent infarcts including micro-infarcts), integrity of fibre tracts, extensively used for tractography assessing the structural integrity of connecting white matter tracts |
| 1H-MRS | Measurement of neuronal damage, inflammation, gliosis, differentiation between pathology and normal aging |
1H-MRS, proton magnetic resonance spectroscopy; CT, computed tomography; DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging.
Atrial fibrillation and cognitive function
Atrial fibrillation, overt stroke, and cognitive function
Evidence suggests that AF is associated with a higher risk for cognitive impairment and dementia, with or without a history of clinical stroke. Two meta-analyses that included both cross-sectional and prospective studies specifically examined the incidence of dementia in patients with AF and strokes.24,25 These meta-analyses found similar estimates of the risk ratios of cognitive impairment or dementia of 2.4324 and 2.7025 (Table 6).
Table 6.
Meta-analyses relating atrial fibrillation to dementia and cognitive impairment
| Author | Study design | Outcome | Inclusions/exclusions | Risk |
|---|---|---|---|---|
| Kwok et al.24 | Meta-analysis cross-sectional and prospective studies | Dementia | Patients with H/o stroke, 7 studies; n = 2425 | OR 2.43; 95% CI 1.70–3.46; P < 0.001; I2 = 10% |
| Kalantarian et al.25 | Meta-analysis cross-sectional and prospective studies | Cognitive impairment and dementia | Patients with H/o stroke, 7 studies; n = 2409 | RR 2.70; 95% CI 1.82–4.00; I2 = 32.3%; P = 0.18 |
| Excluding patients with or adjusting for H/o stroke 10 studies | RR 1.34; 95% CI 1.13–1.5 |
H/o, history of; OR, odds ratio; RR, relative risk.
It is uncertain whether or not the risk of cognitive impairment and dementia varies in paroxysmal compared with persistent AF. Many of the studies examining AF type were small and underpowered and the factors that impact progression, such as rhythm control approaches and physician approach to the patient management, can introduce study biases. In a small hypothesis generating cross-sectional study from the Atherosclerosis Risk in Communities (ARIC) Cohort55 persistent but not paroxysmal AF classified by ambulatory telemetry monitoring was associated with lower cognitive function. Another small cross-sectional study reported that cognitive performance did not significantly differ by AF burden, but the number of subclinical cerebral ischaemia areas was higher in individuals with persistent compared with paroxysmal AF.56 More conclusive understanding of the relation of AF burden to cognitive decline and dementia will require larger and longitudinal studies. The relation between AF type and cognitive impairment and dementia is further complicated by the sometimes arbitrary definition of the AF type in the individual patient.
Unfortunately there are no randomized data examining the efficacy of therapies and in particular of individualized management to prevent dementia in individuals with AF.57 Of interest, the Framingham Heart Study has examined temporal trends in the incidence of dementia and noted that the risk of dementia associated with AF declined over three decades (1970s to the early 2010s).58 One speculation is that improved anticoagulation and treatment of risk factors were responsible for the declining incidence of dementia in individuals with AF. Another piece of inferential evidence, supporting the benefit of preventing stroke as a strategy to prevent dementia in individuals with AF, are observational meta-analyses (Table 6). In individuals with AF but without stroke at baseline the risk of dementia and cognitive decline is more modest [relative risk (RR) 1.37, 95% confidence interval (CI) 1.08–1.73] than in individuals with both AF and a history of stroke (RR 2.7, 95% CI 1.82–4.00).25
Systemic anticoagulation remains the cornerstone of stroke prevention treatment. By meta-analysis, adjusted-dose warfarin is associated with a 64% (95% CI 49–74%) significantly lower risk of stroke (Table 7), whereas aspirin alone was associated with a 19% (95% CI −1 to 35%) non-significant lower stroke risk.59 In studies comparing warfarin and aspirin, warfarin was associated with a 38% (95% CI 18–52%) stroke reduction, when compared with aspirin alone.59
Table 7.
Meta-analyses examining anti-coagulation strategies in atrial fibrillation relating to stroke
| Author | Study design | Outcome | Inclusions/exclusions | Risk |
|---|---|---|---|---|
| Hart et al.59 | Meta-analysis adjusted-dose warfarin and aspirin | Stroke | 6 RCTs warfarin vs. placebo; n = 2900 | RR 64% reduction, 95% CI 49–74%; Absolute reduction: 1° prevention 2.7% per year, 2° prevention 8.48% per year |
| 7 RCTs aspirin vs. placebo or no Rx; n = 3900 | RR 19% reduction, 95% CI −1 to 35%; 1° prevention 0.8% per year, 2° prevention 2.5% per year | |||
| 8 RCTs warfarin vs. aspirin Rx; n = 3647 | RR 38% reduction, 95% CI 18–52%; 1° prevention 0.7% per year, 2° prevention 7.0% per year | |||
| Ruff et al.60 | Meta-analysis phase 3 RCTs: | Stroke and systemic emboli | n = 29 312 NOAC; n = 29 272 warfarin | RR 0.81, 95% CI 0.73–0.91; P < 0.0001; I2 = 47%; P = 0.13 |
| RE-LY, ROCKET AF, ARISTOTLE, ENGAGE AF–TIMI 48 | n = 41 257, no prior stroke; n = 17 269, prior stroke | RR 0.85, 95% CI 0.72–1.01 RR 0.89; 95% CI 0.77–1.02; Pinteraction = 0.30 | ||
| Ischaemic stroke | n = 29 292 NOAC; n = 29 221 warfarin | RR 0.92, 95% CI 0.83–1.02; P = 0.10; I2 = 32%; P = 0.22 | ||
| Haemorrhagic stroke | n = 29 292 NOAC; n = 29 221 warfarin | RR 0.49, 95% CI 0.38–0.64; P < 0.0001; I2 = 34%; P = 0.21 |
H/o, history of; NOAC, non-vitamin K antagonist oral anticoagulant; RCT, randomized clinical trial; RR, relative risk.
A meta-analysis of the four randomized trials comparing the non-vitamin K antagonist oral anticoagulants (NOACs) to warfarin, demonstrated that the NOACs were associated with a significant risk reduction (RR 0.81, 95% CI 0.73–0.91) in overall stroke and systemic emboli, in part driven by the significant risk reduction (RR 0.48, 95% CI 0.39–0.59) in haemorrhagic stroke.60
Since a prior stroke represents the strongest predictor of stroke recurrence, all patients who have AF and have had an ischaemic stroke should be anticoagulated, unless an absolute contraindication exists.61 Of interest, a recent observational study using a propensity score-matched analysis reported that in individuals with a history of AF and dementia, persistent use of warfarin therapy was uncommon (16%), but was associated with the prevention of stroke [hazard ratio (HR) HR 0.74, 95% CI 0.54–0.996; P = 0.047] and death (HR 0.72, 95% CI 0.67–0.87; P < 0.001).62 A recent updated meta-analysis reported a significant reduction of stroke, stroke or systemic embolism, haemorrhagic stroke, and intracranial bleeding in AF patients with previous stroke or transient ischaemic attack (TIA) receiving NOACs compared with warfarin.63
Atrial fibrillation, silent stroke, and cognitive function
It is well established that AF increases the risk of clinical stroke by four- to five-fold, and patients with a clinical history of stroke are at increased risk of developing dementia.64–67 However, AF is also associated with cognitive dysfunction ranging from mild impairment to overt dementia, independently of clinical stroke as well as multiple shared risk factors.64,67 It is also well established that AF and cognitive impairment share common risk factors, including advanced age, diabetes, hypertension, sleep apnoea, and chronic heart failure. Moreover, data have demonstrated a significant (34%) increase in the risk of cognitive impairment in patients with AF in the absence of clinical stroke, even after adjustment for shared risk factors.25,64 Thus, additional mechanisms beyond clinically recognized stroke and shared risk factors may link AF and cognitive impairment. One of the leading potential mechanisms is the occurrence of silent cerebral infarcts, which occur significantly more frequently than clinical stroke and are particularly common in patients with AF. 68,69
Detection of cerebral ischaemic events on MRI is based on acute hyperintense lesions on DWI. Brain MRIs reveal evidence of silent cerebral infarcts in a significant percentage of patients with AF.69 The incidence is related to specifications of MRI and depends on the definition applied.70 Atrial fibrillation is associated with a more than two-fold increase in the risk of developing silent cerebral infarcts.69 Although silent infarcts are not associated with clinically apparent acute neurologic deficits, data suggest a significant association between silent infarcts and the development of cognitive decline and dementia.56,71,72 Silent infarcts in patients with AF are believed to be micro-embolic in origin and are identified as small, well-demarcated lesions, often in clusters, and are most prevalent in the frontal lobes.56 The pattern of silent infarct distribution is similar to that seen in vascular dementia, in which most silent strokes affect frontal circuit components (frontal cortex, basal ganglia, and thalamus) that play an important role in executive functioning.73 Thus, the term ‘silent infarct’ is probably a misnomer. Because of their small size and location away from speech and motor centres, these micro-injuries do not cause clinically apparent acute focal neurological deficits. However, with the accumulation of silent infarcts and associated repetitive brain injuries over time, micro-injuries may contribute to the development of cognitive impairment. At least one study has specifically addressed the role of subclinical cerebrovascular disease as a mediator between AF and cognitive impairment. In a subset of stroke-free participants in the ARIC study who underwent repeat brain MRI after approximately 12 years, AF was associated with cognitive decline only in those patients who had developed incident silent cerebral infarcts.74
There is a paucity of evidence regarding the effect of anticoagulation on silent cerebral infarcts and the risk of cognitive impairment. One recent study addressed this issue by evaluating the time in therapeutic range (TTR) as an indicator of the effectiveness of warfarin anticoagulation in patients with AF. These investigators observed a consistent increase in the risk of dementia as the percentage of TTR decreased.75 The association between warfarin therapy and dementia was ‘U’-shaped, with increased risk of dementia among patients with overexposure and underexposure to warfarin [i.e. supra-therapeutic and sub-therapeutic international normalized ratios (INRs)].75 This may be due to cumulative brain injury from cerebral micro-bleeds and silent infarcts, respectively. Recent observational data also suggest that delaying warfarin therapy in patients with AF and no history of dementia, including patients at low as well as high risk for stroke, significantly increases the risk for developing incident dementia.12,76 Whether the use of the NOACs will offer greater protection than warfarin in preventing AF-related cognitive impairment and dementia remains to be determined. The significantly lower intracranial haemorrhage and micro-haemorrhage rates,77 the lower risk of mortality with intracranial haemorrhage with use of NOACs compared with warfarin,78 coupled with comparable degrees of protection against thromboembolic stroke and substantially lower variability in therapeutic anticoagulation effect over time with NOACs, offer reasons to hypothesize that these agents may be advantageous to warfarin regarding protection against cognitive impairment in patients with AF but this requires confirmation. Initial findings seem to confirm this hypothesis.79
Atrial fibrillation and cognitive function in the absence of stroke
Longitudinal studies have shown that dementia is more common in patients diagnosed with AF80,81 even in the absence of stroke. A meta-analysis of eight prospective studies evaluating the relationship between AF and incident dementia in patients without stroke and baseline normal cognitive function included a total of 77 668 patients of whom 15% had AF. After a mean follow-up of about 8 years, 6.5% of patients developed dementia. Atrial fibrillation was independently associated with increased risk of incident dementia (HR 1.42, 95% CI 1.17–1.72; P < 0.001).82 This result was confirmed by a longitudinal analysis from the Cardiovascular Health Study including 5150 participants without baseline history of stroke.83 Incident AF occurred in 11% of patients, with faster decline in mean cognitive function scores, measured using the Modified Mini Mental State Examination (3MSE), compared with patients in sinus rhythm. Although both AF and dementia are diseases of aging, in two large observational studies the highest RR of dementia was observed in younger AF patients <70 years of age.84,85 A recent cross-sectional study indicated that in individuals with heart failure with reduced and preserved systolic ejection fraction, AF was associated with an adjusted higher odds of presence and severity of prevalent cognitive impairment.86 (≥80 years) the relationship between AF and dementia seems to be mostly mediated by concomitant risk factors.87
The relationship between AF and cognitive decline may occur through a variety of pathological mechanisms. Given the relationship between AF and stroke, vascular dementia may be an obvious contributor to cognitive decline, encompassing both multi-infarct dementia and SVD dementia.80–83,88 The second form of dementia in AF patients is Alzheimer’s disease, which is the most common type of dementia overall. Atrial fibrillation has been identified as a risk factor for Alzheimer’s disease.84,89 Alzheimer’s disease is the result of accumulation of abnormally folded beta-amyloid and tau proteins forming cerebral plaques which exert cytotoxic effects leading to cerebral atrophy. Interestingly, misfolded atrial natriuretic peptides may lead to development of amyloid fibrils and deposits in the atria of elderly patients with AF causing a specific atrial cardiomyopathy classified as EHRAS IVa.90,91 However, if AF and Alzheimer’s disease share a common link with regards to protein misfolding and amyloidgenesis, it does not appear to be through the APOE ε4 allele.92 Other studies suggest that the occurrence of Alzheimer’s disease is related to hypoperfusion, inflammation, oxidative stress, and endothelial dysfunction.93–95 All these factors may be induced by several non-cardiac diseases resulting in an atrial cardiomyopathy which in turn, leads to AF91 in the sense of both AF and Alzheimer’s disease being the result of third confounding factors. Additionally, several circulating biomarkers of oxidative stress, inflammation, and endothelial dysfunction are elevated during AF.91,96,97 These factors are also linked to cerebral SVD; therefore, AF may provide a specific milieu for non-stroke related cognitive decline and dementia. For example, hippocampal atrophy in AF patients may be mediated by altered cerebral perfusion due to irregular R-R intervals, abnormal or rapid heart rate, and reduced blood pressure caused by AF, since the hippocampus is one of the most perfusion-sensitive structures of the brain.94,98–101
Interestingly, patients with AF had lower total brain volume when compared with those without AF, independent of cerebral emboli in a large cross-sectional study.102 In addition, recently, AF was associated with a decrease in total cerebral blood flow and brain perfusion in an unselected elderly cohort.103 These results may, at least in part, explain the association of AF with reduced relative brain volume and cognitive impairment.
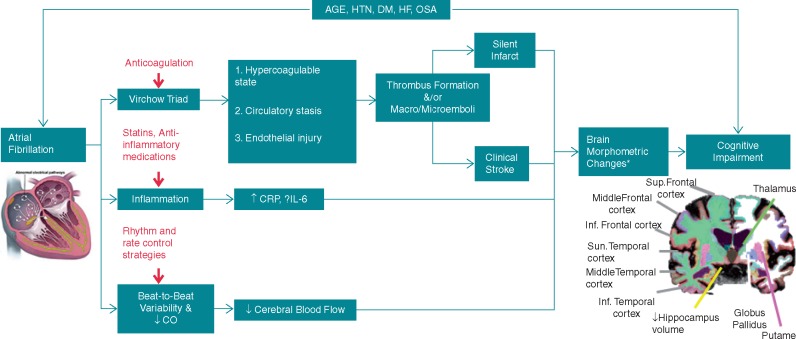
A schematic overview of the various mechanisms, through which AF may lead to cognitive impairment is illustrated in Figure 1.
Figure 1.
Different mechanisms through which atrial fibrillation may contribute to cognitive impairment. Potential interventions are shown in red. aSome of the reported brain morphometric changes include: hippocampus atrophy, white matter hyperintensities, and frontal medial lobe atrophy. Reproduced with modification after permission from Ref.64 CO, cardiac output; CRP, C-reactive protein; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; IL, interleukin; OSA, obstructive sleep apnoea.
A number of trials are currently examining, as the primary or secondary outcome, the effect of different therapies including anticoagulation and of different interventions on cognitive function in patients with AF. A non-exhaustive list of such studies is found in Table 8.
Table 8.
Studies that are currently examining the effect of different therapies and interventions on cognitive function in patients with AF or atrial tachyarrhythmias
| Study name | Target population | Intervention | Cognitive function as outcome |
|---|---|---|---|
| Impact of Anticoagulation Therapy on the Cognitive Decline and Dementia in Patients With Non-Valvular Atrial Fibrillation (CAF), NCT03061006 | Non-valvular AF | Randomization to dabigatran or warfarin | Primary outcome: incident dementia and moderate decline in cognitive function |
| Comparison of Brain Perfusion in Rhythm Control and Rate Control of Persistent Atrial Fibrillation, NCT02633774 | Persistent AF | Randomization to rhythm or rate control | Primary outcome: cognitive assessment |
| Cognitive Impairment Related to Atrial Fibrillation Prevention Trial (GIRAF), NCT01994265 | AF patients >65 years old and CHA2DS2-VASc >1 | Randomization to dabigatran or warfarin | Primary outcome: cognitive impairment |
| Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST), NCT01288352 | AF patients | Randomization to early standardized rhythm control or usual care | Secondary outcome: cognitive function |
| Apixaban During Atrial Fibrillation Catheter Ablation: Comparison to Vitamin K Antagonist Therapy (AXAFA), NCT02227550 | Patients undergoing catheter ablation of non-valvular AF | Randomization to vitamin K antagonists or apixaban | Secondary outcome: cognitive function change |
| NOACs for Stroke Prevention in Patients With Atrial Fibrillation and Previous ICH (NASPAF-ICH), NCT02998905 | Patients with a high-risk of AF and previous intracerebral haemorrhage | Randomization to non-vitamin K antagonist oral anticoagulant or acetylsalicylic acid | Secondary outcome: cognitive function |
| Non-vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes (NOAH), NCT02618577 | patients with atrial high rate episodes and at least two stroke risk factors but without AF | Randomization to edoxaban or acetylsalicylic acid or placebo | Secondary outcome: cognitive function |
| Optimal Anticoagulation for Higher Risk Patients Post-Catheter Ablation for Atrial Fibrillation Trial (OCEAN), NCT02168829 | Patients having undergone a successful AF catheter ablation | Randomization to rivaroxaban or acetylsalicylic acid | Secondary outcome: neuropsychological testing |
| Blinded Randomized Trial of Anticoagulation to Prevent Ischaemic Stroke and Neurocognitive Impairment in AF (BRAIN-AF), NCT02387229 | Patients with non-valvular AF and with low risk of stroke | Randomization to rivaroxaban or acetylsalicylic acid | Primary outcome: composite endpoint of stroke, TIA and neurocognitive decline |
| Secondary outcomes: neurocognitive decline, new onset of cognitive impairment |
AF, atrial fibrillation; NOACs, non-vitamin K antagonist oral anticoagulant; TIA, transient ischaemic attack.
The results of these studies will help to improve our understanding of the relationship between AF and cognitive function and provide us with more data for possible prevention of cognitive decline by treatment of AF.
It should also be noted that, conversely, impairment of cognitive function per se might have a negative impact on therapy adherence and medication intake104,105 and might thus adversely affect treatment effectiveness and outcome in patients with arrhythmias.
Assessment of cognitive function in atrial fibrillation patients in clinical practice
Despite increasing awareness about the relationship between AF and cognitive decline,74,98,106,107 clinical guidelines for the management of AF do not specifically include assessment of cognitive function in the diagnostic work-up. With increasing prevalence of cognitive impairment in the elderly108 and given that the highest RR of cognitive decline is in AF patients >70 years of age, healthcare professionals who treat AF patients should be able to diagnose, and assess risk factors for cognitive impairment appropriately.
Assessment of cognitive function should be multifaceted (see Table 3), and psychometric testing is just one component. Numerous validated tools are available to assess cognitive function, varying from brief screening tools, which take 1–8 min to complete among elderly patients, to more complex time-consuming neuropsychological batteries (see Table 4). Brief screening tools may be most applicable when cognitive impairment is suspected among AF patients, whereas more comprehensive assessments may be performed after appropriate referral to a geriatrician or neurologist. Other factors determining the choice of test include the time available with the patient, the setting (office-based or inpatient), the patient’s ability to speak English (some tools are not translated and/or validated in other languages), and the purpose of the assessment (screening vs. confirmatory). In practical terms, any of the brief tests could be used, although the most common is the GPCOG.42 In research settings, the Mini Mental State Examination (MMSE) and Montreal Cognitive Assessment (MOCA) have been commonly used.43,44,109 Informant questionnaires, such as the second step of GPCOG or the IQCODE,50 provide important additive information, since they assess a patient’s change over time from someone who knows the person well. This level of detail may not always be feasible, however, and may be more suited for comprehensive geriatric or neurological assessment.
Prevention of cognitive dysfunction in atrial fibrillation patients
Since the precise mechanism(s) of cognitive disorders in patients with AF is not fully known, the optimal way to prevent cognitive dysfunction for a given patient remains to be established. Considering the mechanisms of cognitive impairment described in the sections above, several therapies may be considered (see ‘Recommendations’). Both disease states share common risk factors that include aging, smoking, hypertension, diabetes, sleep apnoea, physical inactivity, vascular disease, inflammation, and heart failure. Many of these risk factors represent modifiable targets for preventative therapies and if treated early may lower the risk of both diseases.
Stroke prevention is the principal priority in the management of AF and integrated approaches such as the Atrial fibrillation Better Care (ABC) pathway (Avoid stroke, Better symptom management, Cardiovascular and comorbidity risk reduction) may improve AF management.110 Stroke prevention therapy, particularly oral anticoagulation, applied to the appropriate patients according to risk stratification proposed in scientific guidelines107 may reduce the risk of dementia. Fridberg and Rosenqvist111 studied 444 106 AF patients over 1.5 million years at risk. Anticoagulation use was in 202 946 (46%) of the patients with the primary anticoagulant used warfarin (94%). In AF patients not treated with anticoagulation, 60% were on aspirin. In multivariate analysis, the strongest predictors of dementia were in order: age (HR per decade 2.19, 95% CI 2.16–2.22), Parkinson’s disease (HR 2.46, 95% CI 2.25–2.69), absence of oral anticoagulation treatment (HR 2.08, 95% CI 1.73–2.53), and alcohol abuse (HR 1.53, 95% CI 1.41–1.66).
In patients managed long term with vitamin K antagonists (VKAs), for example, TTR is inversely associated with new-onset dementia.75 Risk of dementia is augmented in AF patients who are frequently over anticoagulated or receiving antiplatelet therapy.112 However, dementia can have a confounding effect on maintenance of TTR, and oral anticoagulation in AF patients has not been consistently associated with either improved cognitive function or less hippocampal atrophy.98,109,113 Anticoagulation with warfarin neither influenced the reduction of total brain volume nor cognitive function in individuals with AF.102 Non-vitamin K antagonist oral anticoagulant therapy may reduce the incidence of brain micro-haemorrhage compared with VKAs,60 but whether NOACs improve long-term cognitive function is currently unknown. A recent community-based study provided some optimism in this regard and found that NOAC therapies were associated with lower stroke and dementia rates compared with warfarin.114 Considering the incidence of dementia in AF, only trials with large numbers of patients and extended long-term follow-up would be able to firmly establish the possible benefit of oral anticoagulation on the subsequent risk of cognitive decline.
Preventing early onset of AF through lifestyle or risk factor modification could delay the onset and progression of cognitive decline. Prevention and early management of smoking, excess alcohol consumption, hypertension, obesity, diabetes, and sleep apnoea may reduce the onset and/or progression of AF115 with concomitant reductions in stroke and possibly cognitive function. However, such risk factor modifications may have independent positive effects on cognitive function regardless of the development of AF. It is also unclear if aggressive modification should start at the time of onset of AF. Lifestyle modification may also reduce the risk of cognitive decline in AF patients. Prevention of cognitive dysfunction may include general measures proposed in the treatment and management of vascular dementia or Alzheimer’s disease. Several trials have tested the effects of physical activity and cognitive training in Alzheimer’s disease and have shown some evidence of efficacy on cognitive endpoints.116 Most of the trials, however, had short follow-up periods. Further evidence is needed to confirm the optimal design and dose of interventions, the appropriate target population, and the efficacy of such interventions. Innovations such as the development of multi-domain interventions and the use of biomarkers or genetic profiles to better target higher-risk patients are being assessed in ongoing trials. However, differentiating the AF-dependent or AF-independent effects of lifestyle and risk factor modifications remains a major challenge.
There are no robust data to affirm that therapy for rhythm control with medication or ‘successful’ AF catheter ablation can prevent cognition disorders in AF patients. Atrial fibrillation catheter ablation may not eliminate AF in the majority of patients, but rather attenuate overall AF burden. Follow-up data beyond 5 or 10 years are limited, and suggest that 2–5% of ‘successfully’ ablated patients will have recurrences annually.117–120 Furthermore, many of these recurrences may be asymptomatic and the prognostic implication of asymptomatic episodes on both stroke risk and cognitive function is unknown.121–123 Catheter ablation as a specific therapeutic approach to lower risk of stroke and dementia is discussed in the Catheter Ablation section.
In patients with persistent AF for whom which rhythm control is not pursued, atrioventricular (AV) node ablation with pacemaker implantation that restores a predictable R-R interval and heart rate has been shown, in a small study, to improve frontal and temporal blood flow and improve memory and learning.124
Recommendations on the prevention of cognitive dysfunction in AF patients are made in the Recommendations section. Most of these recommendations are consistent with those of international guidelines107 and are not necessarily unique to those patients with AF and cognitive dysfunction.
Other arrhythmias and cognitive dysfunction
Cognitive dysfunction in patients with regular supraventricular tachycardias
Recurrent supraventricular tachycardias in children and adolescents, mediated by AV nodal re-entry or by accessory pathways, were shown to be associated with cognitive deficits in 48% of such patients, when assessed prior to catheter ablation.125 Whether an early catheter ablation of supraventricular arrhythmia would affect the cognitive status of such patients needs further investigation.
Cognitive impairment after cardiac arrest
Brain injury after non-fatal cardiac arrest
Cardiac arrest occurs in two different settings, in-hospital and out-of-hospital, with completely different prognosis, for obvious reasons. As cardiac arrests that occur in a hospital context are usually immediately attended, the primary focus of the study of brain injury after cardiac arrest has been among survivors of out-of-hospital cardiac arrest (OHCA).126 In this setting, brain damage is caused by cerebral hypoperfusion and its severity depends on the time of such deficit127; the proportion of cardiac arrest survivors who present with some degree of brain damage ranges from 35% to 100%.128,129 The working group of Chun-Lim and colleagues has delineated three scenarios that are clearly related to the duration of brain hypoperfusion: (i) patients with early recovery of brain function without any sequelae, usually associated with opportune resuscitation and/or early recovery of consciousness (<3 days after OHCA); (ii) patients with extensive damage, associated with prolonged coma (>7 days after OHCA); and (iii) an intermediate group between those extremes.130 They report that a coma duration of less than 3 days results in a better quality of life at 3- and 12-month follow-up, and that the manifestation of severe cognitive impairment early on in recovery results in higher risk for permanent memory and motor impairment.
Clinical sequelae of brain damage after OHCA may range from mild memory impairment to severe physical and mental disability. As expected, if brain damage persists, it negatively impacts patients’ quality of life.130,131 Cognitive impairment could include limited attention span, personality disturbances, movement disorders (i.e. Parkinsonism), and even dementia; however, memory seems to be the cognitive function most affected in survivors of cardiac arrest. Neuropsychological studies have shown deficits in different cognitive areas including memory (64.3%), executive functioning (21.4%), language (21.4%), and perception (14.3%).132 In 1990, the Utstein style was developed in order to standardize the results of resuscitation studies; this includes neurological evaluation using either the Cerebral Performance Category (CPC) or the Modified Rankin Scale (mRS).133,134 Cerebral Performance Category classifies patients on a scale from 1 to 5 (1 = good cerebral performance; 5 = deceased) while the mRS classifies patients on a 0–6 scale (0 = asymptomatic; 6 = deceased); CPC scores of 1–2 and mRS scores of 0–3 are considered favourable neurological outcomes.134 These criteria are included as a reminder of the risk of neurological dysfunction among survivors of cardiac arrest.
Memory impairment after cardiac arrest
In patients successfully treated for an OHCA in a rapid emergency response program, the long-term survival and quality of life are similar to age- and gender-matched controls.135 However, if cognitive assessment is evaluated in detail, memory loss is prevalent.136 Alexander et al.137 reported that among 30 selected patients (1 day of coma, with responsiveness after 24 h but with remaining confusion for 7 days), only one-third of the sample suffered from motor impairment after the event, but the total population showed at least a mild degree of memory impairment. Torgersen et al.138 also reported that even after therapeutic hypothermia, 52% of the patients who suffered cardiac arrest showed cognitive impairment, especially episodic memory dysfunction. This finding is not new, in 1996, Grubb et al.139 demonstrated in a population of 35 patients that up to 37% of the patients suffered chronic memory impairment after cardiac arrest, and that memory dysfunction was inversely proportional to the duration of the event.
Memory impairment after cardiac arrest does not seem to improve over time; a case-control study comparing OHCA patients with patients who suffered acute coronary syndrome without OHCA (controls) showed that memory impairment recorded at 3-month follow-up remained unchanged after 12 months, with just mild improvement of other functions.130 Further, only 16% returned to work after the cardiac arrest whereas more than 94% of controls returned to work.130 This study also evaluated quality of life among cardiac arrest survivors and controls; physical quality of life was not perceived as impaired in either group, however, the ‘cases’ perceived a worse quality of life as a result of memory impairment.130
Therapeutic hypothermia to prevent cognitive impairment after cardiac arrest
Although the effects of hypothermia have been evaluated in humans and experimental models before,140,141 in 1956 Marchand and Allan142 developed an experimental model to measure the effects of hypothermia on the heart and brain. The first studies of therapeutic hypothermia in cardiac arrest patients were performed in the 1950s. Later, Zola-Morgan et al.143 reported that ischaemic episodes damaged CA1 hippocampal cells. The benefits of induced hypothermia were demonstrated in animal models in the nineties.144 At the present time, compelling evidence supports the use of targeted therapeutic hypothermia. Effectiveness of therapeutic hypothermia to prevent cognitive impairment has been reported with varying results among different authors.145 For example, Fugate et al.145 followed 56 survivors of cardiac arrest treated with therapeutic hypothermia. Twenty-month follow-up interviews yielded results favouring the use of this therapy: 33 (60%) patients were reported as being ‘cognitively normal’, with 79% of working patients returning to their normal activities after the event. In contrast, a randomized clinical trial (RCT) of 70 patients comparing therapeutic hypothermia to a normothermic control group (without further intervention), found no statistically significant difference in cognitive function between the two groups, although the authors suggests that differences in the neuropsychological tests employed (previous prospective studies focusing on memory functions rather than executive functions) might explain the neutral findings in this RCT.146 Current guidelines for hospital care after cardiac arrest recommend the use of targeted therapeutic hypothermia between 32 and 34°C for 48 h.147 After publication of these guidelines, a systematic review by Schenone et al.148 reported that mortality was halved [odds ratio (OR) = 0.51, 95% CI 0.4–0.64] and neurological impairment caused by arrest-induced hypoxia was significantly lower (good neurological outcome; OR = 2.48, 95% CI 1.91–3.22) in patients who underwent therapeutic hypothermia compared with those who did not.
In summary, minimising and treating the complications originating from a cardiac arrest are almost as important as treating the arrest itself. Physicians should perform appropriate follow-up and referral to a specialized centre that can offer appropriate post-cardiac arrest care in order to minimise the extent of brain damage and to avoid adverse outcomes, since the ultimate success of medical therapy is not only survival, but preservation of quality of life.
Cardiac implantable electronic devices and cognitive dysfunction
Patients requiring cardiac implantable electronic devices (CIEDs) are generally older and as such may have associated cognitive dysfunction. It has also been shown that patients with severe bradycardia or high-grade AV block may show impaired cognitive function.149–151
Bradycardia is also more common in patients treated for dementia with cholinesterase inhibitor drugs (adjusted HR 1.4, 95% CI 1.1–1.6) and increases the risk of syncope, CIED need, and falls.152 A retrospective study showed that patients with cognitive dysfunction were more likely than those without cognitive deficits to be implanted with a pacemaker, even after adjusting for clinical risk factors.153 Treatment of both permanent or transient bradycardia with CIEDs has been shown to improve cognition in a number of small trials.150,151,154
Although it may be inferred that patients with cognitive impairment and standard device indications may be at increased risk for device complications, this was not demonstrated in a study by Jama et al.155 However, the survival was lower than in matched controls suggesting that these patients may have more co-morbidities. A small study suggested that the increase in cerebral blood flow after pacemaker implantation for symptomatic bradycardia resulted in improvement in cognitive function.151 Another small study showed that the improvement was, however, not significant over a 6–12 months follow-up after pacemaker implantation.150 Ventricular pacing may result in impaired haemodynamics and has been associated with AF, which itself has been associated with multi-infarct dementia. Ventricular pacing was noted to show a trend towards a detrimental effect on the visual memory score.156 It is well known that CIED may have psychological side effects; this is particularly true for ICD and especially shock therapy.157,158 Apart from this psychological effect, there may be a direct effect on cognitive function. Implantable cardioverter-defibrillator implantation with defibrillation testing has been reported to initially result in cognitive dysfunction in 31–39% of patients, as determined by neuropsychological testing before and after ICD implantation in 52 patients, however most patients improve within a year.159 Another study in 115 ICD patients observed that cognitive function in memory was poor at baseline and decreased over 12 months post-ICD implantation.160 In a small Polish study of 51 patients with primary prevention ICDs, seven patients who received ICD shocks for ventricular fibrillation scored worse in neuropsychological measurements compared with patients without such shocks, suggesting greater cognitive impairment,161 which could be multifactorial. However, further studies are needed to demonstrate whether shock or prevention of ventricular fibrillation will prevent decline in cognitive function.
In contrast, there are several reports that cardiac resynchronization therapy may be associated with neurocognitive functional improvement.162–166 An early systematic review of three studies reported improvements in executive functioning and attention.
Catheter ablation
With mounting evidence to suggest an association between arrhythmias and cognitive decline and dementia, treatment of these arrhythmias has been considered as an option to lower risk of cognitive decline. As antiarrhythmic drug therapies have variable efficacy and are associated with many side effects, and medications can directly influence quality of life, mood, and function,167 catheter ablation is often employed as a durable non-pharmacological option.
The majority of evidence that catheter ablation may impact cognition is derived from observational studies of AF management. Outcomes in a consecutive series of 4212 patients who underwent AF ablation were compared (1:4) with 16 848 age/gender-matched controls with AF (no ablation) and 16 848 age/gender-matched controls without AF. In this analysis, stroke outcomes of patients with AF, and an ablation, were better than patients with AF and no ablation, but similar to patients without AF.168 Similarly, long-term outcomes of cognition were better in AF ablation patients compared with AF patients who did not undergo ablation, including lower rates of Alzheimer’s, senile, and vascular dementia. Cognitive outcomes between those patients that received an ablation were similar to patients without a history of AF, including all subtypes of dementia. In patients with atrial flutter stroke rates post-ablation are significantly lower compared with AF patients treated with ablation.169 However, as these were not RCTs, better outcomes could have been related to selection bias rather than impact of ablation.
Dementia has not been a traditional endpoint in observational studies of outcomes after AF ablation. However, stroke and TIA are commonly reported endpoints. Long-term cognitive deficits are common after stroke with up to 10% of patients developing dementia after their first stroke with an incidence that increases to 30–40% with recurrent stroke.23 In a propensity-matched study of 969 consecutive AF ablation patients with a CHA2DS2-VASc score ≥2, AF ablation was associated with a long-term reduced risk of stroke (HR 0.62, 95% CI 0.47–0.82) and TIA (HR 0.47, 95% CI 0.20–0.78).170 In a separate observational study, AF ablation was associated with lower rates of stroke/TIA compared with AF patients not treated with ablation across all age and CHADS2 strata including patients considered at high risk for stroke and patients with prior stroke.171 In this study, stroke rates in all groups increased with higher CHADS2 scores, including non-AF patients, consistent with the influence of systemic risk factors on stroke risk beyond that of AF.
Catheter ablation of all arrhythmias has peri-procedural risks that may have long-term significant consequences with regard to cognition and dementia risk. Procedural risk with all cardiac left sided procedures may impact long-term cognition due to the presence of peri-procedural thrombus, atheroemboli, cerebral hypoperfusion, sheath and wire manipulation and management, and anaesthesia. In patients that undergo right sided cardiac procedures the risk is anticipated to be lower although paradoxical thromboembolism can occur in the presence of a septal defect.169 The risk of stroke during left atrial catheter ablation is estimated at approximately 0.5–1%.121 However asymptomatic or subclinical ischaemic lesions develop in up to 41% of AF ablation patients with an incidence that varies with anticoagulation approach, ablation tool used, and cranial scan protocol.121,122,172,173 In addition, when peri-procedural transcranial Doppler analysis is used during AF ablation to monitor for emboli, sheath manipulation, removal and insertion of tools, and using of multiple tools within the left atrium are significantly associated with micro-embolic events.174 The risk of these lesions is higher (up to 63%) during ventricular arrhythmia ablation with retro-aortic access and long sheaths in the aorta being unique risk factors.175
To put these incidences in context with other cardiovascular procedures, the estimated incidence of new brain lesions has been reported to be 8–18% after AF ablation, 11–17% after coronary angiography or percutaneous coronary intervention, 16–51% after coronary artery bypass graft, 38–47% after surgical aortic valve replacement, 68–91% after transaortic valve implantation, 4–34% after carotid endarterectomy, 15–67% after carotid artery stenting, 11–20% after cerebral angiography, and 10–64% after endovascular aneurysm procedures.176
The long-term consequences of asymptomatic or subclinical cerebral ischaemic events are unknown. It stands to reason that cranial injury of any type, if persistent or accumulative can impact function. However, cognition is not often tested serially after ablation and the mechanisms that underlie the genesis of these cranial lesions are not fully understood. In the prospective Mesh Ablator vs. Cryoballoon Pulmonary Vein Ablation of Symptomatic Paroxysmal Atrial Fibrillation (MACPAF) study, high-resolution diffusion-weighted MRI imaging, performed within 48 h after ablation, showed that new brain lesions (range 1–17) were present in 43.2% of patients.122 Follow-up MRI at 6 months found that 12.5% of the acute brain lesions after ablation formed a persistent scar. Neuropsychological assessment at 6 months found that there was not a significant consequence of these lesions on attention or executive functions, short-term memory, or learning.122 However, other studies such as the ERACE study reported much lower rates of permanent scar indicating the uncertainty in this area.177
Based on the limited available data, post-AF ablation cognitive dysfunction seems to be common. In a study of 150 patients who underwent ablation, cognitive dysfunction was evident in 28% with paroxysmal AF, 27% with persistent AF, 13% with supraventricular tachycardia, and 0% in control patients with AF who did not undergo ablation. Although these incidences decreased to 13%, 20%, and 3% at 90 days, measurable cognitive dysfunction persisted; access time in the left atrium was the most significant procedural variable of risk.123 Unfortunately in this study, MRI imaging was not performed to determine and correlate this dysfunction with peri-procedural cranial lesions. Neuropsychological outcomes were sought at 3 months in a small study of 23 patients who underwent ablation with post-procedure diffusion-weighted MRI cranial imaging. New cranial lesions were detected in three (14%) patients and one patient suffered a clinical stroke.161 Residual cognitive defects were noted at 3 months with neuropsychological testing, in particular, in verbal memory (one of five cognitive domains); deterioration was observed in 56.5% of ablation patients compared with 17.4% of controls.178
Implications for electrophysiological procedures and cognitive function
As described in the preceding sections, emerging evidence suggests that various electrophysiological procedures may be associated with cerebral injury and the potential of cognitive decline therefore, it is timely to consider current evidence and guidelines to minimise these risks.
Over recent years considerable research has focused on how to minimise these procedural risks during left sided catheter ablation and in particular AF ablation. The routine use of transoesophageal echocardiography (TOE) to identify pre-existing thrombus at the time of AF ablation remains controversial particularly in an era where most electrophysiological procedures will be performed on uninterrupted anti-coagulation. However, TOE studies have demonstrated that up to 2% of anticoagulated AF patients may have left atrial appendage thrombus or sludge with risk varying according to CHA2DS2-VASc score. In the most recent AF ablation HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement guidelines, 51% of the writing group members perform a TOE in all patients presenting for AF ablation regardless of presenting rhythm and anticoagulation status; 71% perform a TOE in patients presenting in AF even on therapeutic anticoagulation, and 78% perform a TOE in patients not previously anticoagulated even in sinus rhythm. Computed tomography and intra-cardiac echocardiography have both also been used to screen for thrombus but evidence from large comparative studies is lacking.
Evidence suggests that patients with an anticoagulation window period or those requiring bridging are at increased risk of peri-procedural events. Uninterrupted anticoagulation ensures the full anticoagulant effect in the early post-procedural phase when embolic events are most likely to occur.179,180 In this context, in recent years it had become routine to perform AF ablation on uninterrupted warfarin with a therapeutic INR. Until recently there were little data to support this practice for NOACs however, emerging data from both meta-analyses and a recent large randomized study support the safety of this approach.179 This practice is likely to gain increasing acceptance particularly as reversal agents become more widely available for all NOACs.
Intraprocedurally, a strategy of more aggressive heparin dosing has evolved over the past decade in the light of data that patients who have an activated clotting time (ACT) of <300 s during the procedure have an increased risk of silent cerebral infarction. The current consensus document recommends an ACT target of 300–400 with repeated checks at 15–20 min intervals. In addition, echocardiographic data have demonstrated that thrombus may form on sheaths immediately following trans-septal puncture; as such 77% of Consensus Document Writing Group181 members give heparin prior to the trans-septal puncture.
Less data exist for ablation of ventricular arrhythmias although a recent study demonstrated the presence of new silent cerebral infarction in 7 of 12 patients having ablation of ventricular tachycardia originating from the left ventricle. The majority of these patients underwent ablation via a retrograde trans-aortic approach and the target ACT was 300–400 s. Whether the incidence would be lower using a trans-septal approach is unknown.
Similarly, limited data exist regarding the impact of device implantation on cognitive function and these have been discussed in the Cognitive Impairment after Cardiac Arrest section. Until more comprehensive data are available it seems prudent to follow recommendations in the 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal ICD programming and testing even though data that such an approach minimises cognitive impact are lacking. These include the 2A recommendation that: ‘It is reasonable to omit defibrillation efficacy testing in patients undergoing initial left pectoral transvenous ICD implantation procedures where appropriate sensing, pacing, and impedance values are obtained with fluoroscopically well-positioned right ventricular leads’; and the Class 3 recommendation that ‘Defibrillation efficacy testing at the time of implantation of a transvenous ICD should not be performed on patients with a documented non-chronic cardiac thrombus, AF or atrial flutter without adequate systemic anticoagulation, critical aortic stenosis, unstable coronary artery disease, recent stroke or TIA, haemodynamic instability, or other known morbidities associated with poor outcomes’.
Regarding the impact of left atrial appendage occlusion (LAAO) procedures on cognitive function, MRI-detected new acute brain lesions were detected in 12 of 23 (52%) patients after LAAO procedures using the Amulet, Occlutech, or LAmbre devices. New brain lesions were associated with a higher number of left atrial appendage angiographies although there was no apparent impact on cognitive testing.182 Procedural stroke, typically related to air embolism, has also been reported with the Watchman device in the PROTECT trial, although impact on long-term cognition is unknown.183,184 However, any long-term impact on cognition has not been reported.
Current knowledge gaps, future directions, and areas for research
Global management of dementia syndromes has been recently set as a public health priority, and the World Health Organization has prioritized seven research domains to reduce the global burden of dementia185: (i) Prevention, identification, and reduction of risk; (ii) Quality of care for people with dementia and their carers; (iii) Delivery of care and services for people with dementia and their carers; (iv) Diagnosis, biomarker development, and disease monitoring; (v) Pharmacological and non-pharmacological clinical–translational research; (vi) Public awareness and understanding; and (vii) Physiology and progression of normal ageing and disease pathogenesis. Other expert groups have also provided recommendations for further progress and improvements in dementia-related research186 and highlighted knowledge gaps in cardiovascular care of the elderly, including those with cognitive impairment.187 Declining incidence and age-specific prevalence of dementia in high-income countries188–191 implies that dementia risk is modifiable, likely through improved management of cardiovascular risk factors192 and psychosocial factors.186,193–195 Although dementia-prevention RCTs failed to confirm many signals from observational studies, those RCTs highlighted some key methodological issues to be considered in contemporary trials (Table 9).
Table 9.
Knowledge gaps and areas for further research
| Knowledge gap(s) | Further research | |
|---|---|---|
| General dementia-related research domainsa | ||
| Distinguishing progression of normal aging from pre-clinical cognitive decline | Physiology of normal aging/pathological neurodegenerative processes. |
|
| Identification of the risk factors and risk reduction | Interactions of shared pathological pathways and risk factors. |
|
| Early diagnosis, biomarkers and disease monitoring | Effective strategies for cognitive surveillance; Early detection of cognitive impairment; Monitoring of disease progression. |
|
| Prevention of cognitive impairment/dementia | Effective preventive strategies in general population. |
|
| Pharmacological and non-pharmacological clinical–translational research on dementia diagnosis and treatment | Effective dementia-specific therapies are not available yet. |
|
| AF-specific research domains | ||
| Association of AF with cognitive impairment or dementia | Underlying mechanisms beyond clinically overt strokes, silent strokes and aging are poorly understood. |
|
| Time-course of cognitive impairment in AF patients | Mechanism(s) of accelerated development of dementia in AF patients. |
|
| Rhythm control and other strategies, including ablation, for AF burden reduction | Short- and long-term effects on cognitive function in AF patients |
|
| Pharmacological rate control therapies in AF; AV node ablation with permanent pacemaker implantation for rate control in AF | Short- and long-term effects on cognitive function in AF patients |
|
| VKA, NOACs and non-pharmacological (LAAO) thromboprophylaxis in AF | Long-term effects on cognitive function in AF patients |
|
| Screening for asymptomatic AF | Asymptomatic AF-associated risk of cognitive impairment |
|
| Early AF detection and treatment | Effects of early aggressive rhythm control on cognitive function |
|
| Primary prevention of AF | Effective interventions for primary prevention (ongoing research) |
|
| Other arrhythmia-specific research domains | ||
| SCD risk assessment and SCD prevention | Effective strategies in individuals with cognitive limitations |
|
| Catheter ablation of ventricular arrhythmias | Short and long-term effects in individuals with cognitive limitations |
|
| ICD for primary and secondary prevention | Short- and long-term effects in patients with cognitive limitations |
|
| CRT | Effects in patients with cognitive limitations |
|
Compiled and modified from the Refs.131–133,185–187 (for details on the quality of care, delivery of care and services for individuals with dementia and public awareness and understanding see the cited documents).
AF, atrial fibrillation; AV, atrioventricular; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; LAAO, left atrial appendage occlusion; NOACs, non-vitamin K antagonist oral anticoagulants; OAC, oral anticoagulation; QALY, quality-adjusted life-years; QoL, quality of life; SCD, sudden cardiac death; VKA, vitamin K antagonist.
Elderly patients with cognitive impairment commonly have mixed pathologies, including cardiovascular disease (e.g. AF and heart failure)80,85,196–198 but the complexity of shared pathological pathways and risk factors is still poorly understood and warrants further research. Better understanding of the mechanisms and determinants of AF-related cognitive impairment/dementia beyond aging and stroke would inform AF-specific preventive strategies to attenuate and/or postpone cognitive deterioration. Prospective studies addressing the long-term effects of AF treatments directed towards rhythm control, reduction of total AF burden and improvement in cardiac function (i.e. catheter ablation and pharmacotherapy) on cognitive function are needed. Inherent to achieving these targets is consistent utilization of well-validated neurocognitive measures. Also, the effects of lifestyle changes resulting in weight loss, improvement in overall cardio-metabolic risk profile and reduced AF burden need to be investigated. Further research is needed to optimise rate control in AF relative to cognitive function.
Cognitive endpoints were not addressed in the recent large RCTs of OAC (oral anticoagulation: VKA or NOACs) for thromboprophylaxis in AF, with one exception, the Birmingham Atrial Fibrillation Treatment of the Aged Study (BAFTA) which reported better cognitive function in elderly patients receiving warfarin compared with aspirin.113 In retrospective observational studies the risk of dementia increased with poor management of VKA (a low TTR),75,112 whilst NOAC use was associated with lower risk for dementia compared with warfarin.114 Observational data on AF patients diagnosed with dementia consistently shows significant VKA underuse or discontinuation62 even post-stroke,199 despite similar VKA-related bleeding risk irrespective of the cognitive status. Large prospective studies with pre-specified cognitive outcomes are needed to identify optimal thrombo-prophylactic strategies for AF patients with cognitive impairment/dementia. Importantly, the effects of early AF treatment or primary AF prevention on cognitive deterioration in patients at risk for both conditions remain to be elucidated.
Elderly patients and those with cognitive impairment/dementia were generally under-represented in catheter ablation and CIED (i.e. ICD and cardiac resynchronization therapy) trials. Studies are needed to better define the risks and benefits of cardiac arrhythmias ablation or CIED implantation, long-term effects of these interventions on cognitive status and optimal strategies for shared decision-making and end-of-life decisions in these patients (Table 9).
Recommendations
Interventions that can be considered for prevention of cognitive dysfunction in AF patients are summarized in Table 10. The writing committee reached consensus on recommendations summarized in Table 11.
Table 10.
Interventions to be considered for prevention of cognitive dysfunction in atrial fibrillation patients
Pharmacological interventions
|
Multifactorial vascular risk factor management
|
Nutritional interventions
|
Others
|
AF, atrial fibrillation; TTR, time in therapeutic range.
Table 11.
Recommendations for measures to prevent cognitive dysfunction in AF patients
| Preventive measures of cognitive dysfunction in patients with AF | Class | Ref. |
|---|---|---|
| Appropriate anticoagulation in patients with AF and stroke risk factors should be applied for the prevention of cognitive dysfunction. |  |
107,111 |
| Consider NOAC instead of VKA when using oral anticoagulation for the prevention of stroke in AF, which may have a beneficial effect on subsequent cognitive disorders |  |
107,114 |
| In patients with AF managed with long-term VKA, a high anticoagulation time in therapeutic range may be beneficial for optimal prevention of new-onset dementia |  |
75,107 |
| General health measures (prevention of smoking, hypertension, obesity and diabetes, sleep apnoea, and appropriate control of all risk factors) may reduce the concomitant risks of AF (new onset or recurrences) and stroke, with a putative benefit on cognitive function. |  |
107,115 |
| Prevention of cognitive dysfunction in AF may include general measures proposed in vascular dementia or Alzheimer’s disease. |  |
116 |
| Cognitive assessment should be performed in AF patients where there is suspicion of cognitive impairment. |  |
204 |
AF, atrial fibrillation; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist.
Supplementary Material
Footnotes
Developed in partnership with and endorsed by the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC), the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS).
Contributor Information
ESC Scientific Document Group:
William-Fernando Bautista-Vargas, Chern-En Chiang, Alejandro Cuesta, Gheorghe-Andrei Dan, David S Frankel, Yutao Guo, Robert Hatala, Young Soo Lee, Yuji Murakawa, Cara N Pellegrini, Claudio Pinho, David J Milan, Daniel P Morin, Elenir Nadalin, George Ntaios, Mukund A Prabhu, Marco Proietti, Lena Rivard, Mariana Valentino, and Alena Shantsila
References
- 1. Benhorin J, Bodenheimer M, Brown M, Case R, Dwyer EM Jr, Eberly S. et al. Improving clinical practice guidelines for practicing cardiologists. Am J Cardiol 2015;115:1773–6. [DOI] [PubMed] [Google Scholar]
- 2. Knopman DS, Ryberg S.. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol 1989;46:141–5. [DOI] [PubMed] [Google Scholar]
- 3. Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corp; 1981. [Google Scholar]
- 4. Lezak MD. Neuropsychological Assessment. 3rd ed.New York: Oxford University Press; 1995. [Google Scholar]
- 5. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO. et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240–6. [DOI] [PubMed] [Google Scholar]
- 6. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 7. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP.. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75. [DOI] [PubMed] [Google Scholar]
- 8. Alexander M, Perera G, Ford L, Arrighi HM, Foskett N, Debove C. et al. Age-stratified prevalence of mild cognitive impairment and dementia in European populations: a systematic review. J Alzheimer's Dis 2015;48:355–9. [DOI] [PubMed] [Google Scholar]
- 9. Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JCS. et al. Incidence and prevalence of dementia in the cardiovascular health study. J Am Geriatr Soc 2004;52:195–204. [DOI] [PubMed] [Google Scholar]
- 10. Schrijvers EMC, Schurmann B, Koudstaal PJ, van den Bussche H, Van Duijn CM, Hentschel F. et al. Genome-wide association study of vascular dementia. Stroke 2012;43:315–9. [DOI] [PubMed] [Google Scholar]
- 11. Ott A, Breteler MM, van Harskamp F, Claus JJ, van der Cammen TJ, Grobbee DE. et al. Prevalence of Alzheimer's disease and vascular dementia: association with education. The Rotterdam study. BMJ 1995;310:970–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fratiglioni L, Paillard-Borg S, Winblad B.. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol 2004;3:343–53. [DOI] [PubMed] [Google Scholar]
- 13. Beydoun MA, Beydoun HA, Wang Y.. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev 2008;9:204–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anstey KJ, von Sanden C, Salim A, O'Kearney R.. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol 2007;166:367–78. [DOI] [PubMed] [Google Scholar]
- 15. Crooks VC, Lubben J, Petitti DB, Little D, Chiu V.. Social network, cognitive function, and dementia incidence among elderly women. Am J Public Health 2008;98:1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR. et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000;21:49–55. [DOI] [PubMed] [Google Scholar]
- 17. Gottesman RF, Schneider ALC, Albert M, Alonso A, Bandeen-Roche K, Coker L. et al. Midlife hypertension and 20-year cognitive change the atherosclerosis risk in communities neurocognitive study. JAMA Neurol 2014;71:1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peila R, Rodriguez BL, Launer LJ.. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies—the Honolulu-Asia Aging Study. Diabetes 2002;51:1256–62. [DOI] [PubMed] [Google Scholar]
- 19. Rawlings AM, Sharrett AR, Schneider ALC, Coresh J, Albert M, Couper D. et al. Diabetes in midlife and cognitive change over 20 years a cohort study. Ann Intern Med 2014;161:785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anstey KJ, Ashby-Mitchell K, Peters R.. Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J Alzheimers Dis 2017;56:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Appleton JP, Scutt P, Sprigg N, Bath PM.. Hypercholesterolaemia and vascular dementia. Clin Sci 2017;131:1561–78. [DOI] [PubMed] [Google Scholar]
- 22. Ivan CS, Seshadri S, Beiser A, Au R, Kase CS, Kelly-Hayes M. et al. Dementia after stroke—the Framingham Study. Stroke 2004;35:1264–8. [DOI] [PubMed] [Google Scholar]
- 23. Pendlebury ST, Rothwell PM.. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009;8:1006–18. [DOI] [PubMed] [Google Scholar]
- 24. Kwok CS, Loke YK, Hale R, Potter JF, Myint PK.. Atrial fibrillation and incidence of dementia: a systematic review and meta-analysis. Neurology 2011;76:914–22. [DOI] [PubMed] [Google Scholar]
- 25. Kalantarian S, Stern TA, Mansour M, Ruskin JN.. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med 2013;158:338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R.. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005;65:545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Unverzagt FW, McClure LA, Wadley VG, Jenny NS, Go RC, Cushman M. et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology 2011;77:1729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laurin D, Masaki KH, White LR, Launer LJ.. Ankle-to-brachial index and dementia—the Honolulu-Asia aging study. Circulation 2007;116:2269–74. [DOI] [PubMed] [Google Scholar]
- 29. Leng Y, McEvoy CT, Allen IE, Yaffe K.. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol 2017;74:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Young J, Meagher D, Maclullich A.. Cognitive assessment of older people. BMJ 2011;343:d5042.. [DOI] [PubMed] [Google Scholar]
- 31. Mijajlovic MD, Pavlovic A, Brainin M, Heiss WD, Quinn TJ, Ihle-Hansen HB. et al. Post-stroke dementia—a comprehensive review. BMC Med 2017;15:11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brodaty H, Connors MH, Loy C, Teixeira-Pinto A, Stocks N, Gunn J. et al. Screening for dementia in primary care: a comparison of the GPCOG and the MMSE. Dement Geriatr Cogn Disord 2016;42:323–30. [DOI] [PubMed] [Google Scholar]
- 33. Milne A, Culverwell A, Guss R, Tuppen J, Whelton R.. Screening for dementia in primary care: a review of the use, efficacy and quality of measures. Int Psychogeriatr 2008;20:911–26. [DOI] [PubMed] [Google Scholar]
- 34. Li F, Jia XF, Jia J.. The Informant Questionnaire on Cognitive Decline in the Elderly individuals in screening mild cognitive impairment with or without functional impairment. J Geriatr Psychiatry Neurol 2012;25:227–32. [DOI] [PubMed] [Google Scholar]
- 35. Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:233–8. [DOI] [PubMed] [Google Scholar]
- 36. Agrell B, Dehlin O.. The clock-drawing test. 1998. Age Ageing 2012;41(Suppl 3):iii41–5. [DOI] [PubMed] [Google Scholar]
- 37. Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC.. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002;40:771–81. [DOI] [PubMed] [Google Scholar]
- 38. Wilber ST, Lofgren SD, Mager TG, Blanda M, Gerson LW.. An evaluation of two screening tools for cognitive impairment in older emergency department patients. Acad Emerg Med 2005;12:612–6. [DOI] [PubMed] [Google Scholar]
- 39. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A.. The mini-cog: a cognitive ′vital signs′ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000;15:1021–7. [DOI] [PubMed] [Google Scholar]
- 40. Buschke H, Kuslansky G, Katz M, Stewart WF, Sliwinski MJ, Eckholdt HM. et al. Screening for dementia with the memory impairment screen. Neurology 1999;52:231–8. [DOI] [PubMed] [Google Scholar]
- 41. Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H.. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry 1983;140:734–9. [DOI] [PubMed] [Google Scholar]
- 42. Brodaty H, Pond D, Kemp NM, Luscombe G, Harding L, Berman K. et al. The GPCOG: a new screening test for dementia designed for general practice. J Am Geriatr Soc 2002;50:530–4. [DOI] [PubMed] [Google Scholar]
- 43. Folstein MF, Folstein SE, McHugh PR.. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 44. Nasreddine ZS, Phillips NA, Bédirian VÃ, Charbonneau S, Whitehead V, Collin I. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- 45. Demeyere N, Riddoch MJ, Slavkova ED, Bickerton WL, Humphreys GW.. The Oxford Cognitive Screen (OCS): validation of a stroke-specific short cognitive screening tool. Psychol Assess 2015;27:883–94. [DOI] [PubMed] [Google Scholar]
- 46. Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, Hodges JR.. A brief cognitive test battery to differentiate Alzheimer's disease and frontotemporal dementia. Neurology 2000;55:1613–20. [DOI] [PubMed] [Google Scholar]
- 47. Teng EL, Chui HC.. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–8. [PubMed] [Google Scholar]
- 48. Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S. et al. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986;149:698–709. [DOI] [PubMed] [Google Scholar]
- 49. Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A. et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 1994;6:45–58; discussion 62. [DOI] [PubMed] [Google Scholar]
- 50. Jorm AF, Jacomb PA.. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med 1989;19:1015–22. [DOI] [PubMed] [Google Scholar]
- 51. Woodford HJ, George J.. Cognitive assessment in the elderly: a review of clinical methods. QJM 2007;100:469–84. [DOI] [PubMed] [Google Scholar]
- 52. Knopman DS, Parisi JE, Boeve BF, Cha RH, Apaydin H, Salviati A. et al. Vascular dementia in a population-based autopsy study. Arch Neurol 2003;60:569–75. [DOI] [PubMed] [Google Scholar]
- 53. Heiss WD, Rosenberg GA, Thiel A, Berlot R, de Reuck J.. Neuroimaging in vascular cognitive impairment: a state-of-the-art review. BMC Med 2016;14:174.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Waldemar G, Dubois B, Emre M, Georges J, McKeith IG, Rossor M. et al. Recommendations for the diagnosis and management of Alzheimer's disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol 2007;14:e1–26. [DOI] [PubMed] [Google Scholar]
- 55. Chen LY, Agarwal SK, Norby FL, Gottesman RF, Loehr LR, Soliman EZ. et al. Persistent but not paroxysmal atrial fibrillation is independently associated with lower cognitive function: ARIC Study. J Am Coll Cardiol 2016;67:1379–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gaita F, Corsinovi L, Anselmino M, Raimondo C, Pianelli M, Toso E. et al. Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. J Am Coll Cardiol 2013;62:1990–7. [DOI] [PubMed] [Google Scholar]
- 57. Dietzel J, Haeusler KG, Endres M.. Does atrial fibrillation cause cognitive decline and dementia? Europace 2017. Apr 6 [Epub ahead of print], doi:10.1093/europace/eux031. [DOI] [PubMed] [Google Scholar]
- 58. Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S.. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med 2016;374:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hart RG, Pearce LA, Aguilar MI.. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. [DOI] [PubMed] [Google Scholar]
- 60. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD. et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet (London, England) 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 61. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr. et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–104. [DOI] [PubMed] [Google Scholar]
- 62. Orkaby AR, Ozonoff A, Reisman JI, Miller DR, Zhao S, Rose AJ.. Continued use of warfarin in veterans with atrial fibrillation after dementia diagnosis. J Am Geriatr Soc 2017;65:249–56. [DOI] [PubMed] [Google Scholar]
- 63. Ntaios G, Papavasileiou V, Diener HC, Makaritsis K, Michel P.. Nonvitamin-K-antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and previous stroke or transient ischemic attack: an updated systematic review and meta-analysis of randomized controlled trials. Int J Stroke 2017;12:589–96. [DOI] [PubMed] [Google Scholar]
- 64. Kalantarian S, Ruskin JN.. Atrial fibrillation and cognitive decline: phenomenon or epiphenomenon? Cardiol Clin 2016;34:279–85. [DOI] [PubMed] [Google Scholar]
- 65. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M. et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 66. Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C. et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Graves KG, May HT, Jacobs V, Bair TL, Stevens SM, Woller SC. et al. Atrial fibrillation incrementally increases dementia risk across all CHADS2 and CHA2DS2VASc strata in patients receiving long-term warfarin. Am Heart J 2017;188:93–8. [DOI] [PubMed] [Google Scholar]
- 68. Vermeer SE, Den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM.. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke 2003;34:392–6. [DOI] [PubMed] [Google Scholar]
- 69. Kalantarian S, Ay H, Gollub RL, Lee H, Retzepi K, Mansour M. et al. Association between atrial fibrillation and silent cerebral infarctions: a systematic review and meta-analysis. Ann Intern Med 2014;161:650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Deneke T, Jais P, Scaglione M, Schmitt R, Di Biase L, Christopoulos G. et al. Silent cerebral events/lesions related to atrial fibrillation ablation: a clinical review. J Cardiovasc Electrophysiol 2015;26:455–63. [DOI] [PubMed] [Google Scholar]
- 71. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM.. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–22. [DOI] [PubMed] [Google Scholar]
- 72. Choudhury NA, DeBaun MR, Rodeghier M, King AA, Strouse JJ, McKinstry RC.. Silent cerebral infarct definitions and full-scale IQ loss in children with sickle cell anemia. Neurology 2018;90:e239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Konno S, Meyer JS, Terayama Y, Margishvili GM, Mortel KF.. Classification, diagnosis and treatment of vascular dementia. Drugs Aging 1997;11:361–73. [DOI] [PubMed] [Google Scholar]
- 74. Chen LY, Lopez FL, Gottesman RF, Huxley RR, Agarwal SK, Loehr L. et al. Atrial fibrillation and cognitive decline-the role of subclinical cerebral infarcts: the atherosclerosis risk in communities study. Stroke 2014;45:2568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jacobs V, Woller SC, Stevens S, May HT, Bair TL, Anderson JL. et al. Time outside of therapeutic range in atrial fibrillation patients is associated with long-term risk of dementia. Heart Rhythm 2014;11:2206–13. [DOI] [PubMed] [Google Scholar]
- 76. Bunch TJ, May HT, Bair TL, Crandall BG, Cutler MJ, Day JD. et al. Dementia rates increase with delays in initiation of anticoagulation treatment for atrial fibrillation. Heart Rhythm 2017;14:S73. [Google Scholar]
- 77. Saito T, Kawamura Y, Sato N, Kano K, Takahashi K, Asanome A. et al. Non-vitamin K antagonist oral anticoagulants do not increase cerebral microbleeds. J Stroke Cerebrovasc Dis 2015;24:1373–7. [DOI] [PubMed] [Google Scholar]
- 78. Inohara T, Xian Y, Liang L, Matsouaka RA, Saver JL, Smith EE. et al. Association of intracerebral hemorrhage among patients taking non-vitamin K antagonist vs vitamin K Antagonist oral anticoagulants with in-hospital mortality. JAMA 2018;319:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bellmann B, Fiebach JB, Guttmann S, Lin T, Haeusler KG, Bathe-Peters R. et al. Incidence of MRI-detected brain lesions and neurocognitive function after electrical cardioversion in anticoagulated patients with persistent atrial fibrillation. Int J Cardiol 2017;243:239–43. [DOI] [PubMed] [Google Scholar]
- 80. Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A.. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke 1997;28:316–21. [DOI] [PubMed] [Google Scholar]
- 81. Kilander L, Andren B, Nyman H, Lind L, Boberg M, Lithell H.. Atrial fibrillation is an independent determinant of low cognitive function: a cross-sectional study in elderly men. Stroke 1998;29:1816–20. [DOI] [PubMed] [Google Scholar]
- 82. Santangeli P, Di Biase L, Bai R, Mohanty S, Pump A, Cereceda Brantes M. et al. Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm 2012;9:1761–8. [DOI] [PubMed] [Google Scholar]
- 83. Thacker EL, McKnight B, Psaty BM, Longstreth WT Jr, Sitlani CM, Dublin S. et al. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology 2013;81:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS. et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer's dementia. Heart Rhythm 2010;7:433–7. [DOI] [PubMed] [Google Scholar]
- 85. de Bruijn RF, Heeringa J, Wolters FJ, Franco OH, Stricker BH, Hofman A. et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015;72:1288–94. [DOI] [PubMed] [Google Scholar]
- 86. Coma M, Gonzalez-Moneo MJ, Enjuanes C, Velazquez PP, Espargaro DB, Perez BA. et al. Effect of permanent atrial fibrillation on cognitive function in patients with chronic heart failure. Am J Cardiol 2016;117:233–9. [DOI] [PubMed] [Google Scholar]
- 87. Proietti M, Recchia A, Riva E, Lucca U, Tettamanti M, Mannucci PM. et al. Relationship between atrial fibrillation and cognitive decline in individuals aged 80 and older. Eur J Int Med 2017;46:6–10. [DOI] [PubMed] [Google Scholar]
- 88. O'Connell JE, Gray CS, French JM, Robertson IH.. Atrial fibrillation and cognitive function: case-control study. J Neurol Neurosurg Psychiatry 1998;65:386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mielke MM, Rosenberg PB, Tschanz J, Cook L, Corcoran C, Hayden KM. et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology 2007;69:1850–8. [DOI] [PubMed] [Google Scholar]
- 90. Rocken C, Peters B, Juenemann G, Saeger W, Klein HU, Huth C. et al. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation 2002;106:2091–7. [DOI] [PubMed] [Google Scholar]
- 91. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA. et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016;18:1455–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rollo J, Knight S, May HT, Anderson JL, Muhlestein JB, Bunch TJ. et al. Incidence of dementia in relation to genetic variants at PITX2, ZFHX3, and ApoE epsilon4 in atrial fibrillation patients. Pacing Clin Electrophysiol 2015;38:171–7. [DOI] [PubMed] [Google Scholar]
- 93. Wardlaw JM, Smith C, Dichgans M.. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Poggesi A, Inzitari D, Pantoni L.. Atrial fibrillation and cognition: epidemiological data and possible mechanisms. Stroke 2015;46:3316–21. [DOI] [PubMed] [Google Scholar]
- 95. Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D.. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: a review. J Cereb Blood Flow Metab 2016;36:72–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Goette A, Bukowska A, Lendeckel U, Erxleben M, Hammwohner M, Strugala D. et al. Angiotensin II receptor blockade reduces tachycardia-induced atrial adhesion molecule expression. Circulation 2008;117:732–42. [DOI] [PubMed] [Google Scholar]
- 97. Goette A, Ittenson A, Hoffmanns P, Reek S, Hartung W, Klein H. et al. Increased expression of P-selectin in patients with chronic atrial fibrillation. Pacing Clin Electrophysiol 2000;23:1872–5. [DOI] [PubMed] [Google Scholar]
- 98. Knecht S, Oelschlager C, Duning T, Lohmann H, Albers J, Stehling C. et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J 2008;29:2125–32. [DOI] [PubMed] [Google Scholar]
- 99. Petersen P, Kastrup J, Videbæk R, Boysen G.. Cerebral blood flow before and after cardioversion of atrial fibrillation. J Cereb Blood Flow Metab 1989;9:422–5. [DOI] [PubMed] [Google Scholar]
- 100. Gomez CR, McLaughlin JR, Njemanze PC, Nashed A.. Effect of cardiac dysfunction upon diastolic cerebral blood flow. Angiology 1992;43:625–30. [DOI] [PubMed] [Google Scholar]
- 101. Totaro R, Corridoni C, Marini C, Marsili R, Prencipe M.. Transcranial Doppler evaluation of cerebral blood flow in patients with paroxysmal atrial fibrillation. Ital J Neurol Sci 1993;14:451–4. [DOI] [PubMed] [Google Scholar]
- 102. Stefansdottir H, Arnar DO, Aspelund T, Sigurdsson S, Jonsdottir MK, Hjaltason H. et al. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke 2013;44:1020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gardarsdottir M, Sigurdsson S, Aspelund T, Rokita H, Launer LJ, Gudnason V. et al. Atrial fibrillation is associated with decreased total cerebral blood flow and brain perfusion. Europace 2017. Aug 9 [Epub ahead of print], doi: 10.1093/europace/eux220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Okuno J, Yanagi H, Tomura S.. Is cognitive impairment a risk factor for poor compliance among Japanese elderly in the community? Eur J Clin Pharmacol 2001;57:589–94. [DOI] [PubMed] [Google Scholar]
- 105. Salas M, In’t Veld BA, van der Linden PD, Hofman A, Breteler M, Stricker BH.. Impaired cognitive function and compliance with antihypertensive drugs in elderly: the Rotterdam Study. Clin Pharmacol Ther 2001;70:561–6. [DOI] [PubMed] [Google Scholar]
- 106. Marzona I, O'Donnell M, Teo K, Gao P, Anderson C, Bosch J. et al. Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. CMAJ 2012;184:E329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 108. Plassman BL, Langa KM, McCammon RJ, Fisher GG, Potter GG, Burke JR. et al. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol 2011;70:418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Moffitt P, Lane DA, Park H, O'Connell J, Quinn TJ.. Thromboprophylaxis in atrial fibrillation and association with cognitive decline: systematic review. Age Ageing 2016;45:767–75. [DOI] [PubMed] [Google Scholar]
- 110. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol 2017;14:627–8. [DOI] [PubMed] [Google Scholar]
- 111. Friberg L, Rosenqvist M.. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2017;39:453–60. [DOI] [PubMed] [Google Scholar]
- 112. Jacobs V, Woller SC, Stevens SM, May HT, Bair TL, Crandall BG. et al. Percent time with a supratherapeutic INR in atrial fibrillation patients also using an antiplatelet agent is associated with long-term risk of dementia. J Cardiovasc Electrophysiol 2015. [DOI] [PubMed] [Google Scholar]
- 113. Mavaddat N, Roalfe A, Fletcher K, Lip GY, Hobbs FD, Fitzmaurice D. et al. Warfarin versus aspirin for prevention of cognitive decline in atrial fibrillation: randomized controlled trial (Birmingham Atrial Fibrillation Treatment of the Aged Study). Stroke 2014;45:1381–6. [DOI] [PubMed] [Google Scholar]
- 114. Jacobs V, May HT, Bair TL, Crandall BG, Cutler MJ, Day JD. et al. Long-term population-based cerebral ischemic event and cognitive outcomes of direct oral anticoagulants compared with warfarin among long-term anticoagulated patients for atrial fibrillation. Am J Cardiol 2016;118:210–4. [DOI] [PubMed] [Google Scholar]
- 115. Gorenek B, Pelliccia A, Benjamin EJ, Boriani G, Crijns HJ, Fogel RI. et al. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Europace 2017;19:190–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Andrieu S, Coley N, Lovestone S, Aisen PS, Vellas B.. Prevention of sporadic Alzheimer's disease: lessons learned from clinical trials and future directions. Lancet Neurol 2015;14:926–44. [DOI] [PubMed] [Google Scholar]
- 117. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS. et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gaita F, Scaglione M, Battaglia A, Matta M, Gallo C, Galata M. et al. Very long-term outcome following transcatheter ablation of atrial fibrillation. Are results maintained after 10 years of follow up? Europace 2017. Feb 28 [Epub ahead of print], doi:10.1093/europace/eux008. [DOI] [PubMed] [Google Scholar]
- 119. Kawaji T, Shizuta S, Morimoto T, Aizawa T, Yamagami S, Yoshizawa T. et al. Very long-term clinical outcomes after radiofrequency catheter ablation for atrial fibrillation: a large single-center experience. Int J Cardiol 2017;249:204–13. [DOI] [PubMed] [Google Scholar]
- 120. Mujovic N, Marinkovic M, Markovic N, Shantsila A, Lip GY, Potpara TS.. Prediction of very late arrhythmia recurrence after radiofrequency catheter ablation of atrial fibrillation: the MB-LATER clinical score. Sci Rep 2017;7:40828.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Haeusler KG, Kirchhof P, Endres M.. Left atrial catheter ablation and ischemic stroke. Stroke 2012;43:265–70. [DOI] [PubMed] [Google Scholar]
- 122. Herm J, Fiebach JB, Koch L, Kopp UA, Kunze C, Wollboldt C. et al. Neuropsychological effects of MRI-detected brain lesions after left atrial catheter ablation for atrial fibrillation: long-term results of the MACPAF study. Circ Arrhythm Electrophysiol 2013;6:843–50. [DOI] [PubMed] [Google Scholar]
- 123. Medi C, Evered L, Silbert B, Teh A, Halloran K, Morton J. et al. Subtle post-procedural cognitive dysfunction after atrial fibrillation ablation. J Am Coll Cardiol 2013;62:531–9. [DOI] [PubMed] [Google Scholar]
- 124. Efimova I, Efimova N, Chernov V, Popov S, Lishmanov Y.. Ablation and pacing: improving brain perfusion and cognitive function in patients with atrial fibrillation and uncontrolled ventricular rates. Pacing Clin Electrophysiol 2012;35:320–6. [DOI] [PubMed] [Google Scholar]
- 125. Maryniak A, Bielawska A, Bieganowska K, Miszczak-Knecht M, Walczak F, Szumowski L.. Does atrioventricular reentry tachycardia (AVRT) or atrioventricular nodal reentry tachycardia (AVNRT) in children affect their cognitive and emotional development? Pediatr Cardiol 2013;34:893–7. [DOI] [PubMed] [Google Scholar]
- 126. Roine RO, Kajaste S, Kaste M.. Neuropsychological sequelae of cardiac arrest. JAMA 1993;269:237–42. [PubMed] [Google Scholar]
- 127. Baron JC. Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc Dis 2001;11(Suppl 1):2–8. [DOI] [PubMed] [Google Scholar]
- 128. Moulaert VR, Verbunt JA, van Heugten CM, Wade DT.. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation 2009;80:297–305. [DOI] [PubMed] [Google Scholar]
- 129. Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW. et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008;118:2452–83. [DOI] [PubMed] [Google Scholar]
- 130. Lim C, Verfaellie M, Schnyer D, Lafleche G, Alexander MP.. Recovery, long-term cognitive outcome and quality of life following out-of-hospital cardiac arrest. J Rehabil Med 2014;46:691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Moulaert VR, Wachelder EM, Verbunt JA, Wade DT, van Heugten CM.. Determinants of quality of life in survivors of cardiac arrest. J Rehabil Med 2010;42:553–8. [DOI] [PubMed] [Google Scholar]
- 132. Polanowska KE, Sarzynska-Dlugosz IM, Paprot AE, Sikorska S, Seniow JB, Karpinski G. et al. Neuropsychological and neurological sequelae of out-of-hospital cardiac arrest and the estimated need for neurorehabilitation: a prospective pilot study. Kardiol Pol 2014;72:814–22. [DOI] [PubMed] [Google Scholar]
- 133. Rewers M, Tilgreen RE, Crawford ME, Hjortso N.. One-year survival after out-of-hospital cardiac arrest in Copenhagen according to the ′Utstein style′. Resuscitation 2000;47:137–46. [DOI] [PubMed] [Google Scholar]
- 134. Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D. et al. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a Statement for Healthcare Professionals From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation 2015;96:328–40. [DOI] [PubMed] [Google Scholar]
- 135. Bunch TJ, White RD, Gersh BJ, Meverden RA, Hodge DO, Ballman KV. et al. Long-term outcomes of out-of-hospital cardiac arrest after successful early defibrillation. N Engl J Med 2003;348:2626–33. [DOI] [PubMed] [Google Scholar]
- 136. Bunch TJ, White RD, Smith GE, Hodge DO, Gersh BJ, Hammill SC. et al. Long-term subjective memory function in ventricular fibrillation out-of-hospital cardiac arrest survivors resuscitated by early defibrillation. Resuscitation 2004;60:189–95. [DOI] [PubMed] [Google Scholar]
- 137. Alexander MP, Lafleche G, Schnyer D, Lim C, Verfaellie M.. Cognitive and functional outcome after out of hospital cardiac arrest. J Int Neuropsychol Soc 2011;17:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Torgersen J, Strand K, Bjelland TW, Klepstad P, Kvale R, Soreide E. et al. Cognitive dysfunction and health-related quality of life after a cardiac arrest and therapeutic hypothermia. Acta Anaesthesiol Scand 2010;54:721–8. [DOI] [PubMed] [Google Scholar]
- 139. Grubb NR, O'Carroll R, Cobbe SM, Sirel J, Fox KA.. Chronic memory impairment after cardiac arrest outside hospital. BMJ 1996;313:143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bigelow WG, Callaghan JC, Hopps JA.. General hypothermia for experimental intracardiac surgery; the use of electrophrenic respirations, an artificial pacemaker for cardiac standstill and radio-frequency rewarming in general hypothermia. Ann Surg 1950;132:531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Osborn JJ. Experimental hypothermia; respiratory and blood pH changes in relation to cardiac function. Am J Physiol 1953;175:389–98. [DOI] [PubMed] [Google Scholar]
- 142. Marchand P, Allan JC.. An experimental study of the effect of hypothermia on the heart and brain. S Afr J Med Sci 1956;21:127–41. [PubMed] [Google Scholar]
- 143. Zola-Morgan S, Squire LR, Amaral DG.. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci 1986;6:2950–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Colbourne F, Corbett D.. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci 1995;15:7250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Fugate JE, Moore SA, Knopman DS, Claassen DO, Wijdicks EF, White RD. et al. Cognitive outcomes of patients undergoing therapeutic hypothermia after cardiac arrest. Neurology 2013;81:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tiainen M, Poutiainen E, Kovala T, Takkunen O, Happola O, Roine RO.. Cognitive and neurophysiological outcome of cardiac arrest survivors treated with therapeutic hypothermia. Stroke 2007;38:2303–8. [DOI] [PubMed] [Google Scholar]
- 147. Neumar RW, Shuster M, Callaway CW, Gent LM, Atkins DL, Bhanji F. et al. Part 1: executive summary: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S315–67. [DOI] [PubMed] [Google Scholar]
- 148. Schenone AL, Cohen A, Patarroyo G, Harper L, Wang X, Shishehbor MH. et al. Therapeutic hypothermia after cardiac arrest: a systematic review/meta-analysis exploring the impact of expanded criteria and targeted temperature. Resuscitation 2016;108:102–10. [DOI] [PubMed] [Google Scholar]
- 149. Dalessio DJ, Benchimol A, Dimond EG.. Chronic encephalopathy related to heart block; its correction by permanent cardiac pacemaker. Neurology 1965;15:499–503. [DOI] [PubMed] [Google Scholar]
- 150. Rockwood K, Dobbs AR, Rule BG, Howlett SE, Black WR.. The impact of pacemaker implantation on cognitive functioning in elderly patients. J Am Geriatr Soc 1992;40:142–6. [DOI] [PubMed] [Google Scholar]
- 151. Koide H, Kobayashi S, Kitani M, Tsunematsu T, Nakazawa Y.. Improvement of cerebral blood flow and cognitive function following pacemaker implantation in patients with bradycardia. Gerontology 1994;40:279–85. [DOI] [PubMed] [Google Scholar]
- 152. Hernandez RK, Farwell W, Cantor MD, Lawler EV.. Cholinesterase inhibitors and incidence of bradycardia in patients with dementia in the veterans affairs New England Healthcare System. J Am Geriatr Soc 2009;57:1997–2003. [DOI] [PubMed] [Google Scholar]
- 153. Fowler NR, Johnson KG, Li J, Moore CG, Saba S, Lopez OL. et al. Use of cardiac implantable electronic devices in older adults with cognitive impairment. JAMA Intern Med 2014;174:1514–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Barbe C, Puisieux F, Jansen I, Dewailly P, Klug D, Kacet S. et al. Improvement of cognitive function after pacemaker implantation in very old persons with bradycardia. J Am Geriatr Soc 2002;50:778–80. [DOI] [PubMed] [Google Scholar]
- 155. Jama A, Rabinstein A, Hodge D, Herges R, Asirvatham S, Cha YM. et al. Cardiac device complications in the cognitively impaired. Pacing Clin Electrophysiol 2013;36:1061–7. [DOI] [PubMed] [Google Scholar]
- 156. Gribbin GM, Gallagher P, Young AH, McComb JM, McCue P, Toff WD. et al. The effect of pacemaker mode on cognitive function. Heart 2005;91:1209–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Thylen I, Moser DK, Stromberg A, Dekker RA, Chung ML.. Concerns about implantable cardioverter-defibrillator shocks mediate the relationship between actual shocks and psychological distress. Europace 2016;18:828–35. [DOI] [PubMed] [Google Scholar]
- 158. Haugaa KH, Potpara TS, Boveda S, Deharo JC, Chen J, Dobreanu D. et al. Patients' knowledge and attitudes regarding living with implantable electronic devices: results of a multicentre, multinational patient survey conducted by the European Heart Rhythm Association. Europace 2018;20:386. [DOI] [PubMed] [Google Scholar]
- 159. Hallas CN, Burke JL, White DG, Connelly DT.. A prospective 1-year study of changes in neuropsychological functioning after implantable cardioverter-defibrillator surgery. Circ Arrhythm Electrophysiol 2010;3:170–7. [DOI] [PubMed] [Google Scholar]
- 160. Kim J, Pressler SJ, Groh WJ.. Change in cognitive function over 12 months among patients with an implantable cardioverter-defibrillator. J Cardiovasc Nurs 2013;28:E28–36. [DOI] [PubMed] [Google Scholar]
- 161. Halas K, Krzyzanowski K, Krzyzanowska E, Smurzynski P, Ryczek R, Michalkiewicz D. et al. Cognitive impairment after appropriate implantable cardioverter-defibrillator therapy for ventricular fibrillation. Kardiol Pol 2014;72:134–9. [DOI] [PubMed] [Google Scholar]
- 162. Dixit NK, Vazquez LD, Cross NJ, Kuhl EA, Serber ER, Kovacs A. et al. Cardiac resynchronization therapy: a pilot study examining cognitive change in patients before and after treatment. Clin Cardiol 2010;33:84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Fumagalli S, Pieragnoli P, Ricciardi G, Mascia G, Mascia F, Michelotti F. et al. Cardiac resynchronization therapy improves functional status and cognition. Int J Cardiol 2016;219:212–7. [DOI] [PubMed] [Google Scholar]
- 164. Duncker D, Friedel K, Konig T, Schreyer H, Lusebrink U, Duncker M. et al. Cardiac resynchronization therapy improves psycho-cognitive performance in patients with heart failure. Europace 2015;17:1415–21. [DOI] [PubMed] [Google Scholar]
- 165. Hoth KF, Poppas A, Ellison KE, Paul RH, Sokobin A, Cho Y. et al. Link between change in cognition and left ventricular function following cardiac resynchronization therapy. J Cardiopulm Rehabil Prev 2010;30:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Conti JB, Sears SF.. Cardiac resynchronization therapy: can we make our heart failure patients smarter? Trans Am Clin Climatol Assoc 2007;118:153–64. [PMC free article] [PubMed] [Google Scholar]
- 167. Roalfe AK, Bryant TL, Davies MH, Hackett TG, Saba S, Fletcher K. et al. A cross-sectional study of quality of life in an elderly population (75 years and over) with atrial fibrillation: secondary analysis of data from the Birmingham Atrial Fibrillation Treatment of the Aged study. Europace 2012;14:1420–7. [DOI] [PubMed] [Google Scholar]
- 168. Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS. et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:839–45. [DOI] [PubMed] [Google Scholar]
- 169. Brachmann J, Lewalter T, Kuck KH, Andresen D, Willems S, Spitzer SG. et al. Long-term symptom improvement and patient satisfaction following catheter ablation of supraventricular tachycardia: insights from the German ablation registry. Eur Heart J 2017;38:1317–26. [DOI] [PubMed] [Google Scholar]
- 170. Saliba W, Schliamser JE, Lavi I, Barnett-Griness O, Gronich N, Rennert G.. Catheter ablation of atrial fibrillation is associated with reduced risk of stroke and mortality: a propensity score-matched analysis. Heart Rhythm 2017;14:635–42. [DOI] [PubMed] [Google Scholar]
- 171. Bunch TJ, May HT, Bair TL, Weiss JP, Crandall BG, Osborn JS. et al. Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm 2013;10:1272–7. [DOI] [PubMed] [Google Scholar]
- 172. Rillig A, Meyerfeldt U, Tilz RR, Talazko J, Arya A, Zvereva V. et al. Incidence and long-term follow-up of silent cerebral lesions after pulmonary vein isolation using a remote robotic navigation system as compared with manual ablation. Circ Arrhythm Electrophysiol 2012;5:15–21. [DOI] [PubMed] [Google Scholar]
- 173. Haines DE, Stewart MT, Ahlberg S, Barka ND, Condie C, Fiedler GR. et al. Microembolism and catheter ablation I: a comparison of irrigated radiofrequency and multielectrode-phased radiofrequency catheter ablation of pulmonary vein ostia. Circ Arrhythm Electrophysiol 2013;6:16–22. [DOI] [PubMed] [Google Scholar]
- 174. Miyazaki S, Watanabe T, Kajiyama T, Iwasawa J, Ichijo S, Nakamura H. et al. Thromboembolic risks of the procedural process in second-generation cryoballoon ablation procedures: analysis from real-time transcranial Doppler monitoring. Circ Arrhythm Electrophysiol 2017;10:e005612. [DOI] [PubMed] [Google Scholar]
- 175. Whitman IR, Gladstone RA, Badhwar N, Hsia HH, Lee BK, Josephson SA. et al. Brain emboli after left ventricular endocardial ablation. Circulation 2017;135:867–77. [DOI] [PubMed] [Google Scholar]
- 176. Gress DR. The problem with asymptomatic cerebral embolic complications in vascular procedures: what if they are not asymptomatic? J Am Coll Cardiol 2012;60:1614–6. [DOI] [PubMed] [Google Scholar]
- 177. Verma A, Debruyne P, Nardi S, Deneke T, DeGreef Y, Spitzer S. et al. Evaluation and reduction of asymptomatic cerebral embolism in ablation of atrial fibrillation, but high prevalence of chronic silent infarction: results of the evaluation of reduction of asymptomatic cerebral embolism trial. Circ Arrhythm Electrophysiol 2013;6:835–42. [DOI] [PubMed] [Google Scholar]
- 178. Schwarz N, Kuniss M, Nedelmann M, Kaps M, Bachmann G, Neumann T. et al. Neuropsychological decline after catheter ablation of atrial fibrillation. Heart Rhythm 2010;7:1761–7. [DOI] [PubMed] [Google Scholar]
- 179. Calkins H, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH. et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med 2017;376:1627–36. [DOI] [PubMed] [Google Scholar]
- 180. Providencia R, Marijon E, Albenque JP, Combes S, Combes N, Jourda F. et al. Rivaroxaban and dabigatran in patients undergoing catheter ablation of atrial fibrillation. Europace 2014;16:1137–44. [DOI] [PubMed] [Google Scholar]
- 181. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L. et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Rillig A, Bellmann B, Skurk C, Leistner DM, Haeusler KG, Lin T. et al. Left atrial appendage angiography is associated with the incidence and number of magnetic resonance imaging-detected brain lesions after percutaneous catheter-based left atrial appendage closure. Heart Rhythm 2018;15:3–8. [DOI] [PubMed] [Google Scholar]
- 183. Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M. et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534–42. [DOI] [PubMed] [Google Scholar]
- 184. Meier B, Blaauw Y, Khattab AA, Lewalter T, Sievert H, Tondo C. et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. Europace 2014;16:1397–416. [DOI] [PubMed] [Google Scholar]
- 185. Shah H, Albanese E, Duggan C, Rudan I, Langa KM, Carrillo MC. et al. Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol 2016;15:1285–94. [DOI] [PubMed] [Google Scholar]
- 186. Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H. et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol 2016;15:455–532. [DOI] [PubMed] [Google Scholar]
- 187. Rich MW, Chyun DA, Skolnick AH, Alexander KP, Forman DE, Kitzman DW. et al. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016;133:2103–22. [DOI] [PubMed] [Google Scholar]
- 188. Qiu C, von Strauss E, Backman L, Winblad B, Fratiglioni L.. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology 2013;80:1888–94. [DOI] [PubMed] [Google Scholar]
- 189. Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM.. Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 2012;78:1456–63. [DOI] [PubMed] [Google Scholar]
- 190. Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L. et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013;382:1405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. de Bruijn RF, Bos MJ, Portegies ML, Hofman A, Franco OH, Koudstaal PJ. et al. The potential for prevention of dementia across two decades: the prospective, population-based Rotterdam Study. BMC Med 2015;13:132.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. de Bruijn RF, Ikram MA.. Cardiovascular risk factors and future risk of Alzheimer's disease. BMC Med 2014;12:130.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Barnes DE, Yaffe K.. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C.. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol 2014;13:788–94. [DOI] [PubMed] [Google Scholar]
- 195. Solomon A, Mangialasche F, Richard E, Andrieu S, Bennett DA, Breteler M. et al. Advances in the prevention of Alzheimer's disease and dementia. J Intern Med 2014;275:229–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Liao JN, Chao TF, Liu CJ, Wang KL, Chen SJ, Tuan TC. et al. Risk and prediction of dementia in patients with atrial fibrillation–a nationwide population-based cohort study. Int J Cardiol 2015;199:25–30. [DOI] [PubMed] [Google Scholar]
- 197. Rusanen M, Kivipelto M, Levalahti E, Laatikainen T, Tuomilehto J, Soininen H. et al. Heart diseases and long-term risk of dementia and Alzheimer's disease: a population-based CAIDE study. J Alzheimers Dis 2014;42:183–91. [DOI] [PubMed] [Google Scholar]
- 198. Ikram MA, van Oijen M, de Jong FJ, Kors JA, Koudstaal PJ, Hofman A. et al. Unrecognized myocardial infarction in relation to risk of dementia and cerebral small vessel disease. Stroke 2008;39:1421–6. [DOI] [PubMed] [Google Scholar]
- 199. McGrath ER, Go AS, Chang Y, Borowsky LH, Fang MC, Reynolds K. et al. Use of oral anticoagulant therapy in older adults with atrial fibrillation after acute ischemic stroke. J Am Geriatr Soc 2017;65:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Miller JW, Harvey DJ, Beckett LA, Green R, Farias ST, Reed BR. et al. Vitamin D status and rates of cognitive decline in a multiethnic cohort of older adults. JAMA Neurol 2015;72:1295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Kern J, Kern S, Blennow K, Zetterberg H, Waern M, Guo X. et al. Calcium supplementation and risk of dementia in women with cerebrovascular disease. Neurology 2016;87:1674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hooshmand B, Mangialasche F, Kalpouzos G, Solomon A, Kareholt I, Smith AD. et al. Association of vitamin B12, folate, and sulfur amino acids with brain magnetic resonance imaging measures in older adults: a longitudinal population-based study. JAMA Psychiatry 2016;73:606–13. [DOI] [PubMed] [Google Scholar]
- 203. Kiliaan AJ, Arnoldussen IA, Gustafson DR.. Adipokines: a link between obesity and dementia? Lancet Neurol 2014;13:913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.This panel of experts.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.