Abstract
Aims
Increases in left ventricular filling pressure are a fundamental haemodynamic abnormality in heart failure with preserved ejection fraction (HFpEF). However, very little is known regarding how elevated filling pressures cause pulmonary abnormalities or symptoms of dyspnoea. We sought to determine the relationships between simultaneously measured central haemodynamics, symptoms, and lung ventilatory and gas exchange abnormalities during exercise in HFpEF.
Methods and results
Subjects with invasively-proven HFpEF (n = 50) and non-cardiac causes of dyspnoea (controls, n = 24) underwent cardiac catheterization at rest and during exercise with simultaneous expired gas analysis. During submaximal (20 W) exercise, subjects with HFpEF displayed higher pulmonary capillary wedge pressures (PCWP) and pulmonary artery pressures, higher Borg perceived dyspnoea scores, and increased ventilatory drive and respiratory rate. At peak exercise, ventilation reserve was reduced in HFpEF compared with controls, with greater dead space ventilation (higher VD/VT). Increasing exercise PCWP was directly correlated with higher perceived dyspnoea scores, lower peak exercise capacity, greater ventilatory drive, worse New York Heart Association (NYHA) functional class, and impaired pulmonary ventilation reserve.
Conclusion
This study provides the first evidence linking altered exercise haemodynamics to pulmonary abnormalities and symptoms of dyspnoea in patients with HFpEF. Further study is required to identify the mechanisms by which haemodynamic derangements affect lung function and symptoms and to test novel therapies targeting exercise haemodynamics in HFpEF.
Keywords: Dyspnoea , Exercise , Heart failure , Pulmonary function
Introduction
Exertional dyspnoea is the dominant symptom reported by people with heart failure (HF) and preserved ejection fraction (HFpEF).1 Dyspnoea interferes with activities of daily living and impairs quality of life, but its causes remain poorly understood. Multiple mechanisms have been linked to dyspnoea in HF patients, including abnormalities in the heart, lungs, and the periphery. These range from abnormalities in metaboreflex-stimulated neural signalling in skeletal muscle to congestion-mediated changes in pulmonary compliance, alterations in lung fluid, irritation of J-receptors, increased dead space ventilation, and impaired alveolar gas diffusion.2–5
Diastolic dysfunction causes an increase in left heart filling pressure during exercise in HFpEF.6,7 High filling pressures are widely considered to be the primary cause of dyspnoea, pulmonary limitations, and exercise intolerance in HFpEF, but this has never been directly demonstrated. In fact, prior studies investigating the effects of increased filling pressure during exercise in patients with HF with reduced EF (HFrEF) have revealed little to no relationship between haemodynamics, symptoms, and pulmonary limitations.8–13
However, there are important and fundamental differences between HFpEF and HFrEF,14 and no data is available in HFpEF directly relating simultaneously-measured central haemodynamics to symptoms or measures of pulmonary function during exercise. Accordingly, we sought to determine the relationships between central haemodynamics, symptoms of dyspnoea and ventilatory abnormalities during exercise in people with HFpEF.
Methods
Consecutive subjects referred to the Mayo Clinic catheterization laboratory for invasive exercise haemodynamic testing for exertional dyspnoea of unknown cause were enrolled in this prospective study between August 2011 and July 2013. Some participant data from this study has been previously published,7,15–17 but not as it relates to the HF symptoms or pulmonary function. Written informed consent was obtained by all patients. The study was approved by the Mayo Clinic Institutional Review Board and the study was registered (NCT01418248). All authors have read and agree to the manuscript as written.
Study population
Heart failure with preserved ejection fraction was defined by typical clinical symptoms (dyspnoea and fatigue), normal left ventricular (LV) EF (≥50%), and elevated left heart filling pressures (pulmonary capillary wedge pressure, PCWP) at rest (>15 mmHg) and/or with exercise (≥25 mmHg).6,7 Subjects with significant valvular disease (>mild stenosis, >moderate regurgitation), infiltrative, restrictive or hypertrophic cardiomyopathy, constrictive pericarditis, significant pulmonary disease including chronic obstructive pulmonary disease, pulmonary embolism, and right ventricular (RV) myopathies were excluded.
Control subjects were also referred to invasive exercise evaluation because of indication of exertional dyspnoea but were found to display no evidence of HF or cardiac cause of dyspnoea after thorough clinical evaluation, imaging and invasive assessment, including normal rest and exercise mean PCWP (criteria above) and normal rest and exercise pulmonary artery (PA) pressures (rest <25 mmHg, exercise <40 mmHg).
Study protocol
History and physical examination and echocardiography were performed in a blinded fashion following informed consent, but prior to transfer to the catheterization laboratory. Invasive haemodynamic data, blood sampling, expired gas measurements, and ventilatory assessments were performed simultaneously at rest in the supine position and then during supine cycle ergometry exercise, starting at 20 W for 5 min, increasing 10 W increments in 3 min stages to subject-reported exhaustion. Symptoms of dyspnoea and fatigue during exercise were rated by subjects during each stage according to the Borg perceived dyspnoea (0–10) and fatigue scales (6–20).18
Catheterization protocol
Subjects underwent maximal-effort supine cycle ergometry testing with simultaneous expired gas analysis on chronic medications as described previously.6,7,15–17 Right heart catheterization was performed through a 9 Fr sheath via the right internal jugular vein. Right atrial pressure, PA pressures, and PCWP were measured at end expiration (mean of ≥3 beats) using 2 Fr high fidelity micromanometer-tipped catheters (Millar Instruments, Houston, TX, USA) advanced through the lumen of a 7 Fr fluid-filled catheter (Balloon wedge, Arrow). Pressure tracings from the entire study were digitized (240 Hz) and stored for offline analysis by one investigator experienced in exercise haemodynamic assessment (B.A.B.). Pulmonary capillary wedge pressure position was confirmed by appearance on fluoroscopy, characteristic pressure waveforms, and oximetry (saturation ≥94%).
A 4–6 Fr radial arterial cannula was used to measure arterial blood pressure (BP) and sampling of arterial blood gases throughout the tests. Arterial-venous O2 difference (AVO2 diff) was measured directly as the difference between systemic arterial and PA O2 content (=saturation * haemoglobin * 1.34). Cardiac output (CO) was calculated by the direct Fick method (CO = VO2 ÷ AVO2 diff) at baseline, 20 W and peak exercise. Stroke volume (SV) was determined from the quotient of CO and heart rate. Pulmonary vascular function were assessed by pulmonary vascular resistance [PVR = (mean PA − PCWP)/CO] and PA compliance [PAC = stroke volume/(PA pulse pressure)].
Ventilation and expired gas analysis
Breath-by-breath oxygen consumption (VO2), carbon dioxide production (VCO2), tidal volume (VT), respiratory rate, minute ventilation (VE = VT × respiratory rate), and inspiratory time (TI) were measured continuously throughout the experiments as previously described (MedGraphics, St. Paul, MN, USA).3,7,15 Objective effort was estimated by the respiratory exchange ratio (RER = VCO2/VO2). Ventilatory efficiency was assessed by the slope of VE to VCO2, and ventilatory drive was estimated by the ratio of VT to TI.3,19 Pulmonary dead space (VD) is the volume that does not participate in gas exchange, and the sum of anatomical dead space (conduit airways such as the mouth and trachea) and alveolar dead space. The ratio of dead space ventilation to tidal volume (VD/VT) was determined from the modified alveolar gas equation using directly measured PaCO2, as described previously.2 All analyses of ventilation and gas exchange data were performed offline in a blinded fashion by one investigator (T.P.O.) with extensive experience in pulmonary and expired gas analysis.
Assessment of right ventricular function
Two-dimensional, Doppler, and tissue Doppler echocardiography was performed according to contemporary guidelines by experienced sonographers as described previously.15,20 Studies were interpreted offline in a blinded fashion. Right ventricular systolic and diastolic function was assessed using tissue velocities at the lateral tricuspid annulus (RV s′ and e′), and was obtained simultaneously with invasive assessment at rest and during all stages of exercise.
Statistical analysis
Data are presented as mean (standard deviation), median (interquartile range), or n (%). Between-group differences were compared by unpaired t test, Wilcoxon rank sum test, χ2 or Fisher’s exact test as appropriate. Pearson’s (normally-distributed) or Spearman’s (non-normal distribution) correlation analyses were used to assess relationships between invasive haemodynamics, subjective symptoms, and ventilatory parameters (see Supplementary material online, Table S1). As an exploratory, ancillary analysis, multivariable linear regression was used to test whether differences in exercise haemodynamic and pulmonary variables remain significant after adjusting for age, body mass index (BMI), and haemoglobin levels.
Results
Subject characteristics
Compared with controls, subjects with HFpEF were older, more obese, and anaemic and had higher creatinine and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels (Table 1). Prevalence of diabetes, hypertension, coronary artery disease, and atrial fibrillation was similar between the groups. Jugular vein distention and peripheral oedema were more common in HFpEF compared with controls, but pulmonary rales were rare in both groups, consistent with the fact that all participants were evaluated as compensated outpatients. Left ventricular dimension and EF were similar in cases and controls, but subjects with HFpEF displayed impaired diastolic function, with higher E/e′ ratio (Table 1).
Table 1.
Baseline characteristics
| Control (n = 24) | HFpEF (n = 50) | P-value | |
|---|---|---|---|
| Age (years) | 61 ± 12 | 70 ± 11 | 0.004 |
| Female | 11 (46) | 27 (54) | 0.6 |
| Body mass index (kg/m2) | 27.2 ± 4.4 | 34.4 ± 6.9 | <0.0001 |
| Comorbidities | |||
| Diabetes mellitus | 5 (21) | 18 (36) | 0.3 |
| Hypertension | 22 (92) | 48 (96) | 0.6 |
| Coronary disease | 6 (25) | 18 (36) | 0.4 |
| Atrial fibrillation | 2 (8) | 8 (16) | 0.5 |
| Current or ever smoking | 3 (13) | 14 (28) | 0.2 |
| Medications | |||
| ACEI or ARB | 11 (46) | 33 (66) | 0.1 |
| Beta-blocker | 11 (46) | 33 (66) | 0.1 |
| Loop diuretic | 5 (21) | 20 (40) | 0.1 |
| Laboratories | |||
| Haemoglobin (g/dL) | 13.9 ± 1.2 | 12.6 ± 1.3 | <0.0001 |
| Creatinine (mg/dL) | 1.0 (0.8–1.1) | 1.2 (0.9–1.6) | 0.01 |
| NT-proBNP (pg/mL) | 90 (44–429) | 406 (139–1257) | 0.0008 |
| Physical exam | |||
| JVP (<8/8–12/12–16/>16 cm) (n) | 24/0/0/0 | 26/15/2/7 | 0.0007 |
| Rales (n) | 0 | 2 | 1.0 |
| Oedema (none/mild/mod-sev) (n) | 23/1/0 | 30/13/7 | 0.006 |
| NYHA class | 2.3 ± 0.7 | 2.7 ± 0.5 | 0.01 |
| Echocardiography | |||
| LV diastolic dimension (mm) | 48 ± 6 | 48 ± 6 | 0.7 |
| LV ejection fraction (%) | 60 ± 9 | 62 ± 8 | 0.4 |
| E/e′ ratio | 7.5 (6.8–10.0) | 14.3 (10.2–17.9) | <0.0001 |
| RV e′ (cm/s) | 11 ± 5 | 12 ± 9 | 0.6 |
| RV s′ (cm/s) | 12 ± 3 | 11 ± 3 | 0.3 |
| Resting haemodynamics | |||
| Heart rate (b.p.m.) | 68 ± 13 | 67 ± 10 | 0.7 |
| Systolic BP (mmHg) | 139 ± 24 | 150 ± 21 | 0.1 |
| Mean BP (mmHg) | 91 ± 13 | 96 ± 13 | 0.2 |
| RA pressure (mmHg) | 4 ± 2 | 10 ± 4 | <0.0001 |
| PA systolic pressure (mmHg) | 27 ± 6 | 41 ± 12 | <0.0001 |
| PA mean pressure (mmHg) | 16 ± 4 | 27 ± 8 | <0.0001 |
| PCWP (mmHg) | 7 ± 3 | 17 ± 6 | <0.0001 |
| Cardiac output (L/min) | 5.2 ± 1.8 | 5.1 ± 1.2 | 0.8 |
| PVR (WU) | 1.9 ± 0.8 | 2.0 ± 1.2 | 0.5 |
| PA compliance (mL/mmHg) | 4.4 ± 1.5 | 3.6 ± 1.3 | 0.03 |
| Ventilatory performance | |||
| VO2 (mL/kg/min) | 2.9 ± 0.6 | 2.5 ± 0.7 | 0.01 |
| VE (L/min) | 7.2 ± 1.8 | 8.1 ± 2.7 | 0.1 |
| VT (mL) | 517 ± 197 | 484 ± 153 | 0.5 |
| Respiratory rate (/min) | 15 ± 4 | 17 ± 5 | 0.03 |
| VE/VCO2 ratio | 37.2 ± 4.5 | 40.0 ± 7.4 | 0.06 |
| VT/TI | 451 ± 139 | 541 ± 274 | 0.07 |
| VD/VT | 0.40 ± 0.09 | 0.44 ± 0.11 | 0.10 |
| Blood gas data | |||
| Arterial pH | 7.41 ± 0.03 | 7.39 ± 0.04 | 0.1 |
| Arterial pCO2 (mmHg) | 39 ± 4 | 40 ± 4 | 0.2 |
| Arterial pO2 (mmHg) | 80 ± 12 | 71 ± 10 | 0.003 |
| PA pH | 7.38 ± 0.03 | 7.37 ± 0.04 | 0.1 |
| PA pCO2 (mmHg) | 42 ± 4 | 44 ± 3 | 0.1 |
| PA pO2 (mmHg) | 39 ± 4 | 35 ± 3 | 0.002 |
Data are mean ± SD, median (interquartile range), or n (%).
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin-receptor blockers; BP, blood pressure; E/e′ ratio, the ratio of early diastolic mitral inflow velocity to early diastolic mitral annular velocity; HFpEF, heart failure with preserved ejection fraction; JVP, jugular venous pressure; LV, left ventricular; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial; RV e′ and s′, early diastolic and systolic tissue velocities at the lateral tricuspid annulus; TI, inspiratory time; VD, pulmonary dead space; VE, minute ventilation; VT, tidal volume; VCO2, carbon dioxide volume; VO2, oxygen consumption volume.
Resting haemodynamics, ventilation, gas exchange, and right ventricular function
Compared with controls, subjects with HFpEF displayed higher biventricular filling pressures and PA pressures, and lower PA compliance at rest (Table 1). Heart rate, BP, CO, RV systolic and diastolic function, and PVR were similar in HFpEF and controls at baseline. VO2 and arterial pO2 were lower and respiratory rate was higher in HFpEF when compared with controls at rest, but there were no differences in other resting ventilatory parameters (Table 1).
Effects with submaximal exercise
During matched submaximal (20 W) exercise, biventricular filling pressures, and PA pressures were again higher in subjects with HFpEF compared with controls (Table 2, Figure 1A). Borg perceived dyspnoea and effort scores were also higher in HFpEF subjects than controls during 20 W exercise (Figure 1B, Table 2). Compared with controls, subjects with HFpEF displayed lower RV systolic function during the exercise while RV diastolic function was similar between the groups (Table 2).
Table 2.
Haemodynamics, symptoms, ventilatory performance, gas exchange, and right ventricular function during submaximal exercise (20 W)
| Control (n = 24) | HFpEF (n = 50) | P-value | |
|---|---|---|---|
| Haemodynamics | |||
| Heart rate (b.p.m.) | 91 ± 14 | 88 ± 14 | 0.4 |
| Systolic BP (mmHg) | 167 ± 29 | 175 ± 26 | 0.3 |
| RA pressure (mmHg) | 8 ± 3 | 21 ± 5 | <0.0001 |
| PA systolic pressure (mmHg) | 37 ± 10 | 66 ± 12 | <0.0001 |
| PA mean pressure (mmHg) | 25 ± 7 | 47 ± 10 | <0.0001 |
| PCWP (mmHg) | 14 ± 5 | 31 ± 5 | <0.0001 |
| Cardiac output (L/min) | 8.2 ± 1.8 | 6.8 ± 2.0 | 0.005 |
| PVR (WU) | 1.4 ± 0.7 | 2.3 ± 1.2 | 0.0002 |
| PA compliance (mL/mmHg) | 4.8 ± 2.3 | 2.6 ± 1.2 | 0.0002 |
| Exertional symptoms | |||
| Borg effort (6–20) | 11.6 ± 2.3 | 13.4 ± 2.7 | 0.004 |
| Borg dyspnoea (0–10) | 3.2 ± 1.7 | 4.5 ± 2.2 | 0.008 |
| Ventilatory performance | |||
| VE (L/min) | 18.8 ± 4.8 | 22.0 ± 7.1 | 0.03 |
| Respiratory rate (/min) | 22 ± 7 | 28 ± 9 | 0.005 |
| VT (mL) | 882 ± 179 | 808 ± 182 | 0.1 |
| VE/VCO2 ratio | 31.8 ± 5.1 | 35.9 ± 7.1 | 0.006 |
| VT/TI | 973 ± 226 | 1186 ± 386 | 0.004 |
| VD/VT | 0.30 ± 0.08 | 0.35 ± 0.11 | 0.03 |
| Metabolism and gas exchange | |||
| Respiratory exchange ratio | 0.91 ± 0.13 | 0.97 ± 0.13 | 0.1 |
| VO2 (mL/kg/min) | 8.3 ± 1.7 | 6.7 ± 1.9 | 0.0007 |
| Arterial pH | 7.39 ± 0.03 | 7.39 ± 0.04 | 1.0 |
| Arterial pCO2 (mmHg) | 39 ± 5 | 38 ± 4 | 0.4 |
| Arterial pO2 (mmHg) | 79 ± 12 | 72 ± 12 | 0.02 |
| PA pH | 7.35 ± 0.03 | 7.33 ± 0.04 | 0.01 |
| PA pCO2 (mmHg) | 45 ± 6 | 48 ± 6 | 0.05 |
| PA pO2 (mmHg) | 30 ± 4 | 24 ± 3 | <0.0001 |
| RV function | |||
| RV e′ (cm/s) | 13 ± 5 | 11 ± 5 | 0.3 |
| RV s′ (cm/s) | 13 ± 4 | 10 ± 3 | 0.004 |
Data are mean ± SD.
BP, blood pressure; HFpEF, heart failure with preserved ejection fraction; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial; RV e’ and s’, early diastolic and systolic tissue velocities at the lateral tricuspid annulus; TI, inspiratory time; VD, pulmonary dead space; VE, minute ventilation; VT, tidal volume; VCO2, carbon dioxide volume; VO2, oxygen consumption volume.
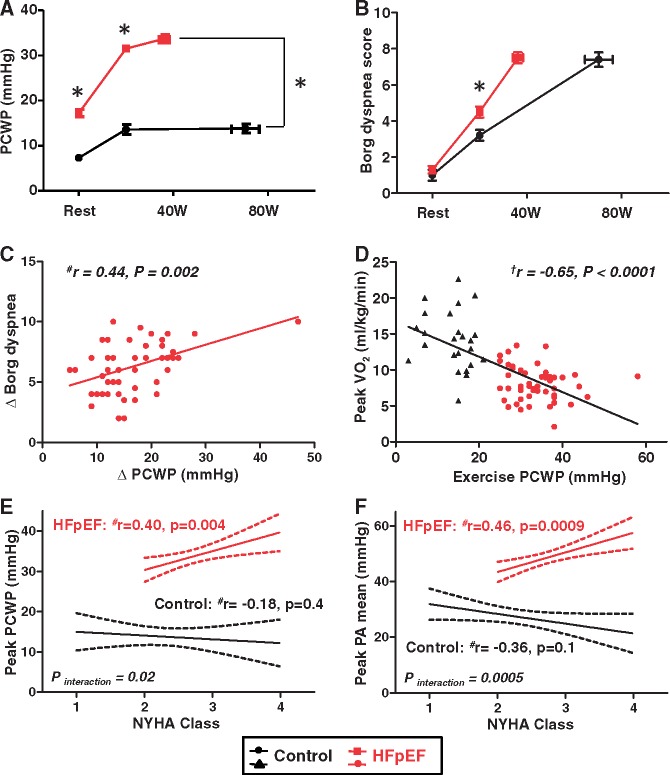
Figure 1.
(A and B) Pulmonary capillary wedge pressure and Borg dyspnoea score as a function of workload in heart failure with preserved ejection fraction and control subjects. (C) The increase in pulmonary capillary wedge pressure from rest to peak exercise was directly correlated with the change in the Borg dyspnoea score in subjects with heart failure with preserved ejection fraction, but not in controls (P = 0.3, data not shown). (D) Peak pulmonary capillary wedge pressure correlated inversely with peak oxygen consumption. (E and F) Elevations in pulmonary capillary wedge pressure and pulmonary artery mean pressure during peak exercise were related to Ney York Heart Association class in heart failure with preserved ejection fraction subjects, but not in control subjects. *P < 0.05 between groups. aDetermined by Pearson’s correlation analysis. bDetermined by Spearman’s correlation analysis.
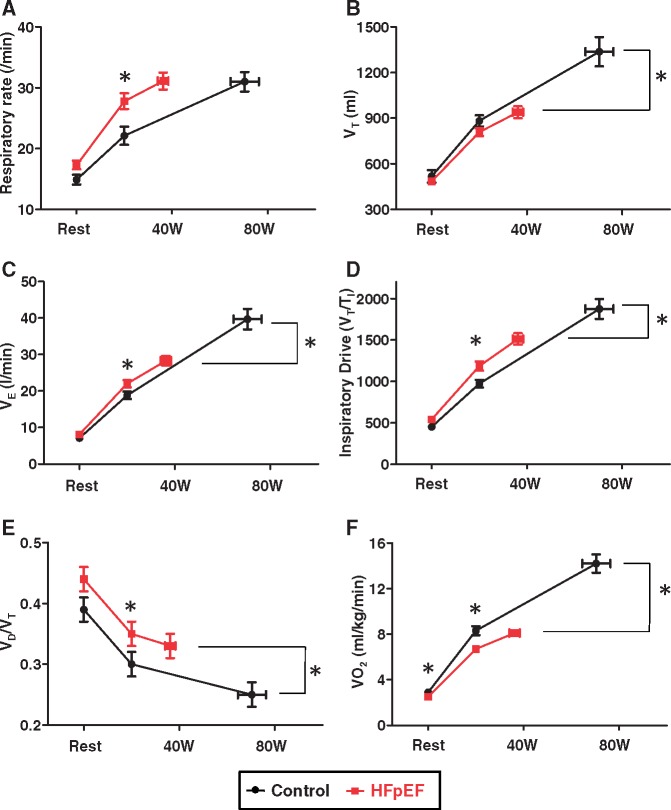
Compared with controls, subjects with HFpEF displayed lower VO2 and greater ventilatory inefficiency (higher VE/VCO2) during submaximal exercise (Table 2). This was manifest by a more rapid, shallow breathing pattern in HFpEF subjects, with greater breathing frequency despite trend to lower VT (Figure 2A–C). The greater tachypnoea in HFpEF subjects was related to an increase in ventilatory drive (VT/TI ratio) during submaximal exercise (Figure 2D).
Figure 2.
Baseline, low-level (20 W) and peak exercise for (A) respiratory rate, (B) tidal volume (VT), (C) minute ventilation (VE), (D) inspiratory drive [tidal volume/inspiration time (VT/TI)], (E) dead space ventilation relative to tidal volume (VD/VT), and (F) peak oxygen consumption in heart failure with preserved ejection fraction and control subjects. *P < 0.05 between groups.
Dead space ventilation (VD/VT ratio) decreased from baseline to 20 W exercise in both groups, but it remained higher in patients with HFpEF than in controls (Figure 2E). Arterial and mixed venous pO2 were lower in HFpEF than in control subjects, but arterial pH and pCO2 were similar between groups during submaximal exercise (Table 2).
Effects with peak exercise
Peak exercise workload (36 ± 15 vs. 70 ± 29 W, P < 0.0001) and peak VO2 were both markedly impaired in HFpEF subjects when compared with controls (Table 3, Figure 2F). Borg perceived effort and dyspnoea scores were similar between subjects with HFpEF and controls at peak workload, indicating that all participants exercised to subjective exhaustion, but dyspnoea relative to intensity of work performed was much higher in patients with HFpEF (P < 0.0001, Figure 1B). Biventricular filling pressures, PA pressures, and PVR were higher and CO and PAC were lower in HFpEF when compared with controls during peak exercise. Subjects with HFpEF displayed impaired RV systolic and diastolic function at peak exercise (Table 3).
Table 3.
Haemodynamics, symptoms, ventilatory performance, gas exchange, and right ventricular function during peak exercise
| Control (n = 24) | HFpEF (n = 50) | P-value | |
|---|---|---|---|
| Haemodynamics | |||
| Heart rate (b.p.m.) | 121 ± 18 | 97 ± 15 | <0.0001 |
| Systolic BP (mmHg) | 184 ± 25 | 185 ± 30 | 0.8 |
| RA pressure (mmHg) | 8 ± 4 | 22 ± 6 | <0.0001 |
| PA systolic pressure (mmHg) | 41 ± 9 | 70 ± 13 | <0.0001 |
| PA mean pressure (mmHg) | 27 ± 6 | 48 ± 8 | <0.0001 |
| PCWP (mmHg) | 14 ± 5 | 34 ± 6 | <0.0001 |
| Cardiac output (L/min) | 11.8 ± 3.9 | 8.1 ± 2.8 | 0.0002 |
| PVR (WU) | 1.3 ± 0.6 | 2.1 ± 1.2 | 0.001 |
| PA compliance (mL/mmHg) | 4.3 ± 1.7 | 2.6 ± 1.4 | 0.0002 |
| Exertional symptoms | |||
| Borg effort (6–20) | 17.1 ± 2.7 | 17.0 ± 1.9 | 0.9 |
| Borg dyspnoea (0–10) | 7.4 ± 1.9 | 7.5 ± 2.0 | 1.0 |
| Ventilatory performance | |||
| VE (L/min) | 39.7 ± 13.8 | 28.3 ± 8.9 | 0.0008 |
| Respiratory rate (/min) | 31 ± 8 | 31 ± 10 | 0.9 |
| VT (mL) | 1337 ± 471 | 939 ± 278 | 0.0006 |
| VE/VCO2 slope | 32.9 ± 5.3 | 36.0 ± 7.7 | 0.04 |
| VT/TI ratio | 1874 ± 119 | 1511 ± 495 | 0.01 |
| VD/VT ratio | 0.25 ± 0.09 | 0.33 ± 0.11 | 0.002 |
| Metabolism and gas exchange | |||
| Respiratory exchange ratio | 1.08 ± 0.10 | 1.03 ± 0.12 | 0.1 |
| VO2 (mL/kg/min) | 14.2 ± 4.0 | 8.1 ± 2.4 | <0.0001 |
| Arterial pH | 7.38 ± 0.04 | 7.38 ± 0.05 | 0.6 |
| Arterial pCO2 (mmHg) | 36 ± 4 | 37 ± 4 | 0.1 |
| Arterial pO2 (mmHg) | 84 ± 12 | 72 ± 14 | 0.0005 |
| PA pH | 7.31 ± 0.04 | 7.31 ± 0.05 | 0.7 |
| PA pCO2 (mmHg) | 49 ± 7 | 51 ± 6 | 0.3 |
| PA pO2 (mmHg) | 27 ± 4 | 23 ± 5 | 0.0007 |
| RV function | |||
| RV e′ (cm/s) | 19 ± 9 | 12 ± 6 | 0.02 |
| RV s′ (cm/s) | 14 ± 4 | 11 ± 3 | 0.01 |
Data are mean ± SD.
BP, blood pressure; HFpEF, heart failure with preserved ejection fraction; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial; RV e’ and s’, early diastolic and systolic tissue velocities at the lateral tricuspid annulus; TI, inspiratory time; VD, pulmonary dead space; VE, minute ventilation; VT, tidal volume; VCO2, carbon dioxide volume; VO2, oxygen consumption volume.
In HFpEF subjects (but not controls), the change in Borg dyspnoea score during exercise was directly correlated with the change in PCWP (Figure 1C). This relationship remained significant after excluding one HFpEF subject with greatest increase in PCWP (r = 0.40, P = 0.004). Aerobic capacity (peak VO2) varied inversely with exercise PCWP (Figure 1D). Exercise PCWP and PA mean pressures were directly correlated with New York Heart Association (NYHA) functional class, but only in HFpEF subjects and not controls (Figures 1E and F).
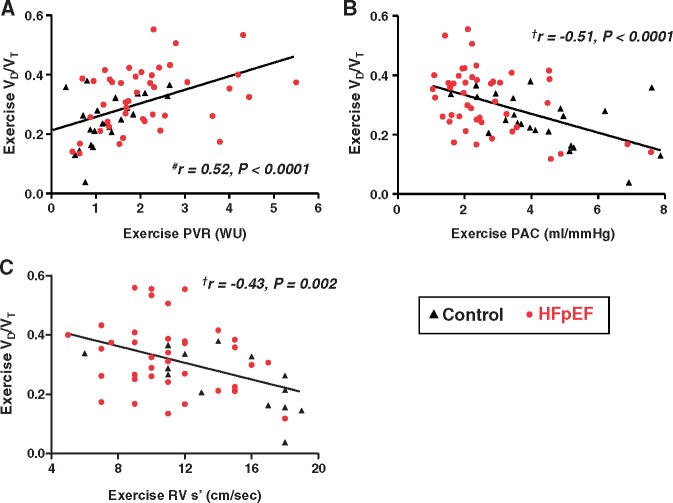
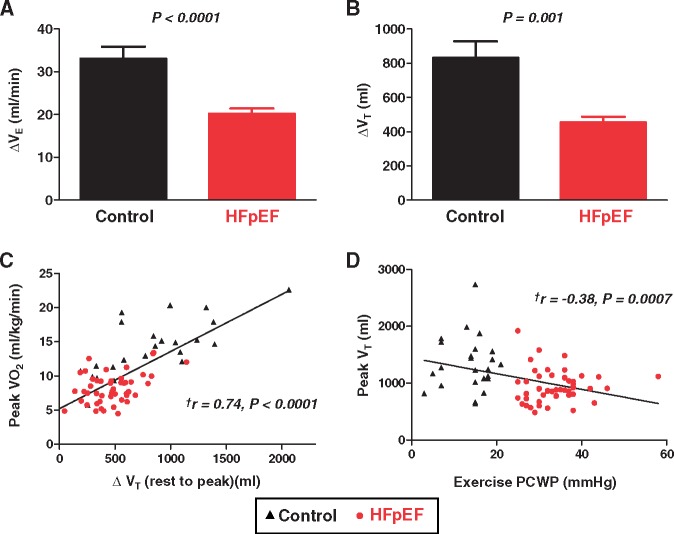
Like Borg dyspnoea scores, respiratory rate at peak exercise was similar in HFpEF and controls, but respiratory rate relative to workload intensity was much higher in HFpEF (Figure 2A). The fraction of dead space ventilation (VD/VT ratio) was higher in HFpEF, and VD/VT ratio correlated with exercise RV s′ (r = −0.43, P = 0.002) as well as exercise PVR and PA compliance (Figure 3). Minute ventilation at peak exercise was lower in HFpEF than controls, due exclusively to blunted increase in VT as respiratory rates were similar (Figures 2 and 4, Table 3). The change in VT with exercise correlated directly with peak VO2 (r = 0.74, P < 0.0001), and peak VT correlated inversely with exercise PCWP (Figure 4).
Figure 3.
Correlation of peak VD/VT ratio with peak pulmonary vascular resistance, pulmonary arterial compliance. aDetermined by Pearson’s correlation analysis. bDetermined by Spearman’s correlation analysis.
Figure 4.
(A) Compared with controls, subjects with heart failure with preserved ejection fraction displayed less increase in VE during peak exercise. (B) This was explained by impaired increase in VT in heart failure with preserved ejection fraction. (C and D) The change in VT varied directly with peak oxygen consumption, and peak VT was correlated inversely with peak pulmonary capillary wedge pressure. Error bars indicate SE. aDetermined by Pearson’s correlation analysis.
Impact of body mass, age, and haemoglobin
Adiposity may influence lung function and haemodynamics. We observed significant relationships between BMI and PCWP, both at rest (r = 0.44, P < 0.0001) and during exercise (r = 0.54, P < 0.0001). In contrast, BMI was unrelated to resting VT (r = 0.16, P = 0.2) or VD/VT (r = −0.01, P = 0.5), and exercise VE (r = −0.11, P = 0.3), VT (r = −0.20, P = 0.1), VD/VT (r = 0.12, P = 0.3), or respiratory rate (r = 0.10, P = 0.4).
Given the older age, increased BMI and greater burden of anaemia in HFpEF subjects, multivariable regression analyses were performed to evaluate whether group differences and relationships between central haemodynamics, ventilation abnormalities, exercise capacity, and symptoms were specific to HFpEF, or explained in part by these comorbidities that are often observed with HFpEF. After adjusting for age, BMI, and haemoglobin levels, all correlations and comparisons remained significant (P < 0.05, Supplementary material online, Table S2) except for correlations with functional class, which became borderline significant (P = 0.10 for PCWP and PA).
Discussion
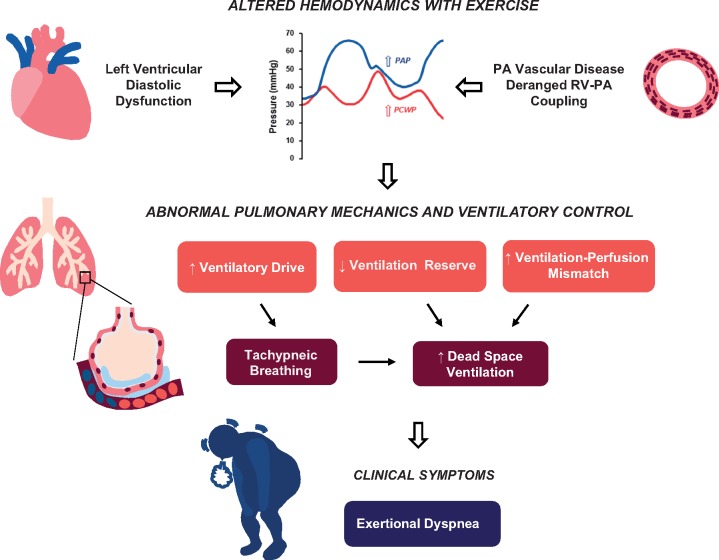
This is the first direct evaluation of the relationships between haemodynamics, symptoms, exercise capacity and ventilatory abnormalities in patients with HFpEF, assessed using high fidelity micromanometers, and expired gas analysis obtained simultaneously at rest and during submaximal and peak exercise. During low-level exercise, at work intensity similar to activities of daily living (20 W), elevation in left heart filling pressures in HFpEF subjects was directly correlated with severity of dyspnoea, heightened ventilatory drive, and altered breathing patterns. At peak exercise, ventilatory reserve in HFpEF was impaired, and there was blunted reduction in dead space ventilation that was coupled with impaired pulmonary vasodilator reserve, suggesting ventilatory-perfusion mismatch related to pulmonary hypoperfusion. Higher left heart filing pressures during exercise were correlated with reduced exercise capacity (peak VO2), as well as the severity of dyspnoea developed during exercise (change in Borg scores) and in chronic everyday life (NYHA functional class). These data provide new insight into the linkages between classical haemodynamic derangements that develop during exercise in the heart, and the symptoms, ventilatory abnormalities and gas exchange limitations that develop in the lungs contributing to the clinical expression of cardiac failure in patients with HFpEF (Take home figure).
Take home figure.
Linkages between haemodynamic derangements, symptoms, ventilatory abnormalities and gas exchange alterations in patients with heart failure with preserved ejection fraction. See text for details.
Cardiac filling pressures and dyspnoea
To date, six studies have examined relationships simultaneously-measured haemodynamics, symptoms and ventilatory abnormalities, and all were performed in patients with HFrEF.8–13 Surprisingly, there was very weak to no relationship between haemodynamics, symptoms, and pulmonary limitations in these studies examining HFrEF.8–12 The exception comes from Lewis and colleagues who did observe a correlation between ventilatory efficiency and PVR in patients with HFrEF.13 The current study represents the first to perform similar analyses in HFpEF. Novel findings in the present study are that elevations in PCWP during exercise were directly correlated with symptoms of dyspnoea, heightened ventilatory drive, tachypneic breathing, impaired exercise capacity, and worse functional class.
The reasons for the discrepant results between prior studies in HFrEF and the current data from HFpEF are not clear, but likely relate in large part to fundamental differences between the HFpEF and HFrEF phenotypes.14 While HFpEF patients are often limited by cardiac output reserve,15,21 this impairment is typically not be as profound as is observed in patients with HFrEF, where exercise capacity may be more constrained by reduced bulk O2 transfer (cardiac output limitation), as shown previously.8,9,13,22,23 In contrast, HFpEF patients may be more likely to cease exercising because of symptoms of dyspnoea related to high filling pressures, as shown in the current study.
The experience of dyspnoea is common to all humans during exercise, and both HFpEF subjects and controls reported significant exertional dyspnoea in this study. However, dyspnoea severity was much greater relative to workload in HFpEF, and importantly, PCWP was correlated with acute and chronic symptoms only in the HFpEF patients, further supporting the hypothesis that excess dyspnoea in these patients is related to central haemodynamic derangements. This is also supported by the fact that exercise capacity (peak VO2) was inversely related to exercise PCWP. Further study is required to better understand how elevated filling pressures with exercise affect afferent signals from airways, lungs, vasculature and chest wall to the brain, leading to the sensation of heightened respiratory effort and dyspnoea that is so pervasive in HFpEF.24
While the current data point to an important role for the heart and lungs in driving symptoms, it is important to remember that HFpEF and HFrEF patients also display significant non-cardiac, peripheral abnormalities in the vasculature and skeletal muscle that also contribute to symptoms and exercise intolerance.4,22,25–29 The current data relating central haemodynamics to symptoms should not be taken as evidence against this well-described and important role of the periphery in HF, regardless of EF.
Haemodynamics and pulmonary reserve
The ventilatory response to exercise is known to be excessive relative to metabolic demand in patients with HFrEF.8,10,30 It has been suggested that this is caused by increases in VD/VT due to ventilation-perfusion mismatching and CO2 production due to HCO3− buffering of lactic acid, but these relationships have never been tested before using simultaneously-measured invasive haemodynamics.2,31–33 Indeed, we found that subjects with HFpEF displayed tachypnoea during low-level exercise which was related to increases in PCWP as well as heightened respiratory drive (VT/TI ratio).
The current data suggest that high filling pressures during exercise directly contribute to the increase in ventilatory drive and tachypnoea in HFpEF. This may be caused by stimulation of pulmonary irritant (J) receptors, decreased lung compliance related to tissue congestion, left atrial distention, impaired alveolar gas diffusion, or worsening ventilation-perfusion mismatch.3,34–37 The inverse correlations between VT and exercise PCWP observed in the current study suggest that the limited rise of VT in HFpEF was related in part to high left heart filling pressure. This ventilatory abnormality may worsen exercise capacity, ventilatory efficiency, and exertional dyspnoea in HFpEF. If this hypothesis is true, PCWP reduction during exercise might also improve ventilatory control in HFpEF, in addition to reducing symptoms.
Haemodynamics and symptoms with submaximal exercise
Prior studies evaluating gas exchange in HFrEF have been restricted to peak exercise,8–12 but most patients with HF rarely achieve this high exertional workload in everyday life. Rather, HF patients typically complain of dyspnoea during lower levels of physical activity, such as walking, bathing, or routine housework. The current data identified important group differences and significant relationships between haemodynamics, symptoms and pulmonary responses during this lower exercise workload (20 W), providing important new insight into the nature of symptoms that are experienced by patients in everyday life, rather than maximal exercise alone.
Worsening ventilation-perfusion mismatch at maximal exercise
Recent studies have shown that subjects with HFpEF have impaired RV-PA coupling with exercise, and that this finding identifies patients with greater lung perfusion abnormalities and increased mortality.15,38 We demonstrate that HFpEF subjects with higher peak PVR displayed greater VD/VT ratio, depressed RV function, and ventilatory inefficiency during peak exercise, and exercise VD/VT ratio was correlated with higher PVR, lower PA compliance, and impaired RV systolic reserve. These findings specifically regarding ventilatory efficiency confirm and extend upon prior data in HFrEF,13 suggesting a linkage between dead space ventilation and pulmonary vasodilator and RV reserve, which is impaired in HFpEF.15 The alterations in right ventricular-pulmonary vascular coupling during exercise might limit lung perfusion and result in ventilation-perfusion mismatching.15 In line with a recent non-invasive study showing the strong contribution of increased VD/VT to ventilatory efficiency in HFpEF,33 this ventilation-perfusion mismatching, along with the aforementioned VT impairment, may lead to increased fractional dead space ventilation and ventilatory inefficiency in patients with HFpEF, which may also contribute to the perception of dyspnoea.
Clinical implications
Current HF guidelines place invasive exercise haemodynamics testing as an alternative approach for the diagnosis of HFpEF.39 In contrast, guidelines for the diagnosis and treatment of pulmonary hypertension strongly recommend invasive haemodynamic testing for HF with pulmonary hypertension (Class I) but do not comment on the use of exercise assessments.40 The current data reinforce the value of invasive exercise haemodynamic testing not only for diagnostic evaluation but also to provide greater insight into pathophysiology.41
Limitations
This is a single-centre study from a tertiary referral centre and as such has inherent limitations relating to both selection and referral bias. The cross-sectional nature precludes assessment of causality in the correlations. The control population was not truly normal in that they had prevalent comorbidities and were referred to invasive exercise stress testing for evaluation of exertional dyspnoea. However, the fact that the control population is more diseased than a truly normal healthy control population only biases our data toward the null. There were baseline differences in age, BMI, and haemoglobin levels between HFpEF and controls, but the key group differences and relationships between central haemodynamics, symptoms, and lung abnormalities remained significant after adjusting for these baseline differences. In addition, older age, obesity, and anaemia are important risk factors for HFpEF, which would make adjustment conceptually less relevant since those factors could be viewed as part of the overall disease process. The haemodynamic differences observed between HFpEF and controls are biased because the HFpEF group was defined by the presence of abnormal haemodynamics. However, this investigation was focused on how haemodynamics affect lung function, symptoms and ventilatory alterations, rather than whether haemodynamic derangements develop during exercise in HFpEF, which is already well-established. The sample size is relatively small, but the assessments of relationships between invasive haemodynamics, symptoms, ventilatory response, and exercise capacity using robust methodologies are unique in the literature and represent a major strength of the current analysis.
Conclusions
Elevation in left heart filling pressure with exercise is correlated with worse symptoms, greater ventilatory drive, more tachypneic breathing, impaired ventilation, and worse exercise capacity in HFpEF. These findings highlight the importance of central haemodynamics as key components in the pathophysiology of HFpEF, and suggest that therapies targeting haemodynamics may help to improve the symptoms, exercise capacity and pulmonary limitations.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This research was supported by a competitive prospective grants award from the Mayo Clinic and Foundation. M.O. is supported by a research fellowship from the Uehara Memorial Foundation, Japan. T.P.O. is supported by RO1 HL126638. Y.N.V.R. is supported by T32 HL007111.V.M. is supported by the Czech Healthcare Research Grant agency (AZV): 17-28784A. B.A.B. is supported by RO1 HL128526, RO1 HL126638, UO1 HL125205, and U10 HL110262.
Conflict of interest: none declared.
Supplementary Material
Footnotes
See page 2822 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy354)
References
- 1. Reddy YN, Borlaug BA.. Heart failure with preserved ejection fraction. Curr Probl Cardiol 2016;41:145–188. [DOI] [PubMed] [Google Scholar]
- 2. Wasserman K, Zhang YY, Gitt A, Belardinelli R, Koike A, Lubarsky L, Agostoni PG.. Lung function and exercise gas exchange in chronic heart failure. Circulation 1997;96:2221–2227. [DOI] [PubMed] [Google Scholar]
- 3. Olson TP, Johnson BD, Borlaug BA.. Impaired pulmonary diffusion in heart failure with preserved ejection fraction. JACC Heart Fail 2016;4:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olson TP, Joyner MJ, Johnson BD.. Influence of locomotor muscle metaboreceptor stimulation on the ventilatory response to exercise in heart failure. Circ Heart Fail 2010;3:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thompson RB, Pagano JJ, Chow K, Sekowski V, Paterson I, Ezekowitz J, Anderson T, Dyck JRB, Haykowsky MJ.. Subclinical pulmonary edema is associated with reduced exercise capacity in HFpEF and HFrEF. J Am Coll Card 2017;70:1827–1828. [DOI] [PubMed] [Google Scholar]
- 6. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM.. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA.. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sullivan MJ, Higginbotham MB, Cobb FR.. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite hemodynamic and pulmonary abnormalities. Circulation 1988;77:552–559. [DOI] [PubMed] [Google Scholar]
- 9. Franciosa JA, Leddy CL, Wilen M, Schwartz DE.. Relation between hemodynamic and ventilatory responses in determining exercise capacity in severe congestive heart failure. Am J Cardiol 1984;53:127–134. [DOI] [PubMed] [Google Scholar]
- 10. Fink LI, Wilson JR, Ferraro N.. Exercise ventilation and pulmonary artery wedge pressure in chronic stable congestive heart failure. Am J Cardiol 1986;57:249–253. [DOI] [PubMed] [Google Scholar]
- 11. Wilson JR, Rayos G, Yeoh TK, Gothard P, Bak K.. Dissociation between exertional symptoms and circulatory function in patients with heart failure. Circulation 1995;92:47–53. [DOI] [PubMed] [Google Scholar]
- 12. Wilson JR, Rayos G, Yeoh TK, Gothard P.. Dissociation between peak exercise oxygen consumption and hemodynamic dysfunction in potential heart transplant candidates. J Am Coll Cardiol 1995;26:429–435. [DOI] [PubMed] [Google Scholar]
- 13. Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM, Semigran MJ.. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail 2008;1:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borlaug BA, Redfield MM.. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 2011;123:2006; discussion 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borlaug BA, Kane GC, Melenovsky V, Olson TP.. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA.. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail 2015;8:41–48. [DOI] [PubMed] [Google Scholar]
- 17. Andersen MJ, Hwang SJ, Kane GC, Melenovsky V, Olson TP, Fetterly K, Borlaug BA.. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail 2015;8:542–550. [DOI] [PubMed] [Google Scholar]
- 18. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–381. [PubMed] [Google Scholar]
- 19. Chua TP, Ponikowski P, Harrington D, Anker SD, Webb-Peploe K, Clark AL, Poole-Wilson PA, Coats AJ.. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol 1997;29:1585–1590. [DOI] [PubMed] [Google Scholar]
- 20. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB.. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713; quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 21. Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA.. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail 2013;15:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD.. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail 2015;8:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zile MR, Kjellstrom B, Bennett T, Cho Y, Baicu CF, Aaron MF, Abraham WT, Bourge RC, Kueffer FJ.. Effects of exercise on left ventricular systolic and diastolic properties in patients with heart failure and a preserved ejection fraction versus heart failure and a reduced ejection fraction. Circ Heart Fail 2013;6:508–516. [DOI] [PubMed] [Google Scholar]
- 24. Manning HL, Schwartzstein RM.. Pathophysiology of dyspnea. N Engl J Med 1995;333:1547–1553. [DOI] [PubMed] [Google Scholar]
- 25. Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW.. Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatr Cardiol 2015;12:205–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD.. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol 2007;50:2136–2144. [DOI] [PubMed] [Google Scholar]
- 27. Wilson JR, Mancini DM.. Factors contributing to the exercise limitation of heart failure. J Am Coll Cardiol 1993;22: 93A–98A. [DOI] [PubMed] [Google Scholar]
- 28. Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS.. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol 2010;55:1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Houstis NE, Eisman A, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD, Lewis GD.. Exercise intolerance in HFpEF: diagnosing and ranking its causes using personalized O2 pathway analysis. Circulation 2018;137:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buller NP, Poole-Wilson PA.. Mechanism of the increased ventilatory response to exercise in patients with chronic heart failure. Br Heart J 1990;63:281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olson TP, Snyder EM, Johnson BD.. Exercise-disordered breathing in chronic heart failure. Exerc Sport Sci Rev 2006;34:194–201. [DOI] [PubMed] [Google Scholar]
- 32. Woods PR, Olson TP, Frantz RP, Johnson BD.. Causes of breathing inefficiency during exercise in heart failure. J Card Fail 2010;16:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Iterson EH, Johnson BD, Borlaug BA, Olson TP.. Physiological dead space and arterial carbon dioxide contributions to exercise ventilatory inefficiency in patients with reduced or preserved ejection fraction heart failure. Eur J Heart Fail 2017;19:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paintal AS. Mechanism of stimulation of type J pulmonary receptors. J Physiol 1969;203:511–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Melenovsky V, Andersen MJ, Andress K, Reddy YN, Borlaug BA.. Lung congestion in chronic heart failure: haemodynamic, clinical, and prognostic implications. Eur J Heart Fail 2015;17:1161–1171. [DOI] [PubMed] [Google Scholar]
- 36. Lloyd TC., Jr. Effect of increased left atrial pressure on breathing frequency in anesthetized dog. J Appl Physiol 1990;69:1973–1980. [DOI] [PubMed] [Google Scholar]
- 37. Roberts AM, Bhattacharya J, Schultz HD, Coleridge HM, Coleridge JC.. Stimulation of pulmonary vagal afferent C-fibers by lung edema in dogs. Circ Res 1986;58:512–522. [DOI] [PubMed] [Google Scholar]
- 38. Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, Temporelli PL, Rossi A, Faggiano P, Traversi E, Vriz O, Dini FL.. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail 2017;19:873–879. [DOI] [PubMed] [Google Scholar]
- 39. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 40. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H, Luis Zamorano J.. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 41. Borlaug BA, Obokata M.. Is it time to recognize a New Phenotype? Heart Failure with preserved ejection fraction with Pulmonary Vascular Disease. Eur Heart J 2017;38:2874–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.