Tetzloff et al. demonstrate that patients who present with agrammatic aphasia without apraxia of speech have a different clinical disease course and different underlying neuroanatomical abnormalities to patients with the more common syndrome of mixed agrammatism and apraxia of speech, supporting the distinction of these clinical syndromes.
Keywords: aphasia, agrammatic, longitudinal, MRI, positron emission tomography
Abstract
Agrammatic aphasia affects grammatical language production and can result from a neurodegenerative disease. Although it typically presents with concomitant apraxia of speech, this is not always the case. Little is known about the clinical course and imaging features of patients that present with agrammatism in the absence of apraxia of speech, which we will refer to as progressive agrammatic aphasia. We aimed to make a detailed description of the longitudinal clinical, linguistic, and neuroimaging features of a cohort of 11 patients with progressive agrammatic aphasia to provide a complete picture of this syndrome. All patients underwent detailed speech and language, neurological and neuropsychological assessments, 3 T structural and diffusion tensor imaging MRI, 18F-fluorodeoxyglucose and Pittsburgh compound B PET. The 11 patients were matched by age and gender to 22 patients who had mixed apraxia of speech and agrammatism. The progressive agrammatic aphasia patients performed abnormally on tests of language, general cognition, executive function, and functional ability at baseline and declined in these measures over time. Only two patients eventually developed apraxia of speech, while parkinsonism was absent-to-mild throughout all visits for all patients. When compared to the patients with mixed apraxia of speech and agrammatism, the patients with progressive agrammatic aphasia performed better on tests of motor speech and parkinsonism but more poorly, and declined faster over time, on tests of general aphasia severity, agrammatism, and naming. The patients with progressive agrammatic aphasia also showed different neuroimaging abnormalities, with greater atrophy, hypometabolism and white matter tract degeneration in the prefrontal and anterior temporal lobes compared to patients with mixed apraxia of speech and agrammatism. These differences were more pronounced as the disease progressed. These results demonstrate that progressive agrammatic aphasia has a different clinical disease course and different underlying neuroanatomical abnormalities than patients with the more common syndrome of mixed agrammatism and apraxia of speech. This supports the distinction of progressive agrammatic aphasia and has implications for the classification of patients with agrammatic aphasia.
Introduction
Agrammatic aphasia is a speech-language disorder that affects language production, resulting in telegraphic speech, grammatical simplification, the omission of function words, and difficulty with syntax and verbs, among other deficits (Grossman et al., 1996; Thompson et al., 1997; Ash et al., 2010; Gorno-Tempini et al., 2011; Thompson and Mack, 2014). These impairments tend to affect both speech and writing, although they may be worse in speech (Tetzloff et al., 2018).
Although agrammatism can be caused by stroke, it can also result from a neurodegenerative disease. Patients with progressive agrammatism are typically diagnosed with the non-fluent/agrammatic variant of primary progressive aphasia (agPPA) (Gorno‐Tempini et al., 2004, 2011; Wilson et al., 2010; Butts et al., 2015; Tetzloff et al., 2017, 2018). However, patients with agrammatic aphasia also frequently have a motor speech disorder known as apraxia of speech (AOS), a disorder of speech motor planning or programming that is characterized by slow speech rate, articulatory distortions, sound substitutions, and word and phrase segmentation (Duffy, 2005, 2006; Josephs et al., 2012). In reality, it is the combination of agrammatism and AOS that most often comprises the syndrome of neurodegenerative agPPA, or Broca’s or non-fluent aphasia when aetiology is non-degenerative. In addition, there is much variability, as either agrammatism or AOS can be dominant in any given patient. It has also been recognized that patients can have isolated neurodegenerative AOS, in the absence of agrammatism, a syndrome termed primary progressive apraxia of speech (PPAOS; Josephs, 2012), and the longitudinal progression and imaging features of this syndrome differ from patients who have agrammatism (Josephs et al., 2012, 2013). Agrammatism (or aphasia classified as Broca’s) without AOS, and isolated apraxia of speech can also occur as a result of stroke (Duffy, 2013; Ballard et al., 2016). Less, however, is known about the clinical course and imaging features of patients who present with agrammatism in the absence of AOS, which we will refer to as progressive agrammatic aphasia (PAA). For example, it is unclear whether these patients will go on to develop AOS and whether the imaging features differ from those of patients with mixed agrammatism and AOS (Tetzloff et al., 2017). Isolated PAA is, in fact, uncommon. For example, of previously published agPPA cohorts, the proportion of patients that had AOS ranged from 75–100% (Ogar et al., 2007; Croot et al., 2012; Harris et al., 2013). Taken together, PAA may account for 1 in 20 patients with all combinations of agrammatic aphasia and AOS. However, given that the diagnostic criteria for agPPA can be met if a patient has one of the two core features, i.e. agrammatism and AOS (Gorno-Tempini et al., 2011), there is no way to know in many studies if patients have PAA, PPAOS or a mixture of agrammatism and AOS.
The primary aim of our study was to describe the clinical and neuroimaging features of a relatively large cohort of 11 patients with PAA who was prospectively recruited and followed over a period of almost a decade. The secondary aim was to determine how the clinical and neuroimaging results of the PAA cohort compare to patients who have mixed AOS and agrammatism (AOS+PAA) to determine whether the identification of PAA as a separate entity makes a difference relative to the clinical course and neuroimaging findings.
Materials and methods
Patients
Fifty-five patients who presented with progressive agrammatism with or without AOS were recruited into the Neurodegeneration Research Group research studies between 1 July 2010 and 12 March 2018. All patients had been recruited from the Department of Neurology at the Mayo Clinic, Rochester, MN, USA. Patients with concurrent illnesses that could account for the speech deficits, such as traumatic brain injury, stroke or developmental syndromes, and patients meeting criteria for another neurodegenerative disease, such as Alzheimer’s dementia (McKhann et al., 2011), dementia with Lewy bodies (McKeith, 2017), behavioural variant frontotemporal dementia (Rascovsky et al., 2007), progressive supranuclear palsy (Höglinger et al., 2017), corticobasal syndrome (Riley et al., 1990; Armstrong et al., 2013), or the logopenic or semantic variants of PPA (Gorno-Tempini et al., 2011) were excluded. All patients underwent a thorough speech-language evaluation by a speech-language pathologist (J.R.D., H.M.C., E.A.S., R.L.U.), a neurological evaluation, neuropsychological evaluation, 3 T volumetric head MRI, 18F-fluorodeoxyglucose PET (FDG-PET) and Pittsburgh compound B (PiB) PET.
Clinical test scores and video recordings from each patient were reviewed by at least two speech-language pathologists, as described in Josephs et al. (2012), and the presence/absence of agrammatism and AOS was recorded by consensus for each patient, as described below. Of the 55 patients, 44 (80%) were determined to have both AOS and agrammatism (AOS+PAA) and 11 (20%) had isolated agrammatism with no AOS (PAA). The 11 PAA patients were the primary focus of this study. Of the 11 PAA patients, three had only one research visit, with four having 2-yearly research visits, three having 3-yearly research visits, and one having 4-yearly research visits. The 11 PAA patients were matched by age, gender, and aphasia severity as measured by the Western Aphasia Battery Aphasia Quotient (WAB-AQ), to a cohort of 22 AOS+PAA patients; eight of these AOS+PAA patients had just one research visit, while 14 had two research visits. They were also matched by age and gender to 62 healthy control subjects who were recruited through the Mayo Clinic Study of Aging and had undergone identical imaging protocols. Of the 11 PAA patients, four were previously included in a study assessing longitudinal neuroimaging in agPPA (Tetzloff et al., 2017).
This study was approved by the Mayo Clinic IRB, and all patients gave consent to participate.
Speech and language evaluation
All patients underwent a thorough speech and language battery. Global aphasia severity and language ability were measured through the testing of lexical content, fluency, repetition, naming, and language comprehension, which are all parts of the WAB (Kertesz, 2007); in the context of this paper, the term fluency refers to the grammatical aspects of language, and not to the motor aspects of speech production. Subscores on these tests were then summed to form the comprehensive WAB-AQ. An aphasia severity score was also rendered by consensus between two speech-language pathologists. The Token Test Part V (De Renzi and Vignolo, 1962) was used to assess auditory comprehension of different sentence structures, and the Boston Naming Test (BNT; Lansing et al., 1999) was used to assess confrontation naming. Patients were also tested on the short form of the Northwestern Anagram Test (NAT; Weintraub et al., 2009), which assesses syntactic performance. The presence of agrammatism was also assessed in speech and writing.
For a patient to meet criteria for having agrammatism, function word omissions or syntactic errors had to be present during the WAB picture description task, in general conversation, or in the narrative writing subtest of the WAB. Apraxia of speech was identified by consensus, based on all spoken language tasks of the WAB plus additional speech tasks that included vowel prolongation, speech alternating motion rates (e.g. rapid repetition of ‘puhpuhpuh’), speech sequential motion rates (e.g. rapid repetition of ‘puhtuhkuh’), word and sentence repetition tasks and a conversational speech sample. The designation of agrammatism was made independent of the motor characteristics of speech. The Apraxia of Speech Rating Scale (ASRS), which rates the presence and prominence of a number of clinical features associated with AOS (Josephs et al., 2012; Strand et al., 2014), and the Motor Speech Disorders (MSD) scale (Yorkston et al., 1993), which rates the effect of any motor speech disorder (AOS or dysarthria) on communication function and speech intelligibility, independent of its specific features, were used as additional tools to measure the presence or severity of AOS. These speech tasks were further used to make a clinical judgement about the presence and severity of dysarthria, a motor speech disorder that reflects weakness, spasticity, incoordination, involuntary movements, or reduced, excessive or variable muscle tone (Duffy, 2013). A measure of non-verbal oral apraxia, a disorder affecting praxis for non-speech movements of speech muscles that can occur with or without AOS (Botha et al., 2014) was also administered.
Neurological and neuropsychological evaluation
All patients underwent thorough neurological and neuropsychological testing, including the Montreal Cognitive Assessment battery (MoCA; Nasreddine et al., 2005) to assess general cognitive function; Clinical Dementia Rating scale (CDR; Morris, 1993) to assess functional ability; Frontal Assessment Battery (FAB; Dubois, 2000), The Sorting Test from the Delis-Kaplan Executive Function System (D-KEFS; Delis et al., 2001), and the Trail Making Test B (Strauss et al., 2006) to assess executive function; Frontal Behavioral Inventory (Kertesz et al., 1997) to assess behavioural dysfunction; Neuropsychiatric Inventory Questionnaire (NPI-Q; Kaufer et al., 2000) to assess neuropsychiatric features; Rey Auditory Verbal Learning Test Delayed Recall Trial (Schmidt, 1996) to assess memory; WAB (Kertesz, 2007) praxis subtest to assess limb apraxia; Movement Disorders Society Sponsored revision of the Unified Parkinson’s Disorder Rating Scale III (MDS-UPDRS III) (Goetz et al., 2008) to assess parkinsonism; Trail Making Test A (Strauss et al., 2006) to assess motor speed; and Visual Object and Space Perception (VOSP; Warrington and James, 1991) cubes and incomplete letters subtests and Rey Osterrieth Complex Figure Test (Osterrieth, 1944) to assess visuospatial and visuoperceptual function.
Language analysis
A linguistic analysis was performed to assess the severity of agrammatism for all patients. All patients were asked to provide a spoken description of a picture of a picnic scene that is part of the WAB, which was video recorded. They were then later asked to provide a written description of the same picnic scene, with the explicit instruction to write in sentences. All language samples from both written and spoken modalities were transcribed and coded in CHAT transcription format for later analysis in the Computerized Language ANalysis (CLAN) software (MacWhinney, 2000).
Word-level codes for grammatical category (e.g. noun, verb, preposition, etc.) were included in the transcriptions, as well as individual codes for each morpheme in multi-morphemic words; all semantic and syntactic errors were also marked. Additionally, each utterance or written response was given a code to indicate if it was (i) grammatical, meaning there were no errors; (ii) ungrammatical, meaning that there were obligatory words omitted or one to three semantic or syntactic errors were present; or (iii) a non-utterance, meaning that the utterance was an isolated noun phrase or that there was no finite or tensed verb in the phrase. All language samples were transcribed and coded by K.A.T., who has substantial experience with linguistic transcriptions (Tetzloff et al., 2018).
CLAN was used to automatically calculate the number of words and utterances, as well as the mean length of utterance for each sample. The number of nouns, verbs, function words (articles, pronouns, and prepositions), and semantic and syntactic errors were also counted. Complex utterances—utterances that had embedding—were further marked as such. Ratios of these variables were manually calculated to account for differences in the number of utterances and number of words produced by different speakers.
Neuroimaging acquisition
Patients all underwent a 3 T volumetric head MRI within 2 days of the clinical assessments at both baseline and follow-up visits, which included a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (repetition time = 2300 ms, echo time = 3 ms, inversion time = 900 ms, flip angle = 8°, field of view = 26 cm, in-plane matrix = 256 × 256, slice thickness = 1.2 mm), as well as a single-shot echo-planar diffusion tensor imaging (DTI) pulse sequence (echo time = 65.9 ms, repetition time = 10 200 ms, in-plane matrix = 128 × 128, phase field of view = 0.66, 2.7 mm resolution, with four volumes without diffusion weighting and 41 directions with b = 1000 s/mm2 diffusion weighting).
PET scans were acquired with a PET/CT scanner (GE Healthcare) while operating in 3D mode. For FDG-PET, participants were injected with 18F-FDG of ∼459 MBq (range 367–576 MBq) and after a 30-min uptake period an 8-min 18F-FDG scan was performed. For PiB-PET, patients were injected with PiB of ∼628 MBq (range, 385–723 MBq) and after 40 min a 20-min PiB scan was obtained. Emission data were reconstructed into a 256 × 256 matrix with a 30-cm field of view (pixel size = 1.0 mm).
Neuroimaging analysis
Voxel-level comparisons of MRI grey matter volumes and FDG-PET metabolism were performed using SPM12 (Ashburner et al., 2014) at both baseline and the first available follow-up visit. All MPRAGE scans were normalized to the Mayo Clinic Adult Lifespan Template (MCALT), segmented via unified segmentation (Ashburner and Friston, 2005) with MCALT priors/settings (Schwarz et al., 2017) and grey matter images were modulated and smoothed at 8 mm full-width at half-maximum (FWHM). FDG-PET images were co-registered to the MPRAGE using 6 degrees-of-freedom registration in SPM12. All voxels in the MPRAGE-space FDG-PET images were divided by median uptake in pons to create standardized uptake value ratio (SUVR) images. SUVR images were normalized to MCALT and smoothed at 6 mm FWHM. To assess change in grey matter volume over time directly, we used an in-house developed tensor-based morphometry with symmetric normalization (TBM-SyN). The baseline and first available follow-up MPRAGE images of each patient were co-registered to their common mean with a 9 degree-of-freedom linear registration, and an in-house developed implementation of differential bias correction was run on each scan-pair. We computed and applied ANTs SyN deformations from the late to the early image, and vice versa, and averaged the deformed image with the stationary image to generate ‘synthetic’ early and late images. We saved the image log of the determinant of the Jacobian for the deformations, which were annualized to provide annualized log Jacobian images that were smoothed at 8 mm FWHM (Vemuri et al., 2015). Voxelwise t-tests in SPM12 were used to compare baseline images, the first available follow-up images and annualized Jacobians between PAA and AOS+PAA against controls and against each other, including age and gender as covariates. The comparisons of the PAA and AOS+PAA groups were also run including disease duration as a covariate. Results are shown after family wise error (FWE) correction for multiple comparisons at P < 0.05 or uncorrected at a threshold of P < 0.001. To visualize individual-level patterns of hypometabolism we created 3D stereotactic surface projections (Minoshima et al., 1995) using CortexID (GE Healthcare) whereby activity at each voxel is Z-scored to an age-segmented normative database.
DTI images were preprocessed by masking out extracranial voxels (Reid et al., 2018), denoising with a version of dwidenoise (Veraart et al., 2016) modified to handle zero padded images, correcting for head motion (in both the images and the diffusion vectors), eddy current distortion, and corrupted slices with FSL’s eddy (Andersson and Sotiropoulos, 2016), and correcting for Gibbs ringing with unring (Kellner et al., 2016). The noise image was used to remove Rician bias in the data (Koay et al., 2009). The intracranial mask was then recalculated and used by diffusion tensor fitting with a non-linear least squares method that works in the space of the data to avoid amplifying noise (Garyfallidis et al., 2014). Fitting the diffusion tensors produced fractional anisotropy, mean diffusivity, axial diffusivity and radial diffusivity images. We excluded voxels with apparent mean diffusivity > 0.002 mm2/s to reduce the effect of partial volume contamination by CSF, and voxels with mean diffusivity < 7.0 × 10−5 mm2/s or a coefficient of variation > 0.25 in their five b = 0 values, to reduce the effect of pulsatile motion. A voxel-based analysis was then performed using a previously published method (Schwarz et al., 2014) based on groupwise registration to a study-specific template using ANTs (Avants et al., 2010) and statistical comparisons using SPM12. Results are shown after FWE correction for multiple comparisons at P < 0.05 or uncorrected at a threshold of P < 0.001.
A global PiB SUVR was calculated as described previously (Jack et al., 2017), using the cerebellar crus grey matter as the reference region, and PiB positivity was defined as >1.42.
Statistical analysis
Demographic and clinical data were compared between PAA and AOS+PAA groups using non-parametric pairwise Wilcoxon rank sum comparisons in R (Team, 2013), using the integrated development environment RStudio (Racine, 2012). All P-values were corrected for multiple comparisons using the false discovery rate (FDR) correction. PAA and AOS+PAA groups were compared at baseline and at their first available follow-up; additionally, to lessen the effect of the AOS+PAA group having significantly longer disease duration, values from the PAA follow-up visit were compared with AOS+PAA baseline values. The same statistical methods were used in analysing the writing and speech language samples, which were compared separately. Additionally, annualized rates of change were calculated for each patient for select clinical variables by subtracting the score of the baseline visit from the score of the follow-up, which was then divided by the time in years that passed between the two visits.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Clinical description of the progressive agrammatic aphasia group
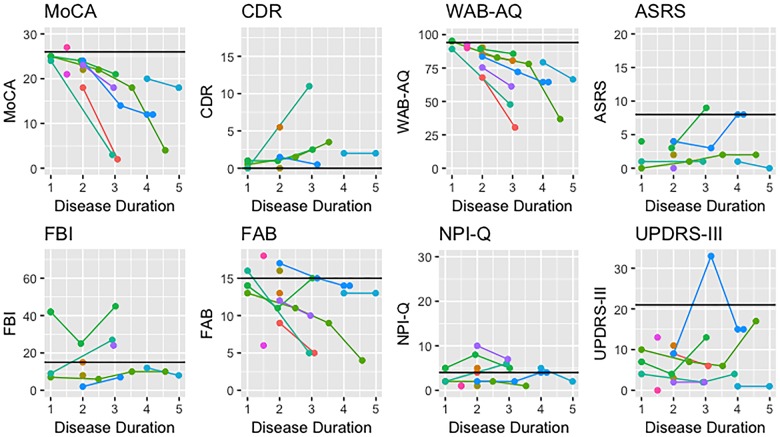
The PAA group consisted of 63% females with an average age of 68.5 years, and disease duration of 1.8 years at baseline. Only one PAA patient showed amyloid-β deposition on PiB-PET. All but two PAA patients showed impairment on WAB-AQ at baseline, scoring <94.1; these two patients, however, had the shortest disease durations at just 1 year. Over time, all PAA patients followed longitudinally showed notable decline in WAB-AQ performance (Fig. 1). Although none of the patients displayed AOS at baseline, mild AOS did emerge after disease duration of 3 years in two patients. The remaining nine patients did not develop AOS, even after disease duration of up to 4.6 years in one patient. Performance on the MDS-UPDRS III was within normal age limits in all PAA patients (Fig. 1).
Figure 1.
Longitudinal performance of all PAA patients on select clinical measures. Normal scores are noted with the solid black line. ASRS = Apraxia of Speech Rating Scale; CDR = Clinical Dementia Rating; FAB = Frontal Assessment Battery; FBI = Frontal Behavioral Inventory; MoCA = Montreal Cognitive Assessment; NPI-Q = Neuropsychiatric Inventory Questionnaire.
All but one PAA patient could be considered to have some cognitive impairment, with MoCA < 26, at baseline. Throughout the first 2 years of disease, cognitive impairment remained just below normal but with disease progression most patients showed a sharp decline in performance. Mild functional impairment was observed on the CDR at baseline, with scores worsening over time (Fig. 1). Only three PAA patients performed abnormally at any visit on the Frontal Behavioral Inventory, whereas executive dysfunction measured on the FAB was more common (Fig. 1). Only four patients performed normally on the FAB at baseline, with two of them showing abnormalities at future visits; the other two were not followed longitudinally. Performance on the FAB tended to decline over time. Most PAA patients performed within the normal range on the NPI-Q, except for three patients who consistently showed mild impairment with little progression.
Clinical comparison of progressive agrammatic aphasia and AOS+PAA
The PAA and AOS+PAA groups did not differ on demographic characteristics such as age at baseline or gender, although AOS+PAA patients had significantly longer disease duration than PAA by ∼2 years (Table 1).
Table 1.
Demographic and clinical variables
| PAA baseline | AOS+PAA baseline | PAA follow-up | AOS+PAA follow-up | P-values (FDR P-values) | |||
|---|---|---|---|---|---|---|---|
| Baseline to baseline | Follow-up to follow-up | PAA follow-up to AOS+PAA baseline | |||||
| Demographic features | |||||||
| Age at exam, years | 68.85 (64.80, 74.18) | 72.21 (68.08, 75.92) | 70.17 (63.43, 78.03) | 73.36 (67.30, 77.49) | 0.369 (0.718) | 0.728 (0.89) | 0.72 (0.845) |
| Gender, % female | 64 | 59 | 71 | 61 | 0.684 (0.97) | 0.876 (0.938) | 0.89 (0.95) |
| Disease duration, years | 2.00 (1.38, 2.00) | 4.00 (2.62, 5.00) | 2.98 (2.81, 3.10) | 5.13 (4.02, 6.42) | 0.002 (0.02) | 0.002 (0.025) | 0.188 (0.3) |
| Global PiB SUVR | 1.31 (1.27, 1.39) | 1.35 (1.27, 1.76) | 1.28 (1.24, 1.31) | 1.29 (1.29, 1.29) | 0.397 (0.728) | 1.00 (1.00) | 0.203 (0.319) |
| Speech/language testing | |||||||
| WAB-AQ | 87.35 (78.32, 90.98) | 85.00 (81.30, 94.00) | 69.30 (57.92, 81.15) | 81.65 (76.75, 86.25) | 0.955 (0.97) | 0.135 (0.393) | 0.016 (0.059) |
| WAB-fluency | 6.00 (5.75, 9.00) | 6.00 (5.00, 9.00) | 4.00 (3.50, 5.00) | 5.00 (4.00, 6.00) | 0.862 (0.97) | 0.265 (0.515) | 0.007 (0.047) |
| WAB-repetition | 9.40 (8.90, 9.45) | 8.80 (7.80, 9.40) | 8.50 (6.75, 9.40) | 8.70 (7.33, 9.45) | 0.17 (0.495) | 0.908 (0.938) | 1 (1) |
| WAB-spontaneous speech | 16.00 (13.50, 17.50) | 15.00 (14.00, 19.00) | 12.00 (9.50, 13.25) | 13.00 (13.00, 15.00) | 0.804 (0.97) | 0.143 (0.393) | 0.006 (0.045) |
| WAB-information content | 9.50 (8.00, 10.00) | 9.50 (8.00, 10.00) | 8.00 (6.00, 8.50) | 9.00 (8.00, 10.00) | 0.784 (0.97) | 0.235 (0.484) | 0.032 (0.078) |
| Aphasia severity | 1.50 (1.00, 2.00) | 1.00 (1.00, 1.62) | 3.00 (2.00, 3.00) | 2.00 (1.38, 2.25) | 0.382 (0.718) | 0.1 (0.331) | 0.003 (0.035) |
| TT | 15.00 (8.25, 17.75) | 18.00 (10.75, 20.00) | 8.00 (4.00, 13.50) | 16.00 (15.00, 19.00) | 0.382 (0.718) | 0.019 (0.089) | 0.041 (0.094) |
| BNT | 12.00 (9.50, 14.00) | 12.50 (10.75, 13.25) | 8.00 (7.50, 9.00) | 13.00 (11.00, 13.00) | 0.96 (0.97) | 0.003 (0.025) | 0.017 (0.059) |
| NAT | 4.50 (2.25, 7.50) | 8.00 (6.00, 8.00) | 5.00 (2.75, 5.75) | 6.00 (4.75, 6.50) | 0.124 (0.402) | 0.316 (0.521) | 0.068 (0.139) |
| Dysarthria severity | 0.00 (0.00, 0.00) | 0.00 (0.00, 1.00) | 0.00 (0.00, 0.00) | 0.50 (0.00, 1.25) | 0.023 (0.148) | 0.061 (0.222) | 0.147 (0.247) |
| ASRS | 2.00 (1.00, 4.00) | 27.00 (20.00, 34.50) | 1.00 (1.00, 3.00) | 29.00 (23.00, 35.00) | <0.001 (0.001) | 0.002 (0.025) | 0.001 (0.019) |
| NVOA | 28.50 (24.00, 29.25) | 20.00 (4.00, 28.00) | 19.00 (10.00, 25.75) | 13.50 (0.50, 19.50) | 0.099 (0.4) | 0.231 (0.484) | 0.864 (0.95) |
| Letter fluency | 14.50 (10.50, 22.00) | 16.00 (8.00, 21.00) | 8.00 (6.50, 11.00) | 11.00 (9.00, 16.00) | 0.29 (0.663) | 0.288 (0.521) | 0.124 (0.22) |
| Action fluency | 5.50 (1.75, 11.00) | 8.00 (6.00, 11.00) | 3.50 (2.00, 5.75) | 4.00 (4.00, 7.00) | 0.427 (0.759) | 0.312 (0.521) | 0.02 (0.062) |
| MSD | 10.00 (10.00, 10.00) | 6.00 (4.00, 7.00) | 9.00 (8.75, 10.00) | 4.50 (2.25, 6.00) | <0.001 (<0.001) | <0.001 (0.004) | <0.001 (0.004) |
| Neurological/neuropsychological testing | |||||||
| FBI | 9.00 (7.50, 13.50) | 11.00 (7.00, 16.00) | 16.00 (7.25, 24.75) | 14.00 (10.00, 22.00) | 0.824 (0.97) | 1.00 (1.00) | 0.574 (0.707) |
| MDS-UPDRS III | 8.00 (2.75, 10.25) | 16.50 (10.00, 22.50) | 4.00 (2.00, 6.50) | 49.00 (21.00, 66.00) | 0.003 (0.022) | 0.004 (0.025) | 0.013 (0.050) |
| WAB-praxis | 54.50 (52.75, 57.25) | 54.00 (45.25, 56.75) | 54.00 (52.00, 54.50) | 37.00 (33.00, 50.00) | 0.247 (0.62) | 0.038 (0.159) | 0.959 (0.990) |
| CDR | 1.00 (0.25, 1.75) | 0.00 (0.00, 2.00) | 1.50 (1.00, 2.00) | 1.50 (0.75, 6.75) | 0.462 (0.778) | 0.909 (0.938) | 0.122 (0.220) |
| MoCA | 23.00 (20.75, 24.25) | 22.50 (21.00, 25.75) | 18.00 (8.50, 20.00) | 18.00 (15.00, 24.00) | 0.856 (0.97) | 0.55 (0.756) | 0.021 (0.062) |
| FAB | 13.50 (12.75, 16.00) | 14.00 (12.00, 16.00) | 11.00 (7.50, 12.00) | 12.00 (7.25, 14.75) | 0.97 (0.97) | 0.681 (0.864) | 0.028 (0.074) |
| NPI-Q | 3.00 (1.75, 5.00) | 1.50 (0.00, 3.75) | 4.00 (2.00, 6.75) | 3.00 (1.00, 4.00) | 0.126 (0.402) | 0.199 (0.484) | 0.069 (0.139) |
| Trail Making Test A MOANS | 9.50 (6.75, 10.75) | 6.00 (3.00, 8.00) | 6.50 (5.75, 7.75) | 7.00 (3.00, 7.75) | 0.293 (0.498) | 0.414 (0.581) | 0.616 (0.747) |
| Trail Making Test B MOANS | 8.50 (6.25, 7.75) | 6.00 (4.50, 8.00) | 3.00 (3.00, 3.00) | 3.00 (2.00, 6.50) | 0.415 (0.581) | 0.412 (0.581) | 0.101 (0.220) |
| AVLT delayed recall | 3.00 (0.50, 5.50) | 7.00 (5.00, 8.00) | 3.00 (2.00, 3.00) | 8.50 (4.00, 10.00) | 0.1 (0.4) | 0.007 (0.039) | 0.01 (0.052) |
| VOSP letters | 20.00 (19.00, 20.00) | 19.00 (18.00, 20.00) | 20.00 (19.00, 20.00) | 20.00 (19.00, 20.00) | 0.252 (0.62) | 0.844 (0.938) | 0.488 (0.624) |
| VOSP cubes | 9.00 (7.00, 10.00) | 9.00 (7.00, 10.00) | 8.00 (8.00, 9.00) | 9.00 (7.50, 9.00) | 0.674 (0.97) | 0.61 (0.805) | 0.739 (0.845) |
| DKEFS Sorting scaled score | 7.00 (7.00, 8.50) | 7.00 (6.00, 10.50) | 6.00 (5.50, 6.75) | 8.00 (7.00, 9.00) | 0.937 (0.97) | 0.435 (0.653) | 0.418 (0.582) |
All bold values were significant after FDR correction for multiple comparisons. Values are shown as median (q1, q3). Missing data: data were available for >70% of patients at baseline visits for all tests, except Trail Making Test B, NAT, DKEFS and AVLT; data were available for >70% of patients at follow-up for all tests, except Trail Making Test A and B, DKEFS, AVLT, VOSP cubes and action and letter fluency.
ASRS = Apraxia of Speech Rating Scale; AVLT = Auditory Verbal Learning Test; BNT = Boston Naming Test; CDR = Clinical Dementia Rating; DKEFS = Delis-Kaplan Executive Function System.; FAB = Frontal Assessment Battery; FBI = Frontal Behavioral Inventory; MoCA = Montreal Cognitive Assessment; MSD = Motor speech disorder rating; NAT = Northwestern Anagram Test; NPI-Q = Neuropsychiatric Inventory Questionnaire; NVOA = Non-Verbal Oral Apraxia rating; PiB SUVR = Pittsburgh Compound B standard uptake value ratio; TT = Token Test; VOSP = Visual Object and Space Perception.
At baseline, as expected by definition, AOS+PAA performed worse on measures of motor speech (ASRS, MSD, dysarthria rating scale); they were also worse on the measure of parkinsonism (MDS-UPDRS III) (Table 1). At follow-up, both cohorts showed decline in performance on the clinical tests, but the ASRS, MSD, and MDS-UPDRS-III, as well as WAB limb apraxia, were worse in AOS+PAA compared to PAA. Additionally, PAA performed significantly worse on AVLT delayed recall. When comparing the follow-up visit of PAA with the baseline visit of AOS+PAA, thus neutralizing the significant difference in disease durations, PAA performed significantly worse on the WAB and its fluency and spontaneous speech subtests, whereas AOS+PAA patients performed worse on ASRS, MSD, dysarthria rating scale, and MDS-UPDRS-III (Table 1).
We investigated rate of change in clinical variables for patients who had multiple visits further (Table 2). PAA had a faster annual rate of change than AOS+PAA on the WAB-AQ, Token Test, and Boston Naming Test. AOS+PAA patients, on the other hand, declined faster on the ASRS and MDS-UPDRS-III.
Table 2.
Annualized rates of change over time for select clinical measures
| PAA | AOS+PAA | P-values (FDR P-values) | |
|---|---|---|---|
| Visit interval, years | 1.04 (0.98, 1.25) | 1.29 (1.10, 1.74) | 0.116 (0.213) |
| WAB-AQ | −11.35 (−16.38, −7.65) | −3.99 (−6.97, −0.26) | 0.012 (0.05) |
| WAB-fluency | −1.93 (−2.23, −1.54) | −0.98 (−1.44, −0.85) | 0.022 (0.059) |
| WAB-repetition | −0.73 (−1.40, −0.10) | −0.10 (−0.87, 0.12) | 0.232 (0.328) |
| WAB-spontaneous speech | −3.17 (−4.18, −2.44) | −0.98 (−2.62, 0.00) | 0.022 (0.059) |
| WAB-information content | −1.04 (−2.04, −0.76) | 0.00 (−1.75, 0.00) | 0.074 (0.155) |
| WAB-praxis | −1.56 (−3.48, 1.02) | −5.90 (−14.53, −3.49) | 0.019 (0.059) |
| Aphasia severity | 0.76 (0.54, 0.91) | 0.00 (0.00, 0.46) | 0.029 (0.067) |
| TT | −4.62 (−6.38, −4.08) | −1.20 (−2.79, −0.24) | 0.006 (0.049) |
| BNT | −2.01 (−2.88, −1.85) | 0.00 (−1.28, 0.00) | 0.009 (0.049) |
| NAT | −3.02 (−3.42, −1.34) | −1.40 (−1.58, 0.02) | 0.268 (0.351) |
| ASRS | −0.85 (−1.01, 0.00) | 1.97 (1.59, 3.80) | 0.002 (0.046) |
| NVOA | −6.12 (−11.58, −3.27) | −5.73 (−7.50, −2.61) | 0.664 (0.734) |
| Letter fluency | −4.20 (−4.58, −2.54) | −3.94 (−4.29, −2.91) | 0.836 (0.843) |
| Action fluency | −2.56 (−3.34, −0.50) | −1.97 (−2.81, −0.85) | 0.607 (0.708) |
| MSD | −0.46 (−1.04, 0.00) | −1.15 (−1.73, −0.60) | 0.094 (0.179) |
| FBI | −0.67 (−4.02, 4.27) | 3.28 (1.50, 6.55) | 0.234 (0.328) |
| MDS-UPDRS III | −1.04 (−2.39, 0.00) | 12.73 (3.94, 17.95) | 0.007 (0.049) |
| CDR | 0.00 (0.00, 0.67) | 0.53 (0.34, 5.10) | 0.173 (0.279) |
| MoCA | −5.20 (−9.73, −2.01) | −1.84 (−7.82, 0.00) | 0.153 (0.268) |
| FAB | −2.08 (−3.44, −1.52) | −1.75 (−4.30, −0.46) | 0.843 (0.843) |
| NPI-Q | 0.00 (−2.26, 1.56) | 0.70 (0.00, 3.18) | 0.29 (0.359) |
Bold values were significant after FDR correction for multiple comparisons.
Values are shown as median (q1, q3).
Only select clinical variables with the most complete longitudinal testing are shown.
ASRS = Apraxia of Speech Rating Scale; BNT = Boston Naming Test; CDR = Clinical Dementia Rating; FAB = Frontal Assessment Battery; FBI = Frontal Behavioral Inventory; MoCA = Montreal Cognitive Assessment; MSD = Motor speech disorder rating; NAT = Northwestern Anagram Test; NPI-Q = Neuropsychiatric Inventory Questionnaire; NVOA = Non-Verbal Oral Apraxia rating; TT = Token Test.
Language results
At baseline, PAA and AOS+PAA performed similarly on writing and speech output, showing no significant differences in the quantitative language analysis (Table 3). However, at follow-up, PAA performed significantly worse than AOS+PAA, particularly in writing. They produced fewer words overall, had a lower mean length of utterance, and an increased ratio of nouns to other words. Additionally, PAA patients produced far fewer grammatical utterances and many more non-utterances in their writing. Verbal production was more similar between the two groups at follow-up, with PAA showing only a greater noun ratio and more non-utterances than AOS+PAA. To minimize the effects of different disease durations between the two groups, the follow-up visit of PAA patients was compared with the baseline visit of AOS+PAA patients. When disease duration did not differ between cohorts, AOS+PAA outperformed PAA. In writing, PAA generated significantly fewer words, had decreased mean length of utterance, increased noun ratio, decreased function word ratio, and more non-utterances. There were no differences in spoken language output between groups at these respective visits.
Table 3.
Linguistic variables
| Language variable | PAA baseline | PAA follow-up | AOS+PAA baseline | AOS+PAA follow-up | Baseline-to-baseline | Follow-up to follow-up | PAA follow-up to AOS+PAA baseline |
|---|---|---|---|---|---|---|---|
| Writing | |||||||
| Word count | 48.00 (36.25, 68.75) | 9.00 (5.50, 15.50) | 38.00 (30.50, 48.50) | 24.00 (16.00, 33.00) | 0.053 (0.344) | 0.015 (0.04) | 0 (0.003) |
| Mean length of utterance | 9.07 (6.83, 9.96) | 2.83 (1.58, 4.75) | 7.12 (6.27, 9.65) | 6.00 (5.50, 7.00) | 0.378 (0.615) | 0.009 (0.028) | 0.001 (0.003) |
| Utterances | 6.00 (6.00, 7.00) | 4.00 (3.00, 6.00) | 6.00 (4.00, 7.00) | 5.00 (4.00, 5.00) | 0.259 (0.615) | 0.687 (0.77) | 0.108 (0.176) |
| Noun ratio | 0.35 (0.33, 0.36) | 0.67 (0.48, 0.80) | 0.33 (0.31, 0.38) | 0.35 (0.31, 0.45) | 0.62 (0.806) | 0.008 (0.028) | 0.004 (0.01) |
| Verb ratio | 0.14 (0.13, 0.16) | 0.17 (0.13, 0.25) | 0.15 (0.14, 0.18) | 0.19 (0.17, 0.22) | 0.345 (0.615) | 0.525 (0.759) | 0.453 (0.589) |
| Function word ratio | 0.38 (0.32, 0.40) | 0.07 (0.00, 0.28) | 0.33 (0.26, 0.39) | 0.31 (0.24, 0.33) | 0.229 (0.615) | 0.073 (0.158) | 0.008 (0.018) |
| Complex utterance ratio | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.527 (0.762) | 0.323 (0.525) | 0.336 (0.485) |
| Semantic error ratio | 0.03 (0.00, 0.04) | 0.00 (0.00, 0.03) | 0.00 (0.00, 0.02) | 0.00 (0.00, 0.00) | 0.014 (0.181) | 0.603 (0.77) | 0.793 (0.859) |
| Syntactic error ratio | 0.03 (0.00, 0.04) | 0.00 (0.00, 0.13) | 0.02 (0.00, 0.05) | 0.04 (0.00, 0.12) | 0.696 (0.823) | 0.966 (0.966) | 1 (1) |
| Correct verb ratio | 0.84 (0.75, 0.97) | 0.75 (0.28, 1.00) | 0.83 (0.67, 1.00) | 0.75 (0.33, 1.00) | 0.782 (0.847) | 0.71 (0.77) | 0.748 (0.859) |
| Grammatical utterance ratio | 0.33 (0.29, 0.63) | 0.00 (0.00, 0.25) | 0.40 (0.20, 0.60) | 0.25 (0.00, 0.50) | 0.96 (0.96) | 0.156 (0.29) | 0.032 (0.06) |
| Non-utterance ratio | 0.17 (0.00, 0.19) | 1.00 (0.58, 1.00) | 0.20 (0.00, 0.40) | 0.00 (0.00, 0.20) | 0.341 (0.615) | 0.002 (0.023) | 0.001 (0.003) |
| Ungrammatical utterance ratio | 0.50 (0.20, 0.63) | 0.00 (0.00, 0.00) | 0.29 (0.14, 0.50) | 0.50 (0.33, 0.75) | 0.204 (0.615) | 0.004 (0.023) | 0.003 (0.01) |
| Speaking | |||||||
| Word count | 78.00 (59.75, 85.25) | 28.50 (13.75, 43.25) | 52.00 (38.00, 75.00) | 39.50 (27.25, 57.00) | 0.084 (0.544) | 0.183 (0.281) | 0.084 (0.544) |
| Mean length of utterance | 8.08 (7.11, 9.77) | 3.65 (2.00, 6.65) | 7.57 (5.80, 9.00) | 5.82 (4.66, 8.38) | 0.428 (0.565) | 0.124 (0.231) | 0.428 (0.565) |
| Utterances | 9.00 (8.00, 13.75) | 8.50 (7.75, 9.50) | 8.00 (7.00, 9.00) | 7.00 (6.00, 10.00) | 0.083 (0.544) | 0.681 (0.738) | 0.083 (0.544) |
| Noun ratio | 0.28 (0.25, 0.34) | 0.48 (0.42, 0.64) | 0.31 (0.27, 0.38) | 0.31 (0.25, 0.38) | 0.356 (0.565) | 0.005 (0.039) | 0.356 (0.565) |
| Verb ratio | 0.15 (0.12, 0.16) | 0.15 (0.12, 0.18) | 0.17 (0.13, 0.18) | 0.17 (0.15, 0.23) | 0.304 (0.565) | 0.195 (0.281) | 0.304 (0.565) |
| Function word ratio | 0.37 (0.34, 0.38) | 0.19 (0.15, 0.39) | 0.35 (0.28, 0.38) | 0.34 (0.24, 0.44) | 0.237 (0.565) | 0.116 (0.231) | 0.237 (0.565) |
| Complex utterance ratio | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.706 (0.706) | 0.305 (0.375) | 0.706 (0.706) |
| Semantic error ratio | 0.02 (0.00, 0.04) | 0.02 (0.00, 0.05) | 0.00 (0.00, 0.02) | 0.01 (0.00, 0.04) | 0.137 (0.565) | 0.971 (0.971) | 0.137 (0.565) |
| Syntactic error ratio | 0.02 (0.00, 0.02) | 0.08 (0.02, 0.15) | 0.02 (0.00, 0.04) | 0.03 (0.00, 0.10) | 0.362 (0.565) | 0.317 (0.375) | 0.362 (0.565) |
| Correct verb ratio | 0.87 (0.83, 1.00) | 0.33 (0.12, 0.66) | 0.87 (0.75, 0.99) | 0.85 (0.67, 0.98) | 0.435 (0.565) | 0.077 (0.2) | 0.435 (0.565) |
| Grammatical utterance ratio | 0.57 (0.41, 0.79) | 0.06 (0.00, 0.13) | 0.50 (0.25, 0.62) | 0.41 (0.10, 0.50) | 0.193 (0.565) | 0.024 (0.078) | 0.193 (0.565) |
| Non-utterance ratio | 0.18 (0.00, 0.31) | 0.94 (0.77, 1.00) | 0.17 (0.00, 0.43) | 0.18 (0.15, 0.46) | 0.616 (0.668) | 0.006 (0.039) | 0.616 (0.668) |
| Ungrammatical utterance ratio | 0.19 (0.14, 0.34) | 0.00 (0.00, 0.09) | 0.25 (0.15, 0.43) | 0.36 (0.21, 0.43) | 0.49 (0.579) | 0.024 (0.078) | 0.49 (0.579) |
Bold values were significant after FDR correction for multiple comparisons. Values are shown as median (q1, q3). Data were available for 91% of PAA patients at baseline and 100% at follow-up, and 100% of AOS-PAA patients at baseline and follow-up.
MRI-grey matter results
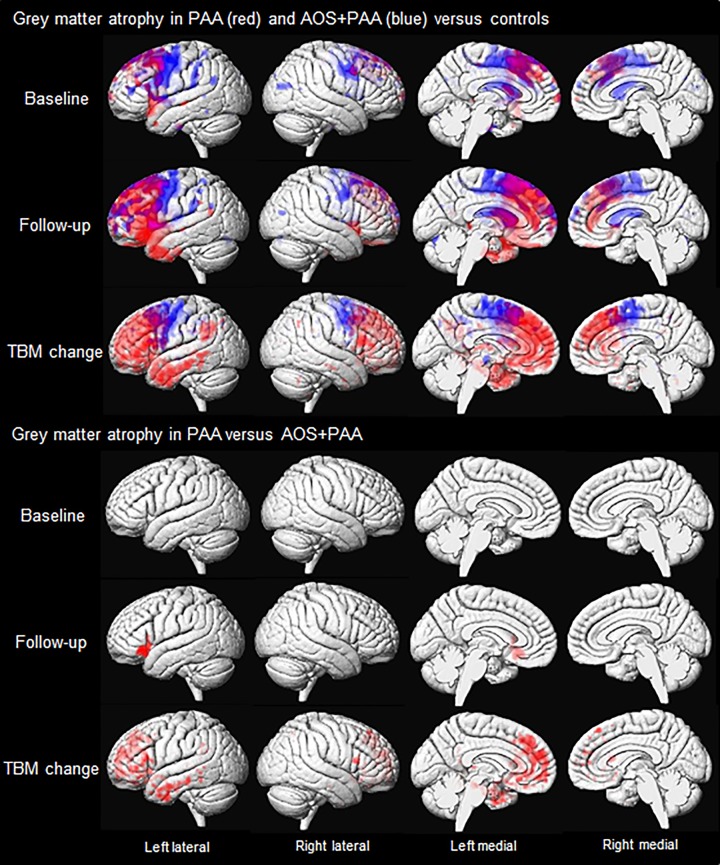
Results of the MRI analyses are shown in Fig. 2. At baseline, PAA showed reduced grey matter volume throughout left prefrontal lobe compared with controls. Reduced volume was most notable in superior and middle frontal gyri, medial frontal lobe, as well as Broca’s area, and was also observed in anterior superior temporal gyrus. Some medial frontal regions of the right hemisphere were also affected. Grey matter volume loss in AOS+PAA was more bilateral, although still left-hemisphere dominant, with most striking involvement of premotor regions compared to controls. Reduced volume was also observed in motor cortex, the superior and middle frontal regions, supramarginal and angular gyri, anterior cingulate gyrus, and thalamus.
Figure 2.
Voxel-level maps of grey matter loss in PAA and AOS+PAA. The top three rows show 3D surface renderings of the brain showing grey matter volume loss at baseline and follow-up, and grey matter atrophy measured using TBM-SyN, in PAA (red) and AOS+PAA (blue) versus controls. The bottom three rows show regions where volume loss or atrophy is greater in PAA than AOS+PAA. All results are FWE corrected at P < 0.05.
At follow-up, the regions of reduced volume in PAA spread diffusely throughout the left prefrontal lobe. The right hemisphere remained relatively spared, with a small degree of loss observed in superior and medial frontal regions. Involvement of the left temporal lobe also expanded to involve anterior superior, middle, and inferior temporal gyri, as well as temporal pole. Bilateral volume loss was observed in anterior cingulate. The AOS+PAA patients also showed more grey matter volume loss at follow-up compared to baseline. However, the extent of progression was less than that observed in PAA. Premotor regions still showed the most significant volume loss bilaterally, with some extension into prefrontal cortices.
In the TBM-SyN analysis of Jacobian change maps, PAA showed greater atrophy than controls throughout left prefrontal cortex and anterior inferior and middle temporal gyri, spreading back into the posterior middle temporal gyrus and inferior parietal lobe, with atrophy in the right hemisphere restricted to the prefrontal cortex. Atrophy in AOS+PAA was observed bilaterally in premotor cortex compared to controls.
When compared directly against each other at baseline, there were no significant differences that survived correction for multiple comparisons between the two groups. However, when uncorrected (P < 0.001), PAA showed greater volume loss in Broca’s area compared to AOS+PAA. At follow-up, PAA showed greater volume loss in left Broca’s area than AOS+PAA after correction for multiple comparisons (Fig. 2). There were no regions that showed more loss in AOS+PAA versus PAA. In the direct comparison of TBM-SyN Jacobians, PAA showed greater atrophy in left prefrontal cortex and anterior temporal lobe compared to AOS+PAA after correction for multiple comparisons. Conversely, AOS+PAA showed greater atrophy than PAA predominantly in the bilateral premotor cortex, although only when uncorrected (P < 0.001).
MRI-DTI results
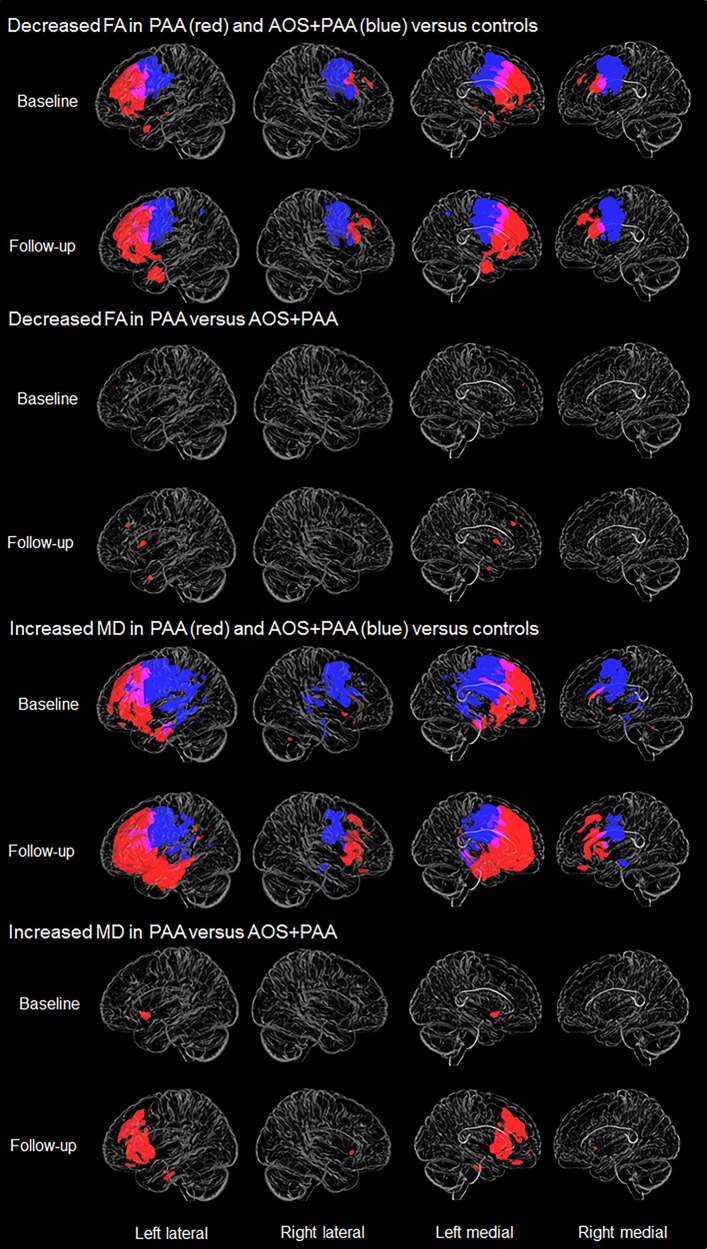
Results of the DTI analysis are shown in Fig. 3. PAA showed lower fractional anisotropy compared to controls at baseline in left anterior inferior and middle frontal regions, genu of the corpus callosum and uncinate fasciculus, and right middle frontal white matter. mean diffusivity results were similar, although more extensive. AOS+PAA showed more bilateral patterns of reduced fractional anisotropy compared to controls at baseline, most notable in motor and premotor regions and body of the corpus callosum; although loss was observed bilaterally, the left hemisphere was more severely affected. Increased mean diffusivity was observed in similar regions, although patterns were more extensive, also involving temporoparietal regions. Patterns observed in AOS+PAA were generally more posterior than what was observed in PAA.
Figure 3.
Voxel-level maps of fractional anisotropy (FA) and mean diffusivity (MD) in PAA and AOS+PAA. The top two rows are 3D transparent renders showing decrease in fractional anisotropy in PAA (red) and AOS+PAA (blue) versus controls at baseline and follow-up, respectively. The third and fourth rows show regions where fractional anisotropy is decreased in PAA compared to AOS+PAA at both time points. Rows five and six show increase in mean diffusivity in PAA (red) and AOS+PAA (blue) versus controls at baseline and follow-up, respectively. The bottom two rows show regions of increased mean diffusivity in PAA compared to AOS+PAA at both time points. All results are FWE corrected at P < 0.05.
At the follow-up visit, PAA again showed reduced fractional anisotropy and increased mean diffusivity in left prefrontal white matter, but showed greater involvement of left anterior temporal lobe and right superior and middle frontal regions and genu of the corpus callosum than at baseline. At follow-up, AOS+PAA showed a pattern of reduced fractional anisotropy and increased mean diffusivity very similar to baseline.
When compared directly against each other at baseline, PAA showed decreased fractional anisotropy compared to AOS+PAA in small regions in left prefrontal and medial frontal regions. The PAA cohort also showed increased mean diffusivity in inferior frontal regions compared to AOS+PAA. AOS+PAA did not show regions of decreased fractional anisotropy compared to PAA after correction for multiple comparisons, but when uncorrected (P < 0.001), they showed decreased fractional anisotropy in the left premotor region and middle corpus callosum. AOS+PAA patients showed increased mean diffusivity in the motor and premotor regions compared to PAA, bilaterally, but only when left uncorrected. Differences between the two cohorts were exaggerated at the follow-up visits, with PAA showing reduced fractional anisotropy in left middle frontal white matter and increased mean diffusivity throughout left inferior frontal and inferior temporal white matter, including the uncinate fasciculate, compared to AOS+PAA. The fractional anisotropy and mean diffusivity findings in AOS+PAA compared to PAA were similar to baseline, but also did not survive correction for multiple comparisons.
At both baseline and follow-up visits, axial diffusivity and radial diffusivity results were also examined but did not differ greatly from the mean diffusivity results. For this reason, their discussion has been omitted.
FDG-PET results
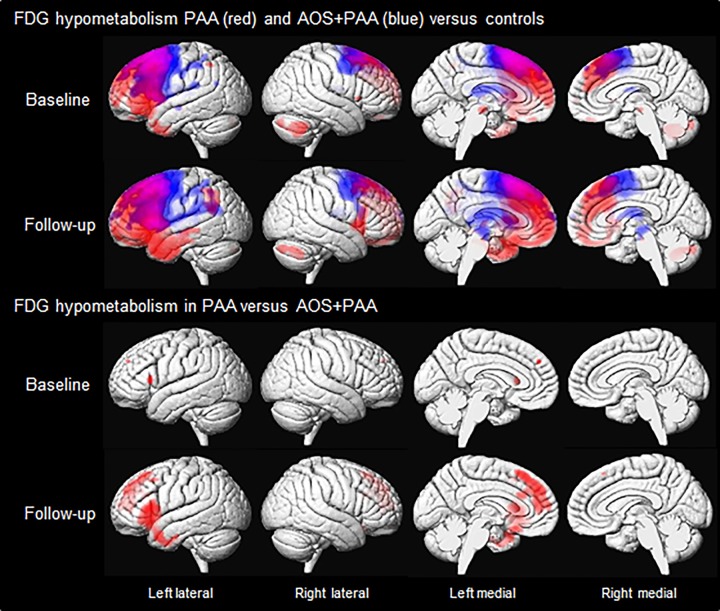
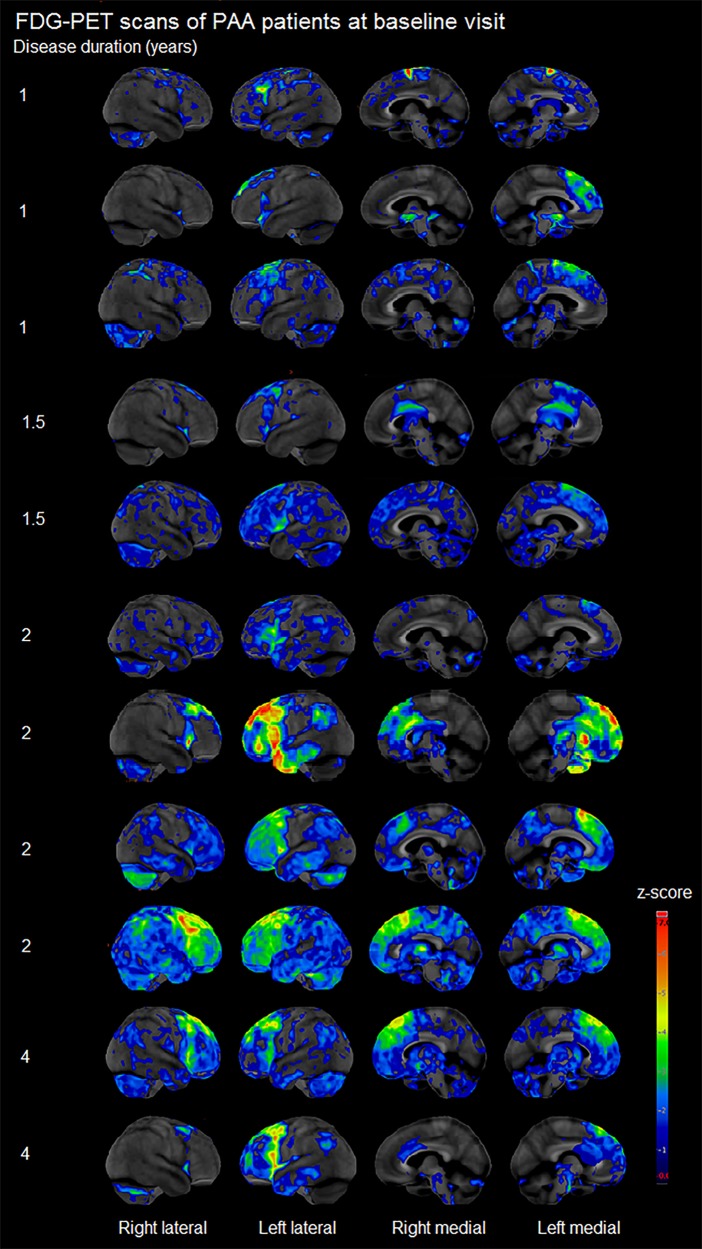
Results of the SPM analysis of FDG-PET are shown in Fig. 4. At baseline, PAA showed hypometabolism diffusely throughout left prefrontal cortex, specifically in superior and inferior regions, compared to controls. The anterior portion of the left temporal lobe was also affected. Although more predominant in the left hemisphere, hypometabolism was also observed in the right prefrontal cortex. The medial frontal lobe and anterior cingulate were affected bilaterally. The amount of hypometabolism observed in individual patients varied greatly within the PAA cohort, although all patients showed hypometabolism of the prefrontal cortex (Fig. 5). At baseline, AOS+PAA also showed hypometabolism in left frontal lobe compared with controls, particularly in lateral and medial premotor regions extending back into motor cortex. Right hemisphere hypometabolism was observed in motor and premotor regions.
Figure 4.
Voxel-level maps of FDG hypometabolism in PAA and AOS+PAA. The top two rows show 3D surface renderings of the brain showing FDG hypometabolism in PAA (red) and AOS+PAA (blue) versus controls at baseline and follow-up, respectively. The bottom two rows show regions where FDG hypometabolism is greater in PAA than AOS+PAA at both time points. All results are FWE corrected at P < 0.05.
Figure 5.
Cortex ID Z-score maps of FDG-PET hypometabolism in individual PAA patients.
At follow-up, hypometabolism spread throughout the left prefrontal and anterior temporal lobes in PAA. Additional hypometabolism was observed in the left supramarginal gyrus. Right hemisphere hypometabolism also increased, with greater involvement of inferior frontal and prefrontal regions. Bilateral medial frontal and anterior cingulate regions showed increased hypometabolism at follow-up. AOS+PAA also showed greater hypometabolism at follow-up compared with controls. Bilateral premotor and motor regions were more affected, as well as the left parietal lobe. Hypometabolism was also observed in the basal ganglia and surrounding midbrain structures at follow-up.
When compared directly to each other at baseline, PAA showed greater hypometabolism in left Broca’s area and in a small region in the medial prefrontal cortex compared to AOS+PAA. At follow-up, PAA showed greater hypometabolism throughout left Broca’s area, prefrontal cortex, anterior temporal regions, and medial frontal/anterior cingulate, and right prefrontal regions, compared to AOS+PAA. AOS+PAA showed greater hypometabolism than PAA in bilateral premotor cortex at both time points, although only uncorrected (P < 0.001).
Additional MRI and PET SPM analyses were run in which disease duration, which differed between the groups, was included as a covariate; the results from these analyses were not qualitatively different from those previously described.
Discussion
In this study, we describe the clinical and imaging characteristics of 11 patients with isolated agrammatism that we have designated as PAA, which is an uncommon and previously under recognized neurodegenerative syndrome. They were compared against healthy control subjects and patients with the more common AOS+PAA clinical presentation. We showed that PAA is associated with a fundamentally different pattern of neurodegeneration than AOS+PAA, involving the prefrontal and anterior temporal lobes, and that PAA patients show a faster decline in language and other neurocognitive functions over time. The majority of previous studies have combined PAA and AOS+PAA patients into a single agPPA group, but our data suggest that doing so adds significant heterogeneity to that cohort, heterogeneity that masks important reliable clinical and neuroimaging distinctions.
Patients diagnosed with PAA presented with agrammatism in the absence of AOS. Speech and language testing also showed mild naming abnormalities and non-verbal oral apraxia. Only 2 of 11 PAA patients went on to develop mild AOS, but their agrammatism remained the predominant impairment. The remaining nine patients did not develop any features of AOS, even after a relatively long follow-up of almost 5 years in one patient. We cannot rule out the possibility that AOS may develop in some of these patients eventually, although it does seem that the development of AOS is uncommon, especially early in the disease course. They also commonly displayed cognitive impairment at baseline which progressed rapidly over the course of the disease. The profile of cognitive impairment was dominated by executive dysfunction with some episodic verbal memory deficits, suggestive of frontotemporal aetiology. Parkinsonism, limb apraxia and dysarthria were not features of the disease course in PAA.
Although the PAA and AOS+PAA cohorts performed similarly on many clinical tests at baseline and follow-up, this may have been expected since they were matched for aphasia severity. Furthermore, the cohort comparison was confounded by a difference in disease duration, with the PAA patients being captured earlier in their disease course. To provide a comparison that was less confounded by disease duration, we compared the second visit of the PAA patients to the AOS+PAA baseline visits. This comparison revealed that PAA performed worse on measures of aphasia severity, including the WAB-AQ, WAB fluency subtest, WAB spontaneous speech subtest, and the aphasia severity rating, for similar disease duration. Similarly, when we examined annualized rates of decline in the clinical test scores, PAA showed a significantly faster rate of decline on measures of aphasia, including the WAB-AQ, Token Test, and BNT, with borderline findings for the WAB fluency and spontaneous speech subtests, compared to AOS+PAA. Therefore, not only do PAA patients show more aphasia than AOS+PAA at a given time point in their disease duration, but their disease progresses at a faster rate. Importantly, this suggests that PAA is not just an earlier or milder form of AOS+PAA in which AOS has not yet developed.
As expected, based on how we defined our groups, PAA patients consistently performed better and progressed at a significantly slower rate on clinical tests related to motor speech impairments. They also showed less parkinsonism and limb apraxia compared to AOS+PAA. We and others have noted that patients with AOS+PAA often evolve into a parkinsonian syndrome over time with overlapping features of corticobasal syndrome and progressive supranuclear palsy, and have a 4R tauopathy, such as progressive supranuclear palsy or corticobasal degeneration, at autopsy (Josephs et al., 2005, 2006; Deramecourt et al., 2010; Adeli et al., 2013; Santos-Santos et al., 2016). In contrast, PAA seems to have a different disease course, with patients rarely developing parkinsonism or limb apraxia and not showing progression of those features over time. This could possibly, therefore, also suggest a different pathology underlying PAA. In fact, autopsy findings have been reported in two PAA patients and both had the TAR DNA binding protein of 43 kDa (TDP-43) pathology (Harris et al., 2013), which differs from the tauopathies that are commonly found in patients with AOS (Josephs et al., 2006; Deramecourt et al., 2010; Harris et al., 2013). We can be confident that Alzheimer’s disease is not the underlying pathology given the negative PiB-PET scans.
Both cohorts showed the expected features of agrammatism (Thompson and Mack, 2014; Tetzloff et al., 2018), including reduced language production, simple sentence structure, omission of function words, verbal and syntactic difficulties, and disproportionate production of nouns. PAA and AOS+PAA groups did not show any significant differences in their language production at their baseline visits, likely because the two groups were matched based on aphasia severity. However, PAA patients’ language declined more rapidly than that of the AOS+PAA patients, as evidenced by their poorer performance at follow-up visits, especially in writing. At follow-up, PAA patients produced fewer words, had shorter utterances, fewer grammatical utterances, and more non-utterances (i.e. utterances that consist of an isolated noun or a phrase that lacks a tensed verb). Again, correcting for disease duration by comparing PAA’s baseline visits with AOS+PAAs’ follow-up visits showed similar increased grammatical impairment in PAA’s writing. These linguistic findings provide further support for the notion that PAA, and in particular features of agrammatism, progresses more rapidly than in AOS+PAA.
Language differences between the two cohorts were more striking in the written than spoken modality. PAA seemed to perform similarly in speech and writing. AOS+PAA, on the other hand, did not; their spoken language production was much more impaired than their written, likely due to the motor speech difficulties caused by the apraxia of speech. Although their aphasia was less severe than PAA when disease duration was controlled, their apraxia of speech hindered their ability to perform well on this task. For this reason, written language production may be a more valid way to evaluate agrammatism in AOS+PAA as long as limb apraxia does not substantially affect the ability to write.
On neuroimaging measures, PAA showed striking involvement of the left prefrontal and anterior temporal lobes on MRI and FDG-PET. These regions are similar to what is observed in the behavioural variant of frontotemporal dementia (Rosen et al., 2002); although none of the PAA patients met clinical criteria for that disorder (Rascovsky et al., 2007) and they did not show worse behaviour or executive dysfunction than the AOS+PAA cohort. The striking left dominant patterns of volume loss and hypometabolism in PAA supports the fact that it is a language disorder, in contrast with the behavioural variant frontotemporal dementia, which usually shows more bilateral neurodegeneration (Whitwell et al., 2013b). AOS+PAA patients, on the other hand, showed most atrophy in premotor and precentral regions and showed more bilateral patterns of volume loss and hypometabolism. These patterns are consistent with those previously associated with patients who have both AOS and agrammatic aphasia (Josephs et al., 2006, 2013; Peelle et al., 2008; Rogalski et al., 2011, 2014), with involvement of superior premotor regions likely associated with the presence of AOS, and involvement of inferior frontal gyrus, i.e. Broca’s area, likely associated with the presence of agrammatism (Whitwell et al., 2013a). We observed significant differences in regional atrophy between PAA and PAA-AOS even after correction for multiple comparisons and despite the fact that these direct contrasts may have had reduced power compared to when each group was contrasted with controls. The difference between the two groups was most striking in Broca’s area, where PAA was significantly more affected, especially at the follow-up visit. This concurs with the fact that the PAA group showed worse agrammatism than the AOS+PAA group.
PAA also showed distinct patterns of white matter tract degeneration compared to AOS+PAA, with greater involvement of the left prefrontal white matter tracts and the uncinate fasciculus. In AOS+PAA, however, degeneration did not include the anterior temporal lobe but instead spanned from the left posterior frontal lobe to the anterior parietal region, along posterior aspects of the superior longitudinal fasciculus. The uncinate fasciculus and superior longitudinal fasciculus form part of the speech-language network (Duffau et al., 2009; Dronkers, 2011; Papagno, 2011; Friederici and Gierhan, 2013). Our results suggest that damage to different subcomponents of the superior longitudinal fasciculus may be occurring in PAA and AOS+PAA, with damage to more posterior sections involved in motor-speech planning and programming rather than grammar. Indeed, we have previously demonstrated involvement of the posterior superior longitudinal fasciculus in patients with AOS (Whitwell et al., 2010, 2013a; Josephs et al., 2012; Botha et al., 2015; Tetzloff et al., 2017). The left uncinate fasciculus was significantly more affected in PAA than in AOS+PAA, perhaps playing a role in the poorer naming and memory observed in the PAA patients (Papagno et al., 2010; Papagno, 2011), and we observed greater degeneration of white matter tracts in the inferior frontal gyrus in PAA, which may be associated with the greater severity of agrammatism (Catani et al., 2013).
A limitation to the present study is the lack of autopsy-confirmed pathology. Additionally, the PAA cohort was not very large, consisting of just 11 patients, which may limit statistical power and generalizability, but given the rarity of the syndrome it is a reasonable sample, and many results survived correction for multiple comparisons despite the small number of patients. Nevertheless, the spatial extent of the neuroimaging differences between PAA and AOS-PAA may have been larger if we had a larger sample. The present study has several strengths, which include the fact that all patients were comprehensively evaluated by a multidisciplinary team that included expertise in neurology, speech-language pathology, neuropsychology, and neuroimaging. Additionally, an analysis of both written and spoken language was performed for all patients, providing a more comprehensive look at the agrammatic features of language (Thompson et al., 1997; Tetzloff et al., 2018). Importantly, our PAA cohort was homogeneous, in that no patient had AOS at baseline.
The clinical, language, and neuroimaging findings for PAA demonstrate that it is a syndrome that affects the prefrontal cortex and anterior temporal lobes with associated agrammatism, naming deficits and cognitive impairment. These features differ from patients with AOS+PAA, suggesting it represents a distinct syndrome with different patterns of progression. Notably, PAA does not evolve into a Parkinsonian syndrome, in contrast to AOS+PAA. Patients with isolated PAA have more involvement of Broca’s area, which is consistent with their more rapid clinical decline in the grammatical aspects of language. These findings have implications for clinical diagnosis and prognosis for these two cohorts, which are most commonly lumped together in a single group, agPPA. In PAA, with no AOS present at baseline, the worsening of aphasia will likely be more rapid and will remain the predominant symptom. These distinctions may also be important when it comes to predicting pathology, although autopsy confirmation is still necessary.
Funding
This study was funded by National Institutes of Health grants R01-DC12519, R21-NS94684, R01-DC010367, R01-DC14942 and U01 AG006786.
Competing interests
The authors report no competing interests.
Glossary
Abbreviations
- agPPA
non-fluent/agrammatic variant of primary progressive aphasia
- AOS
apraxia of speech
- MDS-UPDRS III
Movement Disorders Society Unified Parkinson’s Disorder Rating Scale III
- PAA
progressive agrammatic aphasia
- WAB-AQ
Western Aphasia Battery Aphasia Quotient
References
- Adeli A, Whitwell JL, Duffy JR, Strand EA, Josephs KA. Ideomotor apraxia in agrammatic and logopenic variants of primary progressive aphasia. J Neurol 2013; 260: 1594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016; 125: 1063–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gunawardena D, Avants B, Morgan B, Khan A et al. Speech errors in progressive non-fluent aphasia. Brain Lang 2010; 113: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Barnes G, Chen C, Daunizeau J, Flandin G, Friston K et al. SPM12 manual. London: Wellcome Trust Centre for Neuroimaging; 2014. [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005; 26: 839–51. [DOI] [PubMed] [Google Scholar]
- Avants BB, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage 2010; 49: 2457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard KJ, Azizi L, Duffy JR, McNeil MR, Halaki M, O'Dwyer N et al. A predictive model for diagnosing stroke-related apraxia of speech. Neuropsychologia 2016; 81: 129–39. [DOI] [PubMed] [Google Scholar]
- Botha H, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA. Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology 2014; 82: 1729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H, Duffy JR, Whitwell JL, Strand EA, Machulda MM, Schwarz CG et al. Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex 2015; 69: 220–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts AM, Machulda MM, Duffy JR, Strand EA, Whitwell JL, Josephs KA. Neuropsychological profiles differ among the three variants of primary progressive aphasia. J Int Neuropsychol Soc 2015; 21: 429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C et al. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain 2013; 136: 2619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croot K, Ballard K, Leyton CE, Hodges JR. Apraxia of speech and phonological errors in the diagnosis of nonfluent/agrammatic and logopenic variants of primary progressive aphasia. J Speech Lang Hear Res 2012; 55: S1562–72. [DOI] [PubMed] [Google Scholar]
- De Renzi A, Vignolo LA. Token test: a sensitive test to detect receptive disturbances in aphasics. Brain 1962; 85: 665–78. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system: examiners manual. New York: Psychological Corporation; 2001. [Google Scholar]
- Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani M, Bombois S, Kerdraon O et al. Prediction of pathology in primary progressive language and speech disorders. Neurology 2010; 74: 42–9. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci 2011; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B. The FAB: a frontal assessment battery at bedside. Neurology 2000; 55: 1621–6. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Moritz-Gasser S, Mandonnet E. Is the left uncinate fasciculus essential for language? J Neurol 2009; 256: 382. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Apraxia of speech in degenerative neurologic disease. Aphasiology 2006; 20: 511–27. [Google Scholar]
- Duffy JR. Motor speech disorders: substrates, differential diagnosis and management. St Louis: Elsevier; 2013. [Google Scholar]
- Duffy JR. Motor speech disorders: substrates, differential diagnosis, and management. St Louis: Elsevier Health Sciences; 2005. [Google Scholar]
- Friederici AD, Gierhan SM. The language network. Curr Opin Neurobiol 2013; 23: 250–4. [DOI] [PubMed] [Google Scholar]
- Garyfallidis E, Brett M, Amirbekian B, Rokem A, Van Der Walt S, Descoteaux M et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform 2014; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez‐Martin P et al. Movement disorder society‐sponsored revision of the unified Parkinson’s disease rating scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008; 23: 2129–70. [DOI] [PubMed] [Google Scholar]
- Gorno‐Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004; 55: 335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E, D’Esposito M, Ding X-S et al. Progressive nonfluent aphasia: language, cognitive, and PET measures contrasted with probable Alzheimer’s disease. J Cogn Neurosci 1996; 8: 135–54. [DOI] [PubMed] [Google Scholar]
- Harris JM, Gall C, Thompson JC, Richardson AM, Neary D, du Plessis D et al. Classification and pathology of primary progressive aphasia. Neurology 2013; 81: 1832–9. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 2017; 32: 853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement 2017; 13: 205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Boeve B, Duffy J, Smith G, Knopman D, Parisi J et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase 2005; 11: 283–96. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology 2013; 81: 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain 2012; 135: 1522–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006; 129: 1385–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci 2000; 12: 233–9. [DOI] [PubMed] [Google Scholar]
- Kellner E, Dhital B, Kiselev VG, Reisert M. Gibbs‐ringing artifact removal based on local subvoxel‐shifts. Magn Reson Med 2016; 76: 1574–81. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western aphasia battery (revised). San Antonio: PsychCorp; 2007. [Google Scholar]
- Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementi. Can J Neurol Sci 1997; 24: 29–36. [DOI] [PubMed] [Google Scholar]
- Koay CG, Özarslan E, Basser PJ. A signal transformational framework for breaking the noise floor and its applications in MRI. J Magn Reson 2009; 197: 108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Arch Clin Neuropsychol 1999; 14: 481–7. [PubMed] [Google Scholar]
- MacWhinney B. The CHILDES project: tools for analyzing talk. 3rd edn. Mahwah, New Jersey: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- McKeith I, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017; 89: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 2011; 7: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 1995; 36: 1238. [PubMed] [Google Scholar]
- Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412–4. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–9. [DOI] [PubMed] [Google Scholar]
- Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord 2007; 21: S23–30. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d’une figure complexe; contribution à l’étude de la perception et de la mémoire. Arch Psychol 1944; 30: 286–356. [Google Scholar]
- Papagno C. Naming and the role of the uncinate fasciculus in language function. Curr Neurol Neurosci Rep 2011; 11: 553. [DOI] [PubMed] [Google Scholar]
- Papagno C, Miracapillo C, Casarotti A, Romero Lauro LJ, Castellano A, Falini A et al. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain 2010; 134: 405–14. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Gee J, Moore P, McMillan C, Vesely L et al. Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. J Neurolinguistics 2008; 21: 418–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine JS. RStudio: a platform‐independent IDE for R and Sweave. J Appl Econom 2012; 27: 167–72. [Google Scholar]
- Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, Mendez MF et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord 2007; 21: S14–8. [DOI] [PubMed] [Google Scholar]
- Reid RI, Nedelska Z, Schwarz CG, Ward C, Jack CR, The Alzheimer's Disease Neuroimaging Initiative. Diffusion specific segmentation: skull stripping with diffusion MRI data alone. In: Kaden E, Grussu F, Ning L, Tax C, Veraart J, editors. Computational diffusion MRI. Mathematics and visualization. Cham, Switzerland: Springer; 2018. p. 67–80. [Google Scholar]
- Riley D, Lang A, Lewis Ae, Resch L, Ashby P, Hornykiewicz O et al. Cortical‐basal ganglionic degeneration. Neurology 1990; 40: 1203. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison T, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology 2011; 76: 1804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Martersteck A, Rademaker A, Wieneke C, Weintraub S et al. Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology 2014; 83: 1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno–Tempini ML, Goldman W, Perry R, Schuff N, Weiner M et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 2002; 58: 198–208. [DOI] [PubMed] [Google Scholar]
- Santos-Santos MA, Mandelli ML, Binney RJ, Ogar J, Wilson SM, Henry ML et al. Features of patients with nonfluent/agrammatic primary progressive aphasia with underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurol 2016; 73: 733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. Rey auditory verbal learning test: a handbook. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- Schwarz CG, Gunter JL, Ward CP, Vemuri P, Senjem ML, Wiste HJ et al. The mayo clinic adult lifespan template: better quantification across the lifespan. Alzheimers Dement 2017; 13: 792.28174070 [Google Scholar]
- Schwarz CG, Reid RI, Gunter JL, Senjem ML, Przybelski SA, Zuk SM et al. Improved DTI registration allows voxel-based analysis that outperforms tract-based spatial statistics. Neuroimage 2014; 94: 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand EA, Duffy JR, Clark HM, Josephs K. The apraxia of speech rating scale: a tool for diagnosis and description of apraxia of speech. J Commun Disord 2014; 51: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman E, Spreen O. Trail making test. A compendium of neuropsychological tests administration, norms, and commentary. Oxford: Oxford University Press; 2006. p. 655–77. [Google Scholar]
- Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2013. http://www.R-project.org/. [Google Scholar]
- Tetzloff KA, Duffy JR, Clark HM, Strand EA, Machulda MM, Schwarz CG et al. Longitudinal structural and molecular neuroimaging in agrammatic primary progressive aphasia. Brain 2017; 141: 302–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzloff KA, Utianski RL, Duffy JR, Clark HM, Strand EA, Josephs KA et al. Quantitative analysis of agrammatism in agPPA and DAOS. J Speech Lang Hear Res 2018; 61: 2337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology 1997; 11: 297–321. [Google Scholar]
- Thompson CK, Mack JE. Grammatical impairments in PPA. Aphasiology 2014; 28: 1018–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Senjem ML, Gunter JL, Lundt ES, Tosakulwong N, Weigand SD et al. Accelerated vs. unaccelerated serial MRI based TBM-SyN measurements for clinical trials in Alzheimer’s disease. Neuroimage 2015; 113: 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. NeuroImage 2016; 142: 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, James M. The visual object and space perception battery: VOSP. London: Pearson; 1991. [Google Scholar]
- Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am J Alzheimer’s Dis Dement 2009; 24: 408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J, Avula R, Senjem M, Kantarci K, Weigand S, Samikoglu A et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology 2010; 74: 1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Xia R, Mandrekar J, Machulda MM et al. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang 2013a; 125: 245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Xu J, Mandrekar J, Boeve BF, Knopman DS, Parisi JE et al. Frontal asymmetry in behavioral variant frontotemporal dementia: clinicoimaging and pathogenetic correlates. Neurobiol Aging 2013b; 34: 636–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W et al. Connected speech production in three variants of primary progressive aphasia. Brain 2010; 133: 2069–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston KM, Strand EA, Miller R, Hillel A, Smith K. Speech deterioration in amyotrophic lateral sclerosis: implications for the timing of intervention. J Med Speech-Lang Pathol 1993; 1: 35–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.