See Voets and Plaha (doi:10.1093/brain/awz197) for a scientific commentary on this article.
Puglisi & Howells et al. investigate the white matter pathways that subserve cognitive control using a combination of lesion-symptom analysis, diffusion tractography, and intraoperative brain mapping during the Stroop test in glioma patients. The results reveal critical involvement of inferior cortico-striatal connections, emphasising the importance of their preservation during frontal tumour resection.
Keywords: executive function, direct electrical stimulation, tractography, fronto-striatal, awake neurosurgery
Abstract
A key aspect of cognitive control is the management of conflicting incoming information to achieve a goal, termed ‘interference control’. Although the role of the right frontal lobe in interference control is evident, the white matter tracts subserving this cognitive process remain unclear. To investigate this, we studied the effect of transient network disruption (by means of direct electrical stimulation) and permanent disconnection (resulting from neurosurgical resection) on interference control processes, using the Stroop test in the intraoperative and extraoperative neurosurgical setting. We evaluated the sites at which errors could be produced by direct electrical stimulation during an intraoperative Stroop test in 34 patients with frontal right hemisphere glioma. Lesion-symptom mapping was used to evaluate the relationship between the resection cavities and postoperative performance on the Stroop test of this group compared with an additional 29 control patients who did not perform the intraoperative test (63 patients in total aged 17–77 years; 28 female). We then examined tract disruption and disconnection in a subset of eight patients who underwent both the intraoperative Stroop test and high angular resolution diffusion imaging (HARDI) tractography. The results showed that, intraoperatively, the majority of sites associated with errors during Stroop test performance and concurrent subcortical stimulation clustered in a region of white matter medial to the right inferior frontal gyrus, lateral and superior to the striatum. Patients who underwent the intraoperative test maintained cognitive control ability at the 1-month follow-up (P = 0.003). Lesion-symptom analysis showed resection of the right inferior frontal gyrus was associated with slower postoperative Stroop test ability (corrected for multiple comparisons, 5000 permutations). The stimulation sites associated with intraoperative errors most commonly corresponded with the inferior fronto-striatal tracts and anterior thalamic radiation (over 75% of patients), although the latter was commonly resected without postoperative deficits on the Stroop test (in 60% of patients). Our results show converging evidence to support a critical role for the inferior frontal gyrus in interference control processes. The intraoperative data combined with tractography suggests that cortico-subcortical tracts, over cortico-cortical connections, may be vital in maintaining efficiency of cognitive control processes. This suggests the importance of their preservation during resection of right frontal tumours.
Introduction
From an evolutionary perspective, the capability to transcend instinct and automatisms in favour of deliberate and flexible behaviour has been a fundamental acquisition in human cognitive evolution and corresponds with the progressive development of prefrontal areas (Coolidge and Wynn, 2001; Carlén, 2017). This is referred to as cognitive control and is essential for contextual adjustment, in particular when focusing on specific behaviours despite distractions (Chan et al., 2008; Diamond, 2013). Cognitive control deficits result in an inability to adequately focus attention, which impacts negatively on quality of life (Duncan, 1986; Goel et al., 2013). Moreover, they have been linked to psychopathological conditions such as risky conduct (Dalley et al., 2011), attention deficit hyperactivity disorder (Mullane et al., 2009), obsessive compulsive behaviour (van Velzen et al., 2014), and schizophrenia (Ettinger et al., 2017).
A crucial component of cognitive control is resistance to interference, which is most commonly assessed using the Stroop Colour and Word test in which subjects are required to look at a series of words describing colours (e.g. ‘green’, ‘red’, ‘blue’) and to report the colour of the hue. Given that the words are written in an incongruent hue (e.g. the word ‘green’ written in blue, ‘red’ in green, etc.), the subject must suppress the prepotent tendency to read the word, and instead name the colour of the ink, causing a type of cognitive interference termed the Stroop Effect (Stroop, 1935). Clinical data from the neurological population indicates that frontal lobe lesions are associated with interference control deficits (Stuss et al., 1999, 2001; McDonald et al., 2005). Neuroimaging studies have identified a complex network of cortico-cortical but also cortico-subcortical regions involved (Koechlin et al., 2003; Badre, 2008), with growing evidence to suggest this system is right-lateralized (Chambers et al., 2009). At the cortical level, functional imaging studies highlight the involvement of the inferior frontal gyrus (IFG), dorsolateral prefrontal cortex, anterior cingulate and pre-supplementary motor area (pre-SMA) (MacDonald et al., 2000; Kerns et al., 2004). Many of these regions are connected with each other, and with other lobes, via long-range white matter pathways. However, there is also considerable connectivity with the basal ganglia, which plays a well-established role in inhibitory processing (Derrfuss et al., 2004; Haupt et al., 2009; Aron et al., 2016; Bomyea et al., 2017). Cognitive control may be mediated by striatal connections of the inferior (IFG) and superior frontal (pre-SMA) regions, but also via direct cortico-cortical communication between these regions through the frontal aslant tract (Liston et al., 2006; Aron et al., 2007, 2014). Although numerous brain regions have been demonstrated to be involved in cognitive control, the white matter connections subserving this function are still not clearly defined.
The awake neurosurgical setting offers a unique opportunity for testing cognitive function, as boundaries to tumour resection require the identification of functional limits, which can be assessed using direct electrical stimulation (DES) during task performance. We recently demonstrated that task disruption during an intraoperative Stroop test (iST) occurs when using low frequency (LF) DES on a discrete white matter region beneath the right inferior and middle frontal gyri, lateral to the head of the caudate nucleus (Puglisi et al., 2018). At these sites, patients made no errors during the linguistic or motor tasks tested (naming, counting, hand movement), suggesting a role for this region that may be specific to cognitive control. A hodological approach to neurofunctional organization (Catani et al., 2012) indicates that stimulation of this subcortical region is causing transient disruption to the underlying white matter network mediating cognitive control. In fact, patients who performed this test intraoperatively showed a reduction in cognitive control deficits with respect to those in which it was not used, suggesting that the preserved white matter connections running through this region support these processes.
Besides highlighting the importance of using the iST in awake surgery to maintain cognitive control ability, the main aim of this study was to identify the precise anatomical region involved in interference control. The hypothesis was that subcortical stimulation during the iST that induced positive errors must target specific white matter tracts relevant in the network subserving this function. Identification of these tracts would shed light on the neural circuits involved and, as a clinical impact, enable preservation of cognitive control abilities of patients submitted to surgery of right frontal lobe tumours. To accomplish this we used complementary approaches. First, in the intraoperative setting, we identified subcortical sites where DES had the highest probability of inducing errors in 34 patients with right hemisphere frontal lobe glioma (iST group) performing the iST. Next, we evaluated the impact of using the iST on preserving regions important for cognitive control, by comparing the postoperative neuropsychological Stroop test performance of this iST group with a control group including 29 patients who did not perform the iST. We then performed lesion-symptom analysis (VLSM) using the resection cavities of the entire group (63 patients in total) in order to investigate whether postoperative Stroop test deficits were associated with resection of a specific anatomical region. Finally, in a subset of eight patients who performed both preoperative high angular resolution diffusion imaging (HARDI)-optimized diffusion tractography and the iST we set out to identify the white matter tracts likely to have been stimulated by DES, subserving cognitive control.
Materials and methods
Patients
Sixty-three adult neuro-oncological patients [53 right-handed, 30 females, mean age 43, standard deviation (SD) 14] undergoing surgery for a brain lesion participated in the study in Humanitas Research Hospital between 2014 and 2018 (Table 1). Patients were recruited using the following inclusion criteria: (i) a unilateral right hemisphere lesion; and (ii) absence of any language and visual deficits. Exclusion criteria included a previous history of neurological and psychiatric illness, low education levels (<8 years of formal education) or preoperative deficits on the Stroop test. All patients underwent total or supratotal tumour resection, except four who underwent subtotal resection. All patients gave written informed consent to the surgical and mapping procedure (IRB1299), which followed the principles outlined in the declaration of Helsinki. The study was performed with strict adherence to the routine procedure adopted for surgical tumour removal.
Table 1.
Demographic and clinical information on all patients
| Patient | Group | Histological diagnosis | Grade | Lobe affected | Tumour volume, ml | Gender | Age | Education |
|---|---|---|---|---|---|---|---|---|
| 1a | IST | Ganglioglioma | I | F | 78.2 | Female | 37 | 13 |
| 2a | IST | Anaplastic oligodendroglioma | III | F | 0.5 | Female | 24 | 13 |
| 3a | IST | Astrocytoma | II | F | 1 | Female | 29 | 17 |
| 4a | IST | Anaplastic oligodendroglioma | III | F | 21.1 | Male | 28 | 13 |
| 5a | IST | Oligodendroglioma | II | F | 38.9 | Male | 34 | 13 |
| 6a | IST | Anaplastic astrocytoma | III | T-I | 7.05 | Female | 42 | 8 |
| 7a | IST | Oligodendroglioma | II | F | 5.24 | Male | 45 | 17 |
| 8a | IST | Diffuse astrocytoma | II | F | 4.65 | Female | 42 | 17 |
| 9 | IST | Anaplastic astrocytoma | III | F | 43.4 | Male | 43 | 13 |
| 10 | IST | Cavernous hemangioma | II | F-T | 1 | Female | 25 | 17 |
| 11 | IST | Anaplastic astrocytoma | III | F | 6.304 | Female | 29 | 17 |
| 12 | IST | Oligodendroglioma | II | F | 21.970 | Male | 29 | 17 |
| 13 | IST | Glioblastoma | IV | F-I | 84.878 | Female | 32 | 17 |
| 14 | IST | Dysembryoplastic neuroepithelial tumour | I | F-T | 1.2 | Female | 36 | 13 |
| 15 | IST | Oligodendroglioma | II | F-T | 27.02 | Male | 56 | 13 |
| 16 | IST | Oligodendroglioma | II | F | 45.1 | Female | 43 | 10 |
| 17 | IST | Anaplastic astrocytoma | II | F | 77.823 | Male | 33 | 13 |
| 18 | IST | Anaplastic astrocytoma | III | F | 59.012 | Female | 60 | 13 |
| 19 | IST | Oligodendroglioma | II | F | 1.414 | Female | 56 | 13 |
| 20 | IST | Anaplastic astrocytoma | III | F | 3 | Female | 25 | 17 |
| 21 | IST | Anaplastic oligodendroglioma | III | F | 28.1 | Female | 52 | 13 |
| 22 | IST | Astrocytoma | II | F-I | 28.1 | Female | 37 | 17 |
| 23 | IST | Anaplastic oligodendroglioma | III | F | 10.31 | Male | 40 | 13 |
| 24 | IST | Anaplastic oligodendroglioma | III | F | 25.8 | Female | 57 | 13 |
| 25 | IST | Anaplastic oligodendroglioma | III | F-T-P | 14.335 | Female | 39 | 17 |
| 26 | IST | Diffuse glial tumour | II | F-I | 15.665 | Male | 29 | 17 |
| 27 | IST | Anaplastic astrocytoma | III | F-T-I | 116.093 | Female | 30 | 17 |
| 28 | IST | Oligodendroglioma | II | F-I | 173.3 | Male | 35 | 13 |
| 29 | IST | Astrocytoma | II | T-F-I | 18.808 | Male | 59 | 13 |
| 30 | IST | Oligodendroglioma | II | F-I | 10.77 | Female | 22 | 15 |
| 31 | IST | Oligoastrocytoma | III | F-I | 124.7 | Male | 41 | 13 |
| 32 | IST | Oligodendroglioma | II | F-T-I | 56.86 | Male | 51 | 13 |
| 33 | IST | Oligodendroglioma | II | F | 11.8 | Female | 34 | 17 |
| 34 | IST | Anaplastic astrocytoma | III | F-T-I | 178.3 | Male | 18 | 13 |
| 35 | IST | Diffuse astrocytoma | II | F | 4.814 | Male | 29 | 17 |
| 36 | Control | Anaplastic oligodendroglioma | III | F-I | 66.386 | Female | 29 | 17 |
| 37 | Control | Anaplastic astrocytoma | III | F | 44.7 | Female | 41 | 13 |
| 38 | Control | Glioblastoma | IV | F-T-I | 77.183 | Male | 58 | 8 |
| 39 | Control | Anaplastic astrocytoma | III | T | 1.41 | Female | 47 | 13 |
| 40 | Control | Glioblastoma | IV | P | 16.051 | Male | 65 | 13 |
| 41 | Control | Glioblastoma | IV | F-P | 37.738 | Female | 30 | 17 |
| 42 | Control | Anaplastic oligodendroglioma | III | F | 17.119 | Male | 47 | 13 |
| 43 | Control | Anaplastic astrocytoma | III | F-T-I | 36.627 | Female | 46 | 17 |
| 44 | Control | Anaplastic oligodendroglioma | III | F | 27.681 | Female | 51 | 13 |
| 45 | Control | Anaplastic astrocytoma | III | T | 18.3 | Male | 62 | 8 |
| 46 | Control | Glioblastoma | IV | F-T-I | 20.088 | Male | 61 | 13 |
| 47 | Control | Glioblastoma | IV | T | 28.2 | Female | 37 | 17 |
| 48 | Control | Glioblastoma | IV | P | 22.4 | Male | 67 | 13 |
| 49 | Control | Anaplastic oligodendroglioma | III | F | 103.195 | Female | 16 | 10 |
| 50 | Control | Glioblastoma | IV | F | 2.326 | Male | 31 | 13 |
| 51 | Control | Anaplastic astrocytoma | III | P | 24.4 | Male | 51 | 13 |
| 52 | Control | Oligoastrocytoma | II | F | 10.77 | Male | 77 | 17 |
| 53 | Control | Anaplastic astrocytoma | III | F | 11.945 | Male | 45 | 13 |
| 54 | Control | Glioblastoma | IV | T | 9.9 | Male | 34 | 13 |
| 55 | Control | Anaplastic oligodendroglioma | III | F-T-I | 39.4 | Male | 73 | 8 |
| 56 | Control | Anaplastic astrocytoma | III | F-T-I | 23.6 | Male | 46 | 8 |
| 57 | Control | Glioblastoma | IV | P | 14.1 | Male | 47 | 17 |
| 58 | Control | Glioblastoma | IV | T | 27.1 | Male | 52 | 13 |
| 59 | Control | Glioblastoma | IV | T-P | 90.4 | Female | 67 | 8 |
| 60 | Control | Anaplastic astrocytoma | III | F-T | 28.987 | Male | 46 | 17 |
| 61 | Control | Glioblastoma | IV | P-O | 66.367 | Female | 49 | 13 |
| 62 | Control | Anaplastic astrocytoma | III | F | 53.2 | Male | 65 | 13 |
| 63 | Control | Anaplastic astrocytoma | III | F | 74.085 | Male | 35 | 17 |
aPatients who also underwent HARDI-optimized diffusion tractography.
F = frontal; I = insula; O = occipital; P = parietal; T = temporal.
Surgical procedure and intraoperative brain mapping
All 63 patients underwent awake surgery for tumour removal according to functional boundaries defined using the brain mapping technique. Among this group, 34 patients, enrolled between 2016 and 2018, underwent the standard brain mapping used to preserve language (Bello et al., 2014), motor (Bello et al., 2014) and praxis (Rossi et al., 2018) implemented with an intraoperative version of the Stroop Test (iST) to identify cortical-subcortical ‘eloquent’ sites also for executive functioning (Puglisi et al., 2018). Eight of these 34 patients also underwent a preoperative HARDI-optimized diffusion imaging sequence. Twenty-nine patients, enrolled from 2014 to 2015—thus before the introduction of the iST—were also included in the study as a control group and underwent the standard Brain Mapping technique without the iST.
In the iST group, DES during the Stroop test was usually applied with an LF-DES paradigm (60 Hz) or, in few cases, with a high frequency (HF)-DES paradigm (train of 5, pulse duration 0.5 ms; interstimulus interval: 3–4 ms, repetition rate 3 Hz) set at the same current intensity adopted for language mapping (2.75 ± 0.93 mA). Following identification of the safe point of entry at cortical level (identified with awake mapping with the lowest threshold current intensity needed to interfere with speech production), the surgeon started the subcortical stimulation. Counting and naming were first performed with corresponding LF-DES mapping, as the resection progressed subcortically. If no language error was produced, the iST was initiated and resection continued until an error occurred. Subcortical mapping for the iST was performed by applying DES at the same intensity used on cortex to interfere with speech production, while the patient was performing the Stroop test. The ultrasonic aspirator used for resection was coupled with the stimulation probe, looking for interferences during test performance. A subcortical site was considered positive/eloquent for interference when an error occurred in three non-consecutive stimulation trials. The brain mapping technique implemented with the iST was video-recorded and reviewed offline by clinicians for verification. A stimulation site (cortical or subcortical) was considered ‘positive’ when patients responded with the colour instead of the word (error, more frequent) or when the correct response was given with a delay >1 s (latency, less frequent). During the iST, the patients’ verbal responses were reported real time to the neurosurgeon by the neuropsychologist, fully blinded to the neuroanatomical location of the stimulation site.
Statistical analysis of intraoperative stimulation
The location of positive sites was recorded using neuronavigation (Curve, Brainlab AG) correcting brain shift with intraoperative ultrasound (Hitachi Aloka Medical, Ltd.) at the end of the subcortical mapping procedure before the resection of the tumour. Recorded positive sites in all patients were verified offline using the video recordings and were registered to MNI space using an affine transformation implemented in SPM8 software (Brett et al., 2001). All positive stimulation sites were used (including those patients with more than one site) to calculate a 3D visualization of the anatomical region with the highest probability of inducing interference during the iST (Supplementary Fig. 2), based on the concentration of stimulation sites. This was estimated using an in-house script using non-parametric kernel density estimation from the Statistics toolbox in MATLAB (Jones, 1993).
Neuropsychological assessment and statistical analysis
All patients were submitted to a preoperative (1 week before surgery) and post-acute (1 month following surgery) neuropsychological assessment of cognitive functions including language, praxis ability, attentive and executive function (detailed in Supplementary Table 1). Stroop test performance was used to assess interference control. Scores of all tests were corrected for education and age. Notably, for patients with postoperative neglect (n = 9) or quadrantanopia (n = 2), scores for the tasks that require accurate visual scanning [i.e. the Attentive Matrices, Trail Making Test, Rey Figure (copy), Raven’s Matrices] were not reliable, thus these scores were not considered in the analysis. The Stroop task used for extraoperative assessment requires vertical scanning of single words to be read, thus is not affected by hemianopia or neglect (Caffarra et al., 2002). The test is composed of three subtasks, in which the patient is instructed to answer as fast and as accurately as possible to the relevant stimulus attribute: in the first subtask the patient is asked to read a vertical list of colour names (red, blue, or green), while in the second the patient is asked to name the colour of a vertical series of coloured dots (red, blue, or green). If reading or perceptual difficulties occur in these subtasks, the third subtask is not administered. The third subtask (colour-word subtask) specifically evaluates the interference control process: a series of colour words printed in an incongruent hue (e.g. blue printed in a red hue) is presented to patients who must report the hue colour. The test is scored for accuracy (based on the number of errors made) and time (measured in the number of seconds required to complete the task, weighted for the performance speed on the first two trials).
All statistical analyses were performed using IBM SPSS 24.0 software. We compared the different time points (preoperative and postoperative) using a paired sample t-test for each neuropsychological test, in the entire group. P-values were corrected for multiple comparisons using Bonferroni correction (P < 0.05/14 = P < 0.003). To assess the effect of preserving functional structures by using the iST, we compared the neuropsychological performance of patients who performed the iST with the Control group who did not perform the test. Outliers were defined as having standardized residuals over 2 SD and were not included in the analysis. Two-way mixed ANOVAs were performed for each neuropsychological test, using group as a between-factors measure (iST versus Control) and neuropsychological assessment time point (preoperative versus 1 month) as the within-group measure.
Voxel-based lesion symptom mapping
We performed VLSM analysis and an adapted region of interest-based analysis using the NiiStat toolbox for MATLAB (http://www.nitrc.org/projects/niistat). This approach compares regions of interest rather than individual voxels for lesion-symptom analysis to reduce dimensionality, thus reducing the rate of family-wise errors that can occur in voxel-based approaches (Smith et al., 2013). We limited the analysis to the right hemisphere as this was the focus of our study and used the AAL template to delineate regions of interest, including white matter and subcortical regions (Tzourio-Mazoyer et al., 2002). The proportion of resection in each region was entered into a general linear model to identify regions associated with the corrected neuropsychological scores at 1 month after surgery. The results of this analysis showed, as a z-score, the statistical likelihood of resection of a given region predicting a decline in performance on a behavioural test. Statistical significance was determined by permutation thresholding (5000 permutations) and multiple comparison correction. We only included voxels resected in over 15% of patients in the analysis, as voxels that are less commonly affected have lower statistical power which can influence the false discovery rate (Findlater et al., 2016). As this limit is arbitrary, we ran the same analysis when considering voxels resected also in over 10% and over 25% of patients for comparison.
Diffusion preprocessing and analysis
Diffusion data (Supplementary material) were first visually inspected for outliers, reordered and corrected for head motion and eddy current distortions using ExploreDTI (Leemans et al., 2009). We used spherical deconvolution to model the orientation distribution function, using a damped Richardson-Lucy algorithm (Dell’Acqua et al., 2010). An α-value of 1.5 was used with 200 iterations with an n of 0.001, a v of 16, and an absolute threshold of 0.00138 (Dell’Acqua et al., 2013). Whole brain deterministic tractography was calculated using a step size of 0.5 mm, with a constraint to display streamlines between 15 and 300 mm in length. Euler interpolation was used to track streamlines using an angle threshold of 35 degrees. Spherical deconvolution facilitates the modelling of orientation distribution functions which approximate the distribution of fibres within a voxel, thereby enabling tracking of crossing fibres when performing tractography (Dell’Acqua and Tournier, 2018). This enables tracking of fibre bundles that are not easily visualized using diffusion tensor methods such as the superior longitudinal fascicles (Thiebaut de Schotten et al., 2011). Spherical deconvolution modelling and tractography was performed with StarTrack software (Dell’Acqua et al., 2013) (www.mr-startrack.com). Native to MNI space transformations of structural images and tractography was performed using FLIRT and FNIRT in FSL and Diffusion Toolkit.
Defining tract stimulation and disconnection
Manually guided virtual dissections were performed for white matter pathways using a constrained region of interest-based approach for each patient’s whole brain tractogram. The detailed approach used for tractography dissection is reported in the Supplementary material (Rojkova et al., 2016). The total volume of space occupied by streamlines of each white matter bundle was extracted (measured in cubic millimetres). We overlaid the stimulation site as spherical regions of interest with 5-mm diameter into the TrackVis software. This diameter was set in line with the proposed extent of stimulation of the bipolar probe (Haglund et al. 1993). We then used this sphere as an inclusion region of interest to identify streamlines passing through this site. These streamlines were compared with virtual dissections, and white matter tracts were defined as ‘stimulated’ if streamlines generated were corresponding. The same approach was used by overlaying the resection cavity as an inclusion region of interest. The volume (mm3) of streamlines that were intersecting with the resection cavity was recorded. If this resected volume was over 50% of the entire volume of the white matter tract, we defined it as ‘disconnected’ (Foulon et al., 2018). We also reported the exact percentage of tract volume disconnection.
Data availability
The data used in this study are available on request, in anonymized format, from the corresponding author.
Results
Anatomical and demographic characteristics
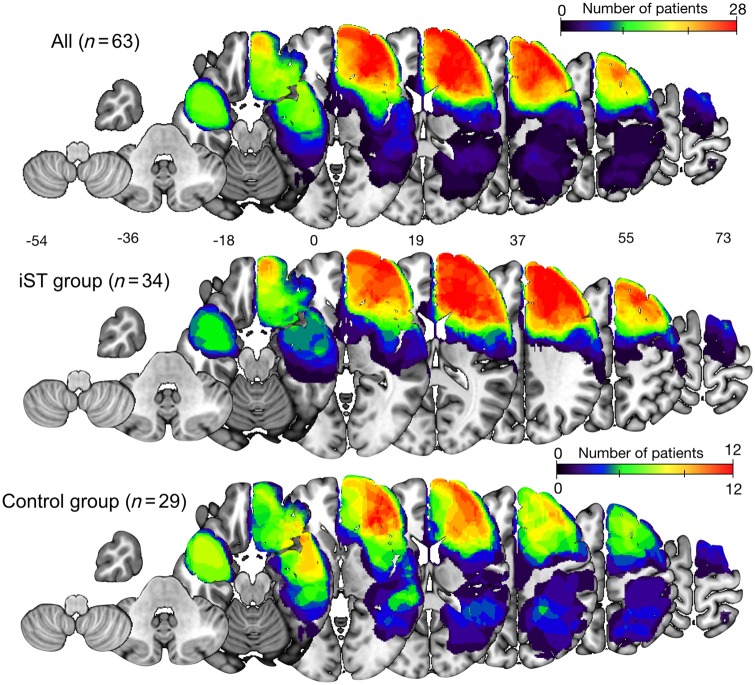
The spatial distribution of the resection cavities in the 34 patients who performed the iST (iST group) and the 29 patients who did not (Control group) are shown in Fig. 1. The region of maximum overlap (n = 12) was located in widespread right frontal regions and the anterior temporal lobe, consistent with the neurosurgical approach (Bello et al., 2014).
Figure 1.
Topography of tumour resection cavities. Topography is shown using a heat map, in the total group (n = 63), and the overlap in patients who performed the iST (n = 34) and the control group (n = 29).
Intraoperative stimulation
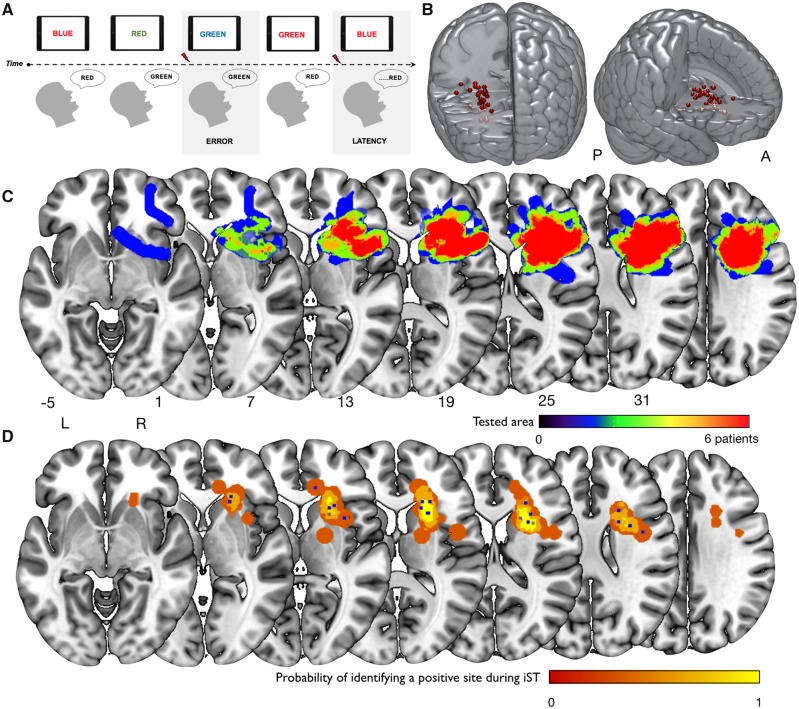
Of the 34 patients who underwent the intraoperative Stroop test, interference in task performance during DES were recorded in 25 patients. In the remaining nine patients DES did not affect task performance during surgery. Thirty-four sites at which task impairment occurred were recorded (‘positive sites’), with four patients making an error at more than one site. Test performance was impaired when DES was delivered to subcortical regions in each of the 25 patients, specifically inducing errors (65%) or latencies (35%) (Fig. 2A). When different DES protocols were delivered (HF-DES and/or LF-DES) on the same site, the same errors were elicited. No positive Stroop sites occurred during stimulation of the cortex of the inferior, medial or superior frontal gyrus (IFG, middle frontal gyrus or superior frontal gyrus), rather they occurred, in all patients, during stimulation of the periventricular white matter medial to the right IFG and lateral and superior to the striatum. When normalizing to a common template, the sites were clustered in a discrete subcortical area within the white matter tracts running under the inferior and middle frontal gyri, in front of the anterior insula, and lateral to the head of the caudate, passing over the putamen and the anterior thalamus to reach the cingulum (Fig. 2B). Kernel density estimation confirmed the Stroop error sites were clustered within this region (Fig. 2C). Four patients produced an error when a site within the more lateral white matter of the IFG was stimulated.
Figure 2.
Intraoperative procedure and positive stimulation sites. (A) The time course of the iST. Red arrows indicate onset of stimulation. Reproduced with permission from Puglisi et al. (2018). (B) The individual stimulation sites of 25 patients who showed an error during brain mapping (of the 34 that underwent the iST) and the region of highest probability of a stimulation error occurring, calculated using kernel density estimation of the individual stimulation sites. (C) The spatial extent of testing in all 34 patients who underwent the iST.
Lesion-symptom analysis
Neuropsychological outcome
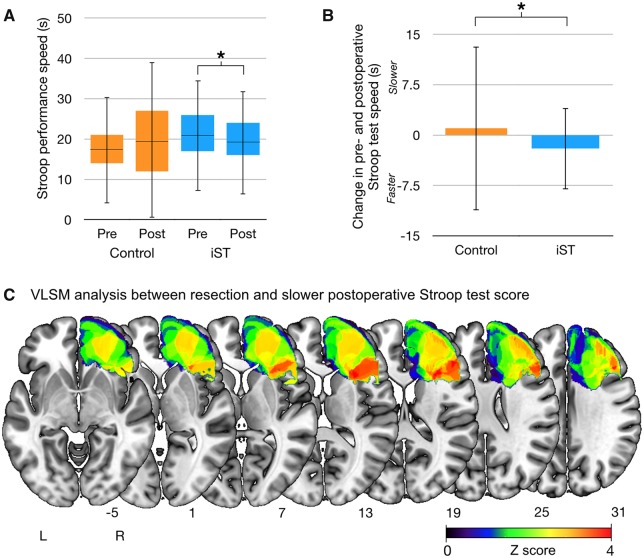
Test performance across the neuropsychological battery at all time points (preoperative and 1 month following surgery) in the entire cohort are reported in Table 2. The result of the paired samples t-tests showed a statistically significant decline in test performance between the preoperative and postoperative phases for phonemic fluency [t(39) = 3.36, P = 0.002]. There was a trend toward a decline in test performance for semantic fluency and the Trail Making Test A and B (Table 1). No significant impairment was recorded for basic language tests for comprehension (Token Test) and picture naming, nor for all the other administered tests. Further analysis was performed using group (iST versus Control) as a between-subjects factor and time as a within-subjects factor using a two-way mixed ANOVA. A significant interaction between group and time was observed [F(1,40) = 5.01, P = 0.03]. This showed that Stroop test performance was modulated differently in the iST and control groups between the pre- and postoperative time points, irrespective of tumor grade and volume. Post hoc analysis showed a significant improvement in performance (based on speed to completion) over time [t(24) = 3.27, P = 0.003, adjusted P-value = 0.006] in the iST group (Fig. 3A). This effect was absent in the control group where there was a trend toward slower postoperative Stroop test performance. The net change in speed in the two groups is shown in Fig. 3B. We performed a one-way ANOVA to assess the difference in this in the two groups, and this was statistically greater in the iST group than the controls [F(1,42) = 5.04, P = 0.03].
Table 2.
Mean corrected scores in the different neuropsychological tests at the different time points with P-value scores after multiple comparisons correction were reported
| Test | Group | Pre | Post | P-value (adjusted) |
|---|---|---|---|---|
| Token Test | Control | 34.34 (2.1) | 34.4 (2.1) | |
| Stroop | 35.1 (1.7) | 34.8 (2.3) | ||
| Total | 34.79 (1.9) | 34.65 (2.2) | 0.595 (8.33) | |
| Naming | Control | 46 (1.36) | 46.12 (1.4) | |
| Stroop | 46.6 (1.4) | 46.7 (1.4) | ||
| Total | 46.38 (1.3) | 46.48 (1.4) | 0.600 (8.4) | |
| Semantic Fluency | Control | 37 (7.8) | 35.7 (9.2) | |
| Stroop | 40.4 (8.2) | 34.95 (9.1) | ||
| Total | 39.01 (8.1) | 35.24 (9) | 0.002 (0.02) | |
| Phonemic Fluency | Control | 29.7 (7.1) | 28.2 (10.6) | |
| Stroop | 33.4 (10.2) | 26 (9.2) | ||
| Total | 31.85 (9.1) | 26.93 (9.7) | 0.005 (0.07) | |
| Attentive Matrices | Control | 46 (5.12) | 45.1 (7.8) | |
| Stroop | 48.1 (6.75) | 45.4 (7.4) | ||
| Total | 47.20 (6.1) | 45.3 (7.4) | 0.060 (0.84) | |
| Trail Making Aa | Control | 33.3 (9.3) | 43.2 (12.3) | |
| Stroop | 39.2 (12) | 46.9 (16.3) | ||
| Total | 37 (11.3) | 45.4 (14.7) | 0.008 (0.11) | |
| Trail Making Ba | Control | 91.1 (24.2) | 112.6 (49.8) | |
| Stroop | 99.6 (29.9) | 121.8 (37.5) | ||
| Total | 96.16 (27.6) | 118 (42.3) | 0.010 (0.14) | |
| Trail Making ABa | Control | 65 (24.3) | 70.54 (45.53) | |
| Stroop | 59.8 (28.64) | 77.6 (37.38) | ||
| Total | 61.94 (26.6) | 74.7 (39.8) | 0.085 (1.19) | |
| Stroop Errorsa | Control | 0.62 (1.1) | 1.35 (2.7) | |
| Stroop | 0.57 (1.2)) | 0.4 (1) | ||
| Total | 0.6 (1.16) | 0.8 (1.9) | 0.542 (7.5) | |
| Stroop Timea | Control | 17.9 (6.5) | 19.9 (9.9) | |
| Stroop | 21.5 (6.9) | 19.25 (6.5) | ||
| Total | 20.1 (7) | 19.5 (8) | 0.593 (8.3) | |
| Digit Span Forward | Control | 5.87 (0.9) | 5.76 (0.7) | |
| Stroop | 7.15 (6.36) | 5.57 (0.8) | ||
| Total | 6.66 (5) | 5.64 (0.8) | 0.227 (3.1) | |
| Digit Span Backward | Control | 4.2 (0.8) | 4.34 (1.2) | |
| Stroop | 4.24 (1) | 4 (1) | ||
| Total | 4.24 (0.9) | 4.13 (1) | 0.509 (7.1) | |
| Rey Complex Figure (copy) | Control | 29.9 (7) | 29.7 (6.9) | |
| Stroop | 33.54 (2.4) | 30.78 (3.9) | ||
| Total | 32 (5.2) | 30.3 (5.3) | 0.47 (6.6) | |
| Raven’s Progressive Matrices | Control | 30.6 (1.3) | 29.83 (1.5) | |
| Stroop | 31.7 (3.5) | 27.75 (9.2) | ||
| Total | 31.3 (3) | 28.6 (7.2) | 0.062(0.9) |
aIn these tests, the greater the score is, the worse the performance.
Figure 3.
The effect of resection on behavioural outcome. (A) The change in Stroop performance time in the two groups (iST and control group) at the different time points. *P = 0.006 (B) Change in performance on the Stroop test in the two groups measured as the difference between postoperative and preoperative test performance. Values > 0 indicate postoperative test performance was slower and values < 0 indicate postoperative test performance was higher *P = 0.03. Both graphs are shown with 95% confidence intervals. (C) The resected brain regions identified by the voxel-based lesion-symptom analysis resulting in slower test performance on the Stroop test at 1 month in the entire group (63 patients). Only regions significant at P < 0.05 (corrected for multiple comparisons based on 5000 permutations) are presented. The MNI z-coordinates for each axial slice are displayed.
Lesion-symptom mapping
To assess the effect of permanent resection of different brain regions on neuropsychological outcome, voxel-based and region of interest-based lesion-symptom analyses were conducted using the entire cohort, constrained to the right hemisphere, for the cognitive control domain (Supplementary Table 1). We considered the group as a whole to identify whether resection of a specific brain region was associated with slower performance time on the Stroop test at the 1-month follow-up (Fig. 3C). When considering regions where only 15% or more of patients had a common resection, VLSM analysis identified a significant association between slower performance time and resection of the white matter of the right IFG in the region surrounding the fundus of the inferior frontal sulcus, including also the white matter lateral to the head of the caudate nucleus (z > 3.55, P < 0.05 using permutation correction) (Fig. 3C). There was no significant association between other brain regions and performance on any other cognitive control tests at the 1-month follow-up.
The region of interest-based analysis confirmed there was a significant association between slower performance time and resection of the posterior region of the right IFG: the pars opercularis (z = 3.122, P < 0.05 using permutation correction) and pars triangularis (z = 3.113, P < 0.05 using permutation correction), and the right middle frontal gyrus (z = 3.024, P < 0.05 using permutation correction). Analysis using higher (25%) and lower (10%) thresholds for common resection yielded the same result.
White matter tractography
Anatomical and neuropsychological characteristics
The spatial distribution of the resection cavities in the eight patients was similar to the group analysis distribution (Supplementary Fig. 3), thus the group can be considered representative of the main group analysis. During the immediate postoperative phase, the most frequent deficit (six of eight patients) was hemispatial neglect syndrome, which progressively recovered in the following months (scores not reported).
Stimulation of white matter tracts
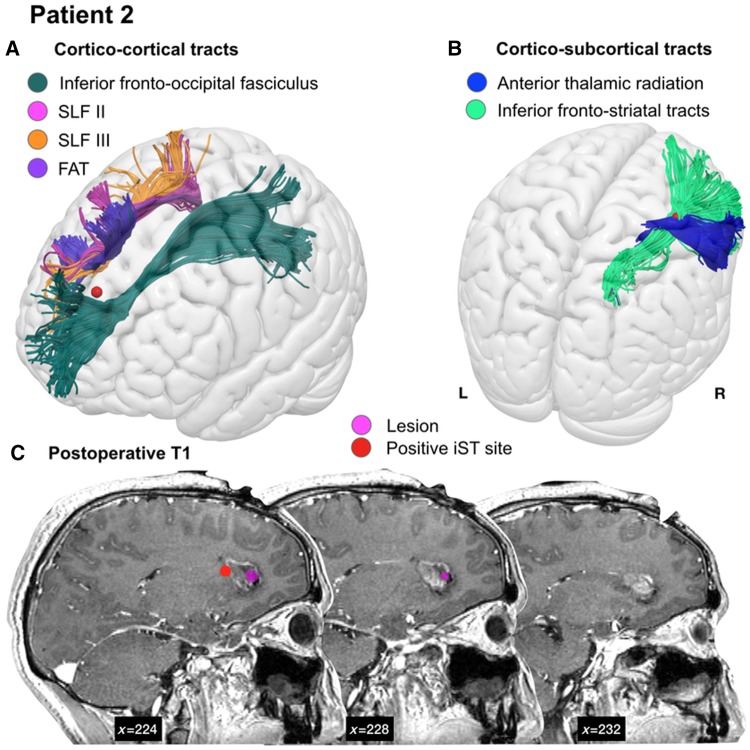
In all eight patients we analysed only the stimulation sites impairing the Stroop test without inducing language (naming, counting, semantic association) or motor (hand manipulation) deficits. These sites all fell within the region identified as most probable for inducing an intraoperative Stroop error (Fig. 2). In each of the eight patients, the tracts highlighted by the group analysis were reconstructed using the individual patient’s tractogram, and the stimulation sites were then overlaid for comparison (Table 3 and Fig. 4). We performed virtual dissections of eight candidate frontal lobe tracts in each patient: the anterior thalamic radiation, the inferior fronto-occipital fasciculus, the superior longitudinal fasciculus (SLF) II, SLF III, frontal aslant tract, inferior and superior fronto-striatal tracts and cortico-pontine tracts. The tracts were present in all patients, with the exception of tumour infiltration of the frontal aslant tract in Patient 5 and SLF II in Patients 1 and 8. In 75% of patients, cortico-subcortical tracts (the anterior thalamic radiation and inferior fronto-striatal tracts) intersected with the site where a Stroop error was induced intraoperatively (Table 3 and see example in Fig. 4). This site intersected with the inferior fronto-occipital fasciculus and frontal aslant tract in only one patient, and did not intersect with the SLF II or SLF III in any of the patients tested.
Table 3.
Tract volumes of the eight tracts dissected in the preoperative stage (in mm3) and the extent of tract disconnection by the neurosurgical procedure (measured as a proportion of total tract volume)
| Volume, (extent of disconnection) | Inferior fronto-striatal | Superior fronto-striatal | Frontal aslant tract | IFOF | Anterior thalamic radiation | SLF II | SLF III | Corticopontine tracts |
|---|---|---|---|---|---|---|---|---|
| P1 | 8.6 (0.32) | 8.1 (0.86)b | 17.3 (0.99)b | 33.5 (0.07) | 32.3a (0.29) | 2.8 (0.4) | 10.5 (0) | 14.3 (0.7)b |
| P2 | 7.6a (0.20) | 6.3 (0.11) | 9.1 (0.07) | 40.8 (0.32) | 22.6a (0.21) | 15.5 (0) | 12.1 (0) | 23.01a (0.3) |
| P3 | 14.3a (0.27) | 6.1a (0.94)b | 10.7a (1)b | 20.9a (0.4) | 36.1a (0.71)b | 11.1 (0.9b) | 8.9 (0) | 17.35a (0.8)b |
| P4 | 17.1a (0.08) | 7.5 (0.99)b | 15.1 (0.99)b | 43.5 (0.9)b | 33.8a (0.92)b | 15.3 (0.9)b | 28.8 (0) | 19.02 (1)b |
| P5 | 15.6a (0.4) | 5.0 (0.99)b | 1.2 (0.92)b | 39.6 (1)b | 23.2a (1)b | 6.1 (0.77)b | 13.8 (0.94)b | 13.19 (1)b |
| P6 | 6.8a (0.39) | 4.5 (0) | 8.9 (0.5)b | 32.7 (0) | 20.6 (0) | 13.0 (0) | 22.5 (0) | 12.4 (0) |
| P7 (deficit) | 8.5 (1) | 10.9a (0.9)b | 23.9 (0.99)b | 46.4 (0.98)b | 44.7a (0.99)b | 22.6 (0.8)b | 25.6 (1)b | 22.03 (1)b |
| P8 | 9.7a (0.1) | 3.4 (0) | 4.2 (0) | 22.7 (0.63)b | 14.5a (0.83)b | - | 6.2 (0.22) | 11.3a (0.5)b |
| Mean (SD) | 11.02 (4) | 6.5 (2.4) | 11.3 (7.23) | 35.0 (9.4) | 28.5 (9.9) | 11.5 (6.5) | 16.9 (8.5) | 16.6 (4.5) |
aTracts that intersected with the stimulation site.
bValues indicate the resection cavity has removed over 50% of total tract volume.
IFOF = inferior fronto-occipital fasciculus.
Figure 4.
Proximity of tracts to stimulation sites. Example of the relationship between a site positive for the iST (red) and (A) the cortico-cortical and (B) cortico-subcortical white matter tracts in Patient 2. The individual patient anatomy has been registered to a standard template (MNI) to improve visualization. (C) This patient underwent neurosurgery for a focal lesion (shown in pink on sagittal slices of the 1-month postoperative T1) within the deep frontal white matter anterior to the caudate nucleus. FAT = frontal aslant tract.
White matter tract disconnection
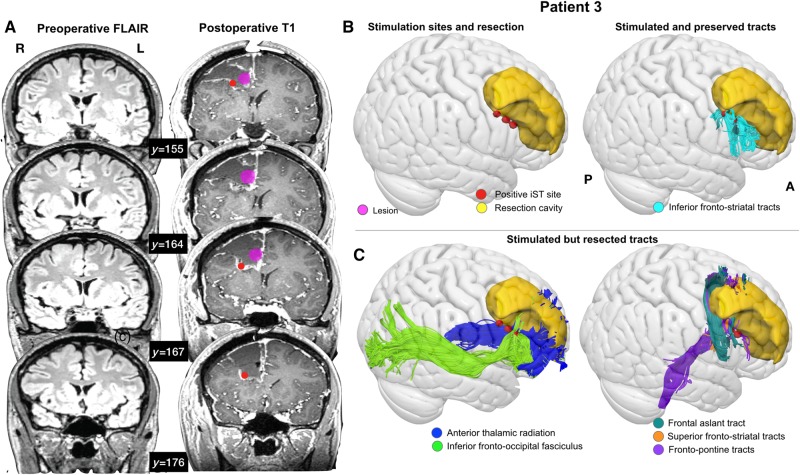
We evaluated which tracts had been permanently disconnected by the neurosurgical procedure, by overlaying each patient’s resection cavity delineated on the 1-month follow-up MRI, aligned with their preoperative diffusion tractography. To assess the effect of the resection, we calculated the volume of streamlines intersecting with the resection cavity, as a proportion of the total tract volume (reported in Table 3). We considered the tract to be permanently disconnected if this proportion was over 50% of the total tract volume (Foulon et al., 2018). The only patient to experience immediate postoperative deficits on the Stroop test was Patient 7, who had all the studied tracts resected. The only tracts to be preserved in all patients with no postoperative Stroop test deficits were the inferior fronto-striatal tracts. In over half of the non-affected patients, the superior fronto-striatal tracts, frontal aslant tract, anterior thalamic radiation and SLF II were disconnected by the surgical intervention (Fig. 5).
Figure 5.
The effect of surgery on white matter. Example of a single patient (Patient 3) showing (A) the location of the stimulation sites (red spheres), the tumour and the resection cavity on coronal slices of the preoperative FLAIR and postoperative T1 (B) the tracts that were both stimulated and preserved of those studied, and (C) the tracts that were stimulated, but were disconnected by the neurosurgical intervention.
Discussion
Here we present direct evidence of a subcortical region subserving cognitive control, emerging from the combined analysis of intraoperative brain mapping in awake surgery, lesion-symptom mapping and HARDI-based diffusion tractography. We investigated the effect of transient DES-induced disruption of white matter tracts on iST performance, and the relationship between permanent disconnection of different white matter regions and postoperative impairment on a well-established cognitive control task, the Stroop test. Results of the intraoperative brain mapping, lesion-symptom analysis and diffusion tractography point to inferior frontal regions as key hubs supporting interference control and demonstrate that their preservation during surgery preserve the cognitive control abilities in patients.
Neuropsychological outcome
We assessed the impact of iST on preserving regions important for interference control performing a comparison of postoperative Stroop test performance between patients who performed the iST with a control group who did not. The iST group performed better at follow-up than in the preoperative phase, consistent with a well-described practice effect on Stroop ability (Dulaney and Rogers, 1994; Edwards et al., 1996; Davidson et al., 2003). In the control group this effect was absent. This may indicate the iST is a useful tool to preserve neural systems subserving cognitive control functions.
Intraoperative findings and lesion symptom mapping
To identify the precise anatomical regions related to interference control processes, on one side, we localized the subcortical sites that, when electrically stimulated with DES to induce a transient lesion, produce performance deficits during the Stroop test. We found such sites were concentrated in a specific lateral subcortical region. The results of the lesion-symptom analysis showed a marked anatomical contiguity between this brain region, a subcortical region beneath the inferior and middle frontal gyrus, and the region where intraoperative stimulation caused performance errors, also located within the periventricular white matter lateral to the caudate nucleus. This region has previously been associated with cognitive control by functional magnetic resonance studies, termed the ‘inferior frontal junction’, where consistent blood oxygen level-dependent activity is present during the Stroop test but also other task-switching tests such as the n-back task. In particular, functional parcellation of the inferior frontal junction has shown there to be a motor to cognitive rostro-caudal gradient, which is in line with the region we identified as causing the highest probability of intraoperative errors on the Stroop test (Muhle-Karbe et al., 2016; Thiebaut de Schotten et al., 2017; Hartwigsen et al., 2019).
The lesion-symptom results suggest that damage to the inferior and middle frontal gyrus impairs performance on the Stroop test; however, intraoperative stimulation failed to disclose a significant number of positive sites for the iST extending toward the cortical surface, and further, we have described previously how stimulation of the cortical surface of the IFG does not produce intraoperative errors on the Stroop test (Puglisi et al., 2018). The explanation may be that a behavioural disruption can be caused only in a region of converging fibres within deeper white matter, rather than diverging projections extending from or toward the inferior frontal cortex. Alternatively the stimulation may cause a transient disconnection of more than one white matter bundle that passes through this location. For this reason, we investigated all major frontal white matter tracts that might have been affected by the stimulation at these sites, without constraining this to solely those extending from the IFG. These included both association tracts (frontal aslant tract, SLF II, SLF III, and the inferior fronto-occipital fasciculus) and cortico-subcortical tracts (the fronto-striatal tracts, anterior thalamic projections and cortico-pontine tracts). We performed diffusion tractography in eight patients to identify which white matter tracts passed through the region causing a positive iST error. Although major white matter pathways are usually fairly conserved in their cortical terminations and subcortical trajectories across individuals, there is a still some variability that should be taken into consideration, particularly in cases of rapid growth of abnormal tissue, such as patients with brain tumours (Concha, 2014). For this reason, identifying likely tracts that have been stimulated intraoperatively using atlases of healthy white matter might not be appropriate (Forkel and Catani, 2018). By combining the individual patient’s stimulation site and resection cavity with the preoperative white matter tractogram, we were able to reproduce, in vivo, likely white matter tracts involved in cognitive control using HARDI-optimized spherical deconvolution tractography.
White matter tractography
We identified the precise tracts involved in transient disconnection (intraoperative stimulation) and permanent disconnection (based on resection cavities) in eight patients. In all the patients tested, individual stimulation sites interfering with iST corresponded either with the anterior thalamic radiation or the inferior fronto-subcortical connections, and with both tracts in five of eight patients.
We examined the extent of permanent tract disconnection, based on the resection cavities, in the same group of patients. Our results suggested that despite the fact that ∼90% of patients did not show any immediate postoperative deficits on the Stroop test, many frontal lobe tracts were resected, with the exception of the inferior fronto-striatal tracts, suggesting this tract may be crucial for interference control as tested using the iST. Supporting this hypothesis is the finding that the only patient with immediate postoperative deficits had this tract resected, although further investigation is needed to confirm this observation in a larger cohort. Taken together, our intraoperative and postoperative results in the eight patients indicate that both temporary and permanent deficits in interference control may occur when connections between the IFG and the striatum are resected.
Fronto-striatal networks, the frontal aslant tract and cognitive control
Our results are consistent with the suggested involvement of cortico-subcortical connections in cognitive control (Derrfuss et al., 2004; Haupt et al., 2009; Aron et al., 2016; Bomyea et al., 2017). Different anatomical hypotheses have been proposed regarding the specific cortico-subcortical connectivity that could account for cognitive control processes. One hypothesis relates to the putative role of cortico-cortical connections between the IFG and pre-SMA, mediated by the frontal aslant tract area (i.e. frontal aslant tract), in cognitive control specifically related to response inhibition and executive function in the right hemisphere (Aron et al., 2007; Dick et al., 2019). Tractography results did not show the frontal aslant tract to be intersected by positive sites except in one patient, and further, was not associated to any postoperative deficits on the Stroop task. Further, our VLSM analysis did not highlight the pre-SMA as a region that, when resected, leads to postoperative deficits in Stroop test performance. Such a result may challenge the role of the right frontal aslant tract in cognitive control, at least for verbal responses.
Another hypothesis is that rather than cortico-cortical IFG connections with the pre-SMA being responsible for cognitive control, it may be hyper-direct pathways between these regions and the basal ganglia may exert inhibitory modulation of motor or verbal responses (Nambu et al., 2002; Liston et al., 2006; Aron et al., 2016). In fact, our intraoperative results showed that stimulation of superior fronto-striatal pathways resulted in Stroop errors in only two patients of seven and lesion-symptom analysis did not show any association between superior frontal regions and postoperative Stroop performance. Further evidence to support this was evident from the diffusion tractography, in that patients with resection of the right superior fronto-striatal tracts did not have experience postoperative performance deficits on the Stroop test. On the other hand, as previously described, our results converge to strongly suggest a crucial role for the inferior fronto-striatal tracts in interference control, the aspect of cognitive control specifically assessed by the Stroop test.
One constraint of the study, due to task design, deserves discussion. The Stroop test used in both the intra- and postoperative setting requires a verbal response rather than a manual keypress, which is used in the majority of functional imaging studies (Banich et al., 2000; Hung et al., 2018). Accordingly, our results suggest that the connections between the IFG and basal ganglia play a crucial role in interference control processes measured by the verbal Stroop test, but we cannot exclude the role of other frontal connections in inhibitory processes related to non-verbal responses (e.g. the manual Stroop test, the go/no-go task or the flanker task). Damage to the pre-SMA and superior frontal regions may specifically affect cognitive control ability when responses are related to motor programming of hand movements. This is supported by recent tractography studies linking the frontal aslant tract and other connections of the superior frontal gyrus to goal-directed hand movements (Budisavljevic et al., 2016; Howells et al., 2018).
Other tracts and cognitive control
Functional MRI studies have highlighted a number of other brain regions are active during interference control tasks such as the Stroop test, including the anterior cingulate and prefrontal cortices (Banich et al., 2000; Gruber et al., 2002; Derrfuss et al., 2004; Liu et al., 2004). We did not find any evidence to suggest tracts connected with the anterior cingulate were involved in cognitive control, and the VLSM analysis did not show any association between resection of this area and postoperative Stroop performance. This is supported by a previous lesion study of stroke patients, which indicated that even bilateral lesions of this region did not impair performance on a range of cognitive control tasks, including the Stroop test (Fellows and Farah, 2005). It is important to highlight that functional imaging studies indicate the brain regions that are involved in a cognitive process, but not those that are fundamental for the brain to be able to perform that function (Rorden and Karnath, 2004). Further investigation is needed to clarify how critical the role of the anterior cingulate is in interference control processes in the intraoperative setting.
The results highlighted tracts connecting the anterior frontal lobe with the thalamus, the anterior thalamic radiation, as possible candidates for transient disconnection during the intraoperative Stroop task, in line with the literature suggesting an important role for the thalamus and anterior thalamic radiation in cognitive control (Wimmer et al., 2015; Wright et al., 2015; Mamiya et al., 2018). The resection of the anterior thalamic radiation in most of our patients, in the absence of significant postoperative deficits on the Stroop test, reasonably excludes a crucial role, at least in the right hemisphere, in interference control processes, or it is possible that it may not play an essential role in the distributed network involved in this cognitive mechanism.
Finally, some additional aspects of the study must be discussed. First, due to clinical constraints, only eight patients performed both iST and diffusion sequence, thus confirmation of the relevance of inferior fronto-striatal tracts in cognitive control is necessary in a larger cohort. Tractography enables indirect visualization of white matter and using an overlaid resection cavity, it is possible to identify tracts disconnected by the surgical intervention. However, defining what constitutes ‘permanent disconnection’ is difficult as it is currently unknown how remaining fibres may compensate for damage. We used an arbitrary cut-off of 50%; however, closer investigation of this should be made to determine whether this is appropriate for white matter bundles connecting more widespread regions, such as the inferior fronto-occipital fasciculus.
The lack of a positive site observed in some iST patients deserves a comment. Many factors may be responsible for this, not just that the probe may be distant from eloquent areas, oedema surrounding the tumour may affect current leakage or reorganization may have occurred during tumour growth. The good neuropsychological performance of the overall iST group indicates that a negative response might not be interpreted as a failure in test accuracy. Should it be so, patients would show postoperative impairment in cognitive control functions. Given that preoperative neuroimaging data is not necessarily reliable in detecting the eloquent tracts with respect to the tumour border, iST brain mapping is always recommended. Moreover, the probability of not finding a positive site when testing a ‘positive region’ (on the cortical level) is estimated in as many as 40% of patients tested (Desmurget et al., 2018). We identified error sites in 26 of 35 patients (74%), well above usual proportions, suggesting a good choice of the intensity used for mapping and high accuracy of the iST.
We did not test the role of the left hemisphere in cognitive control intraoperatively, due to difficulties in determining whether disruption to language processing is the underlying cause of the error. Future tests using manual responses may be useful in identifying left hemisphere networks. We were also unable to identify any associations between lesions and other cognitive control tests. As many patients showed hemispatial neglect syndrome following surgery, their test results were unreliable, thus we may have been underpowered to show lesion-symptom relationships.
Conclusion
The current study sheds light on a crucial network sustaining a key component of cognitive control in the right hemisphere, showing convincing evidence that the right IFG and its connections with the striatum may be fundamental for this function. This suggests that preserving cortico-striatal rather than cortico-cortical connections may be critical for maintaining cognitive control abilities in the surgery of right frontal lobe tumours.
Supplementary Material
Acknowledgements
We would like to acknowledge the patients who participated in the study.
Glossary
Abbreviations
- HARDI
high angular resolution diffusion imaging
- HF/LF-DES
high frequency/low frequency-direct electrical stimulation
- IFG
inferior frontal gyrus
- iST
intraoperative Stroop test
- pre-SMA
pre-supplementary motor area
- SLF
superior longitudinal fasciculus
- VLSM
voxel-based lesion-symptom mapping
Funding
This work was supported by grant from Associazione Italiana per la Ricerca sul Cancro (AIRC) to L.B. M.C. is the recipient of a Wellcome Trust Investigator Award No. 103759/Z/14/Z.
Competing interests
The authors report no competing interests.
References
- Aron AR, Behrens TEJ, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 2007; 27: 3743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Herz DM, Brown P, Forstmann BU, Zaghloul K. Frontosubthalamic circuits for control of action and cognition. J Neurosci 2016; 36: 11489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 2014; 18: 177–85. [DOI] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci 2008; 12: 193–200. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T et al. fMRI studies of stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cogn Neurosci 2000; 12: 988–1000. [DOI] [PubMed] [Google Scholar]
- Bello L, Riva M, Fava E, Ferpozzi V, Castellano A, Raneri F et al. Tailoring neurophysiological strategies with clinical context enhances resection and safety and expands indications in gliomas involving motor pathways. Neurol Oncol 2014; 16: 1110–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomyea J, Taylor CT, Spadoni AD, Simmons AN. Neural mechanisms of interference control in working memory capacity. Hum Brain Mapp 2017; 39: 772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 2001; 14: 486–500. [DOI] [PubMed] [Google Scholar]
- Budisavljevic S, Dell’Acqua F, Zanatto D, Begliomini C, Miotto D, Motta R et al. Asymmetry and structure of the fronto-parietal networks underlie visuomotor processing in humans. Cereb Cortex 2016; 12: 1532–44. [DOI] [PubMed] [Google Scholar]
- Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A. Una versione abbreviata del test di Stroop: dati normativi nella popolazione italiana. Nuova Rivis di Neurol 2002; 12: 111–5. [Google Scholar]
- Carlén M. What constitutes the prefrontal cortex? Science 2017; 358: 478–82. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell’Acqua F, Bizzi A, Forkel SJ, Williams SC, Simmons A et al. Beyond cortical localization in clinico-anatomical correlation. Cortex 2012; 48: 1262–87. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev 2009; 33: 631–46. [DOI] [PubMed] [Google Scholar]
- Chan RCK, Shum D, Toulopoulou T, Chen EYH. Assessment of executive functions: Review of instruments and identification of critical issues. Arch Clin Neuropsychol 2008; 23: 201–16. [DOI] [PubMed] [Google Scholar]
- Concha L. A macroscopic view of microstructure: using diffusion-weighted images to infer damage, repair, and plasticity of white matter. Neuroscience 2014; 276: 14–28. [DOI] [PubMed] [Google Scholar]
- Coolidge FL, Wynn T. Executive functions of the frontal lobes and the evolutionary ascendancy of Homo sapiens. Camb Archaeol J 2001; 2: 255–60. [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron 2011; 69: 680–94. [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Zacks RT, Williams CC. Stroop interference, practice, and aging. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2003; 10: 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Acqua F, Scifo P, Rizzo G, Catani M, Simmons A, Scotti G et al. A modified damped Richardson-Lucy algorithm to reduce isotropic background effects in spherical deconvolution. Neuroimage 2010; 49: 1446–58. [DOI] [PubMed] [Google Scholar]
- Dell’Acqua F, Simmons A, Williams SCR, Catani M. Can spherical deconvolution provide more information than fiber orientations? Hindrance modulated orientational anisotropy, a true-tract specific index to characterize white matter diffusion. Hum Brain Mapp 2013; 34: 2464–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Acqua F, Tournier J-D. Modelling white matter with spherical deconvolution: how and why? NMR Biomed 2018; 32: e3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Yves Von Cramon D. Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. Neuroimage 2004; 23: 604–12. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Richard M, Beuriat PA, Szathmari A, Mottolese C, Duhamel JR, Sirigu A. Selective inhibition of volitional hand movements after stimulation of the dorsoposterior parietal cortex in humans. Curr Biol 2018; 28: 3303–09. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu Rev Psychol 2013; 64: 135–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AS, Garic D, Graziano P, Tremblay P. The frontal aslant tract (frontal aslant tract) and its role in speech, language and executive function. Cortex 2019; 111: 148–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulaney CL, Rogers WA. Mechanisms underlying reduction in Stroop interference with practice for young and old adults. J Exp Psychol: Learn Mem Cogn 1994; 20: 470–84. [DOI] [PubMed] [Google Scholar]
- Duncan J. Disorganization of behaviour after frontal lobe damage. Cogn Neuropsychol 1986; 3: 271–90. [Google Scholar]
- Edwards S, Brice C, Craig C, Penri-Jones R. Effects of caffeine, practice, and mode of presentation on Stroop task performance. Pharmacol Biochem Behav 1996; 54: 309–15. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Aichert DS, Wöstmann N, Dehning S, Riedel M, Kumari V. Response inhibition and interference control: effects of schizophrenia, genetic risk, and schizotypy. J Neuropsychol 2017; 12: 434–510. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain 2005; 128: 788–96. [DOI] [PubMed] [Google Scholar]
- Findlater SE, Desai JA, Semrau JA, Kenzie JM, Rorden C, Herter TM et al. Central perception of position sense involves a distributed neural network: evidence from lesion-behavior analyses. Cortex 2016; 79: 42–56. [DOI] [PubMed] [Google Scholar]
- Forkel SJ, Catani M. Lesion mapping in acute stroke aphasia and its implications for recovery. Neuropsychologia 2018; 115: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulon C, Cerliani L, Kinkingnéhun S, Levy R, Rosso C, Urbanski M et al. Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. Gigascience 2018; 7: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V, Vartanian O, Bartolo A, Hakim L, Maria Ferraro A, Isella V et al. Lesions to right prefrontal cortex impair real-world planning through premature commitments. Neuropsychologia 2013; 51: 713–24. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Holcomb P, Soraci S, Yurgelun-Todd D. Stroop performance in normal control subjects: an fMRI study. Neuroimage 2002; 16: 349–60. [DOI] [PubMed] [Google Scholar]
- Haglund MM, Ojemann GA, Blasdel GG. Optical imaging of bipolar cortical stimulation. J Neurosurg 1993; 78: 785–93. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Neef NE, Camilleri JA, Margulies DS, Eickhoff SB. Functional segregation of the right inferior frontal gyrus: evidence from coactivation-based parcellation. Cereb Cortex 2019; 29: 1532–46. [DOI] [PubMed] [Google Scholar]
- Haupt S, Axmacher N, Cohen MX, Elger CE, Fell J. Activation of the caudal anterior cingulate cortex due to task-related interference in an auditory Stroop paradigm. Hum Brain Mapp 2009; 30: 3043–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells H, Thiebaut de Schotten M, Dell’Acqua F, Beyh A, Zappalà G, Leslie A et al. Frontoparietal tracts linked to lateralized hand preference and manual specialization. Cereb Cortex 2018; 28: 2482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Y, Gaillard SL, Yarmak P, Arsalidou M. Dissociations of cognitive inhibition, response inhibition, and emotional interference: voxelwise ALE meta-analyses of fMRI studies. Hum Brain Mapp 2018; 39: 4065–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MC. Simple boundary correction for kernel density estimation. Stat Comput 1993; 3: 135–46. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science 2004; 303: 1023–6. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science 2003; 302: 1181–5. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jeurissen B, Sijbers J, Jones D. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc Intl Soc Mag Reson Med 2009; 17: 3537. [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex 2006; 16: 553–60. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage 2004; 22: 1097–106. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Andrew Stenger V, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000; 288: 1835–8. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull 1991; 109: 163–203. [DOI] [PubMed] [Google Scholar]
- Mamiya PC, Richards TL, Kuhl PK. Right forceps minor and anterior thalamic radiation predict executive function skills in young bilingual adults. Front Psychol 2018; 9: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Bauer RM, Filoteo JV, Grande L, Roper SN, Gilmore R. Attentional inhibition in patients with focal frontal lobe lesions. J Clin Exp Neuropsychol 2005; 27: 485–503. [DOI] [PubMed] [Google Scholar]
- Muhle-Karbe PS, Derrfuss J, Lynn MT, Neubert FX, Fox PT, Brass M et al. Co-activation-based parcellation of the lateral prefrontal cortex delineates the inferior frontal junction area. Cereb Cortex 2016; 26: 2225–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane JC, Corkum P V, Klein RM, McLaughlin E. Interference control in children with and without ADHD: a systematic review of Flanker and Simon task performance. Child Neuropsychol 2009; 15: 321–42. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res 2002; 43: 111–7. [DOI] [PubMed] [Google Scholar]
- Puglisi G, Sciortino T, Rossi M, Leonetti A, Fornia L, Conti Nibali M et al. Preserving executive functions in non-dominant frontal lobe glioma surgery: an intraoperative tool. J Neurosurg 2018; 28: 1–7. [DOI] [PubMed] [Google Scholar]
- Rojkova K, Volle E, Urbanski M, Humbert F, Dell’Acqua F, Thiebaut de Schotten M. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Struct Funct 2016; 221: 1751–66. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nat Rev Neurosci 2004; 5: 813–9. [DOI] [PubMed] [Google Scholar]
- Rossi M, Fornia L, Puglisi G, Leonetti A, Zuccon G, Fava E et al. Assessment of the praxis circuit in glioma surgery to reduce the incidence of postoperative and long-term apraxia: a new intraoperative test. J Neurosurg 2018; 23: 1–11. [DOI] [PubMed] [Google Scholar]
- Smith D V, Clithero JA, Rorden C, Karnath H-O. Decoding the anatomical network of spatial attention. Proc Natl Acad Sci 2013; 110: 1518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18: 643. [Google Scholar]
- Stuss DT, Floden D, Alexander MP, Levine B, Katz D. Stroop performance in focal lesion patients: dissociation of processes and frontal lobe lesion location. Neuropsychologia 2001; 39: 771–86. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Toth JP, Franchi D, Alexander MP, Tipper S, Craik FIM. Dissociation of attentional processes in patients with focal frontal and posterior lesions. Neuropsychologia 1999; 37: 1005–27. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DGM et al. A lateralized brain network for visuospatial attention. Nat Neurosci 2011; 14: 1245–6. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Batrancourt B, Levy R, Dubois B, Cerliani L et al. Rostro-caudal architecture of the frontal lobes in humans. Cereb Cortex 2017; 27: 4033–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–89. [DOI] [PubMed] [Google Scholar]
- van Velzen LS, Vriend C, de Wit SJ, van den Heuvel OA. Response inhibition and interference control in obsessive compulsive spectrum disorders. Front Hum Neurosci 2014; 11: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM. Thalamic control of sensory selection in divided attention. Nature 2015; 526: 705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NF, Vann SD, Aggleton JP, Nelson AJD. A critical role for the anterior thalamus in directing attention to task-relevant stimuli. J Neurosci 2015; 35: 5480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are available on request, in anonymized format, from the corresponding author.