See Scheffer (doi:10.1093/brain/awz208) for a scientific commentary on this article.
In a prospective population-based cohort study, Symonds et al. perform high-throughput genetic testing in children presenting with seizures before 3 years of age, and provide incidence estimates for the most common single-gene epilepsies. One quarter of cases are found to have a genetic cause, and 80% of the genetic diagnoses are found to have potential treatment implications.
Keywords: epilepsy, genetics, epidemiology, incidence, precision
Abstract
Epilepsy is common in early childhood. In this age group it is associated with high rates of therapy-resistance, and with cognitive, motor, and behavioural comorbidity. A large number of genes, with wide ranging functions, are implicated in its aetiology, especially in those with therapy-resistant seizures. Identifying the more common single-gene epilepsies will aid in targeting resources, the prioritization of diagnostic testing and development of precision therapy. Previous studies of genetic testing in epilepsy have not been prospective and population-based. Therefore, the population-incidence of common genetic epilepsies remains unknown. The objective of this study was to describe the incidence and phenotypic spectrum of the most common single-gene epilepsies in young children, and to calculate what proportion are amenable to precision therapy. This was a prospective national epidemiological cohort study. All children presenting with epilepsy before 36 months of age were eligible. Children presenting with recurrent prolonged (>10 min) febrile seizures; febrile or afebrile status epilepticus (>30 min); or with clusters of two or more febrile or afebrile seizures within a 24-h period were also eligible. Participants were recruited from all 20 regional paediatric departments and four tertiary children’s hospitals in Scotland over a 3-year period. DNA samples were tested on a custom-designed 104-gene epilepsy panel. Detailed clinical information was systematically gathered at initial presentation and during follow-up. Clinical and genetic data were reviewed by a multidisciplinary team of clinicians and genetic scientists. The pathogenic significance of the genetic variants was assessed in accordance with the guidelines of UK Association of Clinical Genetic Science (ACGS). Of the 343 patients who met inclusion criteria, 333 completed genetic testing, and 80/333 (24%) had a diagnostic genetic finding. The overall estimated annual incidence of single-gene epilepsies in this well-defined population was 1 per 2120 live births (47.2/100 000; 95% confidence interval 36.9–57.5). PRRT2 was the most common single-gene epilepsy with an incidence of 1 per 9970 live births (10.0/100 000; 95% confidence interval 5.26–14.8) followed by SCN1A: 1 per 12 200 (8.26/100 000; 95% confidence interval 3.93–12.6); KCNQ2: 1 per 17 000 (5.89/100 000; 95% confidence interval 2.24–9.56) and SLC2A1: 1 per 24 300 (4.13/100 000; 95% confidence interval 1.07–7.19). Presentation before the age of 6 months, and presentation with afebrile focal seizures were significantly associated with genetic diagnosis. Single-gene disorders accounted for a quarter of the seizure disorders in this cohort. Genetic testing is recommended to identify children who may benefit from precision treatment and should be mainstream practice in early childhood onset epilepsy.
Introduction
It is estimated that the lifetime prevalence of epilepsy is 7.60 per 1000 individuals (Fiest et al., 2017) and that 50–65 million individuals are affected worldwide (Ngugi et al., 2010; Fiest et al., 2017). Epilepsy incidence is age-dependent, with the highest incidences (>60 per 100 000) found in those under the age of 5 years and those over the age of 65 years (Hauser et al., 1993). Even in high resource health systems seizure control is not achieved for one-third of subjects with epilepsy (Kwan and Brodie, 2000).
Children presenting with epilepsy before the age of 3 years experience a high burden of cognitive and behavioural comorbidity (Berg et al., 2008). Comorbidities are more prevalent among children who develop drug-resistant seizures (Wirrell et al., 2012) and those with a high seizure burden (Berg et al., 2012; Wilson et al., 2012). The concept of the ‘developmental and epileptic encephalopathy’ (DEE) recognizes that in children presenting with early-onset epilepsy, neurodevelopmental comorbidity may be attributable to both the underlying cause and to the detrimental effects of uncontrolled epileptic activity (Scheffer et al., 2017). Single-gene causes of childhood-onset epilepsy, such as Dravet syndrome due to SCN1A mutations, typify this concept. Families affected by, and clinicians treating, such epilepsies strive for therapies that more precisely target the syndrome and/or the underlying disease mechanisms, in the hope that seizure control and developmental comorbidity can be simultaneously addressed. Precision therapy approaches have driven randomized therapeutic trials including stiripentol (Chiron et al., 2000) and cannabidiol (Devinsky et al., 2017) in Dravet syndrome, and quinidine in KCNT1-associated epilepsy (Mullen et al., 2018).
The application of next generation sequencing (NGS) technology has facilitated a fundamental change in aetiological diagnosis of epilepsy. When cohorts of children with a suspected genetic cause are tested using NGS, between 18% and 48% receive a diagnosis. The variation in yield may be explained by differences in case inclusion, testing methodology, and number of genes tested (Lemke et al., 2012; Appenzeller et al., 2014; Helbig et al., 2016; Trump et al., 2016; Lindy et al., 2018). Patients included in such studies may have been selected on the basis of the nature of the epilepsy and/or a suspected genetic aetiology. Therefore, these studies may misrepresent the incidence and phenotypic spectrum of the single-gene epilepsies. To appreciate the scope for genetically-guided precision medicine in childhood-onset epilepsy, and to prioritize therapy development, we must understand the epidemiology. This demands a prospective population-based approach to genetic testing.
Materials and methods
Cohort recruitment
Participants were recruited from all 20 regional paediatric departments and four tertiary children’s hospitals in Scotland, from 8 May 2014 to 7 May 2017. Only children who met the inclusion criteria during this period, and who were under 36 months of age, were included.
Inclusion criteria were any of: (i) child receiving a new diagnosis of epilepsy (recurrent unprovoked seizures); (ii) child presenting with an episode of febrile or afebrile status epilepticus (seizure >30 min); (iii) child presenting with two or more febrile or afebrile epileptic seizures within a 24-h period; and (iv) child presenting with a second prolonged (>10 min) febrile seizure, over any time period.
Patients were excluded if an aetiology that would fully explain seizures was identified either prior to or at first presentation with seizures. Examples of such aetiologies were meningitis, hypoxic ischaemic encephalopathy in the neonate, or focal seizures in an infant with a perinatal stroke.
Children presenting with prolonged and clustering febrile seizures were included because in certain genetic epilepsies, including those associated with SCN1A, PCDH19 and PRRT2 variants, epilepsy can be preceded by febrile seizures (Brunklaus et al., 2012; Higurashi et al., 2013; Ebrahimi-Fakhari et al., 2015). Our aim was to optimize case identification and avoid any delay in genetic diagnosis. Early genetic diagnosis may inform treatment and potentially alter disease course (Brunklaus et al., 2013; Lange et al., 2018).
Maximum case ascertainment was ensured by weekly e-mail reminders throughout the study period to the eight paediatric neurophysiology departments, a link clinician in each of the 24 centres and all 17 epilepsy specialist nurses in Scotland. Research nurses throughout Scotland reviewed admissions to intensive care and high dependency units; and a national continuing education program maintained the profile of the study.
We are not aware of any private medical services in Scotland where young children would present with seizures, and given the geographical location of hospitals in the border regions of England and Scotland we would expect that children in Scotland would access both paediatric and paediatric neurology services from hospitals of the Scottish National Health Service (NHS).
Denominator data for births over the study period were taken from National Records of Scotland birth records (National Records of Scotland, 2018). Incidence estimates were rounded to three significant figures and 95% confidence intervals (CIs) were calculated using the Poisson distribution.
Genetic testing
DNA was extracted from whole blood and tested on a custom designed 104 gene epilepsy panel (Supplementary material, part C) at the Scottish Genetic Epilepsy service in Glasgow, unless a genetic diagnosis had already been made through single-gene testing. Accelerated single-gene testing [Sanger sequencing and multiplex ligation probe amplification (MLPA)] of 10 genes (Supplementary material, part D) was offered, with clinicians advised to request these prior to panel testing if clinically indicated. The gene panel was designed to include the early onset childhood genetic epilepsies for which brain imaging was unlikely to give a diagnosis and for which there is evidence for specific therapeutic approaches. Genes to be included on the panel were selected by a team of clinicians (J.D.S., S.M.Z., S.J., D.T.P.) who reviewed the literature extensively. All potentially causative variants identified were validated through Sanger sequencing. Cases negative on the 104 gene panel with typical phenotypes for epilepsy related to SCN1A, KCNQ2, SLC2A1, PCDH19, CDKL5, or MECP2 underwent dosage analysis through MLPA of the relevant gene. All variants of uncertain significance and likely pathogenic/pathogenic variants were discussed in the context of the clinical phenotype by a multidisciplinary team of paediatric neurologists, clinical geneticists and molecular geneticists. Variants were reported with reference to UK Association of Clinical Genetic Science (ACGS) guidelines (Association for Clinical Genetic Science, 2017). Pathogenic and likely pathogenic results were considered diagnostic. Where DNA samples and/or phenotype details from other family members were considered relevant to variant interpretation these were requested. Chromosomal microarray studies were not routinely performed because results were not thought likely to guide therapeutic management.
Clinical information
At the time of case recruitment, clinicians completed a structured proforma detailing clinical features and investigation findings (Supplementary material, part E). A panel of three paediatric neurologists reviewed clinical details of all cases to ensure eligibility criteria were met. A minimum of 12 months after initial presentation and 6 months after the recruiting clinician had been informed of the final genetic test result, clinicians completed a structured clinical follow-up questionnaire (Supplementary material, part F). For the purposes of this study, therapy-resistant seizures were defined as ongoing epileptic seizures (one or more seizure per 6 months) despite adequate trials of two or more appropriately chosen, appropriately dosed (or administered) and taken antiepileptic therapies (including non-drug therapies such ketogenic diet and vagus nerve stimulation). The study was approved by the United Kingdom NHS National Research Ethics Service.
Statistical analysis
IBM® SPSS® Statistics Version 24 was used to determine associations between clinical features and identification of a genetic cause. Only patients who had completed genetic testing were included in this analysis. Patients were defined as having completed genetic testing if either a diagnostic result was identified through accelerated single-gene testing, or gene panel testing was completed. Age of seizure onset was divided into four categorical groups: <6 months, 6–12 months, 12–24 months, and 24–36 months. Type of first seizure was divided into eight categories: febrile generalized (excluding status, which was defined as a seizure lasting >30 min), febrile focal (excluding status), febrile status (focal or generalized), afebrile focal (excluding status), afebrile generalized (excluding status), afebrile status (focal or generalized), afebrile unclassified, and infantile spasms. Univariate analysis was performed using Fisher’s exact equation and multivariate analysis used a Hosmer-Lemeshow binomial regression model. Statistical advice was from Cunyi Wang of Glasgow University.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.
Results
The mean number of births per year in Scotland for the years 2011 to 2016 inclusive was 56 490, making the estimated denominator for this population 169 470. During the 3-year study period, 343 children under the age of 3 years were recruited to this study.
Phenotypes
Of 214 patients recruited because of a diagnosis of epilepsy, 101 (47%) presented with focal seizures, 42 (20%) with tonic-clonic seizures, 23 (11%) with myoclonic seizures, 22 (10%) with epileptic spasms, 11 (5%) with absence seizures, eight (4%) with generalized tonic seizures, three (1%) with atonic seizures, and four with unclassified seizures. Ninety-four patients were recruited because of febrile seizures, of whom 45 presented with febrile status, 36 with a cluster of two or more febrile seizures within 24 h, and 13 with recurrent prolonged febrile seizures (>10-min duration). Twenty-three patients were recruited because of an episode of afebrile status epilepticus, and 12 were recruited because of a single cluster of two or more afebrile seizures within a 24-h period. At the time of recruitment, 129 patients did not have a diagnosis of epilepsy, but by the end of the study period, 49 (38%) of these had acquired an epilepsy diagnosis (Table 1). Seventeen different epilepsy syndrome diagnoses were made. The numbers for epilepsy syndrome diagnosis are shown in Table 2. Of 263 patients, 125 (47.5%) remained without an epilepsy syndrome that could be fully classified. Seventy-six (22.1%) patients in the cohort developed therapy-resistant seizures. Finally, 106 (30.1%) had concerns about development expressed at the time of recruitment, and 115 (33.5%) had developmental concerns raised at most recent follow-up.
Table 1.
Diagnoses at recruitment and diagnoses at most recent follow-up
| Diagnosis at recruitment | Diagnosis at most recent follow-up | % Progression to epilepsy | ||
|---|---|---|---|---|
| Group | n | Epilepsy | Not epilepsy | |
| Recurrent unprovoked seizures (epilepsy) | 214 | 214 | 0 | N/A |
| Afebrile status | 23 | 13 | 10 | 56.5 |
| Afebrile cluster of seizures within 24 h | 12 | 6 | 6 | 50.0 |
| Febrile status | 45 | 13 | 32 | 28.9 |
| Febrile cluster of seizures within 24 h | 36 | 12 | 24 | 33.3 |
| Recurrent prolonged febrile seizures (>10 min) | 13 | 5 | 8 | 38.5 |
| Total | 343 | 263 | 80 | |
| Total (%) with a genetic cause identified | 80 (23.3) | 76 (28.9) | 4 (5.0) | |
N/A = not applicable.
Table 2.
Final phenotypes and genetic diagnoses made within each group
| Phenotype at most recent follow up, n | n (%) with a genetic cause identified | Genes implicated (n) |
|---|---|---|
| Epilepsy, 263 | ||
| DEE, 62 | ||
| West syndrome, 27 | 3/27 (11.1) | CDKL5 (2), DEPDC5 |
| Dravet syndrome, 11 | 11/11 (100) | SCN1A (11) |
| Other DEEs, 24 | 13/24 (54.1) | PCDH19 (3), CDKL5 (2), KCNQ2 (2), GABRA1, KCNT1, MECP2, SCN2A, SCN8A, STXBP1 |
| Alper-Huttenlocher syndrome, 1 | 1/1 (100) | POLG |
| Absences with eyelid myoclonia, 1 | 1/1 (100) | CHD2 |
| Early onset absence epilepsy, 5 | 0/5 | |
| Epilepsy with myoclonic-atonic seizures, 8 | 2/8 (25) | STX1B, SLC6A1 |
| Familial focal epilepsy, 1 | 1/1 (100) | DEPDC5 |
| Febrile seizures plus, 6 | 0/6 | |
| Genetic epilepsy with febrile seizures plus, 2 | 1/2 | SCN1A |
| Glut1 deficiency syndrome, 7 | 7/7 (100) | SLC2A1 (7) |
| Myoclonic epilepsy of infancy, 3 | 0/3 | |
| Panayioutopoulos syndrome, 1 | 0/1 | |
| Self-limited familial infantile epilepsy, 5 | 5/5 (100) | PRRT2 (5) |
| Self-limited infantile epilepsy, 27 | 11/27 (40.7) | PRRT2 (10), KCNQ2 |
| Self-limited familial neonatal seizures, 7 | 7/7 (100) | KCNQ2 (5), KCNQ3 (2) |
| Self-limited neonatal seizures, 1 | 1/1 (100) | KCNQ2 |
| Unclassified myoclonic epilepsy, 5 | 0/5 | |
| Unclassified generalized epilepsy, 10 | 1/10 (10) | CACNA1A |
| Unclassified focal epilepsy, 59 | 5/59 (8.5) | DEPDC5 (2), KCNA2, KCNQ2, PRRT2 |
| Unclassified focal and generalized epilepsy, 8 | 0/8 | |
| Unclassified epilepsy, 44 | 6/44 (13.6) | SLC6A1 (3), COL4A1, PCDH19, PRRT2 |
| Not epilepsy, 80 | ||
| Febrile seizures only, 64 | 3/64 (4.7) | SCN1A (2), KCNA2 (mosaic, 20) |
| Single episode of afebrile status, 10 | 0/10 | |
| Single cluster of afebrile seizures in 24 h, 6 | 1/6 (16.7) | CACNA1A |
DEEs = developmental and epileptic encephalopathies.
Genetic diagnoses
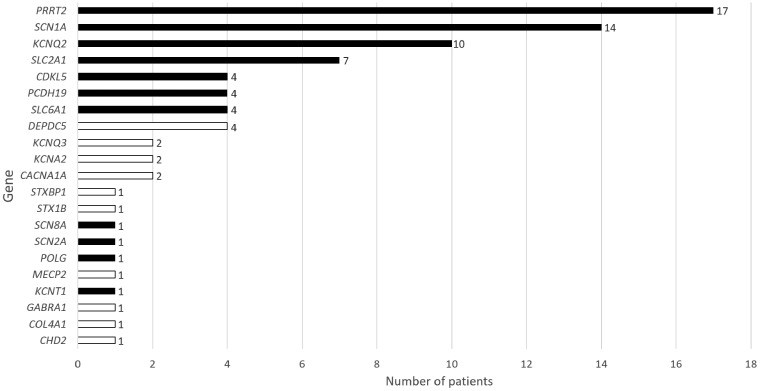
Three hundred and thirty-three patients completed genetic testing. There was insufficient DNA to complete panel testing for 10 patients. Genetic diagnoses were made for 80 children (24%) (Fig. 1). Supplementary material, part B details all the genetic diagnoses made, along with the phenotypic details for each patient. All but four of the genetic diagnoses were in patients who had a diagnosis of epilepsy by the end of the study period (Table 1). No patient had more than one diagnostic finding. The incidence of single-gene seizure disorders in this population is at least 1 per 2120 live births (47.2/100 000; 95% CI 36.9–57.5). Twenty-six patients had a diagnostic result from accelerated single-gene testing (Sanger and/or MLPA), therefore, these did not undergo panel testing. Of the remaining 307 patients tested on the panel, 52 patients had a diagnostic result. Finally, two patients, following multidisciplinary team discussion, underwent KCNQ2 MLPA testing after a negative panel result and were found to have pathogenic copy number variants. The causative variant was de novo in 34 patients, inherited from an affected parent in 15 patients, inherited from an unaffected parent in nine patients, undetermined in 21 cases, and compound heterozygous in one patient (with POLG-related seizures). Table 2 shows the genetic diagnoses made in relation to phenotype at the end of the study period. High yields from genetic testing were observed in patients classified into the following groups: Dravet syndrome; other developmental and epileptic encephalopathies; self-limited infantile seizures.
Figure 1.
Genetic results. No case had more than one diagnostic result. Shaded bars represent genes for which there is evidence for precision therapy.
The most common single-gene epilepsies were PRRT2 (17 patients), SCN1A (14 patients), KCNQ2 (10 patients) and SLC2A1 (seven patients). Eighty-four per cent (67/80) of genetic diagnoses were in the most frequently-implicated 10 genes. Through interrogating clinical data obtained at presentation and follow-up, we were able to characterize the phenotypic spectrum of several single-gene epilepsies (Table 3 and Fig. 2) and define high yield groups for specific single-gene epilepsies (Table 5). In Table 6 we present univariate and multivariate analysis for associations between presenting features and identification of a single-gene cause. Genetic diagnosis was positively associated with presentation before the age of six months, and with presentation with afebrile focal seizures. These associations remained significant in a multivariate model.
Table 3.
Summary of the clinical findings from the eight most common single-gene epilepsies
| Genetic cause | ||||
|---|---|---|---|---|
| PRRT2 | SCN1A | KCNQ2 | SLC2A1 | |
| Number of patients in this cohort and whether related | 17 (8 female) | 14 (5 female) | 10 (5 female) | 7 (3 female) |
| All unrelated | All unrelated | All unrelated | All unrelated | |
| Incidence | 1 per 9970 live births | 1 per 12 200 live births | 1 per 17 000 live births | 1 per 24,300 live births |
| (10.0/100 000; 95% CI 5.26–14.8). | (8.26/100 000; 95% CI 3.93–12.6). | (5.89/100 000; 95% CI 2.24–9.56). | (4.13/100 000; 95% CI 1.07–7.19). | |
| Age range at presentation in months (median) | 3–19 (7) | 1.5–19 (6.5) | 0.17–4 (0.24) | 11–18 (12) |
| Most common presentation(s) | Afebrile focal seizures: 71% (12/17) | Febrile seizures: 50% (7/14) | Afebrile focal seizures: 70% (7/10) | Afebrile generalized seizures: 86% (6/7) |
| Afebrile focal seizures: 36% (5/14) | ||||
| Status epilepticus: 36% (5/14) | ||||
| Diagnosis at latest follow-up | Self-limited infantile epilepsy: 88% (15/17) | Dravet syndrome: 79% (11/14) | Self-limited neonatal epilepsy: 60% (6/10) | Glut1-deficiency with epilepsy: 100% (7/7) |
| Unclassified focal epilepsy: 6% (1/17) | Febrile seizures only: 14% (2/14) | KCNQ2 encephalopathy: 20% (2/10) | ||
| Unclassified epilepsy: 6% (1/17) | Genetic epilepsy with febrile seizures plus: 7% (1/14) | Self-limited infantile epilepsy: 10% (1/10) | ||
| Unclassified focal epilepsy: 10% (1/10) | ||||
| Developmental concerns at presentation | 24% (4/17) | 29% (4/14) (29%) | 20% (2/10) | 57% (4/7) |
| Developmental concerns at follow-up | 12% (2/17) | 64% (9/14) | 30% (3/10) | 43% (3/7) |
| Therapy-resistant seizures | None (0/17) | 86% (12/14) | 20% (2/10) | 14% (1/7) |
| Recommended treatment(s) (Table 4) | Carbamazepine | Stiripentol | Carbamazepine | Ketogenic diet |
| Fenfluramine | Phenytoin | |||
| Cannabidiol | ||||
| Avoidance of sodium channel blocking medications | ||||
| Zygosity | 100% heterozygous (17/17) | 100% heterozygous (14/14) | 100% heterozygous (10/10) | 100% heterozygous (7/7) |
| Inheritance of causative variant | 12% de novo (2/17) | 70% de novo (10/14) | 30% de novo (3/10) | 70% de novo (5/7) |
| 71% inherited (12/17) | 30% inherited (3/14) | 50% inherited (5/10) | 15% inherited (1/7) | |
| 18% unknown (3/17) | 10% unknown (1/14) | 20% unknown (2/10) | 15% unknown (1/7) | |
| Genetic cause | ||||
| CDKL5 | PCDH19 | DEPDC5 | SLC6A1 | |
| Number of patients in this cohort and whether related | 4 (3 female) | 4 (all female) | 4 (2 female) | 4 (all female) |
| All unrelated | All unrelated | All unrelated | 3 were siblings | |
| Incidence | 1 per 42 400 live births | 1 per 20 600 live born females | 1 per 42 400 live births | N/A |
| 2.36/100 000 (95% CI 0.805–5.59) | 4.85/100 000 (95% CI 1.97–9.15) | 2.36/100 000 (95% CI 0.81–5.59) | ||
| Age range at presentation in months (median) | 0.5–6 (1.65) | 6–18 (11.5) | 2.5–26 (20) | 12–31 (19) |
| Most common presentation(s) | Infantile spasms: 50% (2/4) | Afebrile focal seizures: 75% (3/4) | Afebrile focal seizures: 50% (2/4) | Afebrile focal seizures: 75% (3/4) |
| Afebrile focal seizures: 50% (2/4) | ||||
| Diagnosis at latest follow-up | CDKL5 developmental and epileptic encephalopathy: 100% (4/4) | PCDH19 related developmental and epileptic encephalopathy: 75% (3/4) | Unclassified focal epilepsy: 75% (3/4) | Unclassified epilepsy 75% (3/4) |
| Unclassified epilepsy: 25% (1/4) | Infantile spasms (West syndrome) 25% (1/4) | Epilepsy with myoclonic-atonic seizures 25% (1/4) | ||
| Developmental concerns at presentation | 50% (2/4) | None (0/4) | None (0/4) | 75% (3/4) |
| Developmental concerns at follow-up | 100% (4/4) | 75% (3/4) | 25% (1/4) | 100% (4/4) |
| Therapy-resistant seizures | 100% (4/4) | 100% (4/4) | 50% (2/4) | 50% (2/4) |
| Recommended treatment(s) (Table 4) | Ketogenic diet | Clobazam | No specific recommendation | Sodium valproate |
| Zygosity | 75% heterozygous (3/4) | 100% heterozygous (4/4) | 100% heterozygous (4/4) | 100% heterozygous (4/4) |
| 25% hemizygous (1/4) | ||||
| Inheritance of causative variant | 75% de novo (3/4) | 50% paternally inherited (2/4) | 50% de novo (2/4) | 100% inherited (4/4) |
| 25% unknown (1/4) | 50% de novo (2/4) | 50% inherited (2/4) | ||
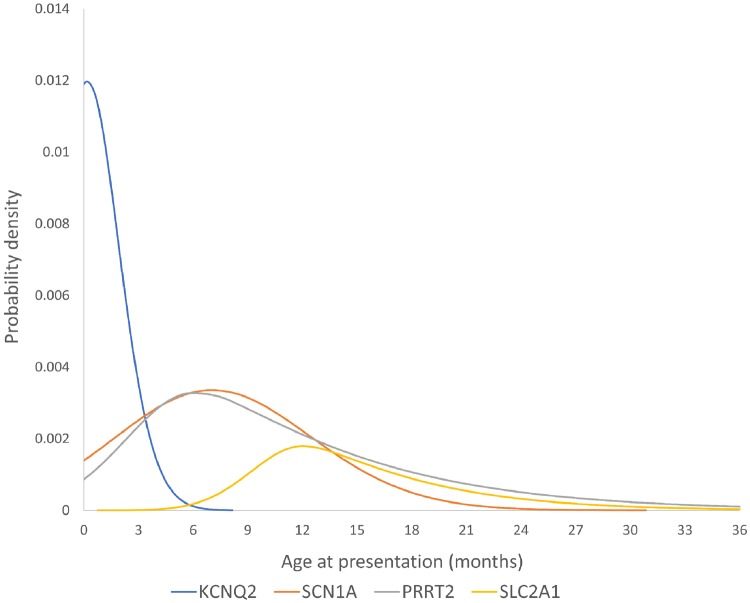
Figure 2.
Age of presentation for four genetic epilepsies. These skewed Gaussian plots are hypothetical distributions based on the mean, median and standard deviations of the age at presentation for these four genetic epilepsies from our data. Each plot has been scaled according to the number of cases identified in this cohort so that the area under each curve represents the total probability of finding a causative variant in each gene in our cohort.
Table 5.
Presentation types that had high yield for specific genetic diagnoses
| Presentation | Afebrile seizures <6 months | Afebrile focal seizures <12 months | Febrile or afebrile status epilepticus <24 months | Afebrile generalized seizures ≥6 months and <24 months |
|---|---|---|---|---|
| n | 63 | 68 | 59 | 74 |
| Genetic diagnosis (n) | KCNQ2 (10) | PRRT2 (11) | SCN1A (5) | SLC2A1 (6) |
| PRRT2 (5) | KCNQ2 (7) | KCNA2 (1) | PRRT2 (2) | |
| SCN1A (4) | SCN1A (5) | POLG (1) | CHD2 (1) | |
| CDKL5 (2) | CDKL5 (2) | PRRT2 (1) | KCNA2 (1) | |
| KCNQ3 (2) | KCNQ3 (2) | SLC2A1 (1) | PCDH19 (1) | |
| COL4A1 (1) | COL4A1 (1) | POLG (1) | ||
| GABRA1 (1) | DEPDC5 (1) | SCN1A (1) | ||
| KCNT1 (1) | GABRA1 (1) | |||
| STCBP1 (1) | PCDH19 (1) | |||
| SCN2A (1) | SCN2A (1) | |||
| SCN8A (1) | SLC2A1 (1) | |||
| Total with genetic diagnosis (%) | 29 (46.0) | 33 (48.5) | 9 (15.2) | 13 (17.6) |
Table 6.
Associations between features at presentation and genetic diagnosis
| n (%) with genetic cause identified | Two-tailed Fisher’s exact probabilityψ | OR (95% CI) | Multivariate model probability (Homser- Lemeshow) | Multivariate OR (95% CI) | |
|---|---|---|---|---|---|
| Total cohort | 80/333 (24.0) | ||||
| Age at presenting seizure | |||||
| <6 months | 34/74 (45.9) | <0.005** | 3.9 (2.3–6.9) | 0.004 | 4.9 (1.9–12.8) |
| 6–12 months | 22/89 (24.7) | n.s. | n.s. | ||
| 12–24 months | 17/117 (14.5) | <0.005* | 0.4 (0.2–0.7) | n.s. | |
| 24–36 months | 7/53 (13.2) | n.s. | Reference category | ||
| Presenting seizure type | |||||
| Febrile generalized, not including status | 2/36 (5.6) | <0.005* | 0.2 (0.0–0.7) | Reference category | |
| Febrile focal, not including status | 3/10 (33.3) | n.s. | n.s. | ||
| Febrile status, generalized or focal | 7/45 (15.6) | n.s. | n.s | ||
| Afebrile focal, not including status | 40/100 (40.0) | <0.005** | 3.2 (1.9–5.3) | 0.012 | 6.9 (1.5–31.7) |
| Afebrile generalized, not including status¥ | 21/96 (21.9) | n.s. | n.s | ||
| Afebrile status, generalized or focal | 3/21 (14.3) | n.s. | n.s | ||
| Afebrile unclassified | 1/4 (25.0) | n.s. | n.s. | ||
| Infantile spasms | 3/21 (14.3) | n.s. | n.s | ||
| Afebrile generalized tonic-clonic | 9/50 (18.0) | n.s. | Subtypes of afebrile generalized seizures were not included in the mutivariate model | ||
| Afebrile generalized myoclonic | 4/23 (17.4) | n.s. | |||
| Afebrile generalized tonic | 4/9 (44.4) | n.s. | |||
| Afebrile generalized atonic | 1/3 (33.3) | n.s. | |||
| Afebrile generalized absence | 3/11 (27.3) | n.s. | |||
ΨFisher’s exact statistic calculated on a contingency table where the null hypothesis was that there would be equal proportions of patients with and without a genetic diagnosis in each subgroup as there were in the entire tested cohort who completed genetic testing (n = 333).
*Negative association.
**Positive association.
¥Composite group of all presentations with afebrile generalized seizures.
n.s. = not significant; OR = odds ratio.
Gene-associated phenotypes
PRRT2: self-limited (familial) infantile seizures
We identified 17 patients and calculate the minimum incidence as 1 per 9970 live births (10.0/100 000; 95% CI 5.26–14.8). Two patients had missense variants and 15 patients had frameshift variants, of whom 13 patients had the recurrent frameshift variant (c.649dup, p.Arg217Profs*8). This is the most frequently observed disease-associated PRRT2 variant in the literature (Ebrahimi-Fakhari et al., 2015). The observation that this variant appears over 400 times in the exome aggregation consortium (ExAC) database (Lek et al., 2016) would initially suggest that it is a relatively common population variant. However, the same variant appears just eight times in the genome aggregation consortium (gnomAD) database, which includes the same participants as ExAC but applies a different sequencing methodology (The Broad Institute, 2018). We therefore suspect that the variants seen in ExAC are artefacts that have appeared during the DNA amplification process. In our study we validated all variants through Sanger sequencing and are confident that we have not reported sequencing artefacts. Among 29 718 participants in gnomAD for whom there is PRRT2 data, eight were heterozygous for the c.649dup variant. In our study there were 10 patients among 333 tested, making this variant >100 times more common in our study population than in the healthy population.
A clear age pattern of PRRT2-related epilepsy presentation was observed, with peak onset of seizures at 7 months. The phenotype we observed was in keeping with previously published literature on PRRT2-related epilepsy, which describes a self-limited infantile epilepsy which has median age of onset at 6.0 months (Ebrahimi-Fakhari et al., 2015). Outcomes were generally good in our cohort. At most recent follow-up no patients had developed therapy-resistant seizures, and all had been seizure-free for >6 months. Interestingly four patients had developmental concerns highlighted at presentation but only two continued to present developmental concerns at most recent follow-up. This provides a suggestion that with this genetic epilepsy, developmental trajectory may improve along with seizure control as the child gets older. This hypothesis would need to be confirmed by studying more prospective developmental assessments. Effective therapies reported were carbamazepine (n = 7), levetiracetam (n = 5), and sodium valproate (n = 3).
SCN1A: Dravet syndrome, febrile seizures plus
We identified 14 patients and calculate the minimum incidence of SCN1A-related seizures as 1 per 12 200 live births (8.26/100 000; 95% CI 3.93–12.6). A spectrum of disease severity was seen. Of 14 patients with SCN1A variants, 11 (79%) were ultimately diagnosed with Dravet syndrome, making the incidence of SCN1A-related Dravet syndrome 1 per 15 500 live births. One patient, who had a de novo variant (Patient 90), received a diagnosis of genetic epilepsy with febrile seizures plus (GEFS+). De novo SCN1A variants do not necessarily imply a severe prognosis and have previously been associated with GEFS+ phenotypes (Myers et al. 2017). Two patients had febrile seizures only, one of whom (Patient 5) had an inherited variant from a father with a history of recurrent febrile seizures. For the other (Patient 317), inheritance status could not be determined. Six patients had variants predicted to result in truncation and eight had missense variants, but no genotype-phenotype association was observed in this cohort. One of the patients with a truncating variant (Patient 5) had febrile seizures only. Age at presentation had a similar distribution to that of the PRRT2-related seizures, with median onset at 6.5 months. In contrast to patients with PRRT2 variants, those with SCN1A variants were likely to present with febrile seizures and with status epilepticus. Half of the SCN1A patients presented with a febrile seizure and half with an afebrile seizure. The SCN1A phenotype observed in our cohort was in line with the previous literature on this genetic seizure disorder, which reports a tendency for early seizures to be associated with fever, a median initial seizure presentation at 6.0 months and a spectrum of disease severity. While the majority go on to develop a drug-resistant epilepsy and cognitive stagnation or decline (Harkin et al., 2007; Brunklaus et al., 2012) some, even if their variant has arisen de novo, may have mostly febrile seizures and a good cognitive outcome (Myers et al., 2017). While it is well established that there is a spectrum of SCN1A-related phenotypes, until now the relative proportions of mild versus severe cases has not been established because no study has used a population-based approach to SCN1A testing. Here we show that the majority of SCN1A phenotypes are in fact at the severe end of the spectrum. In our cohort, 11/14 patients were reported to have normal development at the time of presentation. At most recent follow-up five continued to have normal cognitive development, three had mild cognitive concerns and six had moderate cognitive concerns. Eighty-six per cent (12/14) of patients developed therapy-resistant seizures, making SCN1A the most common genetic cause of therapy resistant seizures in this cohort. The most frequently reported effective therapy was stiripentol (n = 4).
KCNQ2: self-limited (familial) neonatal seizures, early infantile developmental and epileptic encephalopathy
We identified 10 patients and calculate the minimum incidence as 1 per 17 000 live births (5.89/10 000; 95% CI 2.24–9.56). Seizures associated with KCNQ2 variants presented significantly earlier than the other single-gene epilepsies in this cohort (median 7 days). Five patients had a frameshift variant, two had a duplication of exons 2–12, two had whole gene deletions, and one had a de novo missense variant. The most severe epilepsy and developmental impairment was observed in the patient with the missense variant. The literature describes two distinct phenotypes associated with KCNQ2 variants: a self-limited familial neonatal seizure phenotype (Biervert et al., 1998; Singh et al., 2003) and a severe neonatal-onset developmental and epileptic encephalopathy (Weckhuysen et al., 2012; Kato et al., 2013; Olson et al., 2017). We also observed these two phenotypes but found that the majority of patients had a mild phenotype. Seven of 10 patients (70%) had self-limited neonatal or infantile-onset seizures, all of whom had normal cognitive development at most recent follow-up. Two patients had KCNQ2 encephalopathy, and one patient had an unclassified drug-resistant focal epilepsy of onset at 3 months and developed mild cognitive impairment.
Previous studies have identified that more severe phenotypes are observed in patients who carry missense variants in KCNQ2 (Weckhuysen et al., 2012; Kato et al., 2013; Olson et al., 2017). In contrast, the majority of familial self-limited cases are associated with truncating variants (Singh et al., 2003). We did not entirely observe this pattern. In our cohort only one patient had a missense variant (Patient 177). This patient had a severe developmental and epileptic encephalopathy, presenting with seizures at 30 days of age. The other patient with a severe developmental and epileptic encephalopathy had a whole gene deletion. We observed a number of patients presenting with focal seizures beyond the neonatal period (one at 3 months and two at 4 months). Post-neonatal presentation with KCNQ2-related seizures has been reported before (Millichap et al., 2017; Zeng et al., 2018). In our cohort early onset of seizures appeared to be associated with better outcomes. All six of those who presented at under 1 month of age had self-limited seizures and normal cognitive development. Inheritance status did not fully correlate with severity of phenotype. One patient with an inherited variant and an extensive family history of self-limited familial neonatal seizures had profound cognitive impairment (Patient 177), suggesting modifying factors were at play. Another patient (Patient 336) with a de novo variant, had self-limited neonatal seizures and a good cognitive outcome.
The most frequently-reported effective therapy was carbamazepine (n = 4), followed by phenobarbitone (n = 3).
SLC2A1: generalized seizures with gait ataxia (Glut1 deficiency)
We identified seven patients and calculate the minimum incidence as 1 per 24 300 live births (4.13/100 000; 95% CI 1.07–7.19). Four patients had truncating variants and three had missense variants. In contrast to all the other genetic causes, these patients were more likely to present with generalized seizures than with focal seizures. Six of seven patients presented with generalized seizures (three tonic-clonic, two myoclonic, one absence). Age at presentation was later than that observed with the other genetic causes, with a median seizure-onset of 12 months. This is slightly later than the median age of 8 months reported by Pong et al. (2012) in their study of 78 patients with SLC2A1-related seizures. The literature on SLC2A1-related phenotypes tends to emphasize the coexistence of early childhood seizures with other clinical features, most notably developmental delay, chorea, dystonia and microcephaly (Di Georgis and Veggiotti, 2013). Nonetheless, the observation that initial seizure presentation in these children is often with absence seizures or myoclonic seizures (Hully et al., 2015) prompted some authors to screen children with early onset absence epilepsy (EOAE) and epilepsy with myoclonic atonic seizures (EMA), for pathogenic variants in the SLC2A1 gene. The conclusions from these studies was that glucose 1 transporter (Glut1) deficiency was associated with a significant minority of these presentations, 12% (Arsov et al., 2012) and 5% (Mullen et al., 2011), respectively. In the seven patients with causative SLC2A1 variants in our cohort only two had myoclonic seizures at any time in their history, and only one has absence seizures. The most frequent presenting seizure type was generalized tonic clonic seizures, an observation supported by the study by Pong et al. (2012), which reported that a generalized tonic clonic seizure was the presenting seizure in 53% of patients with SLC2A1-related seizures.
Beyond the seizures, additional phenotypic features at initial presentation were uncommon. In only two patients was Glut1 deficiency suspected prior to genetic result and only one patient underwent diagnostic lumbar puncture. One patient had a marked four limb dystonia at presentation. All the others were thought to have normal motor function at the time of their seizure presentation. By the time their genetic result was known, and they were clinically re-reviewed, all six of these had developed a subtle gait ataxia. All seven patients are currently on the ketogenic diet, the established treatment of choice for Glut1 deficiency. Ketogenic diet was perceived to be effective in all cases. At most recent follow-up all seven patients were seizure-free. Four had normal cognition and three had mild cognitive concerns. Long-term follow-up of this cohort and comparison with historical cohorts may help determine whether early instigation of the ketogenic diet is associated with improved outcomes.
Therapy-response and precision therapy:
Seventy-six patients developed therapy resistant seizures, 36 of whom (47%) had a single-gene cause identified. Therapy-resistance was not observed in any patients with PRRT2 variants, and was seen in only 2/10 patients with KCNQ2 variants. Just one patient with SCL2A1-related seizures developed therapy-resistance. The good seizure outcome observed in Glut1 deficiency may be related to early establishment of the ketogenic diet. The only single-gene epilepsies for which more than two patients developed therapy-resistance were SCN1A (12 patients), CDKL5 (four patients), and PCDH19 (four patients). Between them, these three genes accounted for 20/76 (26%) of all therapy-resistant cases in this cohort. Literature review identified evidence to support specific treatment approaches for 64 (80%) of our 80 children with genetic diagnoses (Table 4).
Table 4.
Evidence from the literature to support gene-specific treatment approaches
| Gene | Recommendation(s) | Evidence base | Reference | ||||
|---|---|---|---|---|---|---|---|
| n | Study details | P-value | Evidence level | Recommendation grade | |||
| PRRT2 | Consider carbamazepine | 64 | Retrospective uncontrolled clinician-reported subjective treatment response analysis. | NC | III | C | Huang et al. (2015) |
| 24 | Retrospective uncontrolled clinician-reported subjective treatment response analysis. | NC | III | Ebrahimi-Fakhari et al. (2015) | |||
| SCN1A | Consider stiripentol | 36 | Placebo-controlled RCT of add-on therapy in Dravet syndrome (number with SCN1A variant not reported). | <0.0001 | 1B | A | Chiron et al. (2000) |
| 41 | Prospective observational study of long-term efficacy of add-on stiripentol in patients with Dravet syndrome (39 with SCN1A variant). | NC | III | Myers et al. (2018) | |||
| Consider cannabidiol | 120 | Multicentre double-blinded placebo-controlled RCT of add-on therapy in Dravet syndrome (114 with SCN1A variant). | 0.08 | 1B | A | Devinsky et al. (2017) | |
| Consider fenfluramine | 11 | Retrospective uncontrolled clinician-reported seizure-freedom. | NC | III | C | Ceulemans et al. (2012) | |
| Consider ketogenic diet | 20 | Retrospective uncontrolled clinician-reported seizure reduction in Dravet syndrome (number with SCN1A variant not reported). | NC | III | C | Caraballo et al. (2005) | |
| Consider levetiracetam | 28 | Open label uncontrolled trial of add-on lamotrigine in Dravet syndrome (16 with SCN1A variant). | 0.0001 | III | C | Striano et al. (2007) | |
| Consider topiramate | 18 | Open label uncontrolled trial of add-on topiramate in Dravet syndrome (number with SCN1A variant not reported). | NC | III | C | Coppola et al. (2002) | |
| Consider sodium valproate | 160 | Retrospective uncontrolled clinician-reported subjective treatment response analysis. | NC | III | C | Brunklaus et al. (2012) | |
| Avoid carbamazepine | 60 | Retrospective uncontrolled clinician-reported subjective treatment response analysis. | NC | III | C | Brunklaus et al. (2012) | |
| Avoid lamotrigine | 60 | Retrospective uncontrolled clinician-reported subjective treatment response analysis. | NC | III | C | Brunklaus et al. (2012) | |
| 21 | Uncontrolled unblinded trial of add-on lamotrigine in Dravet syndrome (number with SCN1A variant not reported). | NC | III | Guerrini et al. (1998) | |||
| KCNQ2 | Consider carbamazepine | 15 | Retrospective uncontrolled clinician report of seizure-freedom. | NC | III | C | Pisano et al. (2015) |
| Consider phenytoin | 15 | Retrospective uncontrolled clinician report of seizure-freedom. | NC | III | C | Pisano et al. (2015) | |
| SLC2A1 | Use ketogenic diet | 104 | Retrospective uncontrolled family-reported subjective treatment response analysis. | NC | III | C | Kass et al. (2016) |
| CDKL5 | Consider ketogenic diet | 82 | Retrospective uncontrolled family-reported subjective treatment response analysis. | NC | III | C | Lim et al. (2017) |
| PCDH19 | Consider clobazam | 58 | Retrospective uncontrolled clinician-reported treatment response analysis, 3 months after commencing therapy. | NC | III | C | Lotte et al. (2016) |
| SLC6A1 | Consider sodium valproate | 15 | Retrospective uncontrolled clinician-reported treatment response analysis. | NC | III | C | Johannesen et al. (2018) |
| SCN8A | Consider phenytoin | 0 | Functional study. Single cell patch clamp testing in ND/7 cells transfected with the epilepsy- associated variant (I1327V). | NC | III | C | Barker et al. (2016) |
| 4 | Retrospective uncontrolled clinician-reported subjective treatment response analysis. | NC | III | Boerma et al. (2016) | |||
| SCN2A (with seizure onset <3 mo of age) | Consider sodium channel blocking (SCB) drugs | 158 | Retrospective clinician-reported seizure-freedom. | 1 × 10−6 | III | C | Wolff et al. (2017) |
| POLG | Avoid sodium valproate | 43 | Retrospective clinician-reported hepatotoxicity. | NC | III | C | Anagnostou et al. (2016) |
| KCNT1 (in patients aged <4 y) | Consider trial of quinidine | 0 | Functional study. Single cell patch clamp testing in Xenopus laevis oocyte cells. | NC | III | None (conflicting evidence) | Milligan et al. (2014) |
| 6 | Single centre, inpatient, order randomized, blinded, placebo-controlled trial. | 0.15 | NA | Mullen et al. (2018) | |||
Papers were included if they were either randomized-controlled trials, provided supportive evidence from in vitro functional studies, or analysed a cohort of >10 patients specifically in relation to treatment response. Where multiple treatments were evaluated in the same cohort, evidence is presented in favour of the most efficacious therapy identified. See Supplementary material for a more detailed version of this table.
mo = months; NC = not calculated; y = years.
Discussion
In this study we have used a whole population prospective cohort design to determine the incidence of the more common single-gene epilepsies of early childhood. Recruitment to our cohort was consistent across all 24 centres, which represent all health facilities where young children are expected to present with seizures. Patient ascertainment and exclusion of duplicate reporting were well managed in the recruitment process by cross-referencing within clinical departments, attending physicians, specialist epilepsy nurses, EEG departments and the central genetic laboratory.
The panel of 104 established epilepsy-associated genes was designed to capture all the more commonly-implicated genes, with a specific focus on those for which precision treatment approaches exist. As some genetic epilepsies initially present with prolonged febrile seizures (Brunklaus et al., 2012; Higurashi et al., 2013; Ebrahimi-Fakhari et al., 2015) children with status, clusters of febrile seizures, and recurrent prolonged febrile seizures were included.
The approach to determination of diagnostic genetic results involved comprehensive review of genetic and phenotype data within a multidisciplinary environment. Genetic results were reported in accordance with UK best practice guidelines (Association for Clinical Genetic Science, 2017). Where DNA samples and/or phenotype details from other family members were considered relevant to variant interpretation these were requested. We agree with Anderson and Lassmann (2018) that variants cannot be considered in isolation from phenotypes and relevant variant interpretation requires a multidisciplinary approach.
The incidence of epilepsy in children under 36 months of age has previously been estimated in a US population-based cohort as 1 per 613 live births (Wirrell et al., 2012). Our cohort represents 1 per 495 live births in Scotland. Direct comparison between these cohorts is not appropriate as our study included children presenting with certain febrile seizure presentations and excluded those with established non-genetic causes. In our cohort, 163 patients presented with recurrent afebrile seizures before the age of 12 months, giving an estimated incidence of infantile-onset epilepsy of 1 per 1041 live births (96.0/100 000; 95% CI 81.8–112.0). This is comparable to the figure of 1 per 1240 live births derived from a 20-year population-based cohort in Helsinki (patients with structural-metabolic causes removed) (Gaily et al., 2016) and the figure of 1 per 1220 from a North London cohort (Eltze et al., 2013).
It is established that single-gene aetiology can be identified in a substantial proportion of patients presenting with early-childhood onset epilepsy. Berg et al. (2017) carried out a prospective study in which they aimed to determine aetiology in all patients with epilepsy presenting before the age of 3 years, regardless of severity. Participants were recruited from 17 epilepsy centres in the USA (Berg et al., 2017). Their study was not population-based and used a variety of testing methods that were not consistent between centres. They reported a diagnostic yield of 29.2% for those tested on epilepsy gene panels, and 27.8% for those tested by whole-exome sequencing. These yields are slightly higher than in our study. In Berg et al.’s study the majority of patients (266/446) without determined aetiology did not undergo any form of genetic testing so those results are likely to have been affected by a degree of ascertainment bias.
In comparison with some recently published studies of genetic testing in epilepsy (Heyne et al., 2018; Lindy et al., 2018) ours includes a smaller cohort of patients. The strength of the present study relates to case ascertainment. The broad inclusion criteria and proactive recruitment strategy applied allowed a better understanding of both the full phenotypic spectrum and the incidence of the single-gene epilepsies in childhood. For the most frequently encountered single-gene epilepsies in this cohort—namely, PRRT2, SCN1A, KCNQ2 and SLC2A1—we observed phenotypic spectra that were largely in keeping with the literature published previously. However, we demonstrated that these are more common than has been previously described. Previous reports have estimated the incidence of SCN1A-related Dravet syndrome in California (1 per 20 900 live births) (Wu et al., 2015) and in Denmark (1 per 22 000 live births) (Bayat et al., 2015) but neither study used prospective case ascertainment strategies. Our study estimates the incidence of SCN1A-related Dravet syndrome to be 1 per 15 500 live births (11 patients with SCN1A-related Dravet syndrome). The incidence of Glut1 deficiency has previously been estimated as 1 per 90 000 live births in Queensland (Coman et al., 2006) and 1 per 83 000 live births in Denmark (Larsen et al., 2015) compared with 1 per 24 300 live births in this study. These figures are not likely to represent a Scottish population-specific phenomenon since the majority of cases of Dravet syndrome and Glut1 deficiency are caused by de novo variants. Estimated incidences for PRRT2, CDKL5, DEPDC5, and PCDH19-related epilepsies are provided here for the first time.
Previous studies investigating the yield of NGS in epilepsy have found that the majority of diagnoses are concentrated in a small number of recurrently-implicated genes. Lindy et al. reported the results of testing >8500 patients using a 70-gene epilepsy panel. They quoted a yield of 15.4% (Lindy et al., 2018). As with our study >80% of their diagnoses were in the most frequently-implicated seven genes. Six of our seven most frequently-implicated genes were among their seven most frequently-implicated (DEPDC5 was not included in their panel). We identified a substantially higher rate of PRRT2 variants in this cohort than have been reported in previous studies (Helbig et al., 2016; Trump et al., 2016; Lindy et al., 2018). This is likely to reflect our inclusion of self-limited and pharmaco-responsive epilepsies that would not have previously been considered candidates for high throughput genetic testing.
For a number of reasons this study is likely to have underestimated the incidence of single-gene epilepsies in this group, so our incidence figures are best considered as minimum incidences. Some genes associated with epilepsy are not on our panel, and for some genes (e.g. SCN1A and KCNQ2) we were able to offer more comprehensive testing than for others through MLPA. Chromosomal microarray for deletions and duplications was not part of our routine testing strategy due to the low reported yield in this group, low penetrance of many epilepsy-associated variants, and absence of evidence that identification of chromosomal lesions supports a precision medicine approach. Though the inclusion criteria for our study were broad, we are likely to have missed some patients with very mild phenotypes—e.g. recurrent simple febrile seizures—who did not meet eligibility criteria. The identical twin of Patient 334 had the same PRRT2 variant as her sister but was not eligible for inclusion since all her seizures were febrile and <10 min duration. Similarly, SCN1A-related disease can present with simple febrile seizures only (Escayg et al., 2000). A complex febrile seizure has been defined by some authors as a febrile seizure that has any one of the following elements: focal features, duration >15 min, recurring more than once in 24 h, or associated with postictal palsy or previous neurological deficits (Capovilla et al., 2009). Our criteria did not include children with febrile seizures with focal features lasting <10 min or those with a single febrile seizure lasting between 10 and 30 min as duration and frequency of febrile seizure were considered more reliable clinical predictors of SCN1A, PCDH19 and PRRT2 variants.
We are likely to have underestimated the incidence of SLC2A1-related disease as not all patients with Glut1 deficiency will present with seizures in the first 3 years of life. The same is true for several other genetic epilepsies and neurodevelopmental disorders, including CDKL5, DEPDC5, POLG, SCN2A, MECP2, KCNT1 and GABRA1. According to our protocol patients were not recruited to the study if they had an aetiology identified either prior to or at initial presentation with seizures. As a result, patients are likely to have not been recruited if they had acute neuroimaging findings that were deemed to explain their epilepsy, even if such findings may indeed have an underlying genetic basis. The most notable example of this would be tuberous sclerosis, caused by TSC1/TSC2 variants, where neuroimaging findings are often highly typical for this genetic disorder and the diagnosis may be known prior to onset of seizures. Patients with other genetically determined developmental brain malformations may also not have been recruited. Neonates with symptomatic seizures secondary to hypoxic ischaemic encephalopathy (HIE) at birth were not recruited. Rarely, genetic metabolic disorders such as pyridoxine dependency, sulphite oxidase deficiency, or molybdenum co-factor deficiency may mimic HIE (Baxter, 1999). We would expect these children to continue to have seizures beyond the neonatal period and to be seen within one of Scotland’s tertiary child neurology centres. Finally, we only tested DNA samples derived from blood and it has been shown that some genetic epilepsies are due to somatic variants (Nellist et al., 2015).
Evidence from randomized controlled trials (RCTs), open label trials, retrospective case series, and from in vitro functional studies, informs clinicians’ treatment choice when they make a diagnosis of a single-gene epilepsy. Such evidence exists for SCN1A, PRRT2, KCNQ2, SLC2A1, PCDH19, POLG, SCN2A, and SCN8A (Table 4). On this basis we estimate that 64/80 (80%) of the single-gene diagnoses made in this study had potential treatment implications. In the case of Glut1 deficiency, early diagnosis and implementation of the ketogenic diet may have an impact on developmental and motor comorbidity as well as seizure control (Kass et al., 2016). In SCN1A-related epilepsy, duration of use of contraindicated sodium-channel blocking medication is associated with adverse developmental outcome (de Lange et al., 2018). Additional benefits of early genetic diagnosis in epilepsy include providing information for genetic counselling (Krabbenborg et al., 2016), giving answers for affected families (Brunklaus et al., 2013; Sawyer et al., 2016; Wynn et al., 2018), and avoidance of additional costly and invasive investigations. Recent economic analyses have demonstrated that the application of early high throughput genetic testing could save $5236 Australian dollars (Palmer et al., 2018), or $7047 US dollars (Howell et al., 2018) per diagnosis when compared with investigation programs that involved extensive imaging and metabolic testing prior to genetic testing.
Evidence in support of precision therapy in epilepsy varies in level and nature. In 2019, the majority of truly medically ‘actionable’ genetic diagnoses in epilepsy relate to inherited disorders of metabolism such as Glut1 deficiency, and pyridoxine dependency (Peng et al., 2019). Questions remain unanswered in relation to targeted treatment of other genetic causes of epilepsy. Much of the evidence that we have presented to support gene-specific therapy approaches in Table 4 is at level III. Non-RCT evidence is compromised by the absence of control groups, variability in timing and objectivity of response analysis, and inconsistent reporting of concomitant drug use. As exemplified by KCNT1-related seizures, evidence can be conflicting. Here, despite positive anecdotal reports of benefit from quinidine (Bearden et al., 2014; Fukuoka et al., 2017; Abdelnour et al., 2018) and supportive in vitro functional studies (Milligan et al., 2014) a small randomized-controlled crossover trial demonstrated no significant benefit in adult patients with KCNT1-related frontal lobe epilepsy (Mullen et al., 2018). Nevertheless, in reality, many clinicians if faced with a child with unremitting seizures associated with a KCNT1 variant may be inclined to at least give a trial of quinidine—a drug that they would be unlikely to use for seizures in any other scenario. The recommendation grade for specific therapy in most of the genetic epilepsies is grade C. Nonetheless it is important to note that such evidence is the basis of therapy choice in almost all epilepsy syndromes and may provide a key lead in to definitive trials, as has been the case with fenfluramine in Dravet syndrome (NIH US National Library of Medicine, 2019). Findings from RCTs must also be interpreted in context. In Dravet syndrome cannabidiol demonstrates efficacy (Devinsky et al., 2017); however, there is no biological reason why cannabidiol should be specifically effective in this condition because it does not appear to act on sodium channels or GABA receptors (Devinsky et al., 2014). Cannabidiol also has efficacy in Lennox Gastaut syndrome (French et al., 2017; Devinsky et al., 2018), an epilepsy with varied aetiologies. Several other broadly-acting anti-epileptic therapies including levetiracetam, topiramate, and the ketogenic diet have performed just as well as cannabidiol in open label uncontrolled studies of Dravet syndrome, but as they have not been tested in RCT format they are considered less evidence-based (Coppola et al., 2002; Caraballo et al., 2005; Striano et al., 2007; Devinsky et al., 2018). In contrast the ketogenic diet is regarded as the gold standard therapy in Glut1 deficiency in the absence of any RCT data. Obtaining good quality evidence for gene-specific treatment approaches in epilepsy is perceived as a challenge, since many of these disorders are exceedingly rare. To this end, defining the incidence of the more common difficult to treat genetic epilepsies of childhood is an important step. Orphan medicinal products have been developed and licensed for many rarer conditions than the genetic epilepsies (European Joint Programme Rare Diseases, 2019). Gene therapy approaches, which may provide definitive precision therapy, are being trialled in rodent and non-human primate models of human genetic epilepsies (Berkovic et al., 2015). In this study, 36/80 patients with single-gene epilepsy had therapy-resistant seizures. Of these 20 (56%) were associated with just three genes, SCN1A, CDKL5, and PCDH19. For maximum benefit, these are the genetic epilepsies that should be prioritized in the development of precision therapy.
Study limitations
Although we aimed to include all children with epilepsy presenting in Scotland under the age of 3 years, it is possible that some patients were unreported and not included. Therefore, all incidences reported in this study should be viewed as minimum estimates.
Children with epilepsy due to other identifiable causes such as hypoxic ischaemic encephalopathy, meningitis, metabolic disorders, etc. were excluded. However, some of these patients may have had genetic ‘mimics’ of acquired causes, genetic causes of structural brain abnormalities, or compound genetic-acquired aetiologies. Their exclusion may have reduced the yield of genetic diagnoses in this study.
The health economics of genetic testing in this group of children were not examined, but it is possible that early identification of a genetic diagnosis would save other costly investigations.
This cohort is being followed up to determine whether there is a positive impact of early genetic diagnosis and treatment on a child’s neurodevelopment and comorbidities. However, this study will take several years to report its conclusions.
Conclusions
Single-gene epilepsies are more common than previously reported, with a collective minimum incidence of about 1 per 2000 live births. Many of the cases identified in this study are dominant genetic epilepsies due to de novo mutations. Therefore these minimum incidence figures are applicable to other populations and are not specific for Scotland.
Our data suggest that genetic testing should be a primary investigation for epilepsies presenting in early childhood. The nature of genetic testing will depend upon available resources. Eighty per cent of genetic diagnoses in this group relate to eight genes, with other genetic aetiologies likely to be individually extremely rare. A clinically relevant and economically efficient testing paradigm would be to analyse a small panel of genes and if this is unrevealing move to a larger platform such as clinical exome, whole exome or whole genome.
Funding
This study was supported by grants from Epilepsy Research UK and Dravet Syndrome UK. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Competing interests
The authors report no competing interests.
Supplementary Material
References
- Abdelnour E, Gallentine W, McDonald M, Sachdev M, Jiang Y, Mikati MA. Does age affect response to quinidine in patients with KCNT1 mutations? Report of three new cases and review of the literature. Seizure 2018; 55: 1–3. [DOI] [PubMed] [Google Scholar]
- Anagnostou ME, Shiau NY, Taylor RW, McFarland R. Epilepsy due to mutations in the mitochondrial polymerase gamma (POLG) gene: a clinical and molecular genetic review. Epilepsia 2016; 57: 1531–45. [DOI] [PubMed] [Google Scholar]
- Anderson D, Lassmann T. A phenotype centric benchmark of variant prioritisation tools. NPJ Genom Med 2018; 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller S, Balling R, Barisic N, Baulac S, Caglayan H, Craiu D et al. De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies. Am J Hum Genet 2014; 95: 360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsov T, Mullen SA, Damiano JA, Lawrence KM, Huh LL, Nolan M et al. Early-onset absence epilepsy: 1 in 10 cases is caused by GLUT1 deficiency. Epilepsia 2012; 53: e204–7. [DOI] [PubMed] [Google Scholar]
- Association for Clinical Genetic Science 2017. Practice guidelines for the evaluation of pathogenicity and the reporting of sequence variants in clinical molecular genetics. http://www.acgs.uk.com(April 2018, date last accessed).
- Barker BS, Ottolin M, Wagnon JL, Hollander RM, Meisler MH, Patel MK. The SCN8A encephalopathy mutation p.Ile1327Val displays elevated sensitivity to the anticonvulsant phenytoin. Epilepsia 2016; 57: 1458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter P. Epidemiology of pyridoxine dependent and pyridoxine responsive seizures in the UK. Arch Dis Child 1999; 81: 431–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat A, Helle H, Møller RS. The incidence of SCN1A‐related Dravet syndrome in Denmark is 1:22,000: a population‐based study from 2004 to 2009. Epilepsia 2015; 56: e36–9. [DOI] [PubMed] [Google Scholar]
- Bearden D, Strong A, Ehnot J, DiGiovine M, Dlugos D, Goldberg E. Targeted treatment of migrating partial seizures with Quinidine. Ann Neurol 2014; 76: 457–61. [DOI] [PubMed] [Google Scholar]
- Berg AT, Coryell J, Saneto RP, Grinspan ZM, Alexander JJ, Kekis M et al. Early-life epilepsies and the emerging role of genetic testing. JAMA Pediatr 2017; 171: 863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Langfitt JT, Testa FM, Levy SR, Di Mario F, Westerfield M et al. Global cognitive function in children with epilepsy: a community-based study. Epilepsia 2008; 49: 608–14. [DOI] [PubMed] [Google Scholar]
- Berg AT, Zelko FA, Levy SR, Testa FM. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes. Neurology 2012; 79: 1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovic SF, Scheffer IE, Petrou S, Delanty N, Dixon-Salazar TJ, Dlugos TJ et al. A roadmap for precision medicine in the epilepsies. Lancet Neurol 2015; 14: 1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P et al. A potassium channel mutation in neonatal human epilepsy. Science 1998; 279: 403–6 [DOI] [PubMed] [Google Scholar]
- Boerma RS, Braun KP, van den Broek MPH, van Berkestijn FMC, Swinkels ME, Hagebeuk EO et al. Remarkable phenytoin sensitivity in 4 children with SCN8A-related epilepsy: a molecular neuropharmacological approach. Neurotherapeutics 2016; 13: 192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunklaus A, Dorris L, Ellis R, Reavey E, Lee E, Forbes G et al. The clinical utility of an SCN1A genetic diagnosis in infantile-onset epilepsy. Dev Med Child Neurol 2013; 55: 154–61. [DOI] [PubMed] [Google Scholar]
- Brunklaus A, Ellis R, Reavey E, Forbes G, Zuberi SM. Prognostic, clinical and demographic features in SCN1A mutation-positive Dravet syndrome. Brain 2012; 135: 2329–36. [DOI] [PubMed] [Google Scholar]
- Capovilla G, Mastrangelo M, Romeo AVigevano. Recommendations for the management of febrile seizures. Epilepsia 2009; 50 (Suppl 1): 2–6. [DOI] [PubMed] [Google Scholar]
- Caraballo RH, Cersósimo RH, Sakr D, Cresta A, Escobal N, Fejerman N. Ketogenic diet in patients with Dravet syndrome. Epilepsia 2005; 46: 1539–44. [DOI] [PubMed] [Google Scholar]
- Ceulemans B, Boel M, Leyssens K, van Rossem C, Neels P, Jorens PG et al. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia 2012; 53: 1131–9. [DOI] [PubMed] [Google Scholar]
- Chiron C, Marchand MC, Tran A, Rey E, d‘Athis P, Vincent J et al. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet 2000; 356: 1638–42. [DOI] [PubMed] [Google Scholar]
- Coman DJ, Sinclair KG, Burke CJ, Appleton DB, Pelekanos JT, O‘Neil CM et al. Seizures, ataxia, developmental delay and the general paediatrician: glucose transporter 1 deficiency syndrome. J Paediatr Child Health 2006; 42: 263–7. [DOI] [PubMed] [Google Scholar]
- Coppola G, Capovilla G, Montagnini A, Romeo A, Spanò M, Tortorella G et al. Topiramate as add-on drug in severe myoclonic epilepsy of infancy: an Italian multicentre open trial. Epilepsy Res 2002; 49: 45–8. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Cilio MR, Cross JH, Ferdnandez-Ruiz J, French J, Hill C et al. Cannabidiol: Pharmacology and therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014; 55: 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R et al. Trial of cannabidiol for drug-resistant seizures in the Dravet Syndrome. N Engl J Med 2017; 376: 2011–20. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut Syndrome. N Engl J Med 2018; 378: 1888–97. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, Wright S et al. Long-term cannabidiol treatment in patients with Dravet syndrome: an open-label extension trial. Epilepsia 2018; 60: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange IM, Boudewijn G, Sonsma AC, Lisette G, Marjan K, Verbeek NE et al. Influence of contraindicated medication use on cognitive outcome in Dravet syndrome and age at first afebrile seizure as a clinical predictor in SCN1A-related seizure phenotypes. Epilepsia 2018; 59: 1154–65. [DOI] [PubMed] [Google Scholar]
- Di Giorgis V, Veggiotti P. GLUT1 deficiency syndrome in 2013: current state of the art. Seizure 2013; 22: 803–11. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Saffari A, Westenberger A, Klein C. The evolving spectrum of PRRT2-associated paroxysmal diseases. Brain 2015; 138: 3476–95. [DOI] [PubMed] [Google Scholar]
- Eltze CM, Chong WK, Cox T, Whitney A, Cortina-Borja M, Chin RFM et al. A population-based study of newly diagnosed epilepsy in infants. Epilepsia 2013; 54: 437–45. [DOI] [PubMed] [Google Scholar]
- Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nature Genet 2000; 24: 343–5. [DOI] [PubMed] [Google Scholar]
- European Joint Programme Rare Diseases, 2019. www.ejprarediseases.org (June 2019, date last accessed).
- Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon C, Dykeman J et al. Prevalence and incidence of epilepsy. Neurology 2017; 88: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J, Thiele E, Mazurkiewicz-Beldzinska M, Benbadis S, Marsh E, Joshi C et al. Cannabidiol (cannabidiol) significantly reduces drop seizure frequency in Lennox-Gastaut syndrome (LGS): results of a multi-center, randomized, double-blind, placebo controlled trial (GWPCARE4) (S21.001). Neurology 2017; 88: 16. [Google Scholar]
- Fukuoka M, Kuki I, Kawawaki H, Okazaki S, Kin K, Hattori Y et al. Quinidine therapy for West syndrome with KCNT1 mutation: a case report. Brain and Dev 2017; 39: 80–83. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Dravet C, Genton P, Belmonte A, Kaminska A, Dulac O. Lamotrigine and seizure aggravation in Dravet syndrome. Epilepsia 1998; 39: 508–12. [DOI] [PubMed] [Google Scholar]
- Gaily E, Lommi M, Lapatto R, Lehesjoki A. Incidence and outcome of epilepsy syndromes with onset in the first year of life: A retrospective population-based study. Epilepsia 2016; 57: 1594–601. [DOI] [PubMed] [Google Scholar]
- Harkin LA, McMahon JM, Iona X, Dibbens L, Pelekanos JT, Zuberi SM et al. The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain 2007; 130: 843–52 [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 1993; 34: 453–8. [DOI] [PubMed] [Google Scholar]
- Helbig KL, Farwell Hagman KD, Shinde DN, Mroske C, Powis Z, Li S et al. Diagnostic exome sequencing provides a molecular diagnosis for a significant proportion of patients with epilepsy. Genet Med 2016; 18: 898. [DOI] [PubMed] [Google Scholar]
- Heyne HO, Singh T, Stamberger H, Abou Jamra R, Caglayan H, Craiu D et al. De novo variants in neurodevelopmental disorders with epilepsy. Nat Genet 2018; 50: 1048–53. [DOI] [PubMed] [Google Scholar]
- Higurashi N, Nakamura M, Sugai M, Ohfu M, Sakauchi M, Sugawara Y et al. PCDH19-related female-limited epilepsy: further details regarding early clinical features and therapeutic efficacy. Epilep Res 2013; 106: 191–9. [DOI] [PubMed] [Google Scholar]
- Howell KB, Eggers S, Dalziel K, Riseley J, Mandelstam S, Myers CT et al. A population-based cost-effectiveness study of early genetic testing in severe epilepsies of infancy. Epilepsia 2018; 59: 1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Wang T, Wang J, Liu X, Che X, Li J et al. Paroxysmal kinesigenic dyskinesia: clinical and genetic analyses of 110 patients. Neurology 2015; 85: 1546–553. [DOI] [PubMed] [Google Scholar]
- Hully M, Vuillaumier-Barrot S, Le Bizec C, Boaddaert N, Kaminska A et al. From splitting GLUT1 deficiency to overlapping phenotypes. Eur J Med Genet 2015; 58: 443–54 [DOI] [PubMed] [Google Scholar]
- Johannesen KM, Elena G, Tarja L, Carolina C, Saint MA, Lehesjoki A-E et al. Defining the phenotypic spectrum of SLC6A1 mutations. Epilepsia 2018; 59: 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass HR, Winesett SP, Bessone SK, Turner Z, Kossoff EH. Use of dietary therapies amongst patients with GLUT1 deficiency syndrome. Seizure 2016; 35: 83–7. [DOI] [PubMed] [Google Scholar]
- Kato M, Yamagata T, Kubota M, Arai H, Yamashita S, Nakagawa T et al. Clinical spectrum of early onset epileptic encephalopathies caused by KCNQ2 mutation. Epilepsia 2013; 54: 1282–7. [DOI] [PubMed] [Google Scholar]
- Krabbenborg L, Schieving J, Kleefstra T, Vissers LELM, Willemsen MA, Veltman JA et al. Evaluating a counselling strategy for diagnostic WES in paediatric neurology: an exploration of parents’ information and communication needs. Clin Genet 2016; 89: 244–50. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Eng J of Med 2000; 342: 314–9. [DOI] [PubMed] [Google Scholar]
- Larsen J, Johannesen KM, Ek J, Tang S, Marini C, Blichfeldt S et al. The role of SLC2A1 mutations in myoclonic astatic epilepsy and absence epilepsy, and the estimated frequency of GLUT1 deficiency syndrome. Epilepsia 2015; 56: e203–8. [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016; 536: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke JR, Riesch E, Scheurenbrand T, Schubach M, Wilhelm C, Steiner I et al. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia 2012; 53: 1387–98. [DOI] [PubMed] [Google Scholar]
- Lim Z, Wong K, Olson HE, Bergin AM, Downs J, Leonard H. Use of the ketogenic diet to manage refractory epilepsy in CDKL5 disorder: Experience of >100 patients. Epilepsia 2017; 58: 1415–22. [DOI] [PubMed] [Google Scholar]
- Lindy AS, Stosser MB, Bulter E, Downtain‐Pickersgill C, Shanmugham A, Retterer K et al. Diagnostic outcomes for genetic testing of 70 genes in 8565 patients with epilepsy and neurodevelopmental disorders. Epilepsia 2018; 59: 1062–71. [DOI] [PubMed] [Google Scholar]
- Lotte J, Bast T, Borusiak P, Coppola A, Cross JH, Dimova P et al. Effectiveness of antiepileptic therapy in patients with PCDH19 mutations. Seizure 2016; 35: 106–10. [DOI] [PubMed] [Google Scholar]
- Milligan CJ, Li M, Gazina EV, Heron SE, Nair U, Trager C et al. KCNT1 gain of function in 2 epilepsy phenotypes is reversed by quinidine. Ann Neurol 2014; 75: 581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Records of Scotland, 2018. Births time series data 1855 to 2016. www.nrscotland.gov.uk(April 2018, date last accessed).
- Millichap JJ, Miceli F, De Maria M, Keator C, Joshi N, Tran B et al. Infantile spasms and encephalopathy without preceding neonatal seizures causes by KCNQ2 R198Q, a gain-of-function variant. Epilepsia 2017; 58: e10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen SA, Carney PW, Roten A, Ching M, Lightfoot PA, Churilov L et al. Precision therapy for epilepsy due to KCNT1 mutations. Neurology 2018; 90: e67–72. [DOI] [PubMed] [Google Scholar]
- Mullen SA, Marini C, Suls A, Mei D, Della Giustina E et al. Glucose 1 transporter deficiency as a treatable cause of myoclonic astatic epilepsy. Arch Neurol 2011; 68: 1152–5. [DOI] [PubMed] [Google Scholar]
- Myers KA, Burgess R, Afawi Z, Damiano JA, Berkovic SF et al. De novo pathogenic variants in the GEFS+ spectrum: Not always a familial syndrome. Epilepsia 2017; 58: e26–30 [DOI] [PubMed] [Google Scholar]
- Myers KA, Lightfoot P, Patil SG, Cross JH, Scheffer IE. Stiripentol efficacy and safety in Dravet syndrome: a 12 year observational study. Dev Med Child Neurol 2018; 60: 574–8. [DOI] [PubMed] [Google Scholar]
- Nellist M, Schot R, Hoogeveen-Westerveld M, Neuteboom RF, van der Louw EJTM, Lequin MH et al. Germline activating AKT3 mutation associated with megalencephaly, polymicrogyria, epilepsy and hypoglycaemia. Mol Genet Metab 2015; 114: 467–73. [DOI] [PubMed] [Google Scholar]
- Ngugi AK, Christian B, Immo K, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia 2010; 51: 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH US National Library of Medicine, 2019. www.clinicaltrials.gov, 2018. (June 2019, date last accessed).
- Olson HE, McKenna K, LaCoursiere C, Pinsky R, Tambunan D, Shain C et al. Genetics and genotype-phenotype correlations in early onset epileptic encephalopathy with burst suppression. Ann Neurol 2017; 81: 419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer EE, Deborah S, Rupendra S, Tejaswi K, Rebecca M, Lawson JA et al. Integrating exome sequencing into a diagnostic pathway for epileptic encephalopathy: evidence of clinical utility and cost effectiveness. Mol Genet Genomic Med 2018; 6: 189–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Pang N, Wang Y, Wang X-L, Chen J, Xiong J et al. Next-generation sequencing improves treatment efficacy and reduces hospitalization in children with drug-resistant epilepsy. CNS Neursci Therap 2019; 25: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano T, Numis AL, Heavin SB, Weckhuysen S, Angriman M, Suls A et al. Early and effective treatment of KCNQ2 encephalopathy. Epilepsia 2015; 56: 685–91. [DOI] [PubMed] [Google Scholar]
- Pong AW, Geary BR, Engelstad KM, Natarajan A, Yang H, De Vivo DC. Glucose 1 transporter deficiency syndrome: epilepsy phenotypes and outcomes. Epilepsia 2012; 53: 1503–10. [DOI] [PubMed] [Google Scholar]
- Sawyer SL, Hartley T, Dyment DA, Beaulieu CL, Schwartzentruber J, Smith A et al. Utility of whole-exome sequencing for those near the end of the diagnostic odyssey: time to address gaps in care. Clin Genet 2016; 89: 275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia 2017; 58: 512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NA, Westenskow P, Charlier C, Pappas C, Leslie J, Dillon J et al. KCNQ2 and KCNQ3 potassium channel genes in benign familial neonatal convulsions: expansion of the functional and mutation spectrum. Brain 2003; 126: 2726–37 [DOI] [PubMed] [Google Scholar]
- Striano P, Coppola A, Pezzella M, Ciampa C, Specchio N, Ragona F et al. An open-label trial of levetiracetam in severe myoclonic epilepsy of infancy. Neurology 2007; 69: 250–4. [DOI] [PubMed] [Google Scholar]
- The Broad Institute: Cambridge MA 2018. gnomAD Browser. http://gnomad.broadinstitute.org/(April 2018, date last accessed).
- Trump N, McTague A, Brittain H, Papandreou A, Meyer E, Ngoh A et al. Improving diagnosis and broadening the phenotypes in early-onset seizure and severe developmental delay disorders through gene panel analysis. J Med Genet 2016; 53: 310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol 2012; 71: 15–25. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Silvana M, Asawari H, Genevieve R, Wrennall JAClaireet al. Developmental outcomes of childhood-onset temporal lobe epilepsy: a community-based study. Epilepsia 2012; 53: 1587–96. [DOI] [PubMed] [Google Scholar]
- Wirrell E, Wong‐Kisiel L, Mandrekar J, Nickels K. Predictors and course of medically intractable epilepsy in young children presenting before 36 months of age: a retrospective, population‐based study. Epilepsia 2012; 53: 1563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M, Johannesen KM, Hedrich UBS, Masnada S, Rubboli G, Gardella E et al. Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders. Brain 2017; 140: 1316–36. [DOI] [PubMed] [Google Scholar]
- Wu YW, Sullivan J, McDaniel SS, Meisler MH, Walsh EM, Li SX et al. Incidence of Dravet syndrome in a US population. Pediatrics 2015; 136: e1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn J, Ottman R, Duong J, Wilson AL, Ahimaz P, Martinez J et al. Diagnostic exome sequencing in children: a survey of parental understanding, experience and psychological impact. Clin Genet 2018; 93: 1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Yang X, Zhang J, Liu A, Yang Z, Liu Z et al. Genetic analysis of benign familial epilepsies in the first year of life in a Chinese cohort. J Hum Genet 2018; 63: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.