Abstract
Safety of pregnancy in women with history of estrogen receptor (ER)–positive breast cancer remains controversial. In this multicenter case–control study, 333 patients with pregnancy after breast cancer were matched (1:3) to 874 nonpregnant patients of similar characteristics, adjusting for guaranteed time bias. Survival estimates were calculated using the Kaplan-Meier analysis; groups were compared with the log-rank test. All reported P values were two-sided. At a median follow-up of 7.2 years after pregnancy, no difference in disease-free survival was observed between pregnant and nonpregnant patients with ER-positive (hazard ratio [HR] = 0.94, 95% confidence interval [CI] = 0.70 to 1.26, P = .68) or ER-negative (HR = 0.75, 95% CI = 0.53 to 1.06, P = .10) disease. No overall survival (OS) difference was observed in ER-positive patients (HR = 0.84, 95% CI = 0.60 to 1.18, P = .32); ER-negative patients in the pregnant cohort had better OS (HR = 0.57, 95% CI = 0.36 to 0.90, P = .01). Abortion, time to pregnancy, breastfeeding, and type of adjuvant therapy had no impact on patients’ outcomes. This study provides reassuring evidence on the long-term safety of pregnancy in breast cancer survivors, including those with ER-positive disease.
Many physicians and patients remain concerned about the safety of pregnancy in breast cancer survivors, especially in women previously diagnosed with estrogen receptor (ER)–positive disease in whom pregnancy could be regarded as potentially detrimental by means of endocrine stimulation (1–3). Prior results of our study showed that a subsequent pregnancy had no negative impact on breast cancer outcomes irrespective of ER status; however, median follow-up was relatively short (4.7 years after pregnancy) (4). Considering that women with ER-positive disease remain at increased risk of long-term recurrence (5), these results might not have provided the needed reassurance regarding the safety of pregnancy in these patients. Here, we provide updated survival analysis at median follow-up of 7.2 years after pregnancy.
Details of study design and statistical analysis were previously reported (4). Briefly, this is a multicenter case–control study in which women who had a pregnancy after breast cancer (pregnant cohort) were matched (1:3) to nonpregnant patients (nonpregnant cohort) according to ER status, nodal status, adjuvant treatments, age, and year of diagnosis. To adjust for guaranteed time bias, each nonpregnant patient should have been disease-free for a minimum time not inferior to the time elapsing between breast cancer diagnosis and pregnancy in the matched pregnant case. The study was approved by the ethics committees of all participating institutions. No written informed consent was required.
The primary objective was disease-free survival (DFS) in ER-positive patients. DFS was calculated from the date of conception (or a similar time point in the nonpregnant cohort). DFS in ER-negative patients was secondary end point, as was overall survival (OS). Predefined subgroup analysis investigated the impact of abortion, time to pregnancy, and breastfeeding on DFS. Exploratory analyses included DFS according to prior exposure to chemotherapy and duration of adjuvant endocrine therapy in ER-positive patients in the pregnant cohort.
OS and DFS estimates were calculated using Kaplan-Meier analysis, and groups were compared with the log-rank test. Cox regression semiparametric models were used to calculate the hazard ratios (HRs; pregnant vs nonpregnant) with 95% confidence intervals (CIs). The cutoff date for analysis was December 2016. All reported P values were two-sided, with values of less than .05 considered statistically significant.
A total of 333 and 874 women were included in the pregnant and nonpregnant cohorts, respectively, of whom 56.8% (n = 686) had ER-positive breast cancer (4). Baseline characteristics were well balanced, with the exception of younger age and more frequent breast-conserving surgery in the pregnant cohort (4). Table 1 summarizes the characteristics of the pregnant cohort.
Table 1.
Characteristics of pregnancies among patients in the pregnant cohort (n = 333)
| Characteristics | All patients(n = 333) No. (%) | ER-positive patients (n = 194) No. (%) | ER-negative patients (n = 139) No. (%) | P* |
|---|---|---|---|---|
| Pregnancy outcome | ||||
| Completed pregnancy | 188 (56.5) | 110 (56.7) | 78 (56.1) | .69 |
| Abortion | 135 (40.5) | 76 (39.2) | 59 (42.5) | |
| Induced abortion† | 91 (67.4) | 53 (69.7) | 38 (64.4) | |
| Spontaneous abortion† | 22 (16.3) | 12 (15.8) | 10 (17.0) | |
| Unknown† | 22 (16.3) | 11 (14.5) | 11 (18.6) | |
| Unknown | 10 (3.0) | 8 (4.1) | 2 (1.4) | |
| Pregnancy interval | ||||
| <2 y from diagnosis | 140 (42.0) | 73 (37.6) | 67 (48.2) | .06 |
| ≥2 y from diagnosis | 193 (58.0) | 121 (62.4) | 72 (51.8) | |
| 2–5 y‡ | 139 (72.0) | 77 (63.6) | 62 (86.1) | |
| >5 y‡ | 54 (28.0) | 44 (36.4) | 10 (13.9) | |
| Breastfeeding status§ | ||||
| Yes | 25 (13.3) | 21 (19.1) | 4 (5.1) | .05 |
| No | 39 (20.7) | 23 (20.9) | 16 (20.5) | |
| Unknown | 124 (66.0) | 66 (60.0) | 58 (74.4) |
Chi-square test or Fisher’s exact test with two-sided P values for the comparison between ER-positive and ER-negative patients calculated by excluding unknown data. ER = estrogen receptor.
Percentages calculated on the total number of patients with abortion (135 in all patients, 76 in women with ER-positive breast cancer, and 59 in those with ER-negative disease).
Percentages calculated on the total number of patients who became pregnant two or more years from diagnosis (193 in all patients, 121 in women with ER-positive breast cancer, and 72 in those with ER-negative disease).
Percentages calculated on the total number of patients with completed pregnancy (188 in all patients, 110 in women with ER-positive breast cancer, and 78 in those with ER-negative disease).
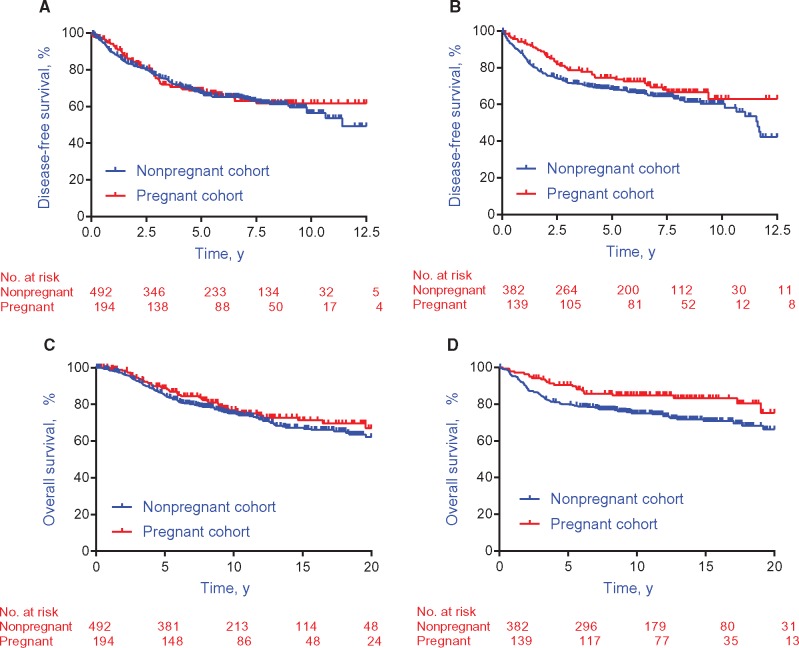
The median follow-up was 9.6 years from breast cancer diagnosis (7.2 years after pregnancy, interquartile range = 4.8 to 8.7 years). No DFS difference was shown between the pregnant and nonpregnant cohorts in ER-positive (HR = 0.94, 95% CI = 0.70 to 1.26, P = .68) (Figure 1A) or ER-negative (HR = 0.75, 95% CI = 0.53 to 1.06, P = .10) (Figure 1B) patients. Similarly, there was no OS difference between the two cohorts in ER-positive patients (HR = 0.84, 95% CI = 0.60 to 1.18, P = .32) (Figure 1C). Yet, a better OS was observed in the pregnant cohort for ER-negative patients (HR = 0.57, 95% CI = 0.36 to 0.90, P = .01) (Figure 1D).
Figure 1.
Survival outcomes in the pregnant and nonpregnant cohorts. A) Disease-free survival in estrogen receptor–positive breast cancer patients (n = 686). B) Disease-free survival in estrogen receptor–negative breast cancer patients (n = 521). C) Overall survival in estrogen receptor–positive breast cancer patients (n = 686). D) Overall survival in estrogen receptor–negative breast cancer patients (n = 521).
Predefined subgroup analyses did not suggest any impact of abortion, time to pregnancy, or breastfeeding on DFS. Pregnancy outcome was available in 323 (97.0%) women in the pregnant cohort. As compared with matched women from the nonpregnant cohort, no difference in DFS was observed in patients who completed their pregnancy (HR = 0.85, 95% CI = 0.63 to 1.14, P = .27), nor in those who had an abortion (HR = 0.80, 95% CI = 0.56 to 1.13, P = .20) (Supplementary Figure 1A, available online), with no statistically significant heterogenity observed (Pinteraction = .80).
An interval of two years was used to evaluate the potential impact of time to pregnancy after breast cancer diagnosis on DFS. As compared with matched women from the nonpregnant cohort, a statistically significantly better DFS was shown in patients who became pregnant less than two years from breast cancer diagnosis (HR = 0.65, 95% CI = 0.47 to 0.90, P = .008), and no difference in DFS was observed in patients who became pregnant two or more years from breast cancer diagnosis (HR = 1.12, 95% CI = 0.82 to 1.54, P = .47) (Supplementary Figure 1B, available online), with statistically heterogeneity observed (Pinteraction = .02). As previously reported (4), patients in the nonpregnant group who were matched with those who became pregnant two or more years from diagnosis tended to have a relatively longer disease-free interval as compared with women who were matched with patients who became pregnant less than two years from diagnosis. On comparing the outcome of patients who became pregnant either less or more than two years from breast cancer diagnosis, no DFS difference was observed between both groups, even after adjusting for tumor and treatment characteristics (unadjusted HR = 1.22, 95% CI = 0.83 to 1.80, P = .31; adjusted HR = 1.10, 95% CI = 0.70 to 1.75, P = .67; data not shown). Hence, the improved outcome of patients who became pregnant earlier seemed to be the result of selection bias rather than a true protective effect of early pregnancy.
Out of 188 patients with completed pregnancy, 64 (34.0%) had available information on breastfeeding. As compared with matched women from the nonpregnant cohort, no difference in DFS was observed in patients who breastfed their newborns (HR = 0.70, 95% CI = 0.26 to 1.94, P = .50) or in those who did not (HR = 1.44, 95% CI = 0.77 to 2.69, P = .25) (Supplementary Figure 1C, available online), with no statistically significant heterogenity observed (Pinteraction = .24). Exploratory subgroup analyses in patients with ER-positive disease in the pregnant cohort did not suggest any impact of adjuvant therapy on DFS (Supplementary Table 1, available online).
This report provides long-term results of the largest study to date addressing the safety of pregnancy according to ER status. In addition, we provide for the first time subgroup analyses showing the lack of apparent detrimental effect of breastfeeding and type of adjuvant therapy on patients’ outcomes. These results reinforce the notion that pregnancy should not be discouraged after breast cancer, even in women with history of ER-positive disease.
Timing of subsequent pregnancy remains a challenging question to address in clinical practice. Previously, two studies showed a trend toward increased risk of recurrence in patients conceiving within six and 12 months after diagnosis (6,7). Our study, which included nearly three times the number of pregnant patients, showed that the timing of pregnancy does not appear to have a major impact on outcomes. These findings suggest that an individualized approach should be adopted taking into account parameters including patient’s age, risk of recurrence, adjuvant therapy, and ovarian reserve. This is of particular relevance for ER-positive patients in whom the need for five to 10 years of adjuvant endocrine therapy may substantially reduce their chance of conception (8). In clinical practice, several of these women are offered temporary interruption of endocrine therapy to allow pregnancy (9). Addressing the safety of such an approach in patients who have received 18 to 30 months of endocrine therapy is the subject of the ongoing IBCSG-BIG-NABCG POSITIVE study (NCT02308085) (9).
Physicians’ and patients’ fear toward a possible detrimental effect of pregnancy after breast cancer might partially explain the high rate of induced abortion observed in our study (approximately 30%) as well as others (6,7). Our results confirm that abortion does not influence patients’ survival, underscoring that it should not be promoted for therapeutic purposes.
This remains the only report addressing the safety of breastfeeding after breast cancer. Despite the limited information, we believe it would help in counseling women inquiring into the safety of this approach. Results of the POSITIVE study are awaited to give further insights on this important issue.
Some limitations should be highlighted, including the retrospective nature of the study, the absence of information on HER2 status for the majority of patients, and the possibility that some pregnant patients could have been subjected to restaging before considering pregnancy. Furthermore, information on the use of assisted reproductive technology (ART) was only available for a minority of patients. The results of this subgroup have been recently reported, showing no apparent detrimental effect of performing ART after completion of breast cancer treatment (10).
In conclusion, our updated results provide reassuring evidence on the long-term safety of pregnancy in breast cancer survivors, including those with ER-positive disease. These findings are of great importance to guiding treating physicians in counseling young breast cancer survivors in this regard.
Funding
This work was partially supported by grants from Les Amis de l’Institut Bordet (grant number 2012-09) and the European School of Oncology (ESO; no grant number). The International Breast Cancer Study Group study, which provided patient information for this study, was partially funded by the National Institutes of Health (grant number CA-753562).
Matteo Lambertini acknowledges the support from the European Society for Medical Oncology for a Translational Research Fellowship at Institut Jules Bordet, Brussels, Belgium.
Notes
The funding organizations (Les Amis de l’Institut Bordet, ESO, and the National Institutes of Health) had no role in the design or conduct of the study, in the collection, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript.
Matteo Lambertini received a 2017 Conquer Cancer Foundation Merit Award for presenting the results of the study at the 2017 American Society of Clinical Oncology Annual Meeting. Niels Kroman has received consulting fees from Roche and GE Medical outside the submitted work. Michail Ignatiadis served as a consultant for Roche and received research grant from Roche (to the insitution) outside the submitted work. Evandro de Azambuja has received honoraria from Roche, travel grants from Roche and GlaxoSmithKline, and a research grant from Roche (to the institute) outside the submitted work. Hatem A. Azim Jr. reports employment at Innate Pharma at the end of this study; this employment is not related in any way to the subject of the current study. All remaining authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Biglia N, Torrisi R, D’Alonzo M, Codacci Pisanelli G, Rota S, Peccatori FA.. Attitudes on fertility issues in breast cancer patients: An Italian survey. Gynecol Endocrinol. 2015;316:458–464. [DOI] [PubMed] [Google Scholar]
- 2. Lambertini M, Di Maio M, Pagani O et al. , A survey on physicians’ knowledge, practice and attitudes on fertility and pregnancy issues in young breast cancer patients. Breast. 2017;32(suppl 1):abstract P189. [DOI] [PubMed] [Google Scholar]
- 3. Senkus E, Gomez H, Dirix L et al. , Attitudes of young patients with breast cancer toward fertility loss related to adjuvant systemic therapies. EORTC study 10002 BIG 3-98. Psychooncology. 2014;232:173–182. [DOI] [PubMed] [Google Scholar]
- 4. Azim HA Jr, Kroman N, Paesmans M et al. , Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: A multicenter retrospective study. J Clin Oncol. 2013;311:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pan H, Gray RG, Davies C et al. , Predictors of recurrence during years 5-14 in 46,138 women with ER+ breast cancer allocated 5 years only of endocrine therapy (ET). J Clin Oncol .2016;34(suppl):abstract 505. [Google Scholar]
- 6. Ives A, Saunders C, Bulsara M, Semmens J.. Pregnancy after breast cancer: Population based study. BMJ. 2007;3347586:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kranick JA, Schaefer C, Rowell S et al. , Is pregnancy after breast cancer safe? Breast J. 2010;164:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lambertini M, Boni L, Michelotti A et al. , Ovarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival: A randomized clinical trial. JAMA. 2015;31424:2632–2640. [DOI] [PubMed] [Google Scholar]
- 9. Pagani O, Ruggeri M, Manunta S et al. , Pregnancy after breast cancer: Are young patients willing to participate in clinical studies? Breast. 2015;243:201–207. [DOI] [PubMed] [Google Scholar]
- 10. Goldrat O, Kroman N, Peccatori FA et al. , Pregnancy following breast cancer using assisted reproduction and its effect on long-term outcome. Eur J Cancer. 2015;5112:1490–1496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.