Abstract
Objective
To test the hypothesis that use of a clinical decision support (CDS) system in a primary care setting can reduce cardiovascular (CV) risk in patients.
Materials and Methods
Twenty primary care clinics were randomly assigned to usual care (UC) or CDS. For CDS clinic patients identified algorithmically with high CV risk, rooming staff were prompted by the electronic health record (EHR) to print CDS that identified evidence-based treatment options for lipid, blood pressure, weight, tobacco, or aspirin management and prioritized them based on potential benefit to the patient. The intention-to-treat analysis included 7914 adults who met high CV risk criteria at an index clinic visit and had at least one post-index visit, accounted for clustering, and assessed impact on predicted annual rate of change in 10-year CV risk over a 14-month period.
Results
The CDS was printed at 75% of targeted visits, and providers reported 85% to 98% satisfaction with various aspects of the intervention. Predicted annual rate of change in absolute 10-year CV risk was significantly better in CDS clinics than in UC clinics (-0.59% vs. +1.66%, −2.24%; P < .001), with difference in 10-year CV risk at 12 months post-index favoring the CDS group (UC 24.4%, CDS 22.5%, P < .03).
Discussion
Deploying to both patients and providers within primary care visit workflow and limiting CDS display and print burden to two mouse clicks by rooming staff contributed to high CDS use rates and high provider satisfaction.
Conclusion
This EHR-integrated, web-based outpatient CDS system significantly improved 10-year CV risk trajectory in targeted adults.
Keywords: cardiovascular risk, clinical decision support, electronic health records, primary care, prioritized treatment opportunities, health informatics
INTRODUCTION
Heart disease and stroke remain a leading cause of death in the United States today and account for approximately $320 billion in healthcare expenditures and related expenses annually.1,2
Driven in part by national health goals and accountability performance measures, adults with diagnosed diabetes and/or cardiovascular disease (CVD) in many areas of the country have experienced great improvements in treatments for glycemic control, statin use, blood pressure control, and aspirin use, with reductions in CV events and mortality rates.3 However, there remains a large opportunity and compelling need for earlier and more effective management of uncontrolled CV risk factors for primary prevention in patients without diabetes or CVD in order to slow the population trends in rates of CVD events.4–6
Several healthcare strategies have been advocated to accomplish this, including the leveraging of electronic health record (EHR) systems to identify patients at high risk for future CVD and to promote evidence-based preventive treatments.4,7
Clinical decision support (CDS) systems may be particularly useful because treatment goals for several CV risk factors (blood pressure, lipids, aspirin use) are patient specific and require consideration of comorbidity, age, and 10-year CV risk to assess patient-specific benefits and risks of various treatment options.8–10 Moreover, time-motion studies show that assessment of CV risk factor status by primary care providers (PCPs) using an EHR takes, on average, 52 mouse clicks and 4 minutes to gather 80% of necessary data, while algorithmic assessment of CV risk can be done in 300 milliseconds and is more complete.11 Previous studies also indicate that both PCPs and patients often inaccurately estimate CV risk and the potential benefits of drug therapy.12
Despite the potential of CDS to identify and recommend treatment for patients with uncontrolled CV risk factors, few CDS systems have been proven to have an impact on clinically meaningful outcomes. Review articles and our experience suggest that this pattern of failure is related to poor integration of CDS systems into clinic workflow, extra time required for PCPs to use the CDS system, failure to personalize and prioritize care recommendations based on the circumstances of each individual patient, and failure to promote shared decision making by providing the CDS to the patient as well as the PCP.10,13
Here, we report the development, implementation, and clinical impact of a web-based, point-of-care CDS system seamlessly integrated within the EHR and primary care workflow. This CDS system was specifically designed to identify adults with moderate to high CV risk but no diagnosis of CVD or diabetes and to facilitate a shared decision-making approach to improve control of major CV risk factors. Clinical algorithms within the CDS system are based on evidence-based national clinical guidelines6,14,15 and provide personalized and prioritized treatment recommendations to both PCPs and patients in high-literacy (professional) and low-literacy (lay person) formats. The objective of this study was to evaluate whether the CDS intervention can improve 10-year CVD risk trajectory in patients in primary care settings.
STUDY DATA AND METHODS
Hypothesis, study design, and study setting
This clinic-randomized trial was designed to test the hypothesis that providing point-of-care CDS to PCPs and patients can improve 10-year CVD risk trajectory compared with usual care (UC). The trial was conducted from August 20, 2012, to August 19, 2014 at 20 HealthPartners Medical Group (HPMG) primary care clinics that implemented the EpiCare© (Verona, WI) EHR in 2003. HPMG is part of a Midwestern, multispecialty, integrated healthcare system.
Protection of human subjects
This study was reviewed, approved in advance, and monitored by the HealthPartners Institutional Review Board (IRB). The IRB approved waiver of written consent for patient study subjects.
Randomization procedure
Of the 20 participating clinics, four strata were created based on the number of eligible patients (<700 vs. ≥700) and the number of PCPs who agreed prior to clinic randomization to complete survey evaluations (<6 vs. ≥6 for large clinics, <4 vs. ≥4 for small clinics) and then sorted into pairs based on a publicly reported quality measure (proportion of diabetes patients with optimal risk factor control).16 Three small clinics staffed by the same PCPs were defined and randomly assigned as one unit to minimize contamination. Within each randomized pair of clinics, the clinic with the higher randomly generated number was assigned to the CDS intervention arm and the other to the UC arm. One intervention clinic closed early in the intervention period and was excluded from analysis.
PCP agreement to use the CDS and complete survey evaluations
Of 186 eligible PCPs (family and internal medicine physicians, physician assistants, and nurse practitioners working at least half time), 102 provided written informed consent to complete pre- and post-intervention surveys for the study and to use the CDS with patients (if they practice in a clinic that was randomly assigned to the CDS intervention). Consented providers who practiced in a clinic randomly assigned to the CDS intervention also received one-time modest compensation of $500 if they achieved an 80% CDS print rate for targeted patients within 3 months of implementation. However, the CDS intervention was triggered for all office encounters at clinics randomized to the CDS intervention, regardless of provider consent status, and safety monitoring and analysis included all eligible patients at randomized clinics.
Patient study subjects
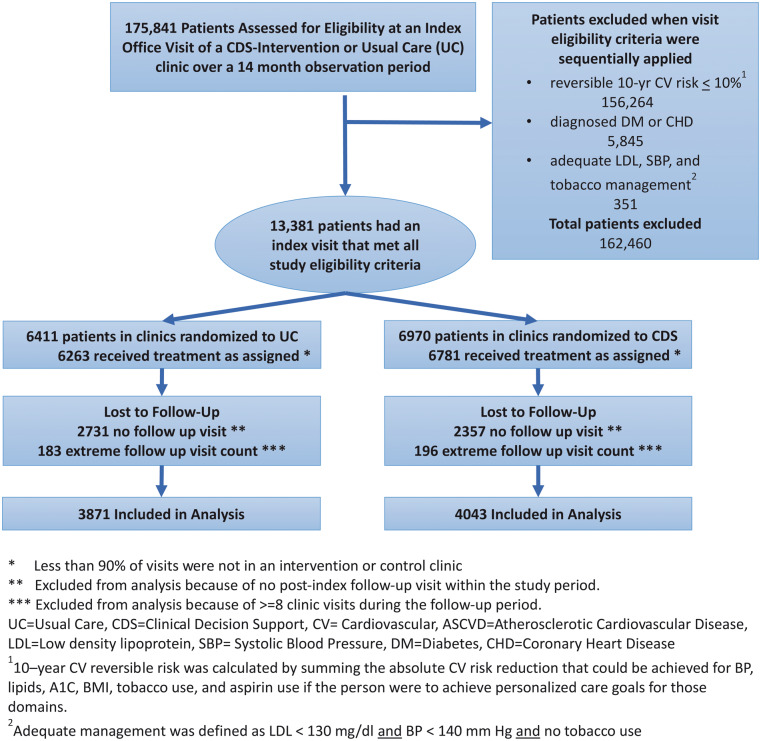
Patients were eligible for the analysis if they met the following criteria at the time of an index visit at a study clinic between June 7, 2013, and August 19, 2014: (a) aged 18 to 75 years; (b) no inpatient or outpatient diagnosis codes for diabetes or CVD before the index date; (c) no hospice care, active cancer treatment, or pregnancy in the last year; (d) high reversible CV risk at the index visit, defined as the potential for an absolute CV risk reduction of 10% or more if uncontrolled CV risk factors were controlled to recommended levels; (e) one or more of the following: (i) systolic blood pressure ≥140 mm Hg, (ii) low-density lipoprotein (LDL) cholesterol ≥130 mg/dl, or (iii) current tobacco smoker. Patients considered clinical outliers due to an extreme number of office visits (eight or more) and those on a federally mandated research opt-out list were deemed ineligible for analysis. All other patients who had an index visit and at least one post-index primary care visit during the 14-month observation period that ended on August 19, 2014, were retained in the analyses (See Figure 1).
Figure 1.
CONSORT Diagram showing identification of study eligible subjects and exclusions for various reasons in this clinic-randomized trial.
Data sources and variable definitions
Ten-year CV risk was calculated at each outpatient visit based on the most recent EHR data at the time of the visit, resulting in as many CV risk measures per patient as there were visits. The Framingham lipid equation was used when a lipid value was available in the 5-year period before the index visit. For patients lacking values required by the lipid equation at any visit, we instead used the Framingham BMI equation17,18 for all visits to keep the method for calculating CV risk constant within patients across visits.
Total cholesterol and high-density lipoprotein (HDL) cholesterol were assayed using standard methods at a single centralized, accredited clinical chemistry laboratory. Blood pressure was measured by rooming staff trained in the proper blood pressure measurement technique using an automated oscillatory blood pressure device (Omron 907). Laboratory test values and dates, blood pressure measurements, smoking status, and other demographic and clinical data were extracted in real time from discrete EHR fields at each encounter when the CDS was triggered.
Description of the CDS intervention
Exchange of selected clinical and demographic data from the EHR to the CDS Web service was automatically triggered in all clinics when rooming staff entered a blood pressure value in the EHR at an office visit. Within the Web service, EHR data were processed through clinical algorithms to (a) identify eligible patients meeting CV risk study criteria, (b) determine evidence-based treatment options for uncontrolled CV risk factors, and (c) prioritize treatment options for any uncontrolled CV risk factor (blood pressure, LDL, weight, tobacco use, appropriate aspirin use) based on reduction in CV risk with improved control.
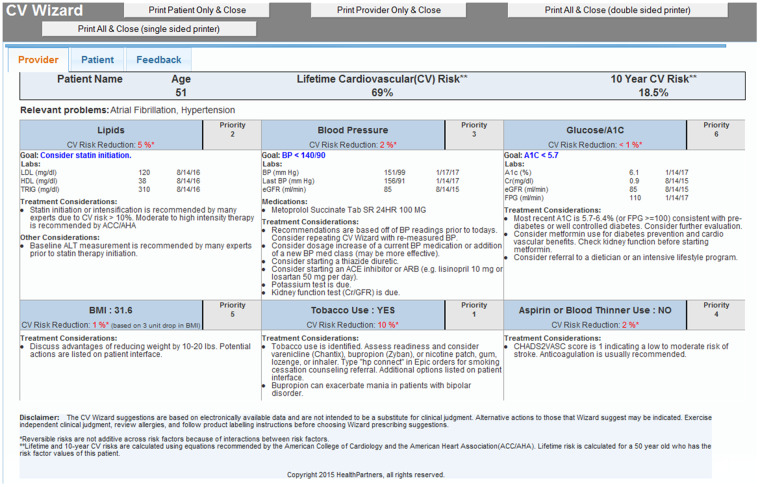
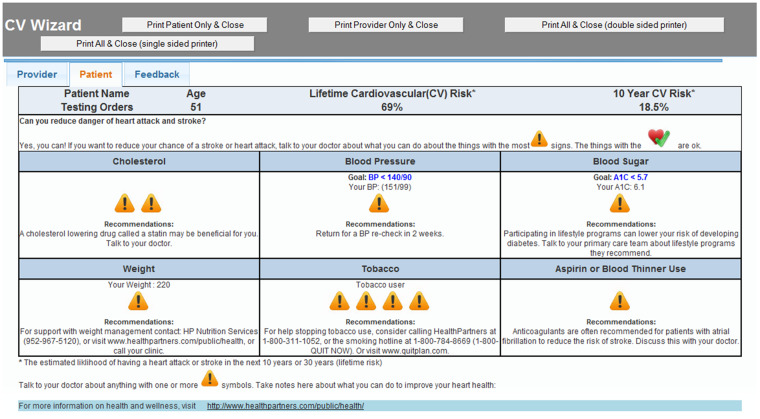
At CDS intervention clinics, an algorithmically generated best-practice alert (BPA) appeared on the EHR screen within 1 or 2 seconds of blood pressure entry for eligible patients who met study criteria for CV risk. The rooming staff clicked once within the BPA to display the CDS and once to print both the lay and professional versions of the CDS. The CDS Professional Version (Figure 2) was given to the PCP to review before entering the exam room. The CDS Lay Version (Figure 3) was given to the patient by the rooming nurse to view while waiting for the PCP with this request: “If you want to reduce your chance of a stroke or heart attack, talk to your doctor about the things with the most caution symbols.” The CDS displays were generated from a web service but were seamlessly viewed within the EHR by clinic staff. If intervention clinic PCPs were interested in viewing the CDS on other patients (regardless of CV risk status) or refreshing the CDS on a study-eligible patient (eg, if the blood pressure was repeated), they could also trigger the CDS display by clicking a button within the EHR visit navigator bar.
Figure 2.
Provider clinical decision support (CDS) display for a fictitious patient. This is displayed on the EHR screen then printed by the rooming nurse and placed on the exam room door for rapid review by the provider just before the start of the visit. Uncontrolled CV risk factors are prioritized by the potential absolute risk reduction that may be achieved by management of that risk factor. The benefit for BMI is predicated on a 3-unit drop in BMI with a floor BMI of 25.
Figure 3.
Clinical decision support (CDS) display for a fictitious patient. This is printed by the rooming nurse and given to the patient to review while waiting in the exam room for the provider, with this message, “If you want to reduce your chance of a stroke or heart attack, talk to your doctor about the things with the most caution symbols.”
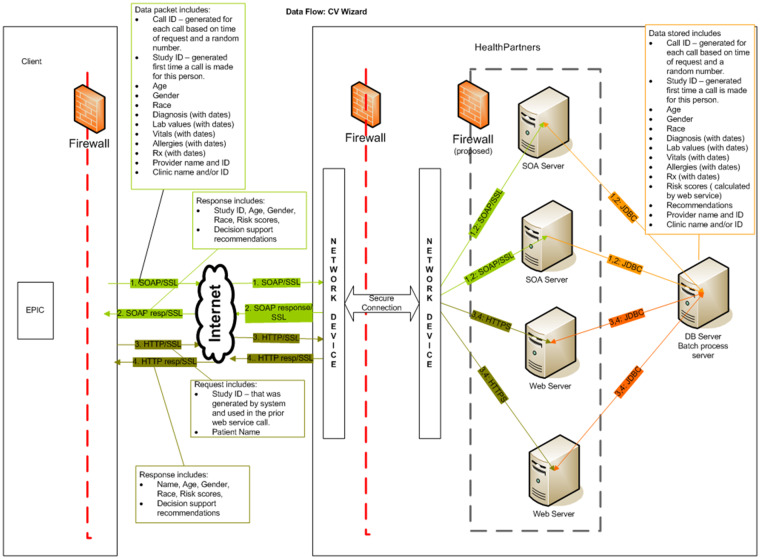
Encryption and firewalls were used to ensure secure flow of data, and all key data and CDS recommendations returned from the web service were stored as discrete data elements within EHR document flow sheets (See Figure 4). All clinical suggestions were based on national guidelines and practice standards,14,15,19,20 and all clinical algorithms were constructed by clinical content experts and approved by physician leaders in the care system for use in routine primary care practice. Treatment recommendations were nonprescriptive and labeled as “considerations.” A disclaimer on all CDS displays reminded PCPs that they were not obligated to accept any CDS-generated clinical suggestions and should use their best clinical judgment and knowledge of each patient to guide all clinical care decisions.
Figure 4.
Schematic diagram illustrating encryption, firewalls, and other measures taken to secure transmission of personally identifiable information between the EMR and the clincial decision support (CDS) web service. Data transfer is governed by business associate agreements.
Training and maintaining intervention fidelity
In addition to conducting 45-minute introductory and training sessions at CDS intervention clinics, other strategies implemented to achieve and maintain high CDS use rates included collaboration with PCP and nursing leaders for workflow integration, triggering of the CDS by rooming staff rather than PCPs, monthly feedback at the clinic and PCP level of CDS use rates to intervention clinic managers and PCPs, and compensation of $500 twice to each intervention clinic nursing pool for clinics that sustained CDS use rates >=75% of targeted patients.
Implementation challenges
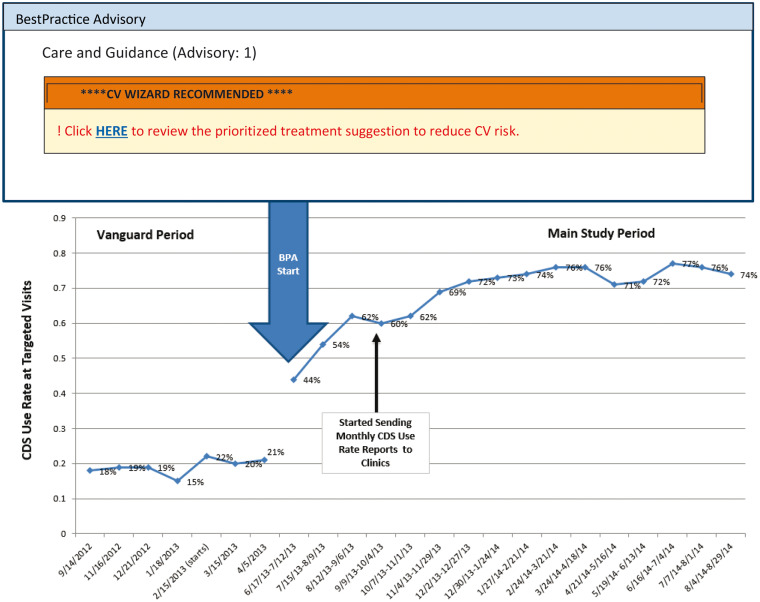
The BPA containing the URL to display and print the CDS tool took longer to develop than we anticipated. To gain more user experience with the CDS tool, we elected to go into the field for a “vanguard” phase in intervention clinics from August 20, 2012, to June 6, 2013, without the BPA that identified study-eligible patients at high CV risk. During this period, rooming staff and PCPs were encouraged to identify patients with uncontrolled CV risk factors and to print materials by clicking the button on the EHR visit navigator bar. During the vanguard phase, staff triggered the CDS system at only approximately 20% of visits with study-eligible patients.
Analytic approach
This was a clinic-randomized nested cohort trial with clinics randomly assigned to UC or CDS intervention arms and repeated outcomes per patient linked to clinics. The link between data from each patient and a primary care clinic was made based on frequency of visits. Clinical outcomes were extracted from clinical measures and laboratory data that were collected as part of routine care delivery; therefore, the number and timing of observations were not standardized and varied among individuals.
To take advantage of all available observations, the primary analysis used a time-by-condition growth curve approach via PROC MIXED in SAS 9.4 to estimate the annual rate of change in post-index CV risk values and 12-month difference in CV risk by treatment group. The time-by-condition models included fixed effects for study arm (CDS vs. UC), time (years elapsed since index), and the study-arm-by-time interaction comparing the rate of change in CDS vs. UC and a random clinic intercept. The intervention effect was the difference in rates of change in CV risk in CDS vs. UC clinics, with the study-arm-by-time interaction assessing statistical significance (P < .05). The analysis followed intent-to-treat principles by including all post-index CV risk values of the 7914 patients in the analysis, regardless of whether the CDS was printed at any of their office visits.
The a priori sample size justification estimated the minimum detectable difference (power=0.80, α2=0.05) for a planned comparison of CV risk at 12 months post-index based on 18 randomized clinics, 1000 patients per clinic, three CV risk values per patient, and ICCCV risk=0.01-03 for observations nested within clinics. It estimated that absolute, between-group differences of ∼2% (ICCCV risk=0.01) to ∼3% (ICCCV risk=0.02) in 10-year CV risk would be detectable.
Secondary analyses assessed the extent to which the intervention effect varied across patient subgroups: those who did and did not have CDS-eligible primary care visits during the vanguard period; those in each quintile of 10-year CV risk at the index visit; smoking status at the index visit; and BMI (kg/m2) category at the index visit. A patient characteristic was considered a treatment modifier if the three-way interaction (study arm by time by subgroup) was statistically significant (P < .05). The intervention effect was calculated in patient subgroups for description.
To assess provider perceptions with regard to confidence and preparedness to address CV risk with patients, PCPs (N = 102) at UC and CDS clinics were surveyed before CDS system implementation (response rate = 90%) and 18 months later (response rate = 78%). Responses to questions were coded as “agree” if one of the top two affirmative of four Likert scale options was selected. Generalized linear mixed models compared the proportions of PCPs who “agreed” at CDS relative to UC clinics at follow-up relative to pre-implementation to estimate the study-arm effect. In the second survey, PCPs at CDS clinics were asked to report their satisfaction and perceptions with the CDS system.
RESULTS
The primary clinical outcomes analysis consisted of available CV risk values obtained at 18 441 post-index visits of N = 7914 patients (Figure 1). Patients were predominantly non-Hispanic white and commercially insured, with a median of two post-index visits (range, one to seven). There were no differences in the number of post-index visits per person or the timing of the first or last post-index visit relative to the index visit between patients in CDS and UC clinics. Additional patient characteristics of study subjects, as well as patients who were excluded from analysis due to lack of a post-index visit, are presented in Table 1.
Table 1.
Characteristics of study subjects at index visit by post-index visits and study arm
| Excluded from analysis due to no follow-up visits |
Included in analysis with 1 to 7 follow-up visits |
||||
|---|---|---|---|---|---|
| Usual care | CDS intervention | Usual care | CDS intervention | ||
| N = 2357 | N = 2731 | N = 3871 | N = 4043 | ||
| Demographics at index visit | |||||
| Age (years) | M (SD) | 57.9 (8.6) | 57.8 (8.5) | 58.5 (8.6) | 58.7 (8.5) |
| Female | (%) | 19.5 | 21.8 | 26.2 | 26.4 |
| White | (%) | 84.0 | 77.2 | 82.9 | 75.7 |
| African American | (%) | 6.9 | 11.7 | 9.2 | 14.9 |
| Other non-white | (%) | 4.2 | 4.5 | 5.5 | 4.4 |
| Race not reported | (%) | 5.0 | 6.6 | 2.4 | 5.1 |
| Hispanic | (%) | 1.4 | 2.2 | 1.3 | 2.1 |
| Insurance type at index visit | |||||
| Commercial | (%) | 88.6 | 85.8 | 88.2 | 84.2 |
| Medicare | (%) | 6.7 | 8.0 | 7.9 | 10.4 |
| Medicaid | (%) | 1.8 | 3.6 | 2.8 | 3.9 |
| Self-pay | (%) | 3.0 | 2.7 | 1.2 | 1.5 |
| Clinical status at index visit | |||||
| Systolic blood pressure (mm Hg) | M (SD) | 140.9 (19.7) | 142.1 (20.0) | 145.3 (21.7) | 144.6 (21.1) |
| Diastolic blood pressure (mm Hg) | M (SD) | 84.1 (12.5) | 85.3 (12.5) | 85.8 (13.4) | 86.2 (12.9) |
| Current smoker | (%) | 59.4 | 61.1 | 54.9 | 58.2 |
| BMI (kg/m2) | M (SD) | 29.8 (6.1) | 29.7 (6.0) | 30.4 (6.4) | 30.1 (6.2) |
| Current statin use | (%) | 19.0 | 19.6 | 21.1 | 21.3 |
| Low-density lipoprotein cholesterol (mg/dl) | M (SD) | 130.4 (36.9) | 129.5 (35.9) | 127.7 (36.9) | 125.0 (36.7) |
| Aspirin use, if indicated | (%) | 21.2 | 22.6 | 25.4 | 25.2 |
| Utilization after index visit | |||||
| Number of post-index visits | M (SD) | − | − | 2.4 (1.6) | 2.3 (1.5) |
| Median | − | − | 2 | 2 | |
| Days to first post-index visit | M (SD) | − | − | 81.5 (86.3) | 85.7 (88.6) |
| Median | − | − | 47 | 51 | |
| Days to last post-index visit | M (SD) | − | − | 166.3 (116.8) | 169.3 (117.7) |
| Median | − | − | 151 | 156 | |
Clinical impact
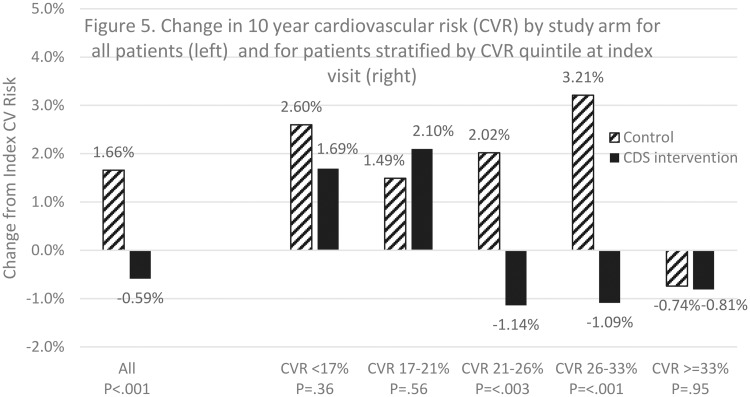
Figure 5 shows that the predicted post-index annual rates of change in 10-year CV risk among all study patients were +1.66% in UC clinics and -0.59% in CDS clinics (P < .001). The predicted difference in 10-year CV risk at 12 months post-index was -2.2%, favoring the CDS group. The annual rate of change differed significantly by treatment group across quintiles of index CV risk, three-way interaction P<.005. The intervention effect was greater in patients who had an index CV risk in the 40th to 60th percentile (UC +2.02%, CDS -1.14%, P < .005) or 60th to 80th percentile (UC +3.21%, CDS -1.09%, P < .001). Table 2 shows that the effect on CV risk was also significant in patient subgroups, including patients who had CDS-eligible visits in the vanguard period (UC +1.81%, CDS −0.74%, P < .005), current smokers (UC +2.38%, CDS -0.54%, P < .001), and those with a BMI of <25 kg/m2 (UC +3.08%, CDS +0.14%, P < .04) or 35 to <40 kg/m2 (UC +2.53%, CDS -2.27%, P < .01). The intervention effect was not different between patients of providers who did and did not consent in advance to complete survey evaluation, P < .46.
Figure 5.
Change in CVR.
Table 2.
Predicted annual rate of change in 10-year cardiovascular risk by study arm among all patients and by patient subgroups, including P-values for rate of change comparisons by study arm (CDS vs. UC) and by study arm and patient subgroup (interaction)
| Usual care | CDS intervention | P, CDS vs. UC | P, interaction | |
|---|---|---|---|---|
| All patients | +1.66 | −0.59 | <.001 | n/a |
| 10-year CVR quintiles at index visit | ||||
| CVR <17% | +2.60 | +1.69 | .36 | <.005 |
| CVR 17−<21% | +1.49 | +2.10 | .56 | |
| CVR 21−<26% | +2.02 | −1.14 | <.003 | |
| CVR 26−<33% | +3.21 | −1.09 | <.001 | |
| CVR >=33% | −0.74 | −0.81 | .95 | |
| CDS-eligible visits in vanguard period | ||||
| Yes | +1.81 | −0.74 | <.005 | .38 |
| No | −0.26 | −1.72 | .10 | |
| Current smoker at index visit | ||||
| Yes | +2.38 | −0.54 | <.001 | .18 |
| No | +2.77 | +1.52 | .20 | |
| BMI (kg/m2) at index visit | ||||
| <25 | +3.08 | +0.14 | .04 | .46 |
| 25−<30 | +2.66 | +1.32 | .20 | |
| 30−<35 | +3.44 | +2.10 | .27 | |
| 35−<40 | +2.53 | −2.27 | <.01 | |
| >=40 | +1.27 | −0.76 | .39 | |
Previous research with this CDS tool in patients with diabetes showed a significant impact on glycemic and blood pressure control.21 In this study, we observed trends in secondary measures (eg, blood pressure control, LDL levels, BMI, aspirin use) that favored the CDS intervention. Although changes in these individual risk factors were not statistically significant, the cumulative effect on CV risk was statistically significant.
CDS use rates and PCP satisfaction
CDS use was measured as a percentage of targeted visits at which rooming staff printed the CDS interfaces for PCPs and patients. CDS use rates at targeted visits in intervention clinics were 42% a month after training and rose steadily to 72% 6 months later (Figure 6). CDS use rates increased significantly when the research team began to provide automated clinic-specific and provider-specific monthly CDS use reports to clinic leadership. Thereafter, mean CDS use rates at all intervention clinics were sustained in the 71% to 77% range until study end and were similar between providers who consented in advance to CDS use and survey evaluation and those who did not.
Figure 6.
Clinical decision support (CDS) use rates at intervention clinic visits of study-eligible patients before the best practice advisory (vanguard) on the left and after the best practice advisory (main study) on the right.
Table 3 shows that, compared with UC clinics, PCPs in CDS clinics reported more frequent discussion of CV risk reduction (60% vs. 30%, P = .06), greater use of CV risk calculations (73% vs. 25%, P = .006), being better prepared to discuss CV risk reduction priorities with patients (98% vs. 78%, P = .03), and greater ability to provide accurate advice on aspirin use for primary prevention (75% vs. 48%, P = .02). Table 3 shows the provider satisfaction and perceived value of CDS features by PCPs in the intervention group. PCPs at CDS clinics reported that the CDS system helped them initiate discussions about CV risk (94%), improved CV risk factor control (98%), saved time when talking about CV risk with patients (93%), enabled efficient elicitation of patient treatment preferences (90%), supported shared decision making (95%), and influenced treatment recommendations (89%). Among PCPs in CDS clinics, 85% reported that their patients liked the CDS patient interface and associated activities.
Table 3.
Provider survey results: first survey was prior to vanguard go-live date, and second survey was 18 months later
| Provider Behaviors | ||||
| The percent of provider survey respondents in usual care and CDS intervention clinics who agreed with statements about clinical practice behaviors related to cardiovascular risk management before and after CDS implementation | ||||
| Usual care (%) | CDS intervention (%) | P, interaction | ||
| Often discuss cardiovascular risk reduction with patient | Before | 38 | 40 | .06 |
| 18 m after | 30 | 60 | ||
| Often use calculated cardiovascular risk while seeing patients | Before | 24 | 26 | .006 |
| 18 m after | 25 | 73 | ||
| Well prepared to discuss cardiovascular risk reduction priorities with patients | Before | 79 | 74 | .03 |
| 18 m | 78 | 98 | ||
| Able to provide accurate advice on aspirin for primary prevention | Before | 45 | 38 | .02 |
| 18 m | 48 | 75 | ||
| Provider Satisfaction | ||||
| The percent of provider respondents (n=47) in intervention clinics who agree or strongly agree (3 or 4 out of 4 Likert scale options) with statements about the CDS 18 months after CDS implementation | ||||
| CDS improved cardiovascular risk factor control in my patients | 18 m | 98 | ||
| CDS saved me time when talking to patients about cardiovascular risk reduction | 18 m | 93 | ||
| CDS efficiently elicited patient treatment preferences | 18 m | 90 | ||
| CDS was useful for shared decision making | 18 m | 95 | ||
| CDS influenced my treatment recommendations | 18 m | 89 | ||
| CDS helped me initiate cardiovascular risk discussions | 18 m | 94 | ||
| My patients liked CV Wizard (the CDS system) | 18 m | 85 | ||
DISCUSSION
Scalable CDS systems that achieve high use rates and high clinician satisfaction, and improve patient outcomes are uncommon.22 The results reported here suggest that these goals are achievable with careful CDS system design, effective integration with clinic workflows, and the use of feedback and, possibly, incentives to maintain high use rates.
While evidence-based algorithms serve to standardize care delivery, presenting personalized evidence-based treatment options to patients provides documentation of patient-centered care and shared decision making. Moreover, this type of system enables rapid translation of new knowledge into clinical practice as evidence and guidelines evolve.
A notable feature of the intervention strategy was the ability to algorithmically identify target patients and proactively bring them to the attention of PCPs at the point of care. If long-term improvement trends in CV event and mortality rates are to be sustained, patients such as those targeted in this study need to be identified early and achieve more effective CV risk factor prevention and control.4,23,24 However, the same strategies can be used to proactively identify other subgroups of patients who may benefit from point-of-care decision support, such as those with opioid use disorder, uncontrolled depression, or deficits in a variety of preventive care services. The CDS system we tested is patient centered (rather than disease specific) because it integrates and prioritizes care recommendations across multiple care domains. This saves PCP time and supports high CDS use rates.
Many previous efforts to implement chronic disease care CDS systems have failed because they were not used on a sustained basis.9,13 To achieve high CDS use rates, we designed the CDS in conjunction with PCP and nurse leaders to fit clinic workflows, targeted the CDS to only about 20% of adult primary care visits, relied on rooming nurses rather than PCPs to trigger the CDS, conducted PCP-led onsite training with lunch at each intervention clinic, offered modest financial incentives, and provided ongoing monthly monitoring and feedback of CDS use rates to care teams and clinic managers.
We have subsequently implemented the same CDS system at all clinics within this study setting and an additional three other large medical groups and are maintaining CDS use rates of >70% at targeted visits by using similar implementation methods but without financial incentives. This suggests that the modest financial incentives offered in this study may not have been necessary to achieve high CDS use rates.
Several factors limit the interpretation of our data. The main study analysis began in June 2013 after a vanguard phase. During the vanguard, there was no BPA to identify high-risk patients and prompt CDS use. Use rates were therefore low during the vanguard. However, the opportunistic vanguard use of the CDS could have improved CV risk factor control for some patients who were later included in the main analysis (in intervention but not UC clinics) leading to possible underestimation of CDS intervention effects. Other consequences of the vanguard phase were (a) a shorter follow-up time interval for the main analysis than we originally anticipated, (b) a larger number of patients without a follow-up visit outcome, and (c) fewer follow-up observations per patient. This study was conducted at a single, relatively high-performing medical group, so generalizability of results to other care delivery systems or patient populations is uncertain. Current studies are underway to ascertain the durability and reproducibility in other study settings, including rural and safety net clinics, and to evaluate the impact of new functionality to facilitate easy ordering within the EHR of tests, referrals, and medications suggested by the CDS.
Data confidentiality remains an important concern in web-based CDS systems. Implementation across care systems is governed by business associate agreements, and data security arrangements include double encryption of data, multiple firewalls, Internet Protocol whitelisting, and other measures that require careful and repeated vetting by data security experts and continuous monitoring for security and function.
The cost and cost effectiveness of this intervention strategy are unknown; however, an earlier version of this CDS system had favorable cost effectiveness in a published analysis.25 This web-based CDS system design is well suited to broad dissemination, and advances in EHR interoperability may further accelerate dissemination and improve future cost effectiveness. Quality-based payments proposed by the Centers for Medicare & Medicaid Services or other entities may offset implementation and maintenance costs of effective CDS systems.26
Despite various limitations, our data provide proof of concept that an EHR-integrated, web-based CDS system that provides personalized and prioritized CDS to both patients and PCPs at the point of care can significantly reduce CV risk in targeted primary care patients. The magnitude of the observed impact is clinically and statistically significant and consistent with previous positive results we achieved in other clinical domains.27–29 In the coming era of personalized medicine, web-based CDS systems that can simultaneously standardize and personalize clinical care will likely become essential tools in both primary and subspecialty care. Integration of CDS recommendations across multiple clinical domains, prioritization of treatment recommendations, and effective communication of CDS output to patients could substantially improve the impact of CDS systems on quality and cost of care.21,27,28
FUNDING
This work was funded by the National Institute's of Health, National Heart, Lung, and Blood Institute by grant R01HL102144 to HealthPartners Institute. This research received no specific grant funding from commercial sectors. Trial Registration: clinicaltrials.gov identifier: NCT01420016. The contents of this article reflect the views of the authors and do not necessarily reflect the position of the funding agency or the U.S. government.
Conflict of interest statement. The authors report no conflicts of interest.
CONTRIBUTORSHIP
JSH, ALC, PJO contributed to the conception and study design.
JSH, ALC, KLM, HLE, DA, JRD, PJO contributed to data collection, analysis, and interpretation.
JSH, KLM, HLE, DA, JRD, GA, RS, PJO contributed to development of CDS software and intervention implementation.
All authors contributed to drafting, review, and final approval of the manuscript.
REFERENCES
- 1. Murphy SL, Kochanek KD, Xu J, Arias E.. Mortality in the United States, 2014. NCHS Data Brief 2015; 229: 1–8. [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS.. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016; 1334: e38–360. [DOI] [PubMed] [Google Scholar]
- 3. Office of Disease Prevention and Health Promotion. Healthy People 2020; 2018. https://www.healthypeople.gov/2020/About-Healthy-People. Accessed Feb 13, 2018.
- 4. Desai JR, Vazquez-Benitez G, Xu Z.. Who must we target now to minimize future cardiovascular events and total mortality?: lessons from the Surveillance, Prevention and Management of Diabetes Mellitus (SUPREME-DM) Cohort Study. Circ Cardiovasc Qual Outcomes 2015; 85: 508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63 (25 Pt B): 2889–934. [DOI] [PubMed] [Google Scholar]
- 6. Whelton PK, Carey RM, Aronow WS.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71: 68. [DOI] [PubMed] [Google Scholar]
- 7. Frieden TR, Berwick DM.. The “Million Hearts” initiative–preventing heart attacks and strokes. N Engl J Med 2011; 36513: e27.. [DOI] [PubMed] [Google Scholar]
- 8. Institute of Medicine. The Computer-Based Patient Record. An Essential Technology for Health Care. Washington, DC: National Academy Press; 1991. [Google Scholar]
- 9. Roshanov PS, Fernandes N, Wilczynski JM, et al. Features of effective computerised clinical decision support systems: meta-regression of 162 randomised trials. BMJ 2013; 346: f657.. [DOI] [PubMed] [Google Scholar]
- 10. Njie GJ, Proia KK, Thota AB, et al. Clinical decision support systems and prevention: a community guide cardiovascular disease systematic review. Am J Prev Med 2015; 495: 784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koopman RJ, Kochendorfer KM, Moore JL, et al. A diabetes dashboard and physician efficiency and accuracy in accessing data needed for high-quality diabetes care. Ann Fam Med 2011; 95: 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pignone M, Phillips CJ, Elasy TA, Fernandez A.. Physicians’ ability to predict the risk of coronary heart disease. BMC Health Serv Res 2003; 31: 13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pal K, Eastwood SV, Michie S, et al. Computer-based diabetes self-management interventions for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev 2013; 3: CD008776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129 (25 Suppl 2): S1–45. [DOI] [PubMed] [Google Scholar]
- 15. Dehmer SP, Maciosek MV, Flottemesch TJ, LaFrance AB, Whitlock EP.. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2016; 16412: 777–86. [DOI] [PubMed] [Google Scholar]
- 16. MN Community Measurement. Measure Development & Refinement; 2017. http://mncm.org/services-solutions/measure-development/. Accessed June 19, 2018.
- 17. Pencina MJ, D’Agostino RB, Larson MG, Massaro JM, Vasan RS.. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation 2009; 11924: 3078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 1176: 743–53. [DOI] [PubMed] [Google Scholar]
- 19. Standards of Medical Care in Diabetes - 2016. Diabetes Care 2016; 39 (Suppl 1): s11–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). J Am Med Assoc 2014; 3115: 507–20. [DOI] [PubMed] [Google Scholar]
- 21. O’Connor PJ, Sperl-Hillen JM, Rush WA, et al. Impact of electronic health record clinical decision support on diabetes care: a randomized trial. Ann Fam Med 2011; 91: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Medicare & Medicaid Services. Million Hearts: Cardiovascular Disease Risk Reduction Model; 2017. https://innovation.cms.gov/initiatives/Million-Hearts-CVDRRM/. Accessed June 19, 2018.
- 23. Garcia MC, Bastian B, Rossen LM, et al. Potentially preventable deaths among the five leading causes of death - United States, 2010 and 2014. MMWR Morb Mortal Wkly Rep 2016; 6545: 1245–55. [DOI] [PubMed] [Google Scholar]
- 24. Heron M, Anderson RN.. Changes in the leading cause of death: recent patterns in heart disease and cancer mortality. NCHS Data Brief 2016; 254: 1–8. [PubMed] [Google Scholar]
- 25. Gilmer TP, O’Connor PJ, Sperl-Hillen JM, et al. Cost-effectiveness of an electronic medical record based clinical decision support system. Health Serv Res 2012; 476: 2137–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sanghavi DM, Conway PH.. Paying for prevention: a novel test of medicare value-based payment for cardiovascular risk reduction. JAMA 2015; 3142: 123–4. [DOI] [PubMed] [Google Scholar]
- 27. O’Connor PJ, Sperl-Hillen JM, Fazio CJ, Averbeck BM, Rank BH, Margolis KL.. Outpatient diabetes clinical decision support: current status and future directions. Diabet Med 2016; 336: 734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kharbanda EO, Nordin JD, Sinaiko AR, et al. TeenBP: development and piloting of an EHR-linked clinical decision support system to improve recognition of hypertension in adolescents. eGEMs 2015; 32: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rindal DB, Rush WA, Schleyer TK, et al. Computer-assisted guidance for dental office tobacco-cessation counseling: a randomized controlled trial. Am J Prev Med 2013; 443: 260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]