Heterozygous variants in SOD1 are a major cause of familial amyotrophic lateral sclerosis. Park et al. report the first known case of SOD1 deficiency, a disorder distinct from ALS characterized by mild cerebellar atrophy and hyperekplexia-like symptoms. The findings may have implications for the use of SOD1 silencing to treat ALS.
Keywords: superoxide dismutase, oxidative damage, amyotrophic lateral sclerosis, hyperekplexia
Abstract
Superoxide dismutase 1 (SOD1) is the principal cytoplasmic superoxide dismutase in humans and plays a major role in redox potential regulation. It catalyses the transformation of the superoxide anion (O2•−) into hydrogen peroxide. Heterozygous variants in SOD1 are a common cause of familial amyotrophic lateral sclerosis. In this study we describe the homozygous truncating variant c.335dupG (p.C112Wfs*11) in SOD1 that leads to total absence of enzyme activity. The resulting phenotype is severe and marked by progressive loss of motor abilities, tetraspasticity with predominance in the lower extremities, mild cerebellar atrophy, and hyperekplexia-like symptoms. Heterozygous carriers have a markedly reduced enzyme activity when compared to wild-type controls but show no overt neurologic phenotype. These results are in contrast with the previously proposed theory that a loss of function is the underlying mechanism in SOD1-related motor neuron disease and should be considered before application of previously proposed SOD1 silencing as a treatment option for amyotrophic lateral sclerosis.
Introduction
Reactive oxygen species were traditionally considered to be detrimental to cell integrity and held responsible for a variety of damages to cellular structures, ultimately resulting either in premature cell death by apoptosis or in cancerogenesis (Lushchak, 2014). Over the course of years of intensive research, a more differentiated view on the role of reactive oxygen species both in health and disease was developed and continues to be refined (LeVine, 1992; Auten and Davis, 2009; Forman et al., 2010; Fang, 2011; Szumiel, 2011).
Among the enzymes regulating reactive oxygen species, superoxide dismutases (SODs) play an important role by facilitating the transformation of the superoxide anion (O2•−) into hydrogen peroxide (H2O2), which is then further processed by a variety of enzymes (Fridovich, 1997). Humans express three distinct SODs, namely SOD1–3. While SOD2 is localized within the mitochondria and SOD3 is located extracellularly, SOD1 is mainly found in the cytoplasm (Fukai and Ushio-Fukai, 2011). However, minor amounts are also localized in the mitochondrial intermembrane space and the nucleus of eukaryotic cells (Higgins et al., 2002; Chung, 2017).
Previously, variants of SOD1 have been implicated in the pathogenesis of familial amyotrophic lateral sclerosis (ALS), a debilitating neurological disorder characterized by the progressive degeneration of motor neurons. SOD1 variants are causative for a significant proportion of familial ALS cases, ranging from about 13% in western to up to 30% in Asian populations (Kaur et al., 2016; Zou et al., 2017). While initially believed to exert mainly gain-of-function effects on the enzyme’s activity, it has become clear that the described variants result in a variety of effects, some of which are suggestive of a prion-like pathomechanism (Vijayvergiya et al., 2005) or mitochondrial dysfunction (Magrane et al., 2009). These emerging new understandings of the pathomechanism of familial ALS and indeed also sporadic ALS (Alexander et al., 2002) continue to give insights into the origins of this debilitating disorder. Interestingly, the overwhelming majority of SOD1 variants associated with familial ALS show an autosomal-dominant inheritance pattern with homozygous variants being a rare exception (Andersen et al., 1995, 1996, 1998). Heterozygous SOD1 variants are therefore well established as a cause of familial ALS. These variants result in varying levels of SOD1 enzyme activity, ranging from severely reduced to levels above those observed in wild-type SOD1 (Borchelt et al., 1994; Keskin et al., 2017). Notably, the observed enzyme activity shows no association with clinical severity (Cleveland et al., 1995).
There is a growing number of Sod1-deficient mouse models. While many were created using ALS-causing mutations observed in humans in order to study the associated phenotype (Dal Canto and Gurney, 1995; Tu et al., 1996), complete knockouts have also been created. Interestingly, no motor neuron disease has been observed in these mice (Reaume et al., 1996). However, they show extensive muscle involvement consisting of progressive motorneuronopathy with axonal denervation (Frey et al., 2000; Hegedus et al., 2007) resulting in secondary muscle pathology (Muller et al., 2006). This phenotype is associated with increased oxidative stress secondary to mitochondrial dysfunction (Fischer et al., 2012).
In addition to neuronal manifestations, these animals exhibit extraneuronal phenotypes with a shortened lifespan, hepatocellular carcinoma (Elchuri et al., 2005) and altered hepatic energy metabolism (Wang et al., 2012) as well as exhibiting endocrinological abnormalities (Matzuk et al., 1998). For an extensive review of Sod1-deficient murine models, see Saccon et al. (2013).
In this study, we report on a homozygous loss-of-function SOD1 variant identified in a patient with a debilitating neurological phenotype. The variant leads to SOD1 activity levels below measurable ranges and is associated with a phenotype marked by hyperekplexia, ataxia and muscular hypotonia in addition to severe psychomotor retardation.
Materials and methods
Subjects
Clinical evaluation was performed on the index patient as well as his parents. In addition, electrophysiological studies were performed on the index patient. All procedures were performed after consent of the patient’s parents was obtained. Written consent for the publication of any photographs was obtained.
Neuroimaging and neuromuscular assessment
Cranial MRI was performed on the index patient at the ages of 2 and 6 years. Furthermore, electromyography and ultrasound of the right deltoid and left vastus lateralis muscle were performed at the age of 6 years.
Genetic analysis
DNA was prepared from EDTA blood samples using the QIAamp DNA Mini Kit (Qiagen). The coding and flanking intronic regions were enriched using in-solution hybridization technology and were sequenced using the Illumina HiSeq/NovaSeq system. Illumina bcl2fastq2 was used to demultiplex sequencing reads. Adapter removal was performed with Skewer. The trimmed reads were mapped to the human reference genome (hg19). Read duplicates that likely resulted from PCR amplification were removed. The remaining high-quality sequences were used to determine sequence variants (single nucleotide changes and small insertions/deletions). Variants were filtered and grouped into the following categories (Supplementary Table 1): de novo variants, homozygous variants, compound heterozygous, and hemizygous. Because of the family history and the absence of a manifest phenotype in the parents, an autosomal-recessive mode of inheritance was deemed most likely. In silico variant evaluation was carried out using the prediction software MutationTaster (Schwarz et al., 2014) and Provean (Choi and Chan, 2015), as well as PolyPhen-2 (Adzhubei et al., 2010). Identified variants were verified using traditional Sanger sequencing as described previously (Park et al., 2015).
Superoxide dismutase functional assay
EDTA blood (10 ml) was mixed with 10 ml ACD-B (acid citrate-dextrose) and incubated for 2 h at room temperature to allow erythrocyte sedimentation. The supernatant was then centrifuged for 15 min at 1550 rpm. The resulting pellet was washed with a mixture of 0.8 ml 0.9% saline solution and 2.4 ml ultrapure water for 90 s, with 0.8 ml 3.6% saline solution added directly after the incubation period. This was followed by a further 10-min centrifugation at 1550 rpm. The supernatant was again discarded, and the pellet repeatedly washed until no erythrocytes were visible. After completion, the pellet was frozen until further processing.
SOD activity was measured using a spectrophotometric approach according to a previously published protocol (Spitz and Oberley, 1989, 2001).
Muscle biopsy
A muscle biopsy was taken from the index patient’s vastus lateralis muscle at the age of 2 years. The biopsy was stained using Periodic acid–Schiff, NADH, ATPase pH 4.3 and 9.4, MADA, phosphofructokinase, and myo-phosphorylase staining. In addition, immunofluorescence for various skeletal muscle proteins and respiratory chain complex activity measurements were carried out.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Case report and radiography studies
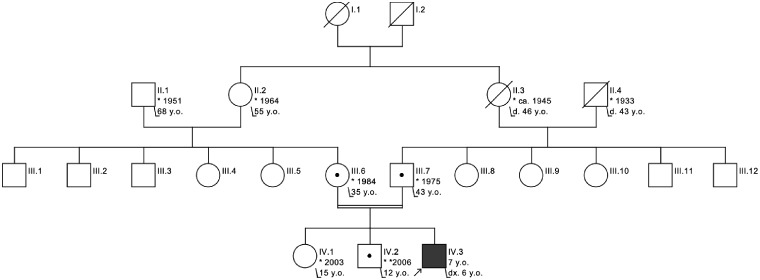
The index patient is the third child of consanguineous parents (first degree cousins) of Afghan origin (Fig. 1). During pregnancy, polyhydramnios was noted. The patient was born at 39 weeks of pregnancy via emergency Caesarean section due to a maternal indication.
Figure 1.
Pedigree of the index patient. His parents are first degree cousins. No other affected individuals were reported. Notably, there are no known cases of (familial) ALS in the kinship, while many of the reported individuals are at or above the mean age of onset observed in SOD1-related familial ALS.
The family reported normal development until the age of 9 months when the patient was able to crawl. Shortly afterwards, progressive psychomotor decline marked by loss of motor abilities and progressive ataxia began.
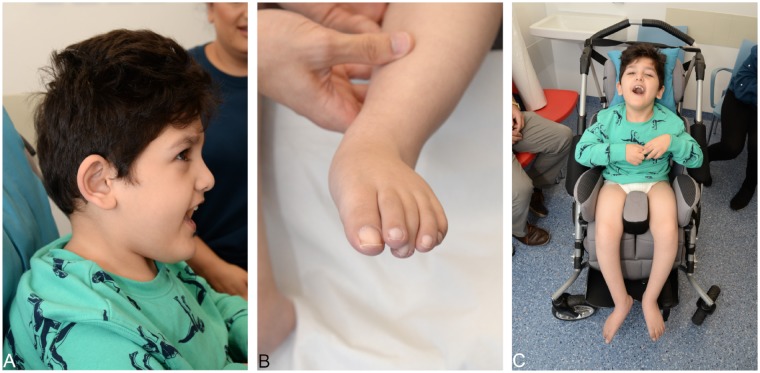
Upon initial presentation at our tertiary care centre at the age of 6 years, he showed a significant combined developmental delay as he was not able to sit or stand unsupported. Additionally, he presented with dysmorphic features such as low set, posteriorly rotated ears (Fig. 2A) and overlapping toes (T2, 3 on the left and T3, 2 on the right) (Fig. 2B).
Figure 2.
Phenotype of the index patient at 6 years of age. Dysmorphic features such as low set, posteriorly rotated ears and overlapping toes are present. A clinical examination demonstrating hyperekplexia-like symptoms is available online.
Clinical examination revealed an increased muscle tone of both upper and lower limbs with persistently bended arms in pronated position whereas muscular mass and distribution appeared normal. Corresponding to the spasticity he demonstrated hyper-reflexive brachioradial reflexes and more pronounced hyperreflexia in patellar reflexes together with enlarged reflex zones. Furthermore, we observed pyramidal path symptoms such as positive Babinski sign and bilateral exhaustible clonus of the feet (Supplementary Video 1). In addition, we stated pronounced symptomatic hyperekplexia with persistent glabellar tap sign as well as an incomplete Moro reflex consisting of the initial abduction, while the typical subsequent adduction reaction was lacking.
Truncal and proximal muscular hypotonia leading to the inability to sit or stand was noted. Therefore, it was not possible to examine for truncal ataxia. The patient showed no nystagmus or oculomotor dysfunction, but slight tremor of both hands and fingers upon movement. Cognitive functions were impaired; however, he was able to understand and/or implement simple correlations but not complexes requirements (e.g. directed pointing). Therefore, detailed assessment of these symptoms remains unanswered. Only non-verbal communication is possible.
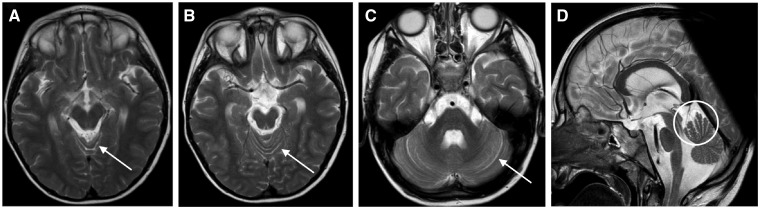
Cranial MRI was performed at the age of 2 and again at 6 years. The initial MRI was inconclusive. At the age of 6 years, mild cerebellar atrophy with discreetly enlarged interfoliar spaces in the region of the anterior vermis was diagnosed (Fig. 3).
Figure 3.
T2-weighted cranial MRI of the index patient at the age of 6 years. In general, mild cerebellar atrophy is present. The cerebellar interfoliar spaces at the anterior vermis are discreetly enlarged (indicated by arrows) as are the cerebellar hemispheres in the lateral view (D).
Extensive metabolic screening, including serum organic acids, serum acylcarnitine profile, urinary oligosaccharides and amino acids, purines/pyrimidines, and serum lactate analysis was performed but results were inconclusive.
Evaluation of antioxidant vitamins and trace elements revealed consistently low levels of blood manganese between 3.1 and 4 ng/ml (reference: 7–11 ng/ml) and zinc [672 µg/l (reference: 750–1400 µg/l)].
Heterozygous carriers identified within this family did not show any overt neurological phenotype. There were no cases of ALS reported in this family.
Genetic analysis
Array-based comparative genomic hybridization of the index patient was carried out without showing any abnormalities. Whole exome sequencing revealed that the patient was homozygous for the frameshift SOD1 variant c.335dupG, resulting in a premature stop codon at position 112 of the resulting polypeptide (p.C112Wfs*11), thus terminating the polypeptide within GK2, i.e. the second β-sheet connection. Importantly, based on previous structural analysis, this disruption can be assumed to affect both a Cu binding site as well as an H2O2 liganding residue, which is believed to exert an important functional role by controlling the active site of the protein (Perry et al., 2010). The parents as well as an older brother were heterozygous for the variant. Additional variants identified in the subjects are presented in the Supplementary material.
Superoxide dismutase functional assay
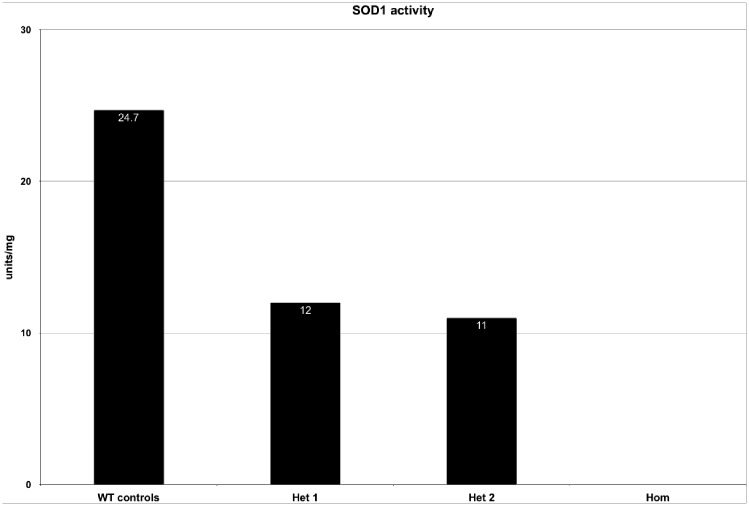
The activity of SOD1 of the index patient was below the detection level of 2 units/mg, whereas SOD2 activity was within normal ranges. Heterozygous carriers of the identified variant had an approximately halved SOD1 activity of 12 and 11 units/mg when compared to wild-type controls (55 and 51% of wild-type SOD1 activity, respectively).
In contrast, the activities measured in three healthy control subjects were all within reference ranges (Fig. 4).
Figure 4.
SOD1 activity measurement of the patient and heterozygous carriers compared to wild-type controls. Measurements were performed on isolated blood leucocytes. The index patient had no detectable SOD1 activity. Heterozygous carriers showed an approximately halved SOD1 activity of 12 and 11 units/mg protein, corresponding to 55% and 51% of the activity measured in wild-type (WT) controls, respectively.
Muscle biopsy and neurophysiological examination
The muscle biopsy obtained at the age of 2 years revealed a pathological finding with increased fibre size variability and atrophic fibres. The number of type 2 fibres was increased but no type grouping was present (Supplementary Fig. 1). Furthermore, focally decreased α-dystroglycan expression was observed, while the function of respiratory chain complexes I–IV was normal.
Muscle ultrasound examination showed multiple fasciculations of the right deltoid, right extensor digitorum, and vastus medialis and tibialis anterior muscles on both sides.
Accordingly, multiple fasciculations of right deltoid and left vastus medialis muscle were identified using electromyography while no other pathologic spontaneous activity was seen. Besides these findings, nerve conduction studies and visual evoked potentials were normal.
Discussion
In this study, we describe a patient carrying a truncating homozygous SOD1 variant resulting in the total absence of SOD1 activity. Human SOD1 deficiency has, to our knowledge, never been described before.
The link between SOD1 variants and familial ALS has been firmly established. Indeed, research on SOD1-related ALS has been an important way of delineating the pathogenesis of this debilitating neurodegenerative disorder.
A vast number of variants in SOD1 have been identified in familial ALS but despite extensive research, the exact pathomechanism by which these alterations cause familial ALS remains elusive. Recently, it has become clear that there are probably several ways in which SOD1 variants exert their causative role in the disorder. It has been firmly established that a vast number of variants leads to a gain-of-function of the gene product SOD1 (Bruijn et al., 1998; Allen et al., 2003). Furthermore, SOD1 aggregation (Jonsson et al., 2008) and mitochondrial dysfunction (De Vos et al., 2007) have been found to play a major role in the disorder.
The role of loss-of-function mutations is somewhat disputed. While this concept was initially believed to be the underlying cause of familial ALS (Deng et al., 1993; Rosen et al., 1993), mounting evidence against this notion has been derived from animal models (Ratovitski et al., 1999; Boillee et al., 2006). In addition, various human SOD1 variants that retain the full dismutase enzyme activity have been described in familial ALS (Borchelt et al., 1994; Hayward et al., 2002). Therapeutic trials of antisense oligonucleotides in murine models carrying human SOD1 variants support this notion (McCampbell et al., 2018).
SOD1 loss-of-function is a new disease with recessive mode of inheritance and heterozygous carriers not affected by the disorder. Although the eldest investigated heterozygous carriers (35 and 44 years, respectively) could still develop symptoms of familial ALS, there have to be several older heterozygous carriers in earlier generations of the family without any symptoms of familial ALS (Fig. 1) (Bali et al., 2017), showing that a reduction in enzyme activity—as in heterozygous carriers—does not lead to familial ALS whereas complete loss-of-function leads to a different disease.
The disorder observed in our patient differs significantly from motor neuron disease. While it shares some similarities, such as signs of muscular denervation observed indirectly in electromyography and muscle ultrasound, the vast majority of symptoms have not been described in ALS before. The age of onset in early childhood is atypical, as is the phenotype indicative of first motor neuron affection. The hyperekplexia-like presentation is a hallmark of the syndrome, which might facilitate diagnosis in the future.
The phenotype bears distinct similarities to the presentation of total murine Sod1 knockouts (Matzuk et al., 1998; Elchuri et al., 2005; Wang et al., 2012; Sakellariou et al., 2018) in which similar features such as tremor, reduced muscle mass, and motor axonopathy (Shefner et al., 1999) were noted.
The total absence of any measurable SOD1 activity suggests a major role of oxidative stress or dysregulation in the pathogenesis of the disease. This is in part supported by the finding of hypomanganesaemia in the index patient, as manganese is a known antioxidant, either as part of SOD2 or independently (Coassin et al., 1992; Aguirre and Culotta, 2012).
Given the severe and debilitating nature of the disorder, the need for therapeutic options is evident. Based on SOD1’s role in metabolism of reactive oxygen species, antioxidant therapy, e.g. by external supplementation, represents an intriguing approach. Indeed, the antioxidant N-acetylcystein was shown to have positive effects on anaemia and autoantibody generation in Sod1−/− mice (Iuchi et al., 2007).
In addition to antioxidant compounds, SOD mimetics represent another promising therapeutic approach. These agents have been suggested as treatment options in the context of Parkinson’s disease (Filograna et al., 2016) and radiation damage (Anderson et al., 2018), among others. In a phase 1b/2a trial in patients undergoing radiation therapy for oral carcinoma, the mimetic GC4419 showed acceptable safety (Anderson et al., 2018), making it a candidate for application in SOD deficiency in the future.
The findings presented in this study not only shed light on the pathomechanism of SOD1-related ALS but are also of high relevance for the intensely investigated therapeutic strategy of SOD1 silencing. Previous studies in various ALS disease models have established the general efficacy of silencing using antisense oligonucleotides, shRNA, miRNA, and other compounds (for a review see van Zundert and Brown, 2017). Due to the lack of ALS in SOD1 knockout mice, safety of silencing of both wild-type and mutant SOD1 is assumed (van Zundert and Brown, 2017).
Given the severe neurological manifestation of SOD1 deficiency in our patient, the possibility of adverse effects following SOD1 silencing must be considered. From a pathomechanistic point of view, silencing of the principal cytoplasmic superoxide dismutase might result in increased oxidative damage as well as altered redox signalling.
Findings from animal as well as early clinical studies did not indicate any adverse events attributable to SOD1 deficiency (Miller et al., 2013; Thomsen et al., 2014; Stoica et al., 2016; Borel et al., 2018). This can in part be explained by the incomplete representation of human (patho)physiology by animal models. Furthermore, currently published findings in humans made use of low doses of silencing agents not resulting in complete suppression of SOD1 activity (Miller et al., 2013). Given the absence of an observable neurological phenotype in heterozygous carriers of the variant described in our study, these results do not exclude adverse events by SOD1 silencing.
In conclusion, our data characterize the effects of a truncating mutation of SOD1, leading to total absence of SOD1 activity in the affected patient. The resulting phenotype is severe with tetraspasticity mainly affecting the upper limbs and hyperreflexia reflecting an affection of the first motor neuron. Antioxidant supplementation may represent a therapeutic approach, although further research is needed to characterize the effects of the variant on a deeper level. The results of this study call for a cautious approach to SOD1 silencing as a therapeutic concept for ALS.
Supplementary Material
Acknowledgements
The authors are deeply indebted to the affected individual, his family, and caretakers for their participation in this study. They are grateful to Prof. Dr. med. Matthias Schilling for fruitful discussions as well as electrophysiological examination of the index patient. Furthermore, technical assistance from members of the Department of Paediatric Neurology of the Children’s University Hospital Münster as well as the Department of Neurology of the University of Münster is gratefully acknowledged.
Glossary
Abbreviations
- ALS
amyotrophic lateral sclerosis
- SOD
superoxide dismutase
Funding
This work was supported by US National Institutes of Health (NIH) grant R01 CA182804 (to D.R.S.).
Competing interests
The authors report no competing interests.
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P et al. A method and server for predicting damaging missense mutations. Nat Methods 2010; 7: 248–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre JD, Culotta VC. Battles with iron: manganese in oxidative stress protection. J Biol Chem 2012; 287: 13541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MD, Traynor BJ, Miller N, Corr B, Frost E, McQuaid S et al. “True” sporadic ALS associated with a novel SOD-1 mutation. Ann Neurol 2002; 52: 680–3. [DOI] [PubMed] [Google Scholar]
- Allen S, Heath PR, Kirby J, Wharton SB, Cookson MR, Menzies FM et al. Analysis of the cytosolic proteome in a cell culture model of familial amyotrophic lateral sclerosis reveals alterations to the proteasome, antioxidant defenses, and nitric oxide synthetic pathways. J Biol Chem 2003; 278: 6371–83. [DOI] [PubMed] [Google Scholar]
- Andersen PM, Forsgren L, Binzer M, Nilsson P, Ala-Hurula V, Keranen ML et al. Autosomal recessive adult-onset amyotrophic lateral sclerosis associated with homozygosity for Asp90Ala CuZn-superoxide dismutase mutation. A clinical and genealogical study of 36 patients. Brain 1996; 119(Pt 4): 1153–72. [DOI] [PubMed] [Google Scholar]
- Andersen PM, Nilsson P, Ala-Hurula V, Keranen ML, Tarvainen I, Haltia T et al. Amyotrophic lateral sclerosis associated with homozygosity for an Asp90Ala mutation in CuZn-superoxide dismutase. Nat Genet 1995; 10: 61–6. [DOI] [PubMed] [Google Scholar]
- Andersen PM, Nilsson P, Forsgren L, Marklund SL. CuZn-superoxide dismutase, extracellular superoxide dismutase, and glutathione peroxidase in blood from individuals homozygous for Asp90Ala CuZu-superoxide dismutase mutation. J Neurochem 1998; 70: 715–20. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Sonis ST, Lee CM, Adkins D, Allen BG, Sun W et al. Phase 1b/2a trial of the superoxide dismutase mimetic GC4419 to reduce chemoradiotherapy-induced oral mucositis in patients with oral cavity or oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2018; 100: 427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res 2009; 66: 121–7. [DOI] [PubMed] [Google Scholar]
- Bali T, Self W, Liu J, Siddique T, Wang LH, Bird TD et al. Defining SOD1 ALS natural history to guide therapeutic clinical trial design. J Neurol Neurosurg Psychiatry 2017; 88: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 2006; 52: 39–59. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Lee MK, Slunt HS, Guarnieri M, Xu ZS, Wong PC et al. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci USA 1994; 91: 8292–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel F, Gernoux G, Sun H, Stock R, Blackwood M, Brown RH Jr et al. Safe and effective superoxide dismutase 1 silencing using artificial microRNA in macaques. Sci Transl Med 2018; 10: eaau6414. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 1998; 281: 1851–4. [DOI] [PubMed] [Google Scholar]
- Choi Y, Chan AP. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 2015; 31: 2745–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WH. Unraveling new functions of superoxide dismutase using yeast model system: beyond its conventional role in superoxide radical scavenging. J Microbiol 2017; 55: 409–16. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Laing N, Hurse PV, Brown RH Jr. Toxic mutants in Charcot's sclerosis. Nature 1995; 378: 342–3. [DOI] [PubMed] [Google Scholar]
- Coassin M, Ursini F, Bindoli A. Antioxidant effect of manganese. Arch Biochem Biophys 1992; 299: 330–3. [DOI] [PubMed] [Google Scholar]
- Dal Canto MC, Gurney ME. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS). Brain Res 1995; 676: 25–40. [DOI] [PubMed] [Google Scholar]
- De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet 2007; 16: 2720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY et al. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science 1993; 261: 1047–51. [DOI] [PubMed] [Google Scholar]
- Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 2005; 24: 367–80. [DOI] [PubMed] [Google Scholar]
- Fang FC. Antimicrobial actions of reactive oxygen species. mBio 2011; 2: e00141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filograna R, Godena VK, Sanchez-Martinez A, Ferrari E, Casella L, Beltramini M et al. Superoxide dismutase (SOD)-mimetic M40403 is protective in cell and fly models of paraquat toxicity: IMPLICATIONS FOR PARKINSON DISEASE. J Biol Chem 2016; 291: 9257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer LR, Li Y, Asress SA, Jones DP, Glass JD. Absence of SOD1 leads to oxidative stress in peripheral nerve and causes a progressive distal motor axonopathy. Exp Neurol 2012; 233: 163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry 2010; 49: 835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci 2000; 20: 2534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem 1997; 272: 18515–7. [DOI] [PubMed] [Google Scholar]
- Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 2011; 15: 1583–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LJ, Rodriguez JA, Kim JW, Tiwari A, Goto JJ, Cabelli DE et al. Decreased metallation and activity in subsets of mutant superoxide dismutases associated with familial amyotrophic lateral sclerosis. J Biol Chem 2002; 277: 15923–31. [DOI] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 2007; 28: 154–64. [DOI] [PubMed] [Google Scholar]
- Higgins CM, Jung C, Ding H, Xu Z. Mutant Cu, Zn superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. J Neurosci 2002; 22: Rc215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi Y, Okada F, Onuma K, Onoda T, Asao H, Kobayashi M et al. Elevated oxidative stress in erythrocytes due to a SOD1 deficiency causes anaemia and triggers autoantibody production. Biochem J 2007; 402: 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson PA, Bergemalm D, Andersen PM, Gredal O, Brannstrom T, Marklund SL. Inclusions of amyotrophic lateral sclerosis-linked superoxide dismutase in ventral horns, liver, and kidney. Ann Neurol 2008; 63: 671–5. [DOI] [PubMed] [Google Scholar]
- Kaur SJ, McKeown SR, Rashid S. Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene 2016; 577: 109–18. [DOI] [PubMed] [Google Scholar]
- Keskin I, Birve A, Berdynski M, Hjertkvist K, Rofougaran R, Nilsson TK et al. Comprehensive analysis to explain reduced or increased SOD1 enzymatic activity in ALS patients and their relatives. Amyotroph Lateral Scler Frontotemporal Degener 2017; 18: 457–63. [DOI] [PubMed] [Google Scholar]
- LeVine SM. The role of reactive oxygen species in the pathogenesis of multiple sclerosis. Med Hypotheses 1992; 39: 271–4. [DOI] [PubMed] [Google Scholar]
- Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 2014; 224: 164–75. [DOI] [PubMed] [Google Scholar]
- Magrane J, Hervias I, Henning MS, Damiano M, Kawamata H, Manfredi G. Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum Mol Genet 2009; 18: 4552–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology 1998; 139: 4008–11. [DOI] [PubMed] [Google Scholar]
- McCampbell A, Cole T, Wegener AJ, Tomassy GS, Setnicka A, Farley BJ et al. Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. J Clin Invest 2018; 128: 3558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TM, Pestronk A, David W, Rothstein J, Simpson E, Appel SH et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol 2013; 12: 435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med 2006; 40: 1993–2004. [DOI] [PubMed] [Google Scholar]
- Park JH, Hogrebe M, Gruneberg M, DuChesne I, von der Heiden AL, Reunert J et al. SLC39A8 deficiency: a disorder of manganese transport and glycosylation. Am J Hum Genet 2015; 97: 894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JJ, Shin DS, Getzoff ED, Tainer JA. The structural biochemistry of the superoxide dismutases. Biochim Biophys Acta 2010; 1804: 245–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratovitski T, Corson LB, Strain J, Wong P, Cleveland DW, Culotta VC et al. Variation in the biochemical/biophysical properties of mutant superoxide dismutase 1 enzymes and the rate of disease progression in familial amyotrophic lateral sclerosis kindreds. Hum Mol Genet 1999; 8: 1451–60. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet 1996; 13: 43–7. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993; 362: 59–62. [DOI] [PubMed] [Google Scholar]
- Saccon RA, Bunton-Stasyshyn RK, Fisher EM, Fratta P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain 2013; 136(Pt 8): 2342–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakellariou GK, McDonagh B, Porter H, Giakoumaki II, Earl KE, Nye GA et al. Comparison of whole body SOD1 knockout with muscle-specific SOD1 knockout mice reveals a role for nerve redox signaling in regulation of degenerative pathways in skeletal muscle. Antioxid Redox Signal 2018; 28: 275–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 2014; 11: 361–2. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Reaume AG, Flood DG, Scott RW, Kowall NW, Ferrante RJ et al. Mice lacking cytosolic copper/zinc superoxide dismutase display a distinctive motor axonopathy. Neurology 1999; 53: 1239–46. [DOI] [PubMed] [Google Scholar]
- Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem 1989; 179: 8–18. [DOI] [PubMed] [Google Scholar]
- Spitz DR, Oberley LW. Measurement of MnSOD and CuZnSOD activity in mammalian tissue homogenates. Curr Protoc Toxicol 2001; Chapter 7: Unit7.5. doi: 10.1002/0471140856.tx0705s08. [DOI] [PubMed] [Google Scholar]
- Stoica L, Todeasa SH, Cabrera GT, Salameh JS, ElMallah MK, Mueller C et al. Adeno-associated virus-delivered artificial microRNA extends survival and delays paralysis in an amyotrophic lateral sclerosis mouse model. Ann Neurol 2016; 79: 687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumiel I. Autophagy, reactive oxygen species and the fate of mammalian cells. Free Radic Res 2011; 45: 253–65. [DOI] [PubMed] [Google Scholar]
- Thomsen GM, Gowing G, Latter J, Chen M, Vit JP, Staggenborg K et al. Delayed disease onset and extended survival in the SOD1G93A rat model of amyotrophic lateral sclerosis after suppression of mutant SOD1 in the motor cortex. J Neurosci 2014; 34: 15587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PH, Raju P, Robinson KA, Gurney ME, Trojanowski JQ, Lee VM. Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc Natl Acad Sci USA 1996; 93: 3155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert B, Brown RH Jr. Silencing strategies for therapy of SOD1-mediated ALS. Neurosci Lett 2017; 636: 32–9. [DOI] [PubMed] [Google Scholar]
- Vijayvergiya C, Beal MF, Buck J, Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J Neurosci 2005; 25: 2463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Jiang Z, Lei XG. Knockout of SOD1 alters murine hepatic glycolysis, gluconeogenesis, and lipogenesis. Free Radic Biol Med 2012; 53: 1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou ZY, Zhou ZR, Che CH, Liu CY, He RL, Huang HP. Genetic epidemiology of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg psychiatry 2017; 88: 540–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.