Abstract
Cancer- and treatment-related cognitive changes have been a focus of increasing research since the early 1980s, with meta-analyses demonstrating poorer performance in cancer patients in cognitive domains including executive functions, processing speed, and memory. To facilitate collaborative efforts, in 2011 the International Cognition and Cancer Task Force (ICCTF) published consensus recommendations for core neuropsychological tests for studies of cancer populations. Over the past decade, studies have used neuroimaging techniques, including structural and functional magnetic resonance imaging (fMRI) and positron emission tomography, to examine the underlying brain basis for cancer- and treatment-related cognitive declines. As yet, however, there have been no consensus recommendations to guide researchers new to this field or to promote the ability to combine data sets. We first discuss important methodological issues with regard to neuroimaging study design, scanner considerations, and sequence selection, focusing on concerns relevant to cancer populations. We propose a minimum recommended set of sequences, including a high-resolution T1-weighted volume and a resting state fMRI scan. Additional advanced imaging sequences are discussed for consideration when feasible, including task-based fMRI and diffusion tensor imaging. Important image data processing and analytic considerations are also reviewed. These recommendations are offered to facilitate increased use of neuroimaging in studies of cancer- and treatment-related cognitive dysfunction. They are not intended to discourage investigator-initiated efforts to develop cutting-edge techniques, which will be helpful in advancing the state of the knowledge. Use of common imaging protocols will facilitate multicenter and data-pooling initiatives, which are needed to address critical mechanistic research questions.
Non–central nervous system (CNS) cancer and its treatments are associated with cognitive declines that can be persistent (1–3), with executive functions, processing speed, and memory especially affected (4–7). Neuroimaging studies have demonstrated alterations in brain structure and function (8–11) that correlate with objective and/or subjective cognitive performance (12–14). Important questions remain regarding the role of cancer disease processes (15–17) and treatments other than chemotherapy (18–20). While most research has focused on breast cancer, other non-CNS cancers have also been studied (21–33). Greater mechanistic understanding of these changes will advance prevention and remediation efforts. Neuroimaging can provide unique and important biomarkers of cognitive changes; however, methodological variability has precluded practice-changing conclusions. Given difficulties inherent in studying cancer patients, multicenter studies and data-pooling initiatives are needed to gain sufficient statistical power to address critical research questions (34).
The International Cognition and Cancer Task Force (ICCTF) was founded in 2006 to facilitate international collaborations around cognitive impairment in cancer patients (35). A specific goal of the ICCTF is to create research recommendations and guidelines that increase the homogeneity of study methods to facilitate between-study comparisons, meta-analyses, and data pooling (36) and provide better estimates of incidence, severity, individual risk factors, and causes of cognitive changes (37). In 2011, the ICCTF published recommendations regarding cognitive assessment methods (37). Here the ICCTF Neuroimaging Working Group offers recommendations for magnetic resonance imaging (MRI) and positron emission tomography (PET). We focus on concerns particularly relevant to studies of cancer populations, including study design, timing of assessments, and choice of control groups(s), and we recommend sequences accessible to most institutions thought likely to be fruitful based on the existing literature (Figure 1).
Figure 1.
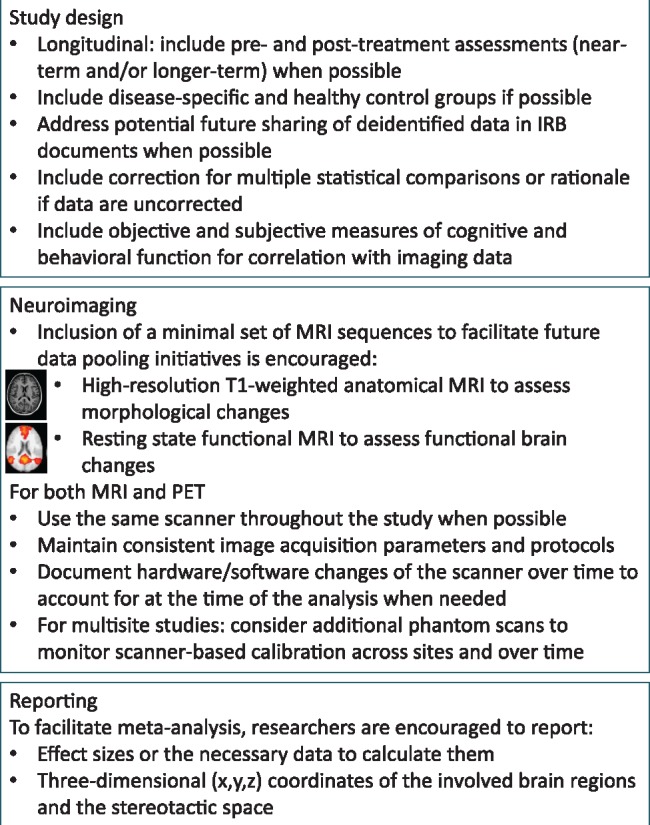
Main recommendations and considerations for incorporation of neuroimaging into studies of cognitive impairment in non–central nervous system cancer patients. IRB = institutional review board; MRI = magnetic resonance imaging; PET = positron emission tomography.
Methodological Considerations
We first discuss general study design concepts formulated in the ICCTF cognitive studies publication (37) that are also critical for neuroimaging studies, then describe additional aspects specific to neuroimaging. Those newly implementing neuroimaging should work with an magnetic resonance imaging (MRI)/positron emission tomography (PET) physicist to optimize sequences (eg, while smaller voxel size increases spatial resolution, other parameters would need to be adjusted to maintain overall signal-to-noise, resulting in increased scan time). Collaboration/consultation with neuroradiology colleagues and/or researchers with neuroimaging expertise in cancer populations may help studies start efficiently and avoid common pitfalls. This Commentary focuses on human neuroimaging studies. Preclinical work is another important component of examination of mechanisms underlying cancer- and treatment-related cognitive changes. Best practices for studies in animal models, including neuroimaging-related considerations, are beyond the scope of this Commentary, but are considered elsewhere (38,39).
General Considerations
Double-blind, randomized, placebo-controlled study designs are optimal, but unethical for cancer patients. Therefore, the optimal approach is a prospective longitudinal design with appropriate control groups, including a minimum of one assessment before and after the treatment under investigation. A longer-term follow-up assessment is also advised to examine persistent or delayed effects. Using intervals similar to those previously published will advance the ability to compare and combine data sets. Acute follow-up intervals have commonly ranged from one to six months postchemotherapy, with longer-term follow-up typically about one year later. Timing of assessments depends on the scientific research questions, taking into consideration potential patient symptoms and side effects and timing of other treatments.
Including control groups is essential, given the absence of appropriate normative data to adequately control for possible confounding factors (eg, variability in neuroimaging equipment and scan sequences). The ideal comparison would be a disease-specific control group differing only in the treatment under investigation. Given current treatment paradigms, patient groups usually also differ in clinical aspects of disease. Such differences should be documented and considered in analyses. The inclusion of an additional control group of individuals without cancer is also recommended to determine the normal pattern of change in outcome variables over time and if observed effects might be due to technical aspects of neuroimaging (eg, scanner drift).
One challenge for data-pooling initiatives is the need to comply with institutional review board (IRB) requirements for data sharing. We strongly recommend including a section specifically addressing the potential future sharing of deidentified data sets in IRB documents, including informed consents. IRB requirements vary across countries and institutions and change over time. Inclusion of such language facilitates the ability to rapidly participate in multicenter initiatives.
Participant Recruitment
Novice neuroimaging researchers have reported difficulty enrolling participants. The authors have found that a critical component of successful recruitment is having research staff who present the study with confidence and enthusiasm and respond to questions in a positive, knowledgeable, reassuring manner. Offering a mock scanner session or visiting/laying in the real scanner can be useful for hesitant individuals. Providing a picture of the brain to take home, having structural scans read by a neuroradiologist for incidental findings, and offering an honorarium for time and effort are also helpful. Flexibility in scheduling study visits can make a great difference in recruitment of patients trying to juggle work, personal responsibilities, and medical care. “Piggybacking” imaging onto other studies in cancer populations can also be effective; for example, participants already enrolled in studies of cognition or quality of life may also consider participating in neuroimaging.
Cancer patients tend to be more receptive to research participation when first approached by a member of their clinical team, so involving surgeons and oncologists in study design and efforts to promote the work is crucial. For prospective studies attempting to obtain pretreatment baseline assessments, working with clinicians as early in the diagnostic and treatment process as possible is important. For example, identifying potentially eligible patients via the surgeon may offer a greater window of time to schedule initial prechemotherapy assessments vs identifying patients through the medical oncologist. Financial support of nurse navigators or other clinic staff to support time spent on recruitment can also be very helpful.
MRI Scanner Considerations
MRI should preferably be conducted on a 3T system, using the most advanced headcoil available for maximum signal detection. It is also important to minimize equipment differences. Consistent scan parameters should be maintained across participants and time points, as slight variations can have great effects. Changes in the scanner or equipment (eg, software or hardware upgrades, coil changes) should be documented. Consideration should be given to scanning phantoms relevant to different sequences at regular intervals, as well as after scanner upgrades, to monitor stability. Regular phantom scans can monitor calibration across sites and over time.
It is ideal to use one scanner for all participants and time points. If acquisition cannot be confined to one scanner (eg, for multicenter studies), individual participants should be assessed on the same scanner over time. Patient and control groups should be intermixed across scanners and over time to avoid confounding group with scanner type or scanner changes over time.
MRI Scan Protocol Considerations
It is beneficial to obtain close to isotropic voxels, particularly for high-resolution structural MRI and diffusion tensor imaging (DTI), to avoid directional bias, reduce partial volume effects and registration errors, and allow more precise and accurate measurements. As cancer treatment effects have been detected in distributed brain regions, whole-brain coverage, including the cerebellum, should be obtained unless contraindicated by the specific scan sequence (eg, arterial spin labeling [ASL]). It is advisable to standardize certain scan parameters across sequences. For instance, using the same field of view (FOV) and angulation increases uniformity of the scan protocol and facilitates multimodal analysis.
Motion is extremely detrimental; minimization of head and body motion should be repeatedly emphasized to the participant before and during scanning. Visual inspection of data at the time of acquisition can be helpful to provide participants with feedback on their level of motion and to repeat sequences as necessary. Remaining in close contact with the participant during scanning is also helpful in reducing anxiety and maintaining motivation and arousal (eg, for fMRI tasks).
It is important to maintain a standardized scan protocol order and document any deviations for consideration in analysis. Series that are particularly susceptible to motion might be better placed early in the scanning session. It is recommended that resting state fMRI (rsfMRI) scans be conducted either before or otherwise at a time removed from task-based scans to reduce the effects of cognitive processes on intrinsic networks (40).
Considerations for MRI Sequence Selection
MR technology can be divided into three tiers: 1) conventional techniques in routine clinical use for structural imaging and available on most 3T and 1.5T scanners, such as T1-weighted and T2-weighted volumetric imaging and fluid attenuated inversion recovery (FLAIR) scans; 2) advanced techniques including specialized sequences such as task-based blood oxygen level–dependent (BOLD) and rsfMRI, diffusion, perfusion, and MR spectroscopy—advanced sequences have greater likelihood of equipment, vendor-specific software feature, and hardware performance differences; 3) emerging techniques including leading-edge prototype applications available at academic centers as “work-in-progress (WIP)” software that may become available commercially if proven useful and there is sufficient demand (eg, multiband acquisition software for BOLD and diffusion imaging). These are the most demanding applications in terms of hardware, software, and specialized expertise.
The Alzheimer’s Disease Neuroimaging Initiative (ADNI) and other consortia (eg, Transforming Research and Clinical Knowledge in TBI [TRACK-TBI], International Neuroimaging Data Sharing Initiative [INDI], Brain Development Cooperative Group) have multivendor 3T MR acquisition sequences that are relatively harmonized. Use of such already-vetted sequences might be particularly helpful for multicenter collaborations or investigators with more limited neuroimaging resources. The ADNI sequences are available at http://adni.loni.usc.edu/methods/documents/mri-protocols/. ADNI-3 includes T1, FLAIR, fMRI, DTI, and ASL perfusion sequences for multiple vendor platforms, both basic (for most available 3T systems) and advanced (for systems with connectome-level hardware gradients and multiband software).
Recommended Imaging Sequences
These recommendations represent a suggested minimum core set of sequences for sites that may not have the capability for more advanced or emerging sequences, as well as additional series likely to be of interest if the necessary resources are available. We recommend using the most current ADNI sequences, unless relevant expertise is available to optimize sequences for a particular research question. Several considerations were taken into account when selecting the minimum core set of MRI sequences. First, the modalities must have proven to be sensitive to cancer- and treatment-related brain changes. Second, the sequences must be available on most clinical scanners and be accessible to a wide range of users regardless of scanner platform. Third, optimized parameters are publicly available via existing cooperative neuroimaging projects, and multicenter reproducibility has been assessed, making data-pooling easier. Based on these considerations, we recommend including the following minimum set of core MRI sequences:
1. A high-resolution, T1-weighted scan with excellent contrast between gray matter, white matter, and cerebrospinal fluid allows precise localization of anatomical structures and permits the usage of postprocessing tools that characterize volumetric changes. This series is also routinely used for anatomical reference and spatial normalization procedures for other imaging modalities and for radiologic review for incidental findings. Inclusion of this sequence is recommended as literature has demonstrated differences in gray matter volume, density, and connectivity related to cancer and its treatment (9,41–43).
2. rsfMRI allows examination of functional brain changes without requiring the stimulus delivery and response recording equipment necessary for task-based fMRI. Previous work has found rsfMRI useful in demonstrating differences in brain network connectivity after cancer treatment (44–47). A sequence of at least six minutes’ duration (ideally 10 minutes or more) is suggested, as is instructing participants to relax, but not to fall asleep. There has been debate about whether eyes should be open or closed (48–51); our consensus recommendation is eyes closed, based on review of the most commonly used procedures.
Investigators are encouraged to consider additional MRI modalities depending on the research questions and available equipment and neuroimaging expertise. Task-based fMRI of working memory has consistently demonstrated alterations in brain function related to cancer and treatment (52–60). Several prior studies have utilized an “N-Back” paradigm, with conditions of increasing working memory load. Use of a visual-verbal N-Back working memory paradigm (eg, the visual analog of the task utilized in [59]) (Supplementary Materials and Supplementary Figure 1, available online) is suggested. fMRI of other cognitive functions of interest should also be considered. Working and episodic memory have been studied most extensively, with some studies examining other aspects of executive functioning (10).
DTI is useful to assess changes in white matter microstructure and has been shown to be sensitive to cancer treatment–related changes (8), though not all scanners may be capable of implementing the parameters necessary for optimal sensitivity. DTI introduces a number of imaging parameters unique to diffusion imaging, including gradient magnitude (b-value) and directions. DTI acquisitions also include nondiffusion T2-weighted images, known as b0 images (61,62), used for artifact removal and tensor fitting. Determination of the number of directions to be acquired and the b-value partly depends on the research question. A minimum of six directions is required for calculating the diffusion tensor (63). A larger number of directions (eg, 30+) is typically required for fiber tracking, with some tracts requiring more directions than others. Multiple sequence runs might be considered to increase signal-to-noise. It is beneficial to acquire as thin a slice thickness and as many directions as possible while being mindful of scan length. DTI was not included in the recommended core minimum set of sequences because it is very sensitive to scanner variability, making it difficult to pool data even when using harmonized sequences.
A 3D FLAIR sequence is useful for detection of white matter hyperintensities as well as radiologic review. Utilization of PET, more advanced diffusion-weighted techniques (eg high angular resolution diffusion-weighted imaging [HARDI], Q-Ball, diffusion spectrum imaging [DSI]), perfusion MRI (eg, ASL, pulsed arterial spin labeling [PASL]), or MR spectroscopy could also be of interest, though, as for DTI, concerns regarding reproducibility between scanners and necessary expertise for reliable prescription and analysis may limit their generalizability across different sites.
Image Processing, Analysis, and Reporting
Quality Assurance
Image quality, and therefore data analysis and interpretation, can be affected by sources of artifact (64,65), including participant motion (resulting in ringing or blurring of the images), experimenter inaccuracy (eg, susceptibility artifacts related to the presence of ferromagnetic objects, incorrect FOV resulting in wrapping artifacts), and hardware failures and image reconstruction problems (eg, large signal dropouts, noise, spikes, signal inhomogeneity, ghosting). Visual inspection of raw images is recommended (66), and automated quality assurance (QA) procedures can be a useful complementary step (67–69). QA is necessary at various stages in the image processing pipeline, as some artifacts may be more evident at different points. Images with excessive distortions that would corrupt further analysis, such as large signal dropouts or excessive motion, can be easily identified, repaired if possible (70,71), or excluded.
Image Processing for Recommended Core Sequences
T1-Weighted Scans
Voxel-based morphometry (VBM) (72,73) is a commonly used method for examining brain volumes, particularly gray matter. An optimized (74) procedure can be semi-automatically executed using existing software tools such as the VBM Toolbox in the SPM software package. VBM statistical analysis is typically done on a whole-brain basis using statistical parametric mapping within a general linear model (GLM) framework (75).
Surface-based methods for volumetric analysis typically involve automated or semi-automated whole-brain or regional parcellation of cortical and subcortical volumes and provide measurements of cortical thickness, surface area, curvature, and volume for discrete cortical and subcortical structures (76–79).
Manual tracing techniques utilize a standardized approach to delineating regions of interest (ROIs), which typically encompass a particular brain structure. Such protocols rely on anatomic landmarks and conventional boundaries to define ROIs, and tracings must have acceptable inter- and intrarater reliability. These methods can be extremely precise for particular ROIs in a given individual, but they are time- and labor-intensive. The many delineation protocols available can also result in substantial differences in reported ROI volumes (80).
Each approach to quantitative structural analysis has strengths and limitations in terms of objectivity, reliability, time and labor intensity, and other factors. One method may be more advantageous than another for a particular scientific question, and the approaches can be used complementarily to provide convergent validation of a finding.
rsfMRI
rsfMRI is typically used to evaluate functional connectivity between brain regions. In addition to the aforementioned QA procedures, fMRI QA should include inspection of the data time courses to assess any abrupt signal changes. In addition to the standard preprocessing steps required for task-based fMRI described below, rsfMRI data require additional corrections (81). Several statistical approaches for rsfMRI functional connectivity exist, including seed-based correlation (SBC), independent components analysis (ICA), and atlas-based ROI. SBC seed sizes and locations are not standardized, leading to variability in results (82), and must therefore be carefully considered.
ICA is a data-driven, multivariable method (83) wherein individual components can represent various brain networks or non-neuronal noise. ICA can therefore serve for artifact correction steps (also see task-based fMRI below) as well as for statistical analysis. Identifying relevant components can be challenging, though several methods exist for this purpose (81).
The magnitude of intrinsic brain activity per region can also be measured from rsfMRI. Several methods exist for obtaining information for ROIs and/or in a whole-brain, voxel-wise manner, including amplitude and fractional amplitude of low-frequency fluctuations (84) and regional homogeneity measurement (85); each has advantages and disadvantages (82).
Image Processing for Additional Imaging Sequences of Interest
Task-Based fMRI
Task-based fMRI can assess changes in brain activation while performing a cognitive task in the scanner, and recommended methods for preprocessing fMRI data have been extensively described (86–89). Many options for further preprocessing exist, including B0 field-map correction to correct for geometric distortions due to magnetic field inhomogeneities (relevant for both fMRI and DTI) (90,91) and ICA for removing motion artifacts (92). Similar to VBM, a GLM approach is widely used for whole-brain voxel-based statistical analysis of fMRI data (93).
The interpretation of fMRI activity becomes complicated when fMRI task performance differs between groups, as between-group activation differences could be the result or the cause of low performance accuracy. It is therefore helpful to consider ways to address this concern, for example, by personalizing task difficulty level to ensure similar performance (94) or covarying analyses for task performance.
DTI
Detailed descriptions of DTI preprocessing steps and an overview of the most commonly used DTI analysis methods can be found elsewhere (8). With the diffusion tensor fitted in every voxel, parameter maps, such as those measuring fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD), can be calculated and analyzed.
The diffusion tensor model is a simple model of water diffusion within the brain, and results must be interpreted with care (95). While DTI has the sensitivity to detect microstructural changes and damage, it is not specific in identifying what changes occurred (eg, demyelination vs axonal degeneration). Additionally, the diffusion tensor model is inadequate in the many regions of the brain that contain “crossing fibers.” Higher-order diffusion models can bring additional insights in this regard (62,96), but they require more advanced diffusion sequences (eg, acquiring multiple shells with different b-values and number of orientations).
FDG-PET
FDG-PET serves as a quantitative biochemical marker of glucose utilization rate. Relative levels of FDG uptake are more broadly acquired to index synaptic activity throughout more metabolically active brain tissues (eg, neocortex, thalamus, cerebellar cortex), while little attention has thus far been given to less metabolically active structures (eg, globus pallidus, white matter tracts).
Similar procedures to those discussed above for MRI are recommended to allow collection of data that can be combined in collaborative analyses. Additional considerations for FDG-PET include the following: 1) the participant must fast for a minimum of four to six hours prior to the scheduled time of FDG administration; 2) prior to administering FDG, blood glucose level must be measured and recorded; 3) an intravenous catheter is required for FDG administration; 4) the participant should be accommodated to an environment with minimal sensory stimulation; and 5) PET is acquired while the participant is awake with eyes open.
Specific PET acquisition protocols depend upon various factors related to instrumentation and are thus highly scanner-dependent. Identifying an expert collaborator who can assist in designing these experiments is key to successful PET studies. Preprocessing and analysis of FDG-PET data are highly similar to processing and analysis of fMRI data, including statistical parametric mapping and ROI methods, as described above.
Connectome Analysis
Mapping the brain network, or connectome, has become a focus of increasing attention. Connectomes can be constructed from gray matter volumes (97), DTI (98), and rsfMRI (44), as well as electroencephalogram (EEG) (99) and magnetoencephalography (MEG) (100). Connectome analyses, typically based on graph theory, provide advantages over traditional neuroimaging approaches, including being inherently multivariable, utilizing multiple neuroimaging data modalities, and improved characterization of the brain’s complexity. Connectome properties include global and local metrics of brain organization, efficiency, and connectivity. Additionally, connectomes can be used for more advanced analyses, including network response to simulated neuropathology (98). Connectome analyses can be computationally intensive, however, and thresholding networks of different sizes for comparison remain an area of ongoing debate (101).
Analytic Considerations
Thresholding and Correction for Multiple Comparisons
Analyses of neuroimaging data are typically done at the voxel level across the whole brain, and therefore involve tens of thousands of univariate statistical tests (eg, a t test is performed at each voxel). Options to correct for multiple comparisons include family-wise error (FWE) correction, typically based on an adjusted Bonferroni method, which accounts for the fact that observations for single voxels are not independent; random field theory (RFT), which better accounts for spatial smoothing and correlation in neuroimaging data (102); and false discovery rate (FDR), which often provides greater sensitivity by applying Bonferroni correction in a descending manner across statistically significant voxels, thus adapting to the level of signal present in the data. FWE and RFT control for the chance of any false positives, while FDR controls the proportion of false positives among suprathreshold voxels (103). Permutation methods may offer advantages, especially for small samples (102,104). Threshold-free cluster enhancement (TFCE) is a permutation-based method that identifies areas of spatial continuity while minimizing problems related to arbitrary cluster thresholds and spatial smoothing (105). Alternate approaches include explicit masking as well as ROI extraction to restrict the number of comparisons; however, these approaches inherently downsample the data.
With regard to the concern of adequate consideration of false positives for fMRI (106), recent work suggests that when an appropriate initial statistical threshold is used, the number of false positive errors is in keeping with what would be predicted statistically (107). With careful consideration of related issues (eg, use of more stringent primary thresholds, transparent reporting practices), methods like cluster-based thresholding can still be useful (108). We recommend that an appropriate method of multiple comparison correction be employed for findings to be considered robust, while also acknowledging the challenges of working with small samples and recognizing that more exploratory analysis can be critical for discovery science.
Considerations for Analyzing Longitudinal Image Data
Two approaches are commonly applied for analysis of longitudinal image data: repeated measures analysis of variance (or within-subject ANOVA) and cross-sectional (GLM-based) analysis of summary measurements such as percent difference (109). If only two time points are being analyzed, baseline measurements can also be included as a covariate when analyzing post-treatment data. Typical methods may be suboptimal, however, as they do not model the covariance structure of serial measurements appropriately, introducing a systematic processing bias (110,111). Some software packages offer solutions for unbiased longitudinal image analysis by using within-subject template creation (110,111). However, commonly used neuroimaging toolboxes make restrictive or unrealistic assumptions when modeling more than two time points per subject (112). More sophisticated methods are being developed to model longitudinal neuroimaging data that account for the within-subject correlation of longitudinal data (109,112–114).
It is important to use the same software version for all analyses to prevent bias. Differences in scanner hardware or software or different acquisition parameters or protocols over time will also influence results (111). A combined scan of subjects and phantoms could allow post hoc correction of these biases, or appropriate covariates reflecting scanner-related differences could be included in the statistical design.
Describing and Reporting Neuroimaging Data
A detailed guide for describing fMRI experiments and results that can be applied to many neuroimaging studies can be found elsewhere (115). It is best to be inclusive regarding details of the study design, data acquisition parameters, QA measures, preprocessing steps, modeling, statistical inference, and any other important methods (eg, ROI definitions). Figures and tables should describe statistics and thresholds used, how anatomical underlays or displayed templates were created, if masking or other such methods were used, the 3D coordinates (x,y,z) of statistically significant clusters, the stereotactic space of these coordinates, statistics for each cluster (eg, Z-value, number of voxels, cluster extent), and anatomical labels for clusters (eg, “left superior frontal gyrus”). To facilitate meta-analyses, we recommend reporting effect sizes or the necessary data to calculate them.
Clinical, Cognitive, and Biomarker Data Considerations
Clinical and Demographic Variables
It is critical to record demographic and clinical data at each study assessment for integration in neuroimaging analyses. Examples include age, education, sex, handedness, race/ethnicity, and socioeconomic (eg, income level), vocational, menopausal, and marital/social support status. Variables related to cancer and treatment should be documented in as much detail as possible. Examples include type/stage of cancer, disease characteristics (eg, size of tumor, number of affected lymph nodes), details of treatments received (eg, types of surgeries; type and length of anesthesia; chemotherapy regimens, doses, number of cycles completed, and concomitant medications; radiation dose; type, dose, and duration of endocrine therapy). Dates of treatments are needed to calculate relevant intervals for use as covariates. Other medical, psychiatric, and psychosocial data should also be collected, including all medications and vitamins/supplements being taken, other medical or mental health conditions, and ratings of variables such as fatigue, sleep quality, depression, and anxiety.
Cognitive Assessment
A variety of self-report and objective cognitive assessment tools have been shown to correlate with neuroimaging findings (2,11,12,23,24,28,41–47,52–56,94,98,116–130), including measures of attention, memory, and executive function. Some studies have demonstrated correlations with specific tests, while others have utilized cognitive domain scores. While the use of domain scores can be very helpful for dimension reduction of cognitive variables, correlations with neuroimaging variables may be stronger for individual test measures. Specific tests may also be grouped into various cognitive domains, making cross-study comparisons challenging. There has recently been increased emphasis, in the form of a program announcement from the National Cancer Institute, encouraging researchers to leverage cognitive neuroscience to improve assessment of cancer- and treatment-related cognitive changes to better identify and characterize these concerns (eg, potentially via assessment tools targeted toward specific components of cognitive function, or those using more sensitive techniques such as electronic administration). While varying approaches may be taken, incorporation of cognitive assessment is strongly recommended to relate neuroimaging findings to functional status.
Biomarkers
A growing number of articles have evaluated the relationship of neuroimaging to various biomarkers to further elucidate the underlying biology of cancer- and treatment-related cognitive changes (23,41,54,116,125). Preliminary work has also shown genetic variables that relate to cognitive functioning after cancer treatment (23,132–135). Investigators are therefore strongly encouraged to collect blood samples for genotyping and to consider banking plasma and serum for fluid biomarker analyses. While funding or sample size considerations may initially preclude running such analyses, if samples are appropriately processed and stored, which is typically relatively inexpensive, the desired assays may be run later when funding is obtained or potentially via consortium analyses (34). Methodological issues are also important when gathering and storing biomarker data. For example, for cytokine analyses, it is critical to carefully consider factors such as method and timing of sample acquisition (eg, time of day, feeding state and acute stress level of the individual, etc.) as well as processing and storage steps utilized (136). While more speculative in this population at present, consideration might also be given to the correlation of neuroimaging variables with RNA (eg, via PAXgene tube or other collection vehicles) or telomere length. For any chosen biomarker, standard collection, storage, and processing procedures are readily available, and close collaboration with colleagues with appropriate lab expertise is essential.
Conclusions
A goal of the ICCTF is to provide research recommendations and guidelines in order to increase the homogeneity of study methods in the field of cancer and cognition. Here we offer approaches and recommendations for neuroimaging studies with regard to study design, data sharing considerations, core neuroimaging sequences, and the processing, analysis, and reporting of neuroimaging data. These recommendations are not meant to be prescriptive, to limit innovation, or to otherwise interfere with individual studies, but instead to facilitate comparing and combining neuroimaging data collected across institutions. As multicenter studies seem increasingly likely to become the most effective method to obtain adequate sample sizes to address core research questions in this area (eg, identification of risk factors for cancer- and treatment-related cognitive changes, examination of relative risk of various treatments), harmonization of imaging approaches could be very beneficial to the field. However, it is also critical to recognize that these are a set of core recommendations; studies focusing on innovative technologies to advance the field beyond existing approaches and data are also needed and encouraged.
Funding
This work was supported in part by the National Cancer Institute (Grants Nos. R01 CA101318, P30 CA082709, R01 CA172145, R03 CA191559), the National Institute of Nursing Research (Grant No. R01 NR014195), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant No. U54 HD071598) at the National Institutes of Health; the Indiana Clinical and Translational Sciences Institute (Grant Nos. UL1 RR025761, RR027710, and RR020128); an Indiana University Melvin and Bren Simon Cancer Center American Cancer Society institutional grant; and the Institute for the Promotion of Innovation by Science and Technology in Flanders (Project No. IWT 130262).
Notes
The funding agencies had no role in the writing of this commentary or the decision to submit it for publication, and the authors have no conflicts of interest to report.
The authors would like to thank the International Cognition and Cancer Task Force (ICCTF) Steering Committee (Drs. Tim Ahles, Sanne Schagen, Janette Vardy, and Jeffrey Wefel) and Drs. Ali Amidi, Beth Chen, Julie Dumas, Michelle Janelsins-Benton, Marta Simó, and Lei Wang for their review of and comments on drafts of this manuscript.
Supplementary Material
References
- 1. Koppelmans V, Breteler MMB, Boogerd W et al. , Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;3010:1080–1086. 10.1200/JCO.2011.37.0189 [DOI] [PubMed] [Google Scholar]
- 2. de Ruiter MB, Reneman L, Boogerd W et al. , Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;328:1206–1219. 10.1002/hbm.21102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wefel JS, Kesler SR, Noll KR et al. , Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;652:123–138. 10.3322/caac.21258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Correa DD, Ahles TA.. Neurocognitive changes in cancer survivors. Cancer J. 2008;146:396–400. 10.1097/PPO.0b013e31818d8769 [DOI] [PubMed] [Google Scholar]
- 5. Dietrich J, Monje M, Wefel J et al. , Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;1312:1285–1295. 10.1634/theoncologist.2008-0130 [DOI] [PubMed] [Google Scholar]
- 6. Wefel JS, Schagen SB.. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;123:267–275. 10.1007/s11910-012-0264-9 [DOI] [PubMed] [Google Scholar]
- 7. Wefel JS, Witgert ME, Meyers CA.. Neuropsychological sequelae of non-central nervous system cancer and cancer therapy. Neuropsychol Rev. 2008;182:121–131. 10.1007/s11065-008-9058-x [DOI] [PubMed] [Google Scholar]
- 8. Deprez S, Billiet T, Sunaert S et al. , Diffusion tensor MRI of chemotherapy-induced cognitive impairment in non-CNS cancer patients: A review. Brain Imaging Behav. 2013;74:409–435. 10.1007/s11682-012-9220-1 [DOI] [PubMed] [Google Scholar]
- 9. McDonald BC, Saykin AJ.. Alterations in brain structure related to breast cancer and its treatment: Chemotherapy and other considerations. Brain Imaging Behav. 2013;74:374–387. 10.1007/s11682-013-9256-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Ruiter MB, Schagen SB.. Functional MRI studies in non-CNS cancers. Brain Imaging Behav. 2013;74:388–408. 10.1007/s11682-013-9249-9 [DOI] [PubMed] [Google Scholar]
- 11. Silverman DH, Dy CJ, Castellon SA et al. , Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Res Treat. 2007;1033:303–311. 10.1007/s10549-006-9380-z [DOI] [PubMed] [Google Scholar]
- 12. Deprez S, Amant F, Smeets A et al. , Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;303:274–281. 10.1200/JCO.2011.36.8571 [DOI] [PubMed] [Google Scholar]
- 13. Stouten-Kemperman MM, de Ruiter MB, Boogerd W et al. , Very late treatment-related alterations in brain function of breast cancer survivors. J Int Neuropsychol Soc. 2015;211:50–61. [DOI] [PubMed] [Google Scholar]
- 14. Stouten-Kemperman MM, de Ruiter MB, Koppelmans V et al. , Neurotoxicity in breast cancer survivors >/=10 years post-treatment is dependent on treatment type. Brain Imaging Behav. 2015;92:275–284. [DOI] [PubMed] [Google Scholar]
- 15. Ahles TA, Saykin AJ, McDonald BC et al. , Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;1101:143–152. 10.1007/s10549-007-9686-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jim HSL, Donovan KA, Small BJ et al. , Cognitive functioning in breast cancer survivors: A controlled comparison. Cancer. 2009;1158:1776–1183. 10.1002/cncr.24192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menning S, de Ruiter MB, Veltman DJ et al. , Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment—the role of fatigue. Neuroimage. Clin. 2015;7:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agrawal K, Onami S, Mortimer JE et al. , Cognitive changes associated with endocrine therapy for breast cancer. Maturitas. 2010;673:209–214. 10.1016/j.maturitas.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collins B, Mackenzie J, Stewart A et al. , Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;182:134–143. 10.1002/pon.1379 [DOI] [PubMed] [Google Scholar]
- 20. Quesnel C, Savard J, Ivers H et al. , Cognitive impairments associated with breast cancer treatments: Results from a longitudinal study. Breast Cancer Res Treat. 2009;1161:113–123. 10.1007/s10549-008-0114-2 [DOI] [PubMed] [Google Scholar]
- 21. Amidi A, Wu LM, Agerbaek M et al. , Cognitive impairment and potential biological and psychological correlates of neuropsychological performance in recently orchiectomized testicular cancer patients. Psychooncology. 2015;249:1174–1180. 10.1002/pon.3804 [DOI] [PubMed] [Google Scholar]
- 22. Amidi A, Wu LM, Pedersen AD et al. , Cognitive impairment in testicular cancer survivors 2 to 7 years after treatment. Support Care Cancer. 2015;2310:2973–2979. 10.1007/s00520-015-2663-3 [DOI] [PubMed] [Google Scholar]
- 23. Amidi A, Agerbaek M, Wu LM et al. , Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. 2017;113:769–783. [DOI] [PubMed] [Google Scholar]
- 24. Amidi A, Hosseini SMH, Leemans A et al. . Changes in brain structural networks and cognitive functions in testicular cancer patients receiving cisplatin-based chemotherapy. J Natl Cancer Inst. 2017;10912:djx085. 10.1093/jnci/djx085 [DOI] [PubMed] [Google Scholar]
- 25. Correa DD, Zhou Q, Thaler HT et al. , Cognitive functions in long-term survivors of ovarian cancer. Gynecol Oncol. 2010;1192:366–369. 10.1016/j.ygyno.2010.06.023 [DOI] [PubMed] [Google Scholar]
- 26. Correa DD, Hess LM.. Cognitive function and quality of life in ovarian cancer. Gynecol Oncol. 2012;1243:404–409. 10.1016/j.ygyno.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 27. Correa DD, Root JC, Baser R et al. , A prospective evaluation of changes in brain structure and cognitive functions in adult stem cell transplant recipients. Brain Imaging Behav. 2013;74:478–490. 10.1007/s11682-013-9221-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Correa DD, Wang Y, West JD et al. , Prospective assessment of white matter integrity in adult stem cell transplant recipients. Brain Imaging Behav. 2016;102:486–496. 10.1007/s11682-015-9423-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hess LM, Huang HQ, Hanlon AL et al. , Cognitive function during and six months following chemotherapy for front-line treatment of ovarian, primary peritoneal or fallopian tube cancer: An NRG oncology/gynecologic oncology group study. Gynecol Oncol. 2015;1393:541–545. 10.1016/j.ygyno.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hodgson KD, Hutchinson AD, Wilson CJ et al. , A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;393:297–304. 10.1016/j.ctrv.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 31. Hsieh TC, Wu YC, Yen KY et al. , Early changes in brain FDG metabolism during anticancer therapy in patients with pharyngeal cancer. J Neuroimaging. 2014;243:266–272. 10.1111/jon.12006 [DOI] [PubMed] [Google Scholar]
- 32. Vardy JL, Dhillon HM, Pond GR et al. , Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal, controlled study. J Clin Oncol. 2015;3334:4085–4092. 10.1200/JCO.2015.63.0905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu LM, Tanenbaum ML, Dijkers MP et al. , Cognitive and neurobehavioral symptoms in patients with non-metastatic prostate cancer treated with androgen deprivation therapy or observation: A mixed methods study. Soc Sci Med. 2016;156:80–89. 10.1016/j.socscimed.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saykin AJ, de Ruiter MB, McDonald BC et al. , Neuroimaging biomarkers and cognitive function in non-CNS cancer and its treatment: Current status and recommendations for future research. Brain Imaging Behav. 2013;74:363–373. 10.1007/s11682-013-9283-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vardy J, Wefel JS, Ahles T et al. , Cancer and cancer-therapy related cognitive dysfunction: An international perspective from the Venice cognitive workshop. Ann Oncol. 2008;194:623–629. [DOI] [PubMed] [Google Scholar]
- 36. Schagen SB, Vardy J.. Cognitive dysfunction in people with cancer. Lancet Oncol. 2007;810:852–853. 10.1016/S1470-2045(07)70287-5 [DOI] [PubMed] [Google Scholar]
- 37. Wefel JS, Vardy J, Ahles T et al. , International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;127:703–708. 10.1016/S1470-2045(10)70294-1 [DOI] [PubMed] [Google Scholar]
- 38. Seigers R, Fardell JE.. Neurobiological basis of chemotherapy-induced cognitive impairment: A review of rodent research. Neurosci Biobehav Rev. 2011;353:729–741. 10.1016/j.neubiorev.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 39. Seigers R, Schagen SB, Van Tellingen O et al. , Chemotherapy-related cognitive dysfunction: Current animal studies and future directions. Brain Imaging Behav. 2013;74:453–459. 10.1007/s11682-013-9250-3 [DOI] [PubMed] [Google Scholar]
- 40. Northoff G, Qin P, Nakao T.. Rest-stimulus interaction in the brain: A review. Trends Neurosci. 2010;336:277–284. 10.1016/j.tins.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 41. Chao HH, Hu S, Ide JS et al. , Effects of androgen deprivation on cerebral morphometry in prostate cancer patients—an exploratory study. PLoS One. 2013;88:e72032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lepage C, Smith AM, Moreau J et al. , A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus. 2014;3:444. 10.1186/2193-1801-3-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simo M, Root JC, Vaquero L et al. , Cognitive and brain structural changes in a lung cancer population. J Thorac Oncol. 2015;101:38–45. 10.1097/JTO.0000000000000345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bruno J, Hosseini SM, Kesler S.. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;483:329–338. 10.1016/j.nbd.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dumas JA, Makarewicz J, Schaubhut GJ et al. , Chemotherapy altered brain functional connectivity in women with breast cancer: A pilot study. Brain Imaging Behav. 2013;74:524–532. 10.1007/s11682-013-9244-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kesler SR, Wefel JS, Hosseini SM et al. , Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci U S A. 2013;11028:11600–11605. 10.1073/pnas.1214551110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kesler SR, Blayney DW.. Neurotoxic effects of anthracycline- vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol. 2016;22:185–192. 10.1001/jamaoncol.2015.4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu D, Dong Z, Zuo X et al. , Eyes-open/eyes-closed dataset sharing for reproducibility evaluation of resting state fMRI data analysis methods. Neuroinformatics. 2013;114:469–476. 10.1007/s12021-013-9187-0 [DOI] [PubMed] [Google Scholar]
- 49. Patriat R, Molloy EK, Meier TB et al. , The effect of resting condition on resting-state fMRI reliability and consistency: A comparison between resting with eyes open, closed, and fixated. Neuroimage. 2013;78:463–473. 10.1016/j.neuroimage.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tan B, Kong X, Yang P et al. , The difference of brain functional connectivity between eyes-closed and eyes-open using graph theoretical analysis. Comput Math Methods Med. 2013;2013:976365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yuan BK, Wang J, Zang YF et al. , Amplitude differences in high-frequency fMRI signals between eyes open and eyes closed resting states. Front Hum Neurosci. 2014;8:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Askren MK, Jung M, Berman MG et al. , Neuromarkers of fatigue and cognitive complaints following chemotherapy for breast cancer: A prospective fMRI investigation. Breast Cancer Res Treat. 2014;1472:445–455. 10.1007/s10549-014-3092-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Conroy SK, McDonald BC, Ahles TA et al. , Chemotherapy-induced amenorrhea: A prospective study of brain activation changes and neurocognitive correlates. Brain Imaging Behav. 2013;74:491–500. 10.1007/s11682-013-9240-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Conroy SK, McDonald BC, Smith DJ et al. , Alterations in brain structure and function in breast cancer survivors: Effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat. 2013;1372:493–502. 10.1007/s10549-012-2385-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Scherling C, Collins B, Mackenzie J et al. , Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: An FMRI study. Front Hum Neurosci. 2011;5:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang L, Apple AC, Schroeder MP et al. , Reduced prefrontal activation during working and long-term memory tasks and impaired patient-reported cognition among cancer survivors postchemotherapy compared with healthy controls. Cancer. 2016;1222:258–268. 10.1002/cncr.29737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cimprich B, Reuter-Lorenz P, Nelson J et al. , Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;323:324–331. 10.1080/13803390903032537 [DOI] [PubMed] [Google Scholar]
- 58. Ferguson RJ, McDonald BC, Saykin AJ et al. , Brain structure and function differences in monozygotic twins: Possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;2525:3866–3870. 10.1200/JCO.2007.10.8639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McDonald BC, Conroy SK, Ahles TA et al. , Alterations in brain activation during working memory processing associated with breast cancer and treatment: A prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;3020:2500–2508. 10.1200/JCO.2011.38.5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nattinger AB, Pezzin LE, Restrepo JA et al. , Cognitive performance among breast cancer survivors treated with aromatase inhibitors. J Cancer Therapeut Res. 2013;2(7). [Google Scholar]
- 61. Alexander AL, Lee JE, Lazar M et al. , Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;43:316–329. 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tournier JD, Mori S, Leemans A.. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;656:1532–1556. 10.1002/mrm.22924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ni H, Kavcic V, Zhu T et al. , Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. Am J Neuroradiol. 2006;278:1776–1781. [PMC free article] [PubMed] [Google Scholar]
- 64. Ling J, Merideth F, Caprihan A et al. , Head injury or head motion? Assessment and quantification of motion artifacts in diffusion tensor imaging studies. Hum Brain Mapp. 2012;331:50–62. 10.1002/hbm.21192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Power JD, Schlaggar BL, Petersen SE.. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536–551. 10.1016/j.neuroimage.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Peeters R, Sunaert S.. Clinical BOLD fMRI and DTI: Artifacts, tips, and tricks In: Stippich C, ed. Clinical Functional MRI Presurgical Functional Neuroimaging Second Edition. Berlin: Springer; 2014:313–336. [Google Scholar]
- 67. Lauzon CB, Asman AJ, Esparza ML et al. , Simultaneous analysis and quality assurance for diffusion tensor imaging. PLoS One. 2013;84:e61737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mortamet B, Bernstein MA, Jack CR Jr et al. , Automatic quality assessment in structural brain magnetic resonance imaging. Magn Reson Med. 2009;622:365–372. 10.1002/mrm.21992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stocker T, Schneider F, Klein M et al. , Automated quality assurance routines for fMRI data applied to a multicenter study. Hum Brain Mapp. 2005;252:237–246. 10.1002/hbm.20096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mazaika PK, Hoeft F, Glover GH et al. , Methods and software for fMRI analysis of clinical subjects. Neuroimage .2009;47(suppl 1):S58. [Google Scholar]
- 71. Patel AX, Kundu P, Rubinov M et al. , A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage. 2014;95:287–304. 10.1016/j.neuroimage.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ashburner J, Friston KJ.. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6 Pt 1):805–821. [DOI] [PubMed] [Google Scholar]
- 73. Ashburner J, Friston KJ.. Why voxel-based morphometry should be used. Neuroimage. 2001;146:1238–1243. 10.1006/nimg.2001.0961 [DOI] [PubMed] [Google Scholar]
- 74. Good CD, Johnsrude IS, Ashburner J et al. , A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 75. Kurth F, Gaser C, Luders E.. A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM). Nat Protoc. 2015;102:293–304. 10.1038/nprot.2015.014 [DOI] [PubMed] [Google Scholar]
- 76. Dale AM, Fischl B, Sereno MI.. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;92:179–194. [DOI] [PubMed] [Google Scholar]
- 77. Fischl B, Sereno MI, Dale AM.. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;92:195–207. 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- 78. Fischl B, Dale AM.. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;9720:11050–11055. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fischl B, Salat DH, Busa E et al. , Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- 80. Konrad C, Ukas T, Nebel C et al. , Defining the human hippocampus in cerebral magnetic resonance images—an overview of current segmentation protocols. Neuroimage. 2009;474:1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kesler SR. Default mode network as a potential biomarker of chemotherapy-related brain injury. Neurobiol Aging. 2014;35(suppl 2):S11–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cole DM, Smith SM, Beckmann CF.. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Calhoun VD, Adali T.. Multisubject independent component analysis of fMRI: A decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev Biomed Eng. 2012;5:60–73. 10.1109/RBME.2012.2211076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zou QH, Zhu CZ, Yang Y et al. , An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J Neurosci Methods. 2008;1721:137–141. 10.1016/j.jneumeth.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zang Y, Jiang T, Lu Y et al. , Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;221:394–400. 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- 86. Friston KJ, Holmes AP, Worsley KJ et al. , Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 87. Nichols TE, Das S, Eickhoff SB et al. , Best practices in data analysis and sharing in neuroimaging using MRI. Nat Neurosci. 2017;203:299–303. 10.1038/nn.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lindquist MA. The statistical analysis of fMRI data. Stat Sci. 2008;234:439–464. 10.1214/09-STS282 [DOI] [Google Scholar]
- 89. Smith SM. Overview of fMRI analysis. Br J Radiol. 2004;77(Spec No 2):S167–S175. [DOI] [PubMed] [Google Scholar]
- 90. Andersson JL, Hutton C, Ashburner J et al. , Modeling geometric deformations in EPI time series. Neuroimage. 2001;135:903–919. 10.1006/nimg.2001.0746 [DOI] [PubMed] [Google Scholar]
- 91. Jezzard P, Balaban RS.. Correction for geometric distortions in echoplanar images from B0 field variations. Magn Reson Med. 1995;34:65–73. 10.1002/mrm.1910340111 [DOI] [PubMed] [Google Scholar]
- 92. Pruim RH, Mennes M, van Rooij D et al. , ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. 10.1016/j.neuroimage.2015.02.064 [DOI] [PubMed] [Google Scholar]
- 93. Soares JM, Magalhaes R, Moreira PS et al. , A hitchhiker's guide to functional magnetic resonance imaging. Front Neurosci. 2016;10:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Deprez S, Vandenbulcke M, Peeters R et al. , Longitudinal assessment of chemotherapy-induced alterations in brain activation during multitasking and its relation with cognitive complaints. J Clin Oncol. 2014;3219:2031–2038. 10.1200/JCO.2013.53.6219 [DOI] [PubMed] [Google Scholar]
- 95. Jones DK, Knosche TR, Turner R.. White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–254. 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- 96. Zhang H, Schneider T, Wheeler-Kingshott CA et al. , NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;614:1000–1016. 10.1016/j.neuroimage.2012.03.072 [DOI] [PubMed] [Google Scholar]
- 97. Tijms BM, Series P, Willshaw DJ et al. , Similarity-based extraction of individual networks from gray matter MRI scans. Cereb Cortex. 2012;227:1530–1541. 10.1093/cercor/bhr221 [DOI] [PubMed] [Google Scholar]
- 98. Kesler SR, Watson CL, Blayney DW.. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol Aging. 2015;368:2429–2442. 10.1016/j.neurobiolaging.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Verweij IM, Romeijn N, Smit DJ et al. , Sleep deprivation leads to a loss of functional connectivity in frontal brain regions. BMC Neurosci. 2014;151:88. 10.1186/1471-2202-15-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stam CJ, de Haan W, Daffertshofer A et al. , Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer's disease. Brain. 2009;132(Pt 1):213–224. [DOI] [PubMed] [Google Scholar]
- 101. van Wijk BCM, Stam CJ, Daffertshofer A.. Comparing brain networks of different size and connectivity density using graph theory. PLoS One. 2010;510:e13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nichols T, Hayasaka S.. Controlling the familywise error rate in functional neuroimaging: A comparative review. Stat Methods Med Res. 2003;125:419–446. 10.1191/0962280203sm341ra [DOI] [PubMed] [Google Scholar]
- 103. Genovese CR, Lazar NA, Nichols TE.. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. 10.1006/nimg.2001.1037 [DOI] [PubMed] [Google Scholar]
- 104. Nichols TE, Holmes AP.. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp. 2002;151:1–25. 10.1002/hbm.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Smith SM, Nichols TE.. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;441:83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- 106. Eklund A, Nichols TE, Knutsson H.. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;11328:7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cox RW, Chen G, Glen DR et al. , FMRI clustering in AFNI: False-positive rates redux. Brain Connect. 2017;73:152–171. 10.1089/brain.2016.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Woo CW, Krishnan A, Wager TD.. Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage. 2014;91:412–419. 10.1016/j.neuroimage.2013.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bernal-Rusiel JL, Greve DN, Reuter M et al. , Statistical analysis of longitudinal neuroimage data with linear mixed effects models. Neuroimage. 2013;66:249–260. 10.1016/j.neuroimage.2012.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ashburner J, Ridgway GR.. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci. 2012;6:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Reuter M, Schmansky NJ, Rosas HD et al. , Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;614:1402–1418. 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Guillaume B, Hua X, Thompson PM et al. , Fast and accurate modelling of longitudinal and repeated measures neuroimaging data. Neuroimage. 2014;94:287–302. 10.1016/j.neuroimage.2014.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bernal-Rusiel JL, Reuter M, Greve DN et al. , Spatiotemporal linear mixed effects modeling for the mass-univariate analysis of longitudinal neuroimage data. Neuroimage. 2013;81:358–370. 10.1016/j.neuroimage.2013.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sabuncu MR, Bernal-Rusiel JL, Reuter M et al. , Event time analysis of longitudinal neuroimage data. Neuroimage. 2014;97:9–18. 10.1016/j.neuroimage.2014.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Poldrack RA, Fletcher PC, Henson RN et al. , Guidelines for reporting an fMRI study. Neuroimage. 2008;402:409–414. 10.1016/j.neuroimage.2007.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kesler S, Janelsins M, Koovakkattu D et al. , Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(suppl):S109–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kesler SR, Kent JS, O'Hara R.. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;6811:1447–1453. 10.1001/archneurol.2011.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kesler SR, Watson C, Koovakkattu D et al. , Elevated prefrontal myo-inositol and choline following breast cancer chemotherapy. Brain Imaging Behav. 2013;74:501–510. 10.1007/s11682-013-9228-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Abraham J, Haut MW, Moran MT et al. , Adjuvant chemotherapy for breast cancer: Effects on cerebral white matter seen in diffusion tensor imaging. Clin Breast Cancer. 2008;81:88–91. 10.3816/CBC.2008.n.007 [DOI] [PubMed] [Google Scholar]
- 120. de Ruiter MB, Reneman L, Boogerd W et al. , Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: Converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;3312:2971–2983. 10.1002/hbm.21422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Deprez S, Amant F, Yigit R et al. , Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;323:480–493. 10.1002/hbm.21033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. McDonald BC, Conroy SK, Smith DJ et al. , Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: A replication and extension study. Brain Behav Immun. 2013;30(suppl):S117–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jung MS, Zhang M, Askren MK et al. , Cognitive dysfunction and symptom burden in women treated for breast cancer: A prospective behavioral and fMRI analysis. Brain Imaging Behav. 2017;111:86–97. 10.1007/s11682-016-9507-8 [DOI] [PubMed] [Google Scholar]
- 124. Nudelman KN, Wang Y, McDonald BC et al. , Altered cerebral blood flow one month after systemic chemotherapy for breast cancer: A prospective study using pulsed arterial spin labeling MRI perfusion. PLoS One. 2014;95:e96713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pomykala KL, Ganz PA, Bower JE et al. , The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013;74:511–523. 10.1007/s11682-013-9243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cheng H, Li W, Gong L et al. , Altered resting-state hippocampal functional networks associated with chemotherapy-induced prospective memory impairment in breast cancer survivors. Sci Rep. 2017;7:45135. 10.1038/srep45135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Miao H, Chen X, Yan Y et al. , Functional connectivity change of brain default mode network in breast cancer patients after chemotherapy. Neuroradiology. 2016;589:921–928. 10.1007/s00234-016-1708-8 [DOI] [PubMed] [Google Scholar]
- 128. Miao H, Li J, Hu S et al. , Long-term cognitive impairment of breast cancer patients after chemotherapy: A functional MRI study. Eur J Radiol. 2016;856:1053–1057. 10.1016/j.ejrad.2016.03.011 [DOI] [PubMed] [Google Scholar]
- 129. Wang L, Yan Y, Wang X et al. , Executive function alternations of breast cancer patients after chemotherapy: Evidence from resting-state functional MRI. Acad Radiol. 2016;2310:1264–1270. 10.1016/j.acra.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 130. Stouten-Kemperman MM, de Ruiter MB, Caan MW et al. , Lower cognitive performance and white matter changes in testicular cancer survivors 10 years after chemotherapy. Hum Brain Mapp. 2015;3611:4638–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ganz PA, Bower JE, Kwan L et al. , Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30(suppl):S99–S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Aboalela N, Lyon D, Elswick RK Jr et al. , Perceived stress levels, chemotherapy, radiation treatment and tumor characteristics are associated with a persistent increased frequency of somatic chromosomal instability in women diagnosed with breast cancer: A one year longitudinal study. PLoS One. 2015;107:e0133380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ahles TA, Saykin AJ, Noll WW et al. , The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;126:612–619. 10.1002/pon.742 [DOI] [PubMed] [Google Scholar]
- 134. Merriman JD, Aouizerat BE, Cataldo JK et al. , Association between an interleukin 1 receptor, type I promoter polymorphism and self-reported attentional function in women with breast cancer. Cytokine. 2014;652:192–201. 10.1016/j.cyto.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Small BJ, Rawson KS, Walsh E et al. , Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;1177:1369–1376. 10.1002/cncr.25685 [DOI] [PubMed] [Google Scholar]
- 136. Zhou X, Fragala MS, McElhaney JE et al. , Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care. 2010;135:541–547. 10.1097/MCO.0b013e32833cf3bc [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


