Abstract
Familial hemophagocytic lymphohistiocytosis (HLH) is an immune hyperactivation syndrome caused by mutations in genes associated with cytotoxic T-cell and NK-cell function. While neurological manifestations frequently accompany systemic inflammation at initial presentation, isolated central nervous system (CNS) involvement is rare, and the histological correlates are not well described. We present 3 patients (ages 5, 6, and 7 years) with CNS-isolated familial HLH, who presented with a variety of neurological symptoms and underwent brain biopsies for multifocal enhancing supratentorial and infratentorial lesions. Biopsy slides from all 3 patients revealed similar findings: perivascular lymphocytes, predominantly CD3+ T-cells (CD4>CD8) with occasional intramural infiltration of small vessels; scattered histiocytes without hemophagocytosis; parenchymal and leptomeningeal inflammation varying from mild and focal to severe and sheet-like with associated destructive lesions. There was no evidence of demyelination, neoplasia, or infection. Genetic testing identified compound heterozygous mutations in PRF1 (Patients 1 and 2) and UNC13D (Patient 3), with no evidence of systemic disease except decreased NK-cell function. All 3 patients were treated with hematopoietic stem cell transplantation with marked improvement of symptoms. These findings combined with the poor outcomes associated with delayed diagnosis and lack of aggressive treatment highlight the need to consider HLH in the differential diagnosis of inflammatory brain lesions.
Keywords: Diagnostic brain biopsy, Familial hemophagocytic lymphohistiocytosis, Inflammatory brain lesion, Nonneoplastic, Perforin, UNC13D
INTRODUCTION
Hemophagocytic lymphohistiocytosis (HLH) is an immune hyperactivation syndrome affecting multiple organs (1). Symptoms are caused by a cytokine storm created by natural killer (NK) and cytotoxic T-cell secretion of cytokines and activation of macrophages. Incidence is 1–10 per million with 20%–40% mortality. Diagnosis is made by clinical findings and laboratory data as outlined by the HLH-2004 guidelines, and treatment includes immunosuppression and hematopoietic stem cell transplantation (HSCT) (2). Primary, or familial, HLH is associated with autosomal recessive mutations in genes associated with T-cell and NK-cell function including target cell pore formation (PRF1), lymphocyte granule priming (UNC13D) and granule fusion (STX11 and STXBP2), while secondary HLH is associated with a variety of precipitating factors including infections, malignancy, autoimmune disease, and drug hypersensitivity (3).
Central nervous system (CNS) manifestations are common in HLH (50%–70%) but rarely precede systemic findings (4–6). Autopsy studies have shown a range of histological findings from predominantly leptomeningeal inflammation with or without hemophagocytosis to diffuse parenchymal involvement with necrosis (7–9). Rare case reports have been published describing patients with presumed secondary HLH restricted to the CNS (10), as well as patients with PRF1 mutations with CNS symptoms and inconsistent systemic features (11–13). We describe the clinical and pathologic findings of 3 patients with familial HLH restricted to the central nervous system.
MATERIALS AND METHODS
This study was conducted with Institutional Board Approval at Boston Children’s Hospital (P00027116; approved 11/10/2017). Three patients treated at Boston Children’s Hospital from 2010 to 2018 were identified with confirmed familial HLH-associated mutations and disease restricted to the central nervous system. Genetic testing was performed by the National Institute of Allergy and Infectious Diseases (targeted sequencing of PRF1 gene; Patient 1), and Cincinnati Children’s Hospital (Hemophagocytic Lymphohistiocytosis [HLH] Panel; Patients 2 and 3 [14]). Brain biopsies were processed and stained at Boston Children’s Hospital, including hematoxylin and eosin (H&E), special stains for myelin and infections, and immunohistochemistry to characterize inflammatory cells. Antibodies used include anti-CD3 monoclonal clone 2GV6 prediluted (Ventana, Oro Valley, AZ), anti-CD20 monoclonal clone L26 prediluted (Ventana), anti-CD4 monoclonal clone SP35 prediluted (Ventana), anti-CD8 monoclonal clone C8/144B prediluted (Cell Marque, Rocklin, CA), and anti-perforin monoclonal clone 5B10 at 1:400 dilution (Leica, Wetzlar, Germany). All slides were reviewed by 2 neuropathologists. Clinical, radiologic, and laboratory data were obtained from electronic medical records. Photographs were taken using an Olympus BX41 microscope and an Olympus DP25 camera.
RESULTS
Clinical Presentation and Disease Course
Three female patients with confirmed familial HLH-associated mutations and disease restricted to the CNS were identified from 2010 to 2018 at Boston Children’s Hospital. None of the patients met clinical or laboratory criteria for diagnosis of HLH, including fever, splenomegaly, cytopenias, or elevated ferritin, with the exception of decreased NK cell activity. None of the patients underwent bone marrow biopsy. The clinical presentation and disease course are summarized in Table 1.
TABLE 1.
Patient Demographics, Radiological and Molecular Findings, and Clinical Course
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age (years) | 5 | 6 | 7 |
| Sex | F | F | F |
| Presenting symptoms | Headaches, vomiting, mild imbalance, and speech difficulties | Progressive cognitive decline, movement disorder, headaches, right hemiparesis | Diplopia, imbalance, clumsiness, hemiparesis |
| MRI findings | Multiple enhancing lesions in brain, most prominent in cerebellum and periventricular WM | Multiple enhancing lesions in periventricular, cortical, subcortical, and brainstem | Multiple enhancing lesions in brain, brainstem, and spinal cord, most prominent in pons |
| *Increased size of thalamic lesion | *New left frontal lobe lesion | ||
| Gene mutations | PRF1 | PRF1 | UNC13D |
| c.452A>T (p.H151L) c.666C>A (p.H222Q) | c.443C>G (p.A148G) c.666C>A (p.H222Q) | c.2346_2349delGGAG (p.R782fs*12) c.2588G>A (p.G863D) | |
| Outcome | HSCT 5 years after initial presentation | HSCT 2 years after initial presentation and 1.5 years later after relapse | HSCT 6.5 years after initial presentation |
| Improvement of cranial nerve function, strength, coordination, and tremor | Improvement in dystonia, mental status, and strength | Resolution of diplopia, improved gait and strength, decreased seizure frequency |
Abbreviations: MRI, magnetic resonance imaging; HSCT, hematopoietic stem cell transplantation; WM, white matter.
MRI findings prior to second biopsies.
Patient 1
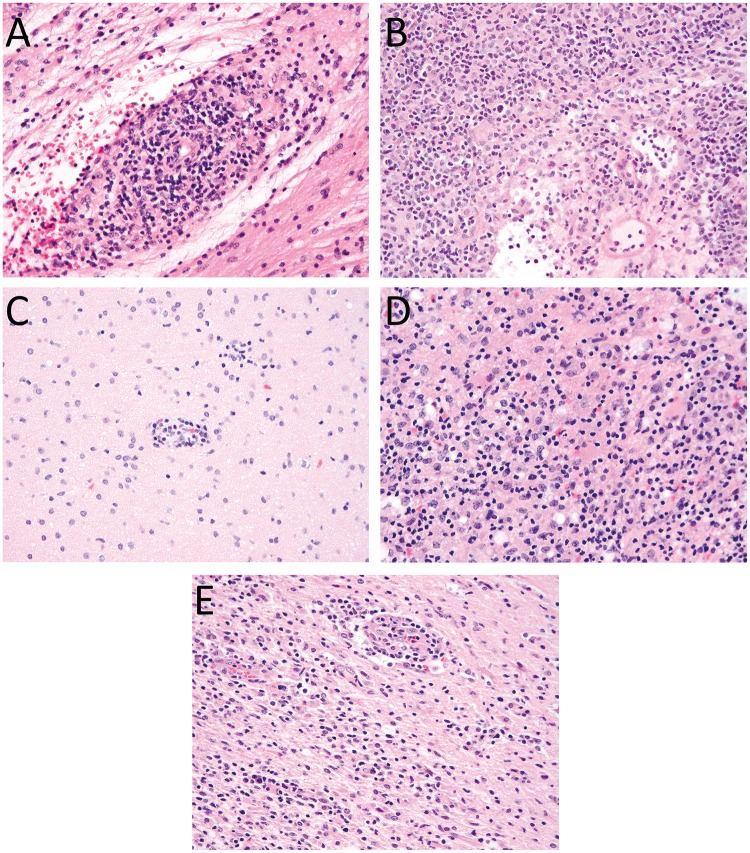
The patient first presented at age 5 years with headache, vomiting, lethargy, and mild ataxia. She was found to have cerebrospinal fluid (CSF) pleiocytosis, MRI revealed multi-focal contrast enhancing cerebral and cerebellar T2-hyperintense lesions, and she was initially diagnosed with demyelinating clinically isolated syndrome. Despite a variety of immunosuppressive treatments including high-dose glucocorticoids, intravenous immunoglobulin (IVIG), cyclophosphamide, mycophenolate mofetil, and natalizumab, her symptoms continued to progress. Diagnostic brain biopsies of the cerebellum (Fig. 1A) and thalamus (Fig. 1B) were collected at 4 and 18 months after initial presentation, respectively, to further guide therapy. CSF immunophenotyping and soluble biomarker analysis revealed activation of the innate immune system leading to PRF1 gene sequencing and identification of 2 pathogenic mutations c.452A>T (p.H151L) and c.666C>A (p.H222Q). She underwent unrelated donor HSCT at age 10 years with improvement of cranial nerve function, strength, coordination, and tremor.
FIGURE 1.
Histological features present in brain biopsies. (A) Biopsy of the cerebellum from Patient 1 reveals a moderate perivascular, predominantly lymphocytic infiltrate. Lymphocytes infiltrate into small blood vessel walls causing some structural alteration and are associated with surrounding parenchymal edema. (B) Biopsy of the thalamus from Patient 1 shows a dense, predominantly lymphocytic perivascular and parenchymal inflammatory infiltrate with some structural alteration of the blood vessel walls and surrounding edema. (C) The initial frontal lobe biopsy from Patient 2 shows mild focal perivascular inflammatory infiltrates. (D) Frontal lobe biopsy of Patient 2 in the setting of recurrent disease shows severe destructive parenchymal lesions. (E) Similar diffuse parenchymal chronic inflammatory infiltrates are present in the frontal lobe biopsy of Patient 3. All images are stained with H&E and were taken with a 40× objective.
Patient 2
The patient presented at 6 years old with vomiting, headache, psychiatric symptoms, cognitive decline, and a focal seizure. MRI showed contrast-enhancing cerebral and cerebellar white matter T2 hyperintense lesions, and she was initially diagnosed with acute disseminated encephalomyelitis. Treatments included high-dose glucocorticoids, IVIG, cyclophosphamide, and plasmapheresis. She underwent diagnostic biopsy of a frontal lobe lesion 20 months after initial presentation (Fig. 1C). Familial HLH gene testing was performed 3 years after symptom onset and identified biallelic PRF1 mutations c.443C>G (p.A148G) and c.666C>A (p.H222Q). She received HLA-matched sibling donor HSCT at age 9 years with improvement in dystonia, mental status, and strength. However, 13 months following HSCT, she experienced seizures, headache, and worsening dystonia, and MRI showed a new frontal lobe white matter enhancing lesion. A brain biopsy was performed (Fig. 1D), and she was diagnosed with relapsed HLH. It was determined that the patient’s sibling donor possessed a mono-allelic PRF1 mutation and NK-cell testing revealed absent function. She then underwent unrelated donor HSCT and has had no recurrence of contrast enhancing lesions on MRI.
Patient 3
The patient presented at 7 years old with diplopia, right hemiparesis, and ataxia. She was found to have CSF pleiocytosis, MRI showed multifocal brain lesions including a large brainstem lesion, and she was initially diagnosed as a demyelinating clinically isolated syndrome with subsequent diagnosis of chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS). Biopsy of a frontal lobe lesion was taken at 10 months after initial presentation (Fig. 1E). Treatments included high-dose glucocorticoids, infliximab, rituximab, azathioprine, and IVIG. Familial HLH gene testing performed 7 years after symptom onset identified bi-allelic UNC13D mutations c.2346_2349delGGAG (p.R782fs*12) and c.2588G>A (p.G863D). She underwent unrelated donor HSCT at age 14 with resolution of diplopia, improved gait and strength, and markedly decreased seizure frequency.
Pathological Findings
A total of 5 biopsies were collected from the 3 patients in this series (Table 2). Two biopsies from Patient 1 and 1 biopsy each from Patients 2 and 3 were taken prior to the establishment of HLH as a definitive diagnosis, and 1 biopsy from Patient 2 was in the setting of recurrent disease.
TABLE 2.
Brain Biopsy Findings Prior to HLH Diagnosis and in Setting of Recurrent Disease
| Patient 1 |
Patient 2 |
Patient 3 | |||
|---|---|---|---|---|---|
| Biopsy | 1 | 2 | 1 | 2 | 1 |
| Time since initial presentation | 4 months | 18 months | 20 months | 39 months | 10 months |
| Treatment prior to biopsy | Steroids, IVIG | Steroids, Cellcept | Steroids, IVIG, Cytoxan | HSCT 16 months earlier | Steroids |
| Biopsy location | Cerebellum | Thalamus | Frontal lobe | Frontal lobe | Frontal lobe |
| Tissue involved | WM, GM, dura leptomeninges | WM | WM, GM, leptomeninges | WM, GM, leptomeninges | WM, GM leptomeninges |
| Inflammatory pattern | Nodular/diffuse parenchymal, perivascular, and small vessel walls | Dense parenchymal, perivascular, and small vessel walls | Mild, focal parenchymal, perivascular, and small vessel walls | Dense, severe destructive parenchymal, perivascular, and small vessel walls | Dense, diffuse parenchymal, perivascular, and small vessel walls |
| Lymphocytes, few histiocytes, plasma cells | Lymphocytes, plasma cells histiocytes (more than prior) | Lymphocytes and microglia | Lymphocytes and histiocytes | Lymphocytes, few plasma cells | |
| Demyelination | Absent | Absent | Absent | Absent | Absent |
| Infection | Negative | Negative | Negative | Negative | Negative |
| Neoplasia | Negative | Negative | Negative | Negative | Negative |
| Hemophagocytosis | Absent | Absent | Absent | Absent | Absent |
| CD3:CD20 | CD3>CD20 | CD3>CD20 | CD3>CD20 | CD3>CD20 | CD3>>CD20 |
| CD4:CD8 | CD4>CD8 | CD4>CD8 | CD4>CD8 | CD4>CD8 | CD4>CD8 |
| Perforin IHC | Not done | Not done | Negative | Scattered | n/a |
Abbreviations: WM, white matter; GM, gray matter; HSCT, hematopoietic stem cell transplantation; IVIG, intravenous immunoglobulin; IHC, immunohistochemistry.
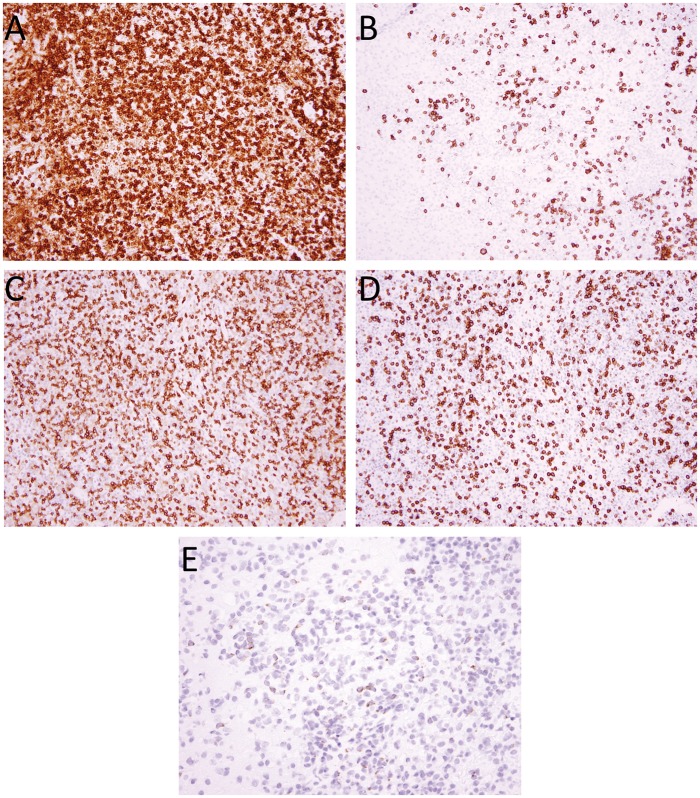
The first biopsy from Patient 1 (Fig. 1A) showed a nodular and diffuse parenchymal and perivascular chronic inflammatory infiltrate, with moderate to severe intramural infiltration of small blood vessels, involving the cerebellar white and gray matter, overlying leptomeninges and dura. The inflammatory infiltrate was composed of a mixed population of CD20-positive B cells and CD3-positive T cells (CD4>CD8), with the latter predominating (Fig. 2A–D), with scattered histiocytes and a minor population of CD138-positive plasma cells. Luxol fast blue (LFB) stain showed no evidence of demyelination. Special stains for microorganisms including Gram, Grocott’s methenamine silver (GMS), Periodic acid-Schiff (PAS), and acid fast bacilli (AFB) were negative for microorganisms. The second biopsy from Patient 1 (Fig. 1B) showed gliotic brain with a dense parenchymal and perivascular chronic inflammatory infiltrate with moderate to severe intramural infiltration of small blood vessels, involving thalamic white matter, consisting of a mixed population of CD20-positive B cells (polytypic for kappa and lambda light chains) and CD3-positive T cells (CD4>CD8), with the latter predominating, with a more prominent population of CD68- and CD163-positive macrophages and plasma cells than the earlier biopsy. Gram, GMS, PAS, and AFB stains and Epstein-Barr Virus (EBV) in situ hybridization were negative for microorganisms.
FIGURE 2.
Characterization of immune cell infiltrate. Immunohistochemistry performed on the cerebellar biopsy from Patient 1 shows predominantly CD3-positive T cells (A), with intermixed CD20-positive B cells. (B) The T cells are a mixed population of CD4 (C) and CD8 (D) cells, with a predominance of CD4-positive cells. The other 4 biopsies showed similar ratios of immune cell staining for CD3, CD20, CD4, and CD8. Perforin 1 immunohistochemistry from Patient 2’s post-transplant biopsy shows scattered positive cells suggestive of partial donor chimerism. (E) Images in panels A–D were taken with 20× objective, and E with 40× objective.
The first biopsy from Patient 2 (Fig. 1C) showed gliotic brain with scattered foci of perivascular chronic inflammatory cells with mild intramural infiltration of small blood vessels and extension into parenchyma involving gray and white matter and leptomeninges. The overlying dura was uninvolved. The inflammatory cells were composed of a mixed population of CD20-positive B cells and CD3-positive T cells (CD4>CD8), with the latter predominating, and scattered CD163-positive microglia. LFB/HE stain showed no evidence of demyelination. The second biopsy from Patient 2 (Fig. 1D) showed a severe and destructive parenchymal and perivascular chronic inflammatory infiltrate with moderate to severe intramural infiltration of small blood vessels involving gray matter, white matter, and leptomeninges with surrounding gliotic brain and microglial activation. The inflammatory infiltrate was composed of a mixed population of small mature lymphocytes including CD20-positive B cells (polytypic for kappa and lambda light chains) and CD3-positive T cells (CD4>CD8), with the latter predominating, and numerous CD163-positive macrophages. Immunohistochemistry for Perforin (Fig. 2E) showed scattered positive lymphocytes while the prior biopsy was negative (not shown). LFB and neurofilament protein immunohistochemistry showed loss of myelin and axonal disruption in destructive lesions. Gram, PAS, AFB, herpes simplex virus (HSV)-1/2, varicella zoster virus (VZV), EBV, cytomegalovirus (CMV), adenovirus, spirochete, and Toxoplasma stains were negative for microorganisms.
The biopsy from Patient 3 (Fig. 1E) showed extensive foci of dense parenchymal and perivascular chronic inflammatory cell infiltrates with moderate to severe intramural infiltration of small blood vessels involving white matter, gray matter, and leptomeninges. The overlying dura was uninvolved. The inflammatory infiltrate was composed of a mixed population of small mature lymphocytes including CD20-positive B cells and CD3-positive T cells (CD4>CD8), with the latter predominating, and occasional plasma cells. LFB stain showed no evidence of demyelination. Gram, GMS, AFB, HSV-1/2, VZV, EBV, CMV, and adenovirus stains were negative for microorganisms.
DISCUSSION
CNS-restricted familial HLH is associated with substantial, but non-specific, histological findings. In all cases in this study, a perivascular lymphocytic infiltrate composed predominantly of CD3+ T cells (CD4>CD8) with intermixed CD20+ B cells was present with associated intramural infiltration of small blood vessels; there was also endothelial reaction and perivascular parenchymal edema, reminiscent of primary small vessel vasculitis described by Elbers et al (15). Plasma cells were present in varying amounts and appeared to be more prevalent in older lesions such as the second biopsy for Patient 1. Scattered histiocytes were also present, although there was no evidence of hemophagocytosis. The degree of parenchymal and leptomeningeal inflammation varied from mild and focal to severe and sheet-like with associated destructive lesions, particularly in the case of recurrent disease. There was no evidence of granulomatous or acute inflammation to suggest an infectious process, and all special and immunohistochemical stains for microorganisms were negative. Stains for myelin and axons did not suggest a primary demyelinating process. There was no evidence of a primary brain neoplasm or of a lymphoproliferative disorder.
The findings in this study are similar to prior reports of systemic HLH secondarily involving the CNS (7–9). In each of the previously published case series, the distribution and extent of inflammatory infiltrates varied widely from mild leptomeningeal involvement to diffuse perivascular cuffing and to dense destructive lesions. Hemophagocytosis, which was not identified in any of the cases in this study, was variably present in the prior series. This discrepancy could be due to greater appreciation of hemophagocytosis on smears of fresh tissue versus histologic sections. However, 3 of the 5 biopsies in this study (1 per patient) included an intraoperative consultation with a smear preparation, and hemophagocytosis was not observed in any of the cases either during the preliminary evaluation by neuropathology or subsequently during hematopathology consultation. A case of presumed secondary HLH restricted to the CNS also showed hemophagocytosis as well as abundant perivascular foamy histiocytes, which was in contrast to the lymphocytic predominance in the current case series (10). A patient with biallelic PRFN1 mutations was reported to have a predominantly lymphocytic vasculitic inflammatory pattern (11). While this report raised the possibility of mild systemic manifestations, the findings are otherwise very similar to the cases in this study and likely represent a continuum of histological appearances of the same disease. No distinctive features were identified between cases with PRF1 mutations (Patients 1 and 2) and UNC13D mutations (Patient 3), although this study is limited by a small number of cases, and the histology was likely impacted by the numerous immunosuppressive treatments administered prior to biopsy.
While there are consistent features present in CNS-restricted familial HLH, there are no pathognomonic histological features to confirm the diagnosis. Once other etiologies including infection, demyelination, and neoplasia have been excluded, HLH should remain in the histological differential diagnosis, and the clinical team alerted about this possibility. Evaluation of NK-cell function and genetic testing for HLH-associated mutations may then be initiated by the treating clinicians for patients with refractory or recurrent CNS inflammation of uncertain etiology. Due to the indication for hematopoietic stem cell transplantation of primary HLH rather than chronic immunosuppression, this diagnosis is critical to make early in disease course. Illustrating the importance of screening asymptomatic siblings of CNS-HLH patients, the younger sister of Patient 3 was also found to have bilallelic UNC13D mutations, and on the basis of brain MRI lesions underwent hematopoietic stem cell transplantation despite being asymptomatic. Once these findings have been confirmed prospectively in additional patients, the need for brain biopsy to rule out alternative diagnoses may be obviated and potential morbidity from surgery can be avoided.
ACKNOWLEDGMENTS
This study would not have been possible without the support of the histology team at Boston Children’s Hospital. We also would like to thank Dr Mark Fleming and the hematopathology service at Boston Children’s Hospital for reviewing these cases.
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. Brisse E, Matthys P, Wouters CH.. Understanding the spectrum of haemophagocytic lymphohistiocytosis: Update on diagnostic challenges and therapeutic options. Br J Haematol 2016;174:175–87 [DOI] [PubMed] [Google Scholar]
- 2. Henter JI, Horne A, Arico M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–31 [DOI] [PubMed] [Google Scholar]
- 3. Morimoto A, Nakazawa Y, Ishii E.. Hemophagocytic lymphohistiocytosis: Pathogenesis, diagnosis, and management. Pediatr Int 2016;58:817–25 [DOI] [PubMed] [Google Scholar]
- 4. Horne A, Trottestam H, Aricò M, et al. Frequency and spectrum of central nervous system involvement in 193 children with haemophagocytic lymphohistiocytosis. Br J Haematol 2008;140:327–35 [DOI] [PubMed] [Google Scholar]
- 5. Deiva K, Mahlaoui N, Beaudonnet F, et al. CNS involvement at the onset of primary hemophagocytic lymphohistiocytosis. Neurology 2012;78:1150–6 [DOI] [PubMed] [Google Scholar]
- 6. Haddad E, Sulis ML, Jabado N, et al. Frequency and severity of central nervous system lesions in hemophagocytic lymphohistiocytosis. Blood 1997;89:794–800 [PubMed] [Google Scholar]
- 7. Henter JI, Nennesmo I.. Neuropathologic findings and neurologic symptoms in twenty-three children with hemophagocytic lymphohistiocytosis. J Pediatr 1997;130:358–65 [DOI] [PubMed] [Google Scholar]
- 8. Soffer D, Okon E, Rosen N, et al. Familial hemophagocytic lymphohistiocytosis in Israel. II. Pathologic findings. Cancer 1984;54:2423–31 [DOI] [PubMed] [Google Scholar]
- 9. Akima M, Sumi SM.. Neuropathology of familial erythrophagocytic lymphohistiocytosis: Six cases and review of the literature. Hum Pathol 1984;15:161–8 [DOI] [PubMed] [Google Scholar]
- 10. Shinoda J, Murase S, Takenaka K, et al. Isolated central nervous system hemophagocytic lymphohistiocytosis: Case report. Neurosurgery 2005;56:187. [PubMed] [Google Scholar]
- 11. Moshous D, Feyen O, Lankisch P, et al. Primary necrotizing lymphocytic central nervous system vasculitis due to perforin deficiency in a four-year-old girl. Arthritis Rheum 2007;56:995–9 [DOI] [PubMed] [Google Scholar]
- 12. Madkaikar M, Gupta M, Dixit A, et al. Predominant neurologic manifestations seen in a patient with a biallelic perforin1 mutation (PRF1; p.R225W). J Pediatr Hematol Oncol 2017;39:143–6 [DOI] [PubMed] [Google Scholar]
- 13. Dias C, McDonald A, Sincan M, et al. Recurrent subacute post-viral onset of ataxia associated with a PRF1 mutation. Eur J Hum Genet 2013;21:1232–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cincinnati Children’s Hospital—Hemophagocytic Lymphohistiocytosis Panel by Next Generation Sequencing (NGS). Available at: https://www.cincinnatichildrens.org/-/media/cincinnati%20childrens/home/service/d/dchi/default/familial-hemophagocytic-lymphohistiocytosis.pdf. Accessed August 21, 2018
- 15. Elbers J, Halliday W, Hawkins C, et al. Brain biopsy in children with primary small-vessel central nervous system vasculitis. Ann Neurol 2010;68:602–10 [DOI] [PubMed] [Google Scholar]