Neurotechnologies, including robotics, muscular electrical stimulation, brain stimulation and brain-computer/machine interfaces, can support upper limb motor recovery in severe chronic stroke. Coscia et al. discuss how use of these technologies within precision stroke medicine, home-based interventions, and novel clinical trial designs can enhance rehabilitative outcomes.
Keywords: stroke rehabilitation, neurotechnologies, severe chronic stroke, upper extremities, clinical trials
Abstract
Upper limb motor deficits in severe stroke survivors often remain unresolved over extended time periods. Novel neurotechnologies have the potential to significantly support upper limb motor restoration in severely impaired stroke individuals. Here, we review recent controlled clinical studies and reviews focusing on the mechanisms of action and effectiveness of single and combined technology-aided interventions for upper limb motor rehabilitation after stroke, including robotics, muscular electrical stimulation, brain stimulation and brain computer/machine interfaces. We aim at identifying possible guidance for the optimal use of these new technologies to enhance upper limb motor recovery especially in severe chronic stroke patients. We found that the current literature does not provide enough evidence to support strict guidelines, because of the variability of the procedures for each intervention and of the heterogeneity of the stroke population. The present results confirm that neurotechnology-aided upper limb rehabilitation is promising for severe chronic stroke patients, but the combination of interventions often lacks understanding of single intervention mechanisms of action, which may not reflect the summation of single intervention’s effectiveness. Stroke rehabilitation is a long and complex process, and one single intervention administrated in a short time interval cannot have a large impact for motor recovery, especially in severely impaired patients. To design personalized interventions combining or proposing different interventions in sequence, it is necessary to have an excellent understanding of the mechanisms determining the effectiveness of a single treatment in this heterogeneous population of stroke patients. We encourage the identification of objective biomarkers for stroke recovery for patients’ stratification and to tailor treatments. Furthermore, the advantage of longitudinal personalized trial designs compared to classical double-blind placebo-controlled clinical trials as the basis for precise personalized stroke rehabilitation medicine is discussed. Finally, we also promote the necessary conceptual change from ‘one-suits-all’ treatments within in-patient clinical rehabilitation set-ups towards personalized home-based treatment strategies, by adopting novel technologies merging rehabilitation and motor assistance, including implantable ones.
Introduction
Stroke constitutes a major public health problem affecting millions of people worldwide with considerable impacts on socio-economics and health-related costs. It is the second cause of death (Langhorne et al., 2011), and the third cause of disability-adjusted life-years worldwide (Feigin et al., 2014): ∼8.2 million people were affected by stroke in Europe in 2010, with a total cost of ∼€64 billion per year (Olesen et al., 2012). Due to ageing societies, these numbers might still rise, estimated to increase 1.5–2-fold from 2010 to 2030 (Feigin et al., 2014).
Improving upper limb functioning is a major therapeutic target in stroke rehabilitation (Pollock et al., 2014; Veerbeek et al., 2017) to maximize patients’ functional recovery and reduce long-term disability (Nichols-Larsen et al., 2005; Veerbeek et al., 2011; Pollock et al., 2014). Motor impairment of the upper limb occurs in 73–88% first time stroke survivors and in 55–75% of chronic stroke patients (Lawrence et al., 2001). Constraint-induced movement therapy (CIMT), but also standard occupational practice, virtual reality and brain stimulation-based interventions for sensory and motor impairments show positive rehabilitative effects in mildly and moderately impaired stroke victims (Pollock et al., 2014; Raffin and Hummel, 2018). However, stroke survivors with severe motor deficits are often excluded from these therapeutic approaches as their deficit does not allow easily rehabilitative motor training (e.g. CIMT), treatment effects are negligible and recovery unpredictable (Byblow et al., 2015; Wuwei et al., 2015; Buch et al., 2016; Guggisberg et al., 2017).
Recent neurotechnology-supported interventions offer the opportunity to deliver high-intensity motor training to stroke victims with severe motor impairments (Sivan et al., 2011). Robotics, muscular electrical stimulation, brain stimulation, brain computer/machine interfaces (BCI/BMI) can support upper limb motor restoration including hand and arm movements and induce neuro-plastic changes within the motor network (Mrachacz-Kersting et al., 2016; Biasiucci et al., 2018).
The main hurdle for an improvement of the status quo of stroke rehabilitation is the fragmentary knowledge about the physiological, psychological and social mechanisms, their interplay and how they impact on functional brain reorganization and stroke recovery. Positive stimulating and negatively blocking adaptive brain reorganization factors are insufficiently characterized except from some more or less trivial determinants, such as number and time of treatment sessions, pointing towards the more the better (Kwakkel et al., 1997). Even the long accepted model of detrimental interhemispheric inhibition of the overactive contralesional brain hemisphere on the ipsilesional hemisphere is based on an oversimplification and lack of differential knowledge and is thus called into question (Hummel et al., 2008; Krakauer and Carmichael, 2017; Morishita and Hummel, 2017).
Here, we take a pragmatic approach of comparing effectiveness data, keeping this lack of knowledge of mechanisms in mind and providing novel ideas towards precision medicine-based approaches to individually tailor treatments to the characteristics and needs of the individual patient with severe chronic stroke to maximize rehabilitative outcome.
Search strategy and selection criteria
The purpose was to identify, for each of the four neurotechnologies (robotics, muscular electrical stimulation, brain stimulation, and BCI/BMI) and their combination, recent (published between January 2014 and June 2017) and relevant (including large samples of patients) reviews/meta-analyses, randomized controlled trials or clinical studies reporting about neurotechnology-aided treatments’ effectiveness.
We searched for references in PubMed and Cochrane Library under the terms ‘effect’ + ‘robotic’, ‘brain computer/machine interface’, ‘functional electrical stimulation’, ‘brain stimulation’ + ‘stroke’ + ‘upper limb’ + ‘motor rehabilitation’ in the above indicated time interval. In addition, reference lists of retrieved articles were reviewed to identify relevant studies.
From the studies found, publications not in English, and reporting small pilot and proof of concepts studies (less than seven individuals) deviating from the above mentioned study’s typology were excluded. Studies addressing neglect, aphasia and somatosensory deficits only were also excluded because this article is focused on motor rehabilitation. Studies where neurotechnologies were used as assessment tools or biomarkers without treatment, or if the aim was to investigate basic mechanisms only, were also excluded. Virtual reality, home-based treatments, treatments including pharmacological agents were not included, because the focus of this article was on novel neuro-technological approaches that can offer promising solutions for the treatment of patients with severe chronic stroke.
The number of papers found and retained is reported in Table 1. The list of the retained studies and their features is reported in Supplementary Table 1.
Table 1.
Summary of the features of the studies included in the review
| Neurotechnology | Total studies found | Number of studies retained | Number of patients | Patient population | Mean difference in upper limb FM pre-post intervention (min–max) | Number of sessions (min–max) |
|---|---|---|---|---|---|---|
| General | - | 4 | 59 186 | - | - | - |
| Robotics | 38 | 8 | 1612 | Subacute–chronic | 2.0–18.0 | 10–40 |
| Moderate–severe | ||||||
| Electrical stimulation | 38 | 11 | 1296 | Acute–subacute–chronic | 4.9–14.8 | 10–120 |
| Moderate–severe | ||||||
| Invasive brain stimulation | 10 | 2 | 94 | Chronic–moderate–severe | 4.3–10.0 | 15–30 |
| tDCs | 42 | 14 | 1334 | Acute–subacute–chronic | 5.2–11.4 | 2–200 |
| Moderate–severe | ||||||
| TMS | 9 | 6 | 648 | Subacute–chronic | 3.0–13.7 | 1–100 |
| Mild–moderate–severe | ||||||
| BCI/BMI | 13 | 10 | 823 | Subacute–chronic | 6.3–13.2 | 1–30 |
| Moderate–severe | ||||||
| tDCs + robotics | 55 | 5 | 295 | Subacute–chronic | 3.0–10.3 | 10–30 |
| Moderate–severe | ||||||
| Electrical stimulation + robotics | 55 | 2 | 50 | Chronic–moderate | 3.9–11.0 | 20 |
| Electrical stimulation + TMS/tDCs | 55 | 3 | 60 | Acute–subacute–chronic | 4.3–12.7 | 5–24 |
| Moderate–severe | ||||||
| BCI/BMI + tDCs + robotics | 52 | 2 | 37 | Chronic | 5.0–6.0 | 10 |
| Moderate–severe |
The upper limb FM score is between 0 and 66. Min = minimum; max = maximum.
For detailed insights about the application and effectiveness of the neurotechnological treatments, studies including acute to chronic and mild to severe patients were screened (Table 1), but discussion and conclusions are focused on severe chronic stroke survivors (Table 2). To identify which studies focused on severe post-stroke patients, we considered the level of motor impairment of the patient population in those studies stating to include moderate to severe chronic stroke patients. We reported the level of motor impairment of the patient population in these studies as the average upper extremities Fugl-Meyer (FM) scores at baseline before the treatments (Supplementary Table 1). The attribution of the level of severity (moderate and severe) was variable across studies: indeed, the level of severity can be attributed according to different factors, such as an arbitrary cut-off of FM scores, or a significantly smaller recovery in comparison to the proportional recovery rule (Prabhakaran et al., 2008; Winters et al., 2015), not allowing one to compare homogenous populations across studies. For this reason, in our analysis, we identified as a population of severe post-stroke individuals one having an average score of the upper limb section of the FM scale (maximum score 66) lower than 30, and we considered as a population of moderate to severely affected individuals, one having an average score of the upper limb FM scale between 30 and 45 (extremes included) (Table 2). We also defined treatment effectiveness with the upper limb section of the FM scale (Fugl-Meyer et al., 1974). We used the FM score as metric to define the severity of the impairment and treatment effectiveness because it was the most adopted clinical outcome across the studies we included (Supplementary Table 1). The definition of severity and impairment was based on the FM scale because, in general, the FM is recognized as a sensitive, reliable, valid and frequently-used measure of recovery at the impairment level and most other measures cannot be used in very severe cases, which constitute the focus of our review (Prabhakaran et al., 2008). To estimate the effectiveness of each intervention, we considered an effect size of 6 FM points in agreement with what is generally reported in the literature for assessing a minimal clinically important difference between groups of stroke patients [5.25 points (Page et al., 2012) or 7 points (Sivan et al., 2011)].
Table 2.
Summary of the features of the studies including chronic moderate to severe and severe stroke patients
| Chronic stroke patients level of impairment | Number of studies retained | Treatment | Mean upper limb FM difference pre-post intervention as min–max (number of patients in the study) | Number of sessions (min–max if multiple studies) |
|---|---|---|---|---|
| Moderate to severe 30 ≤ mean FM ≤ 45 | 2 | Invasive brain stimulation | 4.3 (94)–10.0 | 15–30 |
| 2 | Anodal tDCs | 11.4 (21)–11.4 (24) | 9–12 | |
| 1 | BCI/BMI (robot shoulder-elbow) | 7.2 (21) | 18 | |
| 3 | Combination: BCI/BMI+tDCs (dual mode or anodal) + robotics (shoulder-elbow or orthosis for finger extension) and neuromuscular electrical stimulation | 3.9 (39)–11 (11) | 10–20 | |
| Severe Mean FM <30 | 4 | Robotics (shoulder-elbow) | 3.4 (77)–7.7 (39) | 12–60 |
| 2 | Functional electrical stimulation (upper limb tasks) | 6.5 (11)–14.8 (23) | 10–20 | |
| 1 | tDCs (bilateral) | 6.0 (25) | 24 | |
| 2 | BCI/BMI (shoulder-elbow robot and functional electrical stimulation) | 6.3 (26)–7.8 (30) | 18–20 | |
| 3 | Combination: TMS (repetitive inhibitory)+robotics (shoulder-elbow) or neuromuscular electrical stimulation wrist and BCI, anodal tDCs and othosis for finger extension. | 3.0 (17)–6 (18) | 10–24 |
The upper limb FM score is between 0 and 66. Min = minimum; max = maximum.
Robot-aided rehabilitation
Rehabilitation robots are interactive motorized devices allowing fine-graded limbs mobilization and its precise measurement. They are generally divided into exoskeletons that assist limb movement by controlling the displacement of each segment, and end-effector devices enabling the mobilization of a limb from a distal application point. Both can work in two or three spatial dimensions and in different modes: simple passive, assisted-as-needed ones, providing resistance training or error-augmentation (Marchal-Crespo and Reinkensmeyer, 2009). Despite this variability, motor function gains obtained during robot-assisted therapy seem independent from the type of robot, when the training is provided with a robotic device of the same category (e.g. end effector devices) (Colombo et al., 2017), highlighting the importance for stroke rehabilitation of the use and control of the device over its specific design. Robot-based treatment acts on peripheral nervous system mechanisms by enhancing the (impaired) afferent input to support stroke recovery. Together with the generated motor commands, it drives reorganization in the sensorimotor system, most likely due to Hebbian-like plasticity mechanisms. Furthermore, robot-based treatment allows high task and context-specific training able to stimulate, reactivate and reintegrate the afferents of the somatosensory system involved in the motor control loop, another important component strongly impacting on recovery (Langhorne et al., 2011).
Eight studies (Hesse et al., 2014; Klamroth-Marganska et al., 2014; Orihuela-Espina et al., 2016; Wu et al., 2016; Colombo et al., 2017; Shin et al., 2017; Tomi et al., 2017; Veerbeek et al., 2017) reporting just robot-aided rehabilitation, and nine studies (Ang et al., 2015b; Kasashima-Shindo et al., 2015; Lee et al., 2015; Triccas et al., 2015a,b; Di Lazzaro et al., 2016; Straudi et al., 2016; Rong et al., 2017; Simonetti et al., 2017) including robots combined with other neurotechnologies were compliant with the defined inclusion requirements (Table 1).
Diverse clinical trials have suggested that robotics alone provides intense and safe motor rehabilitation (Klamroth-Marganska et al., 2014; Veerbeek et al., 2017), and phase II trials are appearing (Veerbeek et al., 2017). Current meta-analyses and works including large groups are in agreement with previous older studies (Hesse et al., 2005; Lo et al., 2010), reporting significant, small but marginally clinically meaningful upper limb improvement (e.g. ∼2 FM points) and no effects on upper limb capacity and activity of daily living in stroke patients (Veerbeek et al., 2017). Studies including smaller groups positively report differences up to 18 FM points for mild to moderate impaired individuals (McCabe et al., 2015; Tomi et al., 2017), and up to 8 FM points for severely impaired individuals after an intensive upper limb training (McCabe et al., 2015). Task-oriented training with robotic devices might improve upper limb motor functions in subacute (Orihuela-Espina et al., 2016) and especially chronic severe stroke patients (Klamroth-Marganska et al., 2014) more effectively than physical therapy.
Studies’ results are difficult to compare and summarize because of the lack of common classification in the essential parameters implied in the robotic treatment, such as the amount and type of support and assistance or resistance, the number of joints involved, the features of motor tasks, and the dose (Veerbeek et al., 2017). Therefore, it is currently not possible to draw clear guidelines for this type of intervention except the notion that, in order to achieve clinically significant outcomes, especially in patients with severe stroke, robotic-aided treatments have to be sustained and intense—such as 5 days a week for 12 weeks (Klamroth-Marganska et al., 2014)—and probably personalized.
Muscular electrical stimulation
Electrical stimulation was proposed more than 30 years ago as a rehabilitative technology for hemiparetic/hemiplegic patients. Muscular electrical stimulation also acts on partly similar mechanisms of stroke recovery as robotics, generating force in the affected muscles and respective movements of the paralysed limb stimulating the afferents of the somatosensory system involved and integrated in the motor control loop. Especially, brain-controlled functional electrical stimulation strengthens the brain’s connections to the paretic muscles. Functional electrical stimulation-induced action potentials travel antidromically to the motor cortex with its motor neurons, which may coincide systematically with descending or sensory spinal cord inputs, impacting on their connectivity to motor neurons. Furthermore, functional electrical stimulation-induced afferent activity may coincide with the central motor cortical activity related to the voluntary efforts leading for neuroplastic changes and respective functional reorganization in the respective motor-cortical networks (for review see Ethier et al., 2015). Electrical-induced contractions of paretic musculature lead to reciprocal inhibition of spastic antagonists through the stimulation of spinal interneurons (Robinson, 1995). In particular, depending on the kind of stimulation, it is possible to elicit an inhibitory effect on spasticity through influencing the excitability of the alpha motor neurons and triggering sensorimotor reorganization (Peurala et al., 2002). The simple use of currents has been enriched by elaborated stimulation protocols, such as coupling detection and stimulation of motor synergies (Laffont et al., 2014), allowing the coordination of muscle activity and synergies during functional tasks towards a physiological pattern (Vafadar et al., 2015) and brain-controlled application leveraging the interplay of the respective motor command with the electrical stimulation-induced afferent input.
Here, 11 studies reporting muscular electrical stimulation effectiveness for stroke upper limb rehabilitation were selected (Dorsch et al., 2014; Kim et al., 2014; Quandt and Hummel, 2014; Knutson et al., 2015a, b; Liu et al., 2015; Vafadar et al., 2015; Wilson et al., 2016; Carda et al., 2017; Eraifej et al., 2017; Schick et al., 2017), and seven where muscular electrical stimulation was used in combination with other treatments (Koyama et al., 2014; Lee et al., 2015; Sattler et al., 2015, Jang et al., 2016; Kim et al., 2016; Rong et al., 2017; Tosun et al., 2017) (Table 1). In almost all of these studies, the muscular electrical stimulation elicits a movement and only in few cases, it is used solely for sensory stimulation (Wilson et al., 2016). It is applied mainly to treat shoulder subluxation (Vafadar et al., 2015) or to allow hand opening (Knutson et al., 2015a; Wilson et al., 2016; Carda et al., 2017; Schick et al., 2017), but also wrist dorsiflexion (Liu et al., 2015) or multiple joints actuation (Dorsch et al., 2014). Depending on the muscles stimulated, the task and individual sensitivity, the stimulation parameters are highly variable: the stimulation frequency can be set between 10 and 50 Hz, the pulse amplitude from 0 to 100 mA and pulse width from 0 to 300 μs (Knutson et al., 2015b; Vafadar et al., 2015).
A Cochrane review with a meta-analysis indicates functional electrical stimulation as an effective treatment for shoulder subluxation especially early after stroke (Vafadar et al., 2015). In this case, functional electrical stimulation is used to stimulate the muscles responsible to maintain the head of the humerus in the glenoid fossa (supraspinatus and posterior deltoid) to prevent or restore subluxation, reduce pain, and improve function (Vafadar et al., 2015). However, for pain and functional improvement, it does not have superior effects to physical therapy (Vafadar et al., 2015). In all cases, evidence shows short-term effects, inconclusive in long-term studies (Vafadar et al., 2015).
For hand and finger function, strong evidence of the advantage of muscular electrical stimulation over physical therapy or no therapy is still missing (Dorsch et al., 2014; Quandt and Hummel, 2014; Eraifej et al., 2017). It has been suggested to be effective if combined with other approaches such as mirror therapy (Schick et al., 2017), motor imagery (Liu et al., 2015), intensive goal-oriented (Carda et al., 2017) and BCI-based motor training (Biasiucci et al., 2018). This might indicate that the modality of administration of muscular electrical stimulation, and in particular the task contingent sequence of the stimulation, is an important factor as much as the appropriate stimulation parameters. Interestingly, variations of muscular electrical stimulation, such as cyclic neuromuscular electrical stimulation (NMES), switch-triggered NMES, EMG-triggered NMES, and sensory stimulation on paretic upper-extremities might have comparable effects, with no advantages of complex stimulation over the simple one (Wilson et al., 2016). For chronic severe patients, evidence is limited, but muscular electrical stimulation seems to favour significant clinical improvements, even when it is administrated in low dose (10 sessions) (Carda et al., 2017), and when dose is increased, it seems to favour larger improvements (McCabe et al., 2015).
Except for shoulder subluxation, guidelines cannot be derived from this intervention since the optimal dose and administration of muscular electrical stimulation has not been established yet. Protocols across studies are heterogeneous (Knutson et al., 2015a) and optimal stimulation parameters are highly individual and influenced by the pathology (Quandt and Hummel, 2014).
Brain stimulation
Brain stimulation uses local magnetic fields or electric currents to facilitate or inhibit targeted brain areas. Among non-invasive brain stimulation techniques, transcranial magnetic stimulation (TMS) and transcranial direct-current stimulation (tDCs) are the most adopted (for review, see Hummel and Cohen, 2005). Non-invasive brain stimulation is used to modulate the excitability of the stimulated neuropil. The mentioned techniques act on different specific central mechanisms: TMS most likely stimulates axons at their bends and can induce action potentials (Maccabee et al., 1993); TDCs polarize neuronal elements (somas, dendrites, axons) based on their orientation and polarity of the induced electric field (Rahman et al., 2013). Both can produce changes in cortical excitability in healthy subjects and stroke patients (Hummel and Cohen, 2005; Nitsche et al., 2008; Triccas et al., 2016). They can stimulate or inhibit according to the applied parameters, for instance, pulse frequency, stimulation-train timing, or stimulus intensity for TMS, and current polarity, current intensity, and stimulation duration for tDCs (Hummel and Cohen, 2005; Nitsche et al., 2008; Laffont et al., 2014; Rossini et al., 2015). When net effects are measured via TMS-induced motor-evoked potentials, anodal tDCs increases, whereas cathodal tDCs decreases cortical excitability (Nitsche and Paulus, 2000). It is important to note that this simple dichotomy oversimplifies the complex interactions within the brain, especially of non-motor areas (Hummel et al., 2008; Morishita and Hummel, 2017). More complex inhibition and excitation patterns have been identified, via investigations of cortical gyration, microstructural properties of neurons (e.g. orientation of the somato-dendritic axis), or higher-order behavioural functions (Rahman et al., 2014).
Brain stimulation in stroke is aimed at enhancing adaptive brain plasticity during rehabilitative training, by locally modifying cortical excitability, enhancing focal and remote neuroplastic properties and/or correcting maladaptive brain plasticity induced by the cerebrovascular accident (Lefaucheur et al., 2014). Its use in stroke rehabilitation was initiated more than 10 years ago (Hummel et al., 2005; Khedr et al., 2005; Hummel and Cohen, 2006), and its efficacy is still under investigation with several studies conducted in the past 3 years. We included evidence from 19 publications, where brain stimulation is used alone (Fusco et al., 2014; Lefaucheur et al., 2014; O’Shea et al., 2014; Plow and Machado, 2014; Elsner et al., 2015; Tretriluxana et al., 2015; Allman et al., 2016; Chang et al., 2016; D'Agata et al., 2016; Del Felice et al., 2016; Ilić et al., 2016; Kubis, 2016; Levy et al., 2016; Rocha et al., 2016; Triccas et al., 2016; Cho et al., 2017; Figlewski et al., 2017; Koh et al., 2017; Lefaucheur et al., 2017), and 10 studies (Koyama et al., 2014; Ang et al., 2015b; Kasashima-Shindo et al., 2015; Sattler et al., 2015; Triccas et al., 2015a, b; Di Lazzaro et al., 2016; Straudi et al., 2016; Simonetti et al., 2017; Tosun et al., 2017) where brain stimulation is used in combination with other interventions (Table 1).
Guidelines for use in stroke rehabilitation have been provided for repetitive TMS (rTMS) (Lefaucheur et al., 2014; Rossini et al., 2015). Probable efficacy (Level B) has been reported for low-frequency rTMS applied to the contralesional motor cortex (M1) to improve motor performance in the chronic phase by downregulation of excitatory tone in the contralesional hemisphere. Additionally, high-frequency rTMS stimulation of the ipsilesional M1 reached a Level C recommendation (possibly useful) for improving motor function for the acute and post-acute, and maybe the chronic phase. Active stimulation modes (dual M1 tDCs 1.5 mA versus low-frequency rTMS at 1 Hz) might lead to comparable results in chronic stroke patients using the Action Research Arm Test as functional outcome (D'Agata et al., 2016). Task-complexity is relevant for the effectiveness of the stimulation, for example low-frequency rTMS (1 Hz) applied to contralesional M1 facilitated reach-to-grasp action only for small objects (1.2 versus 7.2 cm cylindrical dowels (Tretriluxana et al., 2015), as well as patient characteristics (such as functional integrity of the corticospinal tract and the brain-derived neurotrophic factor genotype), which may influence the response to a high-frequency rTMS (10 Hz, ipsilesional M1) (Chang et al., 2016).
Recent evidence-based guidelines for the therapeutic use of tDCs found heterogeneous evidence for motor recovery after stroke (Lefaucheur et al., 2017) and some meta-analyses reported small not significant effects on upper limb impairments and activities of daily living post-intervention (Kang et al., 2016; Triccas et al., 2016). Recent publications discussed some of the issues underlying this result, including which modality to use, how the stimulation should be coupled with the motor training, which are the underlying mechanisms, how clinical stroke characteristics affect the efficacy of the different stimulation protocols and which ‘biomarkers’ might help to predict outcome and the magnitude of treatment response (for reviews see Morishita and Hummel, 2017; Raffin and Hummel, 2018).
Concerning the preferential stimulation modality, O’Shea et al. (2014) could show, using a simple reaction time task, that anodal, facilitatory ipsilesional M1 tDCs (1 mA) and cathodal, inhibitory contralesional M1 tDCs (1 mA) improved reaction time in chronic stroke, when compared to sham. However, bilateral tDCs had no effect. Additionally, in a recent randomized controlled trial investigating the effect of anodal ipsilesional M1 tDCs (1 mA) or cathodal contralesional M1 tDCs (1 mA) coupled with modified constraint-induced therapy, anodal stimulation had a more lasting impact on the motor outcomes than cathodal stimulation in chronic stroke patients (Rocha et al., 2016). Figlewski et al. (2017) provided additional evidence for the additive value of ipsilesional M1 anodal tDCs (1.5 mA) when coupled with CIMT. Focusing on post stroke spasticity, Del Felice et al. (2016) showed a superior effectivity for cathodal contralesional M1 tDCs (1 mA), when compared to dual M1 tDCs (1 mA). For the selection of the best time window for application of tDCs, Fusco et al. (2014) found that anodal ipsilesional M1 tDCs (1.5 mA) when applied before motor rehabilitative training, improved hand dexterity but not rehabilitation effectiveness in a cohort of patients with subacute stroke. This is in line with data from healthy subjects pointing towards enhancement of learning, when anodal tDCs (1 mA) is applied during motor training and rather disturbance, when applied before motor training (Stagg et al., 2011).
Currently, there is limited knowledge on which mechanisms mediate the tDCs effects (Fritsch et al., 2010). Allman et al. (2016) provided new insights using structural and functional MRI. In their study, anodal ipsilesional M1 (1 mA) tDCs was paired with motor training in chronic stroke patients. Patients in the anodal stimulation group showed increased activity during movement of the paretic hand in the ipsilesional motor and premotor cortex. In addition, patients had intervention-related increases in grey matter volume of ipsilesional motor and premotor areas.
Because of their different mechanistic properties, TMS and tDCs can be used in combination to achieve synergistic effects. Cho et al. (2017) hypothesized that cathodal contralesional M1 tDCS (2 mA) can balance interhemispheric interactions and hereby enhance effects of high-frequency ipsilesional M1 rTMS (10 Hz). In their study, combined stimulation had a synergistic effect being more effective than TMS alone (Cho et al., 2017).
Stroke characteristics such as lesion site or type of stroke determine the responsiveness to a brain stimulation interventional protocol. For instance, high-frequency rTMS (10 Hz) applied to ipsilesional M1 only improved finger- and hand-movement kinematics in patients with subcortical, but not with additional cortical stroke (Ameli et al., 2009). Patient stratification for treatment protocols may maximize effects in future trials (Morishita and Hummel, 2017; Raffin and Hummel, 2018). Currently, there is insufficient evidence to draw final conclusions on the potential benefit of stratification for the treatment protocol; however, the identification of potential biomarkers constitutes one of the core future areas in the field (Guggisberg et al., 2019). Potential biomarkers are based on neuroimaging, such as lesion location, structural, functional information and connectivity parameters (Ameli et al., 2009; Lindenberg et al., 2012; Diekhoff-Krebs et al., 2017; Koch and Hummel, 2017; Schulz et al., 2017; for reviews see Koch and Hummel, 2017; Guggisberg et al., 2019) ipsilesional intracortical inhibition (GABAergic) (Liuzzi et al., 2014), and clinical characteristics (Stinear, 2010; O'Shea et al., 2014).
The use of invasive implants to deliver brain stimulation could overcome some limitations of non-invasive brain stimulation, e.g. only superficial areas can be reached, topographic resolution and the long-term chronic use are limited. Invasive interventions will allow the application of brain stimulation with high temporal and spatial resolution, sufficient intensity and continuous stimulation throughout task-oriented long-term motor training for home-based use. The first larger trial in this direction, the ‘Everest’ trial, evaluated safety and efficacy of epidural cortical electrical stimulation in clinic rehabilitation (Levy et al., 2016). The epidural brain implant was tested on 94 moderate to severe chronic stroke patients and compared to standard rehabilitation administered for 6 weeks. The primary efficacy endpoints (4.5 points in the upper FM and 0.21 points in the Arm Motor Ability Test) at 4 weeks post-treatment were not accomplished. However, post hoc comparisons showed that a greater proportion of experimental (39%) than control (15%) patients maintained or achieved the primary endpoints at 24 weeks post-treatment.
Despite the fact that guidelines are available for upper limb rehabilitation (Lefaucheur et al., 2014, 2017), knowledge and evidence of brain stimulation for stroke rehabilitation in patients with severely impaired upper extremity function is still not sufficient. This also raises the unsolved question, which patient population might benefit most from brain stimulation. A review focused on mechanisms of synaptic and functional reorganization after stroke suggesting a bimodal balance–recovery model that links interhemispheric balancing and functional recovery to the structural reserve spared by the lesion that could enable brain stimulation to be tailored to the needs of individual patients (Di Pino et al., 2014). This is still quite a simplified model (Hummel et al., 2008). As the functional relevance of secondary motor areas become more and more clear, novel stimulation targets gain attention (Koch and Hummel, 2017; Morishita and Hummel, 2017). In a recent case study, deep brain stimulation of the dentate nucleus resulted in reduced tremor and ataxia in a patient with cerebellar stroke (Teixeira et al., 2015). A clinical trial (EDEN trial) is evaluating if deep brain stimulation in the dentate nucleus area is safe for the treatment of stroke. It will include 12 chronic stroke patients with estimated termination in 2019. Additionally, novel non-invasive stimulation techniques are developed, like non-invasive deep brain stimulation via temporally interfering electric fields (Grossman et al., 2017), which is an exciting development with the potential that stroke patients might benefit in future.
As with many treatment modalities used for stroke, knowledge of the neurophysiological basis of non-invasive brain stimulation (rTMS, tDCS) effects and mechanisms of action, especially in patients compared to healthy subjects, is still limited. Thus, crucial steps towards larger treatment effects and personalized patient-tailored applications are to understand heterogeneous responses (from responders to non-responders) to brain stimulation, and parameters, which determine and predict the treatment response.
Brain computer/machine interfaces
BCI/BMIs activate or deactivate assistive or rehabilitative devices directly by brain activity of the user (usually neuroelectric or neurometabolic) without a motor output. The non-invasive recording of brain activity for a BCI can be achieved with different imaging techniques: for their relative portable nature and low cost, EEG and near-infrared spectroscopy are mostly applied for stroke rehabilitation (van Dokkum et al., 2015), with EEG used more frequently (Pfurtscheller et al., 2008), but other techniques have also been adopted, such as a magnetoencephalography-based or real-time functional MRI-based BCI (Buch et al., 2008; Sitaram et al., 2008). A pattern change in one EEG feature (amplitudes of a particular evoked oscillation, composition of slow cortical potentials or spectral features) can be used to trigger an external device to display real-time feedback or to execute the intended action (van Dokkum et al., 2015). Indeed, BCI/BMI systems are used in stroke to restore the lost motor functions acting on central and peripheral mechanisms: they reactivate and reorganize the central command structures and through their feedback-based learning close the interrupted central-peripheral loop leading to Hebbian-like plasticity-based cortical mechanisms. They are used to train ‘healthy’ brain activity and/or to operate assistive devices (van Dokkum et al., 2015). In the first case, BCI/BMIs are coupled to an auditory or visual feedback system to visualize the effects of brain activity changes, facilitate and enhance the learning of recruiting brain areas and their activation (Laffont et al., 2014): these approaches are often termed neurofeedback. In the second case, BCI/BMIs control passive or active limb mobilization through an external device (such as robots or muscular electrical stimulation) to help patients to improve brain plasticity, based on associative Hebbian learning principles (Soekadar et al., 2015), ‘closing the sensorimotor loop’ and thus promoting the relearning of voluntary motor control (Laffont et al., 2014). Further details about its mechanisms of action are reported in Soekadar et al. (2015) and Biasiucci et al. (2018).
The application of BCI/BMIs for stroke rehabilitation is relatively recent; the number of studies in the field is limited and mostly restricted to single cases or case reports. We included 10 studies reporting its effectiveness when it is used alone (Ang et al., 2014, 2015a; Li et al., 2014; Ono et al., 2014; Morone et al., 2015; Soekadar et al., 2015; van Dokkum et al., 2015; Jang et al., 2016; Kim et al., 2016; Remsik et al., 2016) (Table 1), and two when used in combination with other treatments (Ang et al., 2015b; Kasashima-Shindo et al., 2015). The type of actuator used to provide feedback varied among studies, with three using robotic or orthotic devices (Ang et al., 2014, 2015a; Ono et al., 2014), three using muscular electrical stimulation alone or with visual feedback of a virtual gaming instructing about what to imagine during motor imagery tasks (Li et al., 2014; Jang et al., 2016; Kim et al., 2016), one using visual feedback of the movement of a virtual hand during a motor imagery task only (Morone et al., 2015), and two using robotic and tDCS (Ang et al., 2014; Kasashima-Shindo et al., 2015). Both self-guided (motor imagery and neurofeedback) and assistive movements (using orthotics and muscular electrical stimulation) show significant effects with a limited effect size. The enhancement of treatment effects is achieved when BCI/BMIs are coupled with robots and muscular electrical stimulation (Li et al., 2014; Ang et al., 2015a) with results comparable to conventional physiotherapy or with an improvement of up to 9.4 FM points in moderate to severe patients (Morone et al., 2015). BCI/BMIs seem particularly suitable for the severe cases (Morone et al., 2015), as also shown by a previous study (Ramos‐Murguialday et al., 2013) and a recent report (Biasiucci et al., 2018) with evidence of restoration of individual finger extension in severe chronic stroke survivors using the detection of motor intention with BCI to drive electrical stimulation in hand muscles (Soekadar et al., 2015).
There is currently insufficient evidence to suggest guidelines about BCI/BMIs administration and effectiveness. Few sessions (a minimum of 18) seem enough to provide clinical significant improvements (Ang et al., 2015a), but it is necessary to perform larger randomized controlled trials to have further evidences about its administration, its effectiveness and which stroke population might mostly benefit from this intervention. Enlarging size, neurological and demographic range of participants, adopting novel neuroimaging measures such as near-infrared spectroscopy, functional MRI and real-time functional MRI and invasive and/or hybrid brain-body computer/machine interfaces, developing portable systems for in-home use and increasing personalized treatments have been already identified as the next steps for BCI/BMI-aided rehabilitation (Remsik et al., 2016).
Combinations of interventions
As single interventions’ effect size might not be large enough, combination of interventions might enhance significantly the magnitude of functional improvement and recovery by additive or even supra-additive effects (Laffont et al., 2014; Hatem et al., 2016) conceptually combining to target central and peripheral mechanisms of stroke recovery. However, this statement needs experimental-clinical verification because a combination of effective strategies does not necessarily lead to more effective functional improvement: combining two effective treatment modalities may potentially worsen outcome, as it was found frequently in psychotherapy research. For this reason, we review post-stroke upper limb rehabilitation based on the combination of neurotechnologies. Twelve papers were selected (Koyama et al., 2014; Ang et al., 2015b; Kasashima-Shindo et al., 2015; Lee et al., 2015; Sattler et al., 2015; Triccas et al., 2015a, b; Di Lazzaro et al., 2016; Straudi et al., 2016; Rong et al., 2017; Simonetti et al., 2017; Tosun et al., 2017) (Table 1).
Robotics is the treatment most frequently combined with others (Ang et al., 2015b; Kasashima-Shindo et al., 2015; Triccas et al., 2015a, b; Di Lazzaro et al., 2016; Jang et al., 2016; Straudi et al., 2016; Rong et al., 2017). As shown, its combination with BCI/BMI is promising, probably because of optimal learning conditions, where a brain-driven voluntary movement is paired in time with visual and proprioceptive feedback of that movement and its intention facilitating adaptive motor reorganization. A similar positive synergy has been observed also for BCI/BMI with muscular electrical stimulation (Soekadar et al., 2015).
The effectiveness of the addition of muscular electrical stimulation to robotic training has been addressed in a study using the Bi-Manu-Track robot to target wrist flexion-extension and forearm pronation-supination (Lee et al., 2015). In one group, muscular electrical stimulation was contingent to wrist extension and forearm pronation-supination with symmetrical biphasic square waveform, a frequency of 30 pulses per second, a pulse duration of 200 µs and intensity at muscle contraction level. In the other group (sham group), the intensity of stimulation was zero and the participants were notified that it was set below sensory threshold (Lee et al., 2015). Both groups significantly improved their motor impairment (3.9 versus 3.8 FM points on average) and motor functions, but without significant group differences that were only found for muscle spasticity of wrist flexors, in hand functions and in the quality of life measures. In a more complex design, robotics and neuromuscular electrical stimulation have been combined to provide multi-joint coordinated upper limb physical training, assisting elbow, wrist and fingers to achieve reaching, hand opening and grasping (Rong et al., 2017). An exoskeleton with two modules for the elbow and wrist was combined with neuromuscular electrical stimulation of biceps brachii, triceps brachii, flexor carpi radialis, extensor carpi ulnaris and extensor digitorum communis to control elbow and wrist flexion/extension, and of the extensor carpi ulnaris and extensor digitorum communis for hand opening/closing. In this case, the pre-post training improvement was on average of 11 FM points (Rong et al., 2017), indicating that relevant clinical results might be achieved if the combination of interventions is provided in a learning principles based context, and if the functionality of the whole limb and not just of a single joint is practiced.
An interesting approach is the combination of brain stimulation to enhance neuroplasticity with robotics and electrical stimulation, which has been done so far in few studies with few patients. The combination of robotics and tDCs is currently evaluated (Simonetti et al., 2017); however, the heterogeneity of methodology and patients, and the restricted number of studies and patients do not allow a specific statement about efficacy, but rather a general one regarding feasibility. Indeed, studies adopt 20 min to 1 h of robotic treatment, with devices assisting only wrist, wrist and elbow, or shoulder and elbow, with the combination of tDCs administered in the first 7–20 min of the robotic treatment as anodal, cathodal or bilateral stimulation, with the number of sessions also varying from one to 30. Overall, single studies share the same conclusions of the review (Triccas et al., 2015a, b; Di Lazzaro et al., 2016; Simonetti et al., 2017). Small significant changes are observable only after adjusting statistical analysis for lesion site (cortical versus subcortical), timing from the stroke onset (chronic versus subacute) and type of stroke (ischaemic versus haemorrhagic) (Simonetti et al., 2017). Patients with subacute stroke show on average almost double the improvement compared to chronic stroke patients (10.3 versus 5.8 FM points), after receiving 20 min anodal tDCs before 1 h of training with an upper limb exoskeleton for 18 sessions (Triccas et al., 2015a, b). This might point to the importance of tailoring this treatment to the individual patient, but also it has to be considered that spontaneous remission in acute and subacute patients may carry treatment effects. Bilateral tDCs administrated for 30 min of therapy with a shoulder-elbow robot for 10 sessions seems more effective in the chronic stage and in patients with subcortical lesions (Straudi et al., 2016). Given the heterogeneity in the administration, effectiveness of tDCs and robotics might improve with the best differential choice of interventions’ setup. For example, prolonging their simultaneous presentation (>15 sessions) might enhance effects (Di Lazzaro et al., 2016), as well as choosing the optimal duration and location to apply tDCs (Triccas et al., 2015a, b; Di Lazzaro et al., 2016) or determining the temporal relationship between brain stimulation and robotic therapy (Simonetti et al., 2017). Improvements might be better retained when anodal tDCs is delivered before practice of robotic treatment rather than during or after it. Similar considerations are valid for the combination tDCs and electrical stimulation (Sattler et al., 2015).
The combination of TMS and muscular electrical stimulation appears to have higher efficacy than each intervention alone. Koyama et al. (2014) tried 12 sessions including 880 repetitions of neuromuscular electrical stimulation of wrist extensors (frequency 50 Hz, pulse width of 250 µs, stimulation cycle of 500 ms and intensity matching the level to induce 10° wrist extension, maximum 30 mA) in combination with inhibitory repetitive TMS (biphasic magnetic stimuli at 1 Hz) on the contralesional hemisphere in moderate to severe stroke patients. The results show a pre-post treatment improvement on average of 4.3 FM points. Tosun et al. (2017) showed in a larger group of stroke patients that low frequency repetitive TMS alone and with neuromuscular electrical stimulation of wrist extensors significantly enhance motor recovery in the paretic hand more than conventional therapy.
Finally, first evidence was provided that tDCs (anodal 1 mA over M1 motor cortex of the ipsilesional hemisphere) applied 20 min before motor imagery BCI with a motor feedback provided by a robotic device assisting either shoulder-elbow or finger extension showed that the addition of tDCs elicited significant motor improvement only after long term application (3 months) (Ang et al., 2015b; Kasashima-Shindo et al., 2015). However, tDCs enhanced BCI/BMI features by increasing event-related desynchronization (Ang et al., 2015b; Kasashima-Shindo et al., 2015) and improved online accuracies of the BCI (Ang et al., 2015b). This facilitation may enhance the efficacy of BCI (Ang et al., 2015b), reinforcing adaptive brain plasticity and inhibiting maladaptive reorganization (Laffont et al., 2014).
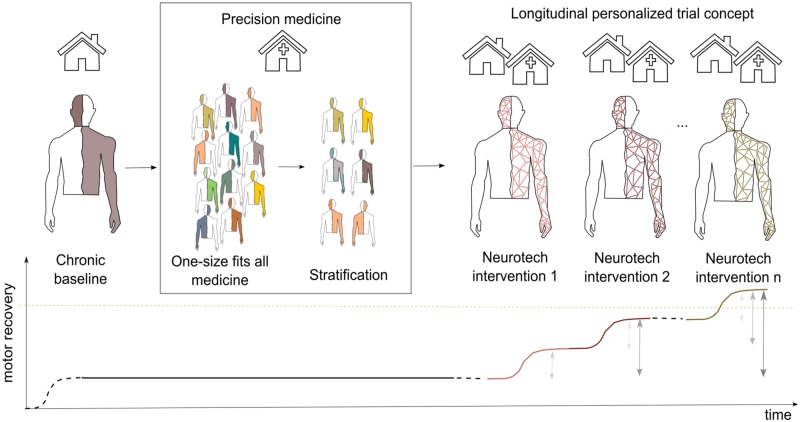
In general, the combination of neurotechnologies for post-stroke upper limb rehabilitation is still in its infancy with few studies comparing the various possibilities of administration of two or more treatments; therefore, no guidelines or indications can be provided so far. In patients with moderate and severe chronic stroke, the combination of interventions seems effective in reducing motor impairments, but not more than single interventions (Table 2), as it has been also shown in a recent study where repetitive peripheral nerve sensory stimulation of the median nerve was provided in combination with anodal tDCs as add-on interventions to training wrist extension with functional electrical stimulation (Menezes et al., 2018). Severe chronic patients might benefit more than moderate ones from the combination of interventions (Ang et al., 2015b; Kasashima-Shindo et al., 2015). The often limited efficacy of the combination of interventions (Table 2) might be also related to a deficient learning context to maximize synergistic effects of single interventions, but also reflects our limited understanding of the physiological mechanisms of brain reorganization after stroke (Fig. 1).
Figure 1.
Conceptualization of longitudinal personalized rehabilitation-treatment designs for patients with severe chronic stroke. Ideally, each patient with severe chronic stroke with a stable motor recovery could be stratified based on objective biomarkers of stroke recovery in order to select the most appropriate/promising neurotechnology-aided interventions and/or their combination for the specific case. Then, these interventions can be administered in the clinic and/or at home in sequence, moving from one to another only when patient’s motor recovery plateaus. In this way, comparisons of the efficacy of each intervention (grey arrows) are still possible, and if the selected interventions and/or their combination are suitable, motor recovery could increase.
Conclusions and future directions
Neurotechnology-aided upper limb rehabilitation has a very promising potential especially for patients with severe stroke, who have very limited opportunities of classical rehabilitative treatments. However, experimental evidence of exclusive benefits of a particular treatment over the others (McCabe et al., 2015), and differential indication of the various treatments (which treatment for which patient), is still lacking (Miller et al., 2010; McCabe et al., 2015). Moreover, the combination of neurotechnology-aided interventions for upper limb stroke rehabilitation does not show cumulative, but rather comparable efficacy to the one achieved with single interventions. A limitation of the present review is that the results are based only on the FM score, which represents a measure of impairment. However, for stroke recovery and the effects of treatment interventions also other aspects like compensation, adaptation or relearning are relevant influential factors. As most of the studies provide mainly data from the FM score (∼59%, Supplementary Table 1) allowing a comparison of the studies, a systematic estimation of the effects of interventions on other relevant parameters such relearning to compensate or adapt for deficits for daily life was unfortunately not possible. In the future, it would be favourable in neuro-rehabilitative treatment studies to have a clinical evaluation, which represents several factors critical for recovery ranging from impairment, adaption, and compensation to quality of life. Compound measures, created out of these parameters, might represent best the individual patients’ recovery or treatment effects.
All the discussed neurotechnology-based interventions for upper limb rehabilitation after stroke and their combination seem to suffer from comparable limitations such as: small sample size, lack of understanding of underlying mechanisms, no patient stratification or tailored-approaches, ‘one-suits-all’ concept applications in a clinical or laboratory environment only, performed in a limited time, with a lack of attention to the motor task that might often be meaningless and far from activities of daily living. In future clinical-scientific efforts, it is mandatory to address these crucial points.
Large homogeneous controlled studies tackling the influence of impairment, timing of intervention and dosage for each intervention are desirable. However, non-invasive technology-aided stroke rehabilitation trials differ from pharmacology and implantable medical device trials. In non-invasive technology-aided stroke rehabilitation, each intervention includes multiple parameters in addition to the variability of dose and timing, and it acts on multiple systems (such as central, peripheral nervous system and muscles) and functional domains. As a result, each intervention is highly variable, especially when it is a combination of multiple interventions. Remarkably, despite generally larger patient numbers and fewer parameters to control than in the case of neurotechnologies, pharmacological treatments also show heterogeneous, and in part, contradictory findings, similar to those found in neurotechnology-aided treatments, leading to insufficient evidence, not allowing one to draw strong and clear conclusions in regard of favourable treatment effects to enhance neuro-rehabilitation and stroke recovery (Scheidtmann et al., 2001; Sprigg et al., 2007; Berends et al., 2009; Clark, 2009; Chollet et al., 2011; Chen et al., 2013; Cramer, 2015; Tran et al., 2016; Graham et al., 2017; Kraglund et al., 2018; Viale et al., 2018). Heterogeneity is an irreducible feature of stroke patients and already many factors have been suggested to possibly influence treatment effectiveness or impact recovery, such as age, gender, type of stroke (ischaemic or haemorrhagic), side of lesion, cortical or subcortical lesion, time since stroke onset, presence of the BDNF Val/Val genotype (Chang et al., 2016), and the structural integrity of corticospinal motor fibres and intracortical connections (Lindenberg et al., 2010; Schulz et al., 2015). Together with the measure of finger extension and shoulder abduction within 72 h after stroke (Nijland et al., 2010) and the integrity of muscle synergy patterns (Cheung et al., 2012; García-Cossio et al., 2014), these features might be more or less relevant for the stratification of stroke patients and for the prediction of motor recovery (Kwakkel et al., 2006; Prabhakaran et al., 2008; Winters et al., 2016b; Wolf et al., 2016; Koch and Hummel, 2017) for each intervention. In addition, double blinding of patients and therapists is not always possible. Placebo effects cannot be completely controlled, but should be attended and carefully measured. A control group where only one critical variable is isolated is almost impossible and heterogeneous groups might cancel individual benefits in both intervention and control groups. To test the effect of a single treatment variation, the effort of a whole research community should be coordinated to carry on clinical trials including hundreds of patients each (Winters et al., 2016a). This implies costly multicentre and international trials that are difficult to control, harmonize and finance.
The personalization of the rehabilitative intervention has been suggested as a critical step to improve the outcome of rehabilitation (Fuhrer and Keith, 1998; Krakauer, 2006; Koch and Hummel, 2017; Raffin and Hummel, 2018). A priori selection and attribution to different groups of patients with particular characteristics (differential indication) could help guiding rehabilitation of individual treatment protocols to achieve larger effects (Klamroth-Marganska et al., 2014; Winters et al., 2016a; Morishita and Hummel, 2017; Raffin and Hummel, 2018). Precision stroke medicine requires the identification of cortical, spinal and muscular correlates of individual stroke recovery (Guggisberg et al., 2019) and an alternative to randomized-controlled trials to move towards within-patient approaches.
A possible solution for patients with severe chronic stroke might be to move towards longitudinal personalized study designs. Such an approach in neurotechnology trials would indicate that each patient is his/her own control in a longitudinal fashion of one or many successive interventions until the patient reaches the individual functional maximum with this specific treatment. Even more interesting, one could consider that the patient starts with a first intervention and when a functional plateau is achieved, treatment moves to another intervention and again leverages it individually until also here the functional plateau is reached. The elegance of this design is that it allows patients to train with different approaches for an extended time, increasing their chance to maximally improve, because each treatment modality will be applied until the personal plateau of functional recovery is reached in the individual patient. This interventional study design also allows one to compare the impact of each therapy stage across that individual, and after completing several patients statistical comparisons between baselines, achieved levels of functioning are possible. Such a design is especially useful for severe chronic stroke patients in whom spontaneous remission is not possible anymore and placebo-expectancy effects are carefully controlled with questionnaires and systematic quantitative behavioural observations.
In conclusion, the available technological solutions have the potential to provide an effective treatment for patients with chronic severe stroke to improve their quality of life and social functioning. It is crucial to provide a prolonged personalized combination of different treatments to maximize individual treatment effects. For this reason, it is necessary to move from classical single case or randomized-controlled trials and towards adopting the concept of individualized precision longitudinal designs. The choice of a trial aiming primarily to improve single patient outcome and only secondarily to allow the comparison of different interventions might significantly increase the present status of therapeutic stroke recovery and maximizes its effects. Finally, innovations towards portable and/or implantable systems to assist, support, control and promote paretic limb use outside the lab or clinic would move upper limb motor training to patients’ life extending high-frequency training duration to life-time.
Funding
The research has been funded by the Wyss Center for Bio- and Neuroengineering (AVANCER WCP030, and WCP024), the Defitech Foundation and by the grant #2017‐205 of the Strategic Focal Area ‘Personalized Health and Related Technologies (PHRT, project PHRT-205)’ of the ETH Domain.
Competing interests
The authors declare that they do not have any competing interest.
Supplementary Material
Glossary
Abbreviations
- BCI
brain-computer interface
- BMI
brain-machine interface
- FM
Fugl-Meyer
- tDCs
transcranial direct-current stimulation
- TMS
transcranial magnetic stimulation
References
- Allman C, Amadi U, Winkler AM, Wilkins L, Filippini N, Kischka U et al. . Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Transl Med 2016; 8: 330re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameli M, Grefkes C, Kemper F, Riegg FP, Rehme AK, Karbe H et al. . Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol 2009; 66: 298–309. [DOI] [PubMed] [Google Scholar]
- Ang KK, Chua KSG, Phua KS, Wang C, Chin ZY, Kuah CWK et al. . A randomized controlled trial of EEG-based motor imagery brain-computer interface robotic rehabilitation for stroke. Clin EEG Neurosci 2015a; 46: 310–20. [DOI] [PubMed] [Google Scholar]
- Ang KK, Guan C, Phua KS, Wang C, Zhou L, Tang KY et al. . Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: results of a three-armed randomized controlled trial for chronic stroke. Front Neuroeng 2014; 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang KK, Guan C, Phua KS, Wang C, Zhao L, Teo WP et al. . Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Arch Phys Med Rehabil 2015b; 96: S79–87. [DOI] [PubMed] [Google Scholar]
- Berends HI, Nijlant JMM, Movig KLL, Van Putten MJAM, Jannink MJA, Ijzerman MJ. The clinical use of drugs influencing neurotransmitters in the brain to promote motor recovery after stroke; a Cochrane systematic review. Eur J Phys Rehabil Med 2009; 45: 621–30. [PubMed] [Google Scholar]
- Biasiucci A, Leeb R, Iturrate I, Perdikis S, Al-Khodairy A, Corbet T et al. . Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun 2018; 9: 2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch E, Weber C, Cohen LG, Braun C, Dimyan MA, Ard T et al. . Think to move: a neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke 2008; 39: 910–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Rizk S, Nicolo P, Cohen LG, Schnider A, Guggisberg AG. Predicting motor improvement after stroke with clinical assessment and diffusion tensor imaging. Neurology 2016; 86: 1924–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byblow WD, Stinear CM, Barber PA, Petoe MA, Ackerley SJ. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol 2015; 78: 848–59. [DOI] [PubMed] [Google Scholar]
- Carda S, Biasiucci A, Maesani A, Ionta S, Moncharmont J, Clarke S et al. . Electrically assisted movement therapy in chronic stroke patients with severe upper limb paresis: a pilot, single-blind, randomized crossover study. Arch Phys Med Rehabil 2017; 98: 1628–35. [DOI] [PubMed] [Google Scholar]
- Chang WH, Uhm KE, Shin Y-I, Pascual-Leone A, Kim Y-H. Factors influencing the response to high-frequency repetitive transcranial magnetic stimulation in patients with subacute stroke. Restor Neurol Neurosci 2016; 34: 747–55. [DOI] [PubMed] [Google Scholar]
- Chen CLH, Young SHY, Gan HH, Singh R, Lao AY, Baroque AC et al. . Chinese medicine neuroaid efficacy on stroke recovery: a double-blind, placebo-controlled, randomized study. Stroke 2013; 44: 2093–100. [DOI] [PubMed] [Google Scholar]
- Cheung VCK, Turolla A, Agostini M, Silvoni S, Bennis C, Kasi P. Muscle synergy patterns as physiological markers of motor cortical damage. Proc Natl Acad Sci USA 2012; 109: 14652–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Lee A, Kim MS, Park E, Chang WH, Shin Y-I et al. . Dual-mode noninvasive brain stimulation over the bilateral primary motor cortices in stroke patients. Restor Neurol Neurosci 2017; 35: 105–14. [DOI] [PubMed] [Google Scholar]
- Chollet F, Tardy J, Albucher J-F, Thalamas C, Berard E, Lamy C et al. . Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol 2011; 10: 123–30. [DOI] [PubMed] [Google Scholar]
- Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother 2009; 10: 839–46. [DOI] [PubMed] [Google Scholar]
- Colombo R, Pisano F, Delconte C, Mazzone A, Grioni G, Castagna M et al. . Comparison of exercise training effect with different robotic devices for upper limb rehabilitation: a retrospective study. Eur J Phys Rehabil Med 2017; 53: 240–8. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Drugs to enhance motor recovery after stroke. Stroke 2015; 46: 2998–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agata F, Peila E, Cicerale A, Caglio MM, Caroppo P, Vighetti S et al. . Cognitive and neurophysiological effects of non-invasive brain stimulation in stroke patients after motor rehabilitation. Front Behav Neurosci 2016; 10: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Felice A, Daloli V, Masiero S, Manganotti P. Contralesional cathodal versus dual transcranial direct current stimulation for decreasing upper limb spasticity in chronic stroke individuals: a clinical and neurophysiological study. J Stroke Cerebrovasc Dis 2016; 25: 2932–41. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Capone F, Di Pino G, Pellegrino G, Florio L, Zollo L et al. . Combining robotic training and non-invasive brain stimulation in severe upper limb-impaired chronic stroke patients. Front Neurosci 2016; 10: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D et al. . Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 2014; 10: 597. [DOI] [PubMed] [Google Scholar]
- Diekhoff-Krebs S, Pool E-M, Sarfeld A-S, Rehme AK, Eickhoff SB, Fink GR et al. . Interindividual differences in motor network connectivity and behavioral response to iTBS in stroke patients. Neuroimage Clin 2017; 15: 559–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch S, Ada L, Canning CG. EMG-triggered electrical stimulation is a feasible intervention to apply to multiple arm muscles in people early after stroke, but does not improve strength and activity more than usual therapy: a randomized feasibility trial. Clin Rehabil 2014; 28: 482–90. [DOI] [PubMed] [Google Scholar]
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (TDCS) for improving activities in patients after stroke. Physiotherapy 2015; 101: e359–60. [Google Scholar]
- Eraifej J, Clark W, France B, Desando S, Moore D. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: a systematic review and meta-analysis. Syst Rev 2017; 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier C, Gallego JA, Miller LE. Brain-controlled neuromuscular stimulation to drive neural plasticity and functional recovery. Curr Opin Neurobiol 2015; 33: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA et al. . Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014; 383: 245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewski K, Blicher JU, Mortensen J, Severinsen KE, Nielsen JF, Andersen H. Transcranial direct current stimulation potentiates improvements in functional ability in patients with chronic stroke receiving constraint-induced movement therapy. Stroke 2017; 48: 229–32. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG et al. . Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 2010; 66: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1974; 7: 13–31. [PubMed] [Google Scholar]
- Fuhrer MJ, Keith RA. Facilitating patient learning during medical rehabilitation: a research agenda1. Am J Phys Med Rehabil 1998; 77: 557–61. [DOI] [PubMed] [Google Scholar]
- Fusco A, Iosa M, Venturiero V, De Angelis D, Morone G, Maglione L et al. . After vs. priming effects of anodal transcranial direct current stimulation on upper extremity motor recovery in patients with subacute stroke. Restor Neurol Neurosci 2014; 32: 301–12. [DOI] [PubMed] [Google Scholar]
- García-Cossio E, Broetz D, Birbaumer N, Ramos-Murguialday A. Cortex integrity relevance in muscle synergies in severe chronic stroke. Front Hum Neurosci 2014; 8: 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham C, Lewis S, Forbes J, Mead G, Hackett ML, Hankey GJ et al. . The FOCUS, AFFINITY and EFFECTS trials studying the effect (s) of fluoxetine in patients with a recent stroke: statistical and health economic analysis plan for the trials and for the individual patient data meta-analysis. Trials 2017; 18: 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk H-J et al. . Noninvasive deep brain stimulation via temporally interfering electric fields. Cell 2017; 169: 1029–41.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggisberg AG, Koch PJ, Hummel FC, Buetefisch CM. Brain networks and their relevance for stroke rehabilitation. Clin Neurophysiol 2019; 130: 1098–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggisberg AG, Nicolo P, Cohen LG, Schnider A, Buch ER. Longitudinal structural and functional differences between proportional and poor motor recovery after stroke. Neurorehabil Neural Repair 2017; 31: 1029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatem SM, Saussez G, della Faille M, Prist V, Zhang X, Dispa D et al. . Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci 2016; 10: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse S, Heß A, Werner CC, Kabbert N, Buschfort R. Effect on arm function and cost of robot-assisted group therapy in subacute patients with stroke and a moderately to severely affected arm: a randomized controlled trial. Clin Rehabil 2014; 28: 637–47. [DOI] [PubMed] [Google Scholar]
- Hesse S, Werner C, Pohl M, Rueckriem S, Mehrholz J, Lingnau ML. Computerized arm training improves the motor control of the severely affected arm after stroke: a single-blinded randomized trial in two centers. Stroke 2005; 36: 1960–6. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu W-H, Gerloff C et al. . Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005; 128: 490–9. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Celnik P, Pascual-Leone A, Fregni F, Byblow WD, Buetefisch CM et al. . Controversy: noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul 2008; 1: 370–82. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Drivers of brain plasticity. Curr Opin Neurol 2005; 18: 667–74. [DOI] [PubMed] [Google Scholar]
- Ilić NV, Dubljanin-Raspopović E, Nedeljković U, Tomanović-Vujadinović S, Milanović SD, Petronić-Marković I et al. . Effects of anodal tDCS and occupational therapy on fine motor skill deficits in patients with chronic stroke. Restor Neurol Neurosci 2016; 34: 935–45. [DOI] [PubMed] [Google Scholar]
- Jang YY, Kim TH, Lee BH. Effects of brain–computer interface‐controlled functional electrical stimulation training on shoulder subluxation for patients with stroke: a randomized controlled trial. Occup Ther Int 2016; 23: 175–85. [DOI] [PubMed] [Google Scholar]
- Kang N, Summers JJ, Cauraugh JH. Transcranial direct current stimulation facilitates motor learning post-stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2016; 87: 345–55. [DOI] [PubMed] [Google Scholar]
- Kasashima-Shindo Y, Fujiwara T, Ushiba J, Matsushika Y, Kamatani D, Oto M et al. . Brain–computer interface training combined with transcranial direct current stimulation in patients with chronic severe hemiparesis: proof of concept study. J Rehabil Med 2015; 47: 318–24. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology 2005; 65: 466–8. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee G, Song C. Effect of functional electrical stimulation with mirror therapy on upper extremity motor function in poststroke patients. J Stroke Cerebrovasc Dis 2014; 23: 655–61. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim S, Lee B. Effects of action observational training plus brain–computer interface‐based functional electrical stimulation on paretic arm motor recovery in patient with stroke: a randomized controlled trial. Occup Ther Int 2016; 23: 39–47. [DOI] [PubMed] [Google Scholar]
- Klamroth-Marganska V, Blanco J, Campen K, Curt A, Dietz V, Ettlin T et al. . Three-dimensional, task-specific robot therapy of the arm after stroke: a multicentre, parallel-group randomised trial. Lancet Neurol 2014; 13: 159–66. [DOI] [PubMed] [Google Scholar]
- Knutson J, Chae J, Gunzler D. Contralaterally controlled FES for chronic upper limb hemiplegia: single site RCT. Arch Phys Med Rehabil 2015a; 96: e4. [Google Scholar]
- Knutson JS, Fu MJ, Sheffler LR, Chae J. Neuromuscular electrical stimulation for motor restoration in hemiplegia. Phys Med Rehabil Clin N Am 2015b; 26: 729–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch PJ, Hummel FC. Toward precision medicine: tailoring interventional strategies based on noninvasive brain stimulation for motor recovery after stroke. Curr Opin Neurol 2017; 30: 388–97. [DOI] [PubMed] [Google Scholar]
- Koh CL, Lin JH, Jeng JS, Huang SL, Hsieh CL. Effect of Transcranial direct current stimulation with sensory modulation on stroke motor rehabilitation: a randomized controlled trial. Arch Phys Med Rehabil 2017; 98: 2477–84. [DOI] [PubMed] [Google Scholar]
- Koyama S, Tanabe S, Warashina H, Kaneko T, Sakurai H, Kanada Y et al. . NMES with rTMS for moderate to severe dysfunction after stroke. NeuroRehabilitation 2014; 35: 363–8. [DOI] [PubMed] [Google Scholar]
- Kraglund KL, Mortensen JK, Damsbo AG, Modrau B, Simonsen SA, Iversen HK et al. . Neuroregeneration and vascular protection by citalopram in acute ischemic stroke (TALOS) a randomized controlled study. Stroke 2018; 49: 2568–76. [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol 2006; 19: 84–90. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Carmichael ST. Broken movement: the neurobiology of motor recovery after stroke. Cambridge, MA: MIT Press; 2017. [Google Scholar]
- Kubis N. Non-invasive brain stimulation to enhance post-stroke recovery. Front Neural Circuits 2016; 10: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke 2006; 37: 2348–53. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Wagenaar RC, Koelman TW, Lankhorst GJ, Koetsier JC. Effects of intensity of rehabilitation after stroke. A research synthesis. Stroke 1997; 28: 1550–6. [DOI] [PubMed] [Google Scholar]
- Laffont I, Bakhti K, Coroian F, van Dokkum L, Mottet D, Schweighofer N et al. . Innovative technologies applied to sensorimotor rehabilitation after stroke. Ann Phys Rehabil Med 2014; 57: 543–51. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011; 377: 1693–702. [DOI] [PubMed] [Google Scholar]
- Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R et al. . Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 2001; 32: 1279–84. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lin K, Cheng H, Wu C, Hsieh Y, Chen C. Effects of combining robot-assisted therapy with neuromuscular electrical stimulation on motor impairment, motor and daily function, and quality of life in patients with chronic stroke: a double-blinded randomized controlled trial. J Neuroeng Rehabil 2015; 12: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur J-P, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH et al. . Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014; 125: 2150–206. [DOI] [PubMed] [Google Scholar]
- Lefaucheur J-P, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F et al. . Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 2017; 128: 56–92. [DOI] [PubMed] [Google Scholar]
- Levy RM, Harvey RL, Kissela BM, Winstein CJ, Lutsep HL, Parrish TB et al. . Epidural electrical stimulation for stroke rehabilitation: results of the prospective, multicenter, randomized, single-blinded everest trial. Neurorehabil Neural Repair 2016; 30: 107–19. [DOI] [PubMed] [Google Scholar]
- Li M, Liu Y, Wu Y, Liu S, Jia J, Zhang L. Neurophysiological substrates of stroke patients with motor imagery-based brain-computer interface training. Int J Neurosci 2014; 124: 403–15. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 2010; 74: 280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Zhu LL, Rüber T, Schlaug G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp 2012; 33: 1040–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Gao C, Wang W, Yu C-S, Wang X, Wu J-L. Effects of motor imagery combined with functional electrical stimulation on upper limb motor function of patients with acute ischemic stroke. Chinese J Contemp Neurol Neurosurg 2015; 15: 209–13. [Google Scholar]
- Liuzzi G, Hörniß V, Lechner P, Hoppe J, Heise K, Zimerman M et al. . Development of movement-related intracortical inhibition in acute to chronic subcortical stroke. Neurology 2014; 82: 198–205. [DOI] [PubMed] [Google Scholar]
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG et al. . Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med 2010; 362: 1772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccabee PJ, Amassian VE, Eberle LP, Cracco RQ. Magnetic coil stimulation of straight and bent amphibian and mammalian peripheral nerve in vitro: locus of excitation. J Physiol 1993; 460: 201–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal-crespo L, Reinkensmeyer DJ. Review of control strategies for robotic movement training after neurologic injury. J Neuroeng Rehabil 2009; 6: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe J, Monkiewicz M, Holcomb J, Pundik S, Daly JJ. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: a randomized controlled trial. Arch Phys Med Rehabil 2015; 96: 981–90. [DOI] [PubMed] [Google Scholar]
- Menezes IS, Cohen LG, Mello EA, Machado AG, Peckham PH, Anjos SM et al. . Combined brain and peripheral nerve stimulation in chronic stroke patients with moderate to severe motor impairment. Neuromodulation 2018; 21: 176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P et al. . Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient a scientific statement from the American Heart Association. Stroke 2010; 41: 2402–48. [DOI] [PubMed] [Google Scholar]
- Morishita T, Hummel FC. Non-invasive brain stimulation (NIBS) in motor recovery after stroke: concepts to increase efficacy. Curr Behav Neurosci Rep 2017; 4: 280–9. [Google Scholar]
- Morone G, Pisotta I, Pichiorri F, Kleih S, Paolucci S, Molinari M et al. . Proof of principle of a brain-computer interface approach to support poststroke arm rehabilitation in hospitalized patients: design, acceptability, and usability. Arch Phys Med Rehabil 2015; 96: S71–8. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Jiang N, Stevenson AJT, Niazi IK, Kostic V, Pavlovic A et al. . Efficient neuroplasticity induction in chronic stroke patients by an associative brain-computer interface. J Neurophysiol 2016; 115: 1410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke 2005; 36: 1480–4. [DOI] [PubMed] [Google Scholar]
- Nijland RHM, van Wegen EEH, Harmeling-van der Wel BC, Kwakkel G, Investigators E. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery early prediction of functional outcome after stroke: the EPOS cohort study. Stroke 2010; 41: 745–50. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A et al. . Transcranial direct current stimulation: state of the art 2008. Brain Stimul 2008; 1: 206–23. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000; 527 (Pt 3): 633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea J, Boudrias M-H, Stagg CJ, Bachtiar V, Kischka U, Blicher JU et al. . Predicting behavioural response to TDCS in chronic motor stroke. Neuroimage 2014; 85: 924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J, Gustavsson A, Svensson M, Wittchen H, Jönsson B. The economic cost of brain disorders in Europe. Eur J Neurol 2012; 19: 155–62. [DOI] [PubMed] [Google Scholar]
- Ono T, Shindo K, Kawashima K, Ota N, Ito M, Ota T et al. . Brain-computer interface with somatosensory feedback improves functional recovery from severe hemiplegia due to chronic stroke. Front Neuroeng 2014; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela-Espina F, Roldán GF, Sánchez-Villavicencio I, Palafox L, Leder R, Sucar LE et al. . Robot training for hand motor recovery in subacute stroke patients: a randomized controlled trial. J Hand Ther 2016; 29: 51–7. [DOI] [PubMed] [Google Scholar]
- Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity fugl-meyer scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther 2012; 92: 791–8. [DOI] [PubMed] [Google Scholar]
- Peurala SH, Pitkänen K, Sivenius J, Tarkka IM. Cutaneous electrical stimulation may enhance sensorimotor recovery in chronic stroke. Clin Rehabil 2002; 16: 709–16. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Scherer R, Neuper C. EEG-based brain-computer interface. In: Parasuraman R, Rizzo M, editors. Neuroergonomics: the brain at work. Oxford series in human-technology interaction. Oxford: Oxford University Press; 2008. [Google Scholar]
- Plow EB, Machado A. Invasive neurostimulation in stroke rehabilitation. Neurotherapeutics 2014; 11: 572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J et al. . Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev 2014; 11. doi: 10.1002/14651858.CD010820.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM et al. . Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair 2008; 22: 64–71. [DOI] [PubMed] [Google Scholar]
- Quandt F, Hummel FC. The influence of functional electrical stimulation on hand motor recovery in stroke patients: a review. Exp Transl Stroke Med 2014; 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffin E, Hummel FC. Restoring motor functions after stroke: multiple approaches and opportunities. Neuroscientist 2018; 24: 416–40. [DOI] [PubMed] [Google Scholar]
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC et al. . Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol 2013; 591: 2563–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Toshev PK, Bikson M. Polarizing cerebellar neurons with transcranial Direct Current Stimulation. Clin Neurophysiol 2014; 125: 435–8. [DOI] [PubMed] [Google Scholar]
- Ramos-Murguialday A, Broetz D, Rea M, Läer L, Yilmaz Ö, Brasil FL et al. . Brain–machine interface in chronic stroke rehabilitation: a controlled study. Ann Neurol 2013; 74: 100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsik A, Young B, Vermilyea R, Kiekhoefer L, Abrams J, Evander Elmore S et al. . A review of the progression and future implications of brain-computer interface therapies for restoration of distal upper extremity motor function after stroke. Expert Rev Med Devices 2016; 13: 445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AJ. NMES for control of spasticity. In: Robinson AJ, Snyder-Kackler L, editors. Clinical electrophysiology. 2nd edn. Baltimore: Lippincott Williams & Wilkins; 1995. [Google Scholar]
- Rocha S, Silva E, Foerster Á, Wiesiolek C, Chagas AP, Machado G et al. . The impact of transcranial direct current stimulation (tDCS) combined with modified constraint-induced movement therapy (mCIMT) on upper limb function in chronic stroke: a double-blind randomized controlled trial. Disabil Rehabil 2016; 38: 653–60. [DOI] [PubMed] [Google Scholar]
- Rong W, Li W, Pang M, Hu J, Wei X, Yang B et al. . A Neuromuscular Electrical Stimulation (NMES) and robot hybrid system for multi-joint coordinated upper limb rehabilitation after stroke. J Neuroeng Rehabil 2017; 14: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R et al. . Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 2015; 126: 1071–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler V, Acket B, Raposo N, Albucher J-F, Thalamas C, Loubinoux I et al. . Anodal tDCS combined with radial nerve stimulation promotes hand motor recovery in the acute phase after ischemic stroke. Neurorehabil Neural Repair 2015; 29: 743–54. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K, Fries W, Müller F, Koenig E. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet 2001; 358: 787–90. [DOI] [PubMed] [Google Scholar]
- Schick T, Schlake H-P, Kallusky J, Hohlfeld G, Steinmetz M, Tripp F et al. . Synergy effects of combined multichannel EMG-triggered electrical stimulation and mirror therapy in subacute stroke patients with severe or very severe arm/hand paresis. Restor Neurol Neurosci 2017; 35: 319–32. [DOI] [PubMed] [Google Scholar]
- Schulz R, Koch P, Zimerman M, Wessel M, Bönstrup M, Thomalla G et al. . Parietofrontal motor pathways and their association with motor function after stroke. Brain 2015; 138: 1949–60. [DOI] [PubMed] [Google Scholar]
- Schulz R, Park E, Lee J, Chang WH, Lee A, Kim Y-H et al. . Interactions between the corticospinal tract and premotor–motor pathways for residual motor output after stroke. Stroke 2017; 48: 2805–11. [DOI] [PubMed] [Google Scholar]
- Shin J-H, Park G, Cho DY. Cognitive-motor interference on upper extremity motor performance in a robot-assisted planar reaching task among patients with stroke. Arch Phys Med Rehabil 2017; 98: 730–7. [DOI] [PubMed] [Google Scholar]
- Simonetti D, Zollo L, Milighetti S, Miccinilli S, Bravi M, Ranieri F et al. . Literature review on the effects of tdcs coupled with robotic therapy in post stroke upper limb rehabilitation. Front Hum Neurosci 2017; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram R, Weiskopf N, Caria A, Veit R, Erb M, Birbaumer N. fMRI brain-computer interfaces. IEEE Signal Process Mag 2008; 25: 95–106. [Google Scholar]
- Sivan M, O’Connor RJ, Makower S, Levesley M, Bhakta B. Systematic review of outcome measures used in the evaluation of robot-assisted upper limb exercise in stroke. J Rehabil Med 2011; 43: 181–9. [DOI] [PubMed] [Google Scholar]
- Soekadar SR, Birbaumer N, Slutzky MW, Cohen LG. Brain–machine interfaces in neurorehabilitation of stroke. Neurobiol Dis 2015; 83: 172–9. [DOI] [PubMed] [Google Scholar]
- Sprigg N, Willmot MR, Gray LJ, Sunderland A, Pomeroy V, Walker M et al. . Amphetamine increases blood pressure and heart rate but has no effect on motor recovery or cerebral haemodynamics in ischaemic stroke: a randomized controlled trial (ISRCTN 36285333). J Hum Hypertens 2007; 21: 616. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Jayaram G, Pastor D, Kincses ZT, Matthews PM, Johansen-Berg H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 2011; 49: 800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol 2010; 9: 1228–32. [DOI] [PubMed] [Google Scholar]
- Straudi S, Fregni F, Martinuzzi C, Pavarelli C, Salvioli S, Basaglia N. tDCS and robotics on upper limb stroke rehabilitation: effect modification by stroke duration and type of stroke. Biomed Res Int 2016; 2016: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MJ, Cury RG, Galhardoni R, Barboza VR, Brunoni AR, Alho E et al. . Deep brain stimulation of the dentate nucleus improves cerebellar ataxia after cerebellar stroke. Neurology 2015; 85: 2075–6. [DOI] [PubMed] [Google Scholar]
- Tomi TJD, Savic AM, Vidakovi AS, Rodic SZ, Isakovic MS, Rodriguez-de-Pablo C et al. . ArmAssist robotic system versus matched conventional therapy for poststroke upper limb rehabilitation: a randomized clinical trial. Biomed Res Int 2017; 2017: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun A, Türe S, Askin A, Yardimci EU, Demirdal SU, Kurt Incesu T et al. . Effects of low-frequency repetitive transcranial magnetic stimulation and neuromuscular electrical stimulation on upper extremity motor recovery in the early period after stroke: a preliminary study. Top Stroke Rehabil 2017; 24: 361–7. [DOI] [PubMed] [Google Scholar]
- Tran DA, Pajaro-Blazquez M, Daneault J-F, Gallegos JG, Pons J, Fregni F et al. . Combining dopaminergic facilitation with robot-assisted upper limb therapy in stroke survivors: a focused review. Am J Phys Med Rehabil 2016; 95: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretriluxana J, Kantak S, Tretriluxana S, Wu AD, Fisher BE. Improvement in paretic arm reach-to-grasp following low frequency repetitive transcranial magnetic stimulation depends on object size: a pilot study. Stroke Res Treat 2015; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triccas LT, Burridge JH, Hughes A-M, Pickering RM, Desikan M, Rothwell JC et al. . Multiple sessions of transcranial direct current stimulation and upper extremity rehabilitation in stroke: a review and meta-analysis. Clin Neurophysiol 2016; 127: 946–55. [DOI] [PubMed] [Google Scholar]
- Triccas LT, Burridge JH, Hughes A-M, Rothwell J, Desikan M, Verheyden G. A mixed-methods study exploring the combination of non-invasive brain stimulation and robot therapy for the impaired upper limb in stroke. Physiotherapy 2015a; 101: e1496–7. [Google Scholar]
- Triccas LT, Burridge JH, Hughes A, Verheyden G, Desikan M, Rothwell J. A double-blinded randomised controlled trial exploring the effect of anodal transcranial direct current stimulation and uni-lateral robot therapy for the impaired upper limb in sub-acute and chronic stroke. NeuroRehabilitation 2015b; 37: 181–91. [DOI] [PubMed] [Google Scholar]
- van Dokkum LEH, Ward T, Laffont I. Brain computer interfaces for neurorehabilitation—its current status as a rehabilitation strategy post-stroke. Ann Phys Rehabil Med 2015; 58: 3–8. [DOI] [PubMed] [Google Scholar]
- Vafadar AK, Côté JN, Archambault PS. Effectiveness of functional electrical stimulation in improving clinical outcomes in the upper arm following stroke: a systematic review and meta-analysis. Biomed Res Int 2015; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerbeek JM, Kwakkel G, van Wegen EEH, Ket JCF, Heymans MW. Early prediction of outcome of activities of daily living after stroke: a systematic review. Stroke 2011; 42: 1482–8. [DOI] [PubMed] [Google Scholar]
- Veerbeek JM, Langbroek-Amersfoort AC, van Wegen EEH, Meskers CGM, Kwakkel G. Effects of robot-assisted therapy for the upper limb after stroke. Neurorehabil Neural Repair 2017; 31: 107–21. [DOI] [PubMed] [Google Scholar]
- Viale L, Catoira NP, Di Girolamo G, González CD. Pharmacotherapy and motor recovery after stroke. Expert Rev Neurother 2018; 18: 65–82. [DOI] [PubMed] [Google Scholar]
- Wilson RD, Page SJ, Delahanty M, Knutson JS, Gunzler DD, Sheffler LR et al. . Upper-limb recovery after stroke. Neurorehabil Neural Repair 2016; 30: 978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters C, Heymans MW, van Wegen EEH, Kwakkel G. How to design clinical rehabilitation trials for the upper paretic limb early post stroke? Trials 2016a; 17: 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters C, Kwakkel G, Nijland R, Van Wegen E, EXPLICIT-stroke consortium. When does return of voluntary finger extension occur post-stroke? A prospective cohort study. PLoS One 2016b; 11: e0160528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters C, van Wegen EEH, Daffertshofer A, Kwakkel G. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil Neural Repair 2015; 29: 614–22. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Kwakkel G, Bayley M, McDonnell MN, Group UESAW. Best practice for arm recovery post stroke: an international application. Physiotherapy 2016; 102: 1–4. [DOI] [PubMed] [Google Scholar]
- Wu X, Guarino P, Lo AC, Peduzzi P, Wininger M. Long-term effectiveness of intensive therapy in chronic stroke. Neurorehabil Neural Repair 2016; 30: 583–90. [DOI] [PubMed] [Google Scholar]
- Wuwei F, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas V-A et al. . Corticospinal tract lesion load: an imaging biomarker for stroke motor outcomes. Ann Neurol 2015; 78: 860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.