Abstract
Objective
To determine whether radiologic extranodal extension (ENE) appearing on pretreatment CT and MRI could predict the prognosis in patients with human papillomavirus (HPV)-related oropharyngeal squamous cell carcinoma (OPSCC).
Materials and Methods
The study population was obtained from a historical cohort diagnosed with HPV-related OPSCC. A total of 134 OPSCC patients who had a metastatic lymph node on pretreatment CT or MRI were included, and radiologic ENE was evaluated by two experienced head and neck radiologists. Kaplan-Meier and multivariate Cox regression analyses were performed to evaluate the impact of radiologic ENE on progression-free survival (PFS). The diagnostic performance of CT and MRI for the diagnosis of ENE was also evaluated in patients who underwent neck dissection.
Results
Seventy patients (52.2%) showed radiologic ENE-positive findings. Although patients showing radiologic ENE had a worse 3-year PFS (83.7% vs. 95.3%, p = 0.023), the association between radiologic ENE and PFS was not statistically significant on multivariate analysis (p = 0.141; hazard ratio, 2.68; 95% confidence interval, 0.72–9.97). CT or MRI had a sensitivity of 62%, specificity of 77.8%, and accuracy of 71.9% for predicting pathologic ENE.
Conclusion
Radiologic ENE on CT or MRI did not predict poor PFS in patients with HPV-related OPSCC, although there was a trend towards worse PFS. Further studies are warranted to determine whether radiologic ENE is a useful imaging biomarker to risk-stratify patients with HPV-related OPSCC.
Keywords: Human papillomavirus, Oropharyngeal squamous cell carcinoma, Extranodal extension, Prognosis
INTRODUCTION
The incidence of human papillomavirus (HPV)-related oropharyngeal squamous cell carcinoma (OPSCC) has markedly increased from approximately 16% in the early 1980s to over 70% of all OPSCC cases at present (1,2). In the 8th edition of the American Joint Committee on Cancer (AJCC 8th edition) primary tumor, lymph node, and metastasis (TNM) staging system, staging of OPSCC is divided into HPV-positive OPSCC and HPV-negative OPSCC, mainly with respect to the lymph node stage (3). Extranodal extension (ENE) has been included in the lymph node staging of head and neck cancer, with the exception of HPV-related OPSCC, and ENE has been shown to significantly influence the prognosis (4,5,6,7,8,9). Generally, patients who develop HPV-positive OPSCC have a better prognosis than those with HPV-negative OPSCC (10). However, in a subset of patients with atypical features of HPV-related OPSCC, including radiologic ENE or matted lymph nodes, the cancer tends to show aggressive behavior, including local recurrence with distant metastases (11,12,13). Recently, two studies that used CT to investigate the associations between radiologic ENE and survival outcomes in patients with HPV-related OPSCC yielded conflicting results: one showed that radiologic ENE had significant prognostic value (14), while the other found that it had no value (15). One previous study showed that contrast-enhanced magnetic resonance (MR) can be useful for lymph node characterization in head and neck squamous cell carcinoma (16). Additionally, to the best of our knowledge, there has been no study investigating associations between radiologic ENE and survival outcomes that used both MRI and CT in patients with HPV-related OPSCC. Therefore, the purpose of this study was to evaluate the associations between MRI- and CT-detected ENE and survival outcomes in a large number of patients with HPV-related OPSCC.
MATERIALS AND METHODS
The Institutional Review Board of our hospital approved this retrospective study, and the requirement for informed consent was waived for data evaluation. Written informed consent for routine neck CT was obtained from all patients before each CT examination. The methods and reporting of results are in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (17). The authors received no external financial support for this study.
Study Population
The study population was obtained from a historical cohort of consecutive patients who were newly diagnosed with OPSCC without evidence of distant metastases at Asan Medical Center between July 2006 and November 2016. The inclusion criteria used to select patients were 1) patients who underwent p16 immunohistochemistry analysis or HPV deoxyribonucleic acid (DNA) detection, and 2) patients who underwent contrast-enhanced neck CT or MRI before treatment. Patients were excluded from the study population if they 1) showed a negative result in p16 immunohistochemistry analysis or HPV DNA detection, 2) had a prior cancer history or synchronous cancers, or 3) showed no metastatic lymph node on pretreatment CT or MRI.
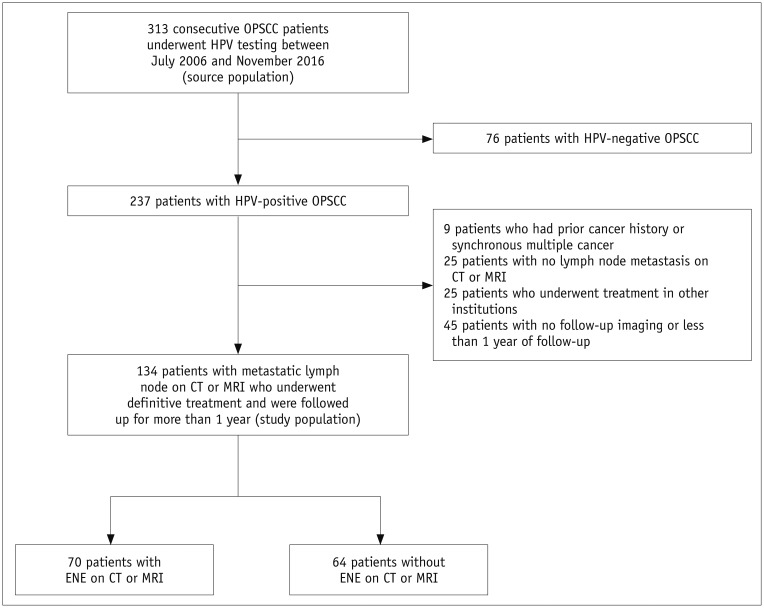
Therefore, 313 consecutive OPSCC patients underwent p16 immunohistochemistry analysis or HPV DNA detection, and 237 were identified as having HPV-positive OPSCC. After excluding 103 patients, 134 patients were included in this study (Fig. 1), which included 118 men (mean age, 59.9 years; age range, 58.2–61.5 years) and 16 women (mean age, 59.9 years; age range, 55.5–64.2 years) with a mean age of 59.9 years (age range, 58.3–61.4 years).
Fig. 1. Flow diagram of patient findings.
ENE = extranodal extension, HPV = human papillomavirus, OPSCC = oropharyngeal squamous cell carcinoma
Patients were staged according to the 8th edition of the AJCC-TNM staging system by using physical and endoscopic exams, CT, MRI, and 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT imaging. Information regarding patient age, sex, smoking status, treatment methods, and survival status at last follow-up were obtained from the medical records.
Analysis of HPV Status
Two methods are mainly used to evaluate HPV infection status in our institution, with one being tumor p16 immunohistochemical analysis using CINtec p16 histology (anti-p16INK4a mouse monoclonal antibody and immunohistochemical detection kit; Roche MTM Laboratories, Heidelberg, Germany). A positive p16 result was defined as diffuse strong staining in the cytoplasm and nucleus. Focal or faint reactivity was considered negative for p16. The other method used was tumor HPV DNA detection by polymerase chain reaction (PCR)/DNA chip scanning, which can detect 43 subtypes of HPV (high-risk subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82 and other lower or undetermined risk subtypes). HPV-related OPSCC was diagnosed when the results of either p16 or HPV DNA PCR were positive (18).
Follow-Up and Outcome Measurement
After completing treatment, patients were evaluated by physical examination, endoscopy, and imaging (e.g., CT, MRI, or 18F-FDG PET/CT) every 2–3 months for the first year, according to the protocols of our hospital, which were based on the guidelines of the National Comprehensive Cancer Network. Follow-up intervals were increased to 3–6 months in the second year and 6–12 months thereafter. To avoid extended follow-up and ensure efficient assessment, progression-free survival (PFS) was chosen as the primary outcome (19). PFS was calculated from the first day of treatment to the date of disease progression (locoregional recurrence or distant metastases), death from any cause, or the date of the last follow-up visit (censored). The minimum follow-up period for ascertaining PFS was 12 months. All locoregional recurrences were diagnosed by physical examination, flexible endoscopy and biopsy, and/or CT or MRI of the neck showing progressive bone erosion and/or a soft tissue mass. Distant metastases were diagnosed using imaging methods that included whole-body bone scan, CT, MRI, or 18F-FDG PET/CT. A secondary outcome was the diagnostic performance of CT or MRI for the diagnosis of ENE among the patients who underwent neck dissection for lymph nodes. The reference standard was surgical pathologic results.
CT and MRI Examinations
A total of 122 patients underwent contrast-enhanced CT scans with one of several different multidetector CT systems with 64–128 channels (Siemens Healthineers, Erlangen, Germany; GE Healthcare, Milwaukee, WI, USA). The typical imaging parameters were as follows: 120-kV tube voltage; 200-mAs effective tube current; 22-cm display field-of-view (FOV); 50-cm large-body scan FOV; and axial and coronal sections with a 3-mm thickness reconstructed using a soft tissue algorithm. The scan range extended from the upper margins of the frontal sinus to the top of the aortic arch. All scans were acquired at 70 seconds after intravenous administration of 140 mL of non-ionic iodinated contrast media (iopamidol, Isovue-370; Bracco Diagnostics, Princeton, NJ, USA) at a rate of 2.5 mL/s.
MRI was performed on 117 patients by using a 3T MR scanner (Achieva; Philips Healthcare, Best, The Netherlands) with a 16-channel neurovascular coil (SENSE NV coil; Philips Healthcare). The neck imaging protocol contained the following sequences: axial T1-weighted imaging, axial T2-weighted imaging, axial fat-suppressed contrast-enhanced T1-weighted imaging (CE-T1WI), coronal T1-weighted imaging, coronal fat-suppressed T2-weighted imaging, and coronal fat-suppressed CE-T1WI. CT or MR images were analyzed on Picture Archiving and Communication System (PACS). The mean interval between staging CT or MRI and the start of treatment was 49 days (range, 7–90 days).
Among 134 patients, 105 patients underwent both CT and MR examinations. Seventeen patients underwent only CT scans and 12 patients underwent only MR scans.
Radiologic Evaluation of ENE
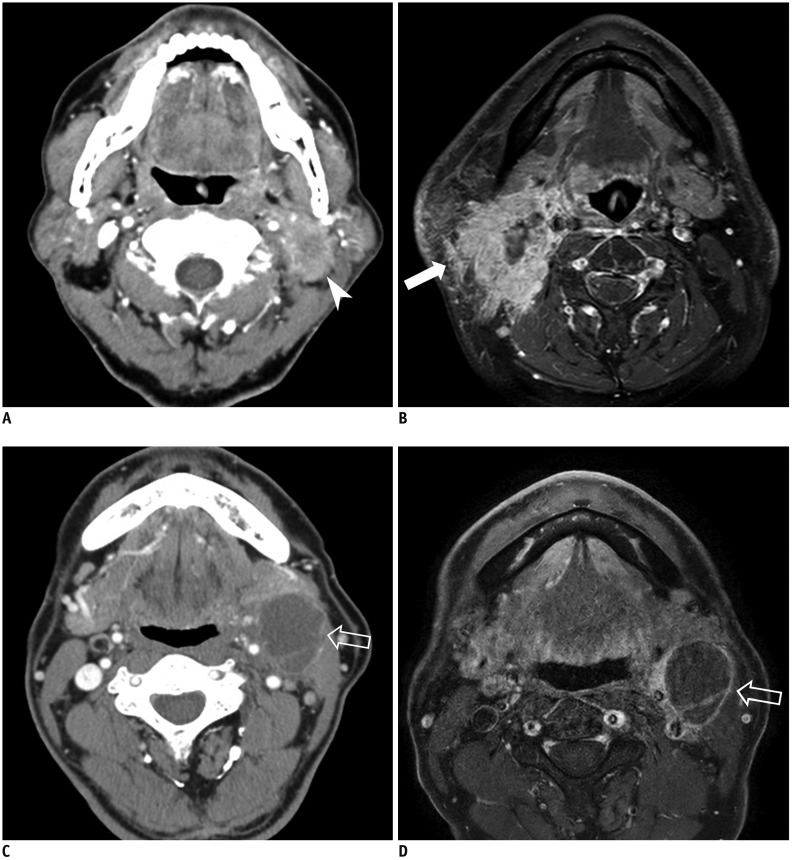
All CT or MRI studies were interpreted in an independent manner by two board-certified radiologists with 5 and 12 years of clinical experience in head and neck imaging. Before evaluation, the two neuroradiologists completed a training session with five patients to help them reach a consensus on evaluation of the imaging findings. Lymph nodes were considered as positive for radiologic ENE when at least one of the following criteria was met (9,20,21,22,23,24): 1) enhancement, thickening, and irregularity of the nodal rim and 2) infiltration of the adjacent fat or other soft tissue planes (Fig. 2). If there was a discrepancy over the presence of radiologic ENE between CT and MRI (i.e., one of the two yielded a positive result), radiologic ENE was considered to be present. If patients underwent lymph node surgery, the images depicting the surgical dissection sites were reviewed. The imaging results, including the presence of radiologic ENE in any cervical lymph node of the patients, were compared with the nodal histopathology results.
Fig. 2. Representative cases of radiologic ENE in patients with HPV-related OPSCC.
Metastatic lymph node with infiltration of adjacent fat or other soft tissue on CT (arrow head) (A) and MRI (arrow) (B). C, D. Metastasis in lymph node with enhancement, thickening, and irregularity of nodal rim on CT and MRI (empty arrows).
Statistical Analysis
The patient's clinicopathological features were analyzed according to their radiologic ENE status by using a chi-squared test or Fisher's exact test for categorical variables, and a t test for continuous variables. Univariate Cox regression analysis was performed to identify predictors of PFS among the following variables: age, sex, smoking status, T-stage, N-stage, overall stage, radiologic ENE, primary tumor site, treatment modality, and interval between imaging and treatment. A multivariate Cox regression analysis was performed using backward elimination. The distribution of PFS was estimated by the Kaplan-Meier method and compared using a log-rank test.
The sensitivity, specificity, accuracy, positive and negative predictive values, and area under the receiver operating characteristic curve of CT and MRI for depicting the presence of radiologic ENE were calculated relative to the histopathologic results.
Interobserver agreement in the image analysis of neck CT or MRI for radiologic ENE was calculated by using Cohen's kappa index. Kappa values were interpreted as indicating poor (κ < 0.1), slight (0.1 ≤ κ ≤ 0.2), fair (0.2 < κ ≤ 0.4), moderate (0.4 < κ ≤ 0.6), substantial (0.6 < κ ≤ 0.8), and nearly perfect (0.8 < κ ≤ 1.0) agreement (25,26,27). Statistical analysis was performed with SPSS version 23 (IBM Corp., Armonk, NY, USA).
RESULTS
Clinical Characteristics of the Patients
The median follow-up period was 38 months (range, 12–107 months). The mean lymph node size (the minimum axial diameter) was 1.9 cm (range, 0.5–4.0 cm). Radiologic ENE showed a statistically significant association with high N-stage and overall stage (p = 0.012 and p = 0.041, respectively), as shown in Table 1.
Table 1. Clinical and Pathologic Characteristics of Patients.
| Characteristic | Radiologic ENE-Negative (n = 64) | Radiologic ENE-Positive (n = 70) | P |
|---|---|---|---|
| Age (year) | 58.5 | 61.1 | 0.092 |
| Male sex | 53 (82.8) | 65 (92.9) | 0.073 |
| Smoking status | 0.426 | ||
| Never smoked | 22 (34.9) | 18 (25.7) | |
| Ever smoked ≤ 10 PY | 11 (17.5) | 17 (24.3) | |
| Ever smoked > 10 PY | 30 (47.6) | 35 (50.0) | |
| T-stage | 0.131 | ||
| Early (T0, T1, T2) | 52 (81.3) | 49 (70.0) | |
| Advanced (T3, T4) | 12 (18.7) | 21 (30.0) | |
| N-stage | 0.012* | ||
| N1 | 58 (90.6) | 51 (72.8) | |
| N2 | 6 (9.4) | 16 (22.9) | |
| N3 | 0 (0) | 3 (4.3) | |
| Overall stage | 0.041* | ||
| Stage I | 48 (75.0) | 38 (54.3) | |
| Stage II | 6 (9.4) | 14 (20.0) | |
| Stage III | 10 (15.6) | 18 (25.7) | |
| Primary tumor site | 0.458 | ||
| Palatine tonsil | 53 (82.8) | 62 (88.6) | |
| Base of tongue | 9 (14.1) | 5 (7.1) | |
| Others | 0 (0) | 1 (1.4) | |
| Unknown | 2 (3.1) | 2 (2.9) | |
| Treatment | 0.223 | ||
| CCRT or RT | 28 (43.8) | 38 (54.3) | |
| Operation and/or MMT | 36 (56.2) | 32 (45.7) | |
| Interval between imaging and treatment | 0.451 | ||
| ≤ 8 weeks | 56 (87.5) | 58 (82.86) | |
| > 8 weeks | 8 (12.5) | 12 (17.14) |
All values are number of patients. Numbers in parenthesis is percentage. *Statistical significance. CCRT = concurrent chemoradiation therapy, ENE = extranodal extension, MMT = multimodality therapy, PY = pack year, RT = radiation therapy
Survival Analyses
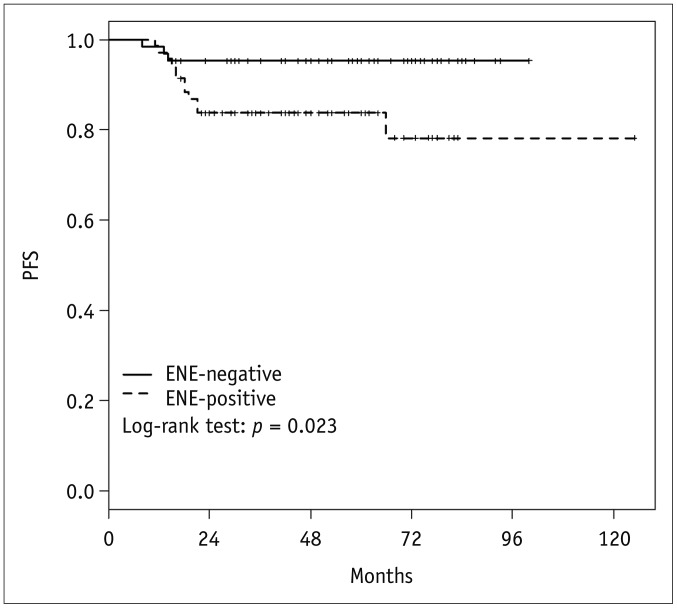
In the univariate Cox regression analysis, the presence of radiologic ENE, increased N-stage (N1 vs. N2, N3), increased T-stage (T0, T1, T2 vs. T3, T4), and overall stage (I, II vs. III) were significant adverse variables for PFS (p = 0.035, p = 0.004, p = 0.026, and p = 0.006, respectively) (Table 2). In the multivariate Cox regression analysis, N-stage and overall stage were statistically associated with PFS (p = 0.041 and p = 0.022, respectively), but the presence of radiologic ENE was not (p = 0.141; hazard ratio, 2.68; 95% confidence interval [CI], 0.72–9.97). The Kaplan-Meier disease-free survival curves show that PFS was significantly lower in patients with radiologic ENE-positive disease, with a 3-year PFS of 95.3% versus 83.7%, respectively (log-rank p = 0.023) (Fig. 3).
Table 2. Univariate and Multivariate Analysis of Progression-Free Survival.
| Characteristic | Radiologic ENE-Negative (n = 64) | Radiologic ENE-Positive (n = 70) | P |
|---|---|---|---|
| Age (year) | 58.5 | 61.1 | 0.092 |
| Male sex | 53 (82.8) | 65 (92.9) | 0.073 |
| Smoking status | 0.426 | ||
| Never smoked | 22 (34.9) | 18 (25.7) | |
| Ever smoked ≤ 10 PY | 11 (17.5) | 17 (24.3) | |
| Ever smoked > 10 PY | 30 (47.6) | 35 (50.0) | |
| T-stage | 0.131 | ||
| Early (T0, T1, T2) | 52 (81.3) | 49 (70.0) | |
| Advanced (T3, T4) | 12 (18.7) | 21 (30.0) | |
| N-stage | 0.012* | ||
| N1 | 58 (90.6) | 51 (72.8) | |
| N2 | 6 (9.4) | 16 (22.9) | |
| N3 | 0 (0) | 3 (4.3) | |
| Overall stage | 0.041* | ||
| Stage I | 48 (75.0) | 38 (54.3) | |
| Stage II | 6 (9.4) | 14 (20.0) | |
| Stage III | 10 (15.6) | 18 (25.7) | |
| Primary tumor site | 0.458 | ||
| Palatine tonsil | 53 (82.8) | 62 (88.6) | |
| Base of tongue | 9 (14.1) | 5 (7.1) | |
| Others | 0 (0) | 1 (1.4) | |
| Unknown | 2 (3.1) | 2 (2.9) | |
| Treatment | 0.223 | ||
| CCRT or RT | 28 (43.8) | 38 (54.3) | |
| Operation and/or MMT | 36 (56.2) | 32 (45.7) | |
| Interval between imaging and treatment | 0.451 | ||
| ≤ 8 weeks | 56 (87.5) | 58 (82.86) | |
| > 8 weeks | 8 (12.5) | 12 (17.14) |
*Statistical significance. CI = confidence interval, Ref = reference
Fig. 3. Kaplan-Meier curve and p values from log-rank test for PFS according to radiologic ENE.
PFS was significantly worse in patients with radiologic ENE than those without, with 3-year PFS of 95.3% versus 83.7%, respectively (log-rank p = 0.023). PFS = progression-free survival
Diagnostic Performance of CT or MRI for Detecting Radiologic ENE
Of the 57 patients who underwent neck dissection and received a pathologic evaluation for ENE, 21 (36.8%) had pathologically confirmed ENE. Among these patients, the area under the receiver operating characteristic curve for diagnosis of radiologic ENE on CT or MRI was 0.70 (range, 0.57–0.83), and the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 62.0%, 77.8%, 61.9%, 77.8%, and 71.9%, respectively (p = 0.002).
The interobserver agreement between the two neuroradiologists for interpreting radiologic ENE was substantial (kappa = 0.79; 95% CI, 0.67–0.89). Specifically, for enhancement, thickening, and irregularity of the nodal rim, it was nearly perfect (kappa = 0.83) while it was substantial (kappa = 0.73) for infiltration of the adjacent fat or other soft tissue planes. The interobserver agreement between the two neuroradiologists for radiologic ENE was substantial on both MRI (kappa = 0.76; 95% CI, 0.64–0.88) and CT (kappa = 0.77; 95% CI, 0.66–0.88).
DISCUSSION
The objective of this study was to evaluate the prognostic value of radiologic ENE in HPV-related OPSCC. We demonstrated that, among patients with HPV-related OPSCC, PFS was significantly lower in patients showing radiologic ENE on pretreatment CT or MRI, although the association between radiologic ENE and PFS did not reach statistical significance in the multivariate analysis (p = 0.141). Additionally, in patients with HPV-related OPSCC, radiologic ENE predicted pathologic ENE with a sensitivity of 62%, specificity of 77.8%, positive predictive value of 61.9%, negative predictive value of 77.8%, and accuracy of 71.9%. To the best of our knowledge, this is the first study to apply both CT and MRI to evaluate associations between radiologic ENE and survival outcomes in patients with HPV-related OPSCC. Recent studies assessing the image findings and diagnostic performance of ENE on CT in patients with HPV-related OPSCC demonstrated sensitivity and specificity of 55% and 95% (28). Since current imaging modalities show limitations in their ability to accurately identify ENE, especially microscopic ENE (3,9,28), it might be difficult to avoid the discord between radiologic and pathologic ENE.
Very few studies have investigated the associations between radiologic ENE on CT and survival outcomes in patients with HPV-related OPSCC. Rath et al. (14) demonstrated that radiologic ENE was an independent worse predictor of disease-free survival in patients with HPV-related OPSCC (hazard ratio = 3.3, p = 0.001). In contrast, Liu et al. (15) showed that radiologic ENE was not a significant prognosticator of PFS in patients with HPV-related OPSCC, although they did find a trend towards worse PFS with radiologic ENE (p = 0.098), as in the present study. The discrepancy in the results between the studies can be explained by the different definitions of radiologic ENE and the differences in imaging modalities and the number of patients included in the studies. In terms of the association between pathologic ENE and prognosis in patents with HPV-related OPSCC, Sinha et al. (7) found that pathologic ENE was not associated with worse disease-free survival in a multivariate analysis (hazard ratio = 3.42, p = 0.23). Furthermore, a recent meta-analysis demonstrated the absence of a negative impact of pathologic ENE in HPV-related OPSCC (29). It is well-known that there is a direct correlation between ENE and a poor prognosis in HPV-unrelated head and neck cancer. In this regard, the AJCC 8th edition TNM staging system introduces radiologic or pathologic ENE as a descriptor for lymph node categorization of HPV-unrelated head and neck cancers, but not for HPV-related OPSCC (3). Our findings are consistent with the existing knowledge, and the clinical significance of our research is that we have verified the AJCC 8th edition TNM staging of HPV-related OPSCC.
In our study, CT or MRI showed a sensitivity of 62%, specificity of 77.8%, and an area under the receiver operating characteristic curve of 0.7 for the evaluation of radiologic ENE, with substantial agreement between the two radiologists (kappa = 0.79). The diagnostic performance shown in this study is within the range of those presented in previous reports studying the diagnostic performance of radiologic ENE in patients with head and neck squamous cell carcinoma (sensitivity: 43.7–65%, specificity: 54–97.7%) (9,20,30). The differences in diagnostic performance could be explained by the use of different imaging criteria for radiologic ENE and the analytical units used (each lymph node, level, and patient).
In this study, when the criteria of the AJCC 8th edition TNM system were analyzed using multivariate Cox regression analysis, increased N-stage (N1 vs. N2, N3) and overall stage (I, II vs. III) were statistically associated with PFS. Mizumachi et al. (31) demonstrated similar results, reporting a significant difference in 3-year overall survival rate between stages I–II and III. Although T-staging is considered more influential for clinical outcome than N-staging in HPV-related OPSCC (18), we did not find a significant association between T-staging and PFS in the multivariate analysis. This may be due to the relatively small number of T3 and T4 cases (n = 33) and progressive T3 and T4 cases (n = 7).
There were several limitations in this study. First, some selection and treatment biases could have been present since this study was performed retrospectively at a single center and used a relatively small number of disease progression events (n = 15). Although the presence of radiologic ENE alone did not influence treatment decisions, patients with higher lymph node stage are treated with more aggressive therapies at our institution. Further studies are needed to validate the study results. Second, we did not assess grading on radiologic ENE and did not analyze any association between grading on radiologic ENE and PFS. Additional investigations are required for this issue.
In conclusion, the presence of radiologic ENE did not predict poor PFS in patients with HPV-related OPSCC, although there was a trend towards poor PFS. Larger-scale longer prospective studies are warranted to determine whether radiologic ENE in HPV-related OPSCC is a useful imaging biomarker to risk-stratify patients for personalized treatment.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380–386. doi: 10.1016/j.oraloncology.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York, NY: springer; 2017. [Google Scholar]
- 4.Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope. 2012;122(Suppl 2):S13–S33. doi: 10.1002/lary.23493. [DOI] [PubMed] [Google Scholar]
- 5.Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 6.Wreesmann VB, Katabi N, Palmer FL, Montero PH, Migliacci JC, Gönen M, et al. Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E1192–E1199. doi: 10.1002/hed.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha P, Lewis JS, Jr, Piccirillo JF, Kallogjeri D, Haughey BH. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer. 2012;118:3519–3530. doi: 10.1002/cncr.26671. [DOI] [PubMed] [Google Scholar]
- 8.Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer. 2001;92:3030–3036. doi: 10.1002/1097-0142(20011215)92:12<3030::aid-cncr10148>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Prabhu RS, Magliocca KR, Hanasoge S, Aiken AH, Hudgins PA, Hall WA, et al. Accuracy of computed tomography for predicting pathologic nodal extracapsular extension in patients with head-and-neck cancer undergoing initial surgical resection. Int J Radiat Oncol Biol Phys. 2014;88:122–129. doi: 10.1016/j.ijrobp.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 11.Kaka AS, Kumar B, Kumar P, Wakely PE, Jr, Kirsch CM, Old MO, et al. Highly aggressive human papillomavirus-related oropharyngeal cancer: clinical, radiologic, and pathologic characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:327–335. doi: 10.1016/j.oooo.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spector ME, Gallagher KK, Light E, Ibrahim M, Chanowski EJ, Moyer JS, et al. University of Michigan Head Neck Specialized Program of Research Excellence (SPORE) Program. Matted nodes: poor prognostic marker in oropharyngeal squamous cell carcinoma independent of HPV and EGFR status. Head Neck. 2012;34:1727–1733. doi: 10.1002/hed.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang SH, Perez-Ordonez B, Liu FF, Waldron J, Ringash J, Irish J, et al. Atypical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:276–283. doi: 10.1016/j.ijrobp.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Rath TJ, Narayanan S, Hughes MA, Ferris RL, Chiosea SI, Branstetter BF., 4th Solid lymph nodes as an imaging biomarker for risk stratification in human papillomavirus-related oropharyngeal squamous cell carcinoma. AJNR Am J Neuroradiol. 2017;38:1405–1410. doi: 10.3174/ajnr.A5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu JT, Kann BH, De B, Buckstein M, Bakst RL, Genden EM, et al. Prognostic value of radiographic extracapsular extension in locally advanced head and neck squamous cell cancers. Oral Oncol. 2016;52:52–57. doi: 10.1016/j.oraloncology.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Lee KC, Moon WK, Chung JW, Choi SH, Cho N, Cha JH, et al. Assessment of lymph node metastases by contrast-enhanced MR imaging in a head and neck cancer model. Korean J Radiol. 2007;8:9–14. doi: 10.3348/kjr.2007.8.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. STROBE Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Lee SW, Park S, Yoon SM, Park JH, Song SY, et al. Refining prognostic stratification of human papilloma-virusrelated oropharyngeal squamous cell carcinoma: different prognosis between T1 and T2. Radiat Oncol J. 2017;35:233–240. doi: 10.3857/roj.2017.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 20.Chai RL, Rath TJ, Johnson JT, Ferris RL, Kubicek GJ, Duvvuri U, et al. Accuracy of computed tomography in the prediction of extracapsular spread of lymph node metastases in squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg. 2013;139:1187–1194. doi: 10.1001/jamaoto.2013.4491. [DOI] [PubMed] [Google Scholar]
- 21.Souter MA, Allison RS, Clarkson JH, Cowan IA, Coates MH, Wells JE. Sensitivity and specificity of computed tomography for detection of extranodal spread from metastatic head and neck squamous cell carcinoma. J Laryngol Otol. 2009;123:778–782. doi: 10.1017/S0022215109004332. [DOI] [PubMed] [Google Scholar]
- 22.Som PM, Brandwein-Gensler MS. Lymph nodes of the neck. In: Som PM, Curtin HD, editors. Head and neck imaging. 5th ed. St. Louis, MO: Mosby; 2011. pp. 2287–2384. [Google Scholar]
- 23.Patel MR, Hudgins PA, Beitler JJ, Magliocca KR, Griffith CC, Liu Y, et al. Radiographic imaging does not reliably predict macroscopic extranodal extension in human papilloma virus-associated oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2018;80:85–95. doi: 10.1159/000487239. [DOI] [PubMed] [Google Scholar]
- 24.Huang YH, Yeh CH, Cheng NM, Lin CY, Wang HM, Ko SF, et al. Cystic nodal metastasis in patients with oropharyngeal squamous cell carcinoma receiving chemoradiotherapy: relationship with human papillomavirus status and failure patterns. PLoS One. 2017;12:e0180779. doi: 10.1371/journal.pone.0180779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003;228:303–308. doi: 10.1148/radiol.2282011860. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Lee JW, Chai JW, Yoo HJ, Kang Y, Seo J, et al. A new MRI grading system for cervical foraminal stenosis based on axial T2-weighted images. Korean J Radiol. 2015;16:1294–1302. doi: 10.3348/kjr.2015.16.6.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JE, Han K, Sung YS, Chung MS, Koo HJ, Yoon HM, et al. Selection and reporting of statistical methods to assess reliability of a diagnostic test: conformity to recommended methods in a peer-reviewed journal. Korean J Radiol. 2017;18:888–897. doi: 10.3348/kjr.2017.18.6.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geltzeiler M, Clayburgh D, Gleysteen J, Gross ND, Hamilton B, Andersen P, et al. Predictors of extracapsular extension in HPV-associated oropharyngeal cancer treated surgically. Oral Oncol. 2017;65:89–93. doi: 10.1016/j.oraloncology.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 29.Mermod M, Tolstonog G, Simon C, Monnier Y. Extracapsular spread in head and neck squamous cell carcinoma: a systematic review and meta-analysis. Oral Oncol. 2016;62:60–71. doi: 10.1016/j.oraloncology.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell JH, Rath TJ, Byrd JK, Albergotti WG, Wang H, Duvvuri U, et al. Accuracy of computed tomography to predict extracapsular spread in p16-positive squamous cell carcinoma. Laryngoscope. 2015;125:1613–1618. doi: 10.1002/lary.25140. [DOI] [PubMed] [Google Scholar]
- 31.Mizumachi T, Homma A, Sakashita T, Kano S, Hatakeyama H, Fukuda S. Confirmation of the eighth edition of the AJCC/UICC TNM staging system for HPV-mediated oropharyngeal cancer in Japan. Int J Clin Oncol. 2017;22:682–689. doi: 10.1007/s10147-017-1107-0. [DOI] [PubMed] [Google Scholar]