Abstract
The specification, maintenance, division and differentiation of stem cells are integral to the development and homeostasis of many tissues. These stem cells often live in specialized anatomical areas, called niches. While niches can be complex, most involve cell-cell interactions that are mediated by adherens junctions. A diverse array of functions have been attributed to adherens junctions in stem cell biology. These include physical anchoring to the niche, control of proliferation and division orientation, regulation of signaling cascades and of differentiation. In this review, a number of model stem cell systems that highlight various functions of adherens junctions are discussed. In addition, a summary of the current understanding of adherens junction function in mammalian tissues and embryonic and induced pluripotent stem cells is provided. This analysis demonstrates that the roles of adherens junctions are surprisingly varied and integrated with both the anatomy and the physiology of the tissue.
15.1. Introduction
Both the development and the homeostasis of many tissues in our body relies on specialized stem cells. In adults, these cells are functionally defined by their ability to self-renew, to exist for extended periods of time and to give rise to daughter cells that contribute to the differentiated cell pool of a tissue. Because of their importance in maintaining tissues, both proliferation and differentiation decisions are highly regulated in these cells. Much of that regulation comes from specialized environments in which many stem cells are found, called niches. Niches may be easily identifiable anatomical structures, like the bulge of the hair follicle or the crypts of the small intestine, but in many cases are less well defined. The complexity of niches is also quite variable. While they can be seemingly simple in some contexts, there may also be contributions from extracellular matrix components, homophilic and heterophilic cell-cell interactions, association with vasculature or neurons and soluble factors. This environment is thought to provide protection for the stem cell, possibly providing a level of insulation from stressors. It also often maintains stem cells in a quiescent state, but this can rapidly change as these cells receive inputs from their environment (Li and Xie 2005; Ohlstein et al. 2004).
There is complex regulation of stem cell behavior by many pathways including cell-cell adhesion. AJs (AJs) play profound roles in controlling cell adhesion, proliferation, polarity and cytoskeletal organization in many cell types, including stem cells.
Below we discuss several roles that AJs play in stem cell and their niches. These include direct anchoring of the stem cell to niche cells, organizing the niche, controlling cell division orientation, regulating signaling pathways and affecting the mechanics of the cells. While β-catenin also plays essential roles in Wnt-signaling pathways, which regulate many facets of stem cell behavior, these non-adhesive functions will not be discussed in this review (Nusse et al. 2008).
Because cell adhesion is integrated into almost all aspects of cell physiology, analysis of adherens junction function in diverse stem cell systems has yielded a great diversity of roles making it difficult to predict the phenotype due to their loss. We begin with a discussion of the roles of AJs in Drosophila stem cell systems where relatively simple niches exist and considerable studies in the role of cell adhesion have been performed. We also discuss the various roles that AJs play in the development, growth and maintenance of embryonic stem cells and induced pluripotent stem cells. In addition, the roles of AJs in the less defined stem cell niches associated with a number of adult mammalian stem cells is considered. Finally, we discuss potential mechanical functions of AJs in regulating cell fate decisions.
15.2. AJs and Niche Association—Drosophila Ovary Germ Stem Cells
The simplest and the first characterized function for AJs in stem cells is association with the niche. As discussed above, the niche can be relatively simple or it can be a complex structure composed of basement membrane, vasculature, nerves and a number of different cell types that can interact directly or indirectly with the stem cell. The anchoring of the stem cell to surrounding cells is important in a number of tissues for both stem cell/niche association as well as maintenance of stemness.
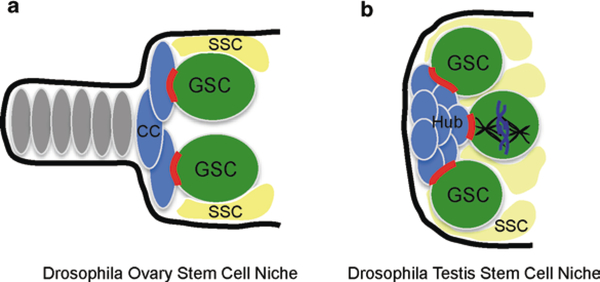
The most elegant example of this can be found in germline stem cells of the Drosophila melanogaster ovary (Gonzalez-Reyes 2003; Xi 2009). The Drosophila ovary is responsible for the continued production of eggs throughout the lifetime of the female fly. It is composed of a number of tubes, the anterior ends of which are termed the germaria (Fig. 15.1). These are the structures that house the stem cells and their supporting cells. The supporting cells are called cap cells and they make direct cell-cell contacts with the two or three germ stem cells (GSCs) that reside in each germarium. These cell contacts include AJs as both the Drosophila homologs of E-cadherin and β-catenin accumulate at the interface between cap and germ cells, and AJ-like structures are visible by electron microscopic analysis (Song et al. 2002). This cadherin-rich interface develops just after specification of the cap cells. At the early stages of germ cell association, only low levels of adherens junction components are seen between the cells. Functionally, cadherin in the germ cells is required both for their efficient initial association with the cap cells as well as their continued attachment to them (Song et al. 2002). Loss of cadherin in primordial germ cells decreases their ability to stably integrate into the germarium. Loss of cadherin in established germ stem cells causes an increased rate of their loss from the niche (Song et al. 2002). In addition to the GSCs, ovaries contain somatic epithelial stem cells that also require cadherin for their maintenance (Song and Xie 2002).
Fig. 15.1.
Drosophila germline stem cell niches. a Ovary stem cell niche. CC cap cell, GSC germline stem cell, SSC somatic stem cell. Adherens junctions between the GSC and CCs are highlighted in red. b Testis stem cell niche. GSCs contact hub cells with adherens junctions and align their mitotic spindles with this site
Additional evidence for cadherin-mediated attachment of GSCs came from analysis of Rab11 GTP-ase mutants (Bogard et al. 2007). Rab11 localizes to and is required for the function of recycling endosomes (Hsu and Prekeris 2010). Cadherins normally undergo recycling in these endosomal compartments (Desclozeaux et al. 2008; Lock and Stow 2005). In Rab11 mutants, cadherin and β-catenin levels are decreased at the GSC-cap cell interface and mutant GSCs are lost from the niche (Bogard et al. 2007).
The association of GSCs with cap cells is required for efficient BMP signal transduction between these two cell types (Chen et al. 2011a). In the ovary, BMP signaling occurs very locally and cells that are not in immediate contact with cap cells do not show active BMP signaling. It is presently not clear whether AJs simply provide close apposition of cell membranes for efficient signaling and communication, or whether there are additional or distinct functions for them. For example, in Drosophila testis, evidence suggests that the AJs may act as scaffolda for signaling molecules as well (see below) (Michel et al. 2011).
A well defined cellular niche like the cap cells have a limited surface area for interaction with stem cells. Examination of GSCs has led to the appreciation that stem cells can compete for position at the niche. Differentiation defective GSCs outcompete wild-type cells for niche occupancy and this occurs in a cadherin-dependent manner (Jin et al. 2008). In fact, simply increasing the cadherin levels in GSCs makes them able to outcompete wild type cells by increasing their area of attachment to the cap cells (Jin et al. 2008). These data suggest that intrinsic and extrinsic cues could regulate stem cell number or activity simply by mediating the physical interaction between cap cells and GSCs. There is experimental evidence for this occurring. When cap cells are mutant for the insulin receptor, there is a decrease in the number of associated GSCs (Hsu and Drummond-Barbosa 2009). This correlates well with the levels of cadherin between the GSC and cap cell. It is not yet known how insulin signaling impinges on adhesion status in these cells and whether this is a direct or indirect effect of the insulin signaling pathway. It does, however, provide an important precedent for extrinsic regulation of cell adhesion controlling stem cell maintenance. This also implies that mutations in stem cells can be positively selected for if they result in more robust niche association.
As flies age, the number of GSCs decreases (Pan et al. 2007; Wallenfang et al. 2006). While this is a complex and poorly understood process, aging is associated with a decrease in the levels of cadherin complexes formed between cap cells and GSCs (Pan et al. 2007). Further, experimentally decreasing cadherin levels resulted in an increased rate of GSC loss with age while over expression of cadherin resulted in fewer lost GSCs. Both the age related control of cadherin levels and their interaction with the insulin signaling pathway point to complex extrinsic regulation of stem-niche association. How well this translates to the function of more complex niches in mammals is still unclear.
15.3. Stem Cells in the Drosophila Testis—Anchoring and Spindle Orientation
Stem cells of the Drosophila testis are similarly dependent upon cadherin for their maintenance (Inaba et al. 2010; Voog et al. 2008). Like the ovary, the testis contains both germline and somatic stem cells termed cyst stem cells (CysSCs). In addition, there is a group of somatic cells, called the hub, that directly interact with the GSCs (Fig. 15.1). The hub cells therefore form part of the physical niche for the GSCs in the testis, with cadherin complexes concentrating at the interface between these cells (Inaba et al. 2010; Voog et al. 2008). The role of cadherin in hub cells and germ cells is distinct. Surprisingly, loss of cadherin by RNAi in the hub alone does not result in niche defects, yet loss of cadherin in the GSCs results in their loss (Voog et al. 2008). The hub cells express both fasciclin II and DN-cadherin, which likely preserves their attachment to one another when DE-cadherin is lost. Although not tested, DN-DE heterodimers or other cell adhesion systems could mediate the association of germ cells with hub cells when DE-cadherin is lost in the hub. When cadherin is lost in the CysSCs, these cells cannot be maintained and are lost (Voog et al. 2008).
Cadherin likely plays a role beyond simple adhesion in the male GSCs. When these cells divide, they orient their mitotic spindle perpendicular to the hub cell (Yamashita et al. 2003). After division, this results in one cell that remains a GSC attached to the hub, and a second cell that has been displaced from the hub and begins to differentiate down the sperm pathway. This process requires capture of one of the centrosomes/spindle poles by the region of the GSC cortex associated with the hub. Because cadherin is localized at this site, it was an excellent candidate for mediating the spindle orientation. However, due to the loss of GSCs from the niche upon loss of DE-cadherin, it has not been possible to look directly at the effect of loss of function mutants on spindle orientation. Instead, expression of a mutant cadherin construct lacking the extracellular domain, and therefore defective in adhesion, has been performed (Inaba et al. 2010). The mutant cadherin is localized around the entire cell cortex rather than being highly enriched at the GSC-hub junction, and results in misoriented mitotic spindles. Proper spindle orientation in GSCs requires the microtubule binding protein, APC2, and mutant cadherin expression causes loss of the normal polarization of APC2 to the GSC-hub junction (Inaba et al. 2010; Yamashita et al. 2003). Therefore, in the GSCs the cortical AJ patch acts as a polarity marker for the cell and allows for spindle orientation. A similar role for AJs has been proposed in the planar polarized divisions of sensory organ precursor cells in the fly (Le Borgne et al. 2002). However, beyond a role for APC2, little is known about how a cortical patch of AJs orients the mitotic spindle. While the assumption is that cadherin is acting in a canonical AJ-like manner, it has not yet been demonstrated that loss of AJ complex components in the hub cell results in spindle orientation defects in the GSCs. DE-cadherin lacking its extracellular domain can exert a dominant influence on spindle orientation in GSCs. Similar experiments in hub cells should determine whether it is the cadherin directly or the AJ complex that mediates the spindle orientation effect. Because AJs have been implicated in controlling spindle orientation in epithelial cells, this is likely a more generalizable phenomenon (den Elzen et al. 2009).
Like in the ovary, there is a decrease in DE-cadherin expression in the hub cells during aging (Boyle et al. 2007). While this is consistent with impaired adhesion affecting the loss of germ cells with age, this has not been directly tested.
In addition to adhesion and spindle orientation, new data suggests that the cortical patch containing AJs that forms between hub cells and GSCs is a signaling platform. Active BMP receptor is found highly enriched at this site and this localized activation correlates with levels of cadherin at the interface (Michel et al. 2011). Signaling complexes are known to associate with AJs in a number of contexts (McLachlan and Yap 2007). In this case, the polarity resulting from hub cell-GSC interaction is transduced into a highly localized activation of the BMP receptor (Michel et al. 2011). What role the AJs play in the localization and activation of BMP receptor signaling requires further analysis.
15.4. Drosophila Intestinal Stem Cells—AJs Role in Signaling in an Epithelial Tissue
While the Drosophila ovary and testis have provided insight into how cadherin anchors stem cells in their niche, the intestine offers insights into cadherin functions in stem cells in an epithelial tissue. The Drosophila gut is a simple epithelium with stem cells that divide to regenerate themselves and give rise to enteroblasts (Ohlstein and Spradling 2006). Differentiation into enteroblasts requires Notch signaling—high levels of the Notch ligand Delta are present in the stem cell and lead to activation of Notch signaling and differentiation of the enteroblast (Ohlstein and Spradling 2006, 2007). In the absence of DE-cadherin, the number of cells with active Notch signaling decreased (Maeda et al. 2008) and normal differentiation was perturbed. This is consistent with either robust cell-cell adhesion being required for Notch signaling, with the cadherin complex playing a more direct role in the signaling process, and/or with DE-cadherin maintaining the stem cells.
15.5. Roles of AJs in Mammalian Embryonic Stem Cells and Induced Pluripotent Cells
E-cadherin plays profound roles not only in intact tissues, but also in cultured cells. A very specialized and important example of this is in embryonic stem cells (ESCs). First cultured from preimplantation mouse blastocysts, these cells are pluripotent and can differentiate in vitro into many cell lineages (Evans and Kaufman 1981; Martin 1981). They can incorporate into blastocysts resulting in the formation of chimeric animals with ESC contribution to all tissue types. Human embryonic stem cells also exist, though they are somewhat distinct from mESCs. While culture of mouse cells relies on leukemia inhibitory factor (LIF) and bone morphogenetic proteins (BMPs), hESCs are often grown with fibroblast growth factor-2 and TGF-β family members activin and nodal. These differences are significant and likely reflect more than just species differences.
There are also epiblast stem cells (EpiSCs) that can be derived from either pre- or post-implantation mouse embryos (mESCs are only derived from the inner cell mass of preimplantation embryos) (Najm et al. 2011; Brons et al. 2007; Tesar et al. 2007). EpiSCs are thought to be a more committed form of the mESCs as they cannot contribute to chimerism. Despite this difference, they still express many markers that are often used to define ESCs—such as Oct-4 and Nanog and can be easily converted back into ESCs. While it has not been possible to test the in vivo pluripotency of human ESCs and determine whether they can contribute to chimerism (as mESCs do), or whether they are more similar to EpiSCs in this assay, it is clear that human ESCs are pluripotent in culture and can be differentiated into many lineages.
E-cadherin is required very early in development. Genetic ablation of E-cadherin results in defects before implantation of the embryo, but compaction, an adhesion-dependent event, does occur (Riethmacher et al. 1995). This is likely due to the presence of a maternal pool of E-cadherin because inhibition of E-cadherin homodimerization with inhibitory antibodies results in failure in compaction, as does antisense RNA inhibition of E-cadherin (Ao and Erickson 1992; Hyafil et al. 1980; Vestweber et al. 1985). These data demonstrate that E-cadherin plays important adhesion roles at very early stages of development. However, it does not offer full insight into the roles that E-cadherin plays in ESCs.
Important roles for AJs/E-cadherin have been reported in three aspects of ESC biology—establishment of ES/iPS cells, maintenance of pluripotency and differential potential. Both human and mouse ESCs are characterized by their formation of tight colonies. Differentiation of these cells is associated with their spreading. E-cadherin is expressed in ESCs, and plays significant roles in their maintenance and differentiation. As this is a young field, there are conflicting reports in the literature making it difficult to fully understand E-cadherin function. Here we discuss some of the findings and suggest possible interpretations and areas that require further clarification.
Cells have been isolated from the inner cell mass of E-cadherin null mouse embryos and used to establish ESC lines (Soncin et al. 2009). These behave as ESCs in that they can be propagated in LIF-containing ESC media and express pluripotent markers like Oct-4. However, distinct from wild-type mESCs, E-cadherin null ESCs do not differentiate when LIF is removed from the media. Similar results have been found with RNAi and peptide-inhibitor mediated loss of E-cadherin function (Soncin et al. 2009). These data suggest that E-cadherin promotes differentiation under some conditions. Although the E-cadherin null cells resemble EpiSCs (not ESCs) in their growth capabilities, they display distinct transcriptional profiles (Soncin et al. 2011). The fact that E-cadherin null cells can switch from a LIF/BMP mode of renewal to an activin/Nodal type suggests that E-cadherin functions in pathways more complex than simple adhesion. This supports a more active role for E-cadherin complexes in regulating signaling in these cells. However, while the E-cadherin null ESC/EpiSCs did not differentiate fully, it is not clear that they are bona fide ESCs either.
In contrast to these findings, other groups have reported an essential function for E-cadherin in maintaining the pluripotency of ESCs (Chen et al. 2011b; Li et al. 2010a; Redmer et al. 2011; Li et al. 2010b). Knockdown of E-cadherin in both hESCs and mESCs was shown to result in decreased expression of the pluripotency marker Oct-4. Whether these cells are actually differentiating or becoming epiblast-like SCs will require further investigation. It may be that their status does not resemble either ESCs or EpiSCs in entirety. Unfortunately, the most relevant assay, the ability to contribute to chimerism cannot be performed because E-cadherin is required during differentiation for cell adhesion. That said, the fact that E-cadherin null ES-like cells could be established and propagated demonstrates that these cells either have alternative mechanisms or adaptive methods of retaining some level of pluripotency even upon loss of E-cadherin.
Similar discrepancies exist for stem cells devoid of β-catenin, which is complicated by β-catenin’s dual roles in cell adhesion and the Wnt pathway. Several lines have been generated with phenotypes more similar to EpiSCs, and others have reported the generation of β-catenin null ESCs (Lyashenko et al. 2011; Wray et al. 2011).
While the detailed effects of loss of E-cadherin in ESCs requires further examination, it is clear that E-cadherin ligation can have significant effects on these cells. When mESCs are plated on substrates of E-cadherin, they no longer grow as colonies (Nagaoka et al. 2006). Despite this, they maintain their growth and their pluripotent ability in contributing to all three germ layers both in culture and when injected into blastocysts. Similarly, mESCs were better maintained on fibroblast feeders that were engineered to express E-cadherin (Horie et al. 2010). These data suggest that ligation of E-cadherin, rather than tight cell-cell association is needed to maintain ESCs. In addition, they provide an alternative way to culture cells that makes them more amenable to manipulation. Similar results have been found in hESCs (Nagaoka et al. 2010). hES cells grew in defined media in the presence of E-cadherin-coated substrates as well as they did on matrigel and maintained their ability to form multiple cell lineages in teratomas. Therefore, in both hESCs and mESCs, exogenous E-cadherin can function to maintain stem cell identity.
E-cadherin ligation can also improve the efficiency of mESC and iPS derivation. A short exposure of early stage blastomeres to E-cadherin resulted in a significant increase in the production of ESC lines, which correlated well with early proliferation of the cells (Gonzalez et al. 2011). Induced pluripotent stem cells are generated by a number of methods—but classically through the transduction of the Yamanaka factors—Oct4, Sox2, Klf4 and myc (Takahashi and Yamanaka 2006). This is an inefficient process with only a fraction of cells becoming iPS cells. E-cadherin and epithelial cell adhesion molecule (EpCAM) are both upregulated in iPS cells, but not in incompletely reprogrammed cells—allowing for an enrichment process. Not only is E-cadherin expressed in iPS cells, but its upregulation promotes the process. Exogenous expression of E-cadherin is able to increase the rate of iPS formation by about 4 fold (Chen et al. 2010). In contrast, loss of E-cadherin (and loss of cell-cell contact) inhibits iPS generation (Chen et al. 2010).
Finally, E-cadherin can also regulate the differentiation of ESCs into defined lineages. Differentiation into neuronal cell types and endoderm and hepatocyte cells is promoted by growth on feeders or matrix with the E-cadherin extracellular domain (Haque et al. 2011; Moore et al. 2011). These data make the situation more complex as it suggests that E-cadherin can have divergent functions depending upon the cells used and the growth environment to which they are exposed.
15.6. Roles of AJs in Tissue Specific Stem Cells
15.6.1. Intestinal Stem Cells
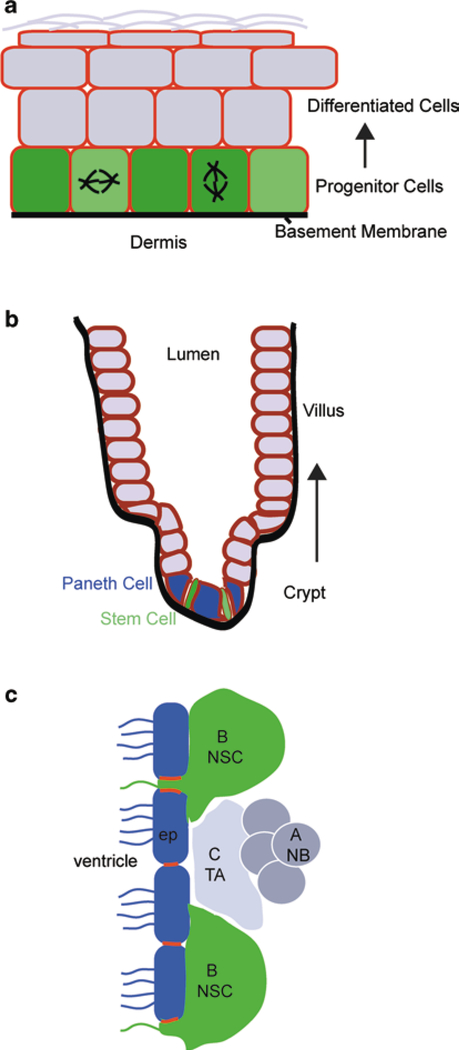
The intestinal epithelium is organized into finger-like projections called villi (which are lined with differentiated enterocytes, goblet cells and enteroendocrine cells) and invaginations into the mesenchyme called crypts of Lieberkühn (Fig. 15.2). The crypts contain not only the stem cells, but also a rapidly proliferating transit amplifying cell population and, in the small intestine, the terminally differentiated Paneth cells. There is still debate about the organization and hierarchy within the stem cell compartment (Snippert et al. 2010; Tian et al. 2011). There are cells at the base of the crypt, interspersed among Paneth cells, that express Lgr5, are proliferative and self renew. Lineage tracing has shown that they can contribute to all intestinal lineages over extended periods of time (Barker et al. 2007). However, there is also a population of cells, sometimes marked by Bmi1 or mTert expression, that are found at a higher position, often termed + 4 (indicating its cell number counting from the base of the crypt) (Montgomery et al. 2011; Sangiorgi and Capecchi 2008). These cells are less proliferative, but also contribute to all lineages over extended periods. Whether these cells are subsets of each other or are hierarchically organized is still under debate. However, all of these cells express E-cadherin and make extensive cell-cell contacts with adjacent cells. Loss of E-cadherin throughout the intestinal epithelium results in severe architectural defects resulting in lethality—making analysis of stem cell function impossible. However, by modulating recombination-inducing doses of tamoxifen, it was possible to perturb E-cadherin function without completely eliminating the protein. In this case, the zone of proliferation in the crypt was expanded. Whether this is a cell autonomous effect of loss of E-cadherin or a tissue response to loss of barrier activity is not yet known. It is also not clear whether the stem cells as well as the transit amplifying cells become more proliferative. In addition, cell lineage specification and organization was altered. There was a decrease in goblet cell number and Paneth cells were localized throughout the villus-crypt axis (Schneider et al. 2011). The mislocalization of Paneth cells suggested that E-cadherin may play a role in cell sorting. Further evidence for this comes from analysis of EphB-based signaling, which is known to pattern the villuscrypt axis (Batlle et al. 2002). Loss of EphB3 results in a similar mislocalization of Paneth cells. Recent data suggest that EphB regulates ADAM10-mediated E-cadherin cleavage (Solanas et al. 2011). Inhibition of ADAM10 also leads to disruption of Paneth cell localization (Solanas et al. 2011). Therefore, E-cadherin may function to properly pattern the stem cell niche and maintain Paneth cells (an important niche component) in an area where they make direct contact with stem cells.
Fig. 15.2.
Mammalian stem cell niches. a Interfollicular epidermis. Progenitor cells ( green) reside in the basal layer, attached to the basement membrane. b Intestinal epithelium. Stem cell reside in the crypt compartment and Paneth cells serve as part of their physical niche. c Neural stem cell niche. Neural stem cells, or Type B cells ( green), are in contact with ependymal cells ( blue) that line the lateral ventricles. They give rise to transit-amplifying ( TA) type C cells which give rise to neurblasts (type A cells). Adherens junctions are highlighted in red between neural stem cells and the ependymal cells
15.6.2. Hematopoietic Stem Cells
While hematopoietic stem cells (HSCs) have served as the best characterized adult mammalian stem cells in many ways, both visualizing them in vivo and identifying their niche has only recently been achieved. Because of this, it has been more difficult to determine their cell-cell contacts in the intact tissue (Singbrant et al. 2011li). These cells normally reside in the bone marrow and are thought to make associations with both osteoblasts and vasculature. Studies on the roles of AJs components in HSCs have yielded some opposing results. Much of the controversy has surrounded the role of N-cadherin. For a full discussion of this, the reader is directed to (Li and Zon 2010). While these studies suggest that N-cadherin does not play a major role in anchoring or proliferation of HSCs, there are likely additional cadherins, including E-cadherin, which could functionally substitute. Evidence against this idea, comes from analysis of mice in which both β-catenin and its homolog γ-catenin (plakoglobin) were eliminated (Jeannet et al. 2008; Koch et al. 2008). In these mice, which should lack functional AJs, the long-term maintenance of the HSCs was normal. However, expression of a dominant-negative N-cadherin in the HSCs resulted in decreased anchoring and long-term engraftment (Hosokawa et al. 2010). Conversely, expression of wild-type N-cadherin in HSCs decreased cell cycling and increased their lodging in the bone marrow. Therefore, while data supporting a strong role for AJs in controlling HSC physiology or niche architecture is under debate, clearly more research is needed to clarify the issue.
15.6.3. Epidermal Stem Cells
The epidermis is a stratified squamous epithelium that acts as a barrier between us and the outside world. Because of its multi-layered architecture the use of cell-cell adhesion structures are somewhat different than in simple epithelia. All the living layers of the epidermis have AJs but there are no clear zonula adherens in these cells (Fig. 15.2). In proliferative basal cells of the epidermis, AJs are not restricted to lateral surfaces, but also cover the apical surface, which is a cell-cell interface in this tissue.
There are a number of stem and progenitor cells in the epidermis. Most well-studied are the stem cells that reside within the bulge region of the hair follicle (Fuchs 2009; Gambardella and Barrandon 2003; Jaks et al. 2010). These cells give rise to all the cell lineages in the hair follicles and can also transiently contribute to the interfollicular epidermis upon wounding. Additional distinct stem cells reside in the region around the bulge and have been reported to have unique properties and perhaps different abilities to contribute long-term to different lineages. In the interfollicular epidermis, progenitor cells lie within the innermost basal layer. There is still debate whether the cells in the basal layer are an essentially homogenous population of progenitors or whether there are distinct groups of stem cells and their progeny (Clayton et al. 2007; Kaur and Potten 2011).
Most loss of function studies on adherens junction components have relied on targeted gene ablation using the keratin 5 or 14 promoter. This promoter is active in all basal cells of the interfollicular epidermis, as well as the bulge and outer root sheath cells of the hair follicle. Because essentially all epidermal cells are derived from the keratin 5/14 population, this complicates analysis as not only stem cells, but also their progeny, which includes some niche cells, have also lost the protein. It is therefore often difficult to unravel direct effects on stem cells verses secondary effects due to changes in tissue architecture or physiology. We present below the effects of ablation of each of the core AJ components which yield surprisingly different phenotypes—highlighting the diverse roles of proteins within this complex.
E/P-cadherin
E-cadherin has been ablated from both adult epidermis (Krox20-Cre) and embryonic epidermis (K14-Cre) (Tinkle et al. 2004; Tunggal et al. 2005; Young et al. 2003). Phenotypes vary somewhat depending on the timing of loss of the protein and genetic background. Early loss in some backgrounds results in perinatal lethality with barrier defects (Tunggal et al. 2005). This is due to an inability to form tight junctions within the differentiated layers of the epidermis, and is unlikely related to defects in progenitor cells. The epidermal cells do not show significant changes in cell shape or adhesion ultrastructurally, suggesting that desmosomes are responsible for the majority of cell adhesion in this tissue. In other strain backgrounds P-cadherin can partially substitute for E-cadherin and the loss of both of these proteins results in a more severe phenotype—including perinatal lethality with barrier defects (Tinkle et al. 2008). In this case, more significant cell architecture and cell-cell adhesion defects were noted, as well as a loss of cell polarity. Therefore, it is likely that P-cadherin (which is upregulated in basal cells upon E-cadherin ablation) can perform these functions in progenitor cells. While no significant changes in proliferation were noted, cadherins are important to protect cells from apoptosis in the progenitor pool (Tinkle et al. 2008). Therefore, within the epidermis, cadherins do not perform an essential anchoring function, and are not primary regulators of proliferation, differentiation or potency.
α-catenin
Loss of α-catenin results in dramatic changes in epidermal architecture, differentiation and proliferation (Vasioukhin et al. 2001). Most notably, the epidermis becomes hyperproliferative resulting in a phenotype closely resembling squamous cell carcinoma. Several explanations have been reported to explain the hyperproliferative phenotype. These include an increase in insulin receptor substrate driven MAP kinase activation, loss of polarity and division orientation and loss of cytoplasmic sequestration of the transcription factor YAP1 (Lechler and Fuchs 2005; Schlegelmilch et al. 2011; Silvis et al. 2011; Vasioukhin et al. 2001). Of these, the influence on YAP1 is perhaps most compelling. α-catenin can directly interact with YAP1, protecting it from dephosphorylation and nuclear accumulation (Schlegelmilch et al. 2011; Silvis et al. 2011). In addition, knockdown of YAP1 prevents some of the transformed phenotypes of α-catenin null cells. Thus, in epidermal progenitors, α-catenin plays profound roles in controlling cell proliferation through affecting signaling pathways. Whether any of the other pleiotropic effects due to α-catenin loss also contribute to the phenotype is still under investigation.
β-catenin
While β-catenin has essential roles in the Wnt pathway in epidermal development and homeostasis (see review, Watt and Collins 2008), it is not clear that its role in AJs is relevant. This is likely due, at least in part, to functional compensation of β-catenin by plakoglobin, a structurally similar protein that is usually a constituent of the desmosome.
p120-catenin
Similar to loss of α-catenin, loss of p120 results in hyperproliferation and tumor formation in mice (Perez-Moreno et al. 2006, 2008). The mechanism underlying this phenotype is quite distinct, however. While adherens junction components are decreased in the tissue, they appear to be sufficient to mediate basic cell adhesion, polarity and tight junction formation. Instead these mice activate the NF-kB pathway through elevated levels of the small GTPase Rho (Perez-Moreno et al. 2006, 2008). The hyperproliferation phenotype of the progenitor cells occurs in response to immune cell stimulation that is caused by NF-kB signaling. In this way, an adherens junction component has a non-cell autonomous effect by regulating epidermal signaling to the immune system.
15.6.4. Neural Stem Cells
The best characterized adult neuronal stem cells are those of the subventricular zone of the lateral ventricles (Fig. 15.2). The ventricle is lined by ependymal cells which form part of the niche for the NSCs. Rosettes are formed by a centrally located NSC surrounded by ependymal cells, with which they make direct contact (Mirzadeh et al. 2008). In addition, NSCs contact astrocytes, neuroblasts and are closely associated with the vasculature through cellular processes. Both in vivo and cultured cell approaches have been used to address roles of cell adhesion molecules in these cells.
Loss of E-cadherin by Cre-mediated recombination in nestin-postive cells results in an increase in proliferation in neuroblasts, but not NSCs (Karpowicz et al. 2009). In young mice, there was no change in DNA label-retaining cell number (marking putative NSCs that are slowly proliferating), but their number decreased as the mice aged. Whether this effect is due to slight changes in proliferation, in anchoring of stem cells, or in symmetric/asymmetric cell divisions is not yet known. These phenotypes were recapitulated in culture—isolated E-cadherin null NSC produced fewer colonies upon repeated passaging (Karpowicz et al. 2009). While affecting stem cell behavior, loss of E-cadherin did not substantially perturb niche architecture, possibly due to compensation by N-cadherin which is also expressed in the niche. In culture, blocking N-cadherin resulted in decreased neurosphere formation (cellular aggregates derived from stem cells) and increased production of glial progeny (Yagita et al. 2009). While additional experiments are required to test the role of N-cadherin in vivo, analysis of β-catenin ablation in the nestin-positive cells suggests that AJs play an important role in stem cell biology. These mice displayed disorganized brains and in neurosphere assays for NSC activity, the cells were unable to adhere to one another or form colonies (Holowacz et al. 2011). Plating cells in collagen matrices prevented their dissociation and under these conditions, β-catenin null cells outperformed their wild-type counterparts. In addition, β-catenin was required for survival of neural progenitors, with increased apoptosis seen in knockout cultures (Holowacz et al. 2011). Additional supporting evidence for cadherin function in the neural stem cell niche comes from analysis of Numb/Numblike and Ankyrin3 mutants. Both of these result in perturbations in lateral membranes and the ability of cadherins to be stably maintained at cell junctions (Kuo et al. 2006; Paez-Gonzalez et al. 2011; Rasin et al. 2007). In both cases, this results in disturbances of the niche architecture. Ablating Ankyrin3, which is expressed in the ependymal cells, results in defects in N-cadherin at the lateral membranes, and in the loss of niche organization and production of neurons. Therefore, misregulation of cadherin impacts the niche and thus affects stem cell activity.
Analysis of α-catenin ablation leads to even more severe phenotypes in the developing brain (Klezovitch et al. 2004). While this has not been performed in the adult brain, developmentally it bears some resemblance to the hyperproliferative phenotypes seen in the developing epidermis. In this case, however, at least some of the phenotype has been ascribed to activation of the Hedgehog signaling pathway (Klezovitch et al. 2004). Whether Yap1 signaling also plays a role in this hyperproliferation has not yet been addressed. Similar hyperproliferative responses were noted after ablation of RhoA (a small GTPase that localizes to AJs) in the developing brain (Katayama et al. 2011).
15.7. AJs and Mechanosensing/Signaling
An area of emerging interest is how mechanical forces are sensed and responded to in tissues. AJs are known mechanosensors and thus serve as ideal candidates for both generating and responding to tissue forces (le Duc et al. 2010; Liu et al. 2010; Yonemura et al. 2010). While there is significant literature demonstrating that mechanical properties of extracellular matrix can control cell fate decisions in stem cells, there is less evidence that this occurs with cell-cell adhesion (Assoian and Klein 2008; Cohen and Chen 2008). Likely, this is due, in part, to the difficulty in teasing apart mechanical aspects of AJs from adhesion and signaling roles. However, our emerging knowledge of how AJs affect cytoskeleton architecture, myosin-dependent contraction, signaling pathways that can independently contribute to cortical tension, and cross-talk between cell-cell and cell-ECM adhesion suggests that mechanical control of stem cell activity by AJs will be important.
15.8. Summary and Future Perspectives
AJs have varied and complex roles in stem cell biology. An important lesson to be taken away from completed studies is that the roles of AJs vary between cell types and the roles of individual AJs components often vary within a single type of stem cell. This suggests an enlightening future for adherens junction research in stem cells. One of the biggest challenges for future researchers will be unraveling the contributions of primary effects and secondary effects of adherens junction perturbation. More thorough descriptions of niches and the ability to specifically perturb AJs in defined cell populations will help in this endeavor. However, equally important is parsing out the effects of AJs on adhesion, polarity, patterning, signaling, proliferation, spindle orientation, and cortical tension—thus discovering the underlying mechanisms by which AJs regulate stem cell behavior.
Acknowledgements
Work on cell adhesion and cytoskeleton organization is supported by a grant to TL from NIH/NIAMS (R01AR055926).
References
- Ao A, Erickson RP (1992) Injection of Antisense RNA specific for E-cadherin demonstrates that E-cadherin facilitates compaction, the first differentiative step of the mammalian embryo. Antisense Res Dev 2:153–163 [DOI] [PubMed] [Google Scholar]
- Assoian RK, Klein EA (2008) Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol 18:347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449:1003–1007 [DOI] [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, Van Den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H (2002) Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111:251–263 [DOI] [PubMed] [Google Scholar]
- Bogard N, Lan L, Xu J, Cohen RS (2007) Rab11 maintains connections between germline stem cells and niche cells in the Drosophila ovary. Development 134:3413–3418 [DOI] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, Jones DL (2007) Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell 1:470–478 [DOI] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448:191–195 [DOI] [PubMed] [Google Scholar]
- Chen HF, Chuang CY, Lee WC, Huang HP, Wu HC, Ho HN, Chen YJ, Kuo HC (2011a) Surface Marker epithelial cell adhesion molecule and E-cadherin facilitate the identification and selection of induced pluripotent stem cells. Stem Cell Rev 7:722–735 [DOI] [PubMed] [Google Scholar]
- Chen S, Wang S, Xie T (2011b) Restricting self-renewal signals within the stem cell niche: multiple levels of control. Curr Opin Genet Dev 6:684–689 [DOI] [PubMed] [Google Scholar]
- Chen T, Yuan D, Wei B, Jiang J, Kang J, Ling K, Gu Y, Li J, Xiao L, Pei G (2010) E-cadherin-mediated cell-cell contact is critical for induced pluripotent stem cell generation. Stem Cells 28:1315–1325 [DOI] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH (2007) A single type of progenitor cell maintains normal epidermis. Nature 446:185–189 [DOI] [PubMed] [Google Scholar]
- Cohen DM, Chen CS (2008) Mechanical control of stem cell differentiation. StemBook [PubMed] [Google Scholar]
- den Elzen N, Buttery CV, Maddugoda MP, Ren G, Yap AS (2009) Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia. Mol Biol Cell 20:3740–3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclozeaux M, Venturato J, Wylie FG, Kay JG, Joseph SR, Le HT, Stow JL (2008) Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am J Physiol Cell Physiol 295:C545–C556 [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156 [DOI] [PubMed] [Google Scholar]
- Fuchs E (2009) The tortoise and the hair: slow-cycling cells in the stem cell race. Cell 137:811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella L, Barrandon Y (2003) The multifaceted adult epidermal stem cell. Curr Opin Cell Biol 15:771–777 [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Ibanez E, Santalo J (2011) Influence of e-cadherin-mediated cell adhesion on mouse embryonic stem cells derivation from isolated blastomeres. Stem Cell Rev 7:494–505 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A (2003) Stem cells, niches and cadherins: a view from drosophila. J Cell Sci 116:949–954 [DOI] [PubMed] [Google Scholar]
- Haque A, Hexig B, Meng Q, Hossain S, Nagaoka M, Akaike T (2011) The effect of recombinant E-cadherin substratum on the differentiation of endoderm-derived hepatocyte-like cells from embryonic stem cells. Biomaterials 32:2032–2042 [DOI] [PubMed] [Google Scholar]
- Holowacz T, Huelsken J, Dufort D, Van Der Kooy D (2011) Neural stem cells are increased after loss of beta-catenin, but neural progenitors undergo cell death. Eur J Neurosci 33:1366–1375 [DOI] [PubMed] [Google Scholar]
- Horie M, Ito A, Kiyohara T, Kawabe Y, Kamihira M (2010) E-cadherin gene-engineered feeder systems for supporting undifferentiated growth of mouse embryonic stem cells. J Biosci Bioeng 110:582–587 [DOI] [PubMed] [Google Scholar]
- Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Hembree M, Yin T, Nakamura Y, Gomei Y, Takubo K, Shiama H, Matsuoka S, Li L, Suda T (2010) Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell Stem Cell 6:194–198 [DOI] [PubMed] [Google Scholar]
- Hsu HJ, Drummond-Barbosa D (2009) Insulin levels control female germline stem cell maintenance via the niche in drosophila. Proc Natl Acad Sci U S A 106:1117–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu VW, Prekeris R (2010) Transport at the recycling endosome. Curr Opin Cell Biol 22:528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyafil F, Morello D, Babinet C, Jacob F (1980) A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell 21:927–934 [DOI] [PubMed] [Google Scholar]
- Inaba M, Yuan H, Salzmann V, Fuller MT, Yamashita YM (2010) E-cadherin is required for centrosome and spindle orientation in drosophila male germline stem cells. PLoS One 5:e12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V, Kasper M, Toftgard R (2010) The hair follicle-a stem cell zoo. Exp Cell Res 316:1422–1428 [DOI] [PubMed] [Google Scholar]
- Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, Malanchi I, Birchmeier W, Leutz A, Huelsken J, Held W (2008) Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood 111:142–149 [DOI] [PubMed] [Google Scholar]
- Jin Z, Kirilly D, Weng C, Kawase E, Song X, Smith S, Schwartz J, Xie T (2008) Differentiationdefective stem cells outcompete normal stem cells for niche occupancy in the drosophila ovary. Cell Stem Cell 2:39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P, Willaime-Morawek S, Balenci L, DeVeale B, Inoue T, Van Der Kooy D (2009) E-Cadherin regulates neural stem cell self-renewal. J Neurosci 29:3885–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Melendez J, Baumann JM, Leslie JR, Chauhan BK, Nemkul N, Lang RA, Kuan CY, Zheng Y, Yoshida Y (2011) Loss of RhoA in neural progenitor cells causes the disruption of adherens junctions and hyperproliferation. Proc Natl Acad Sci U S A 108:7607–7612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Potten CS (2011) The interfollicular epidermal stem cell saga: sensationalism versus reality check. Exp Dermatol 20:697–702 [DOI] [PubMed] [Google Scholar]
- Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V (2004) Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev 18:559–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F (2008) Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood 111:160–164 [DOI] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, Jan YN (2006) Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell 127:1253–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Bellaiche Y, Schweisguth F (2002) Drosophila E-cadherin regulates the orientation of asymmetric cell division in the sensory organ lineage. Curr Biol 12:95–104 [DOI] [PubMed] [Google Scholar]
- le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J (2010) Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol 189:1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E (2005) Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437:275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xie T (2005) Stem cell niche: structure and function. Annu Rev Cell Dev Biol 21:605–631 [DOI] [PubMed] [Google Scholar]
- Li P, Zon LI (2010) Resolving the controversy about N-cadherin and hematopoietic stem cells. Cell Stem Cell 6:199–202 [DOI] [PubMed] [Google Scholar]
- Li D, Zhou J, Wang L, Shin ME, Su P, Lei X, Kuang H, Guo W, Yang H, Cheng L, Tanaka TS, Leckband DE, Reynolds AB, Duan E, Wang F (2010a) Integrated biochemical and mechanical signals regulate multifaceted human embryonic stem cell functions. J Cell Biol 191:631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang BH, Wang S, Moalim-Nour L, Mohib K, Lohnes D, Wang L (2010b) Individual cell movement, asymmetric colony expansion, rho-associated kinase, and E-cadherin impact the clonogenicity of human embryonic stem cells. Biophys J 98:2442–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS (2010c) Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A 107:9944–9949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JG, Stow JL (2005) Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell 16:1744–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyashenko N, Winter M, Migliorini D, Biechele T, Moon RT, Hartmann C (2011) Differential requirement for the dual functions of beta-catenin in embryonic stem cell self-renewal and germ layer formation. Nat Cell Biol 13:753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Takemura M, Umemori M, Adachi-Yamada T (2008) E-cadherin prolongs the moment for interaction between intestinal stem cell and its progenitor cell to ensure notch signaling in adult drosophila midgut. Genes Cells 13:1219–1227 [DOI] [PubMed] [Google Scholar]
- Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 78:7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan RW, Yap AS (2007) Not so simple: the complexity of phosphotyrosine signaling at cadherin adhesive contacts. J Mol Med (Berl) 85:545–554 [DOI] [PubMed] [Google Scholar]
- Michel M, Raabe I, Kupinski AP, Perez-Palencia R, Bokel C (2011) Local BMP receptor activation at adherens junctions in the drosophila germline stem cell niche. Nat Commun 2:415. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A (2008) Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3:265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT (2011) Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A 108:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RN, Cherry JF, Mathur V, Cohen R, Grumet M, Moghe PV (2011) E-Cadherin-expressing feeder cells promote neural lineage restriction of human embryonic stem cells. Stem Cells Dev 21:30–41 [DOI] [PubMed] [Google Scholar]
- Nagaoka M, Koshimizu U, Yuasa S, Hattori F, Chen H, Tanaka T, Okabe M, Fukuda K, Akaike T (2006) E-cadherin-coated plates maintain pluripotent ES cells without colony formation. PLoS One 1:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka M, Si-Tayeb K, Akaike T, Duncan SA (2010) Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum. BMC Dev Biol 10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm FJ, Chenoweth JG, Anderson PD, Nadeau JH, Redline RW, McKay RD, Tesar PJ (2011) Isolation of epiblast stem cells from preimplantation mouse embryos. Cell Stem Cell 8:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Fuerer C, Ching W, Harnish K, Logan C, Zeng A, ten Berge D, Kalani Y (2008) Wnt signaling and stem cell control. Cold Spring Harb Symp Quant Biol 73:59–66 [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Kai T, Decotto E, Spradling A (2004) The stem cell niche: theme and variations. Curr Opin Cell Biol 16:693–699 [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A (2006) The adult drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439:470–474 [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A (2007) Multipotent drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315:988–992 [DOI] [PubMed] [Google Scholar]
- Paez-Gonzalez P, Abdi K, Luciano D, Liu Y, Soriano-Navarro M, Rawlins E, Bennett V, GarciaVerdugo JM, Kuo CT (2011) Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron 71:61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Chen S, Weng C, Call G, Zhu D, Tang H, Zhang N, Xie T (2007) Stem cell aging is controlled both intrinsically and extrinsically in the drosophila ovary. Cell Stem Cell 1:458–469 [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E (2006) p120-catenin mediates inflammatory responses in the skin. Cell 124:631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Song W, Pasolli HA, Williams SE, Fuchs E (2008) Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proc Natl Acad Sci U S A 105:15399–15404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasin MR, Gazula VR, Breunig JJ, Kwan KY, Johnson MB, Liu-Chen S, Li HS, Jan LY, Jan YN, Rakic P, Sestan N (2007) Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci 10:819–827 [DOI] [PubMed] [Google Scholar]
- Redmer T, Diecke S, Grigoryan T, Quiroga-Negreira A, Birchmeier W, Besser D (2011) E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep 12:720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmacher D, Brinkmann V, Birchmeier C (1995) A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc Natl Acad Sci U S A 92:855–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR (2008) Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40:915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD (2011) Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell 144:782–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, Dahlhoff M, Horst D, Hirschi B, Trulzsch K, Muller-Hocker J, Vogelmann R, Allgauer M, Gerhard M, Steininger S, Wolf E, Kolligs FT (2011) A key role for E-cadherin in intestinal homeostasis and paneth cell maturation. PLoS One 5:e14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvis MR, Kreger BT, Lien WH, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V (2011) alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal 4:ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singbrant S, Askmyr M, Purton LE, Walkley CR (2011) Defining the hematopoietic stem cell niche: the chicken and the egg conundrum. J Cell Biochem 112:1486–1490 [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Van Der Flier LG, Sato T, van Es JH, Van Den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143:134–144 [DOI] [PubMed] [Google Scholar]
- Solanas G, Cortina C, Sevillano M, Batlle E (2011) Cleavage of E-cadherin by ADAM10 mediates epithelial cell sorting downstream of EphB signalling. Nat Cell Biol 13:1100–1107 [DOI] [PubMed] [Google Scholar]
- Soncin F, Mohamet L, Eckardt D, Ritson S, Eastham AM, Bobola N, Russell A, Davies S, Kemler R, Merry CL, Ward CM (2009) Abrogation of E-cadherin-mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells 27:2069–2080 [DOI] [PubMed] [Google Scholar]
- Soncin F, Mohamet L, Ritson S, Hawkins K, Bobola N, Zeef L, Merry CL, Ward CM (2011) E-cadherin acts as a regulator of transcripts associated with a wide range of cellular processes in mouse embryonic stem cells. PLoS One 6:e21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xie T (2002) DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the drosophila ovary. Proc Natl Acad Sci U S A 99:14813–14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Zhu CH, Doan C, Xie T (2002) Germline stem cells anchored by adherens junctions in the drosophila ovary niches. Science 296:1855–1857 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448:196–199 [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ (2011) A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478:255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle CL, Lechler T, Pasolli HA, Fuchs E (2004) Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc Natl Acad Sci U S A 101:552–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle CL, Pasolli HA, Stokes N, Fuchs E (2008) New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc Natl Acad Sci U S A 105:15405–15410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, Niessen CM (2005) E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J 24:1146–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E (2001) Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell 104:605–617 [DOI] [PubMed] [Google Scholar]
- Vestweber D, Ocklind C, Gossler A, Odin P, Obrink B, Kemler R (1985) Comparison of two cell-adhesion molecules, uvomorulin and cell-CAM 105. Exp Cell Res 157:451–461 [DOI] [PubMed] [Google Scholar]
- Voog J, D’Alterio C, Jones DL (2008) Multipotent somatic stem cells contribute to the stem cell niche in the drosophila testis. Nature 454:1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenfang MR, Nayak R, DiNardo S (2006) Dynamics of the male germline stem cell population during aging of drosophila melanogaster. Aging Cell 5:297–304 [DOI] [PubMed] [Google Scholar]
- Watt FM, Collins CA (2008) Role of beta-catenin in epidermal stem cell expansion, lineage selection, and cancer. Cold Spring Harb Symp Quant Biol 73:503–512 [DOI] [PubMed] [Google Scholar]
- Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, Smith A (2011) Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol 13:838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi R (2009) Anchoring stem cells in the niche by cell adhesion molecules. Cell Adh Migr 3:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita Y, Sakurai T, Tanaka H, Kitagawa K, Colman DR, Shan W (2009) N-cadherin mediates interaction between precursor cells in the subventricular zone and regulates further differentiation. J Neurosci Res 87:3331–3342 [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT (2003) Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301:1547–1550 [DOI] [PubMed] [Google Scholar]
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M (2010) alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 12:533–542 [DOI] [PubMed] [Google Scholar]
- Young P, Boussadia O, Halfter H, Grose R, Berger P, Leone DP, Robenek H, Charnay P, Kemler R, Suter U (2003) E-cadherin controls adherens junctions in the epidermis and the renewal of hair follicles. EMBO J 22:5723–5733 [DOI] [PMC free article] [PubMed] [Google Scholar]