Summary
Animal models are widely used to study the physiopathology of human diseases. However, the influence of gender on modern society diet style‐induced cardiovascular disease has not thus far been explored in these models. Thus, this study investigated cardiovascular remodelling in C57BL/6J mice fed a diet rich in saturated fat, sucrose and salt, evaluating gender effect on this process. Male and female C57BL/6J mice were fed AIN93M diet or a modified AIN93M rich in fat, sucrose and salt (HFSS) for 12 weeks. Body mass, water and food intake and cardiovascular remodelling were assessed. The HFSS diet did not lead to body mass gain or glucose metabolism disturbance as assessed by serum glucose, insulin and oral glucose tolerance test. However, female mice on a HFSS diet had increased visceral and subcutaneous adiposity. Only male mice displayed heart hypertrophy. The left ventricle was not hypertrophied in either male or female mice, but its lumen was dilated. Intramyocardial arteries and the thoracic aorta showed media thickening in male mice, but in the female it was only observed in the thoracic aorta. Finally, intramyocardial artery dilation was present in both genders, but not in the aorta. Therefore changes in LV dimensions and arterial remodelling were influenced by both gender and the HFSS diet. In conclusion, male and female C57BL/6J mice suffered cardiovascular remodelling after 12 weeks of HFSS feeding, although they did not develop obesity or diabetes. Sexual dimorphism occurred in response to diet for body adiposity, heart hypertrophy and intramyocardial artery remodelling.
Keywords: cardiovascular remodelling, diet, female, gender, sex
1. INTRODUCTION
Animals models are widely used to study human diseases and are important tools to understand the physiopathology of several conditions such as cardiovascular disease (CVD).1 Over the last decades, they have helped the development of drugs and new treatment strategies, since they function as a living system and respond to different approaches. However, much that is currently known was built based on male rodents while the female has been overlooked. For instance, sex‐related differences in pharmacokinetics and pharmacodynamics are known to exist, and this should have prompted the study of gender differences in drug efficacy.2
Premenopausal women display a lower incidence of CVD compared to postmenopausal women and young adult men. This suggests that oestrogen―or the lack of androgens―might play a protective role in CVD development in premenopausal women.3, 4 Male and female mice show several differences regarding coronary artery size, electrophysiological heart properties, gene expression, contractile properties and how the heart responds to injury.4 This kind of gender impact on the occurrence, prognosis and response to treatment of various CVD has recently been a focus of interest,5 and reinforces the necessity to understand how gender impact CVD development in animal models.
Diet is a risk factor for CVD, and it is attributed mainly to the metabolic impact of salt overload, and to sugar and fat quantity and quality.6 Salt overload correlates with higher blood pressure levels, and it changes hemodynamic parameters.7 Saturated fatty acid consumption is strongly correlated with increased CVD incidence, compensatory hyperinsulinaemia, hyperglycaemia and hyperleptinaemia.8 Finally, simple carbohydrates contribute to the high prevalence of obesity and type 2 diabetes, which also contributes to CV outcomes. Among carbohydrates, fructose is one of the main components of the modern occidental diet, especially sucrose, and its consumption is largely associated with insulin resistance and arterial hypertension.9, 10
In animal models, some CVD phenotypes may be induced by diet. Diets rich in sugar or fat, alone or in combination, lead to diabetes and obesity,9, 11, 12 whereas salt alone leads to hypertension and cardiovascular remodelling.13, 14 However, the combination of fat, sugar and salt overload is less widely discussed in the literature, and the response of the different sexes to these nutrients combined. Pigs fed a diet rich in fat, sucrose and salt have increased blood pressure.15 Similar reports in human studies indicate that salt prevents the development of obesity in men.16, 17
Overall, it is extremely important to investigate sex‐specific aspects of cardiovascular remodelling in rodent models of diet‐induced CVD, since it has not been thus far explored for combinations of sugar, fat and salt overload. Paradoxically this represents the realistic dietary style of modern society that has been contributing to the obesity epidemic. Thus, this study investigated cardiovascular remodelling in C57BL/6J mice fed a diet rich in saturated fat, sucrose and salt, and then evaluating the gender effect on this process.
2. MATERIALS AND METHODS
2.1. Ethical approval
The handling and experimental protocols were approved by the local Ethics Committee to Care and Use of Laboratory Animals (CEUA#647/15). The study was performed following the Animal Research Reporting In vivo Experiments ARRIVE guidelines and the Guideline for the Care and Use of Laboratory Animals (US NIH Publication N° 85–23. Revised 1996).18
2.2. Animals and diet
Male and female C57BL/6J mice at two months of age were obtained from colonies maintained at the Universidade Federal Fluminense and kept under standard conditions (collective polypropylene cages, 12 hours light/dark cycles, 21 ± 2°C, humidity 60 ± 10% and air exhaustion cycle 15 min/h). At three months old, mice were randomly allocated into four groups according to diet (n = 12–15/group). Control group received AIN93M diet,19 and the experimental groups received a high‐fat, high‐sucrose and high‐salt diet (HFSS) modified from the AIN93M diet (Pragsolucoes, Jau) for 12 weeks. The AIN93M diet consisted of 3.84 kcal/g and 0.25% NaCl (w/w), and the HFSS diet consisted of 4.39 kcal/g and 8% NaCl (w/w). The main difference is the addition of saturated fat (lard, 36.9% energy/kg), simple carbohydrates (sucrose, 27.3% energy/kg) and sodium chloride to the HFSS diet (Table 1). For 12 weeks, food and water were offered ad libitum, and its ingestion monitored daily and weekly respectively. Body mass was assessed weekly, and body fat evaluated at euthanasia by harvesting visceral and subcutaneous fat depots (genital and inguinal fat pads respectively) (Shimadzu, AUW220D).
Table 1.
Experimental diet
| Ingredients | AIN93M | HFSS |
|---|---|---|
| g/kg | ||
| Casein | 140.0 | 160.0 |
| L‐cystine | 1.8 | 1.8 |
| Corn starch | 620.7 | 140.7 |
| Sucrose | 100.0 | 300.0 |
| Fibres (cellulose) | 50.0 | 50.0 |
| Soy oil | 40.0 | 40.0 |
| Lard | ‐ | 180.0 |
| Vitamin mixa | 10.0 | 10.0 |
| Mineral mixa | 35.0 | 35.0 |
| NaClb | ‐ | 80.0 |
| Choline | 2.5 | 2.5 |
| Antioxidant | 0.008 | 0.06 |
| Energy | ||
| Kcal/g | 3.84 | 4.39 |
| Total protein, % | 14.8 | 14.7 |
| Total lipid, % | 9.4 | 45.1 |
| Total carbohydrate, % | 75.8 | 40.2 |
For vitamin and mineral mix composition, see.19
AIN93M has 0.25% NaCl, and the HFSS diet has 8% NaCl.
2.3. Glucose metabolism
On the day of euthanasia, mice were deeply anaesthetized with ketamine 100.0 mg/kg (Francotar® 10%, Virbac) and xylazine 10.0 mg/kg (Virbaxyl® 2%, Virbac) intraperitoneal, and the heart was exposed for blood collection from the right atrium. Serum was obtained by centrifugation (120 g for 10 minutes) and used to measure glucose and insulin concentrations (rat/mouse insulin ELISA #E6013‐K, Millipore). Oral glucose tolerance test (OGTT) was performed at week 11. After a 6‐hour fast, 50% glucose in sterile saline (0.9% NaCl) with a dose of 1 g/kg was administered by orogastric gavage. Blood samples were milked from the tail tip by a small incision. Plasma glucose concentration was measured (glucometer Accu‐Chek Performa Nano; Roche Diagnostic) before glucose gavage and later at 15, 30, 60 and 90 minutes after glucose administration. The area under the curve (AUC) was calculated using the trapezoid rule to assess glucose intolerance.
2.4. Left ventricle remodelling
The heart was removed and weighed, and the left ventricle (LV) was carefully dissected and isolated for weighing, to evaluate cardiac and LV hypertrophy (Shimadzu, AUW220D). Left tibia length was measured from malleolus to medial condyle to correct cardiac and LV mass, to minimize the effect of animal size on these parameters.20 LV samples were immersed in 4% buffered formalin pH 7.2 for 48 hours and then cut transversally at their half height based on base‐apex axis. The apex fragment underwent routine histological processing, embedded in Paraplast Plus (P3683, Sigma), cut at 5 μm thick and stained with haematoxylin and eosin.
Morphometry focused on the geometric aspects of the LV chamber and its intramyocardial arteries (Figure 1A‐D). Six animals per group and three non‐consecutive sections per animal were used. Images of LV chamber were acquired at 4× magnification and arteries at 40× (Evos XL; Thermo Scientific), and measurements were performed using the Image‐Pro® Plus software v.4.5 (Media Cybernetics). The lumen diameter (LD) of the LV chamber, and the LV free wall (FW) and the interventricular septum (IVS) thickness at the level of papillary muscles were assessed.21 Intramyocardial artery LD and media thickness (MT) were also evaluated.
Figure 1.
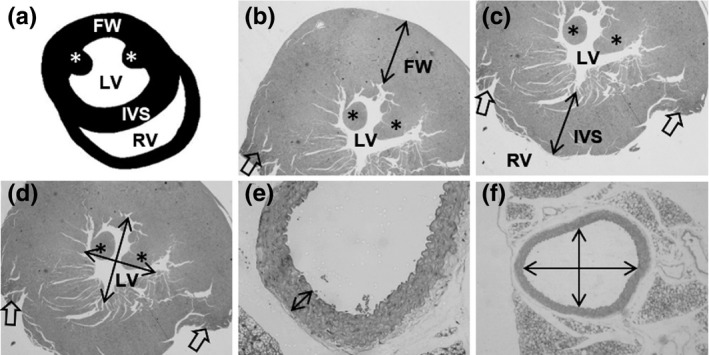
Cardiovascular morphometry. A, Scheme of the heart sectioned in the transverse plane, where the left (LV) and right ventricles (RV) lumen are in evidence, and the free wall (FW) and the interventricular septum (IVS) wall. The papillary muscles are indicated by an asterisk (*). Heart orientation matches photomicrographs in B‐D, and it is not in anatomical orientation. B‐D, Photomicrographs illustrating how the FW (B), IVS (C) and LV lumen diameter (D) were measured. Open arrows indicate the site where RV wall was dissected in euthanasia. E‐F, Photomicrography is illustrating the measure of media thickness (E) and lumen diameter (F) of the thoracic aorta in the transverse plane. The same was performed for intramyocardial arteries
2.5. Aorta remodelling
On the day of euthanasia, the thoracic aorta was harvested and followed the same routine histological procedures as previously mentioned for the LV. For morphometry, six animals were used per group, and three histological sections of the artery were produced per mice. Images were acquired at 4× magnification to assess LD and at 40× to assess MT as previously described and shown in Figure 1E‐F.22
2.6. Statistics
Data are expressed as mean ± SD and analysed for normality and homoscedasticity of variances. Comparison among groups was made by two‐way ANOVA followed by a post hoc test of Tukey. A P‐value <0.05 was considered statistically significant (GraphPad® Prism software v. 6.0).
3. RESULTS
3.1. Diet rich in fat, sucrose and salt does not induce obesity or hyperglycaemia in C57BL/6J mice
As expected, mice fed with the HFSS diet had increased water intake of about 156% for both male and female mice when compared to their respective control AIN93M diet groups (P < 0.0001, Figure 2A). Although food intake was similar among groups, energy consumption was 20% and 14% higher in HFSS male and female mice, respectively, compared to control groups (P < 0.01, Figure 2B‐C). Water and energy intake were influenced by both gender and diet (two‐way ANOVA).
Figure 2.
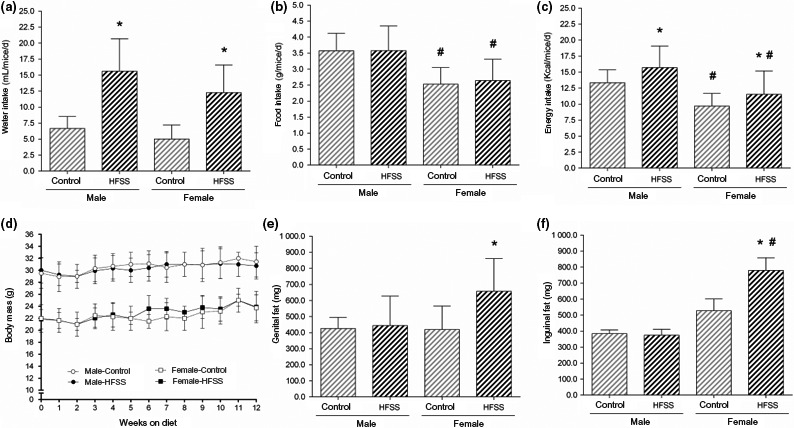
Water (A), food (B) and energy (C) consumption per mice daily. Body mass response to 12 weeks on high‐fat, high‐sucrose and high‐salt (HFSS) diet is shown in (D), and body adiposity represented by visceral (genital) and subcutaneous (inguinal) fat is shown in E‐F. Data are mean ± SD, P < 0.05, * control (same gender), # male (similar diet)
An intriguing result was that HFSS mice did not gain weight along the twelve weeks of HFSS diet ingestion (Figure 2D). Nevertheless, female mice on HFSS diet presented increased body adiposity, characterized by 66% increase in visceral fat (genital, P < 0.05) and 48% in subcutaneous fat (inguinal, P < 0.05) depots (Figure 2E‐F). The same was not true for male mice that showed no increase in fat depots by the HFSS diet. The two‐way ANOVA showed a gender effect on inguinal fat (P < 0.001), but not on genital fat (P = 0.099), which in turn displayed a minor influence by the diet (P < 0.04).
Glucose metabolism was not disturbed in male mice by the HFSS diet since no significant changes were noticed for blood glucose, insulin or glucose tolerance (Figure 3). A similar result was found for female mice, but an intriguing finding was an improvement of glucose tolerance after 12 weeks on the HFSS diet (Figure 3C‐D).
Figure 3.
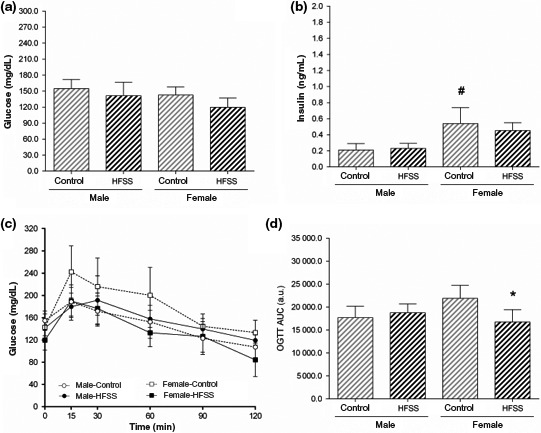
Serum glucose (A) and insulin (B). Oral glucose tolerance test (OGTT) curve is shown in (C) and its area under the curve (AUC) in (D). a.u., arbitrary units. Data are mean ± SD, P < 0.05, * control (same gender), # male (similar diet)
3.2. Gender and diet differently modulate cardiovascular remodelling
Twelve weeks on HFSS diet leads to heart hypertrophy only in male mice (+19% heart/tibia ratio, P < 0.05), and both gender and diet influenced this parameter (Table 2). No significant change in LV mass was noticed when it was assessed the LV/tibia and LV/heart ratio (Table 2). Morphometry of LV wall corroborates with this result since the free wall and the interventricular septum thickness were not increased in male and female mice on the HFSS diet (Table 2), displaying a solely gender‐dependent difference in size as expected (two‐way ANOVA, Table 2). On the other hand, the hyperenergetic and hypersodic diet lead to LV chamber dilation, since lumen diameter increased 38% (P < 0.0001) in male and 34% (P < 0.001) in female mice, a result of both diet and gender interaction (two‐way ANOVA, Table 2 and Figure 4A‐D). Due to LV dilation, the free wall/lumen ratio in male mice fed with the HFSS diet presented a reduction of 36% (P < 0.001, Table 2). In summary, although some LV parameters did not present significant difference between control and HFSS groups, the two‐way analysis of variance showed that LV wall dimension is dependent on gender, and lumen diameter is dependent on both gender and diet intake (Table 2).
Table 2.
Cardiovascular remodelling
| Parameter | Male | Female | Two‐way ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Control | HFSS diet | Control | HFSS diet | Gender | Diet | Interaction | |
| Biometry | |||||||
| Heart/tibia, mg/cm | 7.72 ± 1.32 | 9.19 ± 0.66a | 6.09 ± 0.48b | 6.78 ± 0.52b | <0.0001 | 0.005 | NS |
| LV/tibia, mg/cm | 5.29 ± 0.94 | 5.92 ± 0.60 | 4.32 ± 0.34 | 4.45 ± 0.31b | 0.0002 | NS | NS |
| LV/heart, mg/mg | 0.69 ± 0.021 | 0.66 ± 0.030 | 0.67 ± 0.054 | 0.66 ± 0.037 | NS | NS | NS |
| Left ventricle | |||||||
| Free wall, mm | 1.39 ± 0.11 | 1.25 ± 0.12 | 1.02 ± 0.14b | 1.16 ± 0.16 | 0.0013 | NS | 0.03 |
| Interventricular septum, mm | 1.45 ± 0.09 | 1.58 ± 0.08 | 1.16 ± 0.33 | 1.30 ± 0.10 | 0.0019 | NS | NS |
| Lumen diameter, mm | 1.35 ± 0.07 | 1.86 ± 0.13a | 0.95 ± 0.14b | 1.27 ± 0.05a,b | <0.0001 | <0.0001 | 0.04 |
| Free wall: Lumen | 1.04 ± 0.12 | 0.67 ± 0.07a | 1.07 ± 0.07 | 0.91 ± 0.13b | 0.0084 | <0.0001 | 0.03 |
| IV Septum: Lumen | 1.09 ± 0.12 | 0.85 ± 0.09 | 1.20 ± 0.18 | 1.02 ± 0.08 | 0.015 | 0.0011 | NS |
| Intramyocardial arteries | |||||||
| MT, μm | 7.63 ± 1.54 | 14.68 ± 4.10a | 8.32 ± 0.93 | 10.91 ± 1.82 | NS | 0.0003 | NS |
| Lumen diameter, μm | 44.1 ± 5.9 | 80.7 ± 30.1a | 32.9 ± 3.7 | 70.0 ± 9.8a | NS | <0.0001 | NS |
| MT: Lumen | 0.178 ± 0.052 | 0.197 ± 0.072a | 0.256 ± 0.039 | 0.156 ± 0.015 | NS | NS | NS |
| Thoracic aorta | |||||||
| MT, μm | 43.4 ± 0.41 | 55.2 ± 1.34a | 43.6 ± 3.7 | 52.6 ± 0.59a | NS | <0.0001 | NS |
| Lumen diameter, μm | 522.2 ± 71.0 | 561.9 ± 45.3 | 450.8 ± 64.9 | 469.3 ± 18.6b | 0.0012 | NS | NS |
| MT: Lumen | 0.085 ± 0.013 | 0.099 ± 0.008a | 0.089 ± 0.10 | 0.112 ± 0.004a | 0.02 | <0.0001 | NS |
Data are mean ± SD, P < 0.05.
Abbreviations: HFSS, high‐fat, high‐sucrose and high‐salt diet; LV, left ventricle; MT, media thickness; NS, non‐significant; IV, interventricular.
Control (same gender).
Male (similar diet).
Figure 4.
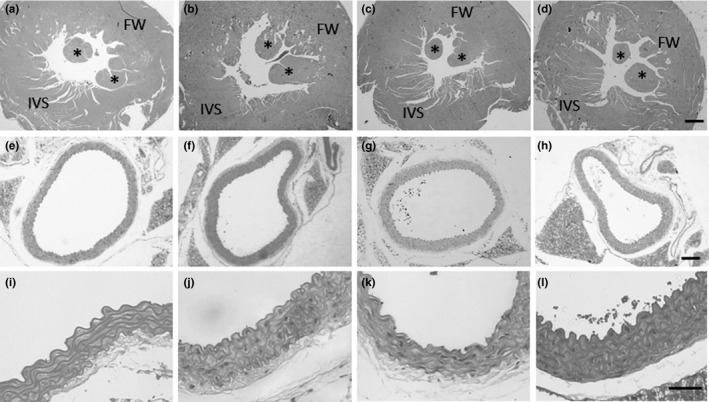
Photomicrographs of the left ventricle (A‐D) and thoracic aorta (E‐L). Each column represents one group (A, E, I: male‐control; B, F, J: male‐HFSS; C, G, K: female‐control; D, H, L: female‐HFSS). Calibration bars represent 500 μm (D), 300 μm (H) and 50 μm (L). FW, free wall (left ventricle); IVS, interventricular septum; * papillary muscles
Intramyocardial arteries displayed wall thickening by HFSS feeding in male mice (+92%, P < 0.01, Table 2) and just a trend in the female. Similar to LV, intramyocardial arteries were dilated in both male (+83%, P < 0.05) and female mice (+113%, P < 0.01, Table 2). These data were influenced solely by diet (two‐way ANOVA, Table 2). The thoracic aorta also showed wall thickening, but not lumen dilation. Media thickness increased 27% and 21% (P < 0.0001) in male and female mice, respectively, when fed for 12 weeks with the HFSS diet (Table 2 and Figure 4I‐L). The ratio between wall thickness and lumen diameter also increased in both gender, being 16% (P < 0.05) and 26% (P < 0.01) higher in male and female mice respectively (Table 2). Two‐way analysis showed that whereas diet modulates aorta wall thickness, both diet and gender influenced MT: lumen ratio (Table 2).
4. DISCUSSION
Although the reasons are not fully elucidated, it is widely accepted that the development and the impact of several diseases are gender‐dependent.23, 24 Despite this, the majority of animal studies were developed and performed in male rats or mice. Thus, female physiology and its response to injury are neglected. In view of this, it is necessary to conduct studies that focus on how gender influences several aspects of body function in the female, such as cardiovascular physiology and metabolism.
In the present study, male and female C57BL/6J mice were fed for 12 weeks with a modified AIN93M diet rich in saturated fat, sugar and salt to investigate the impact of gender and diet intake on cardiovascular remodelling. Surprisingly, despite increased energy consumption, mice did not gain body mass or presented metabolic abnormalities, although female mice had increased body adiposity. Rodent diets rich in fat and sugar are routinely used to induce obesity in the C57BL/6J mice, but the time required to change body mass is still a matter of much controversy, mainly because of the diet chosen. Some authors observe an early effect on body mass after four weeks on high‐fat diet,25, 26, 27 whereas there are reports that longer periods (about 20 weeks) are necessary for a high‐sucrose or a high‐fat diet to induced obesity.26, 28, 29
The diet chosen to carry out the present study was based on the Surwit formulae published in 1995 to induce obesity and diabetes.30 The Surwit diet was previously used by our group to induce obesity and diabetes in male C57BL/6J mice. After one week, mice already presented body mass gain, and after six weeks, glucose and insulin were elevated and mice presented insulin resistance.12 In the present study, the Surwit formula was modified by the addition of 8% salt to induce cardiovascular remodelling, but unexpectedly mice did not gain weight or presented glucose disturbance.
Female C57BL/6J mice showed increased visceral and subcutaneous adiposity after twelve weeks of high‐fat, high‐sucrose and high‐salt feeding. According to Forshee et al., overconsumption of sucrose increases visceral fat deposition, promotes hepatic lipogenesis and lipid peroxidation.31 Also, it promotes the release of very low‐density lipoproteins to blood circulation, which serve as a source of fatty acids to the adipose tissue to synthesize triacylglycerol.31 The increased adiposity might explain in part the improvement of glucose tolerance in HFSS‐fed female mice. Adipocyte proliferation in the white adipose tissue serves as a buffering mechanism, where excessive circulating glucose is taken up by adipocytes that convert it into triacylglycerol for storage. Since glucose is cleared from the bloodstream, the pancreas does not need to liberate a great amount of insulin, and its serum levels also decrease.32, 33 The mechanisms behind the different response between male and female mice need to be further investigated.
Regarding cardiovascular remodelling, only male mice displayed heart hypertrophy. The left ventricle was not hypertrophied in male and female mice, but its lumen was dilated. The intramyocardial arteries and the thoracic aorta of male mice showed media thickening, but in the female, it was only observed in the thoracic aorta. Finally, lumen dilation occurred in the intramyocardial arteries of both genders, but not in the thoracic aorta. Changes in LV dimension and the arterial remodelling were influenced by both gender and the HFSS diet.
The role of salt intake on LV hypertrophy is well established. Salt overconsumption raises blood pressure and leads to local (cardiac) activation of the renin‐angiotensin‐aldosterone system, in which angiotensin stimulates cardiomyocyte and smooth muscle cell hypertrophy through AT1 receptor activation.13, 34, 35 A study conducted in C57Bl/6 mice to evaluate the gender impact on salt sensitivity increased blood pressure after one day on a 4% NaCl diet in male and female mice, and it became significantly high in the sixth day on this diet.36 Not only salt, but also fat overconsumption for nine weeks induces cardiac hypertrophy in male C57BL/6J mice, both alone (high‐fat) or in combination with salt (high‐fat and high‐salt diet), although at different extents.11 Additionally, it was shown that salt added to these diets might potentialize the effect of macronutrients on blood pressure.34
A limitation of the present study is that blood pressure was not assessed. However, it is largely proven that salt overload raises blood pressure in C57BL/6J mice.11, 37 In the present study, mice had increased water intake, and cardiovascular remodelling though dilation is a sign of volume overload.38 Also, increased water intake is a compensatory mechanism that aims to remove the excess of sodium from the body, along with water, through renal glomeruli.11
5. CONCLUSION
Male and female C57BL/6J mice suffered cardiovascular remodelling after 12 weeks of high‐fat, high‐sucrose and high‐salt feeding, although they did not develop obesity or diabetes. Sexual dimorphism occurred in response to diet for body adiposity, heart hypertrophy and intramyocardial artery remodelling.
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
AUTHORS’ CONTRIBUTION
C.F‐S conceived the work. D.C.P‐S, R.P.M.S., C.C‐P and C.F‐S performed the experiments and data collection. D.C.P‐S, R.P.M.S. and C.F‐S performed data analysis and interpretation. D.C.P‐S. drafted the article, and C.F‐S made a critical revision of the article. D.C.P‐S, R.P.M.S., C. C‐P and C.F‐S approved the definitive version to be published.
ACKNOWLEDGEMENTS
Authors are thankful for Dilliane da Paixao Rodrigues Almeida for her technical assistance.
Pereira‐Silva DC, Machado‐Silva RP, Castro‐Pinheiro C, Fernandes‐Santos C. Does gender influence cardiovascular remodeling in C57BL/6J mice fed a high‐fat, high‐sucrose and high‐salt diet?. Int J Exp Path. 2019;100:153–160. 10.1111/iep.12318
Funding information
This study was supported by a grant from the Brazilian agency FAPERJ (E‐26/210.525/2014).
REFERENCES
- 1. Leong XF, Ng CY, Jaarin K. Animal models in cardiovascular research: hypertension and atherosclerosis. Biomed Res Int. 2015;2015:528757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baggio G, Corsini A, Floreani A, Giannini S, Zagonel V. Gender medicine: a task for the third millennium. Clin Chem Lab Med. 2013;51(4):713‐727. [DOI] [PubMed] [Google Scholar]
- 3. Chakrabarti S, Morton JS, Davidge ST. Mechanisms of estrogen effects on the endothelium: an overview. Can J Cardiol. 2014;30(7):705‐712. [DOI] [PubMed] [Google Scholar]
- 4. Deschepper CF, Llamas B. Hypertensive cardiac remodeling in males and females: from the bench to the bedside. Hypertension. 2007;49(3):401‐407. [DOI] [PubMed] [Google Scholar]
- 5. Azevedo PS, Polegato BF, Minicucci MF, Paiva SA, Zornoff LA. Cardiac remodeling: Concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq Bras Cardiol. 2016;106(1):62‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aneja A, El‐Atat F, McFarlane SI, Sowers JR. Hypertension and obesity. Recent Prog Horm Res. 2004;59:169‐205. [DOI] [PubMed] [Google Scholar]
- 7. Van Huysse JW, Dostanic I, Lingrel JB, Hou X, Wu H. Hypertension from chronic central sodium chloride in mice is mediated by the ouabain‐binding site on the Na,K‐ATPase α₂‐isoform. Am J Physiol Heart Circ Physiol. 2011;301(5):H2147‐H2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martha S, Ramreddy S, Pantam N. Study of impaired glucose tolerance, dyslipidemia, metabolic syndrome, and cardiovascular risk in a south Indian population. J Postgrad Med. 2011;57(1):4‐160. [DOI] [PubMed] [Google Scholar]
- 9. Sanders TA. How important is the relative balance of fat and carbohydrate as sources of energy in relation to health? Proc Nutr Soc. 2016;75(2):147‐153. [DOI] [PubMed] [Google Scholar]
- 10. Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta‐analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr. 2014;100(1):65‐79. [DOI] [PubMed] [Google Scholar]
- 11. Costa MV, Fernandes‐Santos C, Faria Tda S, Aguila MB, Mandarim‐de‐Lacerda CA. Diets rich in saturated fat and/or salt differentially modulate atrial natriuretic peptide and renin expression in C57BL/6 mice. Eur J Nutr. 2012;51(1):89‐96. [DOI] [PubMed] [Google Scholar]
- 12. Fernandes‐Santos C, Carneiro RE, de Souza Mendonca L, Aguila MB, Mandarim‐de‐Lacerda CA. Pan‐PPAR agonist beneficial effects in overweight mice fed a high‐fat high‐sucrose diet. Nutrition. 2009a;8:818‐827. [DOI] [PubMed] [Google Scholar]
- 13. Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013;88(9):987‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85(2):679‐715. [DOI] [PubMed] [Google Scholar]
- 15. Myrie SB, McKnight LL, King JC, McGuire JJ, Van Vliet BN, Bertolo RF. Effects of a diet high in salt, fat, and sugar on telemetric blood pressure measurements in conscious, unrestrained adult Yucatan miniature swine (Sus scrofa). Comp Med. 2012;62(4):282‐290. [PMC free article] [PubMed] [Google Scholar]
- 16. Ekinci EI, Clarke S, Thomas MC, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34(3):703‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polonia J, Martins L. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23(11):771‐772. [DOI] [PubMed] [Google Scholar]
- 18. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother. 2010;1(2):94‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reeves PG, Nielsen FH, Fahey GC Jr. AIN‐93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN‐76A rodent diet. J Nutr. 1993;123(11):1939‐1951. [DOI] [PubMed] [Google Scholar]
- 20. Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol. 1982;243(6):H941‐H947. [DOI] [PubMed] [Google Scholar]
- 21. Slawson SE, Roman BB, Williams DS. Koretsky AP Cardiac MRI of the normal and hypertrophied mouse heart. Magn Reson Med. 1998;39(6):980‐987. [DOI] [PubMed] [Google Scholar]
- 22. Fernandes‐Santos C, de Souza Mendonça L, Mandarim‐De‐Lacerda CA. Favorable cardiac and aortic remodeling in olmesartan‐treated spontaneously hypertensive rats. Heart Vessels. 2009b;24(3):219‐227. [DOI] [PubMed] [Google Scholar]
- 23. Onat A, Karadeniz Y, Tusun E, Yüksel H, Kaya A. Advances in understanding gender difference in cardiometabolic disease risk. Expert Rev Cardiovasc Ther. 2016;14(4):513‐523. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization, W . Cardiovascular disease: the atlas of heart disease and stroke. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 25. Anunciado‐Koza RP, Manuel J, Koza RA. Molecular correlates of fat mass expansion in C57BL/6J mice after short‐term exposure to dietary fat. Ann N Y Acad Sci. 2016;1363(1):50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fukuchi S, Hamaguchi K, Seike M, Himeno K, Sakata T, Yoshimatsu H. Role of fatty acid composition in the development of metabolic disorders in sucrose‐induced obese rats. Exp Biol Med (Maywood). 2004;229(6):486‐493. [DOI] [PubMed] [Google Scholar]
- 27. Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet‐induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000;24(5):639‐646. [DOI] [PubMed] [Google Scholar]
- 28. Hariri N, Gougeon R, Thibault L. A highly saturated fat‐rich diet is more obesogenic than diets with lower saturated fat content. Nutr Res. 2010;30(9):632‐643. [DOI] [PubMed] [Google Scholar]
- 29. Krishna S, Lin Z, de La Serre CB, et al. Time‐dependent behavioral, neurochemical, and metabolic dysregulation in female C57BL/6 mice caused by chronic high‐fat diet intake. Physiol Behav. 2016;157:196‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Surwit RS, Feinglos MN, Rodin J, et al. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44(5):645‐651. [DOI] [PubMed] [Google Scholar]
- 31. Forshee RA, Storey ML, Allison DB, et al. A critical examination of the evidence relating high fructose corn syrup and weight gain. Crit Rev Food Sci Nutr. 2007;47(6):561‐582. [DOI] [PubMed] [Google Scholar]
- 32. Hogan S, Canning C, Sun S, Sun X, Kadouh H, Zhou K. Dietary supplementation of grape skin extract improves glycemia and inflammation in diet‐induced obese mice fed a Western high fat diet. J Agric Food Chem. 2011;59(7):3035‐3041. [DOI] [PubMed] [Google Scholar]
- 33. Shao D, Tian R. Glucose transporters in cardiac metabolism and hypertrophy. Compr Physiol. 2015;6(1):331‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mahmud A, Feely J. Arterial stiffness and the renin‐angiotensin‐aldosterone system. J Renin Angiotensin Aldosterone Syst. 2004;5(3):102‐108. [DOI] [PubMed] [Google Scholar]
- 35. Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin‐angiotensin systems. Physiol Rev. 2006;86(3):747‐803. [DOI] [PubMed] [Google Scholar]
- 36. Ma X, et al. Salt sensitivity in male and female C57BL/6J mice: role of renal angiotensin and dopamine receptors and sodium transporters. Hypertension. 2017;70(S1):AP151. [Google Scholar]
- 37. Mills E1, Kuhn CM, Feinglos MN, Surwit R. Hypertension in CB57BL/6J mouse model of non‐insulin‐dependent diabetes mellitus. Am J Physiol. 1993;264(1 Pt 2):R73‐R78. [DOI] [PubMed] [Google Scholar]
- 38. Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol. 2016;97:245‐262. [DOI] [PubMed] [Google Scholar]


