Abstract
Monocytes are classified according to their CD14 and CD16 expression into classical (reparative), intermediate (inflammatory), and non-classical. This study assessed the frequency of monocyte and the relationship between monocyte subset percentages and the levels of blood cytokines in Colombian chagasic patients with different clinical forms. This study included chagasic patients in different clinical stages: indeterminate (IND) n = 14, chronic chagasic cardiomyopathy (CCC) n = 14, and heart transplant chagasic (HTCC) n = 9; controls with non-chagasic cardiopathy (NCC) n = 15, and healthy individuals (HI) n = 15. Peripheral blood mononuclear cells (PBMCs) were isolated, labeled for CD14, CD16, and HLA-DR, and analyzed by flow cytometry. Cytokines were measured with a bead-based immunoassay. Percentages of total CD14+ CD16+ and CD14+ HLA-DR+ monocytes were higher in patients with heart involvement (CCC, HTCC, and NCC) than controls. Percentages of intermediate monocytes increased in symptomatic chagasic patients (CCC and HTCC) compared to asymptomatic chagasic patients (IND) and controls (HI). Asymptomatic chagasic patients (IND) had higher percentages of classical monocytes, an increased production of CCL17 chemokine compared to chagasic symptomatic patients (CCC), and their levels of CCL17 was positively correlated with the percentage of classical monocyte subset. In CCC, the percentages of intermediate and classical monocytes were positively correlated with IL-6 levels, which were higher in this group compared to HI, and negatively with IL-12p40 concentration, respectively. Remarkably, there also was an important increased of classical monocytes frequency in three chronic chagasic patients who underwent cardiac transplant, of which one received anti-parasitic treatment. Our findings suggest that cardiac chagasic patients have an increased percentage of inflammatory monocytes and produce more IL-6, a biomarker of heart failure and left ventricular dysfunction, whereas asymptomatic chagasic individuals present a higher percentage of reparative monocytes and CCL17.
Keywords: cardiomyopathy, Chagas disease, chemokines, cytokines, monocytes, innate immunity
Introduction
Chagas disease (CD) is a public health problem in Latin-America with an estimated prevalence of 10 million cases in 2015 (1), and the major cause of infectious cardiomyopathy worldwide (2). Even though 70% of infected individuals remain asymptomatic during their lifetime (3), 20–30% will develop the chronic chagasic cardiomyopathy (CCC), an inflammatory event in which Trypanosoma cruzi, the parasitic agent, persists in the heart leading to multifocal inflammation, ischemia, and necrosis. The cardiac tissue alterations progress to congestive heart failure, a condition characterized by dilatation and hypertrophy of the heart ventricles associated with a high mortality rate (4). Among the myocardial-infiltrating cells, there are T cells and antigen presenting cells (APCs); CD8+ T cells are the most abundant cells followed by CD4+ T cells and macrophages, favoring heart inflammation, tissue remodeling, and cellular damage (5). Although the role of CD8+ T cells is protective during the acute phase (6, 7), they fail to control T. cruzi during chronic infection (6). T cells from peripheral blood or heart tissue of chagasic patients display an exhaustion phenotype, characterized by alterations in their proliferation capacity, increased expression of inhibitory markers and progressive loss of cytokine secretion (8–13), which are associated with parasite persistence (8, 14, 15). Although less abundant, APCs such as dendritic cells and monocytes/macrophages, can interact with parasite antigens modifying their antigen presentation and their cytokine profile secretion (16–25).
Monocytes are heterogeneous and multifunctional cells that convey great plasticity; as innate immune cells, they phagocyte microbes and produce oxygen reactive species (26, 27), and also participate in cellular processes including tissue repair and regeneration during heart diseases (4, 28). The increase of peripheral blood monocytes has been associated with left ventricular remodeling after acute myocardial infarction (29). Cytokines produced by monocytes including TNF-α and IL-6 and soluble CD14 are correlated with severe congestive heart failure (30–32); also soluble TNF receptor can be a predictor of mortality in the same condition (33, 34). Inflammatory monocytes (CD16+) have been associated with the pathogenesis of several infectious diseases including some caused by parasites (35–40). Currently, monocytes are phenotypically classified into three subsets according to the CD14 (bacterial LPS receptor) and CD16 (IgG low affinity receptor) expression: classical (CD14++ CD16–), intermediate (CD14++ CD16+), and non-classical monocytes (CD14+ CD16++) (41, 42). Each of these subsets can have distinct functions. Intermediate monocytes participate in angiogenesis and have a high expression of MHC class II. Classical monocytes specialize in IL-10 production and phagocytosis (35), and are involved in tissue repair. Non-classical monocytes are characterized by their patrolling activity, and both non-classical and intermediate subsets are the main sources of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8 (35, 43, 44). Several studies support the pivotal role of monocytes-mediated inflammation in the pathogenesis of cardiomyopathy and the progression of congestive heart failure (30).
T. cruzi induces phenotypic and functional alterations in monocytes during the acute and chronic phases of CD. During the acute infection, monocytes present an augmented expression of HLA-DR, TLR2, and costimulatory CD80/CD86 molecules, and increase the production of TNF-α, IL-12/IL-23p40, and IL-10 (45). In chronic stages of the disease, there has been found a correlation between MHC class II, co-stimulatory molecules expression and cytokines production with the development of CCC (16, 17, 19–25).
Only few studies in Chagas disease have characterized the phenotype of the monocyte subsets based on CD14 and the CD16 expression, finding an increase of CD14+ CD16+ monocytes percentage in asymptomatic children (46, 47) and adults during acute and chronic infection (48–50). In this work, we studied the variations in the frequency of the total monocyte population, and the correlation between monocyte subsets and the seric levels of IL-1β, IL-1RA, IL-6, IL-10, IL-12p40, IL-12p70, IL-23, TNF-α, CCL17, and CXCL10 in samples from chronic chagasic patients with different stages of the disease.
Materials and Methods
Ethics Statement
Research protocol and informed consent were approved by the Ethical Committees of Universidad de los Andes (458-2015) and Hospital Universitario San Ignacio (06-2016) from Bogotá, Colombia. The protocol follows the Colombian national regulations and the Declaration of Helsinki. All the included subjects provided written informed consent.
Human Donors
Thirty-eight cardiomyopathy patients were recruited at the Failure and Heart Transplantation Clinic in the Hospital Universitario San Ignacio, of which 23 had chronic Chagas disease. Chagasic patients were classified in two groups, IND or asymptomatic chagasic (stages A and B) and CCC or chronic chagasic cardiomyopathy (stages C and D) according to the American College of Cardiology/American Heart Association staging. IND group included 14 individuals, 6 males and 8 females with ages ranging from 28 to 62 years. CCC group included 14 patients, 7 males and 7 females with ages ranging from 53 to 78 years. A third group of 9 heart transplant chagasic patients (HTCC), 5 males and 4 females with ages ranging from 50 to 71 years, were patients who had received a heart transplant from 3 weeks up to 13 years before entering the study. The control groups included 15 patients with non-chagasic myocardiopathy (NCC), 9 males and 6 females with ages from 42 to 72 years, and 15 healthy individuals (HI), 7 males and 8 females with ages from 34 to 62 years old and seronegative for T. cruzi antibodies. All volunteers were tested for antibodies against T. cruzi by immunofluorescence indirect assay (IFI) with trypomastigotes and an ELISA kit for IgG anti-T. cruzi antibodies (NovaTec, Dietzenbach, Germany) (51). The exclusion criteria were chronic inflammatory conditions or acute infections during the time of sampling. Characteristic of individuals are shown in Table 1.
Table 1.
Demographic and clinical characteristics of patients and individuals enrolled in the study.
| IND | CCC | HTCC | NCC | HI | p-value | |
|---|---|---|---|---|---|---|
| Number of individuals | 14 | 14 | 9 | 15 | 15 | — |
| Age mean (±SD) | 49.7 (9.8) | 63.6 (8.8) | 60.8 (8.5) | 63.9 (10.3) | 53.6 (8.2) | 0.0013* |
| Sex male % | 43 | 50 | 56 | 60 | 47 | — |
| ACC/AHA classification | ||||||
| A No. | 12 | — | — | — | — | — |
| B No. | 2 | — | — | — | — | — |
| C No. | — | 11 | 4 | 15 | — | — |
| D No. | — | 3 | 5 | — | — | — |
| Mean LVEF % (±SD) | 58.8 (6.3) | 27.2 (11.5) | 30.0 (22.8) | 27.3 (11.1) | — | 0.0340** |
| Heart failure etiology | ||||||
| Ischemic heart failure | — | — | — | 10 | — | — |
| Hypertensive heart failure | — | — | — | 2 | — | — |
| Idiopathic heart failure | — | — | — | 2 | — | — |
| Congenital heart failure | — | — | — | 1 | — | — |
Groups. Asymptomatic or indeterminate chagasic patients (IND): those with non-structural cardiac damage (groups A and B); symptomatic or cardiomyopathy chagasic patients (CCC): those with structural cardiac damage (groups C and D); heart transplant chagasic patients (HTCC): chagasic patients who received a new heart in the last 13 years; non-chagasic myocardiopathy (NCC): patients with congestive heart failure associated with an etiology other than CD; healthy individuals (HI). American College of Cardiology/American Heart Association (ACC/AHA) classification. A: normal electrocardiogram (ECG) and echocardiogram (ECHO) findings, and New York Heart Association (NYHA) functional classification I; B: abnormal ECG findings, normal ECHO and NYHA I; C: abnormal ECG findings, increased heart size, decreased left ventricular ejection fraction (LVEF) and NYHA II or III; D: same as class C, but NYHA IV.
The IND group had a lower age than CCC (p = 0.0107) and NCC (p = 0.0068) patients.
The IND had higher LVEF compared to CCC (p = 0.0387) and HTCC (p = 0.0417) groups.
Blood Sample, Isolation of Peripheral Blood Mononuclear Cells and Cell Surface Staining
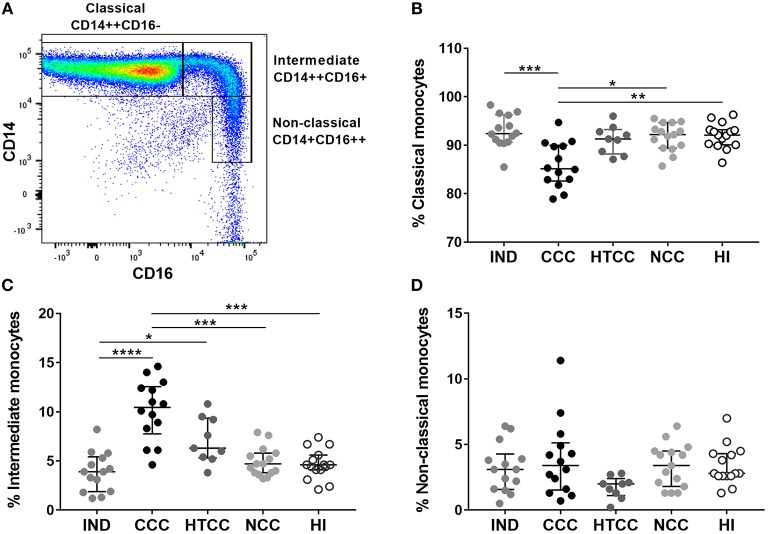
Blood samples were drawn in EDTA vacutainer tubes (BD Biosciences, Franklin Lakes, NJ, USA). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation with Ficoll-Hypaque (GE Healthcare, Chicago, IL, USA) and their viability was evaluated with trypan blue 0.4% (Cell Signaling Technology, Danvers, MA, USA). One million of PBMCs were incubated with anti-CD16/32 (clone 2.4G2) from Tonbo Biosciences (San Diego, CA, USA) and human AB serum to block Fc receptors, and then stained with anti-CD14 PE (HCD14) and anti-CD16 PE-Cy7 (3G8) purchased from BioLegend (San Diego, CA, USA) and anti-HLA-DR FITC (Tu39) from BD Biosciences or with their corresponding isotype controls. Stained samples were incubated for 25 min at 4°C and acquired in a FACS Canto II cytometer with FACSDiva software (BD Biosciences). At least 50,000 events were acquired on the HLA-DR+ gate; over this population it was defined the percentage of monocytes (CD14+ CD16+) and the monocyte subsets according to the CD14 and CD16 expression as follows: CD14++ CD16- (classical), CD14++ CD16+ (intermediate), and CD14+ CD16++ (non-classical) (see Figure 2A). Percentages of HLA-DR+ and HLA-DR mean fluorescence intensity (MFI) were measured in each monocyte subset. Gating strategy is shown in Supplementary Figure 1. Flow cytometry files for monocytes staining can be found in the FlowRepository (https://flowrepository.org/id/FR-FCM-ZYTR).
Figure 2.
Analysis of normalized percentages of monocyte subsets in IND (n = 14), CCC (n = 14), and HTCC (n = 9) patients and NCC (n = 15) and HI (n = 15) controls. The strategy used to differentiate the three monocyte subsets according to CD14 and CD16 expression is shown in (A). The percentages of classical (B), intermediate (C), and non-classical (D) monocytes between the groups were compared with the Kruskal-Wallis test followed by Dunn's post-hoc test. The values are presented by median and interquartile ranges and differences are indicating with: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Detection of Cytokines in Human Plasma
The quantification (pg/mL) of proinflammatory (IL-1β, IL-6, IL-12p40, IL-12p70, IL-23, and TNF-α) and regulatory (IL-1RA and IL-10) cytokines and chemokines (CXCL10 and CCL17) in the plasma of all donors were carried out with LEGENDplex™ Human M1/M2 Macrophage Panel (10-plex) from BioLegend. The assay was done according to the manufacturer's instructions. The samples were acquired on a FACS Canto II flow cytometer and the data was analyzed with the LEGENDplex™ v8.0 software.
Statistical Analysis
Descriptive statistics were used to depict the population and to present the flow cytometry data. Data distribution was evaluated by the Shapiro-Wilk test. Non-parametric analyses were used to compare multiple groups using the Kruskal Wallis test followed by Dunn's post-hoc test. The Mann-Whitney test was used for non-parametric comparison of the cytokines levels. The correlation between the percentage of monocyte subsets of each group and the cytokines concentration were evaluated by the Spearman's coefficient. All statistical analysis was carried out with the GraphPad Prism 7.0 software (GraphPad, San Diego, CA, USA) considering p-values < 0.05 as significant.
Results
Patients Characteristics
Asymptomatic individuals (IND) were younger than patients with heart involvement (p = 0.0013), but have a similar age to the healthy individuals (HI). Left ventricular ejection fraction (LVEF) had normal ranges in IND individuals. All patients with cardiomyopathy (CCC, HTCC, and NCC) had an LVEF <50%. The individuals in the NCC group allows to determine if changes could be related to T. cruzi infection or the heart disease itself (Table 1). The median OD450 of anti-T. cruzi antibodies by ELISA in chagasic patients was similar between CCC, IND, and HTCC groups (OD450 values of 2.857, 2.844, and 2.799, respectively), whereas the controls presented a median OD450 below the cut-off (0.267 and 0.210 for NCC and HI) as expected. All chagasic patients were reactive by indirect immunofluorescence (IFI) with trypomastigotes at 1:40 dilution.
Chagasic Patients With Heart Disease Displayed Higher Percentages of Total CD14+ CD16+ and CD14+ HLA-DR+ Monocytes
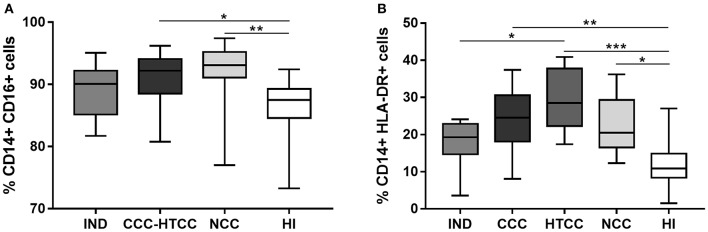
It was assessed the percentages of monocytes and their HLA-DR expression in peripheral blood cells from all donors. The total monocytes percentage, defined as CD14+ and CD16+ cells, increased in donors with heart involvement: CCC-HTCC (p = 0.00164) and NCC patients (p = 0.0034) compared to HI group (Figure 1A). With the aim to compare with previous studies, it was analyzed the percentages of CD14+ HLA-DR+ cells, which were also higher in patients with heart damage, including CCC (p = 0.0030), HTCC (p < 0.0001), and NCC (p = 0.0129), compared to HI group (Figure 1B). When the total percentages of CD14+ CD16+ cells in the CCC (p = 0.1352) and HTCC (p = 0.0765) groups were analyzed separately, there was no significant difference compared to HI.
Figure 1.
Analysis of CD14+ CD16+ (A) and CD14+ HLA-DR+ (B) cells percentages from peripheral blood of chagasic patients classified as IND, CCC, and HTCC, and controls defined as NCC and HI. Multiple comparisons were done with the Kruskal-Wallis test followed by Dunn's post-hoc test. Differences are indicating with: *p < 0.05, **p < 0.01, ***p < 0.001.
Intermediate Monocytes Increased in Cardiac Chagasic Patients
As monocytes increased in chagasic and non-chagasic individuals with heart disease, we determined the frequency of classical, intermediate, and non-classical monocytes according to CD14 and CD16 expression (Figure 2A). It was found that CCC patients had a reduced percentage of classical monocytes compared to IND (p = 0.0007), NCC (p = 0.0121), and HI (p = 0.0074) groups, but without differences with HTCC (Figure 2B). There was an increase of intermediate monocytes percentage in CCC patients compared to IND (p < 0.0001), NCC (p = 0.0009), and HI (p = 0.0004). Also, there was an augmented frequency of intermediate monocytes in HTCC patients only compared to IND individuals (p = 0.0375) (Figure 2C). Non-classical monocytes had similar percentages (p > 0.05) in all groups (Figure 2D). HLA-DR intensity (MFI) of classical, intermediate and non-classical monocytes was similar (p > 0.05) among groups, however, classical monocytes in the IND group had a higher HLA-DR MFI compared to HI (p = 0.0054). Additionally, MFI values for HLA-DR in each group was higher in intermediate monocytes as expect, since this monocyte subset expresses more MHC class II molecules (Supplementary Figure 2).
IL-12p40 and CCL17 Production Decreased in Cardiac Chagasic Patients
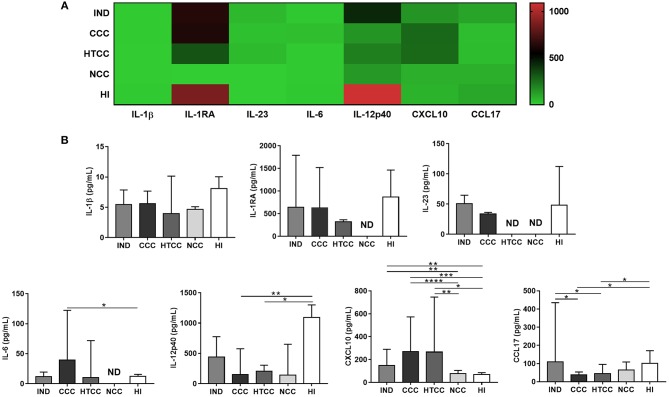
To evaluate if inflammatory and regulatory cytokines and chemokines were differentially secreted, the levels of IL-1β, IL-1RA, IL-6, IL-10, IL-12p40, IL-12p70, IL-23, TNF-α, CCL17, and CXCL10 were measured in plasma. The patterns of cytokine and chemokine production distinguished chagasic patients and controls; for example, HI tend to have more IL-12p40 and IL-1RA, meanwhile chagasic patients (IND, CCC, and HTCC) had higher concentrations of chemokines (CCL17 and CXCL10) (Figure 3A). TNF-α was only detectable in control groups: NCC (mean of 12.73 pg/mL) and HI (mean of 12.45 pg/mL), whereas the concentration of this cytokine in chagasic patients was below the detection limit (3 pg/mL). Similarly, IL-10 and IL-12p70 concentration in all donors were below the detection limit (4.52 and 4.26 pg/mL, respectively), except for two IND donors with a mean IL-12p70 value of 19.55 pg/mL. IL-6 was the only cytokine that showed increased levels in CCC compared to HI (p = 0.0480). In contrast, IL-12p40 levels in CCC and HTCC patients was reduced compared to HI controls (p = 0.0052 and p = 0.0336). Regarding chemokines, CXCL10 concentration was higher in chagasic patients (IND, CCC, and HTCC groups) compared to NCC (p-values of 0.0031, <0.0001, and 0.0055, respectively) and HI (p-values of 0.0083, 0.0001, and 0.0148, respectively). CCL17 had decreased levels in CCC and HTCC patients compared to IND (p = 0.0160 and 0.0111) and HI (p = 0.0246 and 0.0349), respectively (Figure 3B).
Figure 3.
Cytokines and chemokines concentration in plasma from donors of all groups. Heat map that shows the patterns of cytokines and chemokines concentration in chagasic patients (IND, CCC, and HTCC) and controls (NCC and HI) are distinct (A). Comparison of IL-1β, IL-1RA, IL-6, IL-12p40, IL-23, CXCL10, and CCL17 levels was done with Mann-Whitney test. The median and interquartile ranges of cytokines are displayed (B). ND, Not detectable. Differences are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Intermediate and Classical Monocytes Correlated With IL-6 and CCL17 Production in Chagasic Patients
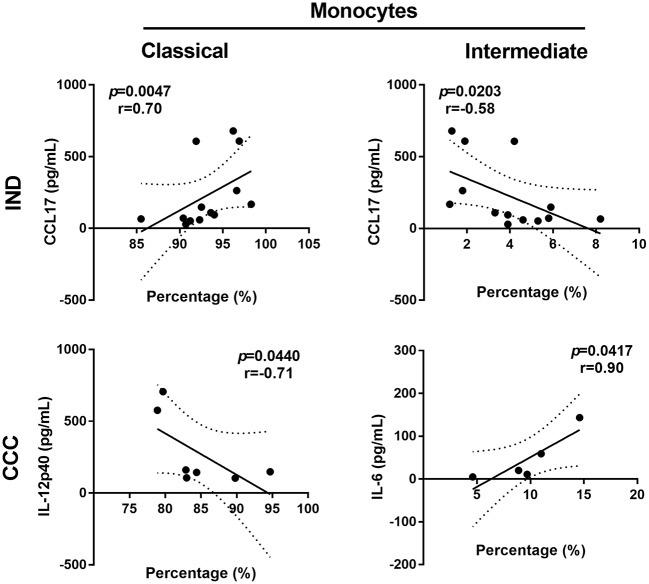
It was evaluated the correlation between monocyte subset percentages and cytokines concentration in all groups. In CCC, it was observed a positive correlation of IL-6 levels and intermediate monocytes (r = 1.00; p = 0.0167), cytokine and monocyte subset that increased in chronic cardiac patients. In IND donors, augmented CCL17 production had a negative correlation with intermediate monocytes (r = −0.58; p = 0.0203) and a positive correlation with classical monocytes (r = 0.70; p = 0.0047) (Figure 4).
Figure 4.
Scatter plot showing the followings correlations: percentages of classical monocytes and CCL17 concentration in IND, percentages of intermediate monocytes and CCL17 concentration in IND, percentages of intermediate monocytes and IL-6 concentration in CCC. Spearman's rank correlation coefficient (r) and p-values were calculated for every association using non-parametric Spearman's correlation test. IND, indeterminate; CCC, chronic chagasic cardiomyopathy.
Percentage of Intermediate Monocytes Decreased After Heart Transplant
Two chagasic patients, CCC16 and CCC18, presented a reduction of intermediate monocytes percentages 6 months and 3 weeks, respectively, after receiving the heart transplant; and it was accompanied with augmented IL-12p40 level in plasma (<97.66 pg/mL and 104.30 pre-transplant vs. 329.26 vs. 234.14 post-transplant, respectively). Similarly, the HTCC21 patient with a 10 years transplant, treated twice for T. cruzi reactivation with anti-parasitic drugs, showed a lower percentage of intermediate monocytes (5.4%) compared to the mean frequency values of HTCC group (7.1%) as shown in Supplementary Figure 3.
Discussion
Monocytes/macrophages which are part of the innate immune response are also implicated in tissue inflammation, repair and fibrosis. Indeed, a single population of monocytes can present pro-inflammatory and pro-repair properties (28). Thus, it is important to define the monocyte subsets in parasitic infectious diseases with an inflammatory component. For instance, the intermediate and the classical monocytes are involved in the pathogenesis of both protozoa and helminth diseases, such as leishmaniasis, malaria, schistosomiasis, and filariasis (36–40). The goal of this study was to determine the monocyte subset percentages in peripheral blood and plasma cytokines levels in chagasic patients with different clinical stages.
In this study, CD14+ CD16+ monocytes were higher in chagasic patients with severe disease and in those with heart transplant. A previous study using CD14 as a unique cellular marker did not show any difference in cells frequencies from chronic chagasic patients (17). As antigen-presenting cells, monocytes process and present T. cruzi peptides onto MHC class II molecules in order to activate T cells (3), therefore we assessed CD14+ HLA-DR+ cells frequency, as described by others (17, 47). It was observed an increased percentage of these cells in all cardiac patients, chagasic or not. One study described a reduction of CD14+ HLA-DR+ cells in indeterminate chagasic compared to healthy individuals (17). Here indeterminate individuals had lower percentages of CD14+ HLA-DR+ cells only compared to transplanted chagasic patients. An increase of CD14+ CD16+ HLA-DR+ monocytes and CD14+ HLA-DR+ cells were described in the early chronic phase in children and in chronic chagasic adults after anti-parasitic treatment (46–48). Despite the fact that transplant chagasic patients had different times of grafting and also had immunosuppression, monocyte subsets depicted similar trends among them.
In two previous works, in which the three monocyte subsets were identified in chronic chagasic patients, it also was found an increase of intermediate monocytes in cardiac patients (49, 50); although, we report different results regarding classical and non-classical monocytes percentages. These differences could be attributed to sample processing, staining reagents and distinct gating strategies for defining the subsets (52). Interestingly, intermediate and classical monocytes are associated with worse prognosis in different myocardial diseases. A higher frequency of intermediate monocytes is correlated with lower LVEF in patients with congestive heart failure (53), elevation of ST segment after myocardial infarction and IL-6 production (54). Similarly, the increased percentage of classical monocytes are correlated with LVEF reduction 6 months after acute myocardial infarction (55).
The intermediate monocytes are characterized by a higher expression of MHC class II molecules as shown here, and CD40 co-stimulatory molecule (43). They also secrete inflammatory cytokines such as IL-6, IL-1β, and mainly TNF-α (56), reflecting their role in inflammation and promotion of T cells activation. Some patterns of cytokines and chemokines in peripheral blood were found in this study. Remarkably, IL-12p40 and CCL17 (TARC), molecules related to T cells response (57, 58), had reduced levels in patients with severe form of Chagas disease. Similarly, these patients had augmented intermediate monocytes and increased production of IL-6, a proinflammatory cytokine (59). CXCL10 or interferon gamma-induced protein 10 (IP-10), a chemokine secreted by monocytes in response to IFN-γ (60), is augmented in all chagasic patients. In contrast, asymptomatic individuals presented an increased percentage of reparative monocytes (classical) and a higher level of CCL17. In chronic chagasic patients IL-12 expression is decreased in antigen-stimulated monocytes. Furthermore, ex vivo expression of IL-12 was higher on classical monocytes of asymptomatic individuals compared with uninfected controls (49). Macrophage-derived IL-12 is crucial to control parasitemia during acute infection (61), as also shown in an IL-12p40 KO murine infection model (62). A study showed CCL17 mRNA was up-regulated in chagasic myocardium, but its genetic polymorphisms were not associated with risk of cardiomyopathy development (63). According to our data, IL-12 and CCL17 levels were higher in asymptomatic patients compared to cardiac chagasic patients. Indeed, it has been proposed that Th2 cytokines reduced production and high levels of Th1 cytokines are associated with chagasic cardiomyopathy severity (64). As reported, IL-12 is crucial for Th1 populations development, and although Th1 role could be controversial in Chagas disease (64), IFN-γ has been associated with protection (65). Here, cardiac chagasic patients displayed higher seric concentration of IL-6 (66), cytokine associated with pro-fibrotic factors and cardiac deterioration in chagasic children and adults (59, 67). Similarly, CXCL10 plasma levels were higher in both asymptomatic and symptomatic chronic chagasic patients compared to controls (68). This chemokine is also considered a predictor of severity in parasitic infections (60). The CXCL10 mRNA expression increased in hearts from chronic T. cruzi infected dogs (69) and humans (63, 70). Interestingly, there is an IFN-γ independent CXCL10 activation pathway, because its gene contains binding sites for multiples pro-inflammatory transcriptions factors (71).
Of note, we had access to samples of two chagasic patients before and after heart transplant. After the transplant and without anti-parasitic treatment, these two patients displayed a reduction of intermediate monocyte percentages (Δ: 3.4 and 5.1%) and IL-12p40 increased production, suggesting the recovery of a protective immune response after heart transplant. Intermediate monocytes frequency changes in adults donors had a lower variation (Δ: 2.3% within 4–6 months), in comparison with the variations in chagasic post-transplant patients showed here (56). Remarkably, one transplant patient who presented reactivation of T. cruzi and treated, had a lower value of intermediate monocytes compared to the mean of transplanted patients, indicating that parasite antigen could be responsible for those changes. Due to the low number of samples, this data must be validated in future studies.
In summary, our findings suggest that all cardiac patients, chagasic or not, have augmented percentages of CD14+ CD16+ monocytes, however, the increase of intermediate monocytes were also found in chronic cardiac chagasic patients. Likewise, the associations between the patterns of variation in monocyte subsets and cytokines levels and differential disease stages suggest the potential involvement of these factors either in protection (classical monocytes and IL-12p40/CCL17) or pathogenesis of Chagas cardiomyopathy (intermediate monocytes and IL-6). Functional studies with monocyte subsets during T. cruzi chronic infection will be crucial to understand their role in the disease pathogenesis.
Ethics Statement
Research protocol and informed consent were approved by the Ethical Committees of Universidad de los Andes and Hospital Universitario San Ignacio from Bogotá, Colombia. The protocol follows the Colombian national regulations and the Declaration of Helsinki. All the included subjects provided written informed consent.
Author Contributions
SG-O recruited the donors, prepared the samples, designed and carried out the experiments, analyzed the data, made the figures, discussed the results, and wrote the paper. NB helped to prepare samples and perform experiments. ME wrote the first protocol of the study and standardized the flow cytometry settings. AR recruit IND patients. AM recruit heart failure patients (CCC and HTCC) and carried out their clinical assessment. CP and AC help with reagents, discussed the results, and reviewed the manuscript. JG acquired and administered funding resources, coordinated the study, designed the experiments, and wrote the manuscript. All authors contributed to manuscript revision and approved the submitted version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors want to thank Professor Jorge Molina from the Department of Biological Sciences of Universidad de los Andes for his support, to Sara Paredes MD for reviewing the manuscript, and to Luisa Aponte and Angie Barrera at the Hospital Universitario San Ignacio for its support in enrolling the patients in the study. Especial thanks to all the donors who participated in the study.
Footnotes
Funding. The project received financial support from Research Vice-Rectory, Department of Biological Sciences and School of Sciences (grant INV-2017-5-1106) from Universidad de los Andes. The funders had no role in study design, data collection, and analysis or preparation of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.01671/full#supplementary-material
Gating strategy for 3-color flow cytometry with a sample from a representative CCC donor. Mononuclear cells were defined according to FSC vs. SSC parameters and gated on the singlets. HLA-DR+ population gate was determined based on the isotype control. On this population, it was defined the percentage of total monocytes (CD14+ CD16+ cells) and monocyte subsets according to CD14 and CD16 expression as follows: CD14++ CD16− (classical), CD14++ CD16+ (intermediate) and CD14+ CD16 ++ (non-classical).
Mean fluorescence intensity (MFI) for HLA-DR expression in monocyte subsets from IND, CCC, HTCC, NCC and HI. Significant differences determined by Kruskal-Wallis test followed by Dunn's post hoc test are shown as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Monocyte subsets and left ventricular ejection fraction (LVEF) variations associated with the time of heart transplantation and reactivation of T. cruzi infection. As shown for CCC16 and CCC18 patients, the percentages of classical monocytes increased and the percentage of intermediate monocytes diminished after heart transplantation, while IL-12p40 blood levels augmented in both patients. In the case of CCC21 patient, it was observed similar monocyte subsets changes upon antiparasitic treatment for parasite reactivation.
References
- 1.WHO Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. (2015) 6:33–43. [PubMed] [Google Scholar]
- 2.Cooper LT, Keren A, Sliwa K, Matsumori A, Mensah GA. The global burden of myocarditis. Part 1: a systematic literature review for the global burden of diseases, injuries, and risk factors 2010 study. Glob Heart. (2014) 9:121–9. 10.1016/j.gheart.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 3.Machado FS, Dutra WO, Esper L, Gollob K, Texeira M, Factor S, et al. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol. (2012) 34:753–70. 10.1007/s00281-012-0351-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apostolakis S, Lip GY, Shantsila E. Monocytes in heart failure: relationship to a deteriorating immune overreaction or a desperate attempt for tissue repair? Cardiovasc Res. (2010) 85:649–60. 10.1093/cvr/cvp327 [DOI] [PubMed] [Google Scholar]
- 5.Álvarez JM, Fonseca R, Borges H, Marinho CR, Bortoluci KR, Sardinha LR, et al. Chagas disease: still many unsolved issues. Mediators Inflamm. (2014) 2014:91296. 10.1155/2014/912965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarleton R. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J Immunol. (1990) 144:717–24. [PubMed] [Google Scholar]
- 7.Tarleton RL, Sun J, Zhang L, Postan M. Depletion of T-cell subpopulations results in exacerbation of myocarditis and parasitism in experimental Chagas' disease. Infect Immun. (1994) 62:1820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasso P, Mateus J, Pavía P, Rosas F, Roa N, Thomas MC, et al. Inhibitory receptor expression on CD8+ T cells is linked to functional responses against Trypanosoma cruzi antigens in chronic Chagasic patients. J Immunol. (2015) 195:3748–58. 10.4049/jimmunol.1500459 [DOI] [PubMed] [Google Scholar]
- 9.Mateus J, Lasso P, Pavia P, Rosas F, Roa N, Valencia-Hernandez CA, et al. Low frequency of circulating CD8+ T stem cell memory cells in chronic chagasic patients with severe forms of the disease. PLoS Negl Trop Dis. (2015) 9:e3432. 10.1371/journal.pntd.0003432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albareda C, Laucella SA, Alvarez G, Armenti H, Bertochi G, Tarleton RL, et al. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas' disease patients. Int Immunol. (2006) 18:465–71. 10.1093/intimm/dxh387 [DOI] [PubMed] [Google Scholar]
- 11.Albareda MC, Olivera GC, Susana A, Alvarez MG, Rodrigo E, Lococo B, et al. Chronic human infection with Trypanosoma cruzi drives CD4+ T cells to immune senescence. J Immunol. (2009) 183:4103–8. 10.4049/jimmunol.0900852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albareda MC, De Rissio AM, Tomas G, Serjan A, Alvarez M, Viotti R, et al. Polyfunctional T cell responses in children in early stages of chronic Trypanosoma cruzi infection contrast with monofunctional responses of long-term infected adults. PLoS Negl Trop Dis. (2013) 7:e2575. 10.1371/journal.pntd.0002575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argüello RJ, Albareda MC, Alvarez MG, Bertocchi G, Armenti AH, Vigliano C, et al. Inhibitory receptors are expressed by Trypanosoma cruzi-specific effector T cells and in hearts of subjects with chronic Chagas disease. PLoS ONE. (2012) 7:e35966. 10.1371/journal.pone.0035966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giraldo A, Bolaños NI, Cuellar A, Roa N, Cucunubá Z, Puerta CJ, et al. T lymphocytes from chagasic patients are activated but lack proliferative capacity and down-regulate CD28 and CD3z. PLoS Negl Trop Dis. (2013) 7:e2038 10.1371/journal.pntd.0002038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mateus J, Pérez-Antón E, Lasso P, Egui A, Roa N, Carrilero B, et al. Antiparasitic treatment induces an improved CD8+ T cell response in chronic chagasic patients. J Immunol. (2017) 198:3170–80. 10.4049/jimmunol.1602095 [DOI] [PubMed] [Google Scholar]
- 16.Souza PEA, Rocha MO, Menezes CA, Coelho JS, Chaves CL, Gollob KJ, et al. Trypanosoma cruzi infection induces differential modulation of costimulatory molecules and cytokines by monocytes and T cells from patients with indeterminate and cardiac Chagas' disease. Infect Immun. (2007) 75:1886–94. 10.1128/IAI.01931-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza PE, Rocha MO, Rocha-Vieira E, Menezes CA, Chaves C, Gollob KJ, et al. Monocytes from patients with indeterminate and cardiac forms of chagas' disease display distinct phenotypic and functional characteristics associated with morbidity. Infect Immun. (2004) 72:5283–91. 10.1128/IAI.72.9.5283-5291.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomes J, Molica A, Souza T, Lima Keesen T, Ferreira Morato M, Fortes de Araujo F, et al. Inflammatory mediators from monocytes down-regulate cellular proliferation and enhance cytokines production in patients with polar clinical forms of Chagas disease. Hum Immunol. (2014) 75:20–8. 10.1016/j.humimm.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 19.Ouaissi A, Guilvard E, Delneste Y, Caron G, Magistrelli G, Herbault N, et al. The Trypanosoma cruzi Tc52-released protein induces human dendritic cell maturation, signals via Toll-like receptor 2, and confers protection against lethal infection. J Immunol. (2002) 168:6366–74. 10.4049/jimmunol.168.12.6366 [DOI] [PubMed] [Google Scholar]
- 20.Brodskyn C, Patricio J, Oliveira R, Lobo L, Arnholdt A, Mendonça-Previato L, et al. Glycoinositolphospholipids from Trypanosoma cruzi interfere with macrophages and dendritic cell responses. Infect Immun. (2002) 70:3736–43. 10.1128/IAI.70.7.3736-3743.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes J, Bahia-Oliveira L, Rocha M, Martins-Filho O, Gazzinelli G, Correa-Oliveira R. Evidence that Development of Severe Cardiomyopathy in Human Chagas' Disease is Due to a Th1-Specific Immune Response. Infect Immun. (2003) 71:1185–1193. 10.1128/IAI.71.3.1185-1193.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes J, Campi-Azevedo AC, Teixeira-Carvalho A, Silveira-Lemos D, Vitelli-Avelar D, Sathler-Avelar R, et al. Impaired phagocytic capacity driven by downregulation of major phagocytosis-related cell surface molecules elicits an overall modulatory cytokine profile in neutrophils and monocytes from the indeterminate clinical form of Chagas disease. Immunobiology. (2012) 217:1005–16. 10.1016/j.imbio.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 23.Soares AK, Neves PA, Cavalcanti MD, Marinho SM, Oliveira WJ, Souza JR, et al. Expression of co-stimulatory molecules CD80 and CD86 is altered in CD14+ HLA-DR+ monocytes from patients with Chagas disease following induction by Trypanosoma cruzi recombinant antigens. Rev Soc Bras Med Trop. (2016) 49:632–6. 10.1590/0037-8682-0149-2016 [DOI] [PubMed] [Google Scholar]
- 24.Cuellar A, Santander SP, Thomas MD, Guzmán F, Gómez A, López MC, et al. Monocyte-derived dendritic cells from chagasic patients vs healthy donors secrete differential levels of IL-10 and IL-12 when stimulated with a protein fragment of Trypanosoma cruzi heat-shock protein-70. Immunol Cell Biol. (2008) 86:255–60. 10.1038/sj.icb.7100146 [DOI] [PubMed] [Google Scholar]
- 25.Gil-Jaramillo N, Motta FN, Favali CF, Bastos IM, Santana JM. Dendritic cells: a double-edged sword in immune responses during Chagas disease. Front Microbiol. (2016) 7:1076. 10.3389/fmicb.2016.01076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villalta F, Kierszenbaum F. Role of inflammatory cells in Chagas' disease. II. Interactions of mouse macrophages and human monocytes with intracellular forms of Trypanosoma cruzi: uptake and mechanism of destruction. J Immunol. (1984) 133:3338–43. [PubMed] [Google Scholar]
- 27.Melo RC, Fabrino DL, D'Avila H, Teixeira HC, Ferreira AP. Production of hydrogen peroxide by peripheral blood monocytes and specific macrophages during experimental infection with Trypanosoma cruzi in vivo. Cell Biol Int. (2003) 27:853–61. 10.1016/S1065-6995(03)00173-2 [DOI] [PubMed] [Google Scholar]
- 28.Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. (2016) 44:450–62. 10.1016/j.immuni.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction: a possible role for left ventricular remodeling. J Am Coll Cardiol. (2002) 39:241–6. 10.1016/S0735-1097(01)01721-1 [DOI] [PubMed] [Google Scholar]
- 30.Wrigley BJ, Lip GY, Shantsila E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail. (2011) 13:1161–71. 10.1093/eurjhf/hfr122 [DOI] [PubMed] [Google Scholar]
- 31.Anker SD, Egerer KR, Volk HD, Kox WJ, Poole-Wilson PA, Coats AJ. Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol. (1997) 79:1426–8. 10.1016/S0002-9149(97)00159-8 [DOI] [PubMed] [Google Scholar]
- 32.Conraads VM, Bosmans JM, Schuerwegh AJ, Goovaerts I, De Clerck LS, Stevens WJ, et al. Intracellular monocyte cytokine production and CD14 expression are up-regulated in severe vs mild chronic heart failure. J Hear Lung Transplant. (2005) 24:854–9. 10.1016/j.healun.2004.04.017 [DOI] [PubMed] [Google Scholar]
- 33.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. (2001) 103:2055–9. 10.1161/01.CIR.103.16.2055 [DOI] [PubMed] [Google Scholar]
- 34.Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. (1998) 31:391–8. 10.1016/S0735-1097(97)00494-4 [DOI] [PubMed] [Google Scholar]
- 35.Wong K, Yeap W, Tai J, Ong S, Dang T, Wong S. The three human monocyte subsets: implications for health and disease. Immunol Res. (2012) 53:41–57. 10.1007/s12026-012-8297-3 [DOI] [PubMed] [Google Scholar]
- 36.Soares G, Barral A, Costa JM, Barral-Netto M, Van Weyenbergh J. CD16+ monocytes in human cutaneous leishmaniasis: increased ex vivo levels and correlation with clinical data. J Leukoc Biol. (2006) 79:36–9. 10.1189/jlb.0105040 [DOI] [PubMed] [Google Scholar]
- 37.Passos S, Carvalho LP, Costa RS, Campos TM, Novais FO, Magalhães A, et al. Intermediate monocytes contribute to pathologic immune response in Leishmania braziliensis infections. J Infect Dis. (2015) 211:274–82. 10.1093/infdis/jiu439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antonelli LR, Leoratti FM, Costa PA, Rocha BC, Diniz SQ, Tada MS, et al. The CD14+CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria. PLoS Pathog. (2014) 10:e1004393. 10.1371/journal.ppat.1004393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tolouei Semnani R, Moore V, Bennuru S, McDonald-Fleming R, Ganesan S, Cotton R, et al. Human monocyte subsets at homeostasis and their perturbation in numbers and function in filarial infection. Infect Immun. (2014) 82:4438–46. 10.1128/IAI.01973-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandes JS, Araujo MI, Lopes DM, de Souza RP, Carvalho EM, Cardoso LS. Monocyte subsets in schistosomiasis patients with periportal fibrosis. Mediators Inflamm. (2014) 2014:1–12. 10.1155/2014/703653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. (2010) 116:5–7. 10.1182/blood-2010-02-258558 [DOI] [PubMed] [Google Scholar]
- 42.Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol. (2013) 4:23. 10.3389/fimmu.2013.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong KL, Tai JJ, Wong W, Han H, Sem X, Yeap W, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. (2011) 118:16–32. 10.1182/blood-2010-12-326355 [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Zhang L, Yu C, Yang X, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. (2014) 2:1–9. 10.1186/2050-7771-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magalhães LM, Viana A, Chiari E, Galvão LM, Gollob KJ, Dutra WO. Differential activation of human monocytes and lymphocytes by distinct strains of Trypanosoma cruzi. PLoS Negl Trop Dis. (2015) 9:e0003816. 10.1371/journal.pntd.0003816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sathler-Avelar R, Vitelli-Avelar DM, Massara RL, de Lana M, Pinto Dias JC, Teixeira-Carvalho A, et al. Etiological treatment during early chronic indeterminate Chagas disease incites an activated status on innate and adaptive immunity associated with a type 1-modulated cytokine pattern. Microbes Infect. (2008) 10:103–13. 10.1016/j.micinf.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 47.Vitelli-Avelar DM, Sathler-Avelar R, Massara RL, Borges JD, Lage PS, Lana M, et al. Are increased frequency of macrophage-like and natural killer (NK) cells, together with high levels of NKT and CD4+CD25high T cells balancing activated CD8+ T cells, the key to control Chagas' disease morbidity? Clin Exp Immunol. (2006) 145:81–92. 10.1111/j.1365-2249.2006.03123.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sathler-Avelar R, Lemos E, Reis D, Medrano-Mercado N, Araujo-Jorge T, Antas P, et al. Phenotypic features of peripheral blood leucocytes during early stages of human infection with Trypanosoma cruzi. Scand J Immunol. (2003) 58:655–63. 10.1111/j.1365-3083.2003.01340.x [DOI] [PubMed] [Google Scholar]
- 49.Pinto BF, Medeiros NI, Teixeira-Carvalho A, Eloi-Santos SM, Fontes-Cal TC, Rocha DA, et al. CD86 expression by monocytes influence an immunomodulatory profile in asymptomatic patients with chronic Chagas disease. Front Immunol. (2018) 9:454 10.3389/fimmu.2018.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Mazliah DE, Castro Eiro MD, Álvarez MG, Lococo B, Bertocchi G, César G, et al. Distinct monocyte subset phenotypes in patients with different clinical forms of chronic Chagas disease and seronegative dilated cardiomyopathy. PLoS Negl Trop Dis. (2018) 12:e0006887. 10.1371/journal.pntd.0006887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panesso-Gómez S, Pavia P, Rodríguez-Mantilla IE, Lasso P, Orozco LA, Cuellar A, et al. Trypanosoma cruzi detection in colombian patients with a diagnosis of esophageal Achalasia. Am J Trop Med Hyg. (2018) 98:717–23. 10.4269/ajtmh.17-0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zawada AM, Fell LH, Untersteller K, Seiler S, Rogacev KS, Fliser D, et al. Comparison of two different strategies for human monocyte subsets gating within the large-scale prospective CARE FOR HOMe Study. Cytom Part A. (2015) 87:750–8. 10.1002/cyto.a.22703 [DOI] [PubMed] [Google Scholar]
- 53.Barisione C, Garibaldi S, Ghigliotti G, Fabbi P, Altieri P, Casale MC, et al. CD14CD16 monocyte subset levels in heart failure patients. Dis Markers. (2010) 28:115–24. 10.1155/2010/236405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tapp LD, Shantsila E, Wrigley BJ, Pamukcu B, Lip GY. The CD14++CD16+ monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. J Thromb Haemost. (2012) 10:1231–41. 10.1111/j.1538-7836.2011.04603.x [DOI] [PubMed] [Google Scholar]
- 55.Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. (2009) 54:130–8. 10.1016/j.jacc.2009.04.021 [DOI] [PubMed] [Google Scholar]
- 56.Boyette LB, MacEdo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, et al. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE. (2017) 12:e0176460. 10.1371/journal.pone.0176460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bettelli E, Kuchroo VK. IL-12– and IL-23–induced T helper cell subsets: figure 1. J Exp Med. (2005) 201:169–71. 10.1084/jem.20042279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. (1997) 272:15036–42. 10.1074/jbc.272.23.15036 [DOI] [PubMed] [Google Scholar]
- 59.López L, Arai K, Giménez E, Jiménez M, Pascuzo C, Rodríguez-Bonfante C, et al. C-reactive protein and interleukin-6 serum levels increase as Chagas disease progresses towards cardiac failure. Rev Española Cardiol. (2006) 59:50–6. 10.1016/S1885-5857(06)60048-0 [DOI] [PubMed] [Google Scholar]
- 60.Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. (2011) 22:121–30. 10.1016/j.cytogfr.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aliberti J, Cardoso M, Martins G, Gazzinelli R, Vieira L, Silva J. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. (1996) 64:1961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graefe SE, Jacobs T, Gaworski I, Klauenberg U, Steeg C, Fleischer B. Interleukin-12 but not interleukin-18 is required for immunity to Trypanosoma cruzi in mice. Microbes Infect. (2003) 5:833–9. 10.1016/S1286-4579(03)00176-X [DOI] [PubMed] [Google Scholar]
- 63.Nogueira LG, Santos RH, Ianni BM, Fiorelli AI, Mairena EC, Benvenuti LA, et al. Myocardial chemokine expression and intensity of myocarditis in chagas cardiomyopathy are controlled by polymorphisms in CXCL9 and CXCL10. PLoS Negl Trop Dis. (2012) 6:e1867. 10.1371/journal.pntd.0001867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guedes PM, Gutierrez FR, Silva GK, Dellalibera-Joviliano R, Rodrigues GJ, Bendhack LM, et al. Deficient regulatory T cell activity and low frequency of IL-17-producing T cells correlate with the extent of cardiomyopathy in human Chagas' disease. PLoS Negl Trop Dis. (2012) 6:e1630. 10.1371/journal.pntd.0001630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodrigues AA, Saosa JSS, da Silva GK, Martins FA, da Silva AA, Souza Neto CP, et al. IFN-γ plays a unique role in protection against low virulent Trypanosoma cruzi strain. PLoS Negl Trop Dis. (2012) 6:e1598. 10.1371/journal.pntd.0001598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poveda C, Fresno M, Gironès N, Martins-Filho OA, Ramírez JD, Santi-Rocca J, et al. Cytokine profiling in Chagas disease: towards understanding the association with infecting Trypanosoma cruzi discrete typing units (A benefit trial sub-study). PLoS ONE. (2014) 9:e91154. 10.1371/journal.pone.0091154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Alba-Alvarado M, Salazar-Schettino PM, Jiménez-Álvarez L, Cabrera-Bravo M, García-Sancho C, Zenteno E, et al. Th-17 cytokines are associated with severity of Trypanosoma cruzi chronic infection in pediatric patients from endemic areas of Mexico. Acta Trop. (2018) 178:134–41. 10.1016/j.actatropica.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 68.Luz PR, Velavan TP, Kremsner PG, Messias-Reason IJ. Association of IP-10 and PDGF-BB levels with clinical forms of chronic Chagas disease. Int J Cardiol. (2013) 169:e53–5. 10.1016/j.ijcard.2013.08.110 [DOI] [PubMed] [Google Scholar]
- 69.de Paula Costa G, Lopes LR, da Silva MC, Horta AL, Pontes WM, Milanezi CM, et al. Doxycycline and benznidazole reduce the profile of Th1, Th2, and Th17 chemokines and chemokine receptors in cardiac tissue from chronic Trypanosoma cruzi-infected dogs. Mediators Inflamm. (2016) 2016:1–11. 10.1155/2016/3694714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cunha-Neto E, Dzau VJ, Allen PD, Stamatiou D, Benvenutti L, Higuchi ML, et al. Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas' disease cardiomyopathy. Am J Pathol. (2005) 167:305–13. 10.1016/S0002-9440(10)62976-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brownell J, Bruckner J, Wagoner J, Thomas E, Loo YM, Gale M, et al. Direct, interferon-independent activation of the CXCL10 promoter by NF- B and interferon regulatory factor 3 during hepatitis C virus infection. J Virol. (2014) 88:1582–90. 10.1128/JVI.02007-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gating strategy for 3-color flow cytometry with a sample from a representative CCC donor. Mononuclear cells were defined according to FSC vs. SSC parameters and gated on the singlets. HLA-DR+ population gate was determined based on the isotype control. On this population, it was defined the percentage of total monocytes (CD14+ CD16+ cells) and monocyte subsets according to CD14 and CD16 expression as follows: CD14++ CD16− (classical), CD14++ CD16+ (intermediate) and CD14+ CD16 ++ (non-classical).
Mean fluorescence intensity (MFI) for HLA-DR expression in monocyte subsets from IND, CCC, HTCC, NCC and HI. Significant differences determined by Kruskal-Wallis test followed by Dunn's post hoc test are shown as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Monocyte subsets and left ventricular ejection fraction (LVEF) variations associated with the time of heart transplantation and reactivation of T. cruzi infection. As shown for CCC16 and CCC18 patients, the percentages of classical monocytes increased and the percentage of intermediate monocytes diminished after heart transplantation, while IL-12p40 blood levels augmented in both patients. In the case of CCC21 patient, it was observed similar monocyte subsets changes upon antiparasitic treatment for parasite reactivation.